Aesthetic Aerogel Window Design for Sustainable Buildings
Abstract
1. Introduction
2. Materials and Methods
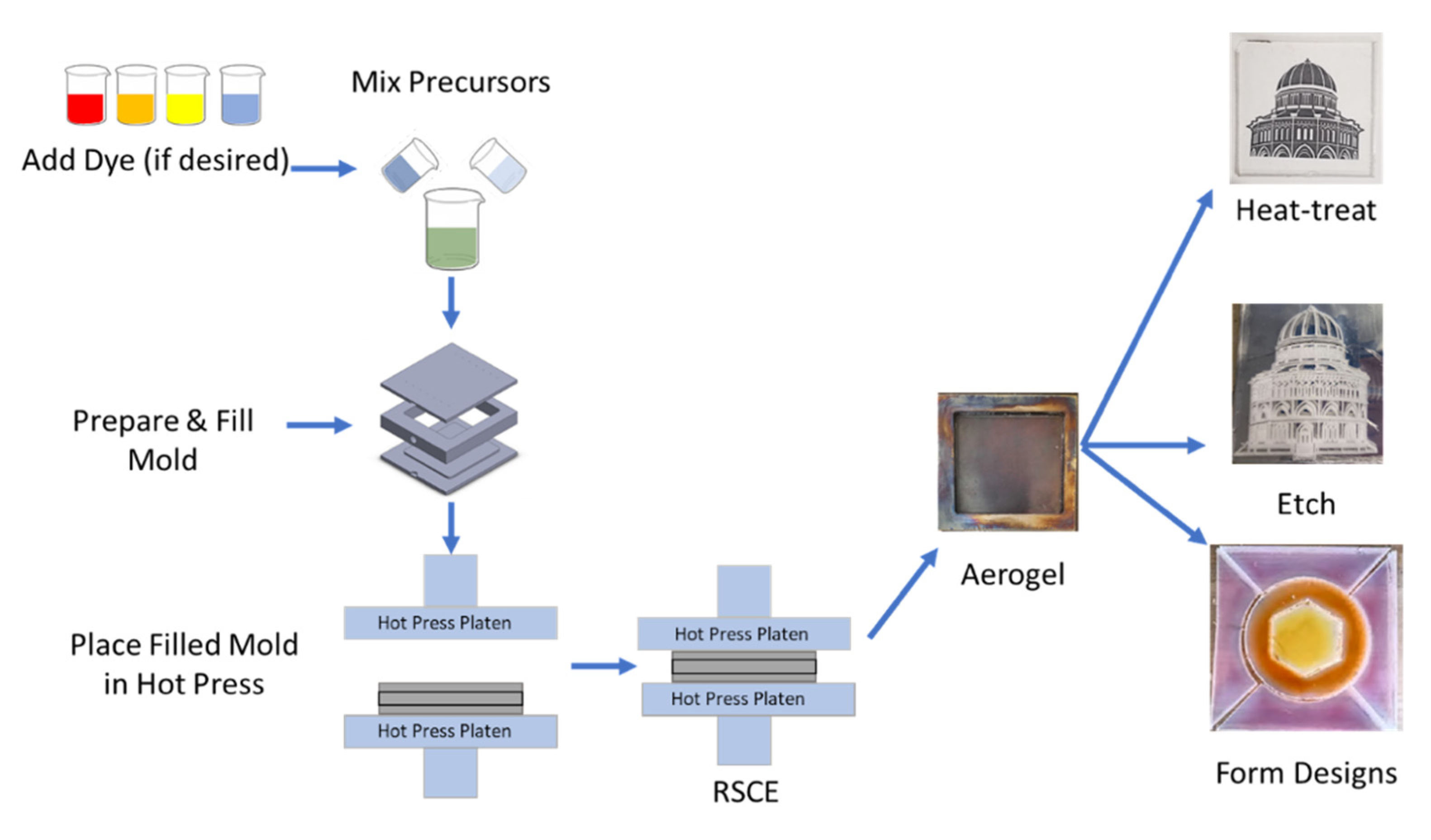
2.1. Aerogel Monolith Preparation
2.1.1. Wet-Gel Synthesis
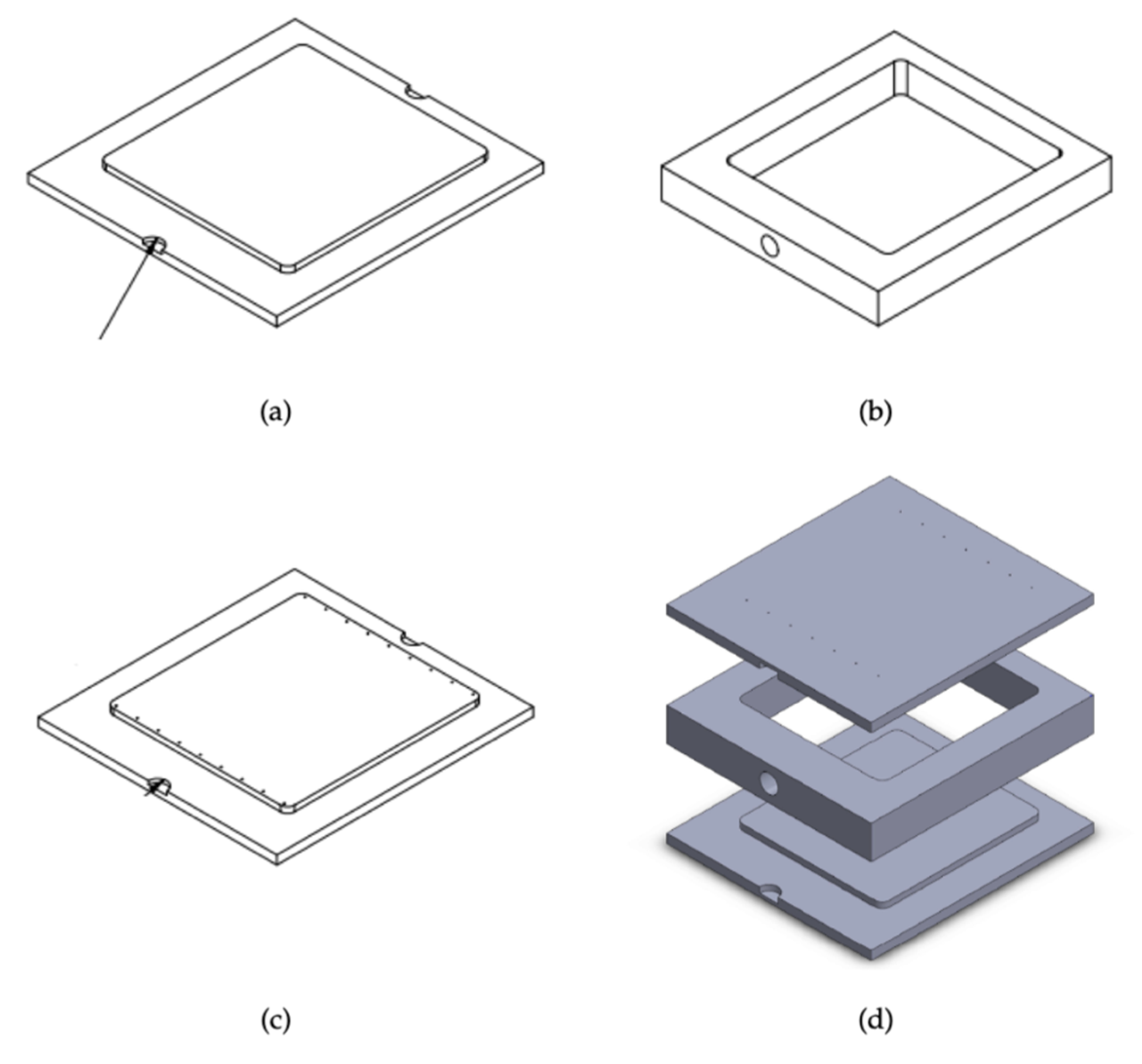
2.1.2. Mold Details
2.1.3. Drying Method
2.2. Aerogel Post-Processing
2.2.1. Heat Treatment
2.2.2. Etching and Cutting
2.2.3. Prototype Assembly
2.3. Characterization Methods
2.3.1. Light Transmittance
