Effects of Black Liquor Shocks on the Stability of Activated Sludge Treatment of Kraft Pulp Mill Effluent: Morphological Alteration in Daphnia magna and Mutagenicity and Genotoxicity Response in Salmonella typhimurium
Abstract
1. Introduction
2. Materials and Methods
2.1. Influent
2.2. Activated Sludge System
2.3. Heterotrophic Biomass Activity
2.4. Analytical Methods
2.5. Mutagenicity Test
2.6. Toxicity Testing in Daphnia magna
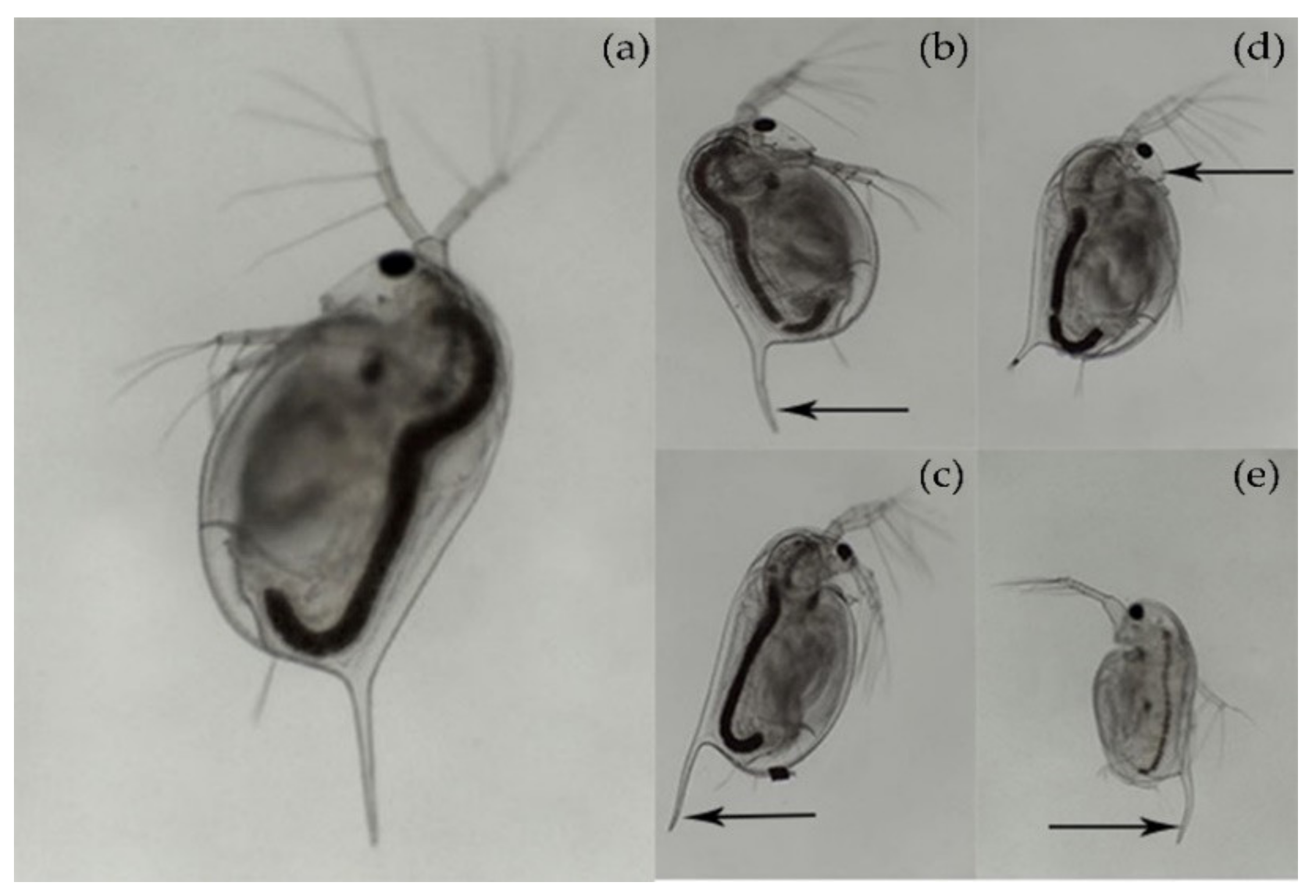
2.7. Morphological Alterations in Daphnia magna
2.8. Statistical Analysis
3. Results and Discussion
3.1. Influent Characterization
3.2. Black Liquor Trials
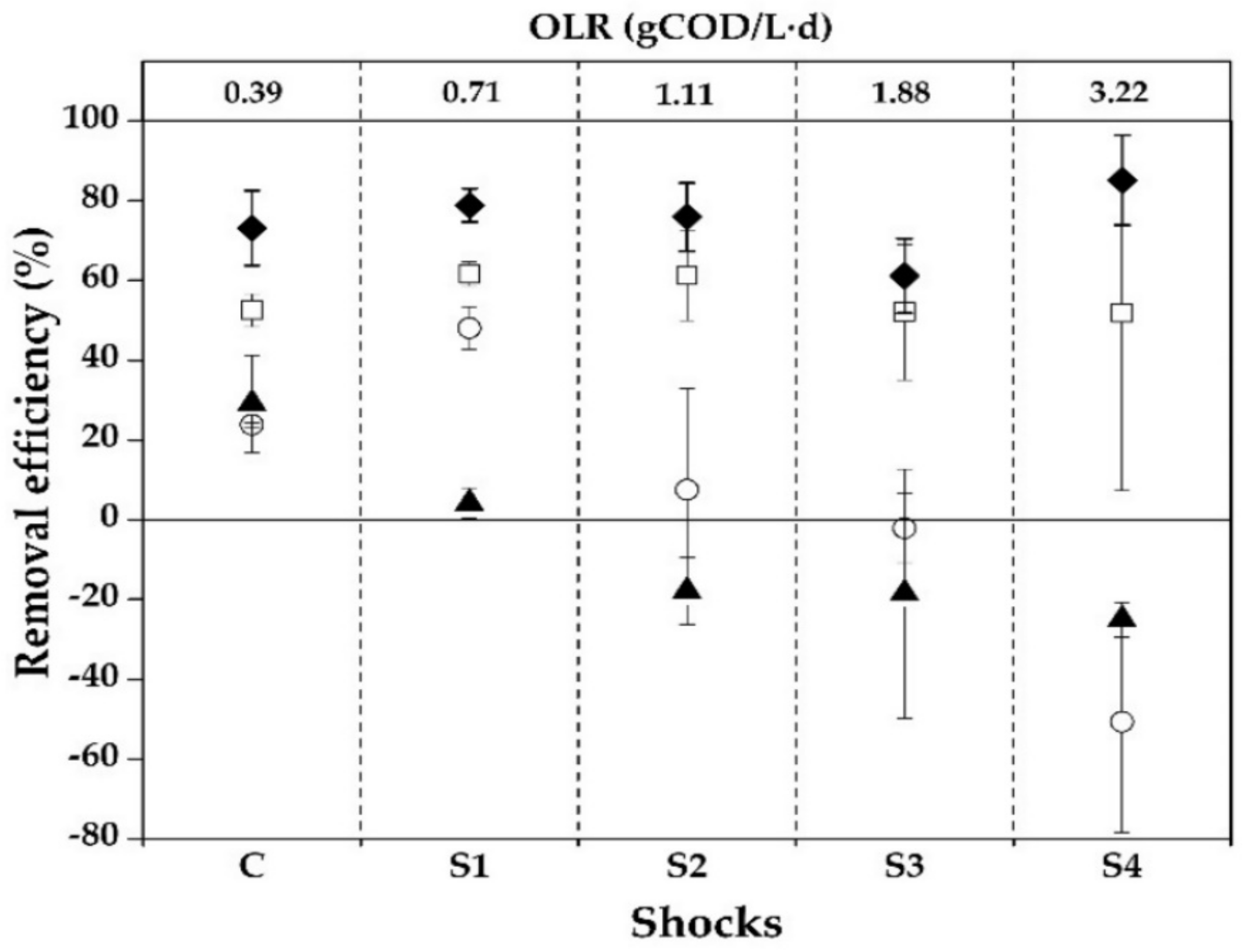
3.3. Activated Sludge Performance
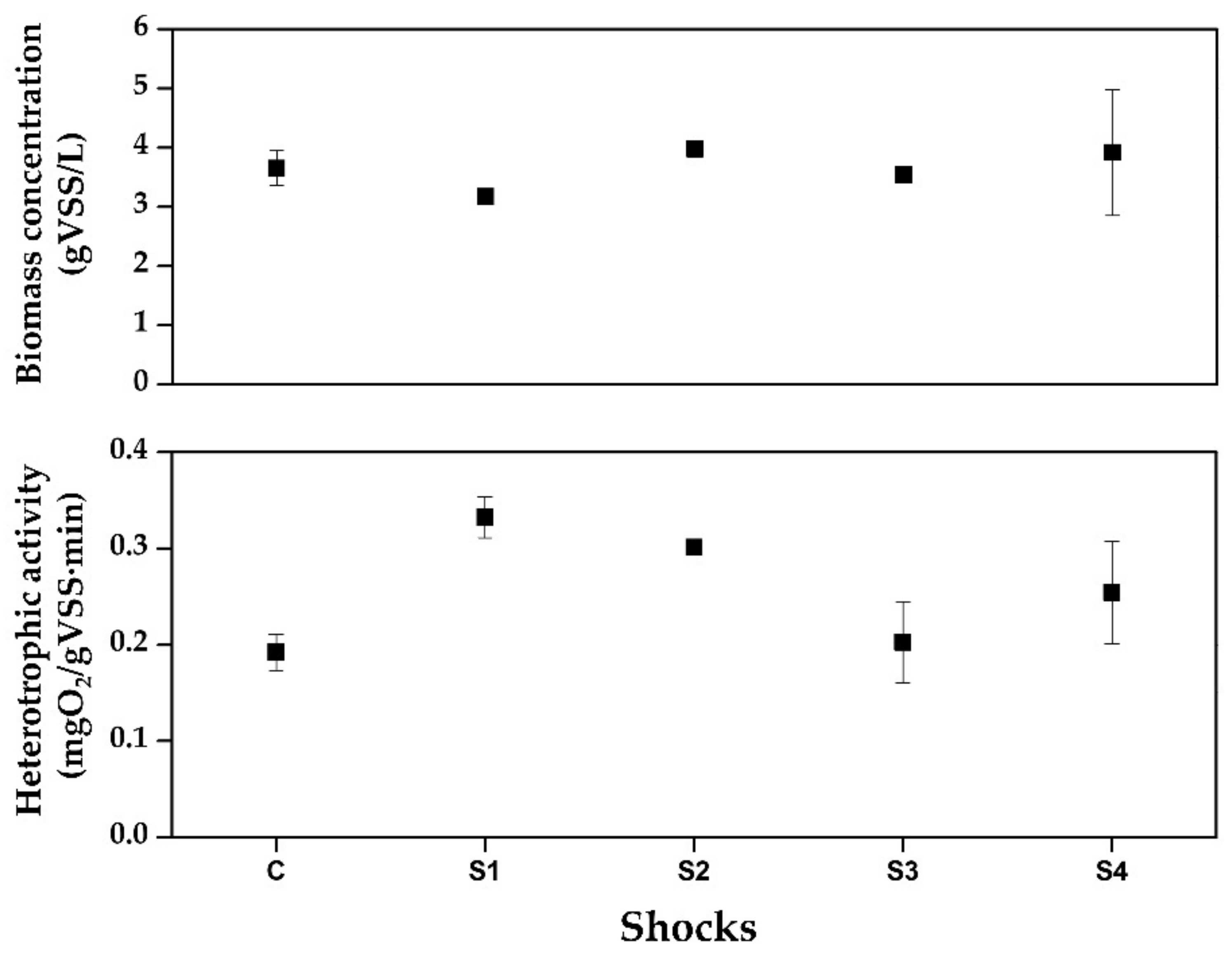
3.4. Biomass and Heterotrophic Activity
3.5. Toxicity Testing in Daphnia magna and Statistical Analysis
3.6. Mutagenicity and Cytotoxicity Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vidal, G.; González, Y.; Piña, B.; Jarpa, M.; Gómez, G. Minimization of environmental impact of kraft pulp mill effluents: Current practices and future perspectives towards sustainability. Sustainability 2021, 13, 9288. [Google Scholar] [CrossRef]
- Singh, U.; Tripathi, Y.C. Characteristics and treatment of pulp and paper mill effluents—A review. Int. J. Eng. Tech. Res. 2020, 10, 19–26. [Google Scholar]
- Morales, G.; Pesante, S.; Vidal, G. Effects of black liquor shocks on activated sludge treatment of bleached kraft pulp mill wastewater. J. Environ. Sci. Health Part A Tox. Hazard. Subst. Environ. Eng. 2015, 50, 627–633. [Google Scholar]
