Circularity of Bioenergy Residues: Acidification of Anaerobic Digestate Prior to Addition of Wood Ash
Abstract
:1. Introduction
2. Materials and Methods
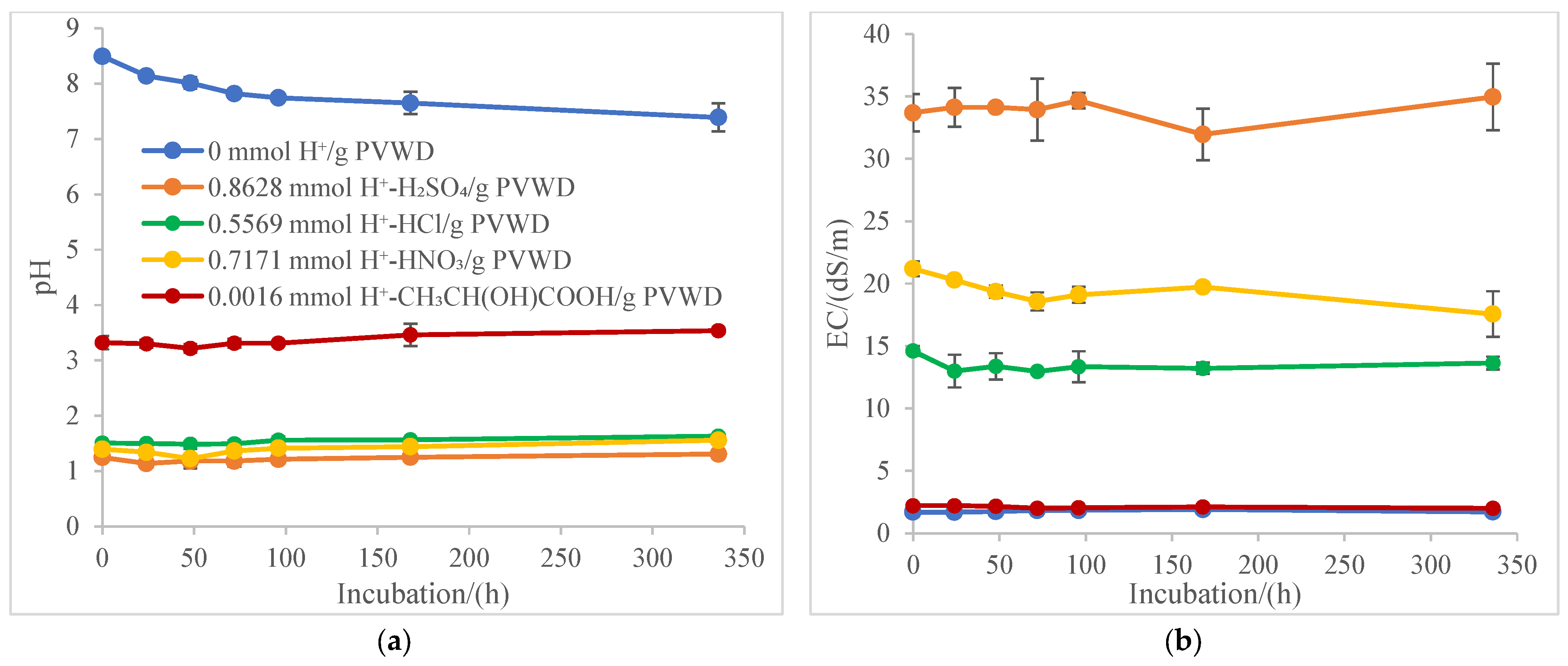
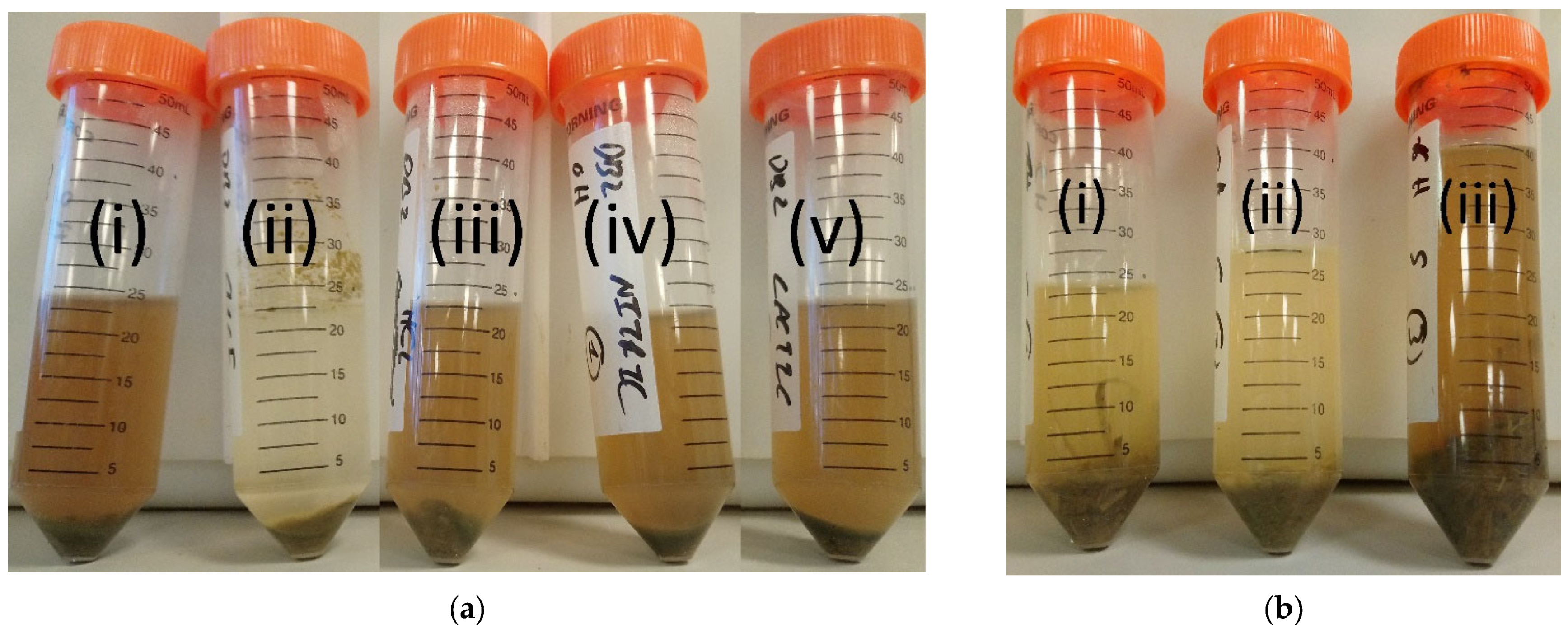
3. Results
4. Discussion
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | anaerobic digestion |
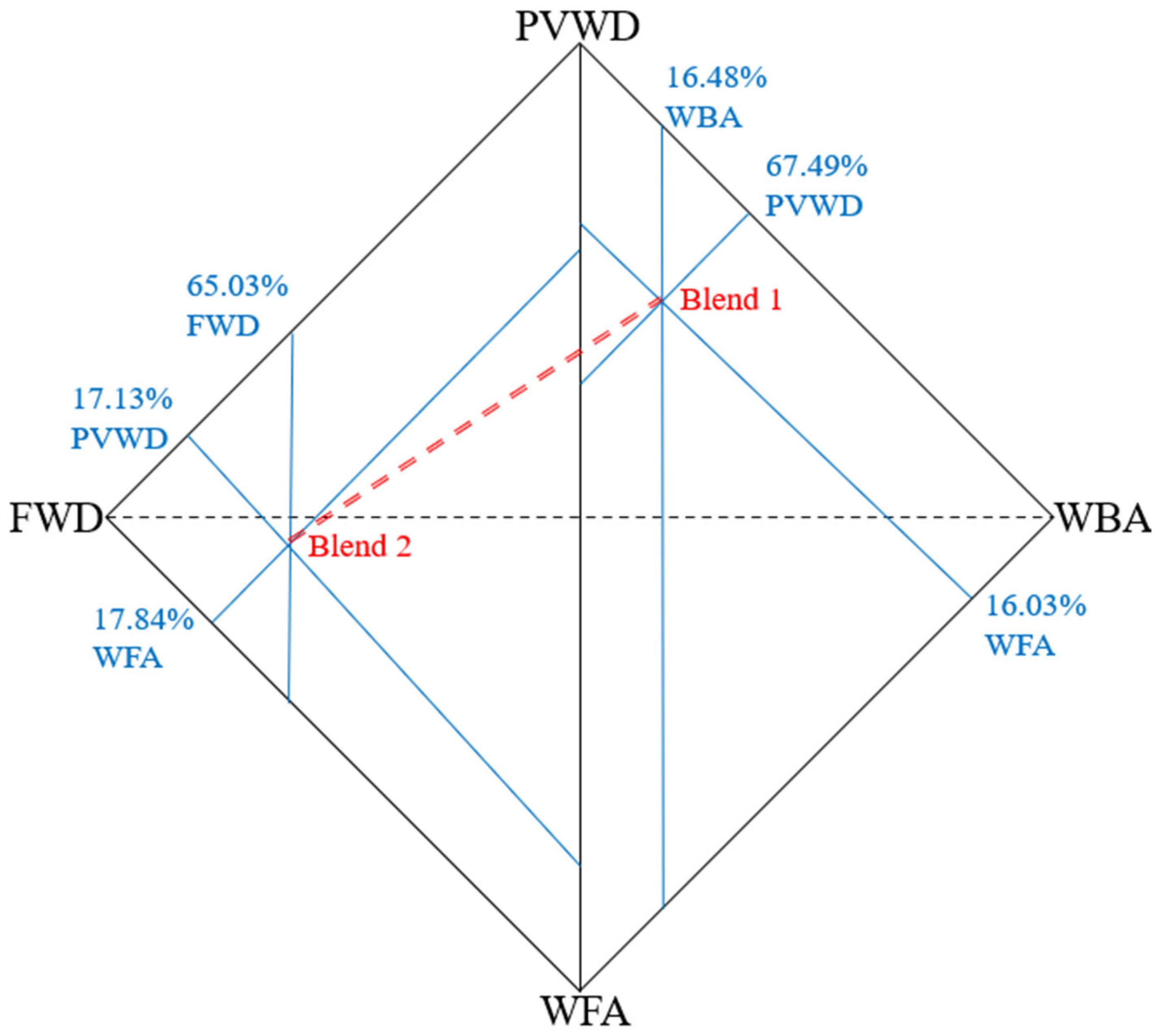
| Blend | mixture of approximately 20% PVWD and 80% FWD |
| BC | black carbon |
| C | carbon |
| Corg | organic carbon |
| CUE | carbon use efficiency |
| C/N/P | nutrient ratio |
| DOC | dissolved organic carbon |
| EoW | end-of-waste |
| FWD | food waste digestate |
| GHGs | greenhouse gases |
| OM | organic matter |
| PVWD | post-harvest vegetable waste digestate |
| PO43−-P | phosphorus in the form of orthophosphate |
| N | nitrogen |
| NH4+-N | ammoniacal nitrogen |
| S/E | sample-to-extractant ratio |
| WBA | wood bottom ash |
| WFA | woof fly ash |
References
- Kavanagh, I.; Burchill, W.; Healy, M.G.; Fenton, O.; Krol, D.J.; Lanigan, G.J. Mitigation of ammonia and greenhouse gas emissions from stored cattle slurry using acidifiers and chemical amendments. J. Clean. Prod. 2019, 237, 117822. [Google Scholar] [CrossRef]
- US Geological Survey. Mineral Commodity Summaries; US Geological Survey: Reston, VA, USA, 2021; pp. 122–123. [Google Scholar] [CrossRef]
- Cisternas, L.A.; Ordóñez, J.I.; Jeldres, R.I.; Serna-Guerrero, R. Toward the Implementation of Circular Economy Strategies: An Overview of the Current Situation in Mineral Processing. Miner. Process. Extr. Metall. Rev. 2021. [Google Scholar] [CrossRef]
- Marshall, R.; Lag-Brotons, A.J.; Inam, E.J.; Herbert, B.M.J.; Hurst, L.; Semple, K.T. From Bioenergy By-products to Alternative Fertilisers: Pursuing a Circular Economy. In Resource Recovery from Wastes: Towards a Circular Economy; Macaskie, L.E., Sapsford, D.J., Mayes, W.M., Eds.; Royal Society of Chemistry: London, UK, 2020; pp. 298–314. [Google Scholar] [CrossRef]
- Kavanagh, I.; Fenton, O.; Healy, M.G.; Burchill, W.; Lanigan, G.J.; Krol, D.J. Mitigating ammonia and greenhouse gas emissions from stored cattle slurry using agricultural waste, commercially available products and a chemical acidifier. J. Clean. Prod. 2021, 294, 126251. [Google Scholar] [CrossRef]
- Seadon, J.K. Sustainable waste management systems. J. Clean. Prod. 2010, 18, 1639–1651. [Google Scholar] [CrossRef]
- Krause, A.; Häfner, F.; Augustin, F.; Udert, K.M. Qualitative Risk Analysis for Contents of Dry Toilets Used to Produce Novel Recycling Fertilizers. Circ. Econ. Sustain. 2021, 1, 1107–1146. [Google Scholar] [CrossRef]
- Welle, F. Investigation into cross-contamination during cleaning efficiency testing in PET recycling. Resour. Conserv. Recycl. 2016, 112, 65–72. [Google Scholar] [CrossRef]
