Contribution of the Order Ericales to Improving Paleoclimate Reconstructions
Abstract
:1. Introduction
2. Materials and Methods
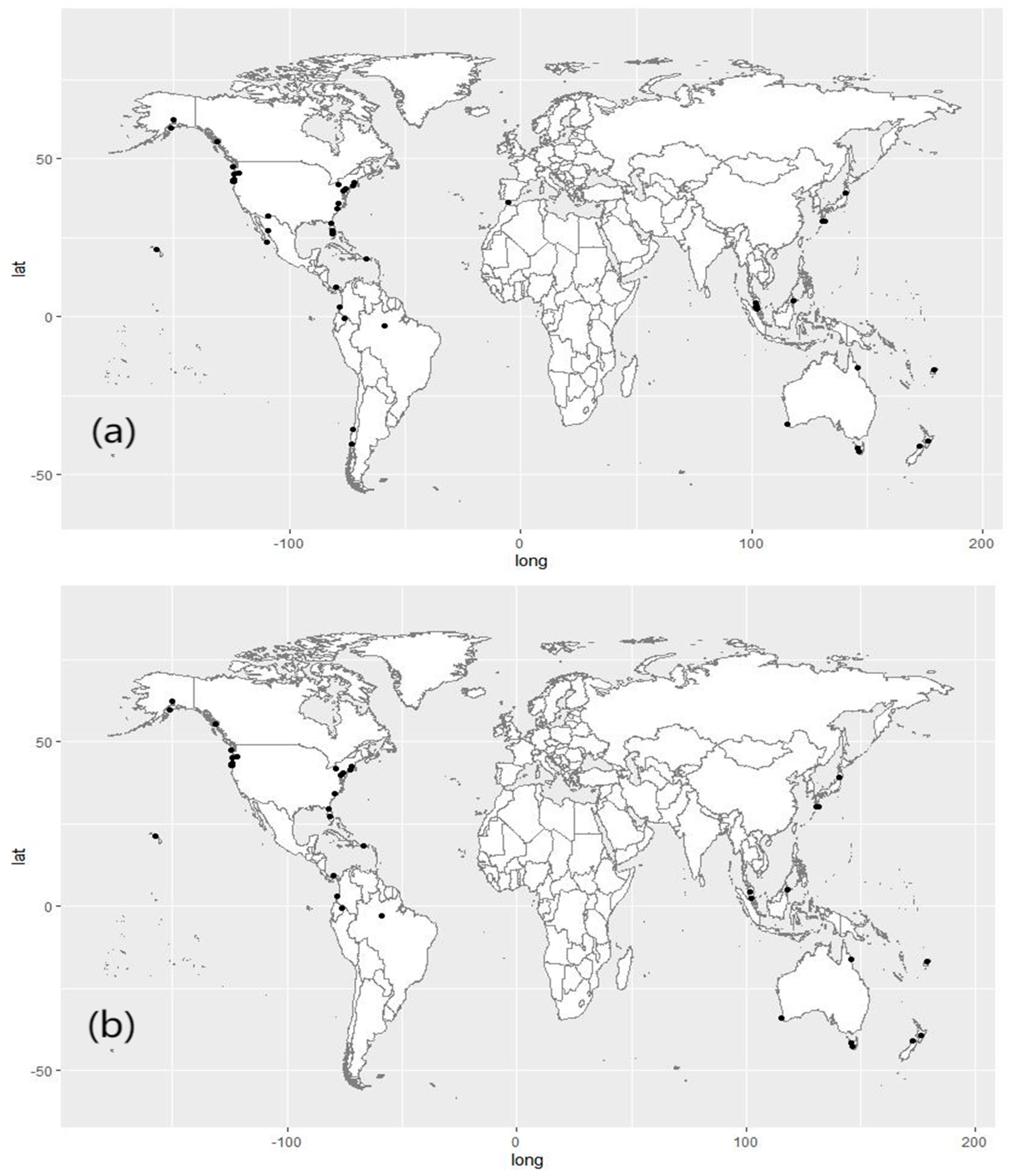
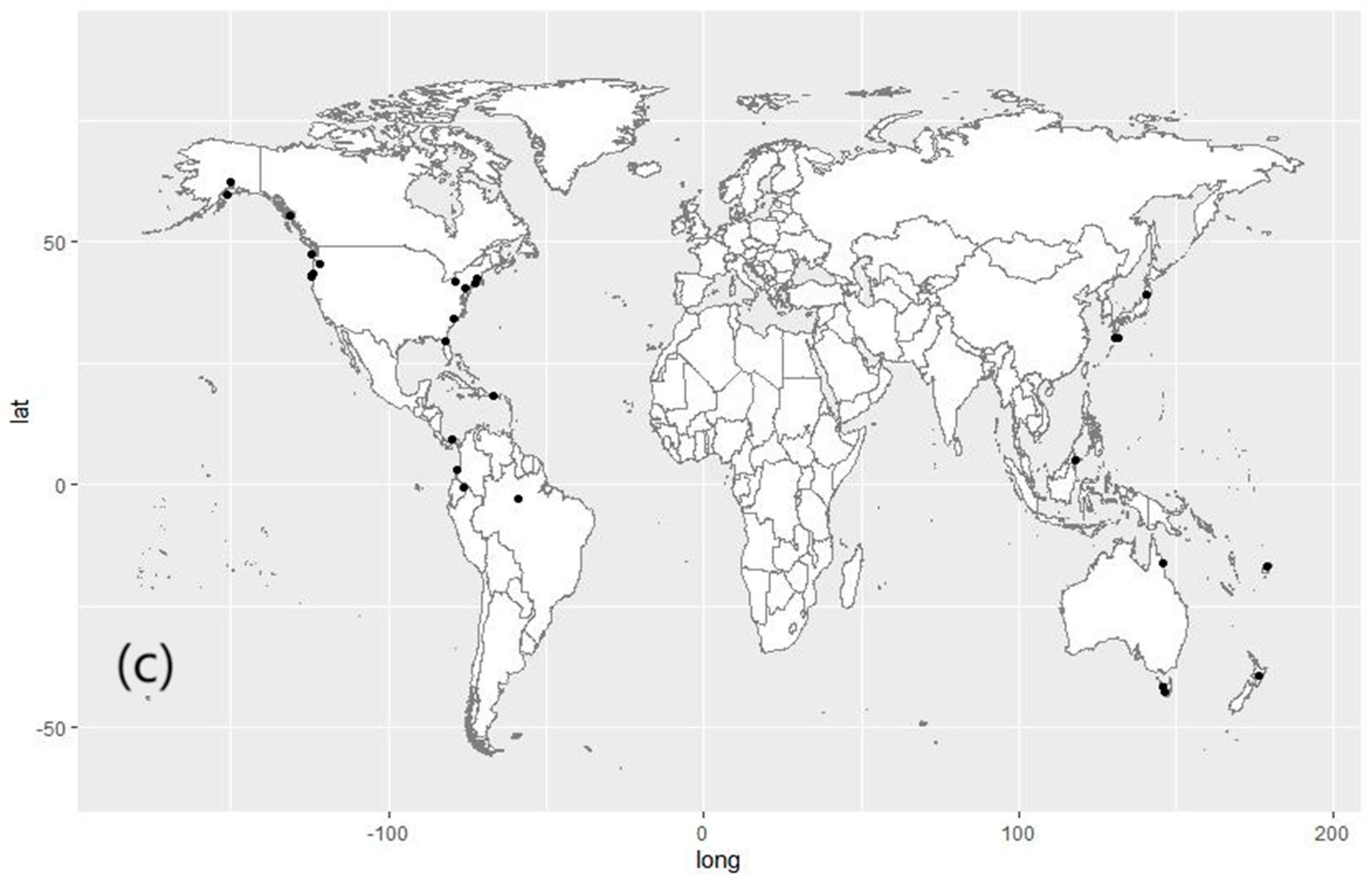
2.1. Dataset
2.2. Correlation Analysis
2.3. Method of Establishing Linear Models
2.3.1. Variable Selection Method for Establishing Multiple Linear Models
2.3.2. Robust Regression
2.4. Evaluation of Model Precision
2.4.1. 10-Fold Cross-Validation
2.4.2. Stratified Random Sampling in Cross-Validation
2.4.3. Evaluation Indexes of Linear Models
3. Results
3.1. Correlations between Physiognomic Variables of the Order Ericales and Climatic Variables
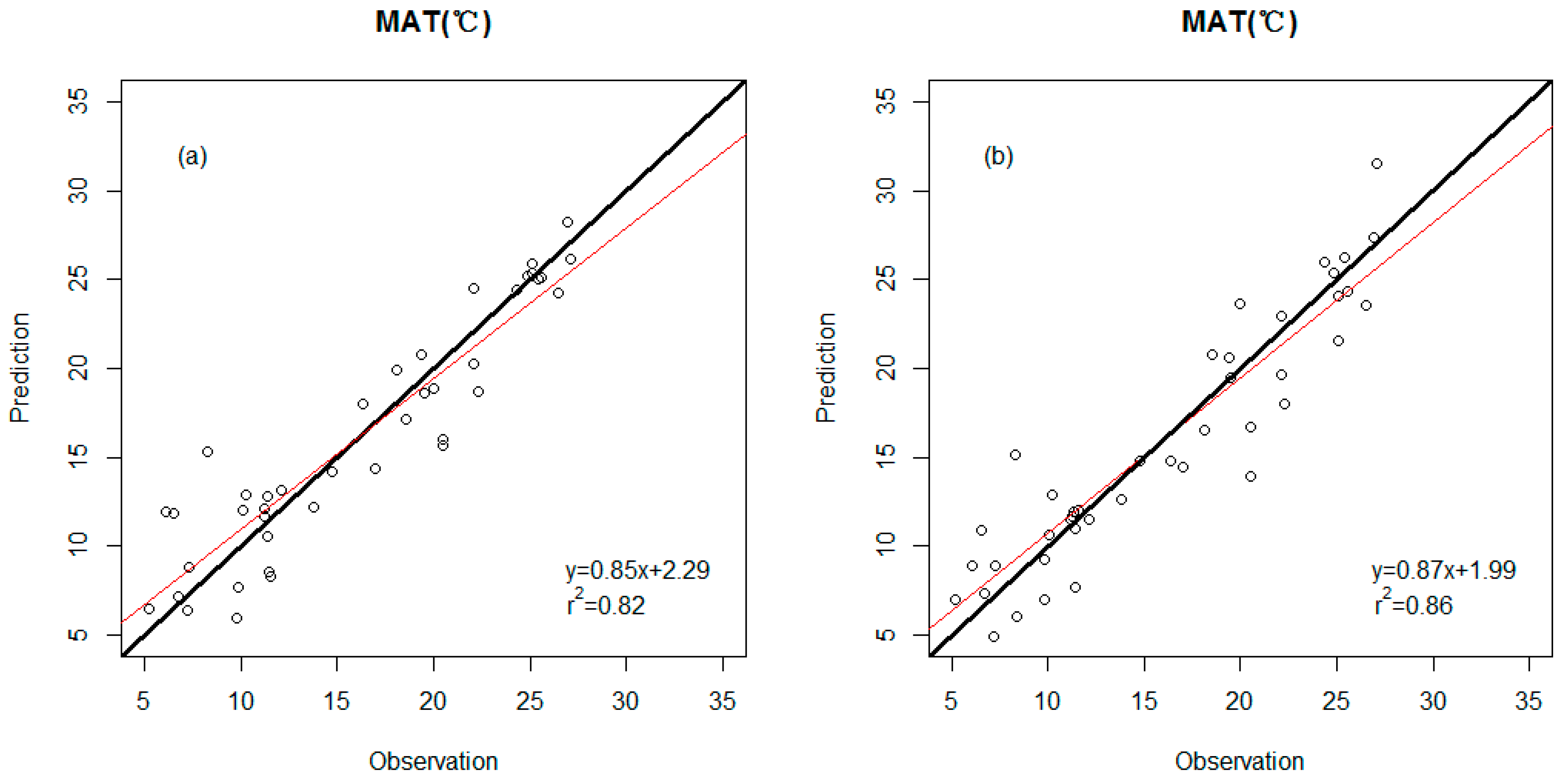
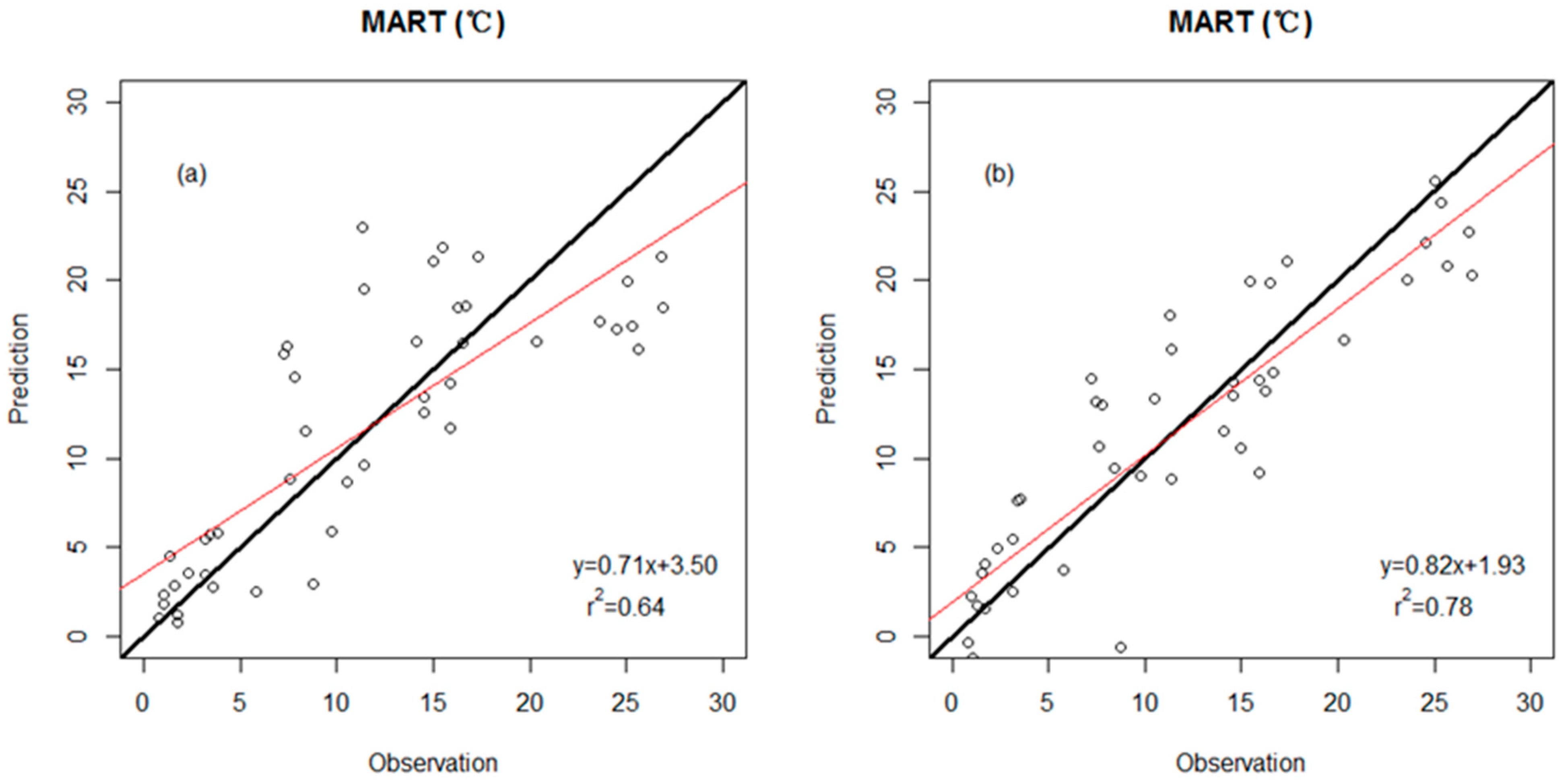
3.2. Precision Comparison of Two Classes of Linear Models
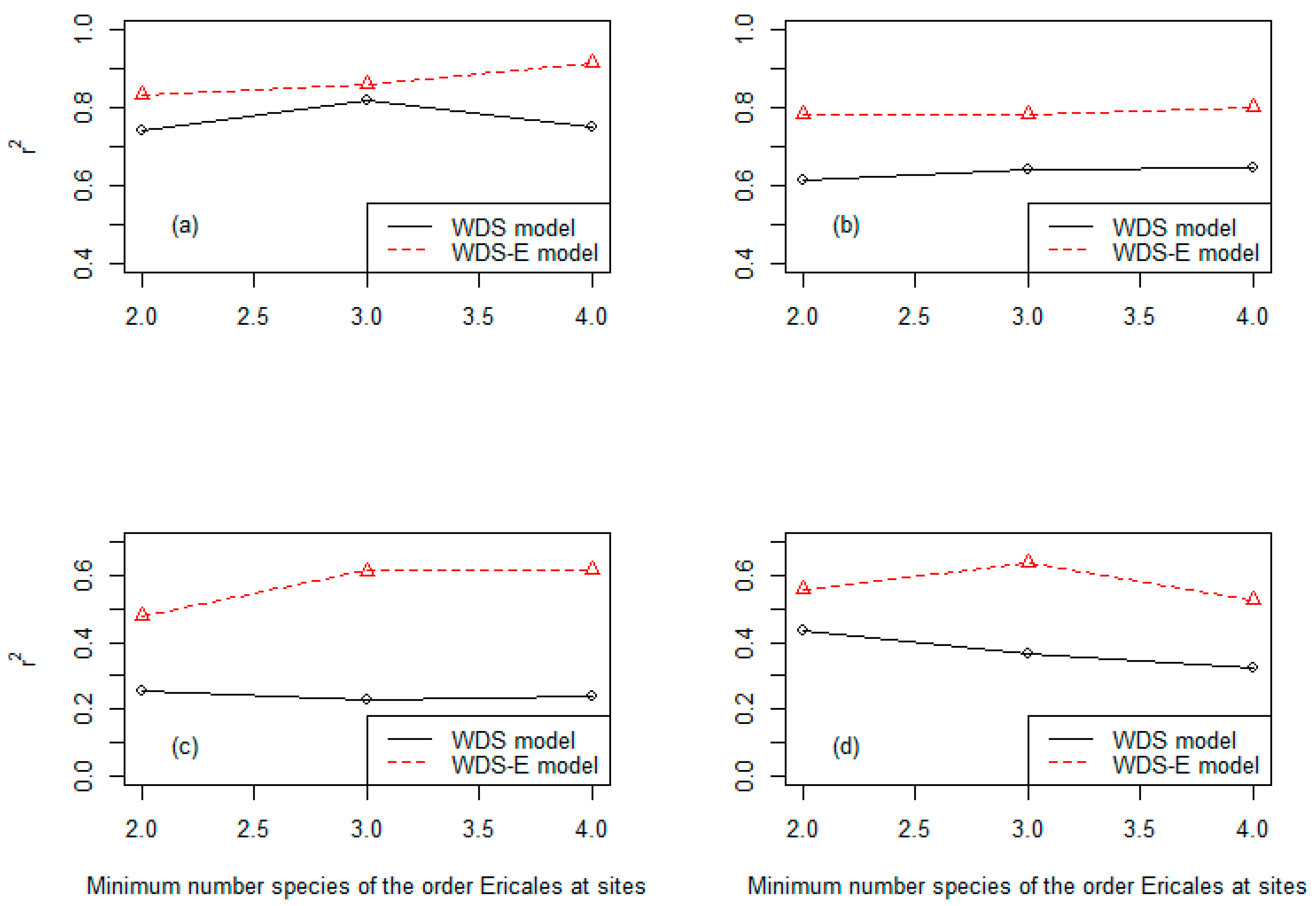
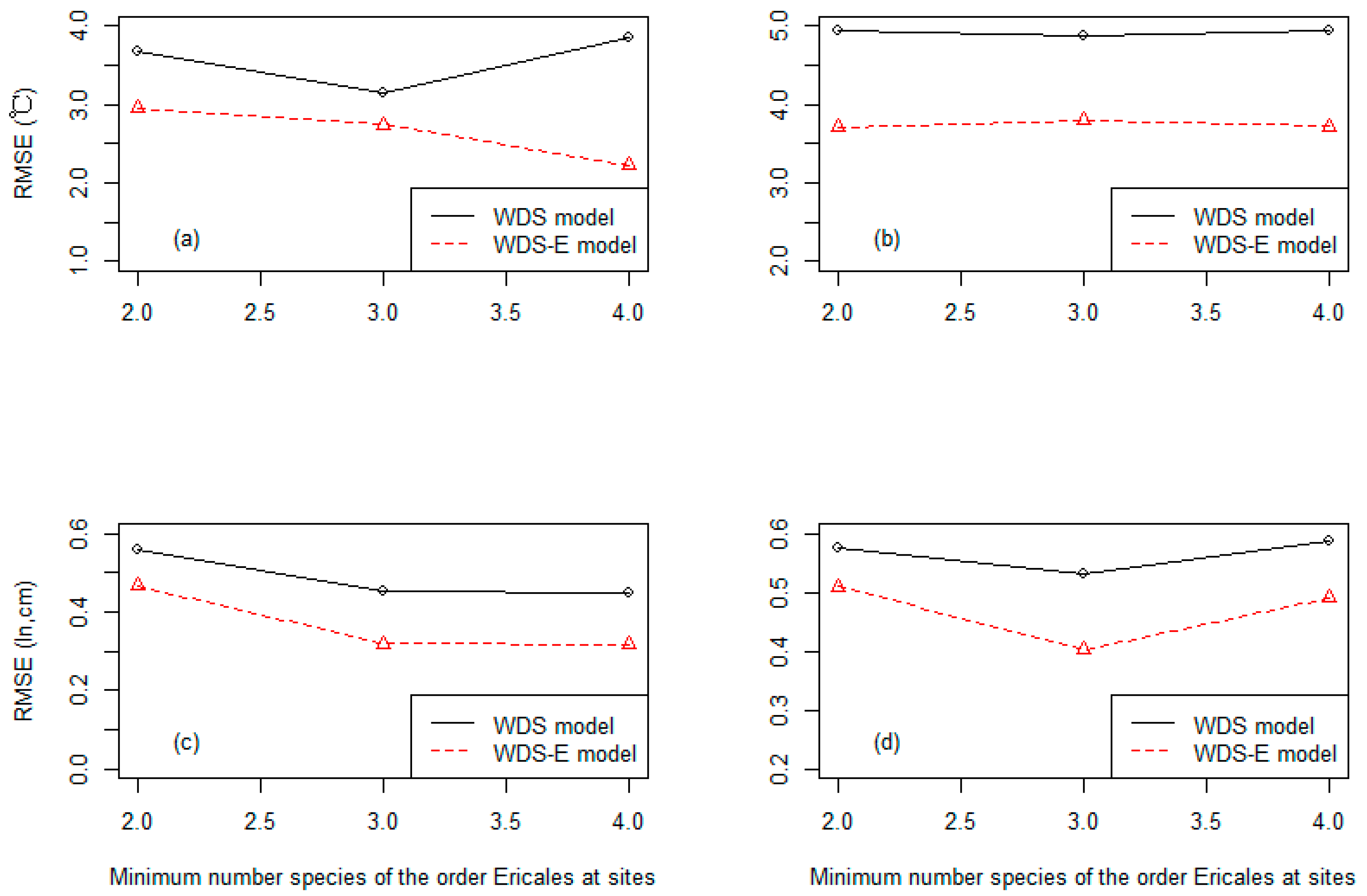
3.3. Precision of the Two Classes of Linear Models at Three Different Levels of the Species Numbers of the Order Ericales
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, L.; Jia, G.; Fang, S.; Shangguan, D.H.; Hu, Y.H.; Zhang, Z.M.; Peng, D. Big Earth Data for UN Sustainable Development Goals: Climate Change and Action. Bull. Chin. Acad. Sci. 2021, 3, 923–931. [Google Scholar]
