Global Warming and Toxicity Impacts: Peanuts in Georgia, USA Using Life Cycle Assessment
Abstract
:1. Introduction
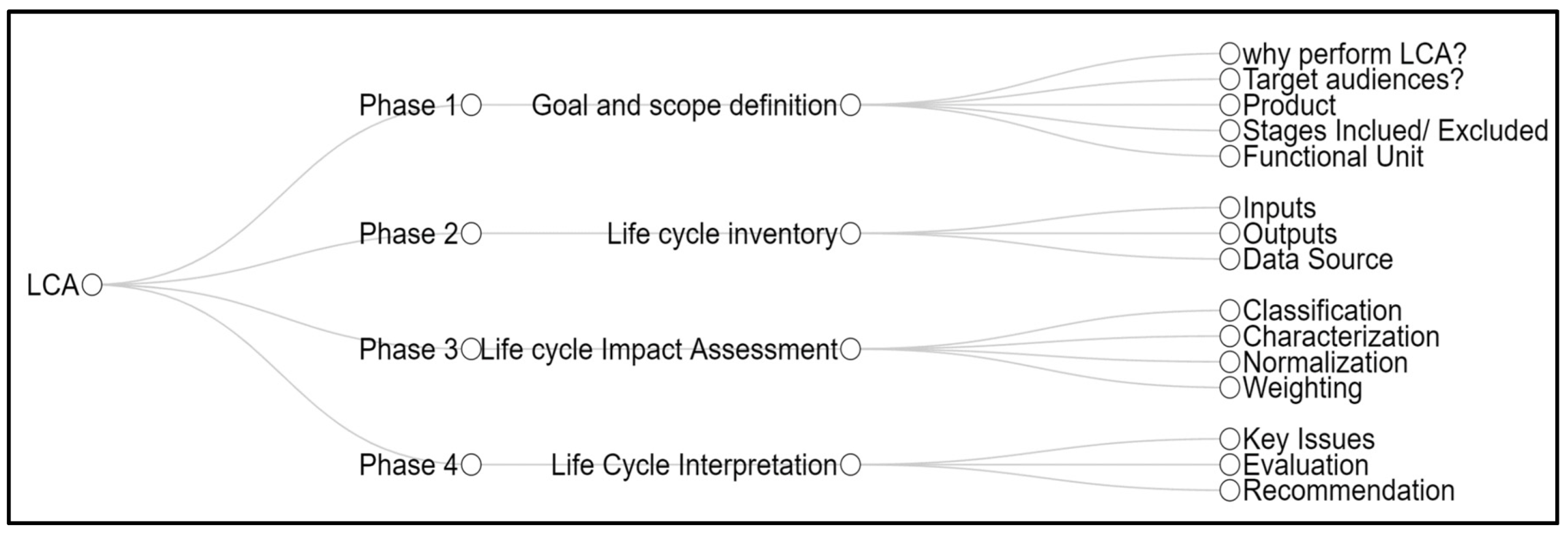
2. Materials and Methods
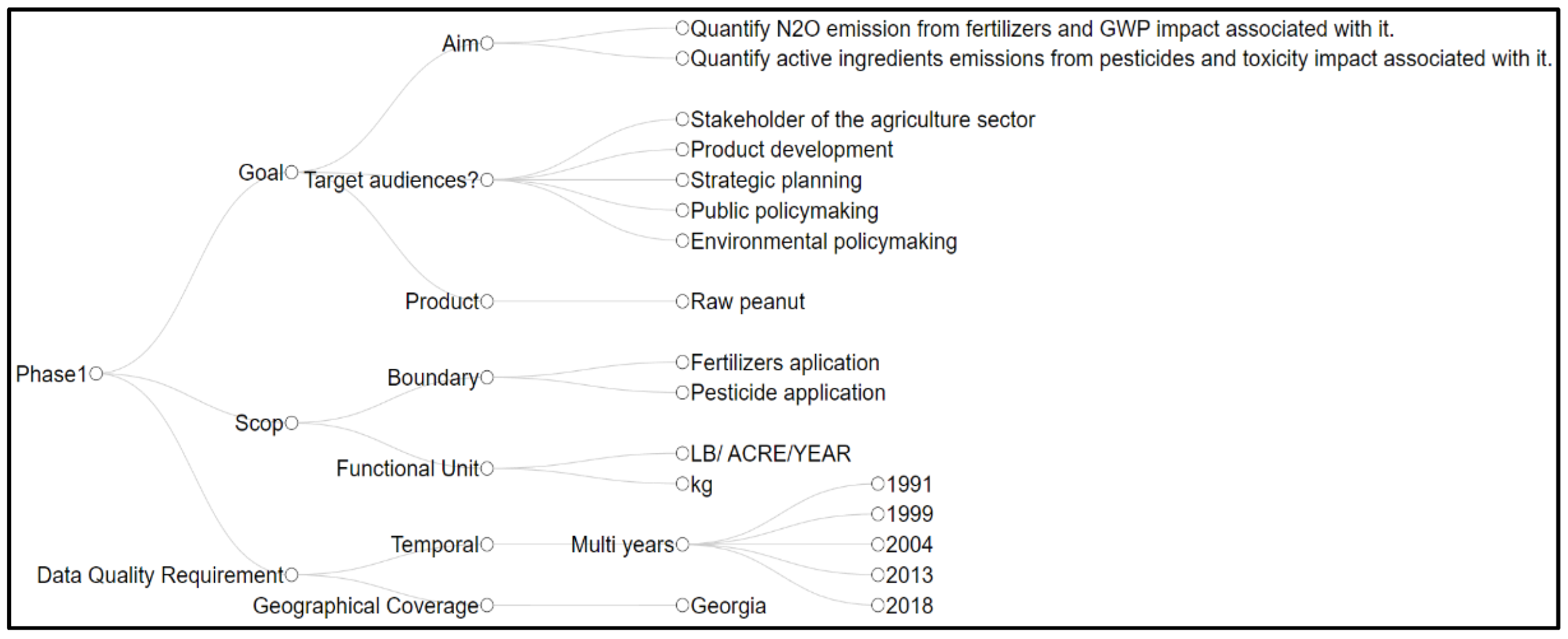
2.1. Phase 1: Goal and Scope Definition
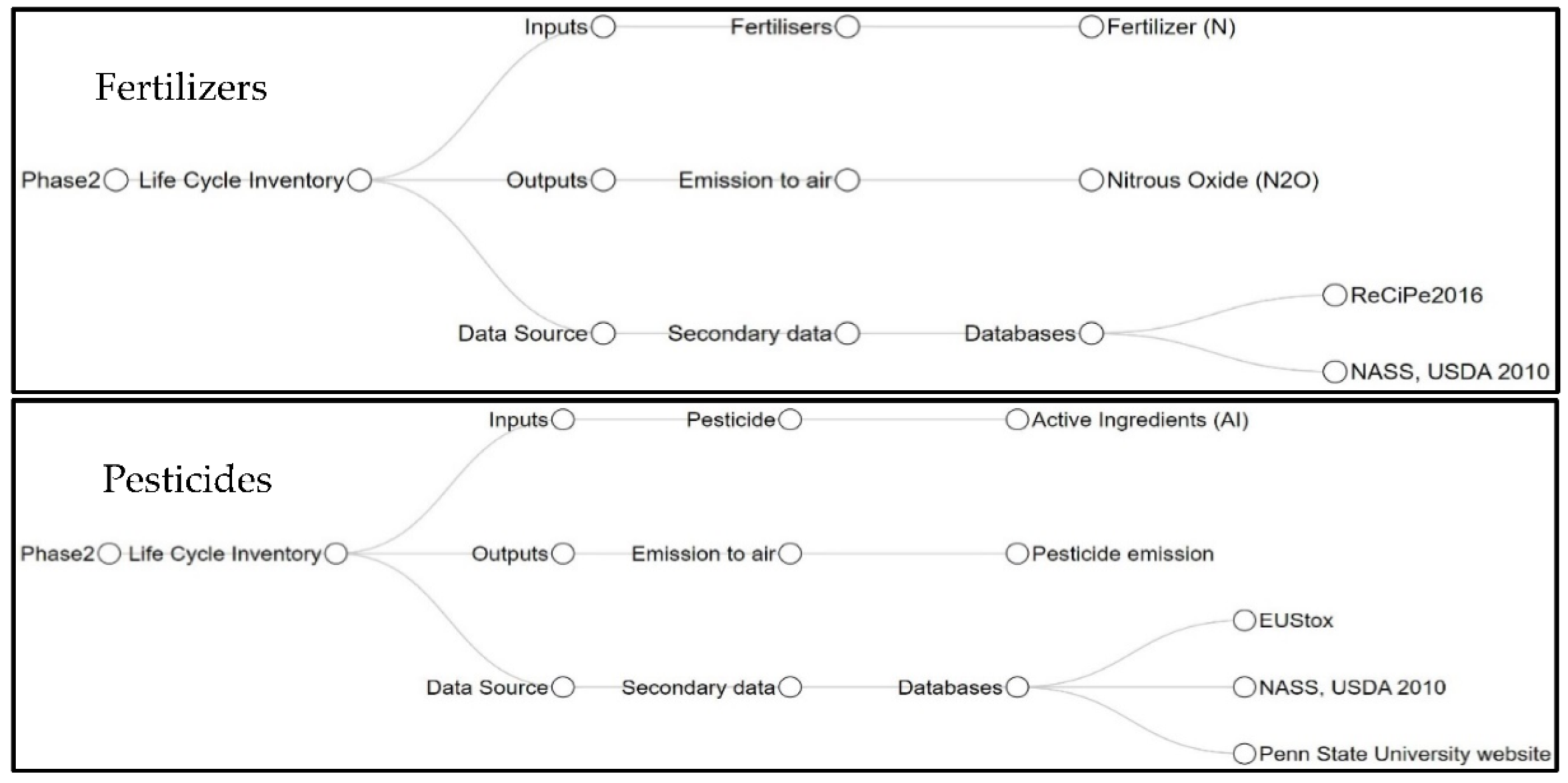
2.2. Phase 2: Life Cycle Inventory (LCI)
2.3. Phase 2: Input Estimation
2.4. Phase 2: Output Estimation
2.5. Phase 2: Data Source (Secondary)
2.6. Phase 3: Life Cycle Impact Assessment (LCIA)
2.7. Phase 3: Classification
2.8. Phase 3: Characterization
2.9. Phase 4: Life Cycle Interpretation
3. Result
3.1. Phase 2 Input and Output Estimation: Amount Applied, and Emissions of Pesticide and Fertilizers to the Air
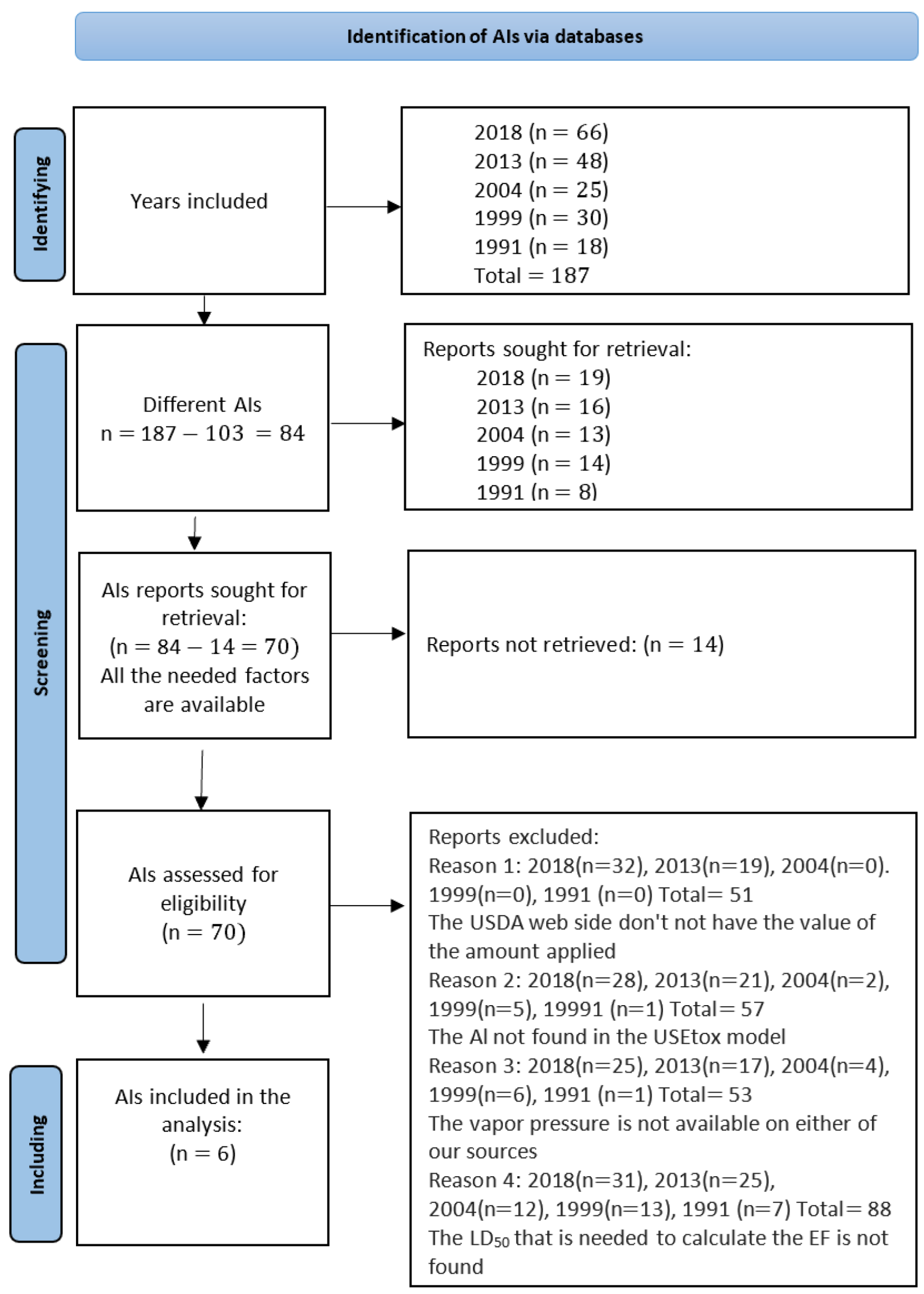
3.2. Phase 3 Characterization: Characteristics of AIs for Oral and Dermal Toxicity from Pesticides with the Six Common AIs
3.3. Calculation of the EF for the Six Common AIs
3.4. Calculation of the CF of the Six Common AIs (Mid-Point)
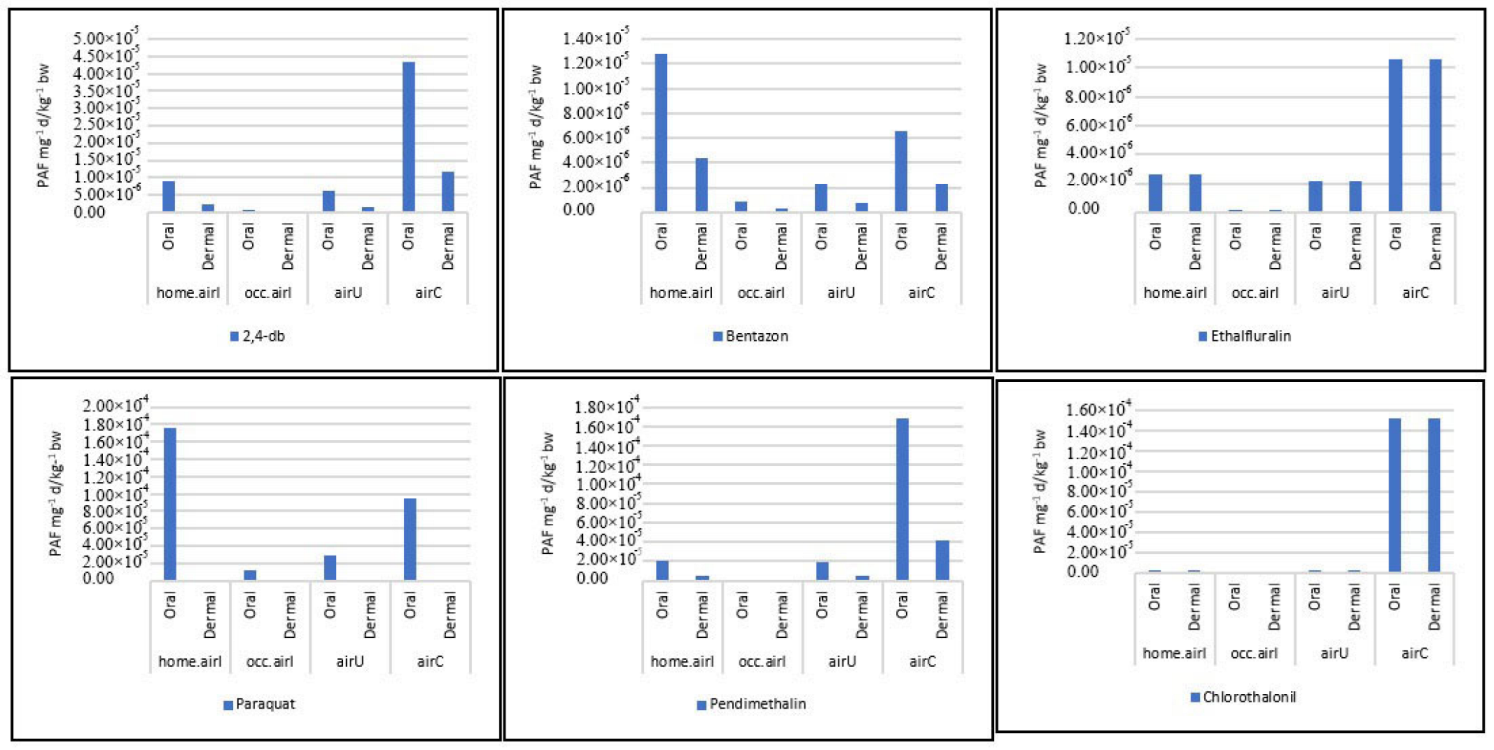
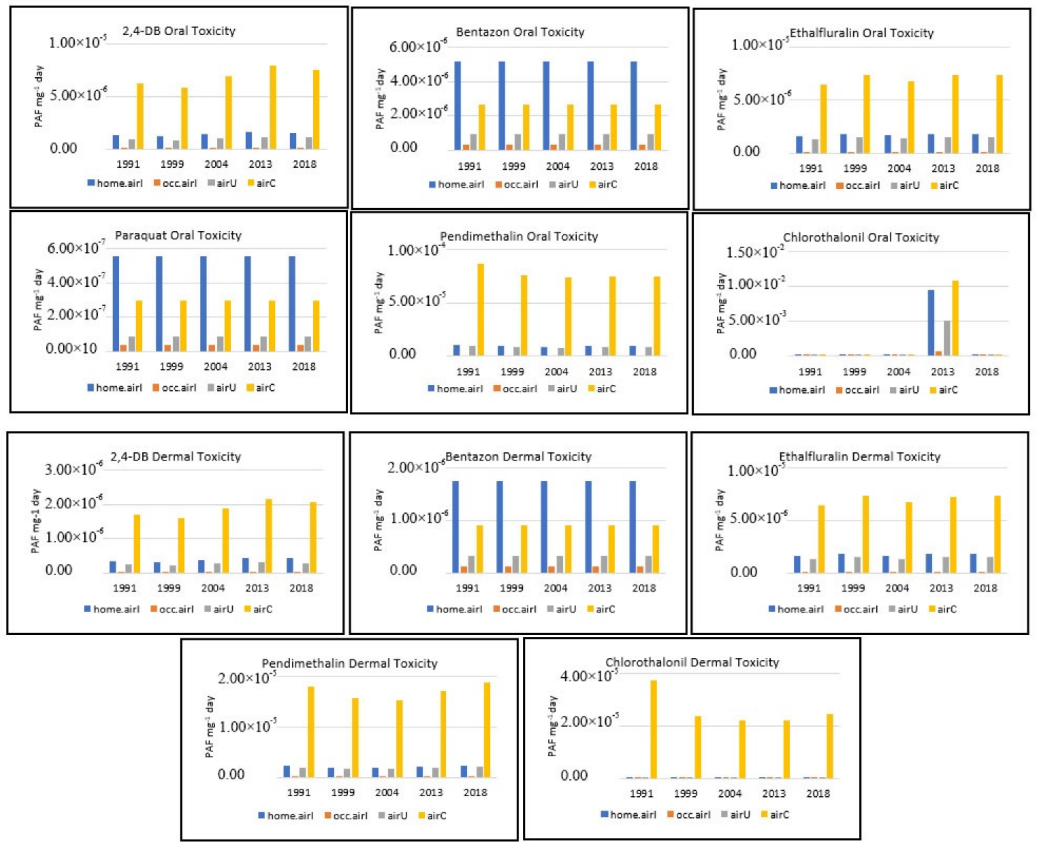
3.5. Potential Oral and Dermal Toxicity of the Six Common AIs (End-Point)
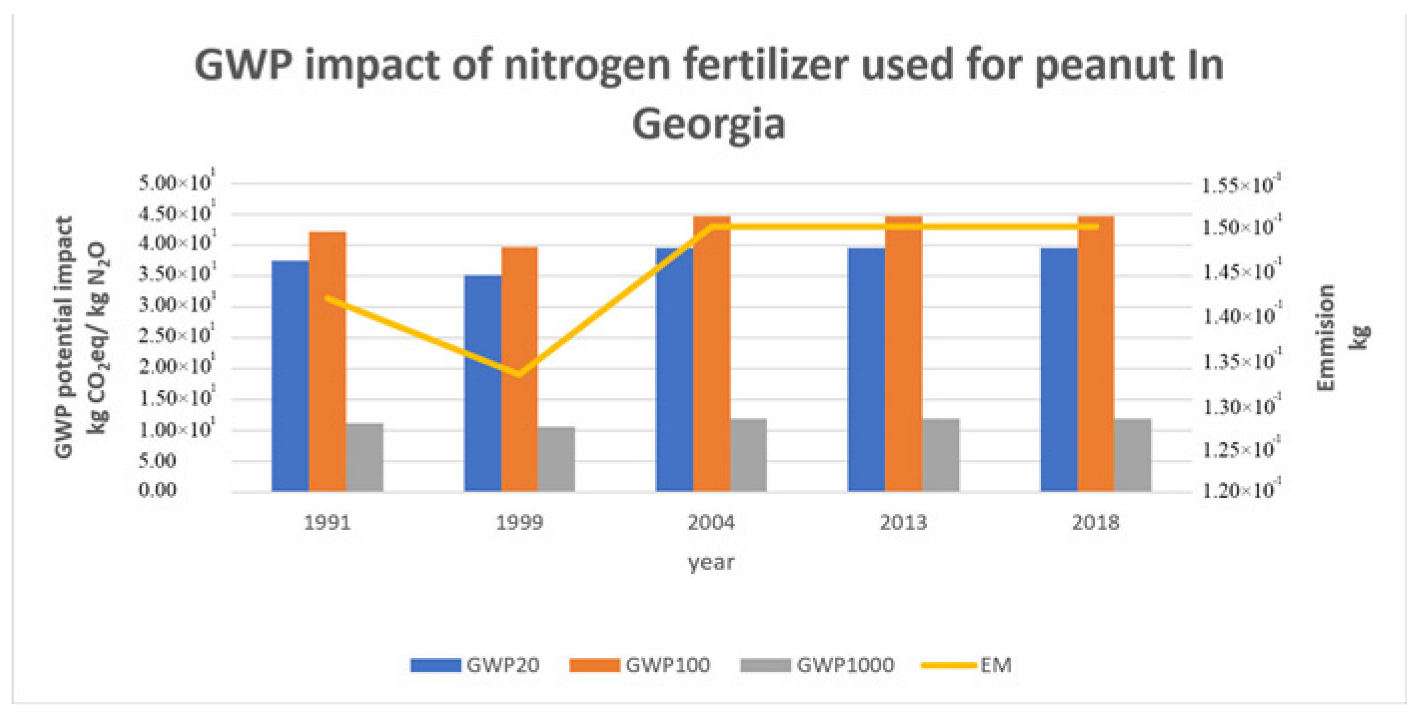
3.6. GWP 20, 100, 1000: Time Horizon of the Fertilizers Used to Produce Peanuts in Georgia (End-Point)
4. Discussion
4.1. Characterization Factor
4.2. Active Ingredient Characteristics
4.3. Potential Uses for This Study
4.4. Key Issues and Recommendations
4.5. Challenges and Limitations
4.6. Assumptions Used Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| AI | 2018 | 2013 | 2004 | 1999 | 1991 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| home.airI | occ.airI | airU | airC | home.airI | occ.airI | airU | airC | home.airI | occ.airI | airU | airC | home.airI | occ.airI | airU | airC | home.airI | occ.airI | airU | airC | |
