The Impact of Pb from Ammunition on the Vegetation of a Bird Shooting Range
Abstract
1. Introduction
2. Materials and Methods
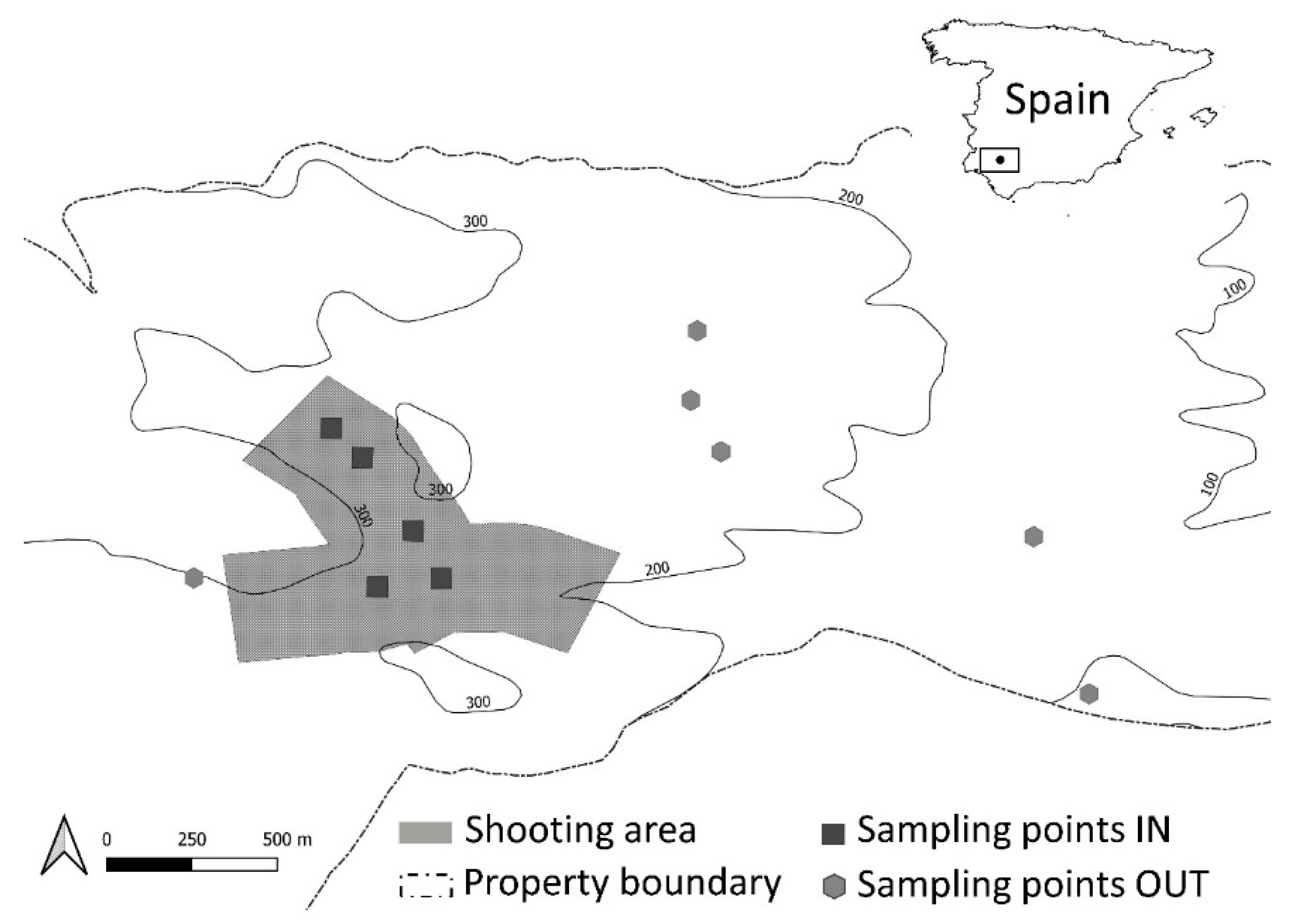
2.1. Study Site
2.2. Sample Collection
2.3. Chemical Analyses
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salomons, W. Environmental impact of metals derived from mining activities: Processes, predictions, prevention. J. Geochem. Explor. 1995, 52, 5–23. [Google Scholar] [CrossRef]
- Dybowska, A.; Farago, M.; Valsami-Jones, E.; Thornton, I. Remediation strategies for historical mining and smelting sites. Sci. Prog. 2006, 89, 71–138. [Google Scholar] [CrossRef] [PubMed]
- De Vos, W.; Tarvainen, T.; Salminen, R.; Reeder, S.; De Vivo, B.; Demetriades, A.; Petersell, V. Geochemical Atlas of Europe. Part 2. Interpretation of Geochemical Maps, Additional Tables, Figures, Maps and Related Publications. Electronic Version. 2006. Available online: http://weppi.gtk.fi/publ/foregsatlas/part2.php (accessed on 8 August 2020).
