Ecological Infrastructures May Enhance Lepidopterans Predation in Irrigated Mediterranean Farmland, Depending on Their Typology and the Predator Guild
Abstract
:1. Introduction
2. Materials and Methods
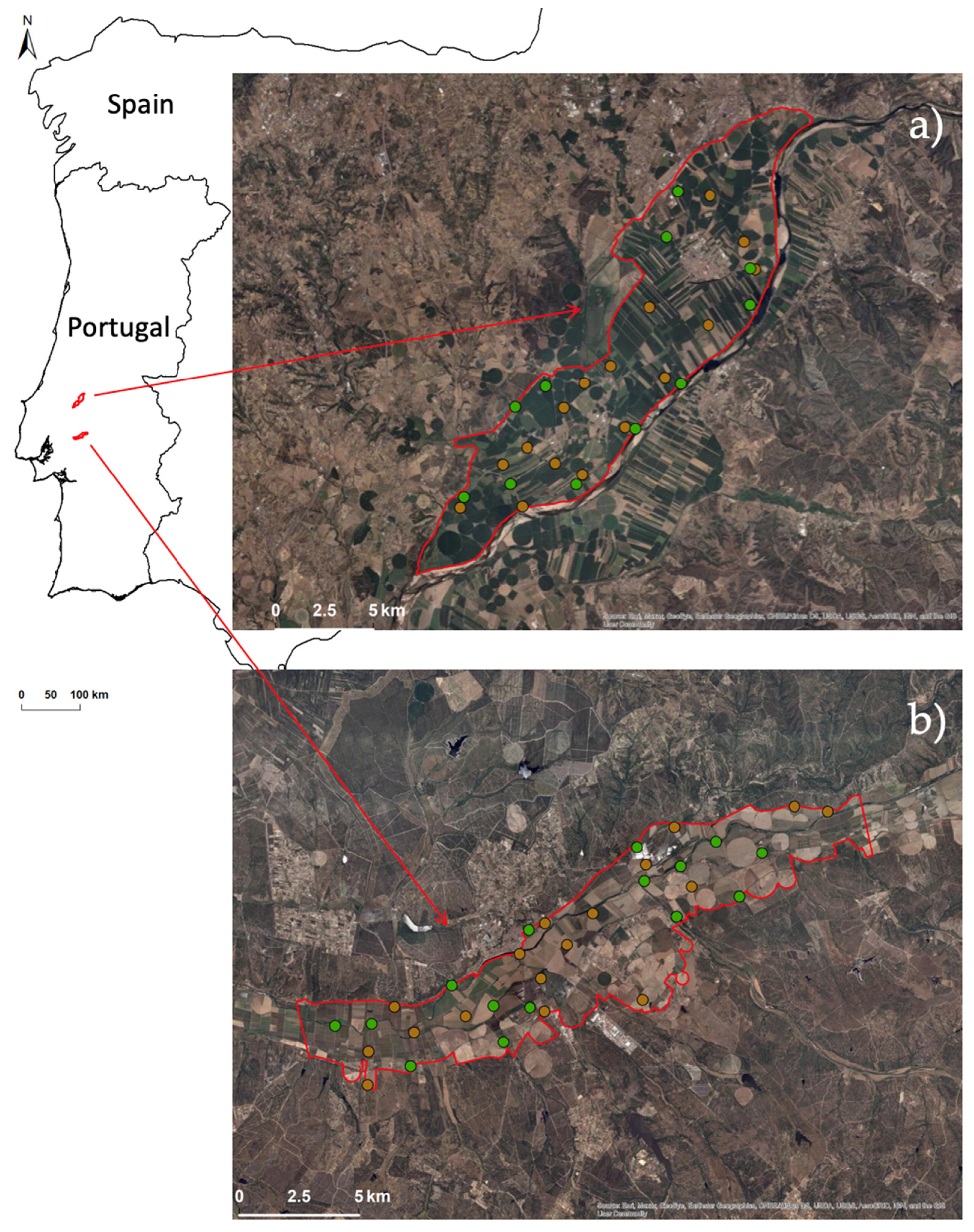
2.1. Study Area
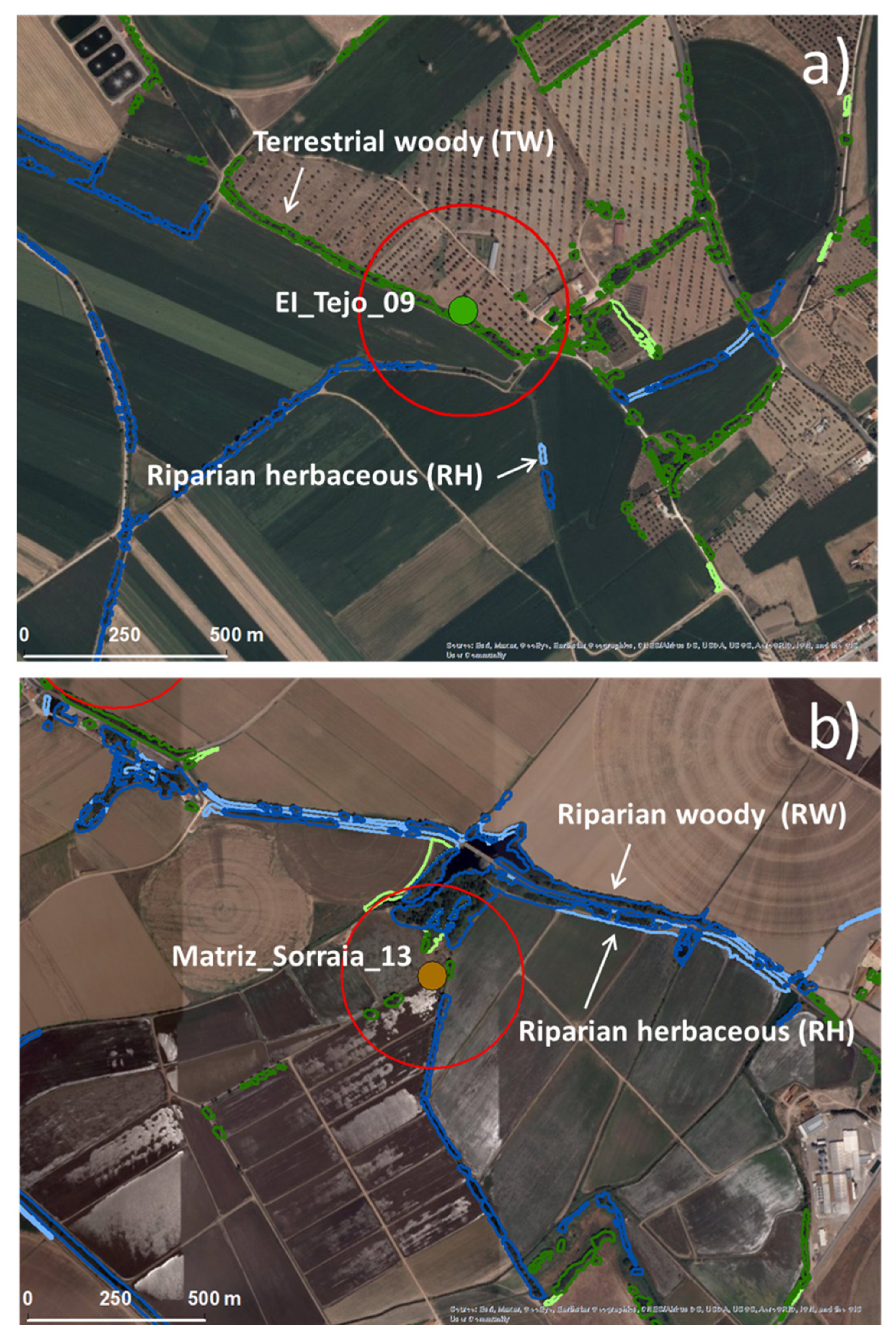
2.2. Ecological Infrastructures Classification System and Mapping
2.3. Dummy Caterpillars
2.4. Field Experimental Design
2.5. Assessment of Bird Species Assemblages
2.6. Identification and Quantification of Predation Marks on Dummy Caterpillars
2.7. Landscape Variables
2.8. Statistical Analyses
3. Results
3.1. Bird Species Observed
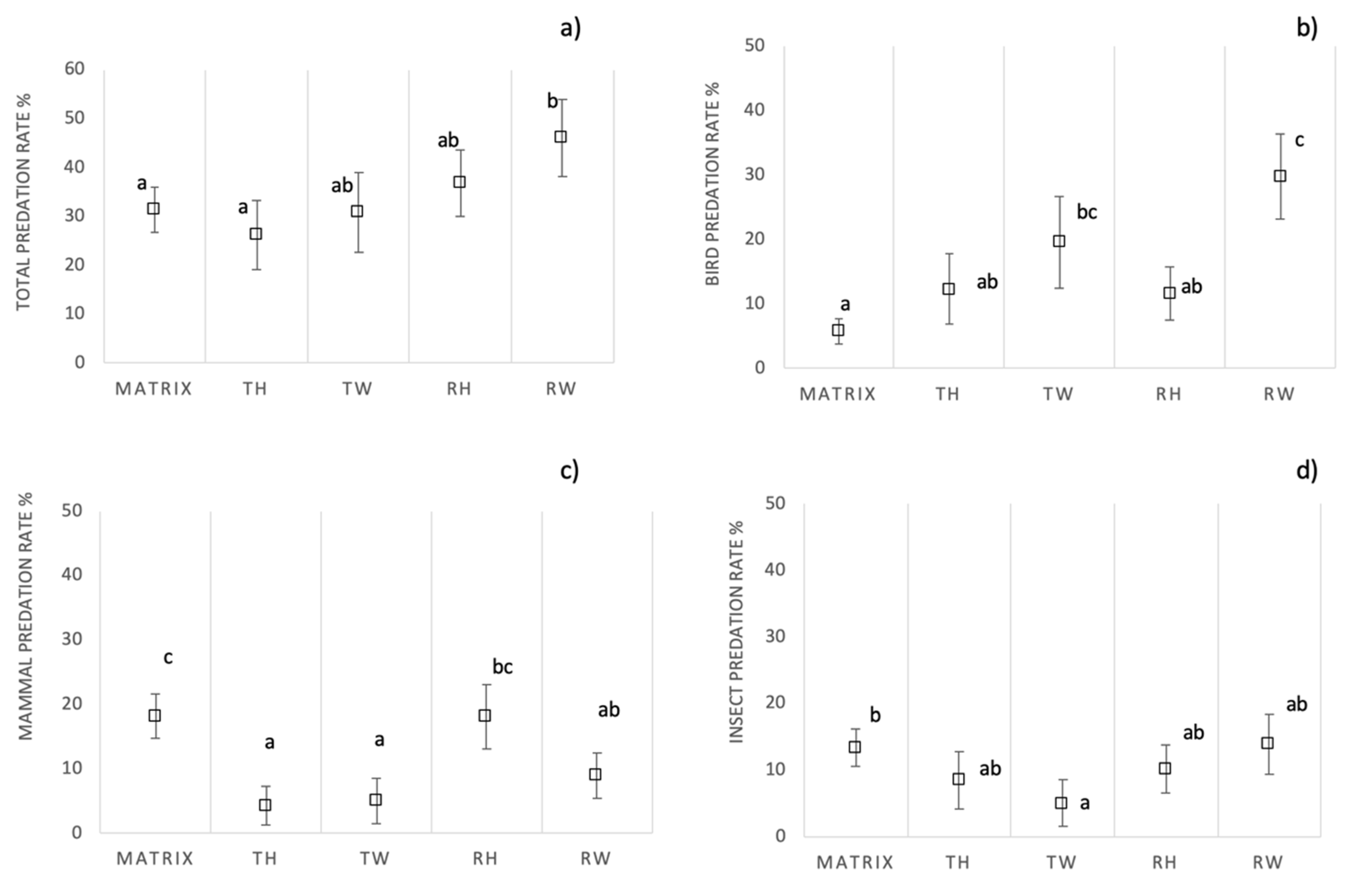
3.2. Overall Predation Pressure
3.3. Predation Rate in Ecological Infrastructures Versus Agricultural Matrix
3.4. Predation Rate in Different Ecological Infrastructures’ Typologies
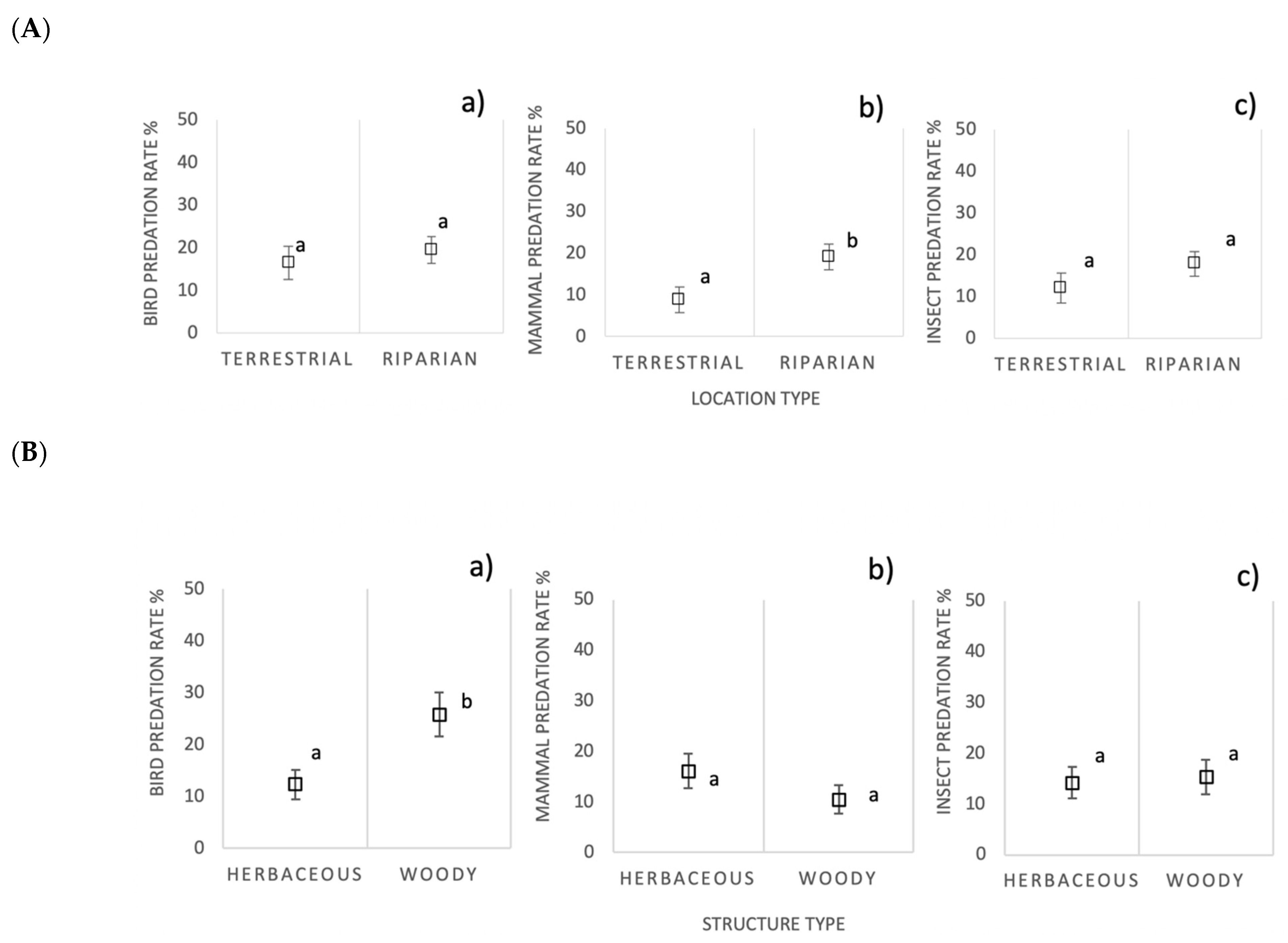
3.5. Predation by Ecological Infrastructure Location and Vegetation Structure
3.6. Landscape Metrics
4. Discussion
4.1. Overall Predation Pressure
4.2. Effects of Ecological Infrastructures and Landscape Metrics on Predation Rate
4.3. Drawbacks and Strengths of the Method
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global consequences of land use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garbach, K.; Milder, J.C.; Montenegro, M.; Karp, D.S.; DeClerck, F.A.J. Biodiversity and Ecosystem Services in Agroecosystems. Encycl. Agric. Food System 2014, 2, 21–40. [Google Scholar] [CrossRef]
- Cole, L.J.; Brocklehurst, S.; Robertson, D.; Harrison, W.; McCracken, D.I. Exploring the interactions between resource availability and the utilisation of semi-natural habitats by insect pollinators in an intensive agricultural landscape. Agric. Ecosyst. Environ. 2017, 246, 157–167. [Google Scholar] [CrossRef]
- Benton, T.G.; Vickery, J.A.; Wilson, J.D. Farmland biodiversity: Is habitat heterogeneity the key? Trends Ecol. Evol. 2003, 18, 182–188. [Google Scholar] [CrossRef]
- Holland, J.M.; Bianchi, F.J.; Entling, M.H.; Moonen, A.-C.; Smith, B.M.; Jeanneret, P. Structure, function and management of semi-natural habitats for conservation biological control: A review of European studies. Pest Manag. Sci. 2017, 72, 1638–1651. [Google Scholar] [CrossRef]
- Hannon, L.E.; Sisk, T.D. Hedgerows in an agri-natural landscape: Potential habitat value for native bees. Biol. Conserv. 2009, 142, 2140–2154. [Google Scholar] [CrossRef]