| 2,4-DB | 1.5 × 10−6 | 1.0 × 10−7 | 1.1 × 10−6 | 7.6 × 10−6 | 1.6 × 10−6 | 1.1 × 10−7 | 1.1 × 10−6 | 8.0 × 10−6 | 1.4 × 10−6 | 9.3 × 10−8 | 9.8 × 10−7 | 6.9 × 10−6 | 1.2 × 10−6 | 7.8 × 10−8 | 8.3 × 10−7 | 5.8 × 10−6 | 1.3 × 10−6 | 8.4 × 10−8 | 8.9 × 10−7 | 6.3 × 10−6 |
| Bentazon | 5.2 × 10−6 | 3.4 × 10−7 | 9.3 × 10−7 | 2.7 × 10−6 | 5.2 × 10−6 | 3.4 × 10−7 | 9.3 × 10−7 | 2.7 × 10−6 | 5.2 × 10−6 | 3.4 × 10−7 | 9.3 × 10−7 | 2.7 × 10−6 | 5.2 × 10−6 | 3.4 × 10−7 | 9.3 × 10−7 | 2.7 × 10−6 | 5.2 × 10−6 | 3.4 × 10−7 | 9.3 × 10−7 | 2.7 × 10−6 |
| Ethalfluralin | 1.8 × 10−6 | 1.2 × 10−7 | 1.5 × 10−6 | 7.4 × 10−6 | 1.8 × 10−6 | 1.2 × 10−7 | 1.5 × 10−6 | 7.3 × 10−6 | 1.7 × 10−6 | 1.1 × 10−7 | 1.4 × 10−6 | 6.7 × 10−6 | 1.8 × 10−6 | 1.2 × 10−7 | 1.5 × 10−6 | 7.4 × 10−6 | 1.6 × 10−6 | 1.0 × 10−7 | 1.3 × 10−6 | 6.4 × 10−6 |
| Paraquat | 5.6 × 10−7 | 3.7 × 10−8 | 9.0 × 10−8 | 3.0 × 10−7 | 5.6 × 10−7 | 3.7 × 10−8 | 9.0 × 10−8 | 3.0 × 10−7 | 5.6 × 10−7 | 3.7 × 10−8 | 9.0 × 10−8 | 3.0 × 10−7 | 5.6 × 10−7 | 3.7 × 10−8 | 9.0 × 10−8 | 3.0 × 10−7 | 5.6 × 10−7 | 3.7 × 10−8 | 9.0 × 10−8 | 3.0 × 10−7 |
| Pendimethalin | 9.4 × 10−6 | 6.2 × 10−7 | 8.4 × 10−6 | 7.5 × 10−5 | 1.0 × 10−5 | 6.8 × 10−7 | 9.2 × 10−6 | 8.2 × 10−5 | 9.3 × 10−6 | 6.1 × 10−7 | 8.3 × 10−6 | 7.4 × 10−5 | 9.5 × 10−6 | 6.2 × 10−7 | 8.5 × 10−6 | 7.6 × 10−5 | 1.1 × 10−5 | 7.1 × 10−7 | 9.7 × 10−6 | 8.7 × 10−5 |
| Chlorothalonil | 4.3 × 10−7 | 2.8 × 10−8 | 4.3 × 10−7 | 2.5 × 10−5 | 3.9 × 10−7 | 2.6 × 10−8 | 3.9 × 10−7 | 2.2 × 10−5 | 3.9 × 10−7 | 2.6 × 10−8 | 3.9 × 10−7 | 2.2 × 10−5 | 4.2 × 10−7 | 2.7 × 10−8 | 4.2 × 10−7 | 2.4 × 10−5 | 6.5 × 10−7 | 4.3 × 10−8 | 6.5 × 10−7 | 3.7 × 10−5 |
| AI | 2018 | 2013 | 2004 | 1999 | 1991 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| home.airI | occ.airI | airU | airC | home.airI | occ.airI | airU | airC | home.airI | occ.airI | airU | airC | home.airI | occ.airI | airU | airC | home.airI | occ.airI | airU | airC | |
