Synergistic Effects of Acacia Prunings-Derived Biochar and Nitrogen Application on the Mineral Profile of Maize (Zea mays L.) Grains
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Site and Biochar Field Trial
Soil Properties (Start of the Experiment)
2.2. Assessment of Soil and Biochar Properties
2.2.1. Soil
Soil pH and Electrical Conductivity
Soil Organic Matter
Plant-Available Phosphorus
Plant-Available Potassium
Soil Texture
2.2.2. Biochar
pH and Electrical Conductivity
Total Carbon and Nitrogen
Alkalinity
Post-Harvest Analyses of Maize Grains
Statistical Analysis
3. Results
3.1. Changes in Soil pH, EC, and OM (at Reproductive Stage and after Harvest)
3.2. Grain Yield
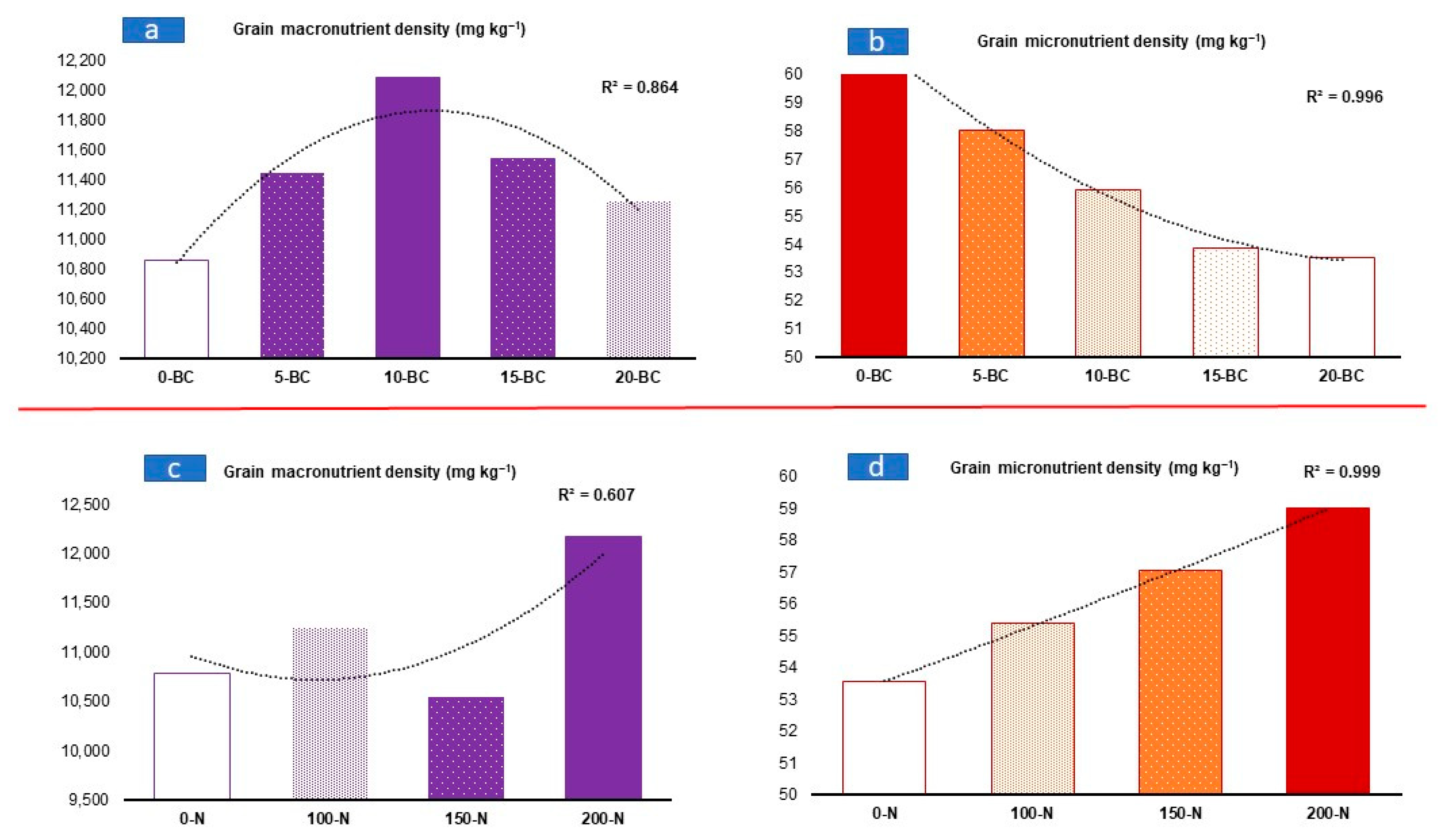
3.3. Effect of Biochar and Nitrogen Application on Macronutrients in Maize Grain
3.4. Effect of Biochar and Nitrogen Application on Micronutrients in Maize Grain
4. Discussion
4.1. Effect of Biochar and Nitrogen Application on Macronutrients (P, K, Ca, and Mg) in Maize Grain
4.2. Effect of Biochar and Nitrogen Application on Micronutrients (Zn, Fe, and Cu) in Maize Grain
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Díaz-Gómez, J.L.; Castorena-Torres, F.; Preciado-Ortiz, R.E.; García-Lara, S. Anti-cancer activity of maize bioactive peptides. Front. Chem. 2017, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Nuss, E.T.; Tanumihardjo, S.A. Maize: A paramount staple crop in the context of global nutrition. Comp. Rev. Food Sci. Food Safe 2010, 9, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Trautvetter, U.; Jahreis, G.; Kiehntopf, M.; Glei, M. Consequences of a high phosphorus intake on mineral metabolism and bone remodeling in dependence of calcium intake in healthy subjects—A randomized placebo-controlled human intervention study. Nutr. J. 2016, 15, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roumelioti, M.E.; Glew, R.H.; Khitan, Z.J.; Rondon-Berrios, H.; Argyropoulos, C.P.; Malhotra, D.; Tzamaloukas, A.H. Fluid balance concepts in medicine: Principles and practice. World J. Nephrol. 2018, 7, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Grober, U.; Schmidt, J.; Kisters, K. Magnesium in prevention and therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef] [Green Version]
- Al Alawi, A.M.; Majoni, S.W.; Falhammar, H. Magnesium and Human Health: Perspectives and Research Directions. Int. J. Endocrinol. 2018, 2018, 9041694. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, K.; Ijaz, A.B.; Muneer, M.; Munawar, I.; Zafar, I. Removal of heavy metals (Zn, Cr, Pb, Cd, Cu and Fe) in aqueous media by calcium carbonate as an adsorbent. Int. J. Chem. Biol. Sci. 2012, 2, 48–53. [Google Scholar]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Ministry of National Health Services Regulation and Coordination (MoNHSR&C) GoP. National Nutrition Survey; MoNHSR&C: Islamabad, Pakistan, 2018. [Google Scholar]