- Cole, L.J.; Brocklehurst, S.; Robertson, D.; Harrison, W.; McCracken, D.I. Riparian buffer strips: Their role in the conservation of insect pollinators in intensive grassland systems. Agric. Ecosyst. Environ. 2015, 211, 207–220. [Google Scholar] [CrossRef]
- Martin, E.A.; Dainese, M.; Clough, Y.; Báldi, A.; Bommarco, R.; Gagic, V.; Garratt, M.P.; Holzschuh, A.; Kleijn, D.; Kovács-Hostyánszki, A.; et al. The interplay of landscape composition and configuration: New pathways to manage functional biodiversity and agroecosystem services across Europe. Ecol. Lett. 2019, 22, 1083–1094. [Google Scholar] [CrossRef] [Green Version]
- Sanaullah, M.; Usman, M.; Wakeel, A.; Cheema, S.A.; Ashraf, I.; Farooq, M. Terrestrial ecosystem functioning affected by agricultural management systems: A review. Soil Tillage Res. 2020, 196, 104464. [Google Scholar] [CrossRef]
- Pecheur, E.; Piqueray, J.; Monty, A.; Dufrêne, M.; Mahy, G. The influence of ecological infrastructures adjacent to crops on their carabid assemblages in intensive agroecosystems. PeerJ 2020, 8, e8094. [Google Scholar] [CrossRef]
- Silva, J.M.C.; Wheeler, E. Ecosystems as infrastructure. Perspect. Ecol. Conserv. 2017, 15, 32–35. [Google Scholar] [CrossRef]
- Boller, E.F.; Hani, F.; Poehling, H.M. Ecological infrastructures. In Ideabook on Functional Biodiversity at the Farm Level; IOBC/wprs: Lindau, Switzerland, 2004. [Google Scholar]
- Rosas-Ramos, N.; Baños-Picón, L.; Tobajas, E.; de Paz, V.; Tormos, J.; Asís, J.D. Value of ecological infrastructure diversity in the maintenance of spider assemblages: A case study of Mediterranean vineyard agroecosystems. Agric. Ecosyst. Environ. 2018, 265, 244–253. [Google Scholar] [CrossRef]
- Rosas-Ramos, N.; Baños-Picón, L.; Trivellone, V.; Moretti, M.; Tormos, J.; Asís, J.D. Ecological infrastructures across Mediterranean agroecosystems: Towards an effective tool for evaluating their ecological quality. Agric. Syst. 2019, 173, 355–363. [Google Scholar] [CrossRef]
- Naiman, R.J.; Decamps, H.; Pollock, M. The role of riparian corridors in maintaining regional biodiversity. Ecol. Appl. 1993, 3, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Riis, T.; Kelly-Quinn, M.; Aguiar, F.C.; Manolaki, P.; Bruno, D.; Bejarano, M.D.; Clerici, N.; Fernandes, M.R.; Franco, J.C.; Pettit, N.; et al. Global overview of ecosystem services provided by riparian vegetation. BioScience 2020, 70, 501–514. [Google Scholar] [CrossRef]