| 2,4-DB | 1.5 × 106 | 1.0 × 10−7 | 1.1 × 10−6 | 7.6 × 10−6 | 1.6 × 10−6 | 1.1 × 10−7 | 1.1 × 10−6 | 8.0 × 10−6 | 1.4 × 10−6 | 9.3 × 10−8 | 9.8 × 10−7 | 6.9 × 10−6 | 1.2 × 10−6 | 7.8 × 10−8 | 8.3 × 10−7 | 5.8 × 10−6 | 1.3 × 10−6 | 8.4 × 10−8 | 8.9 × 10−7 | 6.3 × 10−6 |
| Bentazon | 5.2 × 10−6 | 3.4 × 10−7 | 9.3 × 10−7 | 2.7 × 10−6 | 5.2 × 10−6 | 3.4 × 10−7 | 9.3 × 10−7 | 2.7 × 10−6 | 5.2 × 10−6 | 3.4 × 10−7 | 9.3 × 10−7 | 2.7 × 10−6 | 5.2 × 10−6 | 3.4 × 10−7 | 9.3 × 10−7 | 2.7 × 10−6 | 5.2 × 10−6 | 3.4 × 10−7 | 9.3 × 10−7 | 2.7 × 10−6 |
| Ethalfluralin | 1.8 × 10−6 | 1.2 × 10−7 | 1.5 × 10−6 | 7.4 × 10−6 | 1.8 × 10−6 | 1.2 × 10−7 | 1.5 × 10−6 | 7.3 × 10−6 | 1.7 × 10−6 | 1.1 × 10−7 | 1.4 × 10−6 | 6.7 × 10−6 | 1.8 × 10−6 | 1.2 × 10−7 | 1.5 × 10−6 | 7.4 × 10−6 | 1.6 × 10−6 | 1.0 × 10−7 | 1.3 × 10−6 | 6.4 × 10−6 |
| Paraquat | 5.6 × 10−7 | 3.7 × 10−8 | 9.0 × 10−8 | 3.0 × 10−7 | 5.6 × 10−7 | 3.7 × 10−8 | 9.0 × 10−8 | 3.0 × 10−7 | 5.6 × 10−7 | 3.7 × 10−8 | 9.0 × 10−8 | 3.0 × 10−7 | 5.6 × 10−7 | 3.7 × 10−8 | 9.0 × 10−8 | 3.0 × 10−7 | 5.6 × 10−7 | 3.7 × 10−8 | 9.0 × 10−8 | 3.0 × 10−7 |
| Pendimethalin | 9.4 × 10−6 | 6.2 × 10−7 | 8.4 × 10−6 | 7.5 × 10−5 | 1.0 × 10−5 | 6.8 × 10−7 | 9.2 × 10−6 | 8.2 × 10−5 | 9.3 × 10−6 | 6.1 × 10−7 | 8.3 × 10−6 | 7.4 × 10−5 | 9.5 × 10−6 | 6.2 × 10−7 | 8.5 × 10−6 | 7.6 × 10−5 | 1.1 × 10−5 | 7.1 × 10−7 | 9.7 × 10−6 | 8.7 × 10−5 |
| Chlorothalonil | 4.3 × 10−7 | 2.8 × 10−8 | 4.3 × 10−7 | 2.5 × 10−5 | 3.9 × 10−7 | 2.6 × 10−8 | 3.9 × 10−7 | 2.2 × 10−5 | 3.9 × 10−7 | 2.6 × 10−8 | 3.9 × 10−7 | 2.2 × 10−5 | 4.2 × 10−7 | 2.7 × 10−8 | 4.2 × 10−7 | 2.4 × 10−5 | 6.5 × 10−7 | 4.3 × 10−8 | 6.5 × 10−7 | 3.7 × 10−5 |
References
