Compressed Earth Blocks Using Sediments and Alkali-Activated Byproducts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Dredged Sediments
2.1.2. Binders
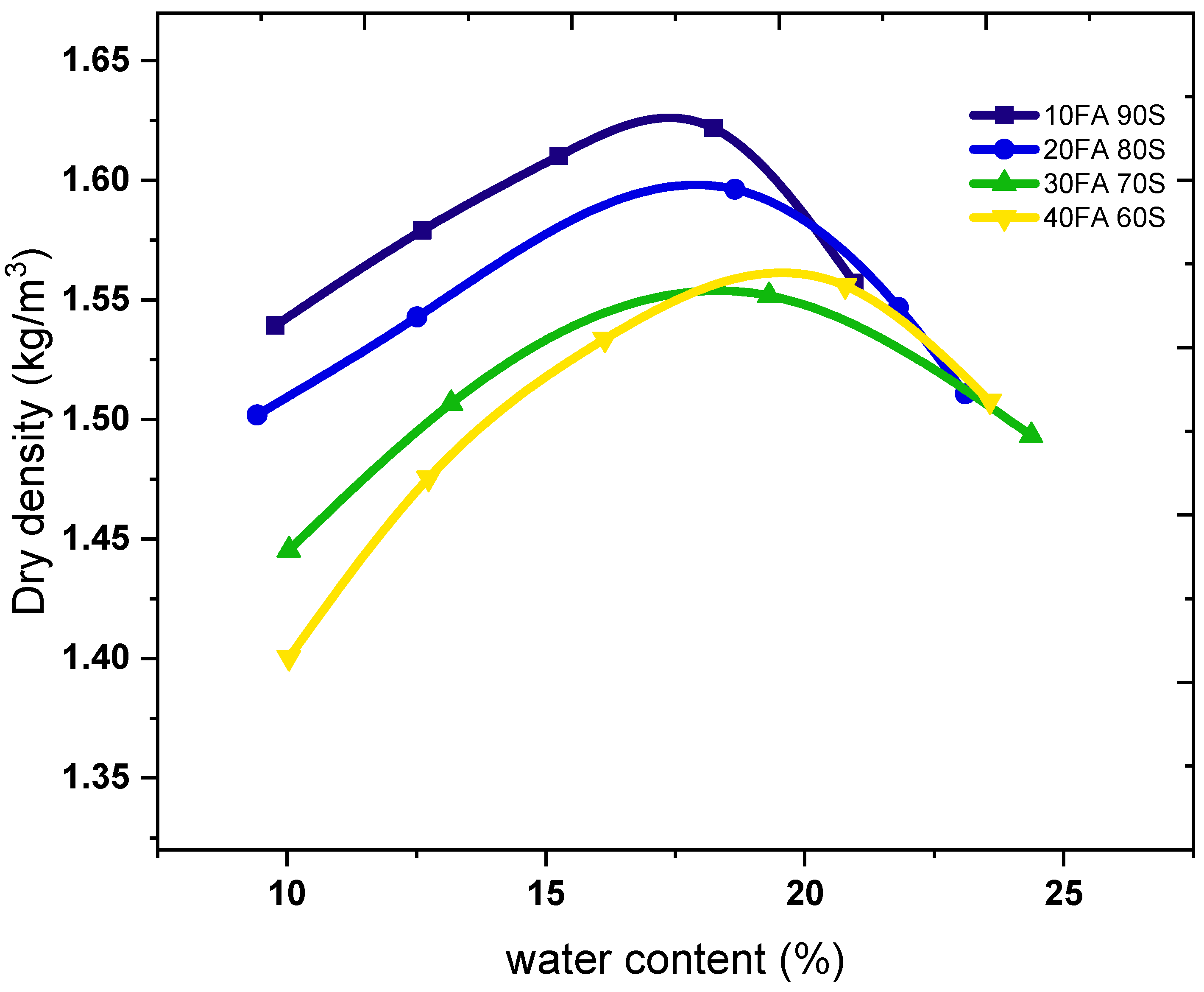
2.2. Mixtrues Design
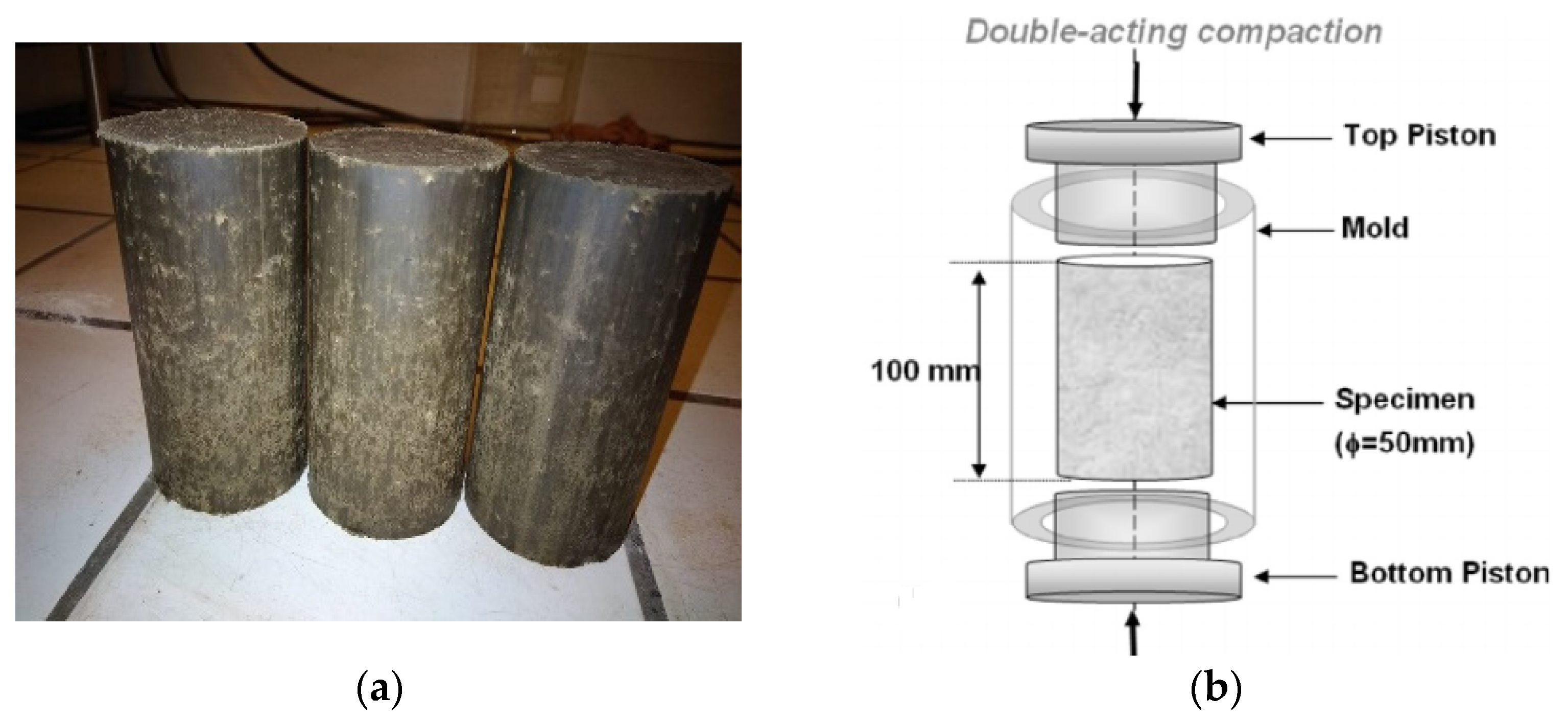
2.3. CEB Preparation and Hardening Conditions