- NFDC (National Fertilizer Development Centre). Soil Fertility and Inputs Use Atlas of Pakistan: The Khyber Pakhtunkhwa Province; Ahmad, W., Khan, M.J., Mustafa, Z., Mahmood, K., Singh, B., Zia, M.H., Ali, A., Jalil, A., Eds.; NFDC (National Fertilizer Development Centre): Islamabad, Pakistan, 2018; p. 115. ISBN 0978-969-78888-00-9. [Google Scholar]
- Baig-Ansari, N.; Badruddin, S.H.; Karmaliani, R.; Harris, H.; Jehan, I.; Pasha, O. Anemia prevalence and risk factors in pregnant women in an urban area of Pakistan. Food Nutr. Bull. 2008, 29, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Parks, S.; Hoffman, M.K.; Goudar, S.S.; Patel, A.; Saleem, S.; Ali, S.A. Maternal anaemia and maternal, fetal, and neonatal outcomes in a prospective cohort study in India and Pakistan. BJOG Int. J. Obs. Gynaecol. 2019, 126, 737–743. [Google Scholar] [CrossRef]
- Ahmad, W.; Ullah, N.; Xu, L.; El Sabagh, A. Global food and nutrition security under changing climates. Front. Agron. 2022, 3, 799878. [Google Scholar] [CrossRef]
- Ahmad, W.; Ullah, N.; Zia, M.H. Soil contamination with metals: Sources, types and implications. In Soil Remediation and Plants: Prospects and Challenges; Academic Press: Cambridge, MA, USA, 2015; pp. 37–61. [Google Scholar]
- Al-Wabel, M.I.; Rafique, M.I.; Ahmad, M.; Ahmad, M.; Hussain, A.; Usman, A.R.A. Pyrolytic and hydrothermal carbonization of date palm leaflets: Characteristics and ecotoxicological effects on seed germination of lettuce. Saudi J. Biol. Sci. 2019, 26, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.Y.; Van Zwieten, V.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of green waste biochar as a soil amendment. Soil Res. 2007, 45, 629–634. [Google Scholar] [CrossRef]
- Major, J.; Rondon, M.; Molina, D.; Riha, S.J.; Lehmann, J. Maize yield and nutrition during 4 years after biochar application to a Colombian Savanna oxisol. Plant Soil 2010, 333, 117–128. [Google Scholar] [CrossRef]
- Uzoma, K.C.; Inoue, M.; Andry, H.; Fujimaki, H.; Zahoor, A.; Nihihara, E. Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manag. 2011, 27, 205–212. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Using poultry litter biochar as soil amendments. Aust. J. Soil Res. 2008, 46, 437–444. [Google Scholar] [CrossRef]