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global Food Demand and the Sustainable Intensification of Agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandt, K.; Barrangou, R. Applications of CRISPR Technologies across the Food Supply Chain. Annu. Rev. Food Sci. Technol. 2019, 10, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.S.; Salve, A.R.; Chauhan, S. Peanuts as Functional Food: A Review. J. Food Sci. Technol. 2016, 53, 31–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Settaluri, V.S.; Kandala, C.V.K.; Puppala, N.; Sundaram, J. Peanuts and Their Nutritional Aspects—A Review. Food Nutr. Sci. 2012, 3, 1644–1650. [Google Scholar] [CrossRef] [Green Version]
- Kiniry, J.R.; Simpson, C.E.; Schubert, A.M.; Reed, J.D. Peanut Leaf Area Index, Light Interception, Radiation Use Efficiency, and Harvest Index at Three Sites in Texas. Field Crops Res. 2005, 91, 297–306. [Google Scholar] [CrossRef]
- Peanut Country, U.S.A. National Peanut Board. Available online: https://nationalpeanutboard.org/peanut-info/peanut-country-usa.htm (accessed on 31 August 2021).
- Zain, M.; Si, Z.; Li, S.; Gao, Y.; Mehmood, F.; Rahman, S.-U.; Mounkaila Hamani, A.K.; Duan, A. The Coupled Effects of Irrigation Scheduling and Nitrogen Fertilization Mode on Growth, Yield and Water Use Efficiency in Drip-Irrigated Winter Wheat. Sustainability 2021, 13, 2742. [Google Scholar] [CrossRef]
- FAO; WFP; IFAD. The State of Food Insecurity in the world—2012. FAO 2012, 5, 65. [Google Scholar]
- Air and Pesticides. Available online: http://npic.orst.edu/envir/air.html (accessed on 20 October 2021).
- Impact of Sustainable Agriculture and Farming Practices. Available online: https://www.worldwildlife.org/industries/sustainable-agriculture (accessed on 4 March 2022).
- Main Sources of Nitrous Oxide Emissions. Available online: https://whatsyourimpact.org/greenhouse-gases/nitrous-oxide-emissions (accessed on 4 March 2022).
- First Aid—Pesticide Environmental Stewardship. Available online: https://pesticidestewardship.org/homeowner/first-aid/ (accessed on 14 March 2022).