- European Commission. Communication to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions: Green Infrastructure (GI)—Enhancing Europe’s Natural Capital; European Commission: Brussels, Belgium, 2013. [Google Scholar]
- European Commission. The EU Guidance Document on Integrating Ecosystems and Their Services in Decision-Making; European Commission: Brussels, Belgium, 2013. [Google Scholar]
- European Commission. Report from the Commission to the European Parliament and the Council on the Implementation of the Ecological Focus Area Obligation under the Direct Payment Scheme; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- European Commission. EU Guidance Document on a Strategic Framework for Further Supporting the Deployment of EU-Level Green and Blue Infrastructure SWD, 193 Final; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Cole, L.J.; Kleijn, D.; Dicks, L.V.; Stout, J.C.; Potts, S.G.; Albrecht, M.; Balzan, M.V.; Bartomeus, I.; Bebeli, P.J.; Bevk, D.; et al. A critical analysis of the potential for EU Common Agricultural Policy measures to support wild pollinators on farmland. J. Appl. Ecol. 2020, 57, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Heyl, K.; Döring, T.; Garske, B.; Stubenrauch, J.; Ekardt, F. The Common Agricultural Policy beyond 2020: A critical review in light of global environmental goals. Rev. Eur. Comp. Int. Environ. Law 2021, 30, 95–106. [Google Scholar] [CrossRef]
- Rotchés-Ribalta, R.; Ruas, S.; Ahmed, K.D.; Gormally, M.; Moran, J.; Stout, J.; White, B.; Ó hUallacháin, D. Assessment of semi-natural habitats and landscape features on Irish farmland: New insights to inform EU Common Agricultural Policy implementation. Ambio 2021, 50, 346–359. [Google Scholar] [CrossRef]
- Tooker, J.F.; O’Neal, M.E.; Rodriguez-Saona, C. Balancing disturbance and conservation in agroecosystems to improve biological control. Annu. Rev. Entomol. 2020, 65, 81–100. [Google Scholar] [CrossRef] [Green Version]
- Luff, M.L. The potential of predators for pest control. Agric. Ecosyst. Environ. 1983, 10, 159–181. [Google Scholar] [CrossRef]