- Gomes, A.; Baptistaa, I.; Sousa, K.; Padilhaa, F.; Toniettoa, J.; Pacheco, A.; Lemos, C.; Barreto, F.; Van Der Kraakc, G. Exposure to a Brazilian pulp mill effluent impacts the testis and liver in the Zebrafish. Comp. Biochem. Physiol. C 2018, 206–207, 41–47. [Google Scholar]
- Chamorro, S.; Monsalvez, E.; Piña, B.; Olivares, A.; Hernández, V.; Becerra, J.; Vidal, G. Analysis of aryl hydrocarbon receptor ligands in kraft mill effluents by a combination of yeast bioassays and CG-MS chemical determinations. J. Environ. Sci. Health Part A Tox. Hazard. Subst. Environ. Eng. 2013, 48, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, S.; Hernández, V.; Matamoros, V.; Domínguez, C.; Becerra, J.; Vidal, G.; Piña, B.; Bayona, J.M. Chemical characterization of organic microcontaminant sources and biological effects in riverine sediments impacted by urban sewage and pulp mill discharges. Chemosphere 2013, 90, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Barra, R.O.; Chiang, G.; Saavedra, M.F.; Orrego, R.; Servos, M.R.; Hewitt, L.M.; McMaster, M.E.; Bahamonde, P.; Tucca, F.; Munkittrick, K.R. Endocrine disruptor impacts on fish from Chile: The influence of wastewaters. Front. Endocrinol. 2021, 12, 611281. [Google Scholar] [CrossRef]
- Chamorro, S.; Xavier, C.; Vidal, G. Behaviour of aromatic compounds contained in the kraft mill effluents measurements by UV-VIS. Biotechnol. Prog. 2005, 21, 1567–1571. [Google Scholar] [CrossRef]