- Sources of Greenhouse Gas Emissions|US EPA. Available online: https://www.epa.gov/ghgemissions/sources-greenhouse-gas-emissions (accessed on 28 July 2021).
- US EPA, O. Overview of Greenhouse Gases. Available online: https://www.epa.gov/ghgemissions/overview-greenhouse-gases (accessed on 28 July 2021).
- Konradsen, F.; van der Hoek, W.; Cole, D.C.; Hutchinson, G.; Daisley, H.; Singh, S.; Eddleston, M. Reducing Acute Poisoning in Developing Countries—Options for Restricting the Availability of Pesticides. Toxicology 2003, 192, 249–261. [Google Scholar] [CrossRef]
- Yusà, V.; Coscollà, C.; Mellouki, W.; Pastor, A.; de la Guardia, M. Sampling and Analysis of Pesticides in Ambient Air. J. Chromatogr. A 2009, 1216, 2972–2983. [Google Scholar] [CrossRef]
- Wania, F.; MacKay, D. Peer Reviewed: Tracking the Distribution of Persistent Organic Pollutants. Environ. Sci. Technol. 1996, 30, 390A–396A. [Google Scholar] [CrossRef]
- Tsai, W.; Cohen, Y.; Sakugawa, H.; Kaplan, I.R. Dynamic Partitioning of Semivolatile Organics in Gas/Particle/Rain Phases during Rain Scavenging. Environ. Sci. Technol. 1991, 25, 2012–2023. [Google Scholar] [CrossRef]
- Bidleman, T.F. Atmospheric Processes. Environ. Sci. Technol. 1988, 22, 361–367. [Google Scholar] [CrossRef]
- Atkinson, R.; Guicherit, R.; Hites, R.A.; Palm, W.; Seiber, J.N.; de Voogt, P. Transformations of Pesticides in the Atmosphere: A State of the Art. Water Air Soil Pollut. 1999, 115, 219–243. [Google Scholar] [CrossRef]
- Muñoz, A.; Vera, T.; Ródenas, M.; Borrás, E.; Mellouki, A.; Treacy, J.; Sidebottom, H. Gas-Phase Degradation of the Herbicide Ethalfluralin under Atmospheric Conditions. Chemosphere 2014, 95, 395–401. [Google Scholar] [CrossRef]
- US EPA, O. Introduction to Pesticide Labels. Available online: https://www.epa.gov/pesticide-labels/introduction-pesticide-labels (accessed on 25 February 2022).