- Symondson, W.O.C.; Sunderland, K.D.; Greenstone, M.H. Can Generalist Predators be Effective Biocontrol Agents? Annu. Rev. Entomol. 2002, 47, 561–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgio, G.; Ferrari, R.; Boriani, L.; Pozzati, M.; van Lenteren, J. The role of ecological infrastructures on Coccinellidae (Coleoptera) and other predators in weedy field margins within northern Italy agroecosystems. Bull. Insectol. 2006, 59, 59–67. [Google Scholar]
- McHugh, N.M.; Moreby, S.; Lof, M.E.; Werf, W.; Holland, J.M. The contribution of semi-natural habitats to biological control is dependent on sentinel prey type. J. Appl. Ecol. 2020, 57, 914–925. [Google Scholar] [CrossRef]
- Ferrante, M.; Lo Cacciato, A.; Lövei, G.L. Quantifying predation pressure along an urbanisation gradient in Denmark using artificial caterpillars. Eur. J. Entomol. 2014, 111, 649–654. [Google Scholar] [CrossRef] [Green Version]
- Low, P.A.; Sam, K.; McArthur, C.; Posa, M.R.C.; Hochuli, D.F. Determining predator identity from attack marks left in model caterpillars: Guidelines for best practice. Entomol. Exp. Appl. 2014, 152, 120–126. [Google Scholar] [CrossRef]
- Sam, K.; Koane, B.; Novotny, V. Herbivore damage increases avian and ant predation of caterpillars on trees along a complete elevational forest gradient in Papua New Guinea. Ecography 2015, 38, 293–300. [Google Scholar] [CrossRef]
- Roels, S.M.; Porter, J.L.; Lindell, C.A. Predation pressure by birds and arthropods on herbivorous insects affected by tropical forest restoration strategy. Restor. Ecol. 2018, 26, 1203–1211. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Huang, C.; Li, M.; Bibi, F.; Zhou, S.; Nakamura, A. Ant assemblage composition explains high predation pressure on artificial caterpillars during early night. Ecol. Entomol. 2020, 45, 547–554. [Google Scholar] [CrossRef]
- Koh, L.P.; Menge, D.N.L. Rapid Assessment of Lepidoptera Predation Rates in Neotropical Forest Fragments. Biotropica 2006, 38, 132–134. [Google Scholar] [CrossRef]
- Howe, A.; Gabor, L.L.; Nachman, G. Dummy caterpillars as a simple method to assess predation rates on invertebrates in a tropical agroecosystem. Entomol. Exp. Appl. 2009, 131, 325–329. [Google Scholar] [CrossRef]
- Loiselle, B.A.; Farji-Brener, A.G. What’s up? An experimental comparison of predation levels between canopy and understory in a tropical wet forest. Biotropica 2002, 34, 327–330. [Google Scholar] [CrossRef]