- Vidal, G.; Navia, R.; Levet, L.; Mora, M.L.; Diez, M.C. Kraft mill anaerobic effluent color enhancement by a fixed-bed adsorption system. Biotechnol. Lett. 2001, 23, 861–865. [Google Scholar] [CrossRef]
- Aro, T.; Fatehi, P. Tall oil production from black liquor: Challenges and opportunities. Sep. Purif. Technol. 2017, 175, 469–480. [Google Scholar] [CrossRef]
- Schmitt, D.; Beiser, N.; Regenbrecht, C.; Zirbes, M.; Waldvogel, S. Adsorption and separation of black liquor-derived phenol derivatives using anion exchange resins. Sep. Purif. Technol. 2017, 181, 8–17. [Google Scholar] [CrossRef]
- Schuijt, L.M.; Peng, F.-J.; van den Berg, S.J.P.; Dingemans, M.M.L.; Van den Brink, P.J. (Eco)toxicological tests for assessing impacts of chemical stress to aquatic ecosystems: Facts, challenges, and future. Sci. Total Environ. 2021, 795, 148776. [Google Scholar] [CrossRef]
- Chamorro, S.; López, D.; Brito, P.; Jarpa, M.; Piña, B.; Vidal, G. Sublethal effects of chlorine-free kraft mill effluents on Daphnia magna. Bull. Environ. Contam. Toxicol. 2016, 97, 843–847. [Google Scholar] [CrossRef]
- Lofrano, G.; Brown, J. Wastewater management through the ages: A history of mankind. Sci. Total Environ. 2010, 5408, 5254–5264. [Google Scholar] [CrossRef]
- Hart, R.C.; Bychek, E.A. Body size in freshwater planktonic crustaceans: An overview of extrinsic determinants and modifying influences of biotic interactions. Hydrobiologia 2011, 668, 61–108. [Google Scholar] [CrossRef]