- Istriningsih; Dewi, Y.A.; Yulianti, A.; Hanifah, V.W.; Jamal, E.; Dadang; Anugrah, I.S.; Darwis, V.; Sarwani, M.; Mardiharini, M.; et al. Farmers’ Knowledge and Practice Regarding Good Agricultural Practices (GAP) on Safe Pesticide Usage in Indonesia. Heliyon 2022, 8, e08708. [Google Scholar] [CrossRef]
- US EPA, O. 2,4-D. Available online: https://www.epa.gov/ingredients-used-pesticide-products/24-d (accessed on 25 February 2022).
- Bentazon. Pesticide Tolerances. Available online: https://www.federalregister.gov/documents/2019/05/01/2019-08785/bentazon-pesticide-tolerances (accessed on 4 March 2022).
- US EPA, O. Paraquat Dichloride. Available online: https://www.epa.gov/ingredients-used-pesticide-products/paraquat-dichloride (accessed on 1 March 2022).
- NASD—Pesticide Exposure. Available online: http://nasdonline.org (accessed on 12 November 2021).
- Lee, K.-M.; Inaba, A. Life Cycle Assessment Best Practices of ISO 14040 Series; Center for Ecodesign and LCA (CEL), Ajou University: Suwon, Korea, 2004; Volume 2004, p. 96. [Google Scholar]
- Alhashim, R.; Deepa, R.; Anandhi, A. Environmental Impact Assessment of Agricultural Production Using LCA: A Review. Climate 2021, 9, 164. [Google Scholar] [CrossRef]
- Weather-Us, Georgia, USA—Climate Data and Average Monthly Weather. Available online: https://www.weather-us.com/en/georgia-usa-climate (accessed on 28 October 2021).
- Fertilizer 101: The Big 3—Nitrogen, Phosphorus and Potassium. Available online: https://www.tfi.org/the-feed/fertilizer-101-big-3-nitrogen-phosphorus-and-potassium (accessed on 3 December 2021).
- Yang, Y. Life Cycle Freshwater Ecotoxicity, Human Health Cancer, and Noncancer Impacts of Corn Ethanol and Gasoline in the U.S. J. Clean. Prod. 2013, 53, 149–157. [Google Scholar] [CrossRef]
- Peña, N.; Knudsen, M.T.; Fantke, P.; Antón, A.; Hermansen, J.E. Freshwater Ecotoxicity Assessment of Pesticide Use in Crop Production: Testing the Influence of Modeling Choices. J. Clean. Prod. 2019, 209, 1332–1341. [Google Scholar] [CrossRef]
- Corrado, S.; Castellani, V.; Zampori, L.; Sala, S. Systematic Analysis of Secondary Life Cycle Inventories When Modelling Agricultural Production: A Case Study for Arable Crops. J. Clean. Prod. 2018, 172, 3990–4000. [Google Scholar] [CrossRef]
- Gentil, C.; Basset-Mens, C.; Manteaux, S.; Mottes, C.; Maillard, E.; Biard, Y.; Fantke, P. Coupling Pesticide Emission and Toxicity Characterization Models for LCA: Application to Open-Field Tomato Production in Martinique. J. Clean. Prod. 2020, 277, 124099. [Google Scholar] [CrossRef]
- Ministry of Agriculture. Food and Fisheries, B.C. Pesticide Toxicity and Hazard. 2/2022, 9. Available online: https://www.cabdirect.org/cabdirect/abstract/19631802537 (accessed on 14 March 2022).
- Corporation (SAIC), S.A.I.; Curran, M.A. Life-Cycle Assessment: Principles and Practice 2006. Available online: people.cs.uchicago.edu/~ftchong/290N-W10/EPAonLCA2006.pdf (accessed on 14 March 2022).
- Huijbregts, M. Uncertainty and Variability in Environmental Life-Cycle Assessment. Int. J. Life Cycle Assess. 2002, 7, 173. [Google Scholar] [CrossRef] [Green Version]
- Bare, J. TRACI 2.0: The Tool for the Reduction and Assessment of Chemical and Other Environmental Impacts 2.0. Clean Technol. Environ. Policy 2011, 13, 687–696. [Google Scholar] [CrossRef]
- US EPA, O. Pesticide Volatilization. Available online: https://www.epa.gov/reducing-pesticide-drift/pesticide-volatilization (accessed on 1 December 2021).
- Hanson, B.; Bond, C.; Buhl, K.; Stone, D. Pesticide Half-Life Fact Sheet. Available online: http://npic.orst.edu/factsheets/half-life.html (accessed on 10 January 2022).
- Pesticide Vapor Pressure. Available online: http://npic.orst.edu/factsheets/vaporpressure.html (accessed on 30 November 2021).
- van de Meent, D.; Huijbregts, M.A.J. Calculating Life-Cycle Assessment Effect Factors from Potentially Affected Fraction-Based Ecotoxicological Response Functions. Environ. Toxicol. Chem. 2005, 24, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Weir, S.M.; Yu, S.; Talent, L.G.; Maul, J.D.; Anderson, T.A.; Salice, C.J. Improving Reptile Ecological Risk Assessment: Oral and Dermal Toxicity of Pesticides to a Common Lizard Species (Sceloporus Occidentalis). Environ. Toxicol. Chem. 2015, 34, 1778–1786. [Google Scholar] [CrossRef]
- Amer, S.M.; Aboul-ela, E.I. Cytogenetic Effects of Pesticides: III. Induction of Micronuclei in Mouse Bone Marrow by the Insecticides Cypermethrin and Rotenone. Mutat. Res. Toxicol. 1985, 155, 135–142. [Google Scholar] [CrossRef]
- Crenna, E.; Jolliet, O.; Collina, E.; Sala, S.; Fantke, P. Characterizing Honey Bee Exposure and Effects from Pesticides for Chemical Prioritization and Life Cycle Assessment. Environ. Int. 2020, 138, 105642. [Google Scholar] [CrossRef]