- Parrish, J.T. Interpreting Pre–Quaternary Climate from the Geologic Record; Columbia University Press: New York, NY, USA, 1998; p. 338. [Google Scholar]
- Bailey, I.W.; Sinnott, E.W. A botanical index of cretaceous and tertiary climates. Science 1915, 41, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Bailey, I.W.; Sinnott, E.W. The climatic distribution of certain types of angiosperm leaves. Am. J. Bot. 1916, 3, 24–39. [Google Scholar] [CrossRef]
- Webb, L.J. Environmental relationships of the structural types of Australian rain forest vegetation. Ecology 1968, 49, 296–311. [Google Scholar] [CrossRef]
- Wilf, P. When are leaves good thermometers? A new case for leaf margin analysis. Paleobiology 1997, 23, 373–390. [Google Scholar] [CrossRef] [Green Version]
- Traiser, C.; Klotz, S.; Uhl, D.; Mosbrugger, V. Environmental signals from leaves—A physiognomic analysis of European vegetation. New Phytol. 2005, 166, 465–484. [Google Scholar] [CrossRef]
- Royer, D.L.; Wilf, P.; Janesko, D.A.; Kowalski, E.A.; Dilcher, D.L. Correlations of climate and plant ecology to leaf size and shape: Potential proxies for the fossil record. Am. J. Bot. 2005, 92, 1141–1151. [Google Scholar] [CrossRef] [Green Version]