- Orrego, R.; Craig, B.; Milestone, L.; Hewitt, L.; Guchardi, J.; Heidd-Furley, T.; Slade, A.; Maclatchy, D.L.; Holdway, D. Evaluating the potential of effluent extracts from pulp and paper mill in Canada, Brazil and New Zealand to affect fish re production: Estrogenics effects in fish. Environ. Toxicol. Chem. 2017, 36, 1547–1555. [Google Scholar] [CrossRef]
- Pereira, J.L.; Gonçalves, F. Daphnia fitness over a food gradient: Is body size the single trait predicting exploitative ability? Ann. Limnol.-Int. J. Limnol. 2008, 44, 169–179. [Google Scholar] [CrossRef][Green Version]
- López, D.; Chamorro, S.; Silva, J.; Bay-Schmith, E.; Vidal, G. Chronic effects of Pinus radiata and Eucalyptus globulus kraft mill effluents and phytosterols on Daphnia magna. Bull. Environ. Contam. Toxicol. 2011, 87, 633–637. [Google Scholar] [CrossRef]
- Preisser, E.L.; Orrock, J.L. The allometry of fear: Interspecific relationships between body size and response to predation risk. Ecosphere 2012, 3, 77. [Google Scholar] [CrossRef]
- Otte, K.; Fröhlich, T.; Arnold, G.; Laforsch, C. Proteomic analysis of Daphnia magna hints at molecular pathways involved in defensive plastic responses. BMC Genom. 2014, 15, 2–16. [Google Scholar] [CrossRef]
- Riessen, H.; Young, J. Daphnia defense strategies in fishless lakes and ponds: One size does not fit all. J. Plankton Res. 2005, 27, 531–544. [Google Scholar] [CrossRef]
- Vidal, G.; Becerra, J.; Hernández, V.; Decap, J.; Xavier, C.R. Anaerobic biodegradation of sterols contained in kraft mill effluents. J. Biosci. Bioeng. 2007, 104, 476–480. [Google Scholar] [CrossRef]
