Climate Change Impacts and Extinction Risk Assessment of Nepeta Representatives (Lamiaceae) in Greece
Abstract
:1. Introduction
2. Materials and Methods
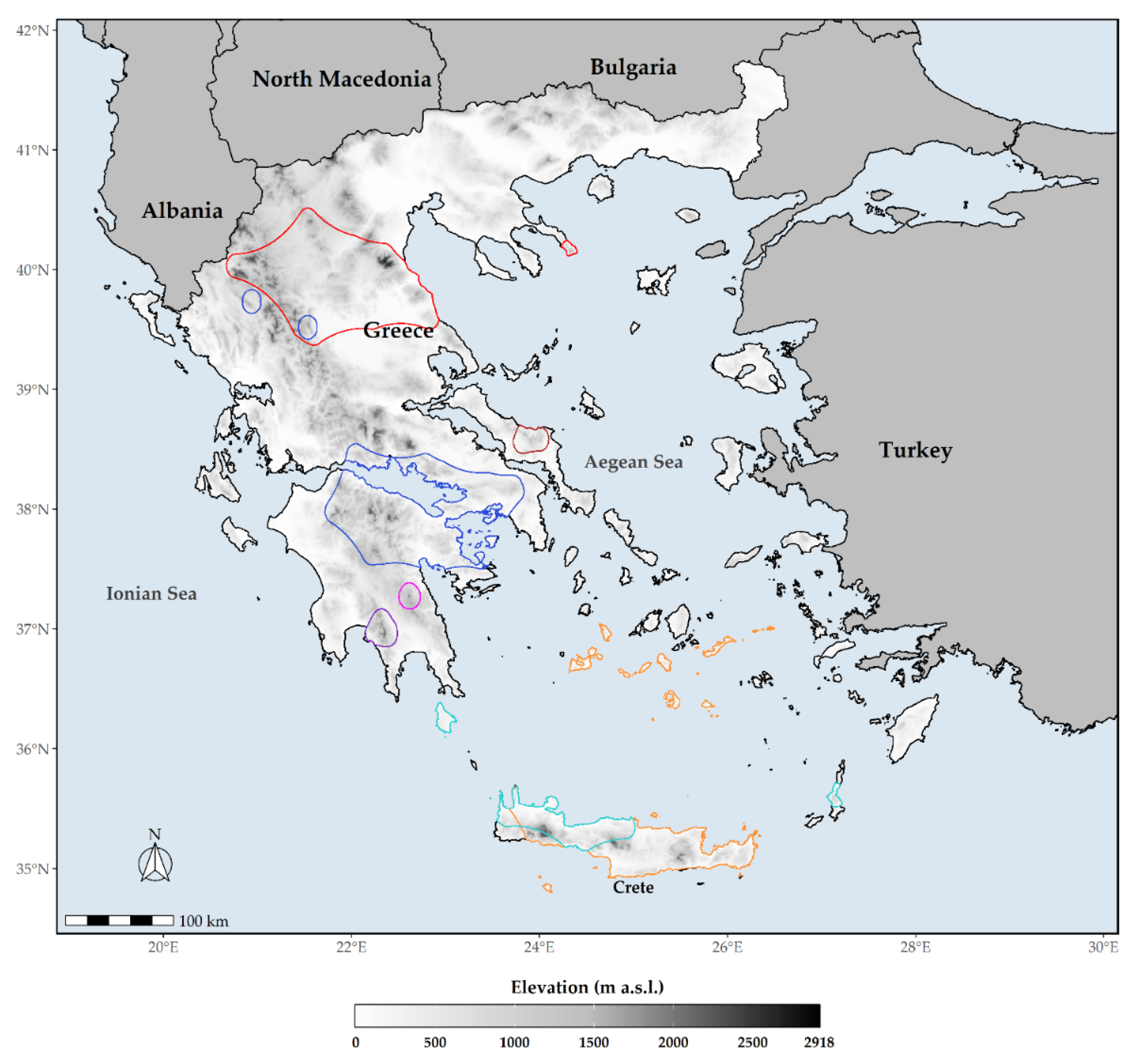
2.1. Species Occurrence Data
2.2. Environmental Data
2.3. Species Distribution Models
2.4. Identifying Biodiversity Hotspots
2.5. Latitudinal and Altitudinal Shifts of the biodiversity Hotspots
2.6. Future IUCN Extinction Risk Assessment
3. Results
3.1. Extent of Occurrence
3.2. Species Distribution Model Performance
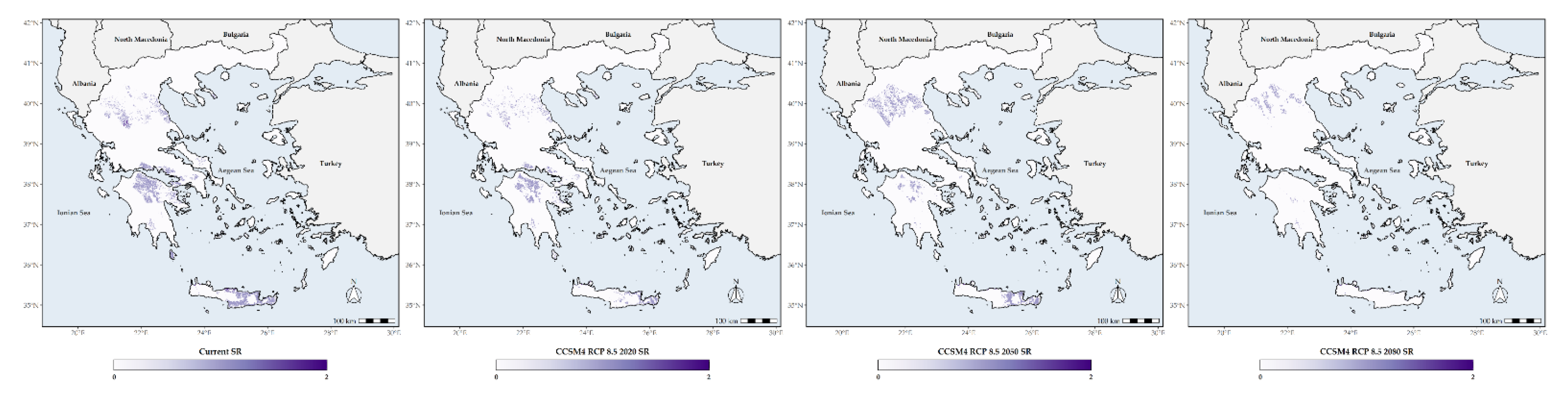
3.3. Habitat Suitability Range Change
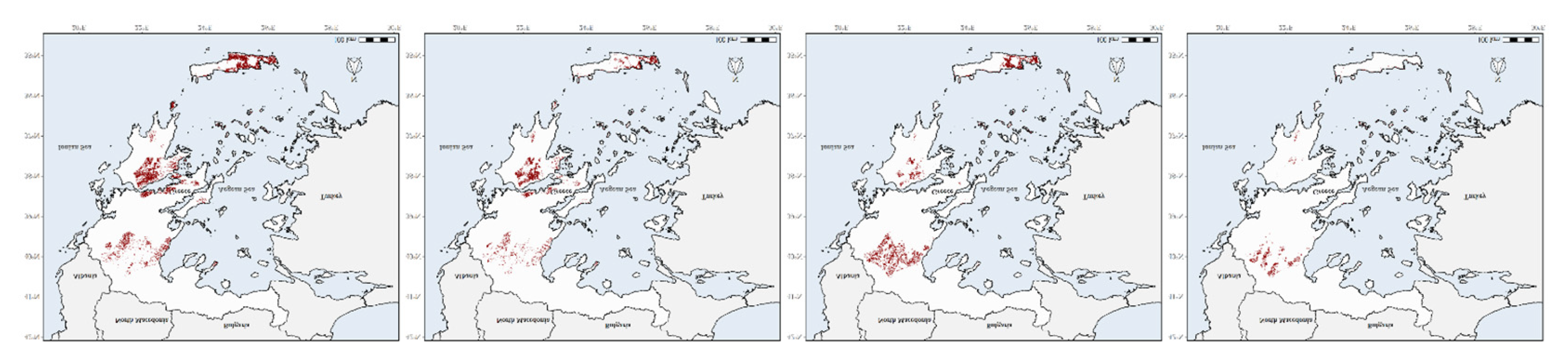
3.4. Species Richness Hotspots
3.5. Altitudinal and Latitudinal Shifts
3.6. IUCN Extinction Risk Assessment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lewis, O.T. Climate Change, Species-Area Curves and the Extinction Crisis. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 163–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thuiller, W.; Lavorel, S.; Araújo, M.B.; Sykes, M.T.; Prentice, I.C. Climate Change Threats to Plant Diversity in Europe. Proc. Natl. Acad. Sci. USA 2005, 102, 8245–8250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; Ferreira De Siqueira, M.; Grainger, A.; Hannah, L.; et al. Extinction Risk from Climate Change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L. Biological Consequences of Global Warming: Is the Signal Already Apparent? Trends Ecol. Evol. 2000, 15, 56–61. [Google Scholar] [CrossRef]
- Araújo, M.B.; New, M. and N.M. Ensemble Forecasting of Species Distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G.; Dawson, T.P. Predicting the Impacts of Climate Change on the Distribution of Species: Are Bioclimate Envelope Models Useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Thuiller, W. BIOMOD—Optimizing Predictions of Species Distributions and Projecting Potential Future Shifts under Global Change. Glob. Change Biol. 2003, 9, 1353–1362. [Google Scholar] [CrossRef]
- Thuiller, W.; Araújo, M.B.; Pearson, R.G.; Whittaker, R.J.; Brotons, L.; Lavorel, S. Uncertainty in Predictions of Extinction Risk. Nature 2004, 430, 34. [Google Scholar] [CrossRef] [PubMed]