- Yamato, M.; Okimori, Y.; Wibowo, I.F.; Anshori, S.; Ogawa, M. Effects of the application of charred bark of Acacia mangium on the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra, Indonesia. Soil Sci. Plant Nutr. 2006, 52, 489–495. [Google Scholar] [CrossRef]
- Walkley, A. A critical examination of a raid method for determining organic carbon in soils: Effect of variations in digestion conditions and of organic soil constituents. Soil Sci. 1947, 63, 251–263. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, S.N.; Dean, L.A. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. In Circular; U.S. Department of Agriculture: Washington, DC, USA, 1954; Volume 939, pp. 1–19. [Google Scholar]
- Berg, M.G.; Ernest, H.G. Methods of Soil Analysis Used in the Soil Testing Laboratory at Oregon State University; Agricultural Experiment Station, Oregon State University: Corvallis, OR, USA, 1978. [Google Scholar]
- Rui, Y.K.; Guo, J.; Huang, K.L.; Jin, Y.H.; Lou, Y.B. Application of ICP-MS to the detection of heavy metals in transgenic corn. Guang Pu Xue Yu Guang Pu Fen Xi = Guang Pu 2007, 27, 796–798. [Google Scholar]
- Dharmakeerthi, R.S.; Chandrasiri, J.A.S.; Edirimanne, V.U. Effect of rubber wood biochar on nutrition and growth of nursery plants of Hevea brasiliensis L. Growth in an ultisol. SpringerPlus 2012, 1, 84. [Google Scholar] [CrossRef] [Green Version]
- Tesfaye, F.; Liu, X.; Zheng, J.; Cheng, K.; Bian, R.; Zhang, X.; Li, L.; Drosos, M.; Joseph, S.; Pan, G. Could biochar amendment be a tool to improve soil availability and plant uptake of phosphorus? A meta-analysis of published experiments. Environ. Sci. Pollut. Res. 2021, 28, 34108–34120. [Google Scholar] [CrossRef] [PubMed]
- Borno, M.L.; Eduah, J.O.; Müller-Stöver, D.S.; Liu, F. Effect of different biochars on phosphorus (P) dynamics in the rhizosphere of Zea mays L. (maize). Plant Soil 2018, 431, 257–272. [Google Scholar] [CrossRef]
- Havlin, J.; Balster, N.; Chapman, S.; Ferris, D.; Thompson, T.; Smith, T. Trends in soil science education and employment. Soil Sci. Soc. Am. J. 2010, 74, 1429–1432. [Google Scholar] [CrossRef] [Green Version]
- Kocsis, T.; Kotroczó, Z.; Kardos, L.; Biró, B. Optimization of increasing biochar doses with soil–plant–microbial functioning and nutrient uptake of maize. Environ. Technol. Innov. 2020, 20, 101191. [Google Scholar] [CrossRef]
- Widowati, W.; Asnah, A. Biochar effect at potassium fertilizer and dosage leaching potassium for two-corn planting season. J. Agric. Sci. 2014, 36, 65–71. [Google Scholar] [CrossRef]