- Royer, D.L.; Wilf, P. Why do toothed leaves correlate with cold climates? Gas exchange at leaf margins provides new insights into a classic paleotemperature proxy. Int. J. Plant Sci. 2006, 167, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Peppe, D.J.; Royer, D.L.; Cariglino, B.; Oliver, S.Y.; Newman, S.; Leight, E.; Enikolopov, G.; Fernandez-Burgos, M.; Herrera, F.; Adams, J.M.; et al. Sensitivity of leaf size and shape to climate: Global patterns and paleoclimatic applications. New Phytol. 2011, 190, 724–739. [Google Scholar] [CrossRef] [Green Version]
- Lewis, M.C. The physiological significance of variation in leaf structure. Sci. Prog. 1972, 60, 25–51. [Google Scholar]
- Givnish, T.J. On the Adaptive Significance of Leaf Form. In Topics in Plant Population Biology, 2nd ed.; Solbrig, T., Jain, S., Johnson, G.B., Raven, P.H., Eds.; Columbia University Press: New York, NY, USA, 1979; pp. 375–407. [Google Scholar]
- Wolfe, J.A. Temperature parameters of humid to mesic forests of eastern Asia and relation to forests of other regions in the Northern Hemisphere and Australasia. United States Geol. Surv. Prof. Pap. 1979, 1106, 1–37. [Google Scholar]
- Wolfe, J.A. A method of obtaining climatic parameters from leaf assemblages. United States Geol. Surv. Bull. 1993, 2040, 1–71. [Google Scholar]
- Wiemann, M.C.; Manchester, S.R.; Dilcher, D.L.; Hinojosa, L.F.; Wheeler, E.A. Estimation of temperature and precipitation from morphological characters of dicotyledonous leaves. Am. J. Bot. 1998, 85, 1796–1802. [Google Scholar] [CrossRef] [PubMed]