- Keith, D.A.; Akçakaya, H.R.; Thuiller, W.; Midgley, G.F.; Pearson, R.G.; Phillips, S.J.; Regan, H.M.; Araújo, M.B.; Rebelo, T.G. Predicting Extinction Risks under Climate Change: Coupling Stochastic Population Models with Dynamic Bioclimatic Habitat Models. Biol. Lett. 2008, 4, 560–563. [Google Scholar] [CrossRef]
- Thuiller, W.; Albert, C.; Araújo, M.B.; Berry, P.M.; Cabeza, M.; Guisan, A.; Hickler, T.; Midgley, G.F.; Paterson, J.; Schurr, F.M.; et al. Predicting Global Change Impacts on Plant Species’ Distributions: Future Challenges. Perspect. Plant Ecol. Evol. Syst. 2008, 9, 137–152. [Google Scholar] [CrossRef]
- Hampe, A. Bioclimate Envelope Models: What They Detect and What They Hide. Glob. Ecol. Biogeogr. 2004, 13, 469–471. [Google Scholar] [CrossRef]
- Shoo, L.P.; Williams, S.E.; Hero, J.M. Potential Decoupling of Trends in Distribution Area and Population Size of Species with Climate Change. Glob. Change Biol. 2005, 11, 1469–1476. [Google Scholar] [CrossRef]
- Corlett, R.T. Safeguarding Our Future by Protecting Biodiversity. Plant Divers. 2020, 42, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Bachman, S.P.; Field, R.; Reader, T.; Raimondo, D.; Donaldson, J.; Schatz, G.E.; Lughadha, E.N. Progress, Challenges and Opportunities for Red Listing. Biol. Conserv. 2019, 234, 45–55. [Google Scholar] [CrossRef]
- Heywood, V.H. Plant Conservation in the Anthropocene—Challenges and Future Prospects. Plant Divers. 2017, 39, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Heywood, V.H. Conserving Plants within and beyond Protected Areas—Still Problematic and Future Uncertain. Plant Divers. 2019, 41, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Cheminal, A.; Kokkoris, I.P.; Strid, A.; Dimopoulos, P. Medicinal and Aromatic Lamiaceae Plants in Greece: Linking Diversity and Distribution Patterns with Ecosystem Services. Forests 2020, 11, 661. [Google Scholar] [CrossRef]
- Kotsiras, K.; Kokkoris, I.P.; Strid, A.; Dimopoulos, P. Integrating Plant Diversity Data into Mapping and Assessment of Ecosystem and Their Services (MAES) Implementation in Greece: Woodland and Forest Pilot. Forests 2020, 11, 956. [Google Scholar] [CrossRef]