- Shehu, H.E.; Kwari, J.D.; Sandabe, M.K. Effects of N, P and K fertilizers on yield content and uptake of N, P and K by sesame (Sesamum indicum L.). Int. J. Agric. Biol. 2010, 12, 845–850. [Google Scholar]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Lehmann, J.; Silva, P.J., Jr.; Steiner, C.; Nehls, T.; Zec, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Dekok, L.J.; Grill, D.; Hawkesford, M.J.; Schnug, E.; Stulen, I. Cost Action 829. Fundamental Agronomical and Environmental Aspects of Sulphur Nutrition and Assimilation in Plants; Office for Official Publications of the European Communities: Luxemburg, 1999; p. 105. [Google Scholar]
- Malvi, U. Interaction of micronutrients with major nutrients with special reference to Potassium. Karnataka J. Agric. Sci. 2011, 24, 106–109. [Google Scholar]
- Esmaili1, E.; Kapourchal, S.A.; Malakouti, M.J.; Homaee, M. Interactive effect of salinity and two nitrogen fertilizers on growth and composition of sorghum. Plant Soil Environ. 2008, 12, 537–546. [Google Scholar] [CrossRef] [Green Version]
- Morrissey, J.; Guerinot, M.L. Iron uptake and transport in plants: The good, the bad, and the ionome. Chem. Rev. 2009, 109, 4553–4567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.H.; Li, X.Y.; Li, X.P.; Yuan, F.M.; Yao, Z.H.; Sun, Y.L.; Zhang, F.D. Crop yield, N uptake and nitrates in a fluvo-aquic soil profile. Pedosphere 2004, 14, 131–136. [Google Scholar]
- Germida, J.J.; Janzen, H.H. Factors affecting the oxidation of elemental sulfur in soils. Fertil. Res. 1993, 35, 101–114. [Google Scholar] [CrossRef]
- Fellet, G.; Marmiroli, M.; Marchiol, L. Elements uptake by metal accumulator species grown on mine tailings amended with three types of biochar. Sci. Total Environ. 2014, 468, 598–608. [Google Scholar] [CrossRef]
- Nishiyama, R.; Kato, M.; Nagata, S.; Yanagisawa, S.; Yoneyama, T. Identification of Zn-nicotianamine and Fe−2 deoxymugineic acid in the phloem sap from rice plants (Oryza sativa L.). Plant Cell Physiol. 2012, 53, 381–390. [Google Scholar] [CrossRef]
- Zuchi, S.; Cesco, S.; Astolfi, S. High S supply improves Fe accumulation in durum wheat plants grown under Fe limitation. Environ. Exp. Bot. 2012, 77, 25–32. [Google Scholar] [CrossRef]
- Kochanek, J.; Rochelle, M.S.; Martinez, C.; Dakuidreketi, A.; Mudge, A.M. Biochar for intensification of plant-related industries to meet productivity, sustainability and economic goals: A review. Resour. Conserv. Recycl. 2022, 179, 106109. [Google Scholar] [CrossRef]
| Property | Mean | |
|---|---|---|
| Soil | pH 1:5 H2O | 8.20 ± 0.28 |
| EC 1:5 H2O (dSm−1) | 0.37 ± 0.04 | |