- Wilf, P.; Wing, S.L.; Greenwood, D.R.; Greenwood, C.L. Using fossil leaves as paleoprecipitation indicators: An Eocene example. Geology 1998, 26, 203–206. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, B.F. Estimation of rainfall variables from leaf characters in tropical Africa. Palaeogeogr. Palaeoecol. Palaeoclimatol. 1999, 145, 231–250. [Google Scholar] [CrossRef]
- Jacobs, B.F. Estimation of low-latitude paleoclimates using fossil angiosperm leaves: Examples from the Miocene Tugen Hills, Kenya. Paleobiology 2002, 28, 399–421. [Google Scholar] [CrossRef]
- Miller, I.M.; Brandon, M.T.; Hickey, L.J. Using leaf margin analysis to estimate Mid-Cretaceous (Albian) paleolatitude of the Baja BC block. Earth Planet. Sci. Lett. 2006, 245, 95–114. [Google Scholar] [CrossRef]
- Wolfe, J.A. Paleoclimatic estimates from Tertiary leaf assemblages. Annu. Rev. Earth Planet. Sci. Lett. 1995, 23, 119–142. [Google Scholar] [CrossRef]
- Huff, P.M.; Wilf, P.; Azumah, E.J. Digital future for paleoclimate estimation from fossil leaves? Preliminary results. Palaios 2003, 18, 266–274. [Google Scholar] [CrossRef]
- Yang, J.; Spicer, R.A.; Spicer, T.E.V.; Arens, N.C.; Jacques, F.M.B.; Su, T.; Kennedy, E.M.; Herman, A.B.; Steart, D.C.; Srivastava, G.; et al. Leaf form-climate relationships on the global stage:anensemble of characters. Glob. Ecol. Biogeogr. 2015, 11, 1113–1125. [Google Scholar] [CrossRef]