- Haq, I.; Kumar, S.; Raj, A.; Lohani, M.; Satyanarayana, G.N.V. Genotoxicity assessment of pulp and paper mill effluent before and after bacterial degradation using Allium cepa test. Chemosphere 2017, 169, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Oanh, N.T.; Bengtsson, B.E.; Reutergardh, L.B.; Hoa, D.T.; Bergqvist, P.A.; Broman, D.; Zebuhr, Y. Persistent organochlorines in the effluents from a chlorine-bleached Kraft integrated pulp and paper mill in Southeast Asia. Arch. Environ. Contam. Toxicol. 1999, 638, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Savant, D.; Abdul-Rahman, R.; Ranade, D. Anaerobic degradation of sorbable organic halides (AOX) from pulp and paper industry wastewater. Bioresour. Technol. 2006, 97, 1092–1104. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association (APHA); American Water Works Association (AWWA); Pollution Control Federation (WPCF). Standard Methods for Examination of Water and Wastewater, 16th ed.; APHA: Washington, DC, USA, 1985. [Google Scholar]
- Chamorro, S.; Xavier, C.; Hernández, V.; Becerra, J.; Vidal, G. Aerobic removal of stigmaterol contained in kraft mill effluents. Electron. J. Biotechnol. 2009, 12, 1–7. [Google Scholar] [CrossRef][Green Version]
- USEPA. Short-Term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Water to Freshwater Organisms; EPA-600-4-91-002; US Environmental Protection Agency, Office of Research and Development: Washington, DC, USA, 1994.