- Lucia, M.; André, J.M.; Gontier, K.; Diot, N.; Veiga, J.; Davail, S. Trace element concentrations (mercury, cadmium, copper, zinc, lead, aluminium, nickel, arsenic, and selenium) in some aquatic birds of the Southwest Atlantic Coast of France. Arch. Environ. Contam. Toxicol. 2010, 58, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Crawford, D.L.; Pometto III, A.L.; Crawford, R.L. Lignin degradation by Streptomyces viridosporus: Isolation and characterization of a new polymeric lignin degradation intermediate. Appl. Environ. Microbiol. 1983, 45, 898–904. [Google Scholar] [CrossRef]
- Needleman, H.L. The removal of lead from gasoline: Historical and personal reflections. Environ. Res. 2000, 84, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Fisher, I.; Pain, D.J.; Thomas, V.G. Are view of lead poisoning from ammunition sources in terrestrial birds. Biol. Conserv. 2006, 131, 421–432. [Google Scholar] [CrossRef]
- Gangoso, L.; Álvarez-Lloret, P.; Rodríguez-Navarro, A.A.; Mateo, R.; Hiraldo, F.; Donázar, J.A. Long-term effects of lead poisoning on bone mineralization in vultures exposed to ammunition sources. Environ. Pollut. 2009, 157, 569–574. [Google Scholar] [CrossRef]
- Ancora, S.; Bianchi, N.; Leonzio, C.; Renzoni, A. Heavy metals in flamingos (Phoenicopterus ruber) from Italian wetlands: The problem of ingestion of lead shot. Environ. Res. 2008, 107, 229–236. [Google Scholar] [CrossRef]
- Fiala, M.; Marveggio, D.; Viganò, R.; Demartini, E.; Nonini, L.; Gaviglio, A. LCA and wild animals: Results from wild deer culled in a northern Italy hunting district. J. Clean. Prod. 2020, 244, 118667. [Google Scholar] [CrossRef]
- Gupta, N.; Yadav, K.K.; Kumar, V.; Kumar, S.; Chadd, R.P.; Kumar, A. Trace elements in soil-vegetables interface: Translocation, bioaccumulation, toxicity, and amelioration—A review. Sci. Total Environ. 2019, 651, 2927–2942. [Google Scholar] [CrossRef]
- Kumar, S.; Prasad, S.; Yadav, K.K.; Shrivastava, M.; Gupta, N.; Nagar, S.; Malav, L.C. Hazardous heavy metals contamination of vegetables and food chain: Role of sustainable remediation approaches—A review. Environ. Res. 2019, 179, 108792. [Google Scholar] [CrossRef]
- Kumar, A.; Cabral-Pinto, M.M.S.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Yadav, K.K.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead toxicity: Health hazards, influence on food chain, and sustainable remediation approaches. Int. J. Environ. Res. 2020, 17, 2179. [Google Scholar] [CrossRef] [PubMed]
- Scheuhammer, A.M.; Norris, S.L. The ecotoxicology of lead shot and lead fishing weights. Ecotoxicology 1996, 5, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, S.S.; Willems, M. The fate of lead in soils: The transformation of lead pellets in shooting-range soils. Ambio 1987, 16, 11–15. [Google Scholar]
- Foy, C.D.; Chaney, R.T.; White, M.C. The physiology of metal toxicity in plants. Annu. Rev. Plant Physiol. 1987, 29, 511–566. [Google Scholar] [CrossRef]
- Sadiq, M. Toxic Metal Chemistry in Marine Environments; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Choi, J.W.; Yoo, E.J.; Kim, J.Y.; Hwang, J.Y.; Lee, K.; Lee, W.S.; Lee, K.S. Pb concentrations and isotopic compositions in the soil and sediments around the abandoned mine in southwest of Korea. J. Agric. Chem. Environ. 2013, 2, 39032. [Google Scholar] [CrossRef][Green Version]