| OM (%) | 0.95 ± 0.06 | |
| Organic C (%) | 0.55 ± 0.03 | |
| Available P (mg kg−1) | 3.45 ± 0.06 | |
| Available K (mg kg−1) | 61.5 ± 4.24 | |
| Biochar | pH 1:10 H2O | 6.46 ± 0.03 |
| EC 1:10 H2O (dS m−1) | 1.06 ± 0.02 | |
| pH NaCl | 6.50 ± 0.02 | |
| EC NaCl (dSm−1) | 1.90 ± 0.01 | |
| Alkalinity (mg L−1) | 8.00 | |
| Total organic C (g kg−1) | 568 ± 2.0 | |
| Total N (g kg−1) | 9.5 ± 0.10 |
| Biochar (t ha−1) | Nitrogen (kg ha−1) | Means | ||||
|---|---|---|---|---|---|---|
| 0 | 100 | 150 | 200 | |||
| pH | 0 | 7.74 ± 0.03 ef | 7.90 ± 0.03 ab | 7.90 ± 0.05 ab | 7.77 ± 0.13 c–f | 7.83 ± 0.09 AB |
| 5 | 7.83 ± 0.06 b–f | 7.78 ± 0.03 c–f | 7.76 ± 0.04 def | 7.80 ± 0.02 bc–f | 7.79 ± 0.03 BC | |
| 10 | 7.72 ± 0.05 f | 7.82 ± 0.07 b–f | 7.77 ± 0.01 c–f | 7.75 ± 0.03 ef | 7.76 ± 0.04 C | |
| 15 | 7.88 ± 0.12 a–d | 7.84 ± 0.03 a–e | 7.91 ± 0.04 ab | 7.82 ± 0.13 bc–f | 7.86 ± 0.04 A | |
| 20 | 7.87 ± 0.03 a–d | 7.95 ± 0.17 a | 7.88 ± 0.03 a–c | 7.75 ± 0.03 ef | 7.86 ± 0.09 A | |
| Mean | 7.81 ± 0.08 AB | 7.86 ± 0.07 A | 7.84 ± 0.07 A | 7.78 ± 0.03 B | ||
| SOM | 0 | 0.46 ± 0.02 fg | 0.54 ± 0.05 abc | 0.71 ± 0.05 abc | 0.55 ± 0.04 efg | 0.57 ± 0.10 C |
| 5 | 0.68 ± 0.02 cd | 0.67 ± 0.12 bc | 0.70 ± 0.07 bc | 0.73 ± 0.10 abc | 0.69 ± 0.03 B | |
| 10 | 0.79 ± 0.04 abc | 0.83 ± 0.12 abc | 0.78 ± 0.03 abc | 0.75 ± 0.03 abc | 0.79 ± 0.03 A | |
| 15 | 0.81 ± 0.06 ab | 0.43 ± 0.04 fg | 0.47 ± 0.03 fg | 0.56 ± 0.03 def | 0.57 ± 0.17 C | |
| 20 | 0.53 ± 0.04 fg | 0.75 ± 0.14 fg | 0.43 ± 0.03 fg | 0.67 ± 0.18 cde | 0.60 ± 0.14 C | |
| Mean | 0.65 ± 0.15 A | 0.64 ± 0.16 A | 0.62 ± 0.15 A | 0.65 ± 0.09 A | ||
| EC | 0 | 0.26 ± 0.02 ab | 0.31 ± 0.04 ab | 0.32 ± 0.04 a | 0.25 ± 003 ab | 0.29 ± 0.04 A |
| 5 | 0.29 ± 0.03 ab | 0.23 ± 0.01 b | 0.27 ± 0.04 ab | 0.29 ± 0.05 ab | 0.27 ± 0.03 A | |
| 10 | 0.31 ± 0.07 ab | 0.27 ± 0.03 ab | 0.27 ± 0.03 ab | 0.28 ± 0.05 ab | 0.28 ± 0.02 A | |
| 15 | 0.30 ± 0.07 ab | 0.30 ± 0.07 ab | 0.32 ± 0.08 a | 0.29 ± 0.08 ab | 0.30 ± 0.01 A | |
| 20 | 0.30 ± 0.07 ab | 0.28 ± 0.03 ab | 0.32 ± 0.05 a | 0.27 ± 0.02 ab | 0.29 ± 0.02 A | |
| Mean | 0.29 ± 0.02 A | 0.28 ± 0.03 A | 0.30 ± 0.03 A | 0.28 ± 0.02 A | ||
| Biochar (t ha−1) | Nitrogen (kg ha−1) | Means | ||||
|---|---|---|---|---|---|---|
| 0 | 100 | 150 | 200 | |||
| pH | 0 | 7.73 ± 0.05 abc | 7.85 ± 0.07 ab | 7.87 ± 0.05 a | 7.73 ± 0.13 abc | 7.80 ± 0.08 AB |
| 5 | 7.75 ± 0.05 abc | 7.76 ± 0.03 abc | 7.73 ± 0.06 abc | 7.76 ± 0.01 abc | 7.75 ± 0.02 BC | |
| 10 | 7.68 ± 0.05 c | 7.80 ± 0.06 abc | 7.72 ± 0.02 abc | 7.72 ± 0.03 bc | 7.73 ± 0.05 C | |
