Strength and Microstructure Characteristics of Metakaolin-Based Geopolymer Mortars with High Water-to-Binder Ratios
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Mixing
2.2. Design of Recipe
2.3. Characterization
3. Results
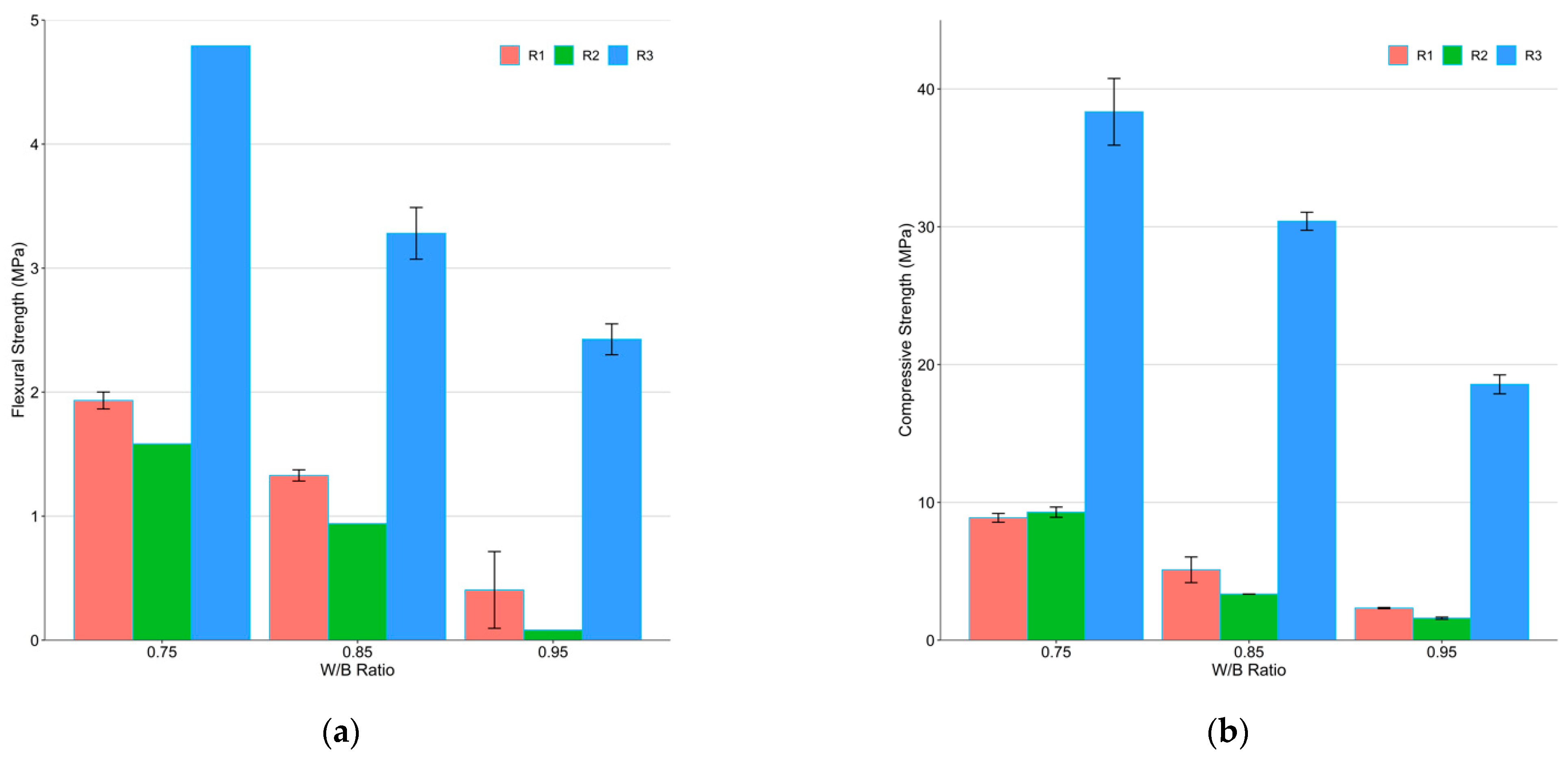
3.1. Mechanical Strength
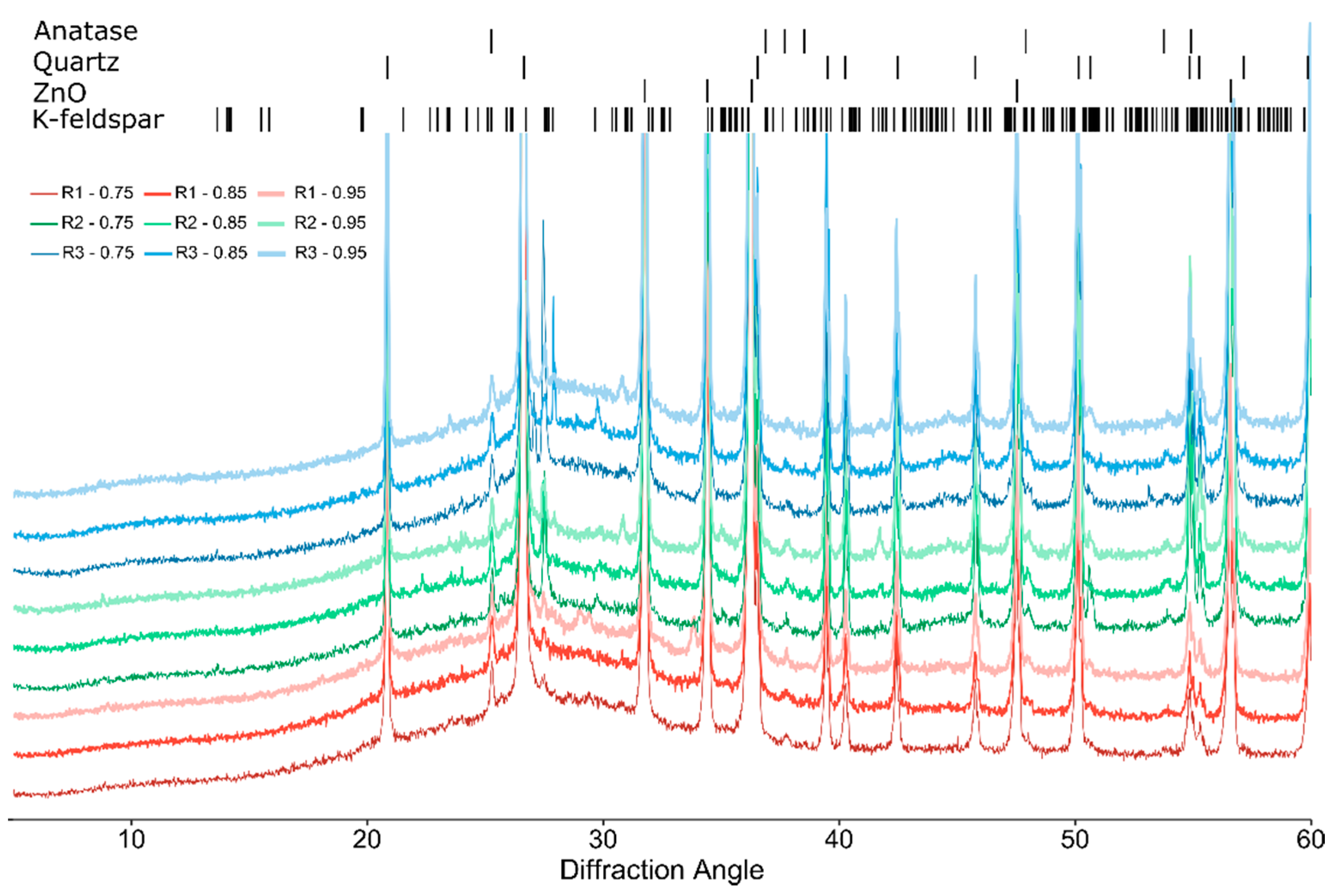
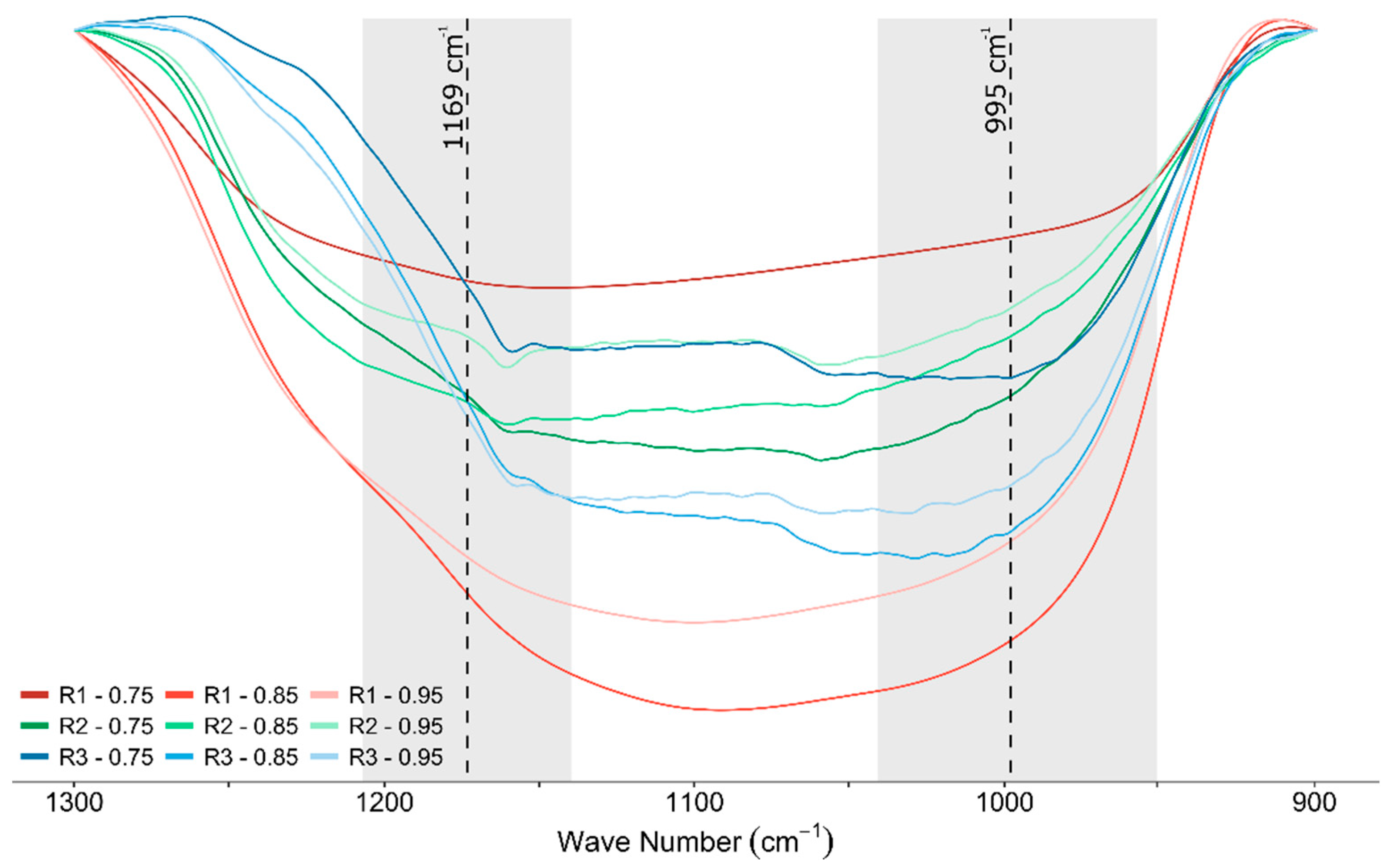
3.2. Mineralogical Analysis
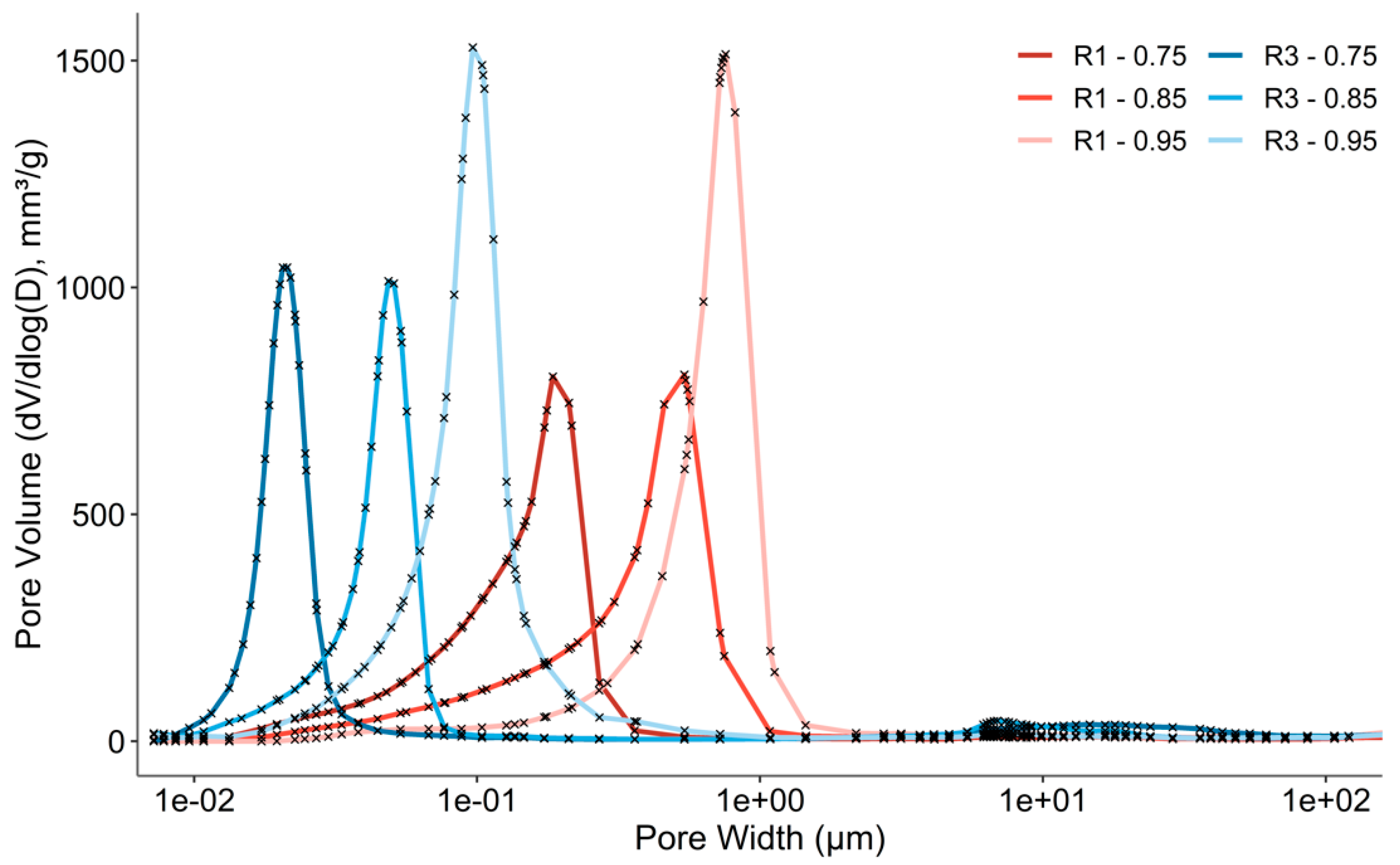
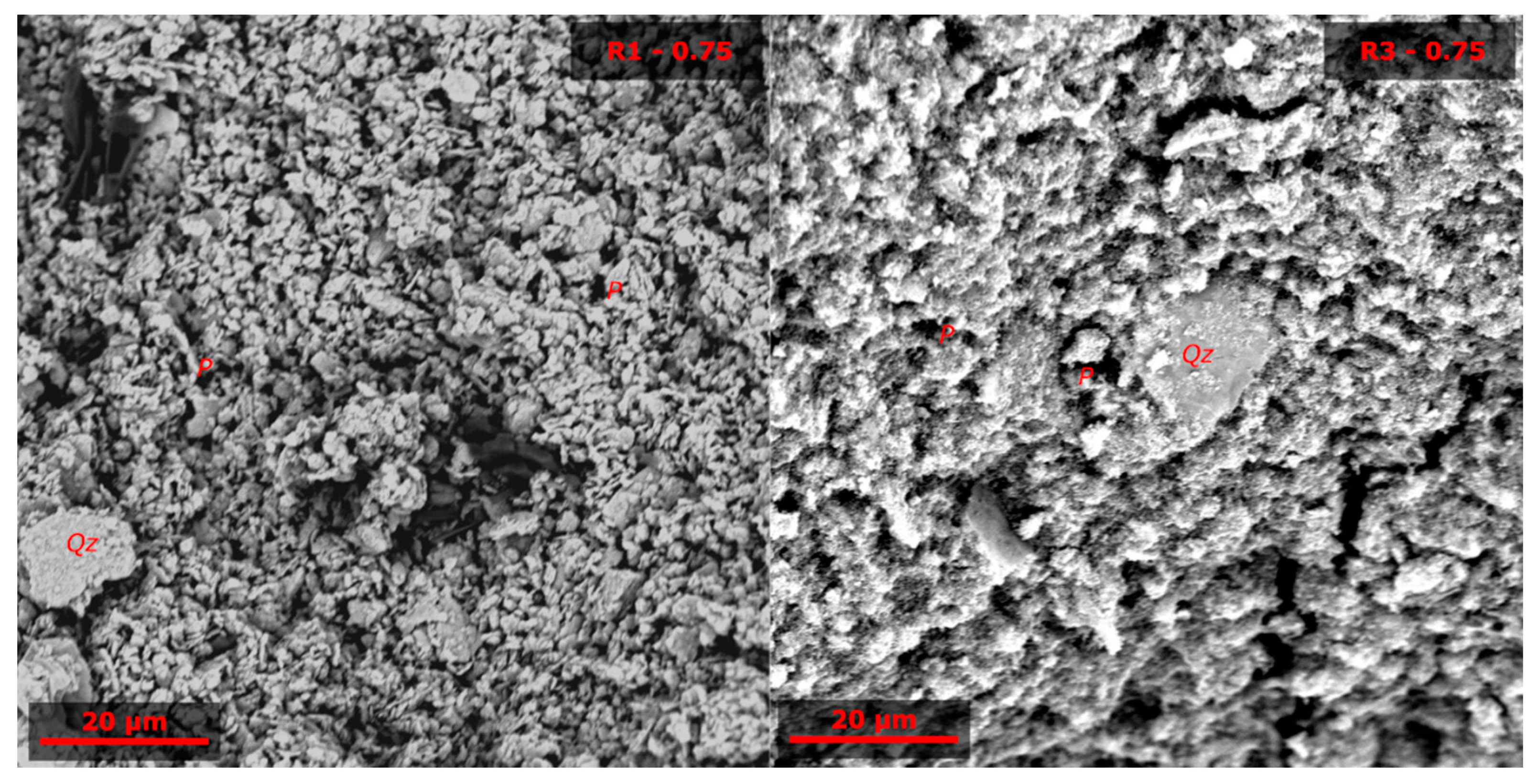
3.3. Microstructural Analysis
4. Discussion
4.1. Factors Influencing the Mechanical Strength