2.3.2. Thermal Performance
3. Results and Discussion
3.1. Approaches to Improve the Translucency of Aerogel Monoliths
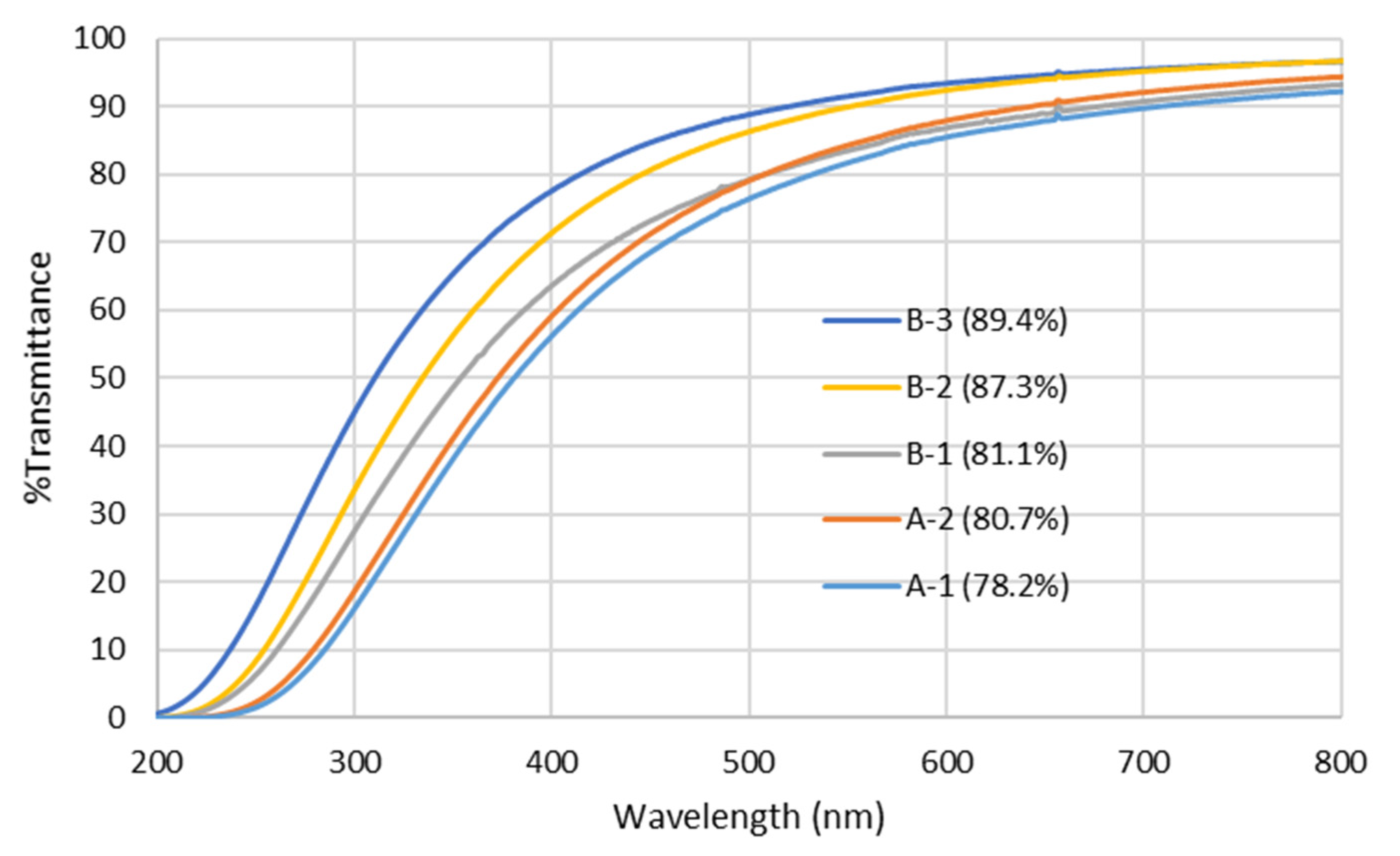
3.1.1. Variation in Chemical Precursor Recipe
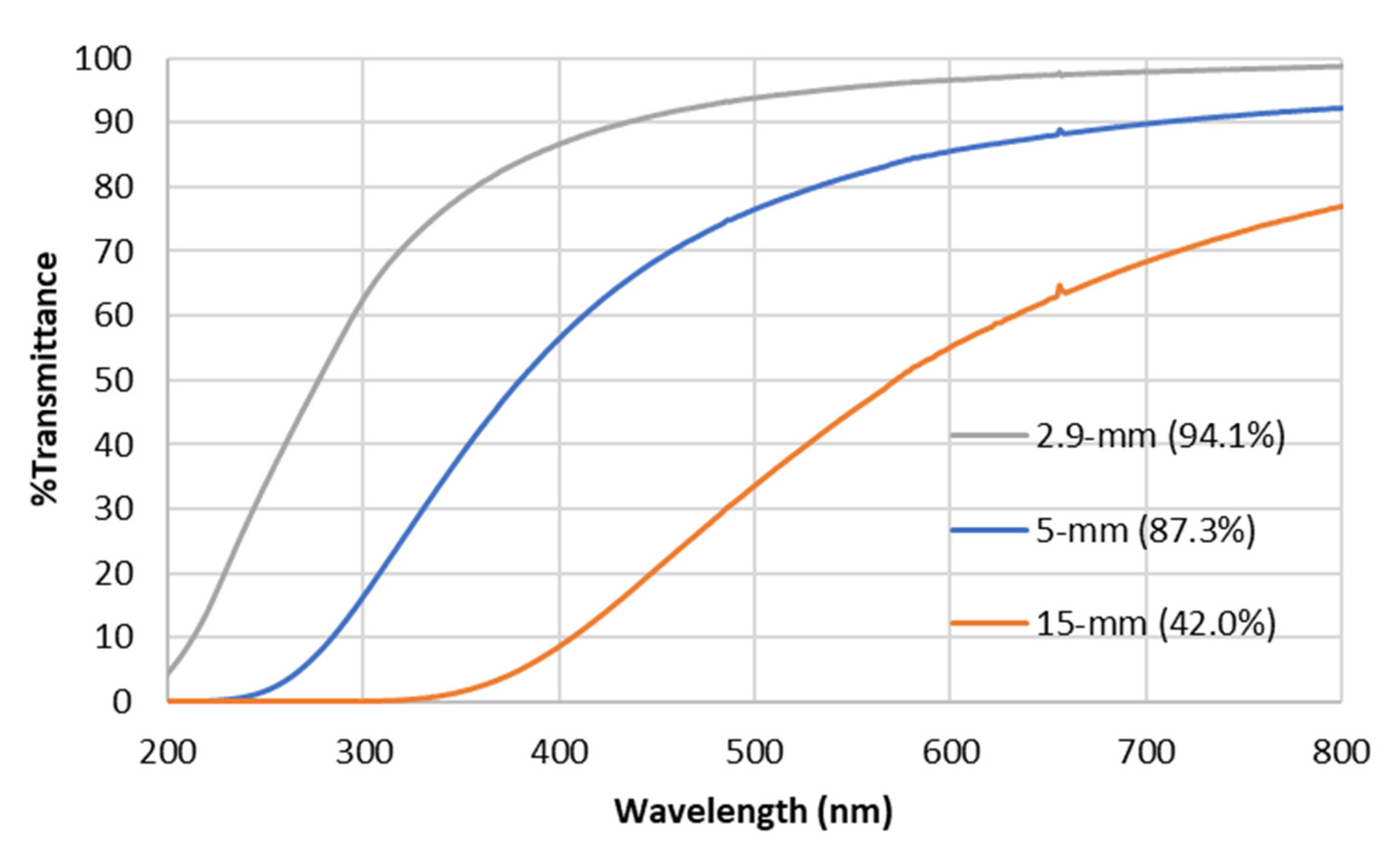
3.1.2. Production of Thinner Aerogels
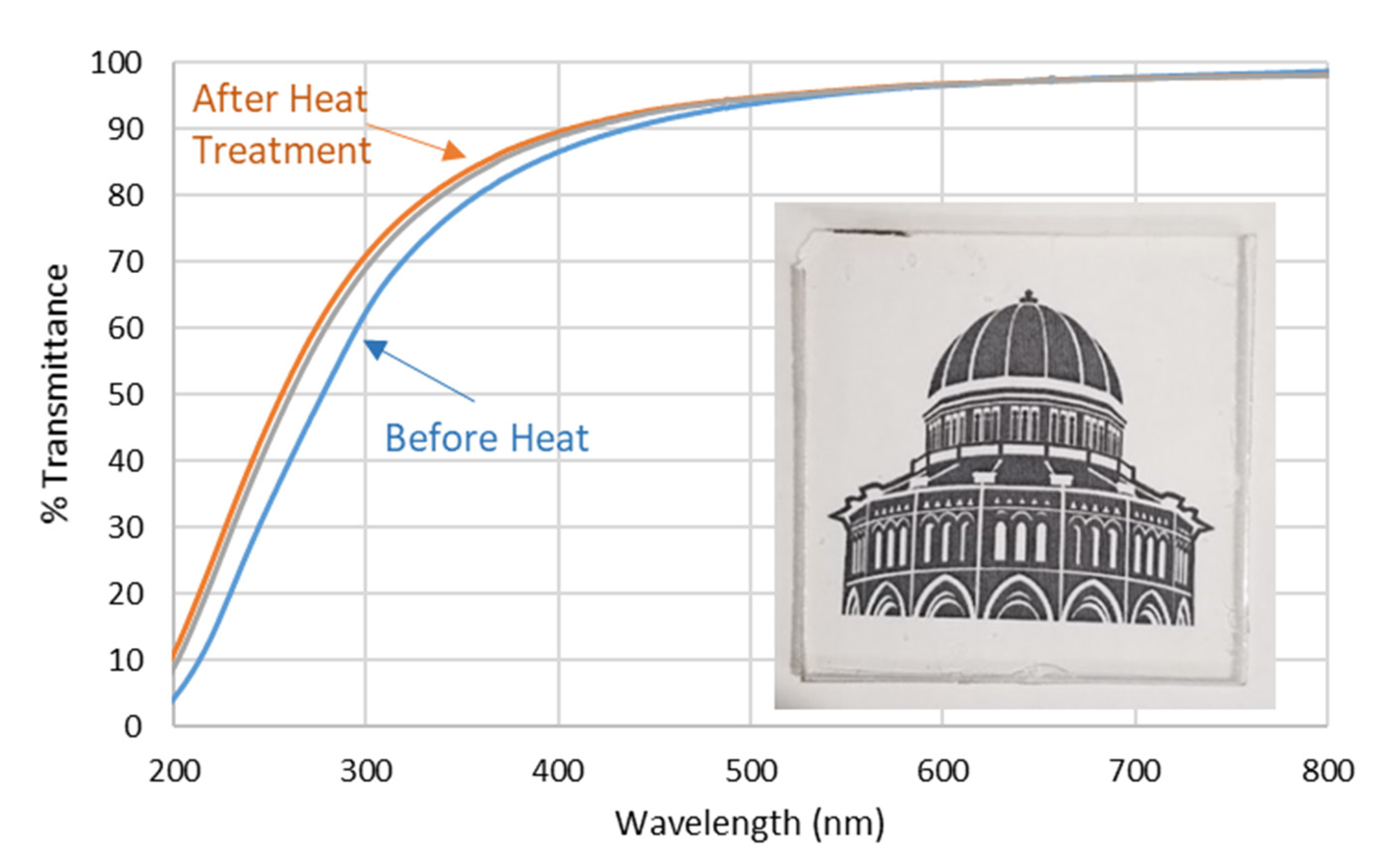
3.1.3. Effect of Heat Treatment
3.2. Approaches to Improve the Aesthetics of Silica Aerogel Monoliths
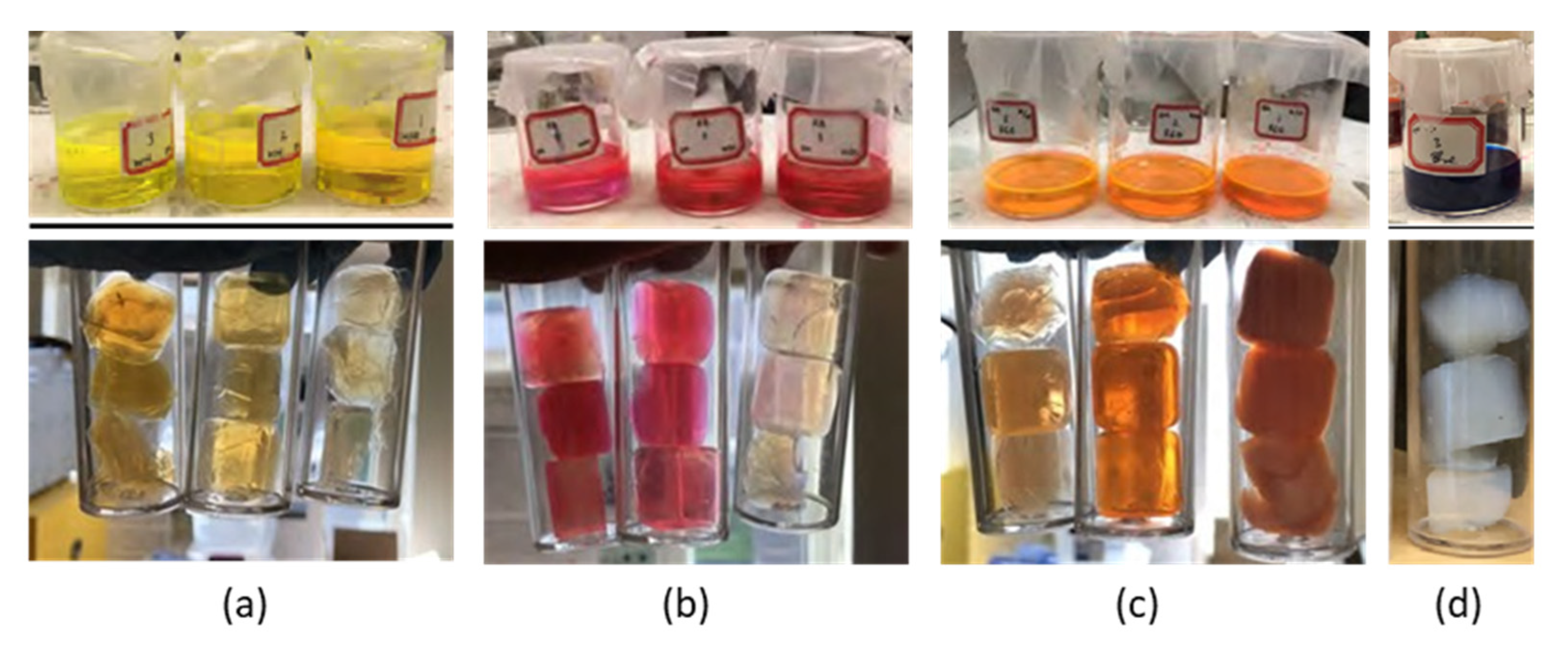