- Fantke, P.; Bijster, M.; Hauschild, M.Z.; Huijbregts, M.; Jolliet, O.; Kounina, A.; Magaud, V.; Margni, M.; McKone, T.E.; Rosenbaum, R.K.; et al. USEtox® 2.0 Documentation (Version 1.00); USEtox® Team: Lyngby, Denmark, 2017; ISBN 978-87-998335-0-4. [Google Scholar] [CrossRef]
- Langenbach, T.; Caldas, L.Q. Strategies for Reducing Airborne Pesticides under Tropical Conditions. Ambio 2018, 47, 574–584. [Google Scholar] [CrossRef]
- Van Scoy, A.R.; Tjeerdema, R.S. Environmental Fate and Toxicology of Chlorothalonil. In Reviews of Environmental Contamination and Toxicology Volume 232; Whitacre, D.M., Ed.; Reviews of Environmental Contamination and Toxicology; Springer International Publishing: Cham, Switzerland, 2014; pp. 89–105. ISBN 978-3-319-06746-9. [Google Scholar]
- Leistra, M.; Van Den Berg, F. Volatilization of Parathion and Chlorothalonil from a Potato Crop Simulated by the PEARL Model. Environ. Sci. Technol. 2007, 41, 2243–2248. [Google Scholar] [CrossRef]
- Deer, H.M. Pesticide Adsorption and Half-Life. AG/Pestic. 1999, 15, 1. [Google Scholar]
- Sonesson, U.; Davis, J.; Ziegler, F. Food Production and Emissions of Greenhouse Gases: An Overview of the Climate Impact of Different Product Groups; SIK Institutet for Livsmedel och Bioteknik: Göteborg, Sweden, 2010; ISBN 978-91-7290-291-6. [Google Scholar]
- Semba, R.D.; Ramsing, R.; Rahman, N.; Kraemer, K.; Bloem, M.W. Legumes as a Sustainable Source of Protein in Human Diets. Glob. Food Secur. 2021, 28, 100520. [Google Scholar] [CrossRef]
| Input | Unit | Reference |
|---|---|---|
Agricultural system practices
| lb./acre/year lb./acre/year | USDA, Quick Stats(https://quickstats.nass.usda.gov/#2B8F5575-0900-3511-8FB4-A942AF602519, accessed on 14 March 2022) |
| Output | Unit | Reference |
| Harvested crop GHG Pesticide emission | lb./acre/year kg CO2 to the air in lb./acre/year, average | USDA, Quick Stats (https://quickstats.nass.usda.gov/#2B8F5575-0900-3511-8FB4-A942AF602519, accessed on 14 March 2022) ReCiPe2016_CFs_v1.1_20180117 USEtox |
| Compartment | Fraction | When Vapor Pressure | Reference |
|---|---|---|---|
| Air | 95% | P > 10 (mPa) | [33,34] |
| 50% | 1 < P < 10 | ||
| 15% | 0.1 < P < 1 | ||
| 5% | 0.01 < P < 0.1 | ||
| 1% | P < 0.01 |
| A | ||||||||
|---|---|---|---|---|---|---|---|---|
| Year | Category | CAS RN | AI | Amount Applied lb./acre/year, avg | VP Pa at 25 °C | VP mPa at 25 °C | emF % | Emission to Air lb./acre/year, avg |
| 2018 | Herbicide | 94-82-6 | 2,4-DB | 0.351 | 8.5 × 10−3 | 8.5 × 10+0 | 0.5 | 1.7 × 10−1 |
| 2018 | Herbicide | 25057-89-0 | Bentazon | 0.426 | 1.7 × 10−1 | 1.7 × 10+2 | 0.95 | 4.0 × 10−1 |
| 2018 | Herbicide | 55283-68-6 | Ethalfluralin | 0.729 | 1.2 × 10−2 | 1.2 × 10+1 | 0.95 | 6.9 × 10−1 |
| 2018 | Herbicide | 4685-14-7 | Paraquat | 0.317 | 1.3 × 10−5 | 1.3 × 10−2 | 0.01 | 3.1 × 10−3 |
| 2018 | Herbicide | 40487-42-1 | Pendimethalin | 0.894 | 4.0 × 10−3 | 4.0 × 10+0 | 0.5 | 4.4 × 10−1 |
| 2018 | Fungicide | 1897-45-6 | Chlorothalonil | 3.253 | 7.6 × 10−5 | 7.6 × 10−2 | 0.05 | 1.6 × 10−1 |
| 2013 | Herbicide | 94-82-6 | 2,4-DB | 0.368 | 8.5 × 10−3 | 8.5 × 10+0 | 0.5 | 1.8 × 10−1 |
| 2013 | Herbicide | 25057-89-0 | Bentazon | 0.426 | 1.7 × 10−1 | 1.7 × 10+2 | 0.95 | 4.0 × 10−1 |
| 2013 | Herbicide | 55283-68-6 | Ethalfluralin | 0.793 | 1.2 × 10−2 | 1.2 × 10+1 | 0.95 | 7.5 × 10−1 |
| 2013 | Herbicide | 4685-14-7 | Paraquat | 0.317 | 1.3 × 10−5 | 1.3 × 10−2 | 0.01 | 3.1 × 10−3 |
| 2013 | Herbicide | 40487-42-1 | Pendimethalin | 0.977 | 4.0 × 10−3 | 4.0 × 10+0 | 0.5 | 4.8 × 10−1 |
| 2013 | Fungicide | 1897-45-6 | Chlorothalonil | 3.245 | 7.6 × 10−5 | 7.6 × 10−2 | 0.05 | 1.6 × 10−1 |
| 2004 | Herbicide | 94-82-6 | 2,4-DB | 0.32 | 8.5 × 10−3 | 8.5 × 10+0 | 0.5 | 1.6 × 10−1 |
| 2004 | Herbicide | 25057-89-0 | Bentazon | 0.426 | 1.7 × 10−1 | 1.7 × 10+2 | 0.95 | 4.0 × 10−1 |
| 2004 | Herbicide | 55283-68-6 | Ethalfluralin | 0.73 | 1.2 × 10−2 | 1.2 × 10+1 | 0.95 | 6.9 × 10−1 |
| 2004 | Herbicide | 4685-14-7 | Paraquat | 0.317 | 1.3 × 10−5 | 1.3 × 10−2 | 0.01 | 3.1 × 10−3 |
| 2004 | Herbicide | 40487-42-1 | Pendimethalin | 0.88 | 4.0 × 10−3 | 4.0 × 10+0 | 0.5 | 4.4 × 10−1 |
| 2004 | Fungicide | 1897-45-6 | Chlorothalonil | 3.25 | 7.6 × 10−5 | 7.6 × 10−2 | 0.05 | 1.6 × 10−1 |
| 1999 | Herbicide | 94-82-6 | 2,4-DB | 0.27 | 8.5 × 10−3 | 8.5 × 10+0 | 0.5 | 1.3 × 10−1 |
| 1999 | Herbicide | 25057-89-0 | Bentazon | 0.426 | 1.7 × 10−1 | 1.7 × 10+2 | 0.95 | 4.0 × 10−1 |
| 1999 | Herbicide | 55283-68-6 | Ethalfluralin | 0.73 | 1.2 × 10−2 | 1.2 × 10+1 | 0.95 | 6.9 × 10−1 |
| 1999 | Herbicide | 4685-14-7 | Paraquat | 0.317 | 1.3 × 10−5 | 1.3 × 10−2 | 0.01 | 3.1 × 10−3 |
| 1999 | Herbicide | 40487-42-1 | Pendimethalin | 0.9 | 4.0 × 10−3 | 4.0 × 10+0 | 0.5 | 4.5 × 10−1 |
| 1999 | Fungicide | 1897-45-6 | Chlorothalonil | 3.48 | 7.6 × 10−5 | 7.6 × 10−2 | 0.05 | 1.7 × 10−1 |
| 1991 | Herbicide | 94-82-6 | 2,4-DB | 0.29 | 8.5 × 10−3 | 8.5 × 10+0 | 0.5 | 1.5 × 10−1 |
| 1991 | Herbicide | 25057-89-0 | Bentazon | 0.426 | 1.7 × 10−1 | 1.7 × 10+2 | 0.95 | 4.0 × 10−1 |