4.2. Conceptual Model for the Development of Mechanical and Microstructural Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Provis, J.L.; Van Deventer, J.S. Geopolymers: Structures, Processing, Properties and Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Van Jaarsveld, J.; Van Deventer, J. The effect of metal contaminants on the formation and properties of waste-based geopolymers. Cem. Concr. Res. 1999, 29, 1189–1200. [Google Scholar] [CrossRef]
- Davidovits, J.; Comrie, D. Long term durability of hazardous toxic and nuclear waste disposals. Geopolymer 1988, 88, 125–134. [Google Scholar]
- Shi, C.; Grattan-Bellew, P.E.; Stegemann, J.A. Conversion of a waste mud into a pozzolanic material. Constr. Build. Mater. 1999, 13, 279–284. [Google Scholar] [CrossRef]
- Cantarel, V.; Nouaille, F.; Rooses, A.; Lambertin, D.; Poulesquen, A.; Frizon, F. Solidification/stabilisation of liquid oil waste in metakaolin-based geopolymer. J. Nucl. Mater. 2015, 464, 16–19. [Google Scholar] [CrossRef]
- Cantarel, V.; Motooka, T.; Yamagishi, I. Geopolymers and Their Potential Applications in the Nuclear Waste Management Field-A Bibliographical Study. JAEA Rev. 2017. Available online: https://jopss.jaea.go.jp/search/servlet/search?5059195 (accessed on 2 January 2022).
- Lahoti, M.; Narang, P.; Tan, K.H.; Yang, E.-H. Mix design factors and strength prediction of metakaolin-based geopolymer. Ceram. Int. 2017, 43, 11433–11441. [Google Scholar] [CrossRef]
- Phung, Q.T.; Ferreira, E.; Seetharam, S.; Nguyen, V.T.; Govaerts, J.; Valcke, E. Understanding hydration heat of mortars containing supplementary cementitious materials with potential to immobilize heavy metal containing waste. Cem. Concr. Compos. 2021, 115, 103859. [Google Scholar] [CrossRef]
- Vandoorne, T.; Coppens, E. Aanpassingen aan de acceptatiecriteria m.b.t tot de immobilisatiematrix op basis van cement voor heterogeen and homogeen geconditioneerd afval (Amendments to the Acceptance Criteria with Regard to the Cement-Based Immobilization Matrix for Heterogeneous and Homogeneous Conditioned Waste). 2016, p. 22. Available online: https://fanc.fgov.be/nl/system/files/8.recumo-deeldossier-afval.pdf (accessed on 2 January 2022).