2.4. Analysis Methods
2.5. Physical and Mechanical Characterization of CEBs
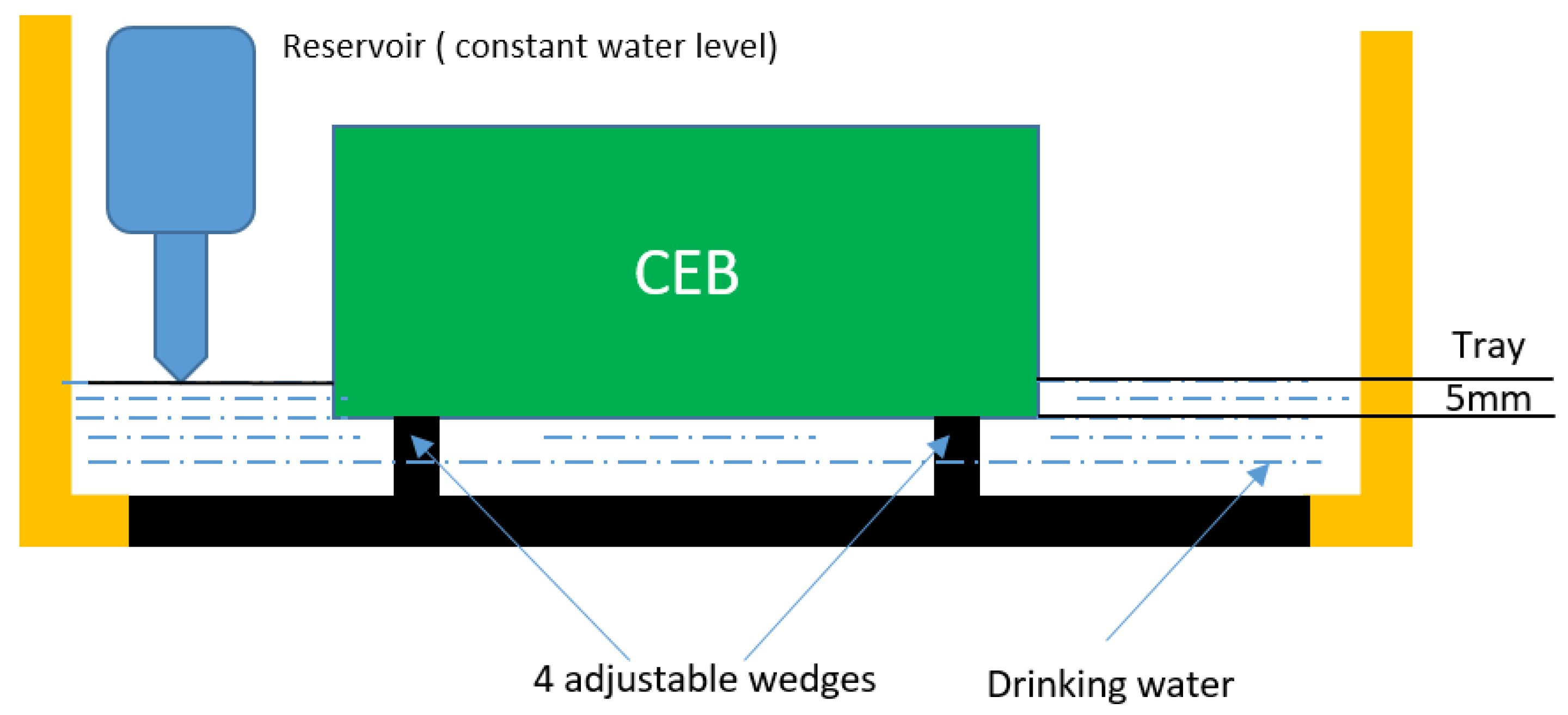
2.5.1. Water Resistance
2.5.2. Compressive Strength
2.5.3. Capillary Water Test
3. Results and Discussion
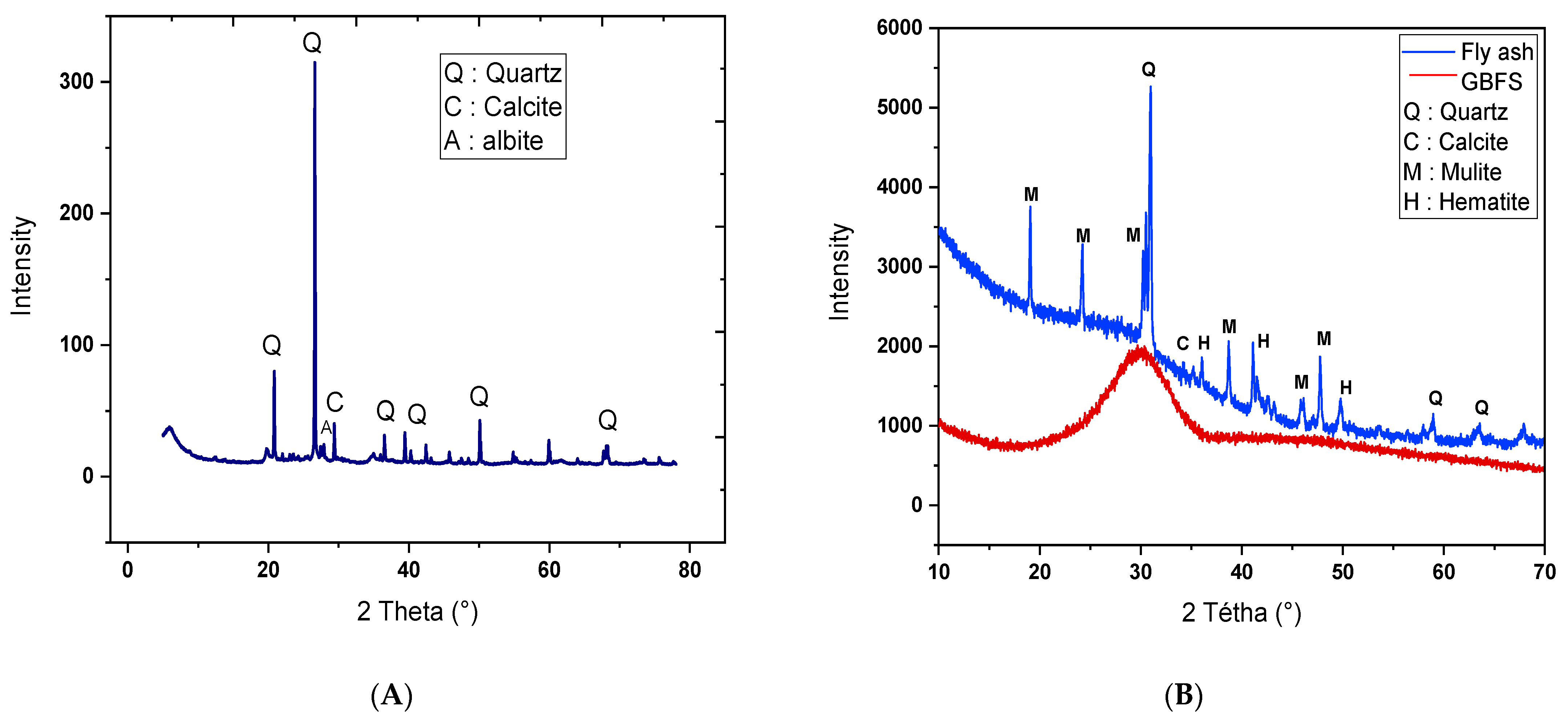
3.1. Basic Material Characterization