- Denan, N.; Zaki, W.M.W.; Norhisham, A.R.; Sanusi, R.; Nasir, D.M.; Nobilly, F.; Ashton-Butt, A.; Lechner, A.M.; Azhar, B. Predation of potential insect pests in oil palm plantations, rubber tree plantations, and fruit orchards. Ecol. Evol. 2020, 10, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Mitter, C.; Davis, D.R.; Cummings, M.P. Phylogeny and Evolution of Lepidoptera. Annu. Rev. Entomol. 2017, 62, 265–283. [Google Scholar] [CrossRef] [PubMed]
- Furlong, M.J.; Zalucki, M.P. Exploiting predators for pest management: The need for sound ecological assessment. Entomol. Exp. Appl. 2010, 135, 225–236. [Google Scholar] [CrossRef]
- Janzen, D.H. Ecological Characterization of a Costa Rican Dry Forest Caterpillar Fauna. Biotropica 1988, 20, 120–135. [Google Scholar] [CrossRef] [Green Version]
- Marković, Č.; Dobrosavljević, J.; Vujičić, P.; Cebeci, H.H. Impact of regeneration by shelterwood cutting on the pedunculate oak (Quercus robur) leaf mining insect community. Biologia 2021, 76, 1197–1203. [Google Scholar] [CrossRef]
- Sánchez-Fernández, J.; Vílchez-Vivanco, A.J.B.; Navarro, F.; Castro-Rodríguez, J. Farming system and soil management affect butterfly diversity in sloping olive groves. Insect Conserv. Divers. 2020, 13, 456–469. [Google Scholar] [CrossRef]
- Barros-Gomes, H.; Rocha, C. Métodos de Previsão e Evolução dos Inimigos das Culturas: Arroz; Serviço Nacional de Avisos Agrícolas, Direcção-Geral de Protecção das Culturas, Ministério da Agricultura, do Desenvolvimento Rural e das Pescas: Oeiras, Portugal, 2006. Available online: https://www.drapc.gov.pt/base/documentos/manual_snna_arroz.pdf (accessed on 3 August 2021).
- DGADR-Direcção-Geral de Agricultura e Desenvolvimento Rural. Produção Integrada Das Culturas De Milho E Sorgo; Ministério da Agricultura, do Desenvolvimento Rural e das Pescas, Direcção-Geral de Agricultura e Desenvolvimento Rural: Lisboa, Portugal, 2010. Available online: https://www.dgadr.gov.pt/mediateca/send/8-protecao-e-producao-integradas/62-producao-integrada-das-culturas-de-milho-e-sorgo (accessed on 3 August 2021).
- Pinto-Correia, T.; Ribeiro, N.; Sá-Sousa, P. Introducing the montado, the cork and holm oak agroforestry system of Southern Portugal. Agroforest. Syst. 2011, 82, 99. [Google Scholar] [CrossRef] [Green Version]
- Atlas do Ambiente. Available online: http://www.iambiente.pt/atlas/ (accessed on 2 August 2021).