- Chenery, S.R.; Izquierdo, M.; Marzouk, E.; Klinck, B.; Palumbo-Roe, B.; Tye, A.M. Soil–plant interactions and the uptake of Pb at abandoned mining sites in the Rookhope catchment of the N. Pennines, UK—A Pb isotope study. Sci. Total Environ. 2012, 433, 547–560. [Google Scholar] [CrossRef]
- Rodríguez-Seijo, A.; Cachada, A.; Gavina, A.; Duarte, A.C.; Vega, F.A.; Andrade, M.L.; Pereira, R. Lead and PAHs contamination of an old shooting range: A case study with a holistic approach. Sci. Total Environ. 2017, 575, 367–377. [Google Scholar] [CrossRef]
- Chrastný, V.; Komárek, M.; Hájek, T. Lead contamination of an agricultural soil in the vicinity of a shooting range. Environ. Monit. Assess. 2010, 162, 37–46. [Google Scholar] [CrossRef]
- Arcega-Cabrera, F.; Noreña-Barroso, E.; Oceguera-Vargas, I. Lead from hunting activities and its potential environmental threat to wildlife in a protected wetland in Yucatan, Mexico. Ecotoxicol. Environ. Saf. 2014, 100, 251–257. [Google Scholar] [CrossRef]
- Vallverdú-Coll, N.; Ortiz-Santaliestra, M.E.; Mougeot, F.; Vidal, D.; Mateo, R. Sublethal Pb exposure produces season-dependent effects on immune response, oxidative balance and investment in carotenoid-based coloration in red-legged partridges. Environ. Sci. Technol. 2015, 49, 3839–3850. [Google Scholar] [CrossRef]
- Ma, W.C. Lead in mammals. In Environmental Contaminants in Biota; CRC Press: Boca Raton, FL, USA, 2011; pp. 595–608. [Google Scholar]
- Reglero, M.M.; Taggart, M.A.; Castellanos, P.; Mateo, R. Reduced sperm quality in relation to oxidative stress in red deer from a lead mining area. Environ. Pollut. 2009, 157, 2209–2215. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Estival, J.; Martinez-Haro, M.; Monsalve-González, L.; Mateo, R. Interactions between endogenous and dietary antioxidants against Pb-induced oxidative stress in wild ungulates from a Pb polluted mining area. Sci. Total Environ. 2011, 409, 2725–2733. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.J. Lead. In Veterinary Toxicology: Basic and Clinical Principles, 2nd ed.; Gupta, R.C., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 522–526. [Google Scholar]
- Goodchild, C.G.; Beck, M.L.; VanDiest, I.; Czesak, F.N.; Lane, S.J.; Sewall, K.B. Male zebra finches exposed to lead (Pb) during development have reduced volume of song nuclei, altered sexual traits, and received less attention from females as adults. Ecotoxicol. Environ. Saf. 2021, 210, 111850. [Google Scholar] [CrossRef] [PubMed]
- Espín, S.; Sánchez-Virosta, P.; Zamora-Marín, J.M.; León-Ortega, M.; Jiménez, P.; Zamora-López, A.; García-Fernández, A.J. Physiological effects of toxic elements on a wild nightjar species. Environ. Pollut. 2020, 263, 114568. [Google Scholar] [CrossRef]
- Descalzo, E.; Camarero, P.R.; Sánchez-Barbudo, I.S.; Martinez-Haro, M.; Ortiz-Santaliestra, M.E.; Moreno-Opo, R.; Mateo, R. Integrating active and passive monitoring to assess sublethal effects and mortality from lead poisoning in birds of prey. Sci. Total Environ. 2021, 750, 142260. [Google Scholar] [CrossRef]
- Vallverdú-Coll, N.; Mougeot, F.; Ortiz-Santaliestra, M.E.; Rodriguez-Estival, J.; López-Antia, A.; Mateo, R. Lead exposure reduces carotenoid-based coloration and constitutive immunity in wild mallards. Environ. Toxicol. Chem. 2016, 35, 1516–1525. [Google Scholar] [CrossRef]
- Descalzo, E.; Mateo, R. La Contaminación por Munición de Plomo en Europa: El Plumbismo Aviary las Implicaciones en la Seguridad de la Carne de Caza; Instituto de Investigación en Recursos Cinegéticos (IREC): Ciudad Real, Spain, 2018; 82p. [Google Scholar]