- Li, S.F.; Jacques, F.M.B.; Spicer, R.A.; Su, T.; Spicer, T.E.V.; Yang, J.; Zhou, Z.K. Artificial neural networks reveal a high-resolution climatic signal in leaf physiognomy. Palaeogeogr. Palaeoecol. Palaeoclimatol. 2015, 442, 1–11. [Google Scholar] [CrossRef]
- Spicer, R.A.; Yang, J.; Spicer, T.E.V.; Farnsworth, A. Woody dicot leaf traits as a palaeoclimate proxy: 100 years of development and application. Palaeogeogr. Palaeoecol. Palaeoclimatol. 2020, 562, 254–267. [Google Scholar] [CrossRef]
- Wei, G.; Peng, C.; Zhu, Q.; Zhou, X.; Yang, B. Application of machine learning methods for paleoclimatic reconstructions from leaf traits. Int. J. Climatol. 2021, 41, E3249–E3262. [Google Scholar] [CrossRef]
- McKee, M.L.; Royer, D.L.; Poulos, H.M. Experimental evidence for species-dependent responses in leaf shape to temperature: Implications for paleoclimate inference. PLoS ONE 2019, 14, e0218884. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z.; Xu, X.; Han, W.; Wang, Q.; Zou, D. Leaf margin analysis of Chinese woody plants and the constraints on its application to palaeoclimatic reconstruction. Glob. Ecol. Biogeogr. 2016, 25, 1401–1415. [Google Scholar] [CrossRef]