- Nag, R.; Russell, L.; Nolan, S.; Auer, A.; Markey, B.K.; Whyte, P.; Flaherty, V.O.; Bolton, D.; Fenton, O.; Richards, K.G.; et al. Quantitative microbial risk assessment associated with ready-to-eat salads following the application of farmyard manure and slurry or anaerobic digestate to arable lands. Sci. Total Environ. 2021, 806, 151227. [Google Scholar] [CrossRef]
- Lag-Brotons, A.J.; Marshall, R.; Herbert, B.M.J.; Hurst, L.; Inam, E.J.; Semple, K.T. Adding Value to Ash and Digestate (AVAnD Project): Elucidating the Role and Value of Alternative Fertilisers on the Soil-Plant System. In Resource Recovery from Wastes: Towards a Circular Economy; Macaskie, L.E., Sapsford, D.J., Mayes, W.M., Eds.; Royal Society of Chemistry: London, UK, 2020; pp. 113–139. [Google Scholar] [CrossRef]
- UK Government. End of Waste Test Submission Forms. Available online: https://www.gov.uk/government/publications/end-of-waste-submission-forms (accessed on 19 February 2022).
- UK Government. Quality protocols: Converting Waste into Non-Waste Products. Available online: https://www.gov.uk/government/collections/quality-protocols-end-of-waste-frameworks-for-waste-derived-products (accessed on 19 February 2022).
- UK Government. Quality Protocol Poultry Litter Ash. End of Waste Criteria for the Production and Use of Treated Ash from the Incineration of Poultry Litter, Feathers and Straw. Available online: https://www.gov.uk/government/publications/quality-protocol-poultry-litter-ash (accessed on 14 December 2021).
- UK Government. Quality Protocol of Anaerobic Digestate. End of Waste Criteria for the Production and Use of Quality Outputs from Anaerobic Digestion of Source-Segregated Biodegradable Waste. Available online: https://www.gov.uk/government/publications/quality-protocol-anaerobic-digestate (accessed on 14 December 2021).
- WRAP. BSI PAS 110:2014 Specification for Whole Digestate, Separated Liquor and Separated Fibre Derived from the Anaerobic Digestion of Source-Segregated Biodegradable Materials. Available online: https://wrap.org.uk/resources/guide/bsi-pas-110-producing-quality-anaerobic-digestate (accessed on 7 January 2022).
- British Standard Institution. PAS 100:2018. Specification for Composted Materials. Available online: https://shop.bsigroup.com/products/specification-for-composted-materials/standard (accessed on 19 February 2022).
- European Commision. Closing the Loop—An EU Action Plan for the Circular Economy. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:52015DC0614&from=ES (accessed on 5 February 2022).
- European Parliament. Directive 2018/851 Amending Directive 2008/98/EC on Waste Framework. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2018.150.01.0109.01.ENG (accessed on 14 December 2021).
- European Parliament. Regulation (EU) 2019/1009 of Fertilising Products. Off. J. Eur. Union 2019. Available online: https://eur-lex.europa.eu/eli/reg/2019/1009/oj (accessed on 22 February 2022).