| 1991 | Herbicide | 55283-68-6 | Ethalfluralin | 0.7 | 1.2 × 10−2 | 1.2 × 10+1 | 0.95 | 6.7 × 10−1 |
| 1991 | Herbicide | 4685-14-7 | Paraquat | 0.317 | 1.3 × 10−5 | 1.3 × 10−2 | 0.01 | 3.1 × 10−3 |
| 1991 | Herbicide | 40487-42-1 | Pendimethalin | 1.03 | 4.0 × 10−3 | 4.0 × 10+0 | 0.5 | 5.2 × 10−1 |
| 1991 | Fungicide | 1897-45-6 | Chlorothalonil | 5.42 | 7.6 × 10−5 | 7.6 × 10−2 | 0.05 | 2.7 × 10−1 |
| B | ||||||||
| Year | Nitrogen Fertilizer Amount Applied (lb./acre/year) | EC of N2O (N/ton N Applied) | Molecular Weight Ratio of N2O | N2O emission (lb.) | ||||
| 2018 | 1.8 × 10+1 | 1.1 × 10−2 | 1.5 × 10+0 | 3.3 × 10−1 | ||||
| 2013 | 1.8 × 10+1 | 1.1 × 10−2 | 1.5 × 10+0 | 3.3 × 10−1 | ||||
| 2004 | 1.8 × 10+1 | 1.1 × 10−2 | 1.5 × 10+0 | 3.3 × 10−1 | ||||
| 1999 | 1.6 × 10+1 | 1.1 × 10−2 | 1.5 × 10+0 | 2.9 × 10−1 | ||||
| 1991 | 1.7 × 10+1 | 1.1 × 10−2 | 1.5 × 10+0 | 3.1 × 10−1 | ||||
| AI | VP mPa at 25 °C | emF | KH Pa·m3·mol−1 at 25 °C | Half-Life in Soil (Days)/Category |
|---|---|---|---|---|
| 2,4-DB | 8.5 × 10+0 | 5.0 × 10+0 | 4.6 × 10+0 | 10/low |
| Bentazone | 1.7 × 10+0 | 9.5 × 10+0 | 2.2 × 10+0 | 20/moderate |
| Ethalfluralin | 1.2 × 10+0 | 9.5 × 10+0 | 1.3 × 10+0 | 60/high |
| Paraquat | 1.3 × 10+0 | 1.0 × 10+0 | 5.6 × 10+0 | 1000/high |
| Pendimethalin | 4.0 × 10+0 | 5.0 × 10+0 | 8.6 × 10+0 | 21/moderate |
| Chlorothalonil | 7.6 × 10+0 | 5.0 × 10+0 | 2.0 × 10+0 | 30/moderate |
| CAS RN | AI | LD50 (mg/kg) | EF = 0.5/LD50 (PAF·Mg−1·kg−1 bw) | ||
|---|---|---|---|---|---|
| Oral | Dermal | Oral EF | Dermal EF | ||
| 94-82-6 | 2,4-DB | >2000 (2001) | >10,000 (10,001) | 2.5 × 10−4 | 5.0 × 10−5 |
| 25057-89-0 | Bentazon | 2063 | >6050 (6051) | 2.4 × 10−4 | 8.2 × 10−5 |
| 55283-68-6 | Ethalfluralin | >10,000 (10,001) | >10,000 (10,001) | 5.0 × 10−5 | 5.0 × 10−5 |
| 4685-14-7 | Paraquat | 150 | - | 3.3 × 10−3 | - |
| 40487-42-1 | PENDIMETHALIN | 1250 | >5000 (5001) | 4.0 × 10−4 | 1.0 × 10−4 |
| 1897-45-6 | Chlorothalonil | >10,000 (10,001) | >10,000 (10,001) | 5.0 × 10−5 | 5.0 × 10−5 |
| AI | Ecotoxicity Effect Factor Efeco (PAF·m3·kg−1) | Exposure Factor XFeco | Fate Factor FF (Days) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Oral | Dermal | Home.AirI | Occ.AirI | AirU | AirC | Home.AirI | Occ.AirI | AirU | AirC | |
| 2,4-DB | 2.5 × 10−4 | 5.0 × 10−5 | 1.0 × 10+0 | 1.0 × 10+0 | 1.0 × 10+0 | 1.0 × 10+0 | 5.2 × 10−2 | 3.4 × 10−3 | 3.6 × 10−2 | 2.5 × 10−1 |
| Bentazon | 2.4 × 10−4 | 8.2 × 10−5 | 1.0 × 10+0 | 1.0 × 10+0 | 9.5 × 10−1 | 9.5 × 10−1 | 5.2 × 10−2 | 3.4 × 10−3 | 1.0 × 10−2 | 2.8 × 10−2 |
| Ethalfluralin | 5.0 × 10−5 | 5.0 × 10−5 | 1.0 × 10+0 | 1.0 × 10+0 | 1.0 × 10+0 | 1.0 × 10+0 | 5.2 × 10−2 | 3.4 × 10−3 | 4.3 × 10−2 | 2.1 × 10−1 |
| Paraquat | 3.3 × 10−3 | - | 1.0 × 10+0 | 1.0 × 10+0 | 1.0 × 10+0 | 1.0 × 10+0 | 5.2 × 10−2 | 3.4 × 10−3 | 8.5 × 10−3 | 2.8 × 10−2 |
| Pendimethalin | 4.0 × 10−4 | 1.0 × 10−4 | 1.0 × 10+0 | 1.0 × 10+0 | 9.9 × 10−1 | 9.9 × 10−1 | 5.2 × 10−2 | 3.4 × 10−3 | 4.7 × 10−2 | 4.2 × 10−1 |
| Chlorothalonil | 5.0 × 10−5 | 5.0 × 10−5 | 1.0 × 10+0 | 1.0 × 10+0 | 1.0 × 10+0 | 1.0 × 10+0 | 5.2 × 10−2 | 3.4 × 10−3 | 5.2 × 10−2 | 3.0 × 10+0 |
| AI | ||||||||||
| Home.AirI | Occ.airI | AirU | AirC | |||||||
| Oral | Dermal | Oral | Dermal | Oral | Dermal | Oral | Dermal | |||
| 2,4-DB | 8.8 × 10−6 | 8.8 × 10−6 | 8.8 × 10−6 | 8.8 × 10−6 | 8.8 × 10−6 | 8.8 × 10−6 | 8.8 × 10−6 | 8.8 × 10−6 | ||
| Bentazon | 1.2 × 10−5 | 1.2 × 10−5 | 1.2 × 10−5 | 1.2 × 10−5 | 1.2 × 10−5 | 1.2 × 10−5 | 1.2 × 10−5 | 1.2 × 10−5 | ||
| Ethalfluralin | 2.6 × 10−6 | 2.6 × 10−6 | 2.6 × 10−6 | 2.6 × 10−6 | 2.6 × 10−6 | 2.6 × 10−6 | 2.6 × 10−6 | 2.6 × 10−6 | ||
| Paraquat | 1.7 × 10−4 | 1.7 × 10−4 | 1.7 × 10−4 | 1.7 × 10−4 | 1.7 × 10−4 | 1.7 × 10−4 | 1.7 × 10−4 | 1.7 × 10−4 | ||
| Pendimethalin | 2.1 × 10−5 | 2.1 × 10−5 | 2.1 × 10−5 | 2.1 × 10−5 | 2.1 × 10−5 | 2.1 × 10−5 | 2.1 × 10−5 | 2.1 × 10−5 | ||
| Chlorothalonil | 2.6 × 10−6 | 2.6 × 10−6 | 2.6 × 10−6 | 2.6 × 10−6 | 2.6 × 10−6 | 2.6 × 10−6 | 2.6 × 10−6 | 2.6 × 10−6 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhashim, R.; Anandhi, A. Global Warming and Toxicity Impacts: Peanuts in Georgia, USA Using Life Cycle Assessment. Sustainability 2022, 14, 3671. https://doi.org/10.3390/su14063671
Alhashim R, Anandhi A. Global Warming and Toxicity Impacts: Peanuts in Georgia, USA Using Life Cycle Assessment. Sustainability. 2022; 14(6):3671. https://doi.org/10.3390/su14063671
Chicago/Turabian StyleAlhashim, Rahmah, and Aavudai Anandhi. 2022. "Global Warming and Toxicity Impacts: Peanuts in Georgia, USA Using Life Cycle Assessment" Sustainability 14, no. 6: 3671. https://doi.org/10.3390/su14063671
APA StyleAlhashim, R., & Anandhi, A. (2022). Global Warming and Toxicity Impacts: Peanuts in Georgia, USA Using Life Cycle Assessment. Sustainability, 14(6), 3671. https://doi.org/10.3390/su14063671