- Kani, E.N.; Allahverdi, A. Effects of curing time and temperature on strength development of inorganic polymeric binder based on natural pozzolan. J. Mater. Sci. 2009, 44, 3088–3097. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y. Relationship between compressive strength of geo-polymers and pre-curing conditions. Appl. Microsc. 2013, 43, 155–163. [Google Scholar] [CrossRef]
- Perera, D.; Uchida, O.; Vance, E.; Finnie, K. Influence of curing schedule on the integrity of geopolymers. J. Mater. Sci. 2007, 42, 3099–3106. [Google Scholar] [CrossRef]
- Samal, S.; Blanco, I. An Application Review of Fiber-Reinforced Geopolymer Composite. Fibers 2021, 9, 23. [Google Scholar] [CrossRef]
- Samal, S.; Thanh, N.P.; Marvalova, B.; Petrikova, I. Thermal characterization of metakaolin-based geopolymer. Jom 2017, 69, 2480–2484. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, W.; Li, Z. Synthesis and microstructural characterization of fully-reacted potassium-poly (sialate-siloxo) geopolymeric cement matrix. ACI Mater. J. 2008, 105, 156. [Google Scholar]
- Provis, J.L.; Van Deventer, J.S. Geopolymerisation kinetics. 1. In situ energy-dispersive X-ray diffractometry. Chem. Eng. Sci. 2007, 62, 2309–2317. [Google Scholar] [CrossRef]
- Kong, D.L.; Sanjayan, J.G.; Sagoe-Crentsil, K. Comparative performance of geopolymers made with metakaolin and fly ash after exposure to elevated temperatures. Cem. Concr. Res. 2007, 37, 1583–1589. [Google Scholar] [CrossRef]
- Liew, Y.M.; Kamarudin, H.; Al Bakri, A.M.; Bnhussain, M.; Luqman, M.; Nizar, I.K.; Ruzaidi, C.; Heah, C. Optimization of solids-to-liquid and alkali activator ratios of calcined kaolin geopolymeric powder. Constr. Build. Mater. 2012, 37, 440–451. [Google Scholar] [CrossRef]
- Lin, K.-L.; Shiu, H.-S.; Shie, J.-L.; Cheng, T.-W.; Hwang, C.-L. Effect of composition on characteristics of thin film transistor liquid crystal display (TFT-LCD) waste glass-metakaolin-based geopolymers. Constr. Build. Mater. 2012, 36, 501–507. [Google Scholar] [CrossRef]
- Hou, L.; Li, J.; Lu, Z.-y. Effect of Na/Al on formation, structures and properties of metakaolin based Na-geopolymer. Constr Build Mater 2019, 226, 250–258. [Google Scholar] [CrossRef]
- Duxson, P.; Mallicoat, S.W.; Lukey, G.C.; Kriven, W.M.; van Deventer, J.S. The effect of alkali and Si/Al ratio on the development of mechanical properties of metakaolin-based geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2007, 292, 8–20. [Google Scholar] [CrossRef]
- Lahalle, H.; Benavent, V.; Trincal, V.; Wattez, T.; Bucher, R.; Cyr, M. Robustness to water and temperature, and activation energies of metakaolin-based geopolymer and alkali-activated slag binders. Constr. Build. Mater. 2021, 300, 124066. [Google Scholar] [CrossRef]
- Wardhono, A. The effect of water binder ratio on strength development of class C fly ash geopolymer mortar prepared by dry geopolymer powder. MATEC Web Conf. 2019, 258, 05032. [Google Scholar]
- Wang, H.; Li, H.; Yan, F. Synthesis and mechanical properties of metakaolinite-based geopolymer. Colloids Surf. A Physicochem. Eng. Asp. 2005, 268, 1–6. [Google Scholar] [CrossRef]
- Pinto, A. Alkali-Activated Metakaolin Based Binders. Ph.D. Thesis, University of Minho, Braga, Portugal, 2004. [Google Scholar]
- Zuhua, Z.; Xiao, Y.; Huajun, Z.; Yue, C. Role of water in the synthesis of calcined kaolin-based geopolymer. Appl. Clay Sci. 2009, 43, 218–223. [Google Scholar] [CrossRef]
- Kamalloo, A.; Ganjkhanlou, Y.; Aboutalebi, S.H.; Nouranian, H. Modeling of compressive strength of metakaolin based geopolymers by the use of artificial neural network. Int. J. Eng. 2010, 23, 143–150. [Google Scholar]