- Ibeto, C.N.; Lag-Brotons, A.J.; Marshall, R.; Semple, K.T. The Nutritional Effects of Digested and Undigested Organic Wastes Combined with Wood Ash Amendments on Carrot Plants. J. Soil Sci. Plant Nutr. 2020, 20, 460–472. [Google Scholar] [CrossRef]
- Hou, Y.; Velthof, G.L.; Case, S.D.C.; Oelofse, M.; Grignani, C.; Balsari, P.; Zavattaro, L.; Gioelli, F.; Bernal, M.P.; Fangueiro, D.; et al. Stakeholder perceptions of manure treatment technologies in Denmark, Italy, the Netherlands and Spain. J. Clean. Prod. 2018, 172, 1620–1630. [Google Scholar] [CrossRef]
- Tabatabaei, M.; Aghbashlo, M.; Valijanian, E.; Kazemi Shariat Panahi, H.; Nizami, A.S.; Ghanavati, H.; Sulaiman, A.; Mirmohamadsadeghi, S.; Karimi, K. A comprehensive review on recent biological innovations to improve biogas production, Part 1: Upstream strategies. Renew. Energy 2020, 146, 1204–1220. [Google Scholar] [CrossRef]
- Tabatabaei, M.; Aghbashlo, M.; Valijanian, E.; Kazemi Shariat Panahi, H.; Nizami, A.S.; Ghanavati, H.; Sulaiman, A.; Mirmohamadsadeghi, S.; Karimi, K. A comprehensive review on recent biological innovations to improve biogas production, Part 2: Mainstream and downstream strategies. Renew. Energy 2020, 146, 1392–1407. [Google Scholar] [CrossRef]
- Appels, L.; Lauwers, J.; Degrve, J.; Helsen, L.; Lievens, B.; Willems, K.; Van Impe, J.; Dewil, R. Anaerobic digestion in global bio-energy production: Potential and research challenges. Renew. Sustain. Energy Rev. 2011, 15, 4295–4301. [Google Scholar] [CrossRef]
- Horta, C.; Carneiro, J.P. Use of Digestate as Organic Amendment and Source of Nitrogen to Vegetable Crops. Appl. Sci. 2021, 12, 248. [Google Scholar] [CrossRef]
- Reuland, G.; Sigurnjak, I.; Dekker, H.; Sleutel, S.; Meers, E. Assessment of the Carbon and Nitrogen Mineralisation of Digestates Elaborated from Distinct Feedstock Profiles. Agronomy 2022, 12, 456. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Prado, J.; Ribeiro, H.; Alvarenga, P.; Fangueiro, D. A step towards the production of manure-based fertilizers: Disclosing the effects of animal species and slurry treatment on their nutrients content and availability. J. Clean. Prod. 2022, 337, 130369. [Google Scholar] [CrossRef]
- de França, A.A.; von Tucher, S.; Schmidhalter, U. Effects of combined application of acidified biogas slurry and chemical fertilizer on crop production and N soil fertility. Eur. J. Agron. 2021, 123, 126224. [Google Scholar] [CrossRef]
- Fangueiro, D.; Hjorth, M.; Gioelli, F. Acidification of animal slurry—A review. J. Environ. Manag. 2015, 149, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Szymula, A.; Wlazło, Ł.; Sasáková, N.; Wnuk, W.; Nowakowicz-Dębek, B. The Use of Natural Sorbents to Reduce Ammonia Emissions from Cattle Faeces. Agronomy 2021, 11, 2543. [Google Scholar] [CrossRef]
- Gonzalez-Perez, A.; Hägg, K.; Duteil, F. Optimizing NOM Removal: Impact of Calcium Chloride. Sustainability 2021, 13, 6338. [Google Scholar] [CrossRef]