3.2. CEB Physical and Mechanical Properties
3.2.1. Compressive Strength
3.2.2. Dynamic Young’s Modulus
3.2.3. Water Sensitivity of Compressed Sediment Block Samples
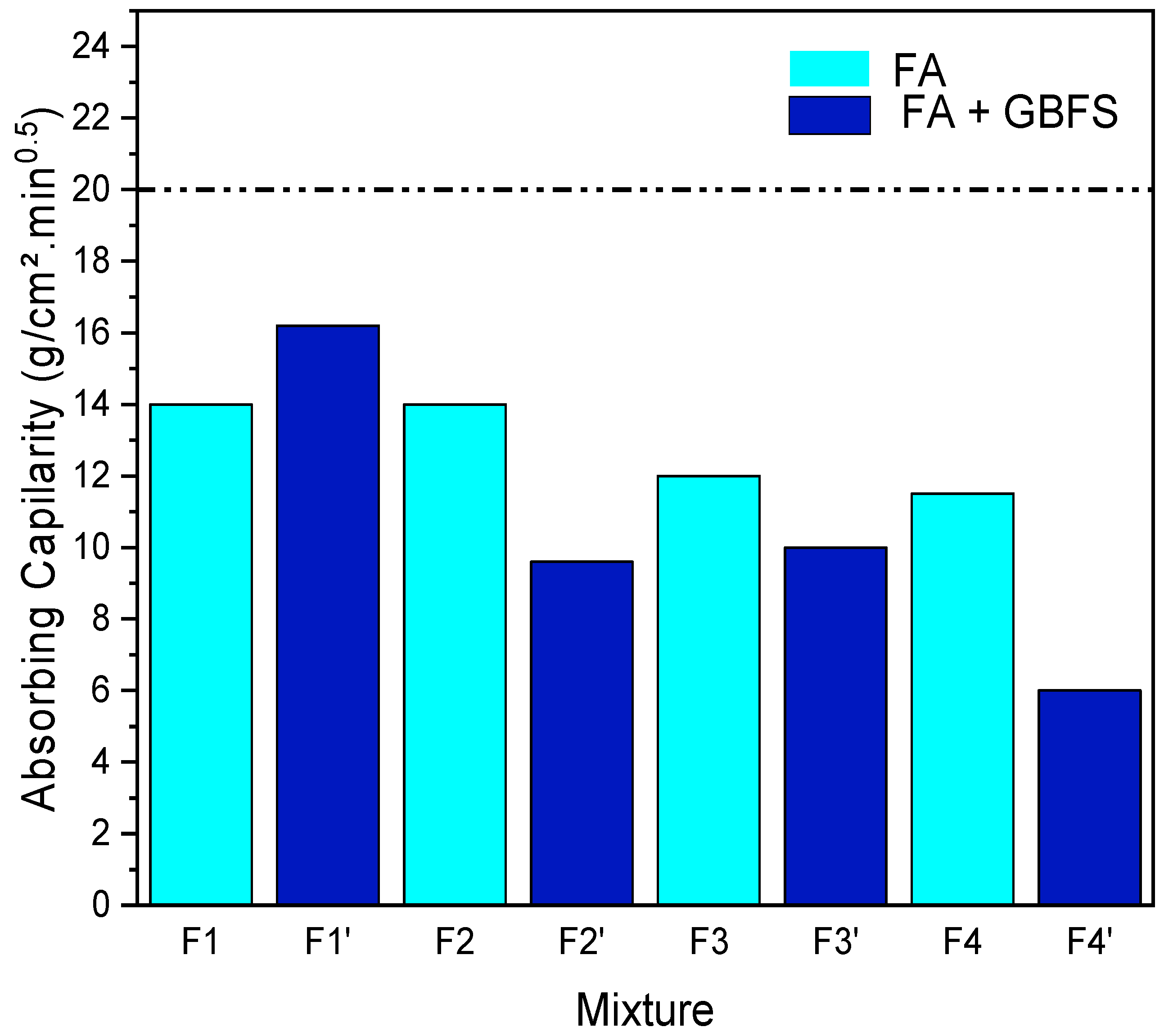
3.2.4. Capillarity of Compressed Block’s
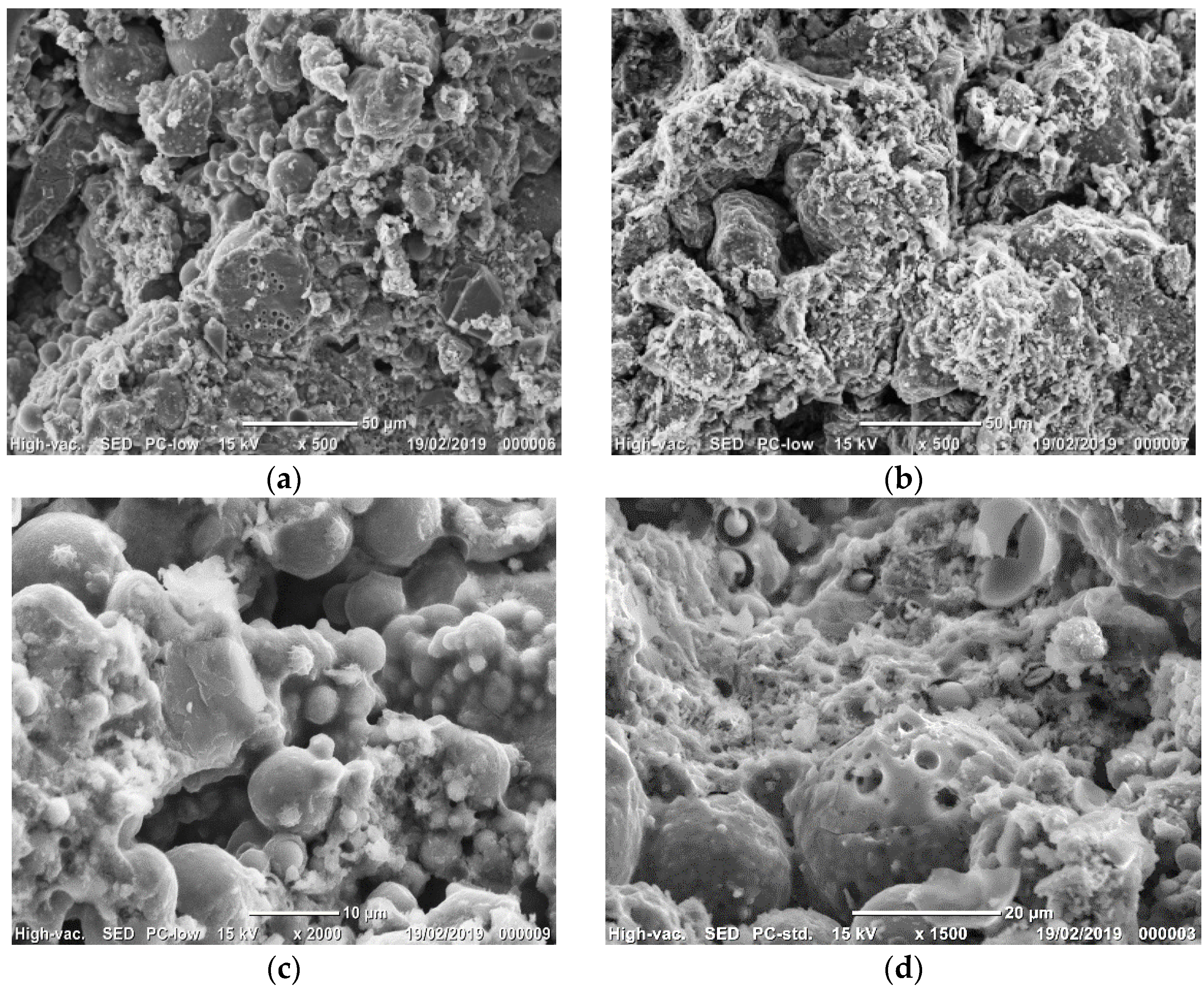
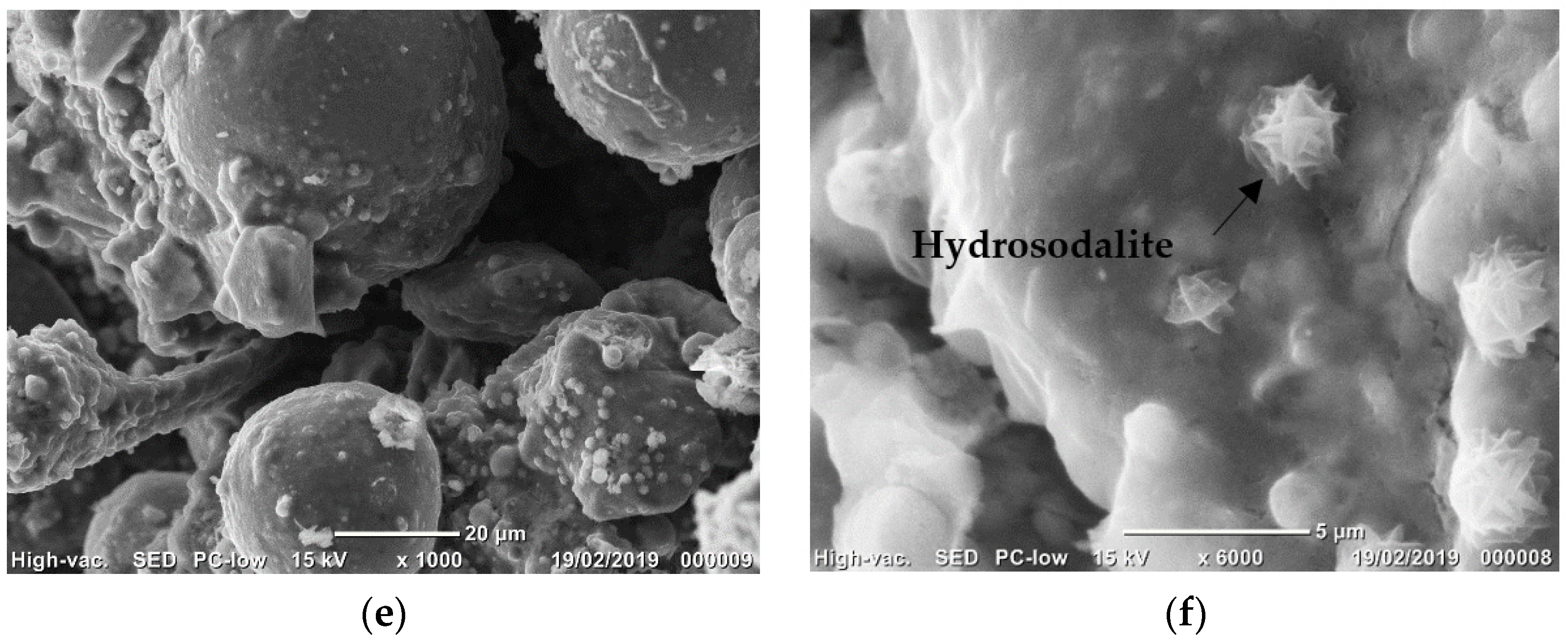