- Manninen, S.; Tanskanen, N. Transfer of lead from shotgun pellets to humus and three plant species in a Finnish shooting range. Arch. Environ. Contam. Toxicol. 1993, 24, 410–414. [Google Scholar] [CrossRef]
- Mateo, R.; Green, A.J.; Lefranc, H.; Baos, R.; Figuerola, J. Lead poisoning in wild birds from southern Spain: A comparative study of wetland areas and species affected, and trends over time. Ecotoxicol. Environ. Saf. 2007, 66, 119–126. [Google Scholar] [CrossRef]
- Younger, P.L. Mine water pollution in Scotland: Nature, extent, and preventative strategies. Sci. Total Environ. 2001, 265, 309–326. [Google Scholar] [CrossRef]
- Reglero, M.M.; Monsalve-González, L.; Taggart, M.A.; Mateo, R. Transfer of metals to plants and red deer in an old lead mining area in Spain. Sci. Total Environ. 2008, 406, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Fernández, D.; Walker, D.J.; Romero-Espinar, P.; Flores, P.; del Río, J.A. Physiological responses of Bituminaria bituminosa to heavy metals. J. Plant Physiol. 2011, 168, 2206–2211. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Fernández, D.; Walker, D.J. The effects of soil amendments on the growth of Atriplex halimus and Bituminaria bituminosa in heavy metal-contaminated soils. Water Air Soil Pollut. 2012, 223, 63–72. [Google Scholar] [CrossRef]
- Eissa, M.A.; Almaroai, Y.A. Phytoremediation capacity of some forage plants grown on a metals-contaminated soil. Soil Sediment Contam. 2019, 28, 569–581. [Google Scholar] [CrossRef]
- Nas, F.S.; Ali, M. The effect of lead on plants in terms of growing and biochemical parameters: A review. J. Ecol. Environ. 2018, 3, 265–268. [Google Scholar]
- Sharma, P.; Dubey, R.S. Lead toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef]
- Mishra, S.; Srivastava, S.; Tripathi, R.D.; Kumar, R.; Seth, C.S.; Gupta, D.K. Lead detoxification by coontail (Ceratophyllum demersum L.) involves induction of phytochelatins and antioxidant system in response to its accumulation. Chemosphere 2006, 65, 1027–1039. [Google Scholar] [CrossRef]
- Seregin, I.V.; Ivanov, V.B. Physiological aspects of cadmium and lead toxic effects on higher plants. Russ. J. Plant Physiol. 2001, 48, 523–544. [Google Scholar] [CrossRef]
- Kumar, R.G.; Dubey, R.S. Glutamine synthetase isoforms from rice seedlings: Effects of stress on enzyme activity and the protective roles of osmolytes. J. Plant Physiol. 1999, 155, 118–121. [Google Scholar] [CrossRef]
- Verma, S.; Dubey, R.S. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2003, 164, 645–655. [Google Scholar] [CrossRef]
- Stevens, R.G.; Creissen, G.P.; Mullineaux, P.M. Cloning and characterisation of a cytosolic glutathione reductase cDNA frompea (Pisum sativum L.) and its expression in response to stress. Plant Mol. Biol. 1997, 35, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Souahi, H. Impact of lead on the amount of chlorophyll and carotenoids in the leaves of Triticum durum and T. aestivum, Hordeum vulgare and Avena sativa. Biosyst. Divers. 2021, 29, 207–210. [Google Scholar] [CrossRef]
- Lane, S.D.; Martin, E.S. A histochemical investigation of lead uptake in Raphanus sativus. New Phytol. 1977, 79, 281–286. [Google Scholar] [CrossRef]
- Ferrandis, P.; Mateo, R.; López-Serrano, F.R.; Martínez-Haro, M.; Martínez-Duro, E. Lead-shot exposure in red-legged partridge (Alectoris rufa) on a driven shooting estate. Environ. Sci. Technol. 2008, 42, 6271–6277. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar]