- Little, S.A.; Kembel, S.W.; Wilf, P. Paleotemperature proxies from leaf fossils reinterpreted in light of evolutionary history. PLoS ONE 2010, 5, e15161. [Google Scholar] [CrossRef]
- Glade-Vargas, N.; Hinojosa, L.F.; Leppe, M. Evolution of climatic related leaf traits in the family Nothofagaceae. Front. Plant Sci. 2018, 9, 152–159. [Google Scholar] [CrossRef]
- Royer, D.L.; McElwain, J.C.; Adams, J.M.; Wilf, P. Sensitivity of leaf size and shape to climate within Acer rubrum and Quercus kelloggii. New Phytol. 2008, 179, 808–817. [Google Scholar] [CrossRef]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, C.; Westoby, M.; Díaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.; Law, E.A.; et al. Global climatic drivers of leaf size. Science 2017, 357, 917–921. [Google Scholar] [CrossRef] [Green Version]
- APG, I.V. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linnean Soc. 2016, 181, 1–20. [Google Scholar]
- Kubitzki, K. Families and Genera of Flowering Plants; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Sally, E.S.; David, J.R. Mycorrhizal Symbiosis, 2nd ed.; Academic Press: Pittsburgh, PA, USA, 1997; pp. 1765–1770. [Google Scholar]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Nettleton, D. Commercial Data Mining. CA: Morgan Kaufmann. 2014. Available online: https://www.sciencedirect.com/book/9780124166028/commercial-data-mining (accessed on 10 March 2022).