- Chamorro, S.; Pozo, G.; Jarpa, M.; Hernández, V.; Becerra, J.; Vidal, G. Monitoring endocrine activity in kraft mill effluent treated by aerobic moving bed bioreactor system. Water Sci. Technol. 2010, 62, 154–161. [Google Scholar] [CrossRef]
- Vadodaria, S.N. Effect of Black Liquor on the Activated Sludge Process: An Experimental Investigation of the Effect of Black Liquor on the Activated Sludge Treatment of Bleached Kraft Mill Effluents. Master’s Thesis, McGill University, Montreal, QC, Canada, 1999. [Google Scholar]
- Xavier, C.; Mosquera-Corral, A.; Becerra, J.; Hernández, V.; Vidal, G. Activated sludge versus aerated lagoon treatment of kraft mill effluents containing β-sitosterol and stigmasterol. J. Toxicol. Environ. Health A 2009, 44, 327–335. [Google Scholar] [CrossRef]
- Diez, M.C.; Castillo, G.; Aguilar, L.; Vidal, G.; Mora, M.L. Operational factors and nutrient effects on activated sludge treatment of Pinus radiata kraft mill wastewater. Bioresour. Technol. 2002, 83, 131–138. [Google Scholar] [CrossRef]
- Tsang, Y.F.; Hua, F.L.; Chua, H.; Sin, S.N.; Wang, Y.J. Optimization of biological treatment of paper mill effluent in a sequencing batch reactor. Biochem. Eng. J. 2007, 34, 193–199. [Google Scholar] [CrossRef]
- Villamar, C.A.; Jarpa, M.; Decap, J.; Vidal, G. Aerobic moving bed bioreactor treating kraft mill effluents from Pinus radiata and Eucalyptus globulus as raw material. Water Sci. Technol. 2009, 59, 507–514. [Google Scholar] [CrossRef][Green Version]
- Torretta, V.; Ragazzi, M.; Trulli, E.; De Feo, G.; Urbini, G.; Raboni, M.; Rada, E. Assessment of biological kinetics in a conventional municipal WWTP by means of the oxygen uptake rate method. Sustainability 2014, 6, 1833–1847. [Google Scholar] [CrossRef]
- Sandberg, M.; Holby, O. Black liquor and alkaline shocks in a multiple stage biological treatment plant. J. Environ. Eng. Sci. 2008, 7, 335–344. [Google Scholar] [CrossRef]
- Olmstead, A.; LeBlanc, G. Effects of endocrine-active chemicals on the development of sex characteristic of Daphnia magna. Environ. Toxicol. Chem. 2000, 19, 2107–2113. [Google Scholar] [CrossRef]
- Clubbs, R.L.; Brooks, B.W. Daphnia magna responses to a vertebrate estrogen receptor agonist and an antagonist: A multigenerational study. Ecotoxicol. Environ. Saf. 2007, 7, 385–398. [Google Scholar] [CrossRef]
- Sperfeld, E.; Wacker, A. Effects of temperature and dietary sterol availability on growth and cholesterol allocation of the aquatic keystone species Daphnia. J. Exp. Biol. 2009, 212, 3051–3059. [Google Scholar] [CrossRef]
- Piepho, M.; Martin-Creuzburg, D.; Wacker, A. Simultaneous Effects of light intensity and phosphorus supply on the sterol content of phytoplankton. PLoS ONE 2010, 5, e15828. [Google Scholar] [CrossRef]
- Martin-Creuzburg, M.; Oexle, S.; Wacker, A. Thresholds for sterol-limited growth of Daphnia magna: A comparative approach using 10 different sterols. J. Chem. Ecol. 2014, 40, 1039–1050. [Google Scholar] [CrossRef]
- Castiglioni, M.; Collins, P.; Paggi, J.C. Detergent-induced cyclomorphosis in Daphnia magna. FABICIB 2008, 12, 137–148. [Google Scholar] [CrossRef]