- Shi, W.; Healy, M.G.; Ashekuzzaman, S.M.; Daly, K.; Leahy, J.J.; Fenton, O. Dairy processing sludge and co-products: A review of present and future re-use pathways in agriculture. J. Clean. Prod. 2021, 314, 128035. [Google Scholar] [CrossRef]
- Brennan, R.B.; Fenton, O.; Rodgers, M.; Healy, M.G. Evaluation of chemical amendments to control phosphorus losses from dairy slurry. Soil Use Manag. 2011, 27, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Brennan, R.B.; Fenton, O.; Grant, J.; Healy, M.G. Impact of chemical amendment of dairy cattle slurry on phosphorus, suspended sediment and metal loss to runoff from a grassland soil. Sci. Total Environ. 2011, 409, 5111–5118. [Google Scholar] [CrossRef] [Green Version]
- Brennan, R.B.; Healy, M.G.; Fenton, O.; Lanigan, G.J. The effect of chemical amendments used for phosphorus abatement on greenhouse gas and ammonia emissions from dairy cattle slurry: Synergies and pollution swapping. PLoS ONE 2015, 10, e0111965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, C.; Soares, A.S.; Coelho, A.C.; Trindade, H.; Teixeira, C.A. Environmental implications of stored cattle slurry treatment with sulphuric acid and biochar: A life cycle assessment approach. Environ. Res. 2021, 194, 110640. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, C.; Liao, W.; Xie, J.; Zhang, X.; Gao, Z. Impact of biochar application on gas emissions from liquid pig manure storage. Sci. Total Environ. 2021, 771, 145454. [Google Scholar] [CrossRef]
- Sullivan, G.L.; Prigmore, R.M.; Knight, P.; Godfrey, A.R. Activated carbon biochar from municipal waste as a sorptive agent for the removal of polyaromatic hydrocarbons (PAHs), phenols and petroleum based compounds in contaminated liquids. J. Environ. Manag. 2019, 251, 109551. [Google Scholar] [CrossRef] [PubMed]
- Leechart, P.; Nakbanpote, W.; Thiravetyan, P. Application of ‘waste’ wood-shaving bottom ash for adsorption of azo reactive dye. J. Environ. Manag. 2009, 90, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Laohaprapanon, S.; Marques, M.; Hogland, W. Removal of organic pollutants from wastewater using wood fly ash as a low-cost sorbent. Clean Soil Air Water 2010, 38, 1055–1061. [Google Scholar] [CrossRef]
- Forbes, M.S.; Raison, R.J.; Skjemstad, J.O. Formation, transformation and transport of black carbon (charcoal) in terrestrial and aquatic ecosystems. Sci. Total Environ. 2006, 370, 190–206. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Sohi, S.P.; Thies, J.E.; O’Neill, B.; Trujillo, L.; Gaunt, J.; Solomon, D.; Grossman, J.; Neves, E.G.; et al. Black carbon affects the cycling of non-black carbon in soil. Org. Geochem. 2010, 41, 206–213. [Google Scholar] [CrossRef]
- Insam, H.; Franke-Whittle, I.H.; Knapp, B.A.; Plank, R. Use of Wood Ash and Anaerobic Sludge For Grassland Fertilization: Effects on Plants and Microbes. Die Bodenkult. 2009, 60, 39–51. Available online: https://diebodenkultur.boku.ac.at/volltexte/band-60/heft-2/insam.pdf (accessed on 11 February 2022).