| 15 | 7.77 ± 0.26 abc | 7.83 ± 0.05 ab | 7.83 ± 0.11 ab | 7.80 ± 0.12 abc | 7.81 ± 0.03 A | |
| 20 | 7.84 ± 0.01 ab | 7.85 ± 0.11 ab | 7.85 ± 0.02 ab | 7.72 ± 0.04 bc | 7.81 ± 0.06 A | |
| Mean | 7.75 ± 0.06 AB | 7.82 ± 0.04 A | 7.80 ± 0.07 A | 7.75 ± 0.03 B | ||
| SOM | 0 | 0.54 ± 0.05 fgh | 0.50 ± 0.01 h | 0.61 ± 0.04 cde | 0.53 ± 0.03 gf | 0.55 ± 0.04 D |
| 5 | 0.65 ± 0.01 bc | 0.65 ± 0.01 bc | 0.60 ± 0.02 de | 0.66 ± 0.02 b | 0.64 ± 0.03 B | |
| 10 | 0.72 ± 0.01 a | 0.74 ± 0.01 a | 0.73 ± 0.02 a | 0.73 ± 0.01 a | 0.73 ± 0.01 A | |
| 15 | 0.71 ± 0.01 a | 0.36 ± 0.07 bc | 0.62 ± 0.03 bcde | 0.58 ± 0.02 efg | 0.57 ± 0.15 D | |
| 20 | 0.59 ± 0.2 de | 0.58 ± 0.02 ef | 0.63 ± 0.03 bcd | 0.66 ± 0.01 b | 0.61 ± 0.04 C | |
| Mean | 0.64 ± 0.08 A | 0.57 ± 0.14 B | 0.64 ± 0.05 A | 0.63 ± 0.08 A | ||
| EC | 0 | 0.26 ± 0.02 ab | 0.31 ± 0.04 ab | 0.32 ± 0.04 a | 0.25 ± 0.03 ab | 0.29 ± 0.04 A |
| 5 | 0.29 ± 0.03 ab | 0.23 ± 0.01 b | 0.27 ± 0.04 ab | 0.29 ± 0.05 ab | 0.27 ± 0.03 A | |
| 10 | 0.31 ± 0.07 ab | 0.27 ± 0.03 ab | 0.27 ± 0.03 ab | 0.28 ± 0.05 ab | 0.28 ± 0.02 A | |
| 15 | 0.30 ± 0.07 ab | 0.30 ± 0.07 ab | 0.32 ± 0.08 a | 0.29 ± 0.08 ab | 0.30 ± 0.01 A | |
| 20 | 0.30 ± 0.07 ab | 0.28 ± 0.03 ab | 0.32 ± 0.05 a | 0.27 ± 0.02 ab | 0.29 ± 0.02 A | |
| Mean | 0.29 ± 0.02 A | 0.28 ± 0.03 A | 0.30 ± 0.03 A | 0.28 ± 0.02 A | ||
| Biochar (t ha−1). | Nitrogen (kg ha−1) | Means | |||
|---|---|---|---|---|---|
| 0 | 100 | 150 | 200 | ||
| 0 | 2602.7 ± 41.04 k | 3106.7 ± 75.11 h | 4147.7 ± 84.52 f | 4678.0 ± 46.2 e | 3633.75 ± 935.9 E |
| 5 | 2709.0 ± 17.32 j | 3145.3 ± 38.9 gh | 4277.7 ± 15.37 ef | 4587.7 ± 27.32 cd | 3679.92 ± 896.2 D |
| 10 | 2926.7 ± 60.28 i | 3425.3 ± 24.73 g | 4507.3 ± 17.04 cd | 4788.3 ± 44.1 cd | 3911.92 ± 881.3 C |
| 15 | 4058.0 ± 86.88 f | 4025.0 ± 34.87 ef | 4741.7 ± 15.28 cd | 4616.0 ± 71.51 bc | 4360.17 ± 371.8 B |
| 20 | 4147.3 ± 105.11 f | 4030.7 ± 30.01 d | 4645.0 ± 45.64 b | 5141.3 ± 96.06 a | 4491.08 ± 508.8 A |
| Mean | 3288.7 ± 752.8 D | 3546.6 ± 456.19 C | 4463.9 ± 248.26 B | 4762.3 ± 225.5 A | |
| Element | Biochar (t ha−1) | Nitrogen (kg ha−1) | Mean | |||
|---|---|---|---|---|---|---|
| 0 | 100 | 150 | 200 | |||
| K | 0 | 4133.09 ± 26.14 h | 4330.52 ± 4.43 fgh | 4342.71 ± 23.37 fgh | 4470.60 ± 7.74 efg | 4319.23 ± 139.33 D |
| 5 | 4225.53 ± 9.511 gh | 4325.20 ± 18.69 fgh | 4369.28 ± 11.65 fgh | 5054.11 ± 6.88 b | 4493.53 ± 378.28 C | |
| 10 | 4520.30 ± 13.44 ef | 4496.77 ± 9.05 ef | 4772.54 ± 3.79 cd | 5549.09 ± 27.45 a | 4834.68 ± 492.36 A | |
| 15 | 4356.38 ± 14.72 de | 4691.64 ± 15.75 de | 4667.69 ± 7.55 de | 4970.18 ± 8.84 bc | 4671.47 ± 250.95 B | |