- Lüdecke, D. ggeffects: Tidy data frames of marginal effects from regression models. J. Open Source Softw. 2018, 3, 772. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis: R Package Version 3.1.1; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Tepe, Y.; Akyurt, I.; Ciminli, C.; Mutlu, E.; Caliskan, M. Protective effect of clinoptilolite on lead toxicity in common carp Cyprinus carpio. Fresenius Environ. Bull. 2004, 13, 639–642. [Google Scholar]
- Has-Schön, E.; Bogut, I.; Strelec, I. Heavy metal profile in five fish species included in human diet, domiciled in the end flow of River Neretva (Croatia). Arch. Environ. Contam. Toxicol. 2006, 50, 545–551. [Google Scholar] [CrossRef]
- Noël, L.; Chekri, R.; Millour, S.; Merlo, M.; Leblanc, J.C.; Guérin, T. Distribution and relationships of As, Cd, Pb and Hg in freshwater fish from five French fishing areas. Chemosphere 2013, 90, 1900–1910. [Google Scholar] [CrossRef]
- Fernández-Trujillo, S.; López-Perea, J.J.; Jiménez-Moreno, M.; Martín-Doimeadios, R.C.R.; Mateo, R. Metals and metalloids in freshwater fish from the floodplain of Tablas de Daimiel National Park, Spain. Ecotoxicol. Environ. Saf. 2021, 208, 111602. [Google Scholar] [CrossRef]
- Mateo, R.; Martínez-Vilalta, A.; Carles Dolz, J.; Belliure, J.; Aguilar Serrano, J.M.; Guitart, R. Estudio de la Problemática del Plumbismo en Aves Acuáticas de Diferentes Humedales Españoles; Ministerio de Medio Ambiente: Madrid, Spain, 1994. [Google Scholar]
- Mateo, R.; Guitart, R.; Green, A.J. Determinants of lead shot, rice, and grit ingestion in ducks and coots. J. Wildl. Manag. 2000, 64, 939–947. [Google Scholar] [CrossRef]
- Tomás, J.; Guitart, R.; Mateo, R.; Raga, J.A. Marine debris ingestion in loggerhead sea turtles, Caretta caretta, from the Western Mediterranean. Mar. Pollut. Bull. 2002, 44, 211–216. [Google Scholar] [CrossRef]
- Guitart, R.; To-Figueras, J.; Mateo, R.; Bertolero, A.; Cerradelo, S.; Martínez-Vilalta, A. Lead poisoning in waterfowl from the Ebro Delta, Spain: Calculation of lead exposure thresholds for mallards. Arch. Environ. Contam. Toxicol. 1994, 27, 289–293. [Google Scholar] [CrossRef]
- Tavecchia, G.; Pradel, R.; Lebreton, J.D.; Johnson, A.R.; Mondain-Monval, J.Y. The effect of lead exposure on survival of adult mallards in the Camargue, southern France. J. Appl. Ecol. 2001, 38, 1197–1207. [Google Scholar] [CrossRef]
- Mateo, R.; Green, A.J.; Jeske, C.W.; Urios, V.; Gerique, C. Lead poisoning in the globally threatened marbled teal and white-headed duck in Spain. Environ. Toxicol. Chem. Int. J. 2001, 20, 2860–2868. [Google Scholar] [CrossRef]
- Krone, O.; Kenntner, N.; Trinogga, A.; Nadjafzadeh, M.; Scholz, F.; Sulawa, J.; Zieschank, R. Lead poisoning in white-tailed sea eagles causes and approaches to solutions in Germany. In Ingestion of Lead from Spent Ammunition: Implications for Wildlife and Humans; The Peregrine Fund: Boise, ID, USA, 2009. [Google Scholar]
- Madry, M.M.; Kraemer, T.; Kupper, J.; Naegeli, H.; Jenny, H.; Jenni, L.; Jenny, D. Excessive lead burden among golden eagles in the Swiss Alps. Environ. Res. Lett. 2015, 10, 034003. [Google Scholar] [CrossRef]
- Taggart, M.A.; Shore, R.F.; Pain, D.J.; Peniche, G.; Martinez-Haro, M.; Mateo, R.; Green, R.E. Concentration and origin of lead (Pb) in liver and bone of Eurasian buzzards (Buteo buteo) in the United Kingdom. Environ. Pollut. 2020, 267, 115629. [Google Scholar] [CrossRef] [PubMed]