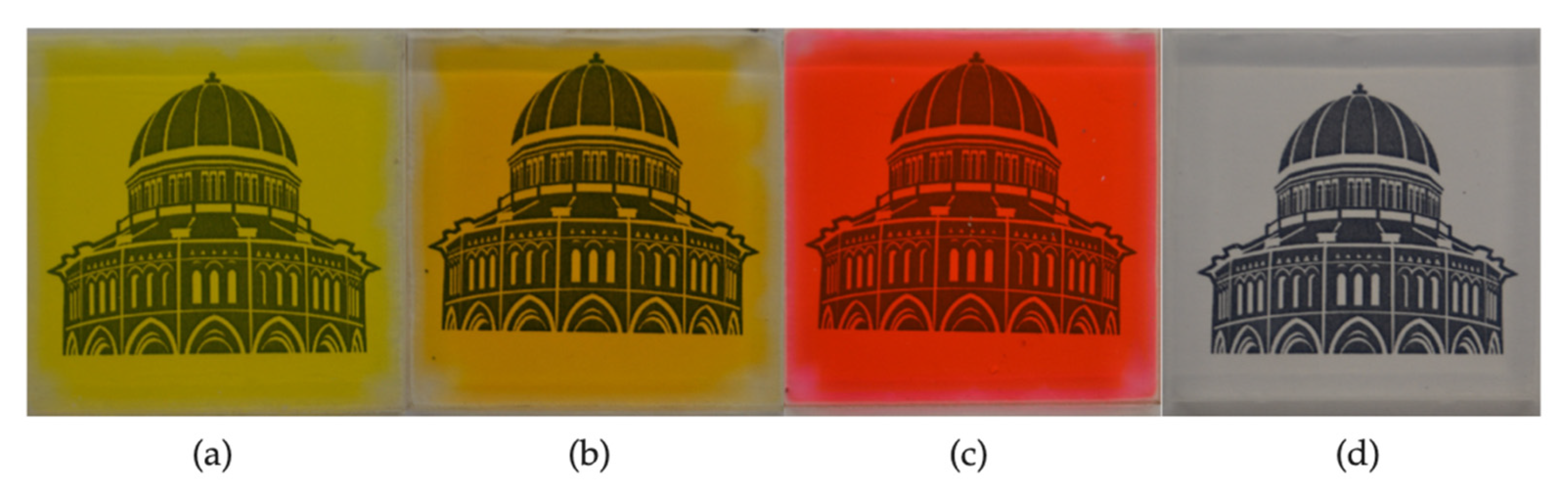
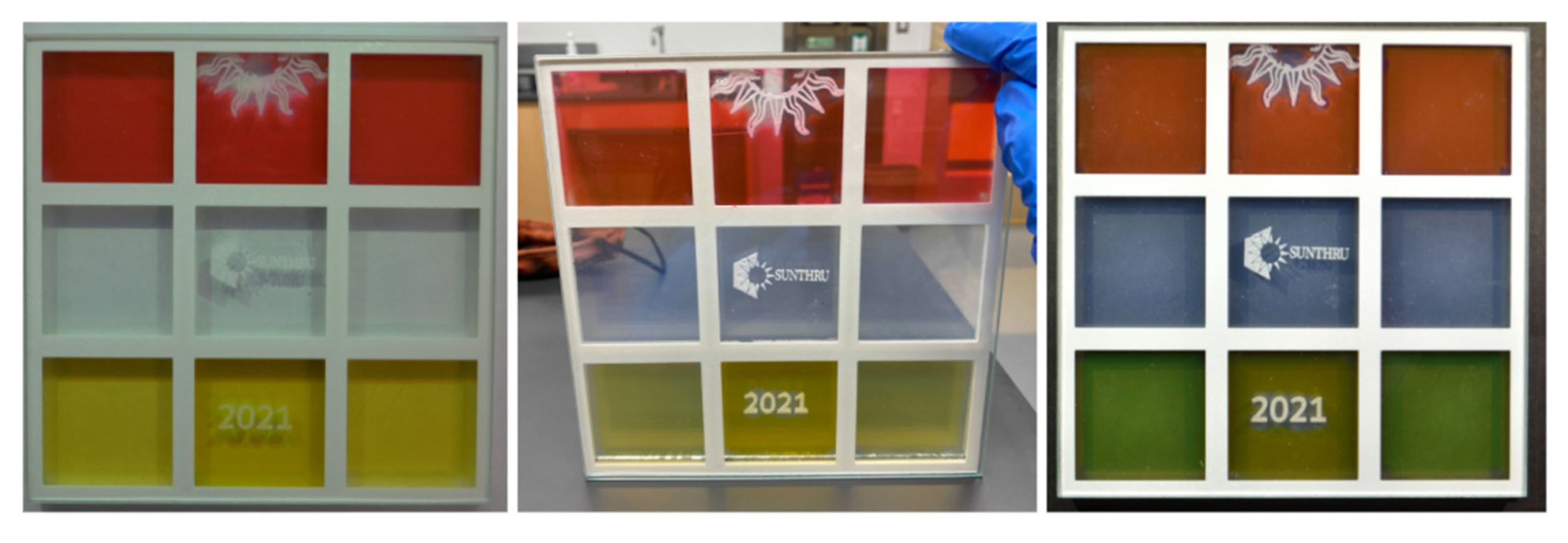
3.2.1. Use of Dyes to Yield Colored Aerogels
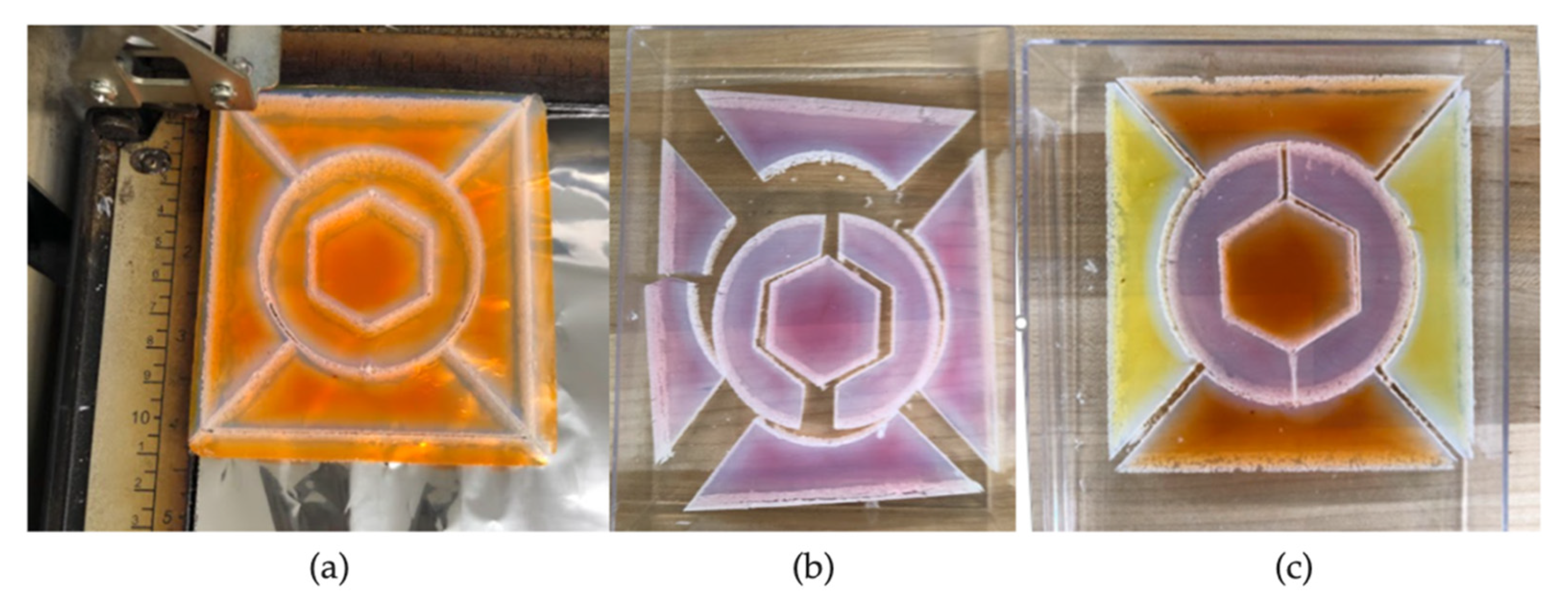
3.2.2. Use of Laser Etching and Cutting to Enhance Aesthetics
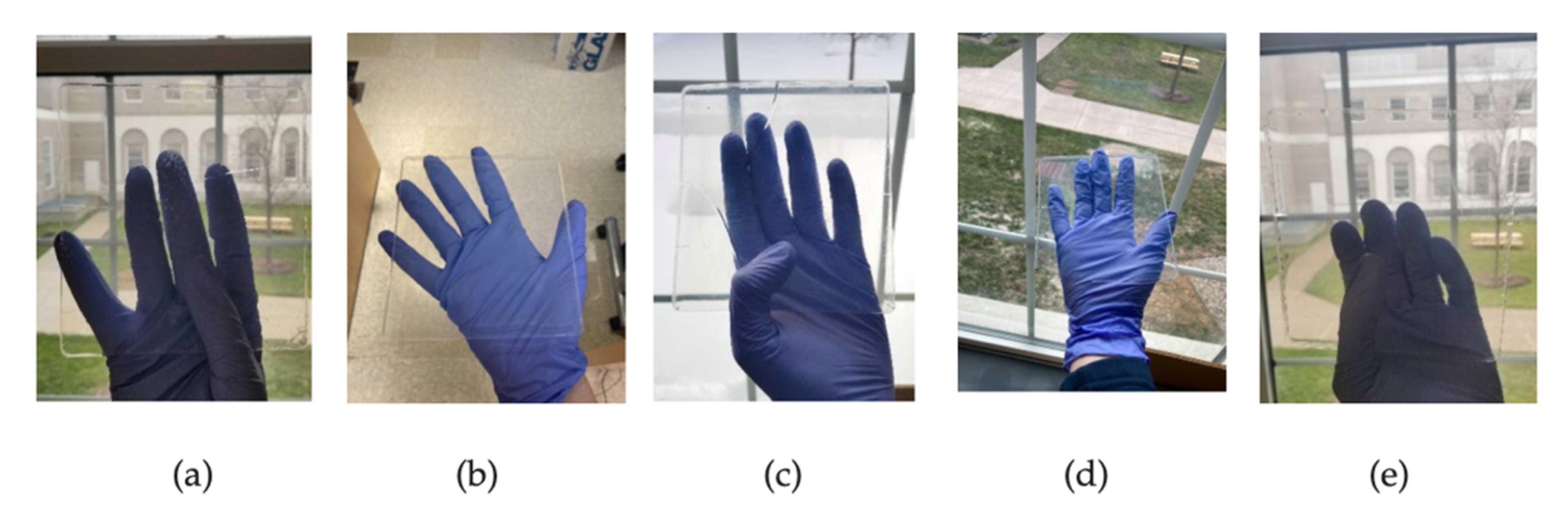
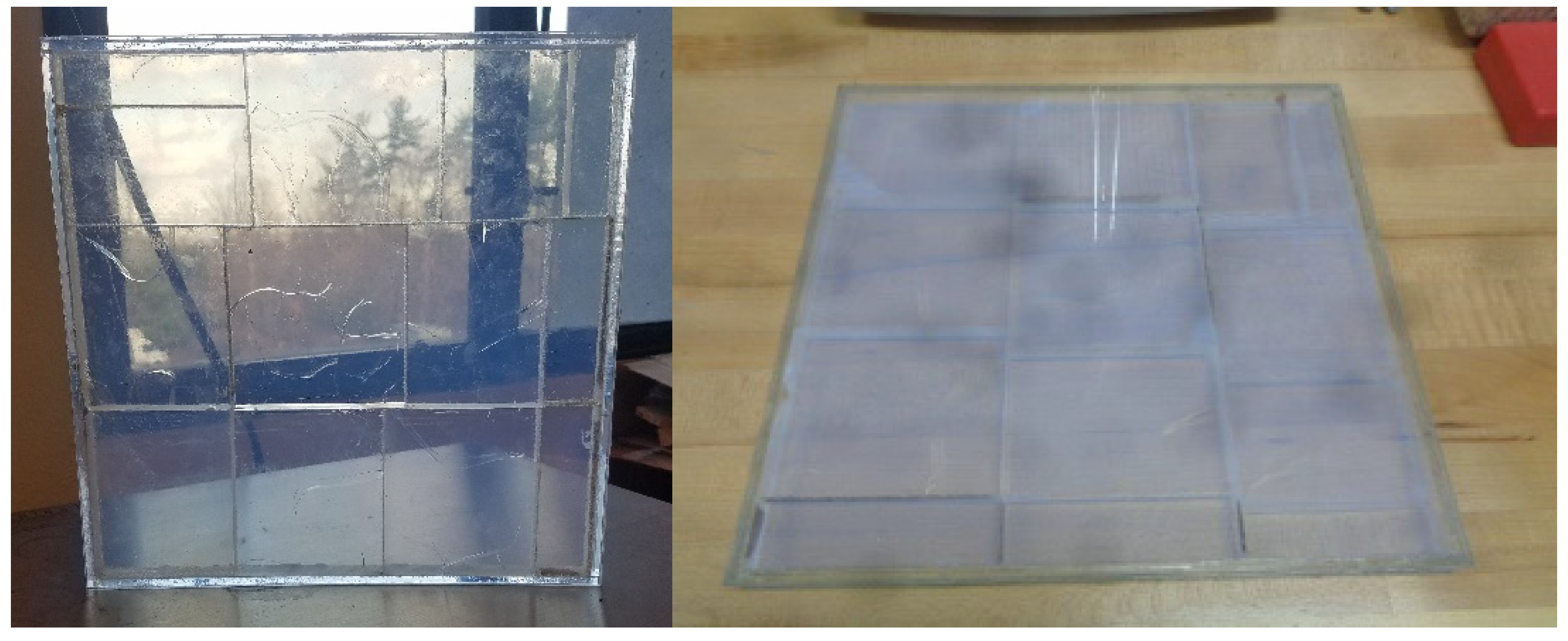
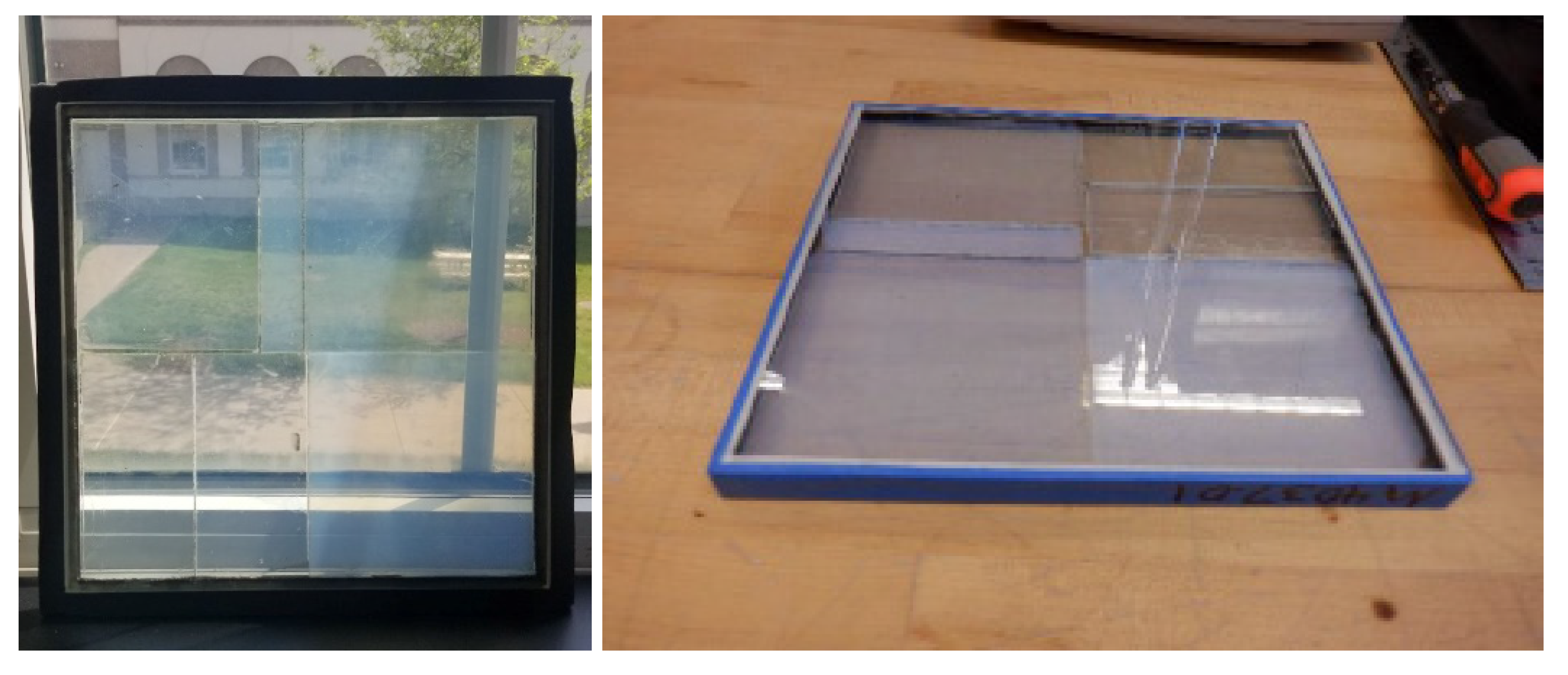
3.3. Approaches to Construction of Aerogel Window Prototypes
3.3.1. Scaled-Up Glazing Units