- Kokkoris, I.P.; Mallinis, G.; Bekri, E.S.; Vlami, V.; Zogaris, S.; Chrysafis, I.; Mitsopoulos, I.; Dimopoulos, P. National Set of MAES Indicators in Greece: Ecosystem Services and Management Implications. Forests 2020, 11, 595. [Google Scholar] [CrossRef]
- Panitsa, M.; Kokkoris, I.P.; Kougioumoutzis, K.; Kontopanou, A.; Bazos, I.; Strid, A.; Dimopoulos, P. Linking Taxonomic, Phylogenetic and Functional Plant Diversity with Ecosystem Services of Cliffs and Screes in Greece. Plants 2021, 10, 992. [Google Scholar] [CrossRef]
- Rana, S.K.; Rana, H.K.; Ranjitkar, S.; Ghimire, S.K.; Gurmachhan, C.M.; O’Neill, A.R.; Sun, H. Climate-Change Threats to Distribution, Habitats, Sustainability and Conservation of Highly Traded Medicinal and Aromatic Plants in Nepal. Ecol. Indic. 2020, 115, 106435. [Google Scholar] [CrossRef]
- Kaky, E.; Nolan, V.; Alatawi, A.; Gilbert, F. A Comparison between Ensemble and MaxEnt Species Distribution Modelling Approaches for Conservation: A Case Study with Egyptian Medicinal Plants. Ecol. Inform. 2020, 60, 101150. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, P.; Lin, F.; Yang, W.; Gaisberger, H.; Christopher, K.; Zheng, Y. MaxEnt Modelling for Predicting the Potential Distribution of a near Threatened Rosewood Species (Dalbergia Cultrata Graham Ex Benth). Ecol. Eng. 2019, 141, 105612. [Google Scholar] [CrossRef]
- Peterson, M.L.; Doak, D.F.; Morris, W.F. Incorporating Local Adaptation into Forecasts of Species’ Distribution and Abundance under Climate Change. Glob. Change Biol. 2019, 25, 775–793. [Google Scholar]
- Yi, Y.; Cheng, X.; Yang, Z.F.; Zhang, S.H. Maxent Modeling for Predicting the Potential Distribution of Endangered Medicinal Plant (H. Riparia Lour) in Yunnan, China. Ecol. Eng. 2016, 92, 260–269. [Google Scholar] [CrossRef]
- Thuiller, W.; Georges, D.; Engler, R. Biomod2: Ensemble Platform for Species Distribution Modeling. R Package Version 3.5.1. 2021. Available online: https://cran.r-project.org/web/packages/biomod2/index.html (accessed on 11 June 2021).