3.2.5. Microstructure Observations
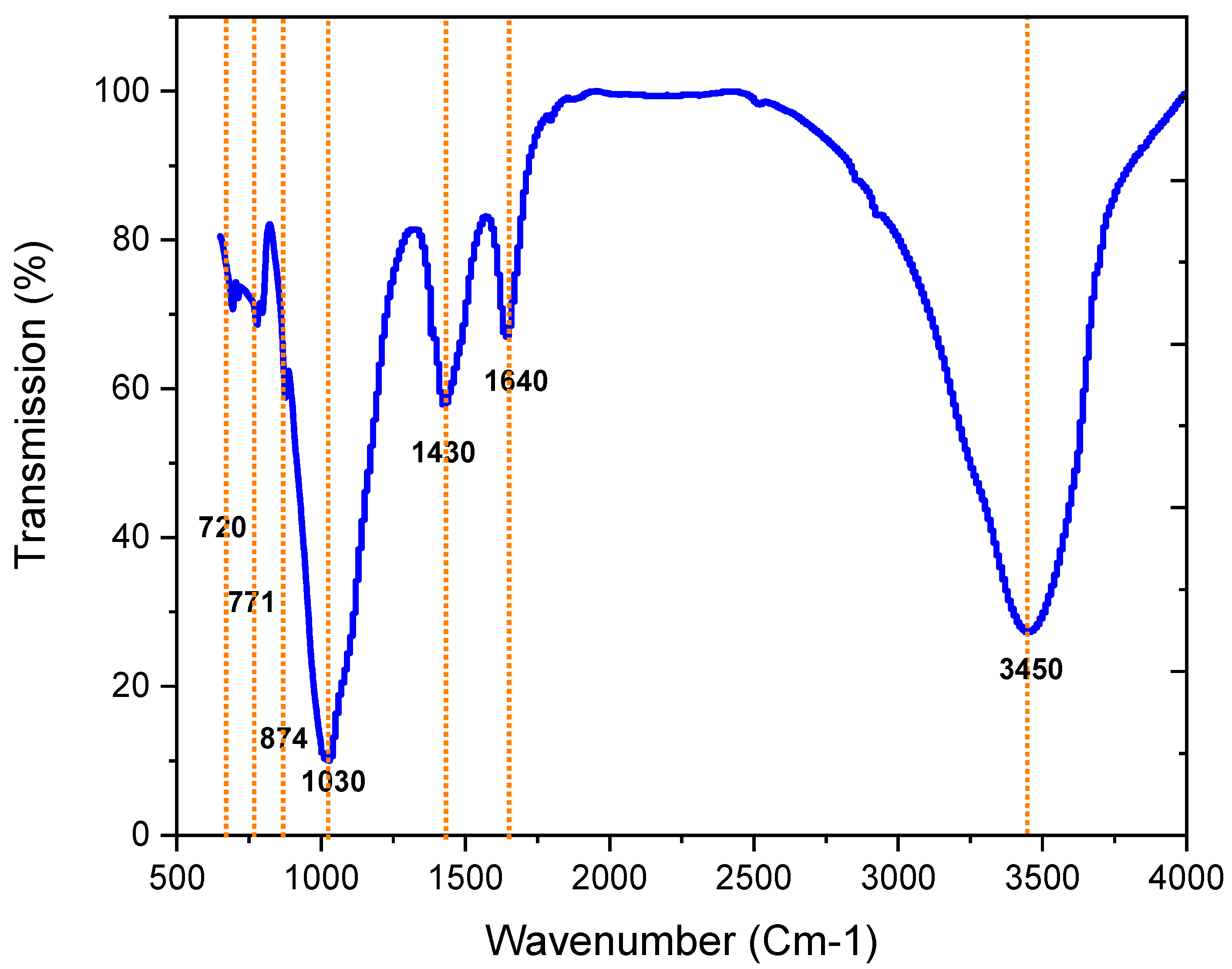
3.2.6. Infrared Red Spectroscopy Analysis
4. Conclusions
- All mixes had higher mechanical strengths than recommended by the standard.
- The obtained highest compressive strength of more than 16.18 MPa represents an optimal value for the compressed earth blocks.
- Water sensitivity is a major parameter because blocks are frequently exposed to rainwater. The sample F1′ had the highest capillarity absorption value. The remaining mixes with or without GBFS did not violate the capillarity limit threshold, which is lower than 40.