3.3.2. Aesthetically Enhanced Glazing Unit Prototype
4. Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jelle, B.P.; Hynd, A.; Gustavsen, A.; Arasteh, D.; Goudey, H.; Hart, R. Fenestration of today and tomorrow: A state-of-the-art review and future research opportunities. Sol. Energy Mater. Sol. Cells 2012, 96, 1–28. [Google Scholar] [CrossRef]
- Buratti, C. (Ed.) Translucent Silica Aerogel: Properties, Preparation and Applications; Nova Science Publishers, Inc.: New York, NY, USA, 2019. [Google Scholar]
- Pajonk, G.M. Transparent silica aerogels. J. Non-Cryst. Solids 1998, 225, 307–314. [Google Scholar] [CrossRef]
- Strohbach, E. Optically Transparent, Thermally Insulating and Soundproofing (OTTIS) Aerogel for High-Efficiency Window Applications. Doctoral Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, February 2020. [Google Scholar]
- Wang, J.; Petit, D.; Ren, S. Transparent thermal insulation silica aerogels. Nanoscale Adv. 2020, 2, 5504. [Google Scholar] [CrossRef]
- Buratti, C.; Belloni, E.; Merli, F.; Zinzi, M. Aerogel glazing systems for building applications: A review. Energy Build. 2021, 231, 110587. [Google Scholar] [CrossRef]
- Aegerter, M.A.; Leventis, N.; Koebel, M.M. (Eds.) Aerogels Handbook; Springer: New York, NY, USA, 2011. [Google Scholar]
- Aegerter, M.A.; Leventis, N.; Koebel, M.; Steiner, S.A., III (Eds.) Springer Handbook of Aerogels; Springer Nature: Cham, Switzerland, 2022. [Google Scholar]
- Bisson, A.; Rigacci, A.; Lecomte, D.; Rodier, E.; Achard, P. Drying of silica gels to obtain aerogels: Phenomenology and basic techniques. Dry. Technol. 2003, 21, 593–628. [Google Scholar] [CrossRef]
- Cabot Aerogel Particles. Available online: https://www.cabotcorp.com/solutions/products-plus/aerogel/particles (accessed on 25 February 2022).