- Elkholy, M.; Mansour, M.; Omar, K. Genetic Variability of Nepeta Septemcrenata Benth. (Lamiaceae) Assessed by RAPD Markers. N. Y. Sci. J. 2011, 4, 97–105. [Google Scholar]
- Pressey, R.L.; Mills, M.; Weeks, R.; Day, J.C. The Plan of the Day: Managing the Dynamic Transition from Regional Conservation Designs to Local Conservation Actions. Biol. Conserv. 2013, 166, 155–169. [Google Scholar] [CrossRef]
- Pfab, M.F.; Victor, J.E.; Armstrong, A.J. Application of the IUCN Red Listing System to Setting Species Targets for Conservation Planning Purposes. Biodivers. Conserv. 2011, 20, 1001–1012. [Google Scholar] [CrossRef]
- Canturk, U.; Kulaç, Ş. The Effects of Climate Change Scenarios on Tilia Ssp. in Turkey. Environ. Monit. Assess. 2021, 193, 771. [Google Scholar] [CrossRef] [PubMed]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Strid, A.; Dimopoulos, P. Extinction Risk Assessment of the Greek Endemic Flora. Biology 2021, 10, 195. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Trigas, P.; Strid, A.; Dimopoulos, P. Spatial Phylogenetics, Biogeographical Patterns and Conservation Implications of the Endemic Flora of Crete (Aegean, Greece) under Climate Change Scenarios. Biology 2020, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Trigas, P.; Strid, A.; Dimopoulos, P. Plant Diversity Patterns and Conservation Implications under Climate-Change Scenarios in the Mediterranean: The Case of Crete (Aegean, Greece). Diversity 2020, 12, 270. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Kallimanis, A.; Strid, A.; Dimopoulos, P. Plant Endemism Centres and Biodiversity Hotspots in Greece. Biology 2021, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Jamzad, Z.; Chase, M.W.; Ingrouille, M.; Simmonds, M.S.J.; Jalili, A. Phylogenetic Relationships in Nepeta L. (Lamiaceae) and Related Genera Based on ITS Sequence Data. Taxon 2003, 52, 21. [Google Scholar] [CrossRef]
- Talebi, S.M.; Nohooji, M.G.; Yarmohammadi, M.; Azizi, N.; Matsyura, A. Trichomes Morphology and Density Analysis in Some Nepeta Species of Iran. Mediterr. Bot. 2018, 39, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Applequist, W.L.; Brinckmann, J.A.; Cunningham, A.B.; Hart, R.E.; Heinrich, M.; Katerere, D.R.; van Andel, T. Erratum: Scientistsʼ Warning on Climate Change and Medicinal Plants. Planta Med. 2020, 86, 10–18. [Google Scholar] [CrossRef]
- Süntar, I.; Nabavi, S.M.; Barreca, D.; Fischer, N.; Efferth, T. Pharmacological and Chemical Features of Nepeta L. Genus: Its Importance as a Therapeutic Agent. Phytother. Res. 2018, 32, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.H.; Alia, A.; Kumar, B.; Mubashir, S. Phytochemical Constituents of Genus Nepeta. Res. Rev. J. Chem. 2018, 7, 31–37. [Google Scholar]
- Sharma, A.; Nayik, G.A.; Cannoo, D.S. Pharmacology and Toxicology of Nepeta Cataria (Catmint) Species of Genus Nepeta: A Review. In Plant and Human Health: Pharmacology and Therapeutic Uses; Springer International Publishing: New York, NY, USA, 2019; Volume 3. [Google Scholar]
- Hadi, N.; Sefidkon, F.; Shojaeiyan, A.; Šiler, B.; Jafari, A.A.; Aničić, N.; Mišić, D. Phenolics’ Composition in Four Endemic Nepeta Species from Iran Cultivated under Experimental Field Conditions: The Possibility of the Exploitation of Nepeta Germplasm. Ind. Crops Prod. 2017, 95, 475–484. [Google Scholar] [CrossRef]
- Tzakou, O.; Harvala, C.; Galati, E.M.; Sanogo, R. Essential Oil Composition of Nepeta Argolica Bory et Chaub. Subsp. Argolica. Flavour Fragr. J. 2000, 15, 115–118. [Google Scholar] [CrossRef]
- Skaltsa, H.D.; Lazari, D.M.; Loukis, A.E.; Constantinidis, T. Essential Oil Analysis of Nepeta Argolica Bory and Chaub. Subsp. Argolica (Lamiaceae) Growing Wild in Greece. Flavour Fragr. J. 2000, 15, 96–99. [Google Scholar] [CrossRef]
- Kalpoutzakis, E.; Aligiannis, N.; Mentis, A.; Mitaku, S.; Charvala, C. Composition of the Essential Oil of Two Nepeta Species and in Vitro Evaluation of Their Activity against Helicobacter Pylori. Planta Med. 2001, 67, 880–883. [Google Scholar] [CrossRef] [PubMed]
- Darras, A.I.; Spiliopoulos, I.; Kartsonas, E.; Assimomitis, P.; Karras, S. Antioxidant Profile, Propagation and Cultivation of Nepeta Camphorata, the Endemic Species of Mt Taygetos (Greece). S. Afr. J. Bot. 2020, 131, 391–397. [Google Scholar] [CrossRef]