- Clausen, B.; Wolstrup, C. Lead poisoning in game from Denmark [mallard, mute swan, waterfowl, lead shot, pellets, Denmark, gizzard]. Dan. Rev. Game Biol. 1979, 11, 1–22. [Google Scholar]
- Martinez-Haro, M.; Taggart, M.A.; Green, A.J.; Mateo, R. Avian digestive tract simulation to study the effect of grit geochemistry and food on Pb shot bioaccessibility. Environ. Sci. Technol. 2009, 43, 9480–9486. [Google Scholar] [CrossRef]
- DeMent, S.H.; Chisolm, J.J., Jr.; Eckhaus, M.A.; Strandberg, J.D. Toxic lead exposure in the urban rock dove. J. Wildl. Dis. 1987, 23, 273–278. [Google Scholar] [CrossRef]
- ClaTavernier, P.; Roels, S.; Baert, K.; Hermans, K.; Pasmans, F.; Chiers, K. Lead intoxication by ingestion of lead shot in racing pigeons (Columba livia). Vlaams Diergeneeskd. Tijdschr. 2004, 73, 307–309. [Google Scholar]
- Walter, H.; Reese, K.P. Fall diet of chukars (Alectoris chukar) in eastern Oregon and discovery of ingested lead pellets. West. N. Am. Nat. 2003, 63, 402–405. [Google Scholar]
- Larsen, R.T.; Flinders, J.T.; Mitchell, D.L.; Perkins, E.R. Grit size preferences and confirmation of ingested lead pellets in Chukars (Alectoris chukar). West. N. Am. Nat. 2007, 67, 152–155. [Google Scholar] [CrossRef]
- Bingham, R.J.; Larsen, R.T.; Bissonette, J.A.; Hall, J.O. Widespread ingestion of lead pellets by wild chukars in northwestern Utah. Wildl. Soc. Bull. 2015, 39, 94–102. [Google Scholar] [CrossRef]
- Soler Rodríguez, F.; Oropesa Jiménez, A.L.; García Cambero, J.P.; Pérez López, M. Lead exposition by gunshot ingestion in red-legged partridge (Alectoris rufa). Vet. Hum. Toxicol. 2004, 46, 133–134. [Google Scholar]
- Butler, D.A.; Sage, R.B.; Draycott, R.A.; Carroll, J.P.; Potts, D. Lead exposure in ring-necked pheasants on shooting estates in Great Britain. Wildl. Soc. Bull. 2005, 33, 583–589. [Google Scholar] [CrossRef]
- Vallverdú-Coll, N.; López-Antia, A.; Martinez-Haro, M.; Ortiz-Santaliestra, M.E.; Mateo, R. Altered immune response in mallard ducklings exposed to lead through maternal transfer in the wild. Environ. Poll. 2015, 205, 350–356. [Google Scholar] [CrossRef]
- Potts, G.R. Incidence of ingested lead gunshot in wild grey partridges (Perdix perdix) from the UK. Eur. J. Wildl. Res. 2005, 51, 31–34. [Google Scholar] [CrossRef]
- Dutton, C.S.; Bolen, E.G. Fall diet of a relict pheasant population in North Carolina. J. Elisha Mitchell Sci. Soc. 2000, 116, 41–48. [Google Scholar]
- Butler, D.A. Incidence of lead shot ingestion in red-legged partridges (Alectoris rufa) in Great Britain. Vet. Rec. 2005, 157, 661. [Google Scholar] [CrossRef]
- Millán, J.; Mateo, R.; Taggart, M.A.; López-Bao, J.V.; Viota, M.; Monsalve, L.; Jiménez, B. Levels of heavy metals and metalloids in critically endangered Iberian lynx and other wild carnivores from Southern Spain. Sci. Total Environ. 2008, 399, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Mulero, R.; Cano-Manuel, J.; Ráez-Bravo, A.; Pérez, J.M.; Espinosa, J.; Soriguer, R.; Fandos, P.; Granados, J.E.; Romero, D. Lead and cadmium in wild boar (Sus scrofa) in the Sierra Nevada Natural Space (southern Spain). Environ. Sci. Pollut. Res. 2016, 23, 16598–16608. [Google Scholar] [CrossRef] [PubMed]