- Aquino, W.; Lange, D.; Olek, J. The influence of metakaolin and silica fume on the chemistry of alkali–silica reaction products. Cem. Concr. Compos. 2001, 23, 485–493. [Google Scholar] [CrossRef]
- Jacops, E.; Phung, Q.T.; Frederickx, L.; Levasseur, S. Diffusive Transport of Dissolved Gases in Potential Concretes for Nuclear Waste Disposal. Sustainability 2021, 13, 10007. [Google Scholar] [CrossRef]
- Washburn, E.W. Note on a method of determining the distribution of pore sizes in a porous material. Proc. Natl. Acad. Sci. USA 1921, 7, 115. [Google Scholar] [CrossRef] [Green Version]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- De Boer, J.; Lippens, B.; Linsen, B.; Broekhoff, J.; Van den Heuvel, A.; Osinga, T.J. The t-curve of multimolecular N2-adsorption. J. Colloid Interface Sci. 1966, 21, 405–414. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernal, S.A.; Provis, J.L.; Brice, D.G.; Kilcullen, A.; Duxson, P.; van Deventer, J.S. Accelerated carbonation testing of alkali-activated binders significantly underestimates service life: The role of pore solution chemistry. Cem. Concr. Res. 2012, 42, 1317–1326. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Provis, J.L. Quantitative study of the reactivity of fly ash in geopolymerization by FTIR. J. Sustain. Cem. Based Mater. 2012, 1, 154–166. [Google Scholar] [CrossRef] [Green Version]
- Rovnaník, P. Effect of curing temperature on the development of hard structure of metakaolin-based geopolymer. Constr. Build. Mater. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]
- Bertier, P.; Schweinar, K.; Stanjek, H.; Ghanizadeh, A.; Clarkson, C.; Busch, A.; Kampman, N.; Prinz, D.; Amann-Hildenbrand, A.; Krooss, B. On the use and abuse of N2 physisorption for the characterization of the pore structure of shales. In The Clay Minerals Society Workshop Lectures Series; Clay Mineral Society: Boulder, CO, USA, 2016; pp. 151–161. [Google Scholar]
- Ghanbari, M.; Hadian, A.; Nourbakhsh, A.; MacKenzie, K. Modeling and optimization of compressive strength and bulk density of metakaolin-based geopolymer using central composite design: A numerical and experimental study. Ceram. Int. 2017, 43, 324–335. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.; Separovic, F.; Van Deventer, J. Effect of alkali cations on aluminum incorporation in geopolymeric gels. Ind. Eng. Chem. Res. 2005, 44, 832–839. [Google Scholar] [CrossRef]
- Wold, H. Soft modelling by latent variables: The non-linear iterative partial least squares (NIPALS) approach. J. Appl. Probab. 1975, 12, 117–142. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Provis, J.L.; Bullen, F.; Reid, A.; Zhu, Y. Quantitative kinetic and structural analysis of geopolymers. Part 1. The activation of metakaolin with sodium hydroxide. Thermochim. Acta 2012, 539, 23–33. [Google Scholar]
- Rifaai, Y.; Yahia, A.; Mostafa, A.; Aggoun, S.; Kadri, E.-H. Rheology of fly ash-based geopolymer: Effect of NaOH concentration. Constr. Build. Mater. 2019, 223, 583–594. [Google Scholar] [CrossRef]
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S. Attenuated total reflectance fourier transform infrared analysis of fly ash geopolymer gel aging. Langmuir 2007, 23, 8170–8179. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; Van Deventer, J.S. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Patel, R.A.; Phung, Q.T.; Seetharam, S.C.; Perko, J.; Jacques, D.; Maes, N.; De Schutter, G.; Ye, G.; Van Breugel, K. Diffusivity of saturated ordinary Portland cement-based materials: A critical review of experimental and analytical modelling approaches. Cem. Concr. Res. 2016, 90, 52–72. [Google Scholar] [CrossRef]