- Hanlidou, E.; Karousou, R.; Lazari, D. Essential Oils of Three Taxa of the Nepeta Argolica Aggregate from Greece. Chem. Biodivers. 2012, 9, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Mišić, D.; Šiler, B.; Gašić, U.; Avramov, S.; Živković, S.; Živković, J.N.; Milutinović, M.; Tešić, Ž. Simultaneous UHPLC/DAD/(+/−)HESI-MS/MS Analysis of Phenolic Acids and Nepetalactones in Methanol Extracts of Nepeta Species: A Possible Application in Chemotaxonomic Studies. Phytochem. Anal. 2015, 26, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Nestorović, J.; Mišić, D.; Šiler, B.; Soković, M.; Glamočlija, J.; Ćirić, A.; Maksimović, V.; Grubišić, D. Nepetalactone Content in Shoot Cultures of Three Endemic Nepeta Species and the Evaluation of Their Antimicrobial Activity. Fitoterapia 2010, 81, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Bedoya, L.M.; Sanchez Palomino, S.; Abad, M.J.; Bermejo, P.; Alcami, J. Screening of Selected Plant Extracts for In Vitro Inhibitory Activity on Human Immunodeficiency Virus. Phytother. Res. 2002, 16, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Rigano, D.; Arnold, N.A.; Conforti, F.; Menichini, F.; Formisano, C.; Piozzi, F.; Senatore, F. Characterisation of the Essential Oil of Nepeta Glomerata Montbret et Aucher Ex Bentham from Lebanon and Its Biological Activities. Nat. Prod. Res. 2011, 25, 614–626. [Google Scholar] [CrossRef]
- Miceli, N.; Taviano, M.F.; Giuffrida, D.; Trovato, A.; Tzakou, O.; Galati, E.M. Anti-Inflammatory Activity of Extract and Fractions from Nepeta Sibthorpii Bentham. J. Ethnopharmacol. 2005, 97, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.M.; de Oliveira, G.; Barreto, B.d.S.; Nabout, J.C.; Rangel, T.F.L.V.B.; Diniz-Filho, J.A.F. Effects of Global Climate Changes on Geographical Distribution Patterns of Economically Important Plant Species in Cerrado. Rev. Árvore 2013, 37, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Gairola, S.; Shariff, N.M.; Bhatt, A.; Kala, C.P. Influence of Climate Change on Production of Secondary Chemicals in High Altitude Medicinal Plants: Issues Needs Immediate Attention. Journal of Medicinal Plants Research 2010, 4, 1825–1829. [Google Scholar]
- Nepeta, B.C. Mountain Flora of Greece; Strid, A., Tan, K., Eds.; Edinburgh University Press: Edinburgh, Scotland, 1991; Volume 2. [Google Scholar]
- Baden, C. Biosystematic Studies in the Nepeta sibthorpii Group (Lamiaceae) in Greece. Opera Bot. 1987, 93, 1–54. [Google Scholar]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular Plants of Greece: An Annotated Checklist; Botanischer Garten und Botanisches Museum Berlin-Dahlem: Berlin, Germany, 2013; pp. 1–371. [Google Scholar]
- Robertson, M.P.; Visser, V.; Hui, C. Biogeo: An R Package for Assessing and Improving Data Quality of Occurrence Record Datasets. Ecography 2016, 39, 394–401. [Google Scholar] [CrossRef] [Green Version]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. SpThin: An R Package for Spatial Thinning of Species Occurrence Records for Use in Ecological Niche Models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- van Proosdij, A.S.J.; Sosef, M.S.M.; Wieringa, J.J.; Raes, N. Minimum Required Number of Specimen Records to Develop Accurate Species Distribution Models. Ecography 2016, 39, 542–552. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Hamann, A.; Wang, T.; Spittlehouse, D.L.; Murdock, T.Q. A Comprehensive, High-Resolution Database of Historical and Projected Climate Surfaces for Western North America. Bull. Am. Meteorol. Soc. 2013, 94, 1307–1309. [Google Scholar] [CrossRef]
- Marchi, M.; Castellanos-Acuña, D.; Hamann, A.; Wang, T.; Ray, D.; Menzel, A. ClimateEU, Scale-Free Climate Normals, Historical Time Series, and Future Projections for Europe. Sci. Data 2020, 7, 428. [Google Scholar] [CrossRef]
- Wang, T.; Hamann, A.; Spittlehouse, D.L.; Murdock, T.Q. ClimateWNA-High-Resolution Spatial Climate Data for Western North America. J. Appl. Meteorol. Climatol. 2012, 51, 16–29. [Google Scholar] [CrossRef] [Green Version]
- Hijmans, R.J. Package ‘Raster’—Geographic Data Analysis and Modeling. CRAN Repos. 2019. Available online: https://cran.r-project.org/web/packages/raster/index.html (accessed on 22 January 2022).