- Ráez-Bravo, A.; Granados, J.E.; Cano-Manuel, F.J.; Soriguer, R.C.; Fandos, P.; Pérez, J.M.; Pavlov, I.Y.; Romero, D. Toxic and essential element concentrations in Iberian ibex (Capra pyrenaica) from the Sierra Nevada Natural Park (Spain): Reference intervals in whole blood. Bull. Environ. Contam. Toxicol. 2016, 96, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Wannaz, E.D.; Carreras, H.A.; Rodriguez, J.H.; Pignata, M.L. Use of biomonitors for the identification of heavy metals emission sources. Ecol. Indic. 2012, 20, 163–169. [Google Scholar] [CrossRef]
- Dinake, P.; Mokgosi, S.M.; Kelebemang, R.; Kereeditse, T.T.; Motswetla, O. Pollution risk from Pb towards vegetation growing in and around shooting ranges—A review. Environ. Pollut. Bioavailab. 2021, 33, 88–103. [Google Scholar] [CrossRef]
- Madejón, P.; Murillo, J.M.; Marañón, T.; Cabrera, F.; López, R. Bioaccumulation of As, Cd, Cu, Fe and Pb in wild grasses affected by the Aznalcóllar mine spill (SW Spain). Sci. Total Environ. 2002, 290, 105–120. [Google Scholar] [CrossRef]
- Madejon, P.; Maranon, T.; Murillo, J.M.; Robinson, B. White poplar (Populus alba) as a biomonitor of trace elements in contaminated riparian forests. Environ. Pollut. 2004, 132, 145–155. [Google Scholar] [CrossRef]
- Hasselbach, L.; Ver Hoef, J.M.; Ford, J.; Neitlich, P.; Crecelius, E.; Berryman, S.; Bohle, T. Spatial patterns of cadmium and lead deposition on and adjacent to National Park Service lands in the vicinity of Red Dog Mine, Alaska. Sci. Total Environ. 2005, 348, 211–230. [Google Scholar] [CrossRef]
- Wilson, B.; Braithwaite, A.; Brian Pyatt, F. An evaluation of procedures for the digestion of soils and vegetation from areas with metalliferous pollution. Toxicol. Environ. Chem. 2005, 87, 335–344. [Google Scholar] [CrossRef]
- Meharg, A.A. Integrated tolerance mechanisms: Constitutive and adaptive plant responses to elevated metal concentrations in the environment. Plant Cell Environ. 1994, 17, 989–993. [Google Scholar] [CrossRef]
- Huang, J.W.; Cunningham, S.D. Lead phytoextraction: Species variation in lead uptake and translocation. New Phytol. 1996, 134, 75–84. [Google Scholar] [CrossRef]
- Chukwuma, C. A comparative study of cadmium, lead, zinc, pH, and bulk density from the Enyigba lead and zinc mine in two different seasons. Ecotoxicol. Environ. Saf. 1995, 31, 246–249. [Google Scholar] [CrossRef]
- Deng, H.; Ye, Z.H.; Wong, M.H. Lead and zinc accumulation and tolerance in populations of six wetland plants. Environ. Pollut. 2006, 141, 69–80. [Google Scholar] [CrossRef] [PubMed]
- An, Y.J. Assessment of comparative toxicities of lead and copper using plant assay. Chemosphere 2006, 62, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, Z.M.; Xu, J.M.; Sun, Y.F. Risk assessment for safety of soils and vegetables around a lead/zinc mine. Environ. Geochem. Health 2006, 28, 37–44. [Google Scholar] [CrossRef]
- Freitas, H.; Prasad, M.N.V.; Pratas, J. Analysis of serpentinophytes from north-east of Portugal for trace metal accumulation—Relevance to the management of mine environment. Chemosphere 2004, 54, 1625–1642. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Bugalho, M.N.; Milne, J.A. The composition of the diet of red deer (Cervus elaphus) in a Mediterranean environment: A case of summer nutritional constraint? For. Ecol. Manag. 2003, 181, 23–29. [Google Scholar] [CrossRef]
- Pugh, R.E.; Dick, D.G.; Fredeen, A.L. Heavy metal (Pb, Zn, Cd, Fe, and Cu) contents of plant foliage near the Anvil Range lead/zinc mine, Faro, Yukon Territory. Ecotoxicol. Environ. Saf. 2002, 52, 273–279. [Google Scholar] [CrossRef]