- Rencher, A.C.; Schaalje, G.B. Linear Models in Statistics, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 476–487. [Google Scholar]
- He, X.Q.; Li, W.Q. Independent Variable Selection and Stepwise Regression. Applied Regression Analysis, 3rd ed.; China Renmin University Press: Beijing, China, 2015; pp. 332–338. [Google Scholar]
- Huberty, C.J. Applied Discriminant Analysis, 2nd ed.; John Wiley and Sons: Hoboken, NJ, USA, 1994; pp. 226–232. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry, 3rd ed.; W. H. Freeman and Company: New York, NY, USA, 1995; pp. 205–212. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows; Version 21; IBM Corp.: Armonk, NY, USA, 2012. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2002; p. 119. [Google Scholar]
- Huber, P.J. Robust estimation of a location parameter. Ann. Stat. 1964, 35, 93–101. [Google Scholar] [CrossRef]
- Lantz, B. Evaluating Model Performance. Machine Learning with R. Machine Learning, 2nd ed.; Packt Publishing: Birmingham, UK, 2013; pp. 278–286. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2011. [Google Scholar]
- Seyfullah, L. Fossil Focus: Using plant fossils to understand past climates and environments. Palaeontol. Online 2012, 2, 7. [Google Scholar]
| Model | r2 | RMSE | Predictor Variables |
|---|---|---|---|
| MAT (response variable) | |||
| WDS model | 0.82 | 3.1 (°C) | Percent untoothed Feret’s diameter ratio Peri/Area |
| WDS-E model | 0.86 | 2.7 (°C) | Percent untoothed Peri/Area ln(#Teeth/IntPeri) E-leaf area E- TA/Peri |