- Fernandez-Delgado Juarez, M.; Prähauser, B.; Walter, A.; Insam, H.; Franke-Whittle, I.H. Co-composting of biowaste and wood ash, influence on a microbially driven-process. Waste Manag. 2015, 46, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Delgado Juarez, M.; Gómez-Brandón, M.; Insam, H. Merging two waste streams, wood ash and biowaste, results in improved composting process and end products. Sci. Total Environ. 2015, 511, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Delgado Juárez, M.; Waldhuber, S.; Knapp, A.; Partl, C.; Gómez-Brandón, M.; Insam, H. Wood ash effects on chemical and microbiological properties of digestate- and manure-amended soils. Biol. Fertil. Soils 2013, 49, 575–585. [Google Scholar] [CrossRef]
- Bougnom, B.P.; Niederkofler, C.; Knapp, B.A.; Stimpfl, E.; Insam, H. Residues from renewable energy production: Their value for fertilizing pastures. Biomass Bioenergy 2012, 39, 290–295. [Google Scholar] [CrossRef]
- Moure Abelenda, A.; Aiouache, F. Wood Ash Based Treatment of Anaerobic Digestate: State-of-the-Art and Possibilities. Processes 2022, 10, 147. [Google Scholar] [CrossRef]
- Regelink, I.C.; Egene, C.E.; Tack, F.M.G.; Meers, E. Speciation of P in Solid Organic Fertilisers from Digestate and Biowaste. Agronomy 2021, 11, 2233. [Google Scholar] [CrossRef]
- Regueiro, I.; Coutinho, J.; Gioelli, F.; Balsari, P.; Dinuccio, E.; Fangueiro, D. Acidification of raw and co-digested pig slurries with alum before mechanical separation reduces gaseous emission during storage of solid and liquid fractions. Agric. Ecosyst. Environ. 2016, 227, 42–51. [Google Scholar] [CrossRef]
- Richards, S.; Marshall, R.; Lag-Brotons, A.J.; Semple, K.T.; Stutter, M. Phosphorus solubility changes following additions of bioenergy wastes to an agricultural soil: Implications for crop availability and environmental mobility. Geoderma 2021, 401, 115150. [Google Scholar] [CrossRef]
- Gebremichael, A.W.; Wall, D.P.; O’Neill, R.M.; Krol, D.J.; Brennan, F.; Lanigan, G.; Richards, K.G. Effect of contrasting phosphorus levels on nitrous oxide and carbon dioxide emissions from temperate grassland soils. Sci. Rep. 2022, 12, 2602. [Google Scholar] [CrossRef]
- Moure Abelenda, A.; Semple, K.T.; Lag-brotons, A.J.; Herbert, B.M.J.; Aggidis, G.; Aiouache, F. Strategies for the production of a stable blended fertilizer of anaerobic digestates and wood ashes. Nat. Based Solut. 2022, 100014. [Google Scholar] [CrossRef]
- Moure Abelenda, A.; Semple, K.T.; Lag-Brotons, A.J.; Herbert, B.M.J.; Aggidis, G.; Aiouache, F. Effects of Wood Ash-Based Alkaline Treatment on Nitrogen, Carbon, and Phosphorus Availability in Food Waste and Agro-Industrial Waste Digestates. Waste Biomass Valorization 2020, 12, 3355–3370. [Google Scholar] [CrossRef]
- Shah, I.; Adnan, R.; Ngah, W.S.W.; Mohamed, N. Iron impregnated activated carbon as an efficient adsorbent for the removal of methylene blue: Regeneration and kinetics studies. PLoS ONE 2015, 10, e0122603. [Google Scholar] [CrossRef] [Green Version]
- Moure Abelenda, A.; Semple, K.T.; Lag-Brotons, A.J.; Herbert, B.M.J.; Aggidis, G.; Aiouache, F. Impact of sulphuric, hydrochloric, nitric, and lactic acids in the preparation of a blend of agro-industrial digestate and wood ash to produce a novel fertiliser. J. Environ. Chem. Eng. 2021, 9, 105021. [Google Scholar] [CrossRef]
- Moure Abelenda, A.; Amaechi, C.V. Manufacturing of a Granular Fertilizer Based on Organic Slurry and Hardening Agent. Inventions 2022, 7, 26. [Google Scholar] [CrossRef]
- Regueiro, I.; Coutinho, J.; Fangueiro, D. Alternatives to sulfuric acid for slurry acidification: Impact on slurry composition and ammonia emissions during storage. J. Clean. Prod. 2016, 131, 296–307. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Conklin, A.R.; Vitha, M.F. Electrical measurements. Introd. Soil Chem. Anal. Instrum. 2014, 178, 188. [Google Scholar] [CrossRef]
- Shcherbakov, V.V.; Artemkina, Y.M.; Ponomareva, T.N.; Kirillov, A.D. Electrical conductivity of the ammonia-water system. Russ. J. Inorg. Chem. 2009, 54, 277–279. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Manzoni, S.; Moorhead, D.L.; Richter, A. Carbon use efficiency of microbial communities: Stoichiometry, methodology and modelling. Ecol. Lett. 2013, 16, 930–939. [Google Scholar] [CrossRef]
- Limoli, A.; Langone, M.; Andreottola, G. Ammonia removal from raw manure digestate by means of a turbulent mixing stripping process. J. Environ. Manag. 2016, 176, 1–10. [Google Scholar] [CrossRef]
- UK Government. Clean Air Strategy 2019. Available online: https://www.gov.uk/government/publications/clean-air-strategy-2019 (accessed on 24 January 2022).
- Drosg, B.; Fuchs, W.; Al Seadi, T.; Madsen, M.; Linke, B. Nutrient Recovery by Biogas Digestate Processing. Available online: http://task37.ieabioenergy.com/technical-brochures.html#form (accessed on 14 December 2021).
- Fuchs, W.; Drosg, B. Assessment of the state of the art of technologies for the processing of digestate residue from anaerobic digesters. Waster Sci. Technol. 2013, 67, 1984–1993. [Google Scholar] [CrossRef] [Green Version]
- UK DEFRA. The Path to Sustainable Farming: An Agricultural Transition Plan 2021 to 2024. Available online: https://www.gov.uk/government/publications/agricultural-transition-plan-2021-to-2024 (accessed on 30 December 2021).
- UK DEFRA. Farming is Changing. Available online: https://www.gov.uk/government/publications/future-farming-changes-to-farming-in-england (accessed on 30 December 2021).
- Zheng, Y.; Ke, L.; Xia, D.; Zheng, Y.; Wang, Y.; Li, H.; Li, Q. Enhancement of digestates dewaterability by CTAB combined with CFA pretreatment. Sep. Purif. Technol. 2016, 163, 282–289. [Google Scholar] [CrossRef]
- Tursi, A. A review on biomass: Importance, chemistry, classification, and conversion. Biofuel Res. J. 2019, 6, 962–979. [Google Scholar] [CrossRef]
- Lencioni, G.; Imperiale, D.; Cavirani, N.; Marmiroli, N.; Marmiroli, M. Environmental application and phytotoxicity of anaerobic digestate from pig farming by in vitro and in vivo trials. Int. J. Environ. Sci. Technol. 2016, 13, 2549–2560. [Google Scholar] [CrossRef]
- Coelho, C.M.M.; Bellato, C.d.M.; Santos, J.C.P.; Ortega, E.M.M.; Tsai, S.M. Quality of plant products from organic agriculture. J. Sci. Food Agric. 2007, 87, 1237–1243. [Google Scholar] [CrossRef]
- Renewable Energy Assurance Ltd. Biofertiliser Certification Scheme. Rules for England, Scotland, Wales & Northern Ireland. 2015. Available online: http://www.biofertiliser.org.uk/pdf/scheme-rules-4-0.pdf (accessed on 22 February 2022).