- The combination of fly ash and slag with well-studied percentages can give impressive results around 16.18 MPa against 8.94 MPa without GBFS
- The gels molecules produced by geopolymer binders have more efficiency than that of traditional CEB communicated by the obtained mechanical strengths of previous work found in the literature.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pierrehumbert, R. There is no Plan B for dealing with the climate crisis. Bull. At. Sci. 2019, 75, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Bellara, S.; Hidjeb, M.; Maherzi, W.; Mezazigh, S.; Senouci, A. Optimization of an Eco-Friendly Hydraulic Road Binders Comprising Clayey Dam Sediments and Ground Granulated Blast-Furnace Slag. J. Build. 2021, 11, 443. [Google Scholar] [CrossRef]
- Apitz, S.E. Waste or resource? Classifying and scoring dredged material management strategies in terms of the waste hierarchy. J. Soils Sediments 2010, 10, 1657–1668. [Google Scholar] [CrossRef]
- Morel, J.-C.; Mesbah, A.; Oggero, M.; Walker, P. Building houses with local materials: Means to drastically reduce the environmental impact of construction. Build. Environ. 2001, 36, 1119–1126. [Google Scholar] [CrossRef]
- Rivera, J.; Coelho, J.; Silva, R.; Miranda, T.; Castro, F.; Cristelo, N. Compressed earth blocks stabilized with glass waste and fly ash activated with a recycled alkaline cleaning solution. J. Clean. Prod. 2021, 284, 124783. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Jalali, S. Earth construction: Lessons from the past for future eco-efficient construction. Constr. Build. Mater. 2012, 29, 512–519. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Kumar, R.; Mehrotra, S.P. Influence of granulated blast furnace slag on the reaction, structure and properties of fly ash based geopolymer. J. Mater. Sci. 2010, 45, 607–615. [Google Scholar] [CrossRef]
- Bagheri, A.; Nazari, A. Compressive strength of high strength class C fly ash based geopolymers with reactive granulated blast furnace slag aggregates designed by Taguchi method. Mater. Des. 2014, 54, 483–490. [Google Scholar] [CrossRef]
- Diaz, E.I.; Allouche, E.N.; Eklund, S. Factors affecting the suitability of fly ash as source material for geopolymers. Fuel 2010, 89, 992–996. [Google Scholar] [CrossRef]
- Phoo-ngernkham, T.; Chindaprasirt, P.; Sata, V.; Pangdaeng, S.; Sinsiri, T. Properties of high calcium fly ash geopolymer pastes containing Portland cement as additive. Int. J. Miner. Metall. Mater. 2013, 20, 214–220. [Google Scholar] [CrossRef]
- Phoo-ngernkham, T.; Chindaprasirt, P.; Sata, V.; Sinsiri, T. High calcium fly ash geopolymer containing diatomite as additive. Indian J. Eng. Mater. Sci. 2013, 20, 310–318. [Google Scholar]
- Palomo, A.; Fernandez-Jimenez, A.; Kovalchuk, G.; Ordonez, L.M.; Naranjo, M.C. Opc-fly ash cementitious systems: Study of gel binders produced during alkaline hydration. J. Mater. Sci. 2007, 42, 2958–2966. [Google Scholar] [CrossRef]
- Williams, P.J.; Biernacki, J.J.; Walker, L.R.; Meyer, H.M.; Rawn, C.J.; Bai, J. Microanalysis of alkali-activated fly ash–CH pastes. Cem. Concr. Res. 2002, 32, 963–972. [Google Scholar] [CrossRef]
- Ravikumar, D.; Peethamparan, S.; Neithalath, N. Structure and strength of NaOH activated concretes containing fly ash or GGBFS as the sole binder. Cem. Concr. Compos. 2010, 32, 399–410. [Google Scholar] [CrossRef]
- Somna, K.; Jaturapitakkul, C.; Kajitvichyanukul, P.; Chindaprasirt, P. NaOH activated ground fly ash geopolymer cured at ambient temperature. Fuel 2011, 90, 2118–2124. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H.; Chen, L.; Dick, W.A. Alkali-activated complex binders from class C fly ash and Ca-containing admixtures. J. Hazard. Mater. 2010, 173, 480–486. [Google Scholar] [CrossRef]
- Aydın, S.; Baradan, B. Effect of activator type and content on properties of alkali activated slag mortars. Compos. B Eng. 2014, 57, 166–172. [Google Scholar] [CrossRef]
- Puertas, F.; Martinez-Ramirez, S.; Alonso, S.; Vazquez, T. Alkali-activated fly ash/slag cements: Strength behavior and hydration products. Cem. Concr. Res. 2000, 30, 1625–1632. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Hamdan, S.; van Deventer, J.S.J. Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem. Concr. Compos. 2014, 45, 125–135. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Fernandez-Jimenez, A.; Palomo, A. Hydration kinetics in hybrid binders: Early reaction stages. Cem. Concr. Compos. 2013, 39, 82–92. [Google Scholar] [CrossRef]
- CRATerre; Houben, H.; Boubekeur, S. Blocs de Terre Comprimée: Normes. Guide CDE, Série Technologie n°11, CRATerre-EAG, Villefontaine/Bruxelles, France/Belgique, 142p. 1998. Available online: https://craterre.hypotheses.org/files/2017/05/7502_BTC_Normes.pdf (accessed on 6 February 2022).
- NF P94-093; Standard Détermination des Références de Compactage d’un Matériau—Proctor Normal et Proctor Modifié. 1999. Available online: https://pdfslide.net/documents/nf-p-94-093-proctor-99.html (accessed on 6 February 2022).
- NF P 94-100; Standard Soils: Reconnaissance and Tests—Materials Treated with Lime and/or Hydraulic Binders—Tests to Assess the Suitability of a Soil for Treatment. Available online: https://www.boutique.afnor.org/en-gb/standard/nf-p94100/soils-investigation-and-testing-lime-and-or-hydraulic-binder-treated-materi/fa059725/45687 (accessed on 6 February 2022).
- X11-667; Standard Particle Size Analysis Laser Optical mEthod Mesasurment of Trasition Period. Available online: https://arenatecnica.com/en/technical-standards/bs_iso_20998-2#last-publication-date (accessed on 6 February 2022).
- NF P94-068; Standard Measurement of the Methylene Blue Adsorption Capacity of a Soil or a Rocky Material—Determination of the Methylene Blue Value of a Soil or a Rocky Material by the Spot Test. Available online: https://www.boutique.afnor.org/en-gb/standard/nf-p94068/soils-investigation-and-testing-measuring-of-the-methylene-blue-adsorption-/fa043689/394 (accessed on 6 February 2022).
- NF EN 1097-7; Standard Tests to Determine the Mechanical and Physical Characteristics of Aggregates—Part 7: Determination of the Absolute Density of Filler—Pycnometer Method. Available online: https://standards.iteh.ai/catalog/standards/cen/f8079242-62c6-45aa-b978-cc250d8a9222/en-1097-7-2008 (accessed on 6 February 2022).
- Omar Sore, S.; Messan, A.; Prud’homme, E.; Escadeillas, G.; Tsobnang, F. Stabilization of compressed earth blocks (CEBs) by geopolymer binder based on local materials from Burkina Faso. Constr. Build. Mater. 2018, 165, 333–345. [Google Scholar] [CrossRef]
- NF EN 196-1; Standard Methods of Testing Cement—Part 1: Determination of Strength. Available online: https://standards.iteh.ai/catalog/standards/cen/37b8816e-4085-4dcc-a642-a383d9bddd6c/en-196-1-2016 (accessed on 6 February 2022).