- Johansen, K.; Phinn, S. Linking riparian vegetation spatial structure in Australian tropical savannas to ecosystem health indicators: Semi-variogram analysis of high spatial resolution satellite imagery. Can. J. Remote Sens. 2006, 32, 228–243. [Google Scholar] [CrossRef]
- Fernandes, M.R.; Aguiar, F.C.; Ferreira, M.T. Assessing riparian vegetation structure and the influence of land use using landscape metrics and geostatistical tools. Landsc. Urban Plan. 2011, 99, 166–177. [Google Scholar] [CrossRef] [Green Version]
- Lövei, G.L.; Ferrante, M. A review of the sentinel prey method as a way of quantifying invertebrate predation under field conditions. Insect Sci. 2017, 24, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Billerman, S.M.; Keeney, B.K.; Rodewald, P.G.; Schulenberg, T.S. Birds of the World; Cornell Laboratory of Ornithology: Ithaca, NY, USA, 2020; Available online: https://birdsoftheworld.org/bow/home (accessed on 3 August 2020).
- Rossi, J.P.; van Halder, I. Towards indicators of butterfly biodiversity based on a multiscale landscape description. Ecol. Indic. 2010, 10, 452–458. [Google Scholar] [CrossRef]
- Samalens, J.C.; Rossi, J.P. Does landscape composition alter the spatiotemporal distribution of the pine processionary moth in a pine plantation forest? Popul. Ecol. 2011, 53, 287–296. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows; Released 2019; Version 26.0; IBM Corp: Armonk, NY, USA, 2019. [Google Scholar]
- Ferrante, M.; Barone, G.; Kiss, M.; Bozóné-Borbáth, E.; Lövei, G.L. Ground-level predation on artificial caterpillars indicates no enemy-free time for lepidopteran larvae. Community Ecol. 2017, 18, 280–286. [Google Scholar] [CrossRef]
- Nason, L.D.; Eason, P.K.; Carreiro, M.M.; Cherry, A.; Lawson, J. Caterpillar survival in the city: Attack rates on model lepidopteran larvae along an urban-rural gradient show no increase in predation with increasing urban intensity. Urban Ecosyst. 2021, 24, 1129–1140. [Google Scholar] [CrossRef]
- Meyer, S.T.; Koch, C.; Weisser, W.W. Towards a standardized rapid ecosystem function assessment (REFA). Trends Ecol. Evol. 2015, 30, 390–397. [Google Scholar] [CrossRef]
- Mänd, T.; Tammaru, T.; Mappes, J. Size dependent predation risk in cryptic and conspicuous insects. Evol. Ecol. 2007, 21, 485. [Google Scholar] [CrossRef]
- Sam, K.; Remmel, T.; Molleman, F. Material affects attack rates of dummy caterpillars in tropical forest where arthropod predators dominate: An experiment using clay and dough dummies with green colourants on various plant species. Entomol. Exp. Appl. 2015, 157, 317–324. [Google Scholar] [CrossRef]
- Cogni, R.; Freitas, A.V.L.; Amaral, B.F. Influence of prey size on predation success by Zelus longipes L. (Het, Reduviidae). J. Appl. Entomol. 2002, 126, 74–78. [Google Scholar] [CrossRef]
- Roger, C.; Coderre, D.; Boivin, G. Differential prey utilization by the generalist predator Coleomegilla maculata lengi according to prey size and species. Entomol. Exp. Appl. 2000, 94, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Franin, K.; Barić, B.; Kuštera, G. The role of ecological infrastructure on beneficial arthropods in vineyards. Span. J. Agric. Res. 2016, 14, e0303. [Google Scholar] [CrossRef] [Green Version]
- Bommarco, R. Reproduction and energy reserves of a predatory carabid beetle relative to agroecosystem complexity. Ecol. Appl. 1998, 8, 846–853. [Google Scholar] [CrossRef]
- Uzman, D.; Entling, M.H.; Leyer, I.; Reineke, A. Mutual and opposing responses of carabid beetles and predatory wasps to local and landscape factors in vineyards. Insects 2020, 11, 746. [Google Scholar] [CrossRef] [PubMed]
- Schilke, P.R.; Bartrons, M.; Gorzo, J.M.; Zanden, M.J.V.; Gratton, C.; Howe, R.W.; Pidgeon, A.M. Modeling a cross-ecosystem subsidy: Forest songbird response to emergent aquatic insects. Landsc. Ecol. 2020, 35, 1587–1604. Available online: https://link.springer.com/article/10.1007%2Fs10980-020-01038-0 (accessed on 4 November 2021). [CrossRef]
- Cubley, E.S.; Bateman, H.L.; Merritt, D.M.; Cooper, D.J. Using Vegetation Guilds to Predict Bird Habitat Characteristics in Riparian Areas. Wetlands 2020, 40, 1843–1862. [Google Scholar] [CrossRef]
- Santos, A.; Fernandes, M.R.; Aguiar, F.C.; Branco, M.R.; Ferreira, M.T. Effects of riverine landscape changes on pollination services: A case study on the River Minho, Portugal. Ecol. Indic. 2018, 89, 656–666. [Google Scholar] [CrossRef]
- Ramberg, E.; Burdon, F.J.; Sargac, J.; Kupilas, B.; Rîşnoveanu, G.; Lau, D.C.P.; Johnson, R.K.; McKie, B.G. The structure of riparian vegetation in agricultural landscapes influences spider communities and aquatic-terrestrial linkages. Water 2020, 12, 2855. [Google Scholar] [CrossRef]
- Maisonneuve, C.; Rioux, S. Importance of riparian habitats for small mammal and herpetofaunal communities in agricultural landscapes of southern Québec. Agric. Ecosyst. Environ. 2001, 83, 165–175. [Google Scholar] [CrossRef]
- Simelane, F.N.; Mahlaba, T.A.M.; Shapiro, J.T.; MacFadyen, D.; Monadjem, A. Habitat associations of small mammals in the foothills of the Drakensberg Mountains, South Africa. Mammalia 2017, 82, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Fischer, C.; Gayer, C.; Kurucz, K.; Riesch, F.; Tscharntkle, T.; Batáry, P. Ecosystem services and disservices provided by small rodents in arable fields: Effects of local and landscape management. J. Appl. Ecol. 2017, 55, 548–558. [Google Scholar] [CrossRef] [Green Version]
- Wijnands, F.; Malavolta, C.; Alaphilippe, A.; Gerowitt, B.; Baur, R. General Technical Guidelines for Integrated Production of Annual and Perennial Crops. IOBC-WPRS Commission IP Guidelines. 2018. Available online: https://www.iobc-wprs.org/ip_practical_guidelines/index.html (accessed on 4 November 2021).