- Esteves, C.; Ribeiro, H.; Braga, R.P.; Fangueiro, D. Remote Sensing (NDVI) and Apparent Soil Electrical Conductivity (ECap) to Delineate Different Zones in a Vineyard. Biol. Life Sci. Forum 2021, 3, 42. [Google Scholar] [CrossRef]
- Daliakopoulos, I.N.; Tsanis, I.K.; Koutroulis, A.; Kourgialas, N.N.; Varouchakis, A.E.; Karatzas, G.P.; Ritsema, C.J. The threat of soil salinity: A European scale review. Sci. Total Environ. 2016, 573, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, M.; Jewiarz, M.; Mudryk, K.; Fraczek, J.; Dziedzic, K. Conceptual design of the mobile granulation line for production fertilizers from digestates and ash mixtures. MATEC Web Conf. 2018, 168, 04003. [Google Scholar] [CrossRef] [Green Version]
- Jewiarz, M.; Wróbel, M.; Fraczek, J.; Mudryk, K.; Dziedzic, K. Digestate, ash and Trichoderm based fertilizer-production line concept design. MATEC Web Conf. 2018, 168, 04004. [Google Scholar] [CrossRef] [Green Version]
- Maresca, A.; Krüger, O.; Herzel, H.; Adam, C.; Kalbe, U.; Astrup, T.F. Influence of wood ash pre-treatment on leaching behaviour, liming and fertilising potential. Waste Manag. 2019, 83, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Winandy, J.; Caia, Z. Potential of Using Anaerobically Digested Bovine Biofiber as a Fiber Source for Wood Composites. BioResources 2008, 3, 1244–1255. Available online: https://www.fs.usda.gov/treesearch/pubs/33689 (accessed on 23 February 2022).
- Rao, J.R.; Watabe, M.; Stewart, T.A.; Millar, B.C.; Moore, J.E. Pelleted organo-mineral fertilisers from composted pig slurry solids, animal wastes and spent mushroom compost for amenity grasslands. Waste Manag. 2007, 27, 1117–1128. [Google Scholar] [CrossRef]
- Pesonen, J.; Kuokkanen, V.; Kuokkanen, T.; Illikainen, M. Co-granulation of bio-ash with sewage sludge and lime for fertilizer use. J. Environ. Chem. Eng. 2016, 4, 4817–4821. [Google Scholar] [CrossRef]
- Wei, X.; Li, Z. Study on hydration of Portland cement with fly ash using electrical measurement. Mater. Struct. Mater. Constr. 2005, 38, 411–417. [Google Scholar] [CrossRef]
- Sakai, E.; Miyahara, S.; Ohsawa, S.; Lee, S.H.; Daimon, M. Hydration of fly ash cement. Cem. Concr. Res. 2005, 35, 1135–1140. [Google Scholar] [CrossRef]
- Demeyer, A.; Voundi Nkana, J.C.; Verloo, M.G. Characteristics of wood ash and influence on soil properties and nutrient uptake: An overview. Bioresour. Technol. 2001, 77, 287–295. [Google Scholar] [CrossRef]