- XP P 13-901; Standard Compressed Earth Blocks for Walls and Partitions—Definitions—Specifications—Test methods—Delivery Acceptance Conditions. Available online: https://standards.globalspec.com/std/811579/XP%20P13-901 (accessed on 6 February 2022).
- Van Jaarsveld, J.G.S.; van Deventer, J.S.J. Effect of the alkali metal activator on the properties of fly ash-based geopolymer. Ind. Eng. Chem. Res. 1999, 38, 32–41. [Google Scholar] [CrossRef]
- Gohan, G.; Kurklu, G. The Influence of NaOH solution on the properties of Fly Ash based geopolymer Mortar cured at Different Temperature. Compos. Part B 2014, 58, 371–377. [Google Scholar] [CrossRef]
- Larbi, S.; Khaldi, A.; Maherzi, W.; Abriak, N.E. Formulation of compressed earth blocks stabilized by glass waste activated with naoh solution. Sustainability 2022, 14, 102. [Google Scholar] [CrossRef]
- Divet, L.; Le Roy, R.; Van Rompaey, G. Hydratation des Laitiers de Haut Fourneau, Rapport LCPC; LCPC, ENPC, HOLCIM Paris: Marne la Vallée, Belgique, 2006. [Google Scholar]
- Casarez, R. Experimental study of XRD, FTIR and TGA techniques in geopolymeric materials. Int. J. Adv. Comput. Sci. Appl. 2014, 4, 221–226. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R. Mechanical activation of fly ash: Effect on reaction, structure and properties of resulting geopolymer. J. Mater. Sci. 2011, 45, 607–615. [Google Scholar] [CrossRef]
- Singh, N. Fly Ash-Based Geopolymer Binder: A Future Construction Material. Minerals 2018, 8, 299. [Google Scholar] [CrossRef] [Green Version]

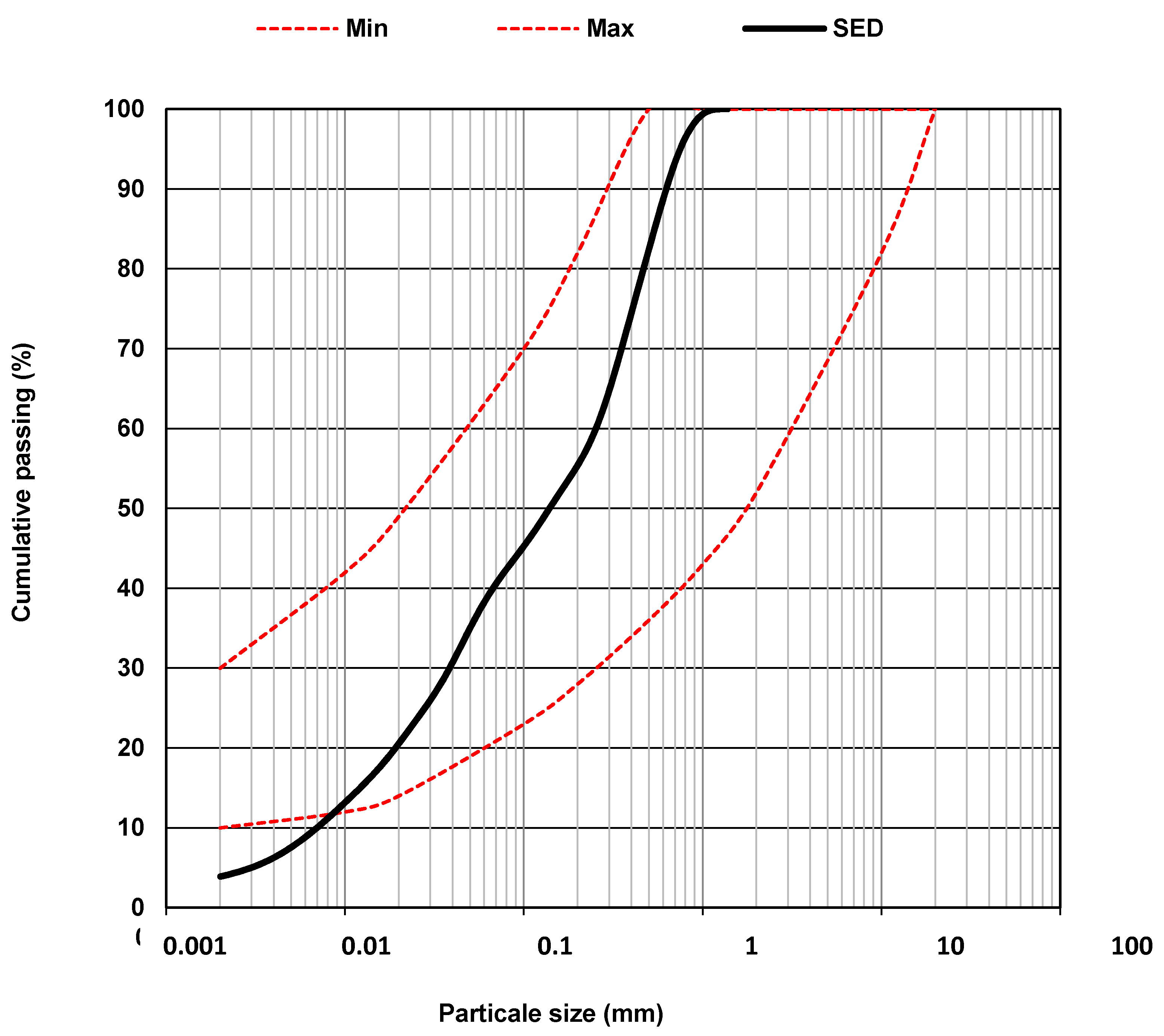



| Particle size distribution (sediment) | Grain size (X11-667 [24]) |
| Clay fraction quantity | Methylene Blue Value—MBV (NF P94-068 [25]) |
| Density | Helium pycnometer (NF EN 1097-7 [26]) |
| Materials | LOI | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | SO3 | Na2O | K2O |
|---|---|---|---|---|---|---|---|---|---|