- Smolders, R.; Baillieul, M.; Blust, R. Relationship between the energy status of Daphnia magna and its sensitivity to environmental stress. Aquat. Toxicol. 2005, 73, 155–170. [Google Scholar] [CrossRef]
- Kim, H.J.; Koedrith, P.; Seo, Y.R. Ecotoxicogenomic approaches for understanding molecular mechanisms of environmental chemical toxicity using aquatic invertebrate, daphnia model organism. Int. J. Mol. Sci. 2015, 16, 12261–12287. [Google Scholar] [CrossRef] [PubMed]
- Knops, M.; Altenburger, R.; Segner, H. Alterations of physiological energetics, growth and reproduction of Daphnia magna under toxicant stress. Aquat. Toxicol. 2001, 53, 79–90. [Google Scholar] [CrossRef]
- Flohr, L.; de Castilhos, J.A.; Gerson, W. Acute and chronic toxicity of soluble fractions of industrial solid wastes on Daphnia magna and Vibrio fischeri. Sci. World J. 2012, 2012, 643904. [Google Scholar] [CrossRef] [PubMed]
- Xavier, C.; Chamorro, S.; Vidal, G. Chronic effects of kraft mill effluents and endocrine active chemicals on Daphnia magna. Bull. Environ. Contam. Toxicol. 2005, 75, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Van den Heuvel, M.; Landman, M.; Finley, M.; David, W. Altered physiology of rainbow trout in response to modified energy intake combined with pulp and paper effluent exposure. Ecotoxicol. Environ. Saf. 2008, 69, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Orrego, R.; Hewitt, L.M.; McMaster, M.; Chiang, G.; Quiroz, M.; Munkittrick, K.; Gavilán, J.; Barra, R. Assessing wild fish exposure to ligands for sex steroid receptors from pulp and paper mill effluents in the Biobio River Basin, Central Chile. Ecotoxicol. Environ. Saf. 2019, 171, 256–263. [Google Scholar] [CrossRef]
- Rao, S.; Burnison, B.K.; Rokosh, D.A.; Taylor, C.M. Mutagenicity and toxicity assessment of pulp mill effluent. Chemosphere 1995, 28, 1859–1870. [Google Scholar] [CrossRef]
- Hewitt, L.M. Effects-directed studies of pulp and paper mill effluents. In Effect-Directed Analysis of Complex Environmental Contamination, 1st ed.; Brack, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 15, pp. 267–283. [Google Scholar]
- Xavier, C.; Oñate, E.; Mondaca, M.A.; Campos, J.; Vidal, G. Genotoxic effects of kraft pulp mill effluents treated by biological aerobic systems. Interciencia 2011, 36, 412–416. [Google Scholar]
- Belmonte, M.; Xavier, C.; Decap, J.; Martínez, M.; Sierra, R.; Vidal, G. Improvement of the abietic acid biodegradation contained in ECF effluent due to biomass adaptation. J. Hazard. Mater. 2006, 135, 256–263. [Google Scholar] [CrossRef]
| Parameter | Unit | Average ± SD |
|---|---|---|
| pH | - | 7.10 ± 3.91 |
| Conductivity | mS/cm | 3.05 ± 0.09 |
| BOD5 | mg/L | 345.00 ± 41.20 |
| COD | mg/L | 477.13 ± 76.10 |
| Color (440 nm) | 1 × 1 cm | 0.15 ± 0.03 |
| Lignosulfnic acid (346 nm) | 1 × 1 cm | 0.05 ± 0.02 |
| Aromatic compounds (254 nm) | 1 × 1 cm | 0.59 ± 0.05 |
| Lignin derives (280 nm) | 1 × 1 cm | 2.12 ± 0.11 |
| Total phenolic compounds | mg/L | 150.30 ± 57.83 |
| TN | mg/L | <0.50 |
| TP | mg/L | 23.92 ± 0.59 |
| Shock | Black Liquor Dose (mL/L) * | Operation Time (d) | pH | EC (mS/cm) | COD (mg/L) | OLR Increase (%) |