- Galan, I.; Glasser, F.P.; Andrade, C. Calcium carbonate decomposition. J. Therm. Anal. Calorim. 2013, 111, 1197–1202. [Google Scholar] [CrossRef]
- Mudryk, K.; Frączek, J.; Wróbel, M.; Jewiarz, M.; Dziedzic, K. Agglomeration of Ash-Based Fertilizer Mixtures from Biomass Combustion and Digestate. In Renewable Energy Sources: Engineering, Technology, Innovation; Krzysztof, M., Sebastian, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 823–834. [Google Scholar] [CrossRef]
- AECOM Inc.; Metcalf & Eddy Inc. Advanced alkaline stabilisation technologies. In Wastewater Engineering: Treatment and Resource Recovery, 5th ed.; Tchobanoglous, G., Stensel, H.D., Tsuchihashi, R., Burton, F., Eds.; Mc Graw Hill: New York, NY, USA, 2014; pp. 1501–1502. [Google Scholar]
- UK Government. Sewage Sludge in Agriculture: Code of Practice for England, Wales and Northern Ireland. Available online: https://www.gov.uk/government/publications/sewage-sludge-in-agriculture-code-of-practice/sewage-sludge-in-agriculture-code-of-practice-for-england-wales-and-northern-ireland (accessed on 14 December 2021).
- Moure Abelenda, A.; Semple, K.T.; Lag-Brotons, A.J.; Herbert, B.M.; Aggidis, G.; Aiouache, F. Alkaline Wood Ash, Turbulence, and Traps with Excess of Sulfuric Acid Do Not Strip Completely the Ammonia off an Agro-waste Digestate. Edelweiss Chem. Sci. J. 2021, 4, 19–24. [Google Scholar] [CrossRef]
- Moure Abelenda, A.; Semple, K.T.; Lag-Brotons, A.J.; Herbert, B.M.J.; Aggidis, G.; Aiouache, F. Kinetic study of the stabilization of an agro-industrial digestate by adding wood fly ash. Chem. Eng. J. Adv. 2021, 7, 100127. [Google Scholar] [CrossRef]
- Ukwuani, A.T.; Tao, W. Developing a vacuum thermal stripping—Acid absorption process for ammonia recovery from anaerobic digester effluent. Water Res. 2016, 106, 108–115. [Google Scholar] [CrossRef]
- Liu, L.; Pang, C.; Wu, S.; Dong, R. Optimization and evaluation of an air-recirculated stripping for ammonia removal from the anaerobic digestate of pig manure. Process Saf. Environ. Prot. 2015, 94, 350–357. [Google Scholar] [CrossRef]
- Georgacakis, D.; Sievers, D.M.; Iannotti, E.L. Buffer stability in manure digesters. Agric. Wastes 1982, 4, 427–441. [Google Scholar] [CrossRef]