- Kruess, A.; Tscharntke, T. Habitat fragmentation, species loss and biological control. Science 1994, 264, 1581–1584. Available online: https://www.jstor.org/stable/2883873 (accessed on 2 February 2022). [CrossRef] [PubMed]
- Wallin, H.; Ekbom, B.S. Movements of carabid beetles (Coleoptera: Carabidae) inhabiting cereal fields: A field tracing study. Oecologia 1988, 77, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Wallin, H.; Ekbom, B. Influence of hunger level and prey densities on movement patterns in three species of Pterostichus beetles (Coleoptera: Carabidae). Environ. Entomol. 1994, 23, 1171–1181. [Google Scholar] [CrossRef]
- Tscharntke, T.; Brandl, R. Plant-insect interactions in fragmented landscapes. Annu. Rev. Entomol. 2004, 49, 405–430. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J. Is dispersal distance of birds proportional to territory size? Can. J. Zool. 2003, 81, 195–202. [Google Scholar] [CrossRef]
- Boesing, A.L.; Nichols, E.; Metzger, J.P. Effects of landscape structure on avian-mediated insect pest control services: A review. Landsc. Ecol. 2017, 32, 931–944. [Google Scholar] [CrossRef]
- Valdés-Correcher, E.; Moreira, X.; Augusto, L.; Barbaro, L.; Bouget, C.; Bouriaud, O.; Branco, M.; Centenaro, G.; Csóka, G.; Damestoy, T.; et al. Search for top-down and bottom-up drivers of latitudinal trends in insect herbivory in oak trees in Europe. Glob. Ecol. Biogeog. 2021, 30, 651–665. [Google Scholar] [CrossRef]
- Eötvös, C.B.; Lövei, G.L.; Magura, T. Predation Pressure on Sentinel Insect Prey along a Riverside Urbanization Gradient in Hungary. Insects 2020, 11, 97. Available online: https://www.mdpi.com/2075-4450/11/2/97 (accessed on 4 November 2021).
- Richards, L.A.; Coley, P.D. Seasonal and habitat differences affect the impact of food and predation on herbivores: A comparison between gaps and understory of a tropical forest. Oikos 2007, 116, 31–40. [Google Scholar] [CrossRef]
- Roslin, T.; Hardwick, B.; Novotny, V.; Petry, W.K.; Andrew, N.R.; Asmus, A.; Barrio, I.C.; Basset, Y.; Boesing, A.L.; Bonebrake, T.C.; et al. Higher predation risk for insect prey at low latitudes and elevations. Science 2017, 356, 742–744. [Google Scholar] [CrossRef] [Green Version]
- Tvardikova, K.; Novotny, V. Predation on exposed and leaf-rolling artificial caterpillars in tropical forests of Papua New Guinea. J. Trop. Ecol. 2012, 28, 331–341. [Google Scholar] [CrossRef] [Green Version]
- Dáttilo, W.; Aguirre, A.; De la Torre, P.L.; Kaminski, L.A.; García-Chávez, J.; Rico-Gray, V. Trait-mediated indirect interactions of ant shape on the attack of caterpillars and fruits. Biol. Lett. 2016, 12, 20160401. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Juricic, E.; Erichsen, J.T.; Kacelnik, A. Visual perception and social foraging in birds. Trends Ecol. Evol. 2004, 19, 25–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boo, K.S.; Chung, I.B.; Han, K.S.; Pickett, J.A.; Wadhams, L.J. Response of the lacewing Chrysopa cognata to pheromones of its aphid prey. J. Chem. Ecol. 1998, 24, 631–643. [Google Scholar] [CrossRef]
- Branco, M.; Lettere, M.; Franco, J.C.; Binazzi, A.; Jactel, H. Kairomonal response of predators to three pine bast scale sex pheromones. J. Chem. Ecol. 2006, 32, 1577–1586. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, T. Chemical cues from Spodoptera litura larvae elicit prey-locating behavior by the predatory stink bug, Eocanthecona furcellata. Entomol. Exp. Appl. 1997, 82, 349–354. [Google Scholar] [CrossRef]
- Turlings, T.; Wäckers, F. Recruitment of predators and parasitoids by herbivore-injured plants. In Advances in Insect Chemical Ecology; Cardé, R., Millar, J., Eds.; Cambridge University Press: Cambridge, UK, 2004; pp. 21–75. [Google Scholar] [CrossRef] [Green Version]
- Goerlitz, H.R.; Siemers, B.M. Sensory ecology of prey rustling sounds: Acoustical features and their classification by wild grey mouse lemurs. Funct. Ecol. 2007, 21, 143–153. [Google Scholar] [CrossRef]
- Castagneyrol, B.; Valdés-Correcher, E.; Bourdin, A.; Barbaro, L.; Bouriaud, O.; Branco, M.; Centenaro, G.; Csóka, G.; Duduman, M.L.; Dulaurent, A.M.; et al. Can school children support ecological research? Lessons from the oak bodyguard citizen science project. Citiz. Sci. Theory Pr. 2020, 5, 10. [Google Scholar] [CrossRef] [Green Version]


| Family | Species | Lepidoptera Predators | Sampling Habitat | |