| 20 | 4158.28 ± 5.93 h | 4491.16 ± 4.75 ef | 4682.32 ± 7.84 de | 4792.81 ± 12.70 cd | 4531.14 ± 278.06 C | |
| Mean | 4278.72 ± 160.42 C | 4467.05 ± 150.16 B | 4566.91 ± 196.91 B | 4967.36 ± 394.71 A | ||
| p | 0 | 3040.76 ± 6.77 g | 3169.06 ± 27.58 de | 3372.95 ± 19.87 c | 3420.17 ± 18.04 b | 3250.73 ± 177.39 E |
| 5 | 3303.52 ± 10.17 fg | 3445.92 ± 13.33 de | 3759.75 ± 27.59 c | 4025.80 ± 3.84 ab | 3633.75 ± 323.48 B | |
| 10 | 3408.00 ± 34.40 def | 3503.58 ± 3.79 d | 3777.04 ± 8.00 c | 4133.48 ± 26.59 a | 3705.52 ± 325.35 A | |
| 15 | 3339.92 ± 3.55 efg | 3417.17 ± 17.14 de | 3447.11 ± 20.89 de | 3409.72 ± 5.96 def | 3403.48 ± 45.35 C | |
| 20 | 3002.72 ± 20.91 h | 3392.79 ± 3.18 def | 3430.98 ± 2.57 de | 3432.94 ± 16.72 g | 3314.86 ± 208.91 D | |
| Mean | 3218.98 ± 184.41 D | 3385.70 ± 127.96 C | 3557.57 ± 194.52 B | 3684.42 ± 362.88 A | ||
| Ca | 0 | 174.74 ± 9.85 fg | 203.38 ± 26.97 b–f | 188.53 ± 3.42 d–g | 192.88 ± 18.50 c–f | 189.88 ± 11.86 D |
| 5 | 214.80 ± 2.78 a–e | 183.90 ± 35.97 e–g | 184.22 ± 6.78 e–g | 216.45 ± 2.01 a–e | 199.84 ± 18.24 CD | |
| 10 | 207.54 ± 15.38 b–f | 238.10 ± 7.50 ab | 231.02 ± 4.68 ab | 247.60 ± 5.06 a | 231.07 ± 17.09 A | |
| 15 | 247.99 ± 9.47 a | 215.33 ± 4.62 a–e | 223.38 ± 3.17 a–d | 214.06 ± 6.77 a–e | 225.19 ± 15.75 AB | |
| 20 | 155.15 ± 6.50 g | 228.67 ± 19.91 a–c | 237.99 ± 6.47 ab | 224.64 ± 12.82 a–c | 211.61 ± 38.05 BC | |
| Mean | 200.05± 36.15 B | 213.88 ± 21.31 A | 213.03 ± 24.92 A | 219.13 ± 19.77 A | ||
| Mg | 0 | 1450.85 ± 2.90 g–j | 1451.42 ± 1.68 f–j | 1427.95 ± 1.14 h–j | 1498.09 ± 6.10 a–h | 1457.08 ± 29.45 B |
| 5 | 1444.91 ± 0.64 e–j | 1468.45 ± 1.06 d–j | 1408.16 ± 4.79 ij | 1520.55 ± 1.37 a–g | 1460.52 ± 47.09 B | |
| 10 | 1495.67 ± 8.03 a–i | 1529.55 ± 3.52 a–f | 1571.94 ± 1.63 ab | 1582.05 ± 1.50 a | 1544.80 ± 39.96 A | |
| 15 | 1447.88 ± 3.32 f–j | 1491.46 ± 0.91 b–i | 1546.84 ± 6.09 a-d | 1550.61 ± 1.28 a–c | 1509.20 ± 49.01 AB | |
| 20 | 1394.71 ± 2.56 j | 1482.04 ± 2.54 c–i | 1542.31 ± 0.88 a–e | 1560.89 ± 13.31 a–c | 1494.99 ± 74.85 AB | |
| Mean | 1446.81 ± 35.79 C | 1484.58 ± 29.05 BC | 1499.44 ± 75.47 AB | 1542.44 ± 33.25 A | ||
| Element | Biochar (t ha−1) | Nitrogen (kg ha−1) | Means | |||
|---|---|---|---|---|---|---|
| 0 | 100 | 150 | 200 | |||
| Zn | 0 | 24.53 ± 0.29 b–f | 26.12 ± 1.62 ab | 27.03 ± 0.16 a | 26.36 ± 0.37 ab | 26.01 ± 1.06 A |
| 5 | 22.30 ± 0.44 fg | 23.13 ± 0.70 de–g | 25.29 ± 0.94 a–d | 26.98 ± 0.17 a | 24.43 ± 2.12 B | |
| 10 | 22.12 ± 0.38 fg | 22.91 ± 0.23 d–g | 25.80 ± 0.08 a–c | 22.73 ± 6.03 e–g | 23.39 ± 1.64 B | |