- Title, P.O.; Bemmels, J.B. ENVIREM: An Expanded Set of Bioclimatic and Topographic Variables Increases Flexibility and Improves Performance of Ecological Niche Modeling. Ecography 2018, 41, 291–307. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.S.; Murphy, M.; Ram, K. Spatial Eco. R Package Version 1.2-0; R Core Team: Vienna, Austria, 2019. [Google Scholar]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where Is Positional Uncertainty a Problem for Species Distribution Modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The Art of Modelling Range-Shifting Species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Breiner, F.T.; Guisan, A.; Bergamini, A.; Nobis, M.P. Overcoming Limitations of Modelling Rare Species by Using Ensembles of Small Models. Methods Ecol. Evol. 2015, 6, 1210–1218. [Google Scholar] [CrossRef]
- Breiner, F.T.; Guisan, A.; Nobis, M.P.; Bergamini, A. Including Environmental Niche Information to Improve IUCN Red List Assessments. Divers. Distrib. 2017, 23, 484–495. [Google Scholar] [CrossRef] [Green Version]
- Breiner, F.T.; Nobis, M.P.; Bergamini, A.; Guisan, A. Optimizing Ensembles of Small Models for Predicting the Distribution of Species with Few Occurrences. Methods Ecol. Evol. 2018, 9, 802–808. [Google Scholar] [CrossRef] [Green Version]
- di Cola, V.; Broennimann, O.; Petitpierre, B.; Breiner, F.T.; D’Amen, M.; Randin, C.; Engler, R.; Pottier, J.; Pio, D.; Dubuis, A.; et al. Ecospat: An R Package to Support Spatial Analyses and Modeling of Species Niches and Distributions. Ecography 2017, 40, 774–787. [Google Scholar] [CrossRef]
- Dauby, G.; Stévart, T.; Droissart, V.; Cosiaux, A.; Deblauwe, V.; Simo-Droissart, M.; Sosef, M.S.M.; Lowry, P.P.; Schatz, G.E.; Gereau, R.E.; et al. ConR: An R Package to Assist Large-Scale Multispecies Preliminary Conservation Assessments Using Distribution Data. Ecol. Evol. 2017, 7, 11292–11303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, L.; Diniz-Filho, J.A.F.; Lohmann, L.G. A Comparison of Hull Methods for Estimating Species Ranges and Richness Maps. Plant Ecol. Divers. 2017, 10, 389–401. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting Pseudo-Absences for Species Distribution Models: How, Where and How Many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Liu, C.; Newell, G.; White, M. The Effect of Sample Size on the Accuracy of Species Distribution Models: Considering Both Presences and Pseudo-Absences or Background Sites. Ecography 2019, 42, 535–548. [Google Scholar] [CrossRef] [Green Version]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the Accuracy of Species Distribution Models: Prevalence, Kappa and the True Skill Statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A Review of Methods for the Assessment of Prediction Errors in Conservation Presence/Absence Models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Hirzel, A.H.; le Lay, G.; Helfer, V.; Randin, C.; Guisan, A. Evaluating the Ability of Habitat Suitability Models to Predict Species Presences. Ecol. Model. 2006, 199, 142–152. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Measuring and Comparing the Accuracy of Species Distribution Models with Presence-Absence Data. Ecography 2011, 34, 232–243. [Google Scholar] [CrossRef]
- Sofaer, H.R.; Hoeting, J.A.; Jarnevich, C.S. The Area under the Precision-Recall Curve as a Performance Metric for Rare Binary Events. Methods Ecol. Evol. 2019, 10, 565–577. [Google Scholar] [CrossRef]
- Broennimann, O.; di Cola, V.; Petitpierre, B.; Breiner, F.; Scherrer, D.; D’Amen, M.; Randin, C.; Engler, R.; Hordijk, W.; Mod, H.; et al. Ecospat: Spatial Ecology Miscellaneous Methods. Package “Ecospat”. R Package Version 3.0. 2018. Available online: https://www.unil.ch/ecospat/home/menuguid/ecospat-resources/tools.html (accessed on 7 January 2022).
- Hamner, B.; Frasco, M.; LeDell, E. Metrics: Evaluation Metrics for Machine Learning. R Package Version 0.1.4. 2018. Available online: https://cran.r-project.org/web/packages/Metrics/index.html (accessed on 9 July 2018).
- Márcia Barbosa, A.; Real, R.; Muñoz, A.R.; Brown, J.A. New Measures for Assessing Model Equilibrium and Prediction Mismatch in Species Distribution Models. Divers. Distrib. 2013, 19, 1333–1338. [Google Scholar] [CrossRef]
- Schwarz, J.; Heider, D. GUESS: Projecting Machine Learning Scores to Well-Calibrated Probability Estimates for Clinical Decision-Making. Bioinformatics 2019, 35, 2458–2465. [Google Scholar] [CrossRef]
- Signorelli, A. DescTools: Tools for Descriptive Statistics. R package Version 0.99.44. 2021. Available online: https://cran.r-project.org/package=DescTools (accessed on 23 November 2021).
- Smith, A.B. Enmsdm: Tools for Modeling Species Niches and Distributions; R Package Version 0.5.1.5; CRAN, R Core Team: Cary, CA, USA, 2020. [Google Scholar]
- Yan, Y. MLmetrics: Machine Learning Evaluation Metrics. R Package Version 1.1.1. 2016. Available online: https://CRAN.R-project.org/package=MLmetrics (accessed on 13 May 2016).