|---|---|---|---|---|---|---|
| S1 | 2 | 13 | 7.5 | 3.47 | 708 ± 3.91 | 180.90 |
| S2 | 4 | 21 | 7.2 | 3.79 | 1113 ± 7.01 | 287.46 |
| S3 | 10 | 27 | 7.3 | 4.53 | 1887 ± 15.29 | 487.36 |
| S4 | 30 | 34 | 7.1 | 6.35 | 3221 ± 12.03 | 832.04 |
| Variable | R2 | Intercept | L | C | Interaction | |
|---|---|---|---|---|---|---|
| Body | 0.662 | Coefficient | 5.09 × 10−2 | 6.71 × 10−5 | 1.24 × 10−4 | 4.42 × 10−6 |
| Standard Error | 1.08 × 10−3 | 7.16 × 10−5 | 2.21 × 10−5 | 1.42 × 10−6 | ||
| Probability | 5.08 × 10−60 | 3.52 × 10−1 | 2.93 × 10−7 | 2.58 × 10−3 | ||
| Abdomen | 0.645 | Coefficient | 3.60 × 10−2 | 4.35 × 10−5 | 7.33 × 10−5 | 3.06 × 10−6 |
| Standard Error | 7.15 × 10−4 | 4.72 × 10−5 | 1.45 × 10−5 | 9.37 × 10−7 | ||
| Probability | 2.12 × 10−62 | 3.60 × 10−1 | 2.82 × 10−6 | 1.59 × 10−3 |
| Treatments | Revertant per Plate (+S9) | Result | |||||
|---|---|---|---|---|---|---|---|
| Day | S Factor | ||||||
| 2 | 3 | 4 | 5 | 6 | |||
| −Control | 0 | 0 | 0 | 0 | 0 | - | - |
| Background | 0 | 13 | 16 | 18 | 20 | - | - |
| +Control | 0 | 44 | 63 | 70 | 72 | - | - |
| S1 24 h | 0 | 21 | 25 | 31 | 37 | 0.514 | Mutagenicity, 99.0% significance |
| S1 48 h | 0 | 16 | 18 | 19 | 21 | 0.292 | Possible cytotoxicity |
| S2 24 h | 0 | 25 | 43 | 43 | 45 | 0.625 | Mutagenicity, 99.9% significance |
| S2 48 h | 0 | 5 | 5 | 7 | 10 | 0.139 | Cytotoxicity |
| S3 24 h | 0 | 4 | 23 | 25 | 27 | 0.375 | Possible cytotoxicity |
| S3 48 h | 0 | 9 | 15 | 19 | 25 | 0.347 | Possible cytotoxicity |
| S4 24 h | 7 | 8 | 8 | 8 | 8 | 0.111 | Cytotoxicity |
| S4 48 h | 7 | 7 | 8 | 10 | 10 | 0.139 | Cytotoxicity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamorro, S.; Hernández, L.; Saéz, K.; Gómez, G.; Vidal, G. Effects of Black Liquor Shocks on the Stability of Activated Sludge Treatment of Kraft Pulp Mill Effluent: Morphological Alteration in Daphnia magna and Mutagenicity and Genotoxicity Response in Salmonella typhimurium. Sustainability 2022, 14, 3869. https://doi.org/10.3390/su14073869
Chamorro S, Hernández L, Saéz K, Gómez G, Vidal G. Effects of Black Liquor Shocks on the Stability of Activated Sludge Treatment of Kraft Pulp Mill Effluent: Morphological Alteration in Daphnia magna and Mutagenicity and Genotoxicity Response in Salmonella typhimurium. Sustainability. 2022; 14(7):3869. https://doi.org/10.3390/su14073869
Chicago/Turabian StyleChamorro, Soledad, Laura Hernández, Katia Saéz, Gloria Gómez, and Gladys Vidal. 2022. "Effects of Black Liquor Shocks on the Stability of Activated Sludge Treatment of Kraft Pulp Mill Effluent: Morphological Alteration in Daphnia magna and Mutagenicity and Genotoxicity Response in Salmonella typhimurium" Sustainability 14, no. 7: 3869. https://doi.org/10.3390/su14073869
APA StyleChamorro, S., Hernández, L., Saéz, K., Gómez, G., & Vidal, G. (2022). Effects of Black Liquor Shocks on the Stability of Activated Sludge Treatment of Kraft Pulp Mill Effluent: Morphological Alteration in Daphnia magna and Mutagenicity and Genotoxicity Response in Salmonella typhimurium. Sustainability, 14(7), 3869. https://doi.org/10.3390/su14073869