- Phung, Q.T.; Maes, N.; De Schutter, G.; Jacques, D.; Ye, G. Determination of water permeability of cementitious materials using a controlled constant flow method. Constr. Build. Mater. 2013, 47, 1488–1496. [Google Scholar] [CrossRef]





| SiO2 | Al2O3 | Fe2O3 | CaO | TiO2 | MgO | Na2O | K2O | P2O5 | LOI | |
|---|---|---|---|---|---|---|---|---|---|---|
| Metakaolin | 52.08 | 44.27 | 0.45 | 0.03 | 1.68 | 0.08 | 0.26 | 0.17 | 0.04 | 0.83 |
| SS (sol.) | 54.5 | 27.5 | 18 | |||||||
| SS (liq.) | 26.78 | 8.97 | 64.25 |
| Recipe | Activator | W/B Ratio | Water/Solids | SiO2/Al2O3 | Na2O/SiO2 | H2O/Na2O | NaOH, M |
|---|---|---|---|---|---|---|---|
| R1 | NaOH + SS (liq.) | 0.75 | 0.51 | 2.75 | 0.17 | 26.8 | 5 |
| NaOH + SS (liq.) | 0.85 | 0.57 | 2.75 | 0.17 | 30.4 | 5 | |
| NaOH + SS (liq.) | 0.95 | 0.62 | 2.75 | 0.17 | 33.9 | 5 | |
| R2 | NaOH + SS (sol.) | 0.75 | 0.43 | 2.81 | 0.18 | 25.7 | 10 |
| NaOH + SS (sol.) | 0.85 | 0.47 | 2.81 | 0.18 | 29.1 | 10 | |
| NaOH + SS (sol.) | 0.95 | 0.51 | 2.81 | 0.18 | 32.5 | 10 | |
| R3 | NaOH + SS (sol.) | 0.75 | 0.48 | 3.79 | 0.26 | 17.0 | 10 |
| NaOH + SS (sol.) | 0.85 | 0.52 | 3.79 | 0.26 | 19.3 | 10 | |
| NaOH + SS (sol.) | 0.95 | 0.57 | 3.79 | 0.26 | 21.5 | 10 |
| Amorph | Ana | Fs | Qz | Trona | |
|---|---|---|---|---|---|
| R1-0.75 | 76 | 0.4 | 0.3 | 23 | 0 |
| R1-0.85 | 79 | 0.4 | 0.2 | 20 | 0 |
| R1-0.95 | 71 | 0.6 | 0.8 | 26 | 2.0 |
| R2-0.75 | 70 | 0.4 | 1.7 | 28 | 0 |
| R2-0.85 | 75 | 0.4 | 1.1 | 24 | 0 |
| R2-0.95 | 70 | 0.4 | 1.1 | 28 | 0 |
| R3-0.75 | 76 | 0.4 | 2.1 | 22 | 0 |
| R3-0.85 | 69 | 0.2 | 1.7 | 29 | 0 |
| R3-0.95 | 71 | 0.2 | 2.1 | 26 | 0 |
| Pore Volume (%) | Bulk Dry Density (kg/m³) | Porosity (%, MIP) | |
|---|---|---|---|
| R1-0.75 | 45 | 1234 | 41 |
| R1-0.85 | 50 | 1159 | 53 |
| R1-0.95 | 46 | 1051 | 48 |
| R2-0.75 | 44 | 1357 | / |
| R2-0.85 | 44 | 1301 | / |
| R2-0.95 | 45 | 1287 | / |
| R3-0.75 | 43 | 1377 | 37 |
| R3-0.85 | 47 | 1263 | 36 |
| R3-0.95 | 52 | 1136 | 67 |
| SSA (m²/g, BET) | SSA (m²/g, t-plot) | Micropore Area (m²/g) | CPV (cm³/g) | |
|---|---|---|---|---|
| R1-0.75 | 10.2 | 10.8 | 0.0 | 0.052 |
| R1-0.85 | 7.6 | 6.7 | 0.9 | 0.027 |
| R1-0.95 | 4.5 | 4.5 | 0.0 | 0.014 |
| R2-0.75 | 6.4 | 6.2 | 0.2 | 0.025 |
| R2-0.85 | 2.5 | 2.1 | 0.5 | 0.013 |
| R2-0.95 | 1.7 | 1.3 | 0.4 | 0.013 |
| R3-0.75 | 5.1 | 4.6 | 0.4 | 0.018 |
| R3-0.85 | 2.4 | 2.4 | 0.0 | 0.007 |
| R3-0.95 | 1.9 | 1.9 | 0.1 | 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frederickx, L.; Nguyen, T.N.; Phung, Q.T. Strength and Microstructure Characteristics of Metakaolin-Based Geopolymer Mortars with High Water-to-Binder Ratios. Sustainability 2022, 14, 3141. https://doi.org/10.3390/su14063141
Frederickx L, Nguyen TN, Phung QT. Strength and Microstructure Characteristics of Metakaolin-Based Geopolymer Mortars with High Water-to-Binder Ratios. Sustainability. 2022; 14(6):3141. https://doi.org/10.3390/su14063141
Chicago/Turabian StyleFrederickx, Lander, Thi Nhan Nguyen, and Quoc Tri Phung. 2022. "Strength and Microstructure Characteristics of Metakaolin-Based Geopolymer Mortars with High Water-to-Binder Ratios" Sustainability 14, no. 6: 3141. https://doi.org/10.3390/su14063141
APA StyleFrederickx, L., Nguyen, T. N., & Phung, Q. T. (2022). Strength and Microstructure Characteristics of Metakaolin-Based Geopolymer Mortars with High Water-to-Binder Ratios. Sustainability, 14(6), 3141. https://doi.org/10.3390/su14063141