| 15 | 22.61 ± 0.51 e–g | 23.45 ± 0.29 c–g | 22.69 ± 0.18 c–g | 24.41 ± 0.35 b–g | 23.29 ± 0.84 B | |
| 20 | 22.01 ± 0.37 g | 21.98 ± 0.37 g | 23.96 ± 0.12 b–g | 24.84 ± 0.54 a–e | 23.19 ± 1.43 B | |
| Mean | 22.71 ± 1.04 B | 23.52 ± 1.55 B | 24.95 ± 1.68 A | 25.06 ± 1.68 A | ||
| Fe | 0 | 30.57 ± 0.67 b–f | 32.51 ± 0.62 ab | 33.10 ± 0.98 a | 32.92 ± 0.20 a | 32.28 ± 1.16 A |
| 5 | 30.66 ± 0.67 b–e | 30.94 ± 0.52 a–d | 31.13 ± 0.57 a–c | 30.25 ± 0.70 c–f | 30.75 ± 0.38 B | |
| 10 | 28.58 ± 0.77 e–h | 28.65 ± 3.03 e–h | 31.35 ± 3.85 a–c | 31.22 ± 1.15 a–c | 29.95 ± 1.54 C | |
| 15 | 27.33 ± 0.56 hi | 25.80 ± 0.70 ij | 27.56 ± 1.54 g–i | 31.63 ± 0.74 a–c | 28.08 ± 2.49 D | |
| 20 | 24.49 ± 0.98 j | 28.43 ± 0.92 f–h | 28.82 ± 0.68 d-h | 29.57 ± 0.26 c–g | 27.83 ± 2.27 D | |
| Mean | 28.33 ± 2.56 B | 29.27 ± 2.57 B | 30.39 ± 2.19 A | 31.12 ± 1.29 A | ||
| Cu | 0 | 2.80 ± 0.58 d–f | 3.30 ± 0.17 a | 3.06 ± 0.03 a–c | 2.32 ± 0.04 hi | 2.87 ± 0.42 A |
| 5 | 2.67 ± 0.24 e–g | 2.64 ± 0.05 fg | 3.15 ± 0.03 ab | 2.90 ± 0.05 b–e | 2.84 ± 0.24 A | |
| 10 | 2.49 ± 0.12 gh | 2.69 ± 0.02 d–g | 2.26 ± 0.03 hi | 2.95 ± 0.10 b–d | 2.60 ± 0.29 B | |
| 15 | 2.51 ± 0.11 gh | 2.17 ± 0.004 i | 2.51 ± 0.01 gh | 2.84 ± 0.11 c–f | 2.51 ± 0.27 B | |
| 20 | 2.06 ± 0.02 i | 2.16 ± 0.03 i | 2.70 ± 0.07 d–g | 3.16 ± 0.01 ab | 2.52 ± 0.51 B | |
| Mean | 2.51 ± 0.28 B | 2.59 ± 0.47 B | 2.74 ± 0.37 A | 2.83 ± 0.31 A | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, H.; Alam, S.; Ahmad, W.; Morrow, S.; Sabir, M.; Hemar, Y. Synergistic Effects of Acacia Prunings-Derived Biochar and Nitrogen Application on the Mineral Profile of Maize (Zea mays L.) Grains. Sustainability 2022, 14, 2995. https://doi.org/10.3390/su14052995
Ullah H, Alam S, Ahmad W, Morrow S, Sabir M, Hemar Y. Synergistic Effects of Acacia Prunings-Derived Biochar and Nitrogen Application on the Mineral Profile of Maize (Zea mays L.) Grains. Sustainability. 2022; 14(5):2995. https://doi.org/10.3390/su14052995
Chicago/Turabian StyleUllah, Habib, Sahib Alam, Waqar Ahmad, Stuart Morrow, Muhammad Sabir, and Yacine Hemar. 2022. "Synergistic Effects of Acacia Prunings-Derived Biochar and Nitrogen Application on the Mineral Profile of Maize (Zea mays L.) Grains" Sustainability 14, no. 5: 2995. https://doi.org/10.3390/su14052995
APA StyleUllah, H., Alam, S., Ahmad, W., Morrow, S., Sabir, M., & Hemar, Y. (2022). Synergistic Effects of Acacia Prunings-Derived Biochar and Nitrogen Application on the Mineral Profile of Maize (Zea mays L.) Grains. Sustainability, 14(5), 2995. https://doi.org/10.3390/su14052995