- Raes, N.; ter Steege, H. A Null-Model for Significance Testing of Presence-Only Species Distribution Models. Ecography 2007, 30, 727–736. [Google Scholar] [CrossRef]
- Araújo, M.B.; Anderson, R.P.; Barbosa, A.M.; Beale, C.M.; Dormann, C.F.; Early, R.; Garcia, R.A.; Guisan, A.; Maiorano, L.; Naimi, B.; et al. Standards for Distribution Models in Biodiversity Assessments. Sci. Adv. 2019, 5, eaat4858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Berry, P.M.; Dawson, T.P.; Pearson, R.G. Selecting Thresholds of Occurrence in the Prediction of Species Distributions. Ecography 2005, 28, 385–393. [Google Scholar] [CrossRef]
- Liu, C.; Newell, G.; White, M. On the Selection of Thresholds for Predicting Species Occurrence with Presence-Only Data. Ecol. Evol. 2016, 6, 337–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; White, M.; Newell, G. Selecting Thresholds for the Prediction of Species Occurrence with Presence-Only Data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A Platform for Ensemble Forecasting of Species Distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Daru, B.H.; Karunarathne, P.; Schliep, K. Phyloregion: R Package for Biogeographical Regionalization and Macroecology. Methods Ecol. Evol. 2020, 11, 1483–1491. [Google Scholar] [CrossRef]
- Daru, B.H.; Farooq, H.; Antonelli, A.; Faurby, S. Endemism Patterns Are Scale Dependent. Nat. Commun. 2020, 11, 2115. [Google Scholar] [CrossRef] [PubMed]
- Daru, B.H.; Elliott, T.L.; Park, D.S.; Davies, T.J. Understanding the Processes Underpinning Patterns of Phylogenetic Regionalization. Trends Ecol. Evol. 2017, 32, 845–860. [Google Scholar]
- Cañadas, E.M.; Fenu, G.; Peñas, J.; Lorite, J.; Mattana, E.; Bacchetta, G. Hotspots within Hotspots: Endemic Plant Richness, Environmental Drivers, and Implications for Conservation. Biol. Conserv. 2014, 170, 282–291. [Google Scholar] [CrossRef]
- Stévart, T.; Dauby, G.; Lowry, P.; Blach-Overgaard, A.; Droissart, V.; Harris, D.J.; Mackinder, A.B.; Schatz, G.E.; Sonké, B.; Sosef, M.S.M.; et al. A Third of the Tropical African Flora Is Potentially Threatened with Extinction. Sci. Adv. 2019, 5, eaax9444. [Google Scholar] [CrossRef] [Green Version]
- Qian, J.; Zhuang, H.; Yang, W.; Chen, Y.; Chen, S.; Qu, Y.; Zhang, Y.; Yang, Y.; Wang, Y. Selecting Flagship Species to Solve a Biodiversity Conservation Conundrum. Plant Divers. 2020, 42, 488–491. [Google Scholar] [CrossRef] [PubMed]
- Hassanpouraghdam, M.B.; Ghorbani, H.; Esmaeilpour, M.; Alford, M.H.; Strzemski, M.; Dresler, S. Diversity and Distribution Patterns of Endemic Medicinal and Aromatic Plants of Iran: Implications for Conservation and Habitat Management. Int. J. Environ. Res. Public Health 2022, 19, 1552. [Google Scholar] [CrossRef] [PubMed]
- Abolmaali, S.M.R.; Tarkesh, M.; Bashari, H. MaxEnt Modeling for Predicting Suitable Habitats and Identifying the Effects of Climate Change on a Threatened Species, Daphne Mucronata, in Central Iran. Ecol. Inform. 2018, 43, 116–123. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, H.; Wang, H. Potential Geographical Distribution of Populus Euphratica in China under Future Climate Change Scenarios Based on Maxent Model. Shengtai Xuebao 2020, 40, 6552–6563. [Google Scholar] [CrossRef]
- Han, L.; Zuo, Y.; He, X.; Hou, Y.; Li, M.; Li, B. Plant Identity and Soil Variables Shift the Colonisation and Species Composition of Dark Septate Endophytes Associated with Medicinal Plants in a Northern Farmland in China. Appl. Soil Ecol. 2021, 167, 104042. [Google Scholar] [CrossRef]
- Fahimirad, S.; Hatami, M. Heavy Metal-Mediated Changes in Growth and Phytochemicals of Edible and Medicinal Plants. In Medicinal Plants and Environmental Challenges; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar]
- Soilhi, Z.; Sayari, N.; Benalouache, N.; Mekki, M. Predicting Current and Future Distributions of Mentha Pulegium L. in Tunisia under Climate Change Conditions, Using the MaxEnt Model. Ecol. Inform. 2022, 68, 101533. [Google Scholar] [CrossRef]
- Borges, C.V.; Minatel, I.O.; Gomez-Gomez, H.A.; Lima, G.P.P. Medicinal Plants: Influence of Environmental Factors on the Content of Secondary Metabolites. In Medicinal Plants and Environmental Challenges; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar]
- Maikhuri, R.K.; Phondani, P.C.; Dhyani, D.; Rawat, L.S.; Jha, N.K.; Kandari, L.S. Assessment of Climate Change Impacts and Its Implications on Medicinal Plants-Based Traditional Healthcare System in Central Himalaya, India. Iran. J. Sci. Technol. Trans. A Sci. 2018, 42, 1827–1835. [Google Scholar] [CrossRef]
- Zhao, G.; Mu, X.; Jiao, J.; Gao, P.; Sun, W.; Li, E.; Wei, Y.; Huang, J. Assessing Response of Sediment Load Variation to Climate Change and Human Activities with Six Different Approaches. Sci. Total Environ. 2018, 639, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Charitonidou, M.; Kougioumoutzis, K.; Halley, J.M. An Orchid in Retrograde: Climate-Driven Range Shift Patterns of Ophrys Helenae in Greece. Plants 2021, 10, 470. [Google Scholar] [CrossRef] [PubMed]