|---|---|---|---|---|
| Ecological Infrastructures | Agricultural Matrix | |||
| Aegithalidae | Aegithalos caudatus1,3 | x | x | x |
| Alaudidae | Calandrella brachydactyla2 | x | ||
| Certhiidae | Certhia brachydactyla2 | x | ||
| Cisticolidae | Cisticola juncidis1,2 | x | x | x |
| Emberizidae | Emberiza calandra2 | x | x | |
| Fringillidae | Carduelis carduelis1,2,3 | x | x | x |
| Fringillidae | Chloris chloris1,2,3 | x | x | x |
| Fringillidae | Fringilla coelebs2,3 | x | x | |
| Fringillidae | Linaria cannabina2 | x | x | |
| Fringillidae | Serinus serinus1,2,3 | x | x | x |
| Motaciliidae | Motacilla alba1,2 | x | x | |
| Motaciliidae | Motacilla flava2 | x | x | |
| Muscicapidae | Saxicola rubicola2 | x | x | |
| Paridae | Cyanistes caeruleus3 | x | x | |
| Paridae | Parus major1,3 | x | x | x |
| Passeridae | Passer domesticus 1,2 | x | x | x |
| Sittidae | Sitta europaea3 | x | x | |
| Sturnidae | Sturnus unicolor1,2,3 | x | x | x |
| Sylviidae | Cettia cetti1,3 | x | x | x |
| Sylviidae | Hippolais polyglotta1,3 | x | x | x |
| Sylviidae | Phylloscopus ibericus1,3 | x | x | x |
| Sylviidae | Sylvia atricapilla1,3 | x | x | x |
| Sylviidae | Sylvia melanocephala3 | x | ||
| Thoglodytidae | Troglodytes troglodytes1,3 | x | x | |
| Turdidae | Erithacus rubecula3 | x | x | |
| Turdidae | Luscinia megarhynchos1,3 | x | x | x |
| Turdidae | Phoenicurus ochruros2 | x | x | |
| Turdidae | Turdus merula1,2,3 | x | x | x |
| Total number of species | 23 | 26 | 18 | |
| Number of predatory species of Lepidoptera | - | 22 | 15 | |
| Number of observed individuals | - | 258 | 126 | |
| Sampled Sites | Distance to the Nearest EI | Total Area Covered by EI on the 200 m Buffer | Total Area Covered by EI on the 500 m Buffer | |||||
|---|---|---|---|---|---|---|---|---|
| Riparian | Woody | Total | Riparian | Woody | Total | Riparian | Woody | |
| A—All Sites | ||||||||
| Mammal | 0.046 (0.730) | 0.277 * (0.033) | −0.105 (0.431) | −0.012 (0.931) | −0.090 (0.498) | −0.066 (0.621) | −0.003 (0.984) | −0.082 (0.536) |
| Bird | −0.297 * (0.022) | −0.276 * (0.034) | 0.205 (0.119) | 0.260 * (0.046) | 0.337 ** (0.009) | 0.367 ** (0.004) | 0.388 ** (0.002) | 0.493 ** (<0.001) |
| Insect | −0.140 (0.289) | 0.022 (0.868) | −0.027 (0.842) | 0.105 (0.429) | −0.035 (0.793) | −0.052 (0.693) | 0.020 (0.881) | −0.073 (0.583) |
| B—Matrix sites | ||||||||
| Mammal | 0.000 (0.998) | 0.273 (0.124) | 0.172 (0.338) | 0.110 (0.542) | 0.113 (0.532) | 0.116 0.521 | 0.089 (0.624) | 0.081 (0.656) |
| Bird | −0.258 (0.147) | −0.242 (0.176) | 0.483 ** (0.004) | 0.343 (0.051) | 0.358 * (0.041) | 0.616 ** (<0.001) | 0.437 * (0.011) | 0.563 ** (0.001) |
| Insect | −0.166 (0.355) | −0.037 (0.836) | 0.215 (0.230) | 0.291 (0.101) | 0.246 (0.168) | 0.060 (0.742) | 0.135 (0.454) | 0.098 (0.587) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, J.C.; Branco, M.; Conde, S.; Garcia, A.; Fernandes, M.R.; Lima Santos, J.; Messina, T.; Duarte, G.; Fonseca, A.; Zina, V.; et al. Ecological Infrastructures May Enhance Lepidopterans Predation in Irrigated Mediterranean Farmland, Depending on Their Typology and the Predator Guild. Sustainability 2022, 14, 3874. https://doi.org/10.3390/su14073874
Franco JC, Branco M, Conde S, Garcia A, Fernandes MR, Lima Santos J, Messina T, Duarte G, Fonseca A, Zina V, et al. Ecological Infrastructures May Enhance Lepidopterans Predation in Irrigated Mediterranean Farmland, Depending on Their Typology and the Predator Guild. Sustainability. 2022; 14(7):3874. https://doi.org/10.3390/su14073874
Chicago/Turabian StyleFranco, José Carlos, Manuela Branco, Sofia Conde, André Garcia, Maria Rosário Fernandes, José Lima Santos, Tainan Messina, Gonçalo Duarte, André Fonseca, Vera Zina, and et al. 2022. "Ecological Infrastructures May Enhance Lepidopterans Predation in Irrigated Mediterranean Farmland, Depending on Their Typology and the Predator Guild" Sustainability 14, no. 7: 3874. https://doi.org/10.3390/su14073874
APA StyleFranco, J. C., Branco, M., Conde, S., Garcia, A., Fernandes, M. R., Lima Santos, J., Messina, T., Duarte, G., Fonseca, A., Zina, V., & Ferreira, M. T. (2022). Ecological Infrastructures May Enhance Lepidopterans Predation in Irrigated Mediterranean Farmland, Depending on Their Typology and the Predator Guild. Sustainability, 14(7), 3874. https://doi.org/10.3390/su14073874