| S | 5.95 | 10.42 | 59.26 | 10.39 | 3.34 | 1.33 | 0.5 | 0.67 | 1.93 |
| FA | 4.19 | 2.66 | 50.49 | 25.32 | 7.58 | 1.33 | 0.75 | 0.54 | 3.73 |
| GBFS | <1.5 | 42.9 | 38.0 | 10.8 | 0.7 | 6.6 | 0.1 | 0.28 | 0.35 |
| Element | Size Distribution of Sediment (%) |
|---|---|
| Sand | 62.39 |
| Silt | 33.91 |
| Clay | 3.91 |
| Materials | Specific Gravity (g/cm3) | Methylene Blue Value (g/100 g) |
|---|---|---|
| S | 2.59 | 2 |
| FA | 2.20 | / |
| GBFS | 2.90 | / |
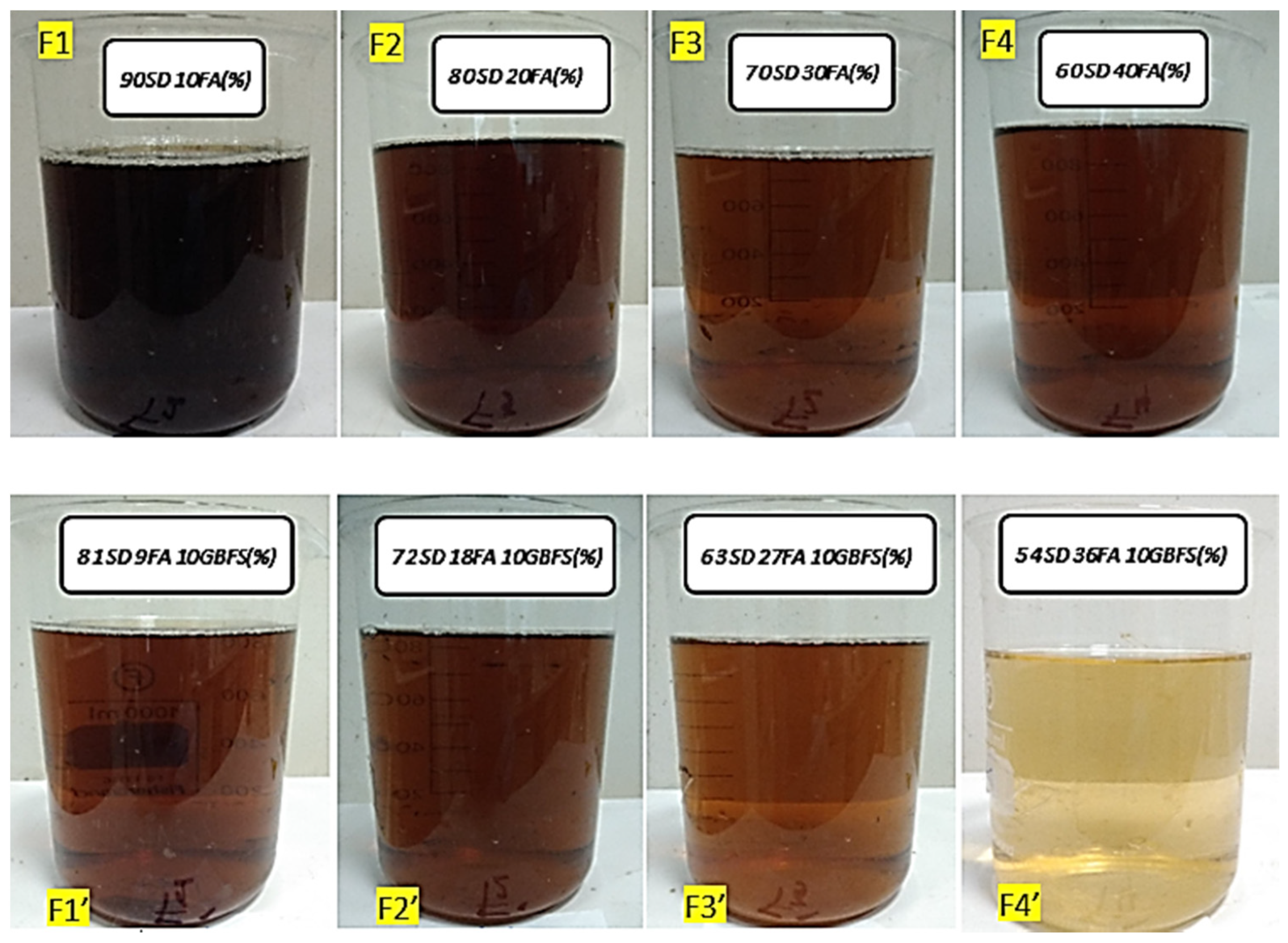
| Mix | Source Materials | S (%) | FA (%) | GBFS (%) | NH (%) |
|---|---|---|---|---|---|
| F1 | S + FA | 90 | 10 | 0 | 18.24 |
| F2 | S + FA | 80 | 20 | 0 | 18.64 |
| F3 | S + FA | 70 | 30 | 0 | 19.30 |
| F4 | S + FA | 60 | 40 | 0 | 20.77 |
| F1′ | S + FA + GBFS | 81 | 9 | 10 | 20.24 |
| F2′ | S + FA + GBFS | 72 | 18 | 10 | 20.64 |
| F3′ | S + FA + GBFS | 63 | 27 | 10 | 21.30 |
| F4′ | S + FA + GBFS | 54 | 36 | 10 | 22.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belayali, F.; Maherzi, W.; Benzerzour, M.; Abriak, N.-E.; Senouci, A. Compressed Earth Blocks Using Sediments and Alkali-Activated Byproducts. Sustainability 2022, 14, 3158. https://doi.org/10.3390/su14063158
Belayali F, Maherzi W, Benzerzour M, Abriak N-E, Senouci A. Compressed Earth Blocks Using Sediments and Alkali-Activated Byproducts. Sustainability. 2022; 14(6):3158. https://doi.org/10.3390/su14063158
Chicago/Turabian StyleBelayali, Fouad, Walid Maherzi, Mahfoud Benzerzour, Nor-Edine Abriak, and Ahmed Senouci. 2022. "Compressed Earth Blocks Using Sediments and Alkali-Activated Byproducts" Sustainability 14, no. 6: 3158. https://doi.org/10.3390/su14063158
APA StyleBelayali, F., Maherzi, W., Benzerzour, M., Abriak, N.-E., & Senouci, A. (2022). Compressed Earth Blocks Using Sediments and Alkali-Activated Byproducts. Sustainability, 14(6), 3158. https://doi.org/10.3390/su14063158