- Drapanauskaite, D.; Handler, R.M.; Fox, N.; Baltrusaitis, J. Transformation of Liquid Digestate from the Solid-Separated Biogas Digestion Reactor Effluent into a Solid NH4HCO3 Fertilizer: Sustainable Process Engineering and Life Cycle Assessment. ACS Sustain. Chem. Eng. 2021, 9, 580–588. [Google Scholar] [CrossRef]



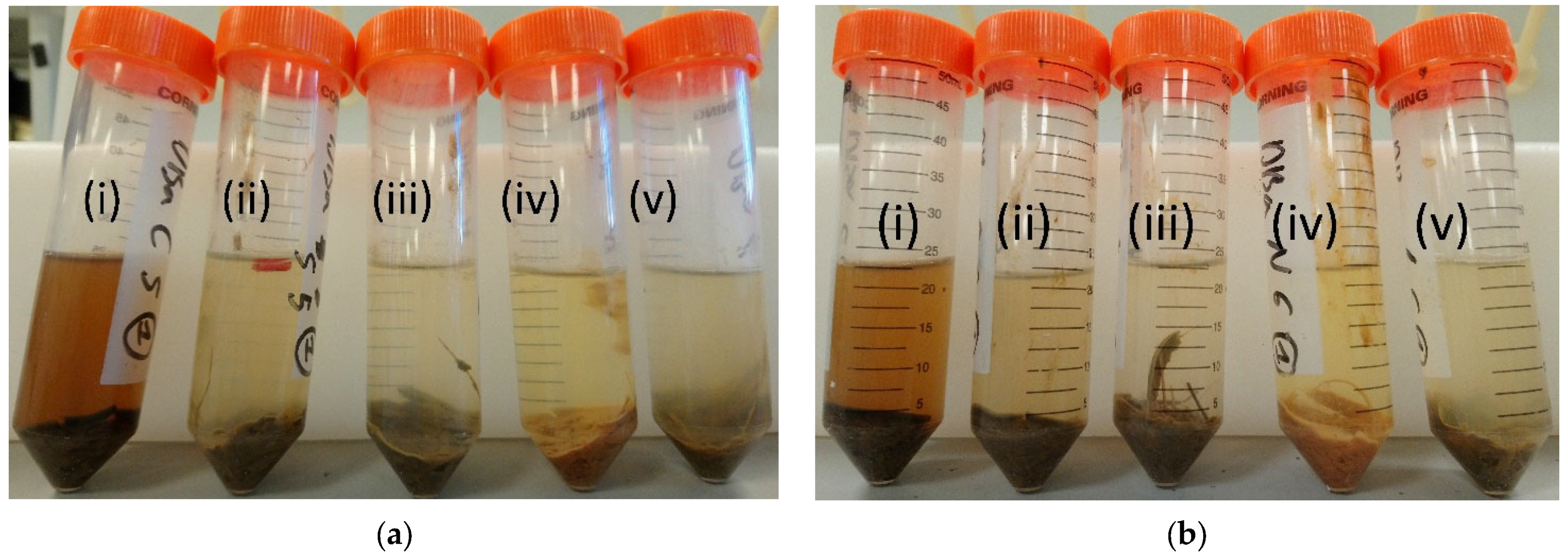

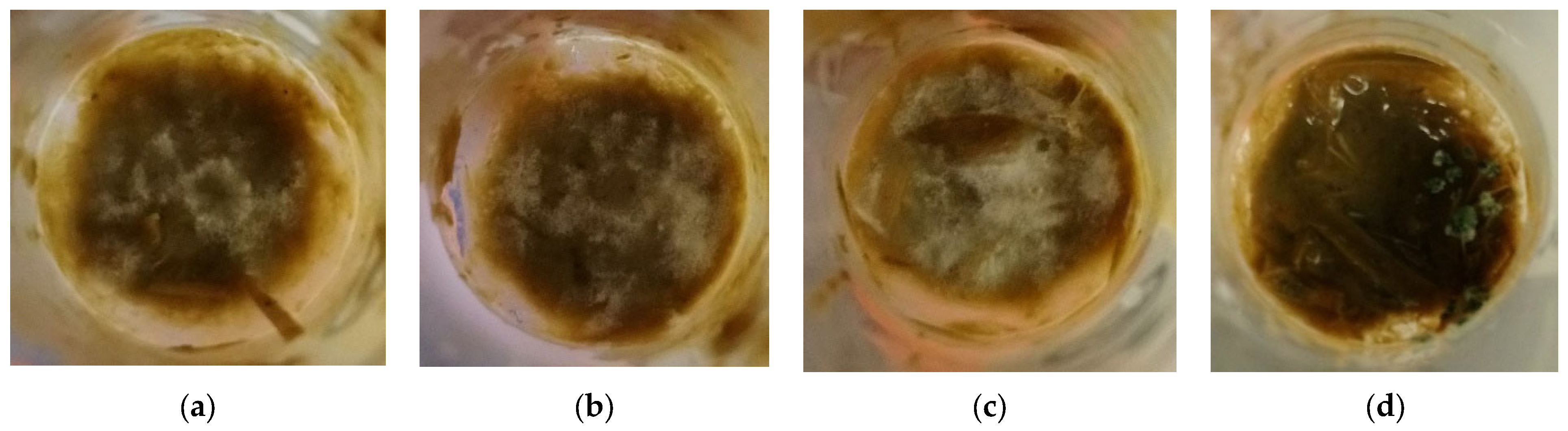
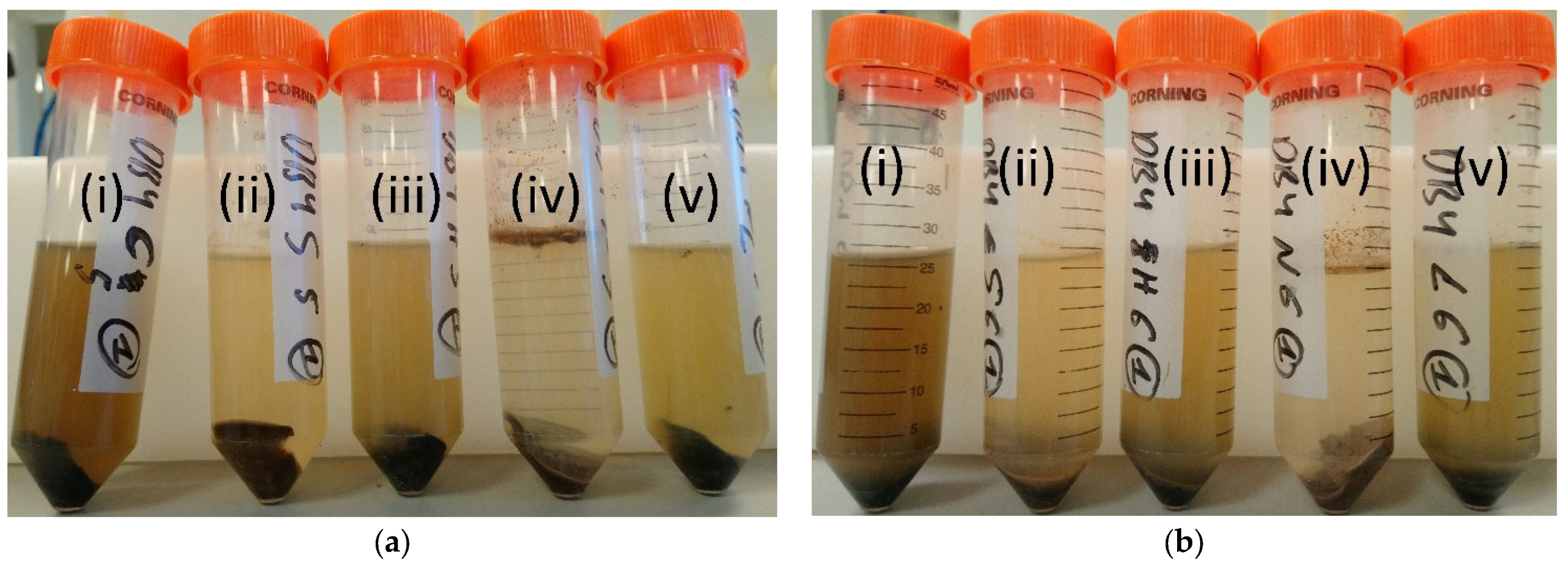
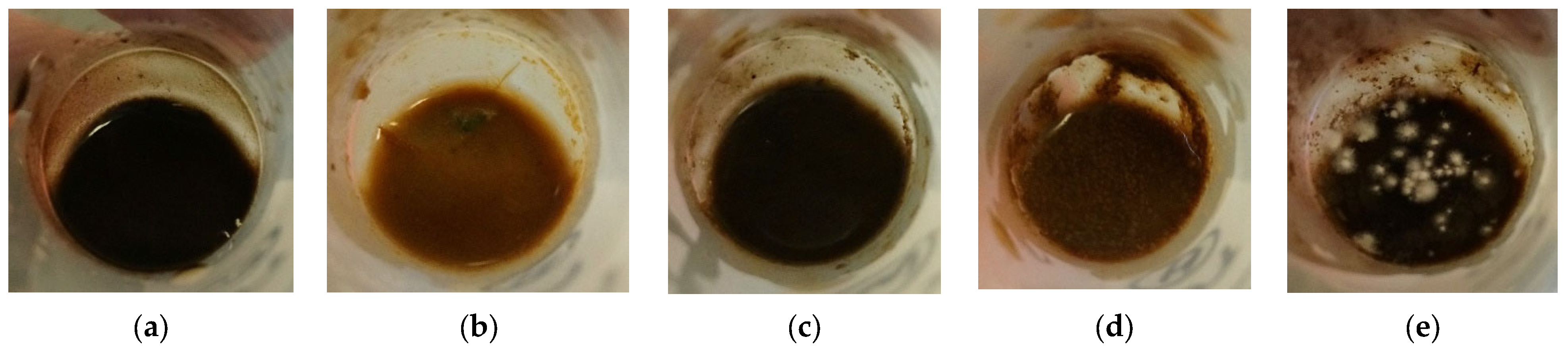

| Material | Purpose | Reference |
|---|---|---|
| Al2(SO4)3 | Coagulation/flocculation, acidification, and mitigation of GHGs after the solid–liquid separation | [51] |
| FeCl2 | Acidification to reduce the NH3 volatilization whilst minimizing the leaching of PO43−-P | [35] |
| CaO | Minimize the leaching of PO43−-P | [36] |
| Wood ashes | Improve the nutrient profile C/N/P | [52] |
| Dose | H2SO4 | HCl | HNO3 | CH3CH(OH)COOH | ||||
|---|---|---|---|---|---|---|---|---|
| µL/g | mmol H+/g Blend | mEq acid/g Blend | mmol H+/g Blend | mEq acid/g Blend | mmol H+/g Blend | mEq acid/g Blend | mmol H+/g Blend | mEq acid/g Blend |
| 10 µL acid/ 2.67 ± 0.55 g PVWD | 0.0687 | 0.1373 | 0.0443 | 0.0443 | 0.0571 | 0.0571 | 0.0001 | 0.0450 |
| 100 µL acid/ 2.12 ± 0.06 g PVWD | 0.8628 | 0.1725 | 0.5569 | 0.0557 | 0.7171 | 0.0717 | 0.0016 | 0.0565 |
| 10 µL acid/ 2.32 ± 0.11 g Blend * | 0.0788 | 0.1574 | 0.0508 | 0.0508 | 0.0655 | 0.0655 | 0.0001 | 0.0516 |
| 100 µL acid/ 2.44 ± 0.11 g Blend * | 0.7488 | 0.1497 | 0.4833 | 0.0483 | 0.6223 | 0.0622 | 0.0014 | 0.0490 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moure Abelenda, A.; Semple, K.T.; Aggidis, G.; Aiouache, F. Circularity of Bioenergy Residues: Acidification of Anaerobic Digestate Prior to Addition of Wood Ash. Sustainability 2022, 14, 3127. https://doi.org/10.3390/su14053127
Moure Abelenda A, Semple KT, Aggidis G, Aiouache F. Circularity of Bioenergy Residues: Acidification of Anaerobic Digestate Prior to Addition of Wood Ash. Sustainability. 2022; 14(5):3127. https://doi.org/10.3390/su14053127
Chicago/Turabian StyleMoure Abelenda, Alejandro, Kirk T. Semple, George Aggidis, and Farid Aiouache. 2022. "Circularity of Bioenergy Residues: Acidification of Anaerobic Digestate Prior to Addition of Wood Ash" Sustainability 14, no. 5: 3127. https://doi.org/10.3390/su14053127
APA StyleMoure Abelenda, A., Semple, K. T., Aggidis, G., & Aiouache, F. (2022). Circularity of Bioenergy Residues: Acidification of Anaerobic Digestate Prior to Addition of Wood Ash. Sustainability, 14(5), 3127. https://doi.org/10.3390/su14053127