| MART (response variable) | |||
| WDS model | 0.64 | 4.9 (°C) | Percent untoothed #Teeth |
| WDS-E model | 0.78 | 3.8 (°C) | Percent untoothed E- ShapFact E- #Teeth/Peri |
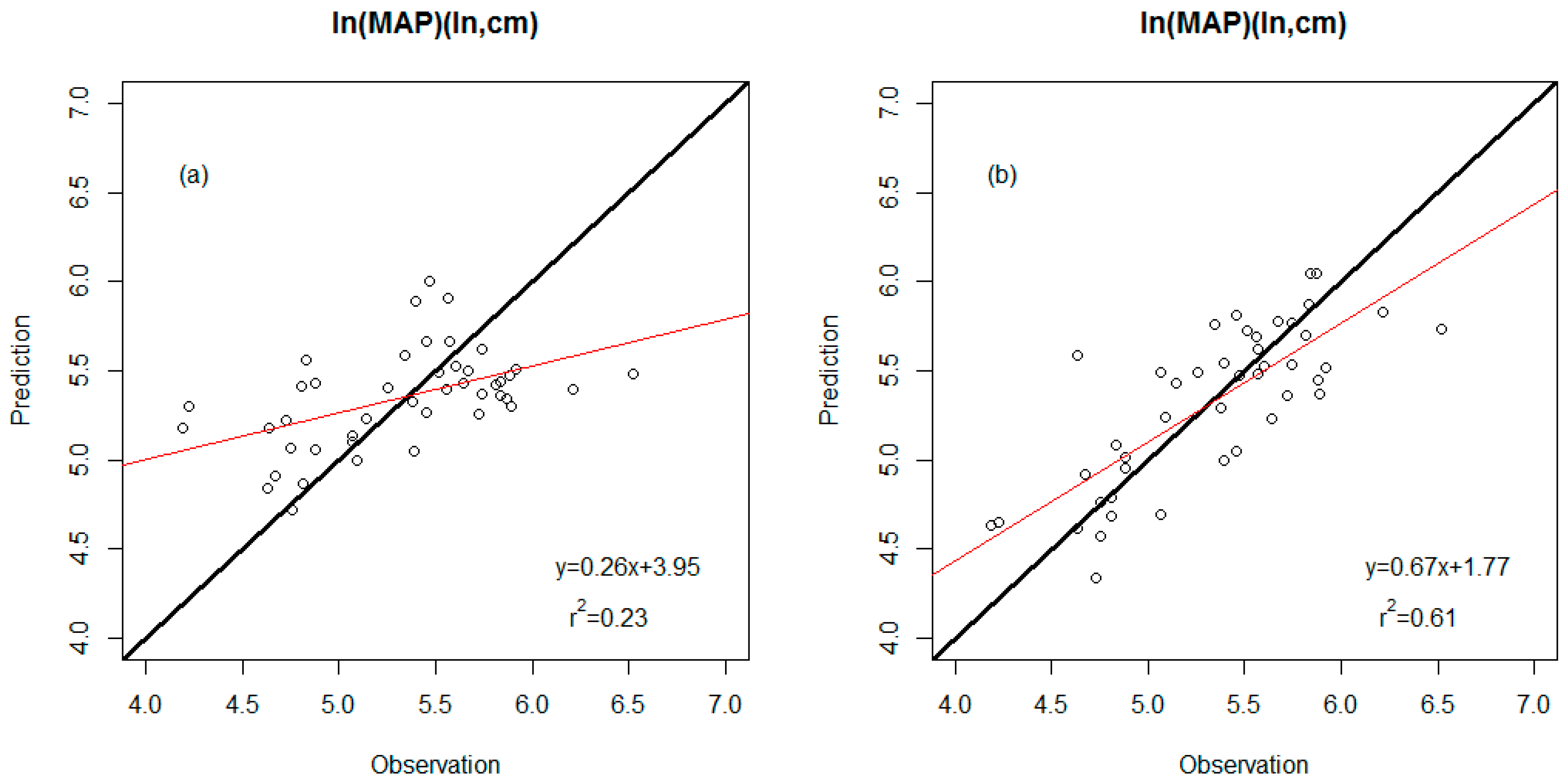
| ln(MAP) (response variable) | |||
| WDS model | 0.23 | 0.45 (ln, cm) | Leaf area TA/IntPeri |
| WDS-E model | 0.61 | 0.32 (ln, cm) | Percent untoothed ShapFact #Teeth E-Percent untoothed E-TA/BA |
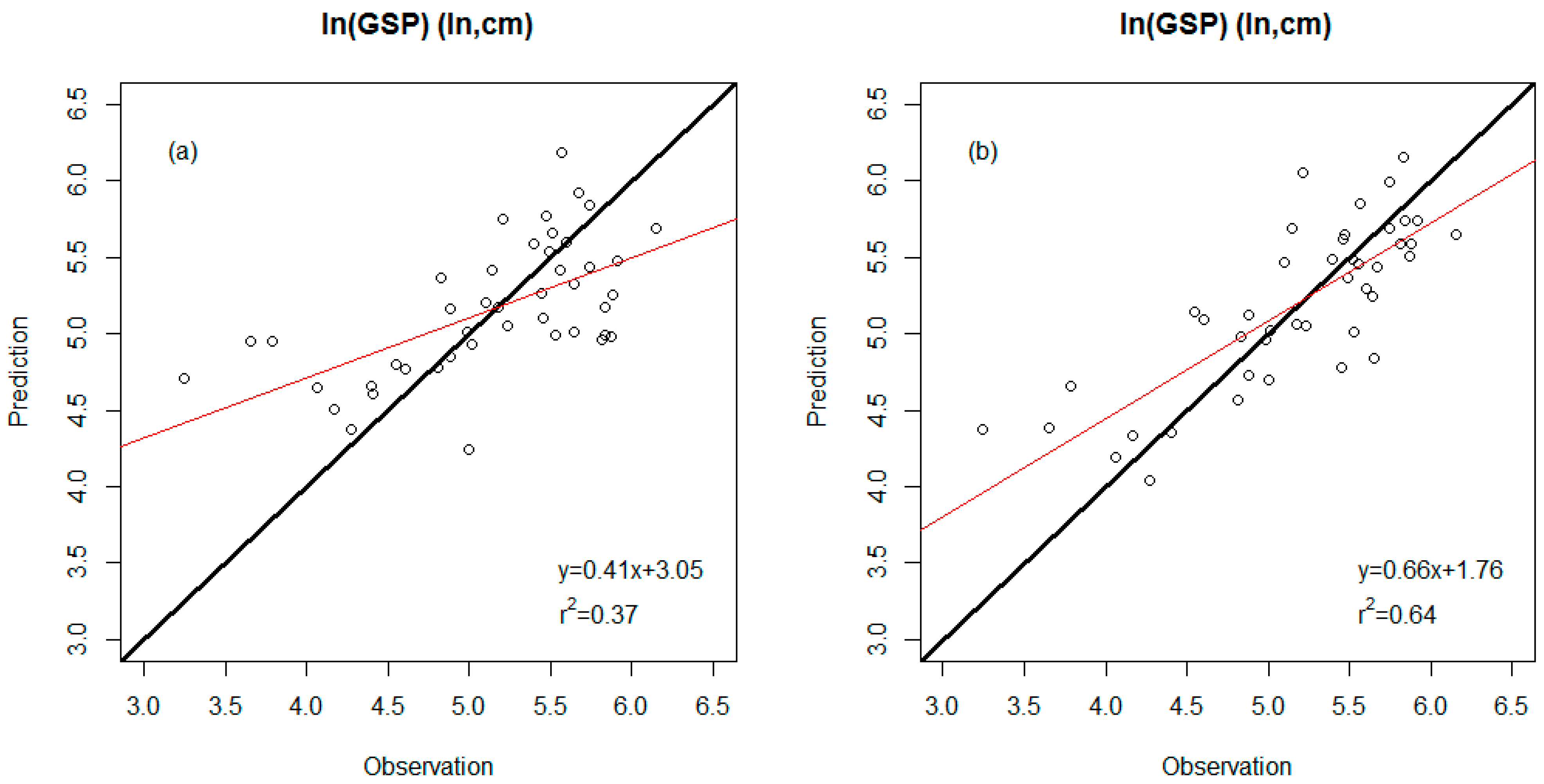
| ln(GSP) (response variable) | |||
| WDS model | 0.37 | 0.53 (ln, cm) | Percent untoothed #Teeth TA/IntPeri |
| WDS-E model | 0.64 | 0.40 (ln, cm) | Percent untoothed #Teeth E-TA/IntPeri |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, G.; Peng, C.; Zhu, Q.; Zhou, X.; Liu, W. Contribution of the Order Ericales to Improving Paleoclimate Reconstructions. Sustainability 2022, 14, 4008. https://doi.org/10.3390/su14074008
Wei G, Peng C, Zhu Q, Zhou X, Liu W. Contribution of the Order Ericales to Improving Paleoclimate Reconstructions. Sustainability. 2022; 14(7):4008. https://doi.org/10.3390/su14074008
Chicago/Turabian StyleWei, Gang, Changhui Peng, Qiuan Zhu, Xiaolu Zhou, and Weiguo Liu. 2022. "Contribution of the Order Ericales to Improving Paleoclimate Reconstructions" Sustainability 14, no. 7: 4008. https://doi.org/10.3390/su14074008
APA StyleWei, G., Peng, C., Zhu, Q., Zhou, X., & Liu, W. (2022). Contribution of the Order Ericales to Improving Paleoclimate Reconstructions. Sustainability, 14(7), 4008. https://doi.org/10.3390/su14074008