- Skerfving, S.; Bergdahl, I. Lead. In Handbook on the Toxicology of Metals, 3rd ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Friberg, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 599–643. [Google Scholar]



| Species | Description | 2017 | 2018 | 2019 | |||
|---|---|---|---|---|---|---|---|
| n = 8 | n = 10 | n = 11 | |||||
| Shooting Area | Non-Shooting Area | Shooting Area | Non-Shooting Area | Shooting Area | Non-Shooting Area | ||
| Cistus salviifolius | Shrub/Perennial | 0 | 0 | 0 | 2 | 1 | 1 |
| Cistus monspeliensis | Shrub/Perennial | 1 | 1 | 0 | 0 | 0 | 0 |
| Nerium oleander | Shrub/Perennial | 0 | 0 | 1 | 0 | 0 | 0 |
| Olea europaea | Tree/Perennial | 0 | 2 | 1 | 1 | 1 | 2 |
| Phillyrea angustifolia | Shrub/Perennial | 0 | 0 | 1 | 0 | 0 | 0 |
| Phlomis purpurea | Shrub/Perennial | 0 | 0 | 1 | 0 | 0 | 0 |
| Pistacia lentiscus | Shrub/Perennial | 0 | 1 | 0 | 0 | 1 | 1 |
| Quercus ilex | Tree/Perennial | 1 | 1 | 1 | 1 | 1 | 1 |
| Rubus ulmifolius | Shrub/Perennial | 1 | 0 | 0 | 1 | 1 | 1 |
| Total sampled plants | 3 | 5 | 5 | 5 | 5 | 6 | |
| Mean (±S.E.) Pb Concentration (mg/Kg) | F-Value | p-Value | ||
|---|---|---|---|---|
| Species | Shooting Area | Non-Shooting | ||
| Cistus salviifolius | 8.48 ± 2.81 | 1.06 ± 0.14 | = 41,64 | <0.001 |
| Cistus monspeliensis | 0.28 ± 0.03 | 0.38 ± 0.08 | = 1.37 | 0.362 |
| Nerium oleander | 0.306 ± 0.071 | No sample | ||
| Olea europaea | 1.23 ± 0.825 | 0.169 ± 0.018 | = 4.737 | 0.049 |
| Phillyrea angustifolia | 0.506 ± 0.010 | No sample | ||
| Phlomis purpurea | 0.677 ± 0.637 | No sample | ||
| Pistacia lentiscus | 0.305 ± 0.145 | 0.208 ± 0.092 | = 0.349 | 0.587 |
| Quercus ilex | 0.746 ± 0.245 | 0.358 ± 0.053 | = 2.393 | 0.153 |
| Rubus ulmifolius | 0.467 ± 0.186 | 0.601 ± 0.250 | = 0.183 | 0.684 |
| Estimate | S.E. | d.f. | t-Value | p-Value | |
|---|---|---|---|---|---|
| Fixed factors | |||||
| Intercept | −0.745 | 0.177 | 4.281 | −4.281 | 0.008 |
| Area (non-shooting area) | 0.227 | 0.093 | 2.447 | 2.447 | 0.022 |
| Sprout (young) | 0.313 | 0.077 | 4.092 | 4.092 | <0.001 |
| Random factors Individual x Species: variance ± SE = 0.012 ± 0.110; Species: 0.072 ± 0.269; Year = 0.049 ± 0.222; Residual = 0.083 ± 0.288 | |||||
| Estimate | S.E. | d.f. | t-Value | p-Value | |
|---|---|---|---|---|---|
| Fixed factors | |||||
| Intercept | −0.243 | 0.171 | 1.031 | −1.418 | 0.385 |
| Area (non-shooting area) | −0.205 | 0.069 | 11.062 | −2.964 | 0.013 |
| Sprout (young) | −0.158 | 0.037 | 16.000 | −4.243 | <0.001 |
| Species | −0.267 | 0.106 | 11.000 | −2.527 | 0.004 |
| Random factors: Individual: variance ± SE = 0.045 ± 0.213; Year = 0.049 ± 0.222; Residual = 0.047 ± 0.217 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Peña, E.; Seoane, J.M.; Carranza, J. The Impact of Pb from Ammunition on the Vegetation of a Bird Shooting Range. Sustainability 2022, 14, 3124. https://doi.org/10.3390/su14053124
de la Peña E, Seoane JM, Carranza J. The Impact of Pb from Ammunition on the Vegetation of a Bird Shooting Range. Sustainability. 2022; 14(5):3124. https://doi.org/10.3390/su14053124
Chicago/Turabian Stylede la Peña, Eva, José Manuel Seoane, and Juan Carranza. 2022. "The Impact of Pb from Ammunition on the Vegetation of a Bird Shooting Range" Sustainability 14, no. 5: 3124. https://doi.org/10.3390/su14053124
APA Stylede la Peña, E., Seoane, J. M., & Carranza, J. (2022). The Impact of Pb from Ammunition on the Vegetation of a Bird Shooting Range. Sustainability, 14(5), 3124. https://doi.org/10.3390/su14053124