- Fassou, G.; Kougioumoutzis, K.; Iatrou, G.; Trigas, P.; Papasotiropoulos, V. Genetic Diversity and Range Dynamics of Helleborus Odorus Subsp. Cyclophyllus under Different Climate Change Scenarios. Forests 2020, 11, 620. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Strid, A.; Raus, T.; Dimopoulos, P. Climate-Change Impacts on the Southernmost Mediterranean Arctic-Alpine Plant Populations. Sustainability 2021, 13, 13778. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kotsakiozi, P.; Stathi, E.; Trigas, P.; Parmakelis, A. Conservation Genetics of Four Critically Endangered Greek Endemic Plants: A Preliminary Assessment. Diversity 2021, 13, 152. [Google Scholar] [CrossRef]
- Stathi, E.; Kougioumoutzis, K.; Abraham, E.M.; Trigas, P.; Ganopoulos, I.; Avramidou, E.V.; Tani, E. Population Genetic Variability and Distribution of the Endangered Greek Endemic Cicer Graecum under Climate Change Scenarios. AoB Plants 2021, 12, plaa007. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.A.; Angueyra, A.; Cleef, A.M.; van Andel, T. Ethnobotany of the Sierra Nevada Del Cocuy-Güicán: Climate Change and Conservation Strategies in the Colombian Andes. J. Ethnobiol. Ethnomedicine 2018, 14, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erfanian, M.B.; Sagharyan, M.; Memariani, F.; Ejtehadi, H. Predicting Range Shifts of Three Endangered Endemic Plants of the Khorassan-Kopet Dagh Floristic Province under Global Change. Sci. Rep. 2021, 11, 9159. [Google Scholar] [CrossRef]
- Schlaepfer, D.R.; Braschler, B.; Rusterholz, H.P.; Baur, B. Genetic Effects of Anthropogenic Habitat Fragmentation on Remnant Animal and Plant Populations: A Meta-Analysis. Ecosphere 2018, 9, e02488. [Google Scholar] [CrossRef]
- Young, A.; Boyle, T.; Brown, T. The Population Genetic Consequences of Habitat Fragmentation for Plants. Trends Ecol. Evol. 1996, 11, 413–418. [Google Scholar] [CrossRef]
- Lee, S.-R.; Choi, J.-E.; Lee, B.-Y.; Yu, J.-N.; Lim, C.E. Genetic Diversity and Structure of an Endangered Medicinal Herb: Implications for Conservation. AoB Plants 2018, 10, ply021. [Google Scholar] [CrossRef] [PubMed]
- Rathore, P.; Roy, A.; Karnatak, H. Predicting the Future of Species Assemblages under Climate and Land Use Land Cover Changes in Himalaya: A Geospatial Modelling Approach. Clim. Change Ecol. 2022, 3, 100048. [Google Scholar] [CrossRef]
- Fois, M.; Cuena-Lombraña, A.; Fenu, G.; Bacchetta, G. Using Species Distribution Models at Local Scale to Guide the Search of Poorly Known Species: Review, Methodological Issues and Future Directions. Ecol. Model. 2018, 385, 124–132. [Google Scholar] [CrossRef] [Green Version]
- Fois, M.; Bacchetta, G.; Cogoni, D.; Fenu, G. Current and Future Effectiveness of the Natura 2000 Network for Protecting Plant Species in Sardinia: A Nice and Complex Strategy in Its Raw State? J. Environ. Plan. Manag. 2018, 61, 332–347. [Google Scholar] [CrossRef]
- Kopsidis, M.; Ivanov, M. Industrialization and De-Industrialization in Southeast Europe, 1870–2010. In The Spread of Modern Industry to the Periphery Since 1871; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Tsani, S.Z. Energy Consumption and Economic Growth: A Causality Analysis for Greece. Energy Economics 2010, 32, 582–590. [Google Scholar] [CrossRef]
- Wang, P.; Yu, P.; Lu, J.; Zhang, Y. The Mediation Effect of Land Surface Temperature in the Relationship between Land Use-Cover Change and Energy Consumption under Seasonal Variations. J. Clean. Prod. 2022, 340, 130804. [Google Scholar] [CrossRef]
- Battisti, C. Unifying the trans-disciplinary arsenal of project management tools in a single logical framework: Further suggestion for IUCN project cycle development. J. Nat. Conserv. 2018, 41, 63–72. [Google Scholar] [CrossRef]
- Cahyaningsih, R.; Phillips, J.; Magos Brehm, J.; Gaisberger, H.; Maxted, N. Climate Change Impact on Medicinal Plants in Indonesia. Glob. Ecol. Conserv. 2021, 30, e01752. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kougioumoutzis, K.; Papanikolaou, A.; Kokkoris, I.P.; Strid, A.; Dimopoulos, P.; Panitsa, M. Climate Change Impacts and Extinction Risk Assessment of Nepeta Representatives (Lamiaceae) in Greece. Sustainability 2022, 14, 4269. https://doi.org/10.3390/su14074269
Kougioumoutzis K, Papanikolaou A, Kokkoris IP, Strid A, Dimopoulos P, Panitsa M. Climate Change Impacts and Extinction Risk Assessment of Nepeta Representatives (Lamiaceae) in Greece. Sustainability. 2022; 14(7):4269. https://doi.org/10.3390/su14074269
Chicago/Turabian StyleKougioumoutzis, Konstantinos, Alexandros Papanikolaou, Ioannis P. Kokkoris, Arne Strid, Panayotis Dimopoulos, and Maria Panitsa. 2022. "Climate Change Impacts and Extinction Risk Assessment of Nepeta Representatives (Lamiaceae) in Greece" Sustainability 14, no. 7: 4269. https://doi.org/10.3390/su14074269
APA StyleKougioumoutzis, K., Papanikolaou, A., Kokkoris, I. P., Strid, A., Dimopoulos, P., & Panitsa, M. (2022). Climate Change Impacts and Extinction Risk Assessment of Nepeta Representatives (Lamiaceae) in Greece. Sustainability, 14(7), 4269. https://doi.org/10.3390/su14074269