- Wittwer, V. Development of Aerogel Windows. J. Non-Cryst. Solids 1992, 145, 233–236. [Google Scholar] [CrossRef]
- Buratti, C.; Moretti, E. Experimental performance evaluation of aerogel glazing systems. Appl. Energy 2012, 97, 430–437. [Google Scholar] [CrossRef]
- Buratti, C.; Moretti, E. Glazing systems with silica aerogel for energy savings in buildings. Appl. Energy 2012, 98, 396–403. [Google Scholar] [CrossRef]
- Cotana, F.; Pisello, A.L.; Moretti, E.; Buratti, C. Multipurpose characterization of glazing systems with silica aerogel: In-field experimental analysis of thermal-energy, lighting and acoustic performance. Build. Environ. 2014, 81, 92–102. [Google Scholar] [CrossRef]
- Gao, T.; Jelle, B.P.; Ihara, T.; Gustavsen, A. Insulating glazing units with silica aerogel granules: The impact of particle size. Appl. Energy 2014, 128, 27–34. [Google Scholar] [CrossRef]
- Garnier, C.; Muneer, T.; McCauley, L. Super insulated aerogel windows: Impact on daylighting and thermal performance. Build. Environ. 2015, 94, 231–238. [Google Scholar] [CrossRef]
- Huang, Y.; Niu, J. Applications of super-insulating translucent silica aerogel glazing system on commercial building envelope of humid subtropical climates—Impact on space cooling load. Energy 2015, 83, 316–325. [Google Scholar] [CrossRef]
- Ihara, T.; Gao, T.; Gyrnning, S.; Jelle, B.P.; Gustavsen, A. Aerogel granulate glazing facades and their application potential from an energy saving perspective. Appl. Energy 2015, 142, 179–181. [Google Scholar] [CrossRef]
- Mujeebu, M.A.; Ashraf, N.; Alsuwayigh, A. Energy performance and economic viability of nano aerogel glazing and nano vacuum insulation panel in multi-story office building. Energy 2016, 113, 949–956. [Google Scholar] [CrossRef]
- Berardi, U. The benefits of using aerogel-enhanced systems in building retrofits. Energy Procedia 2017, 134, 626–635. [Google Scholar] [CrossRef]
- Rubin, M.; Lampert, C.M. Transparent silica aerogels for window insulation. Sol. Energy Mater. 1983, 7, 393–400. [Google Scholar] [CrossRef]
- Duer, K.; Svendsen, S. Monolithic silica aerogel in superinsulating glazings. Sol. Energy 1998, 63, 259–267. [Google Scholar] [CrossRef]
- Jensen, K.L.; Shultz, J.M.; Kristiansen, F.H. Development of windows based on highly insulating aerogel glazings. J. Non-Cryst. Solids 2004, 350, 351–357. [Google Scholar] [CrossRef]
- Shultz, J.M.; Jensen, K.L.; Kristiansen, F.H. Super insulating aerogel glazing. Sol. Mater. Sol Cells 2005, 89, 275–285. [Google Scholar] [CrossRef]
- Schultz, J.M.; Jensen, K.I. Evacuated aerogel glazings. Vacuum 2008, 82, 723–729. [Google Scholar] [CrossRef]
- Berardi, U. The development of a monolithic aerogel glazed window for an energy retrofitting project. Appl. Energy 2015, 154, 603–615. [Google Scholar] [CrossRef]
- Bhuiya, M.M.H.; Anderson, A.M.; Carroll, M.K.; Bruno, B.A.; Ventrella, J.L.; Silberman, B.; Keramati, B. Preparation of monolithic silica aerogel for fenestration applications: Scaling up, reducing cycle time and improving performance. Ind. Eng. Chem. Res. 2016, 55, 6971–6981. [Google Scholar] [CrossRef]
- Merli, F.; Anderson, A.M.; Carroll, M.K.; Buratti, C. Acoustic measurements on monolithic aerogel samples and application of the selected solutions to standard window systems. Appl. Acoust. 2018, 142, 123–131. [Google Scholar] [CrossRef]
- Zinzi, M.; Rossi, G.; Anderson, A.M.; Carroll, M.K.; Moretti, E.; Buratti, C. Optical and visual experimental characterization of a glazing system with monolithic silica aerogel. Sol. Energy 2019, 183, 30–39. [Google Scholar] [CrossRef]
- Buratti, C.; Moretti, E.; Belloni, E.; Zinzi, M. Experimental and numerical energy assessment of a monolithic aerogel glazing unit for building applications. Appl. Sci. 2019, 9, 5473. [Google Scholar] [CrossRef]
- Golder, S.; Narayanan, R.; Hossain, M.R.; Islam, M.R. Experimental and CFD investigation on the application for aerogel insulation in buildings. Energies 2021, 14, 3310. [Google Scholar] [CrossRef]
- SunThru LLC. Available online: https://www.sunthru.biz/ (accessed on 25 February 2022).
- AeroShield. Available online: https://www.aeroshield.tech/ (accessed on 25 February 2022).
- Tiem Factory Inc. Available online: https://www.tiem.jp/en/ (accessed on 25 February 2022).
- Gauthier, B.M.; Bakrania, S.D.; Anderson, A.M.; Carroll, M.K. A fast supercritical extraction technique for aerogel fabrication. J. Non-Cryst. Solids 2004, 350, 238–243. [Google Scholar] [CrossRef]
- Roth, T.B.; Anderson, A.M.; Carroll, M.K. Analysis of a rapid supercritical extraction aerogel fabrication process: Prediction of thermodynamic conditions during processing. J. Non-Cryst. Solids 2008, 354, 3685–3693. [Google Scholar] [CrossRef]
- Carroll, M.K.; Anderson, A.M.; Gorka, C.A. Preparing silica aerogel monoliths via a rapid supercritical extraction method. J. Vis. Exp. 2014, 84, e51421. [Google Scholar] [CrossRef]
- Büttner, B.; Nauschütz, J.; Heinemann, U.; Reichenauer, G.; Scherdel, C.; Weinläder, H.; Weismann, S.; Buck, D.; Beck, A. Evaculated Glazing with Silica Aerogel Spacers. In Proceedings of the EuroSun 2018: 12th International Conference on Solar Energy and Buildings Conference Proceedings, Rapperswil, Switzerland, 10–13 September 2018. [Google Scholar] [CrossRef]
- Rao, A.V.; Pajonk, G.M.; Parvathy, N.N. Effect of solvents and catalysts on monolithicity and physical properties of silica aerogels. J. Mater. Sci. 1994, 29, 1807–1817. [Google Scholar] [CrossRef]
- Tajiri, K.; Igarashi, K. The effect of the preparation conditions on the optical properties of transparent silica aerogels. Sol. Energy Mater. Sol. Cells 1998, 54, 189–195. [Google Scholar] [CrossRef]
- Athmuri, K.; Marinov, V.R. Optically transparent and structurally sound silica aerogels: Insights from a process study. Adv. Mater. Sci. 2012, 12, 5–16. [Google Scholar] [CrossRef][Green Version]
- Strobach, E.; Bhatia, B.; Yang, S.; Zhao, L.; Wang, E.N. High temperature annealing for structural optimization of silica aerogels in solar thermal applications. J. Non-Cryst. Solids 2017, 462, 72–77. [Google Scholar] [CrossRef]
- Michaloudis, I.; Carroll, M.K.; Kupiec, S.; Cook, K.; Anderson, A.M. Facile method for surface etching of silica aerogel monoliths. J. Sol-Gel Sci. Technol. 2018, 87, 22–26. [Google Scholar] [CrossRef]
- Stanec, A.M.; Anderson, A.M.; Avanessian, C.; Carroll, M.K. Analysis and characterization of etched silica aerogels. J. Sol-Gel Sci. Technol. 2020, 94, 406–415. [Google Scholar] [CrossRef]
- Stanec, A.M.; Hajjaj, Z.; Carroll, M.; Anderson, A.M. Aesthetically enhanced silica aerogel via incorporation of etching and dyes. J. Vis. Exp. 2021, 169, e61986. [Google Scholar] [CrossRef] [PubMed]
- Anders, G.D. Windows and Glazing. Whole Building Design Guide. Available online: https://www.wbdg.org/resources/windows-and-glazing (accessed on 25 February 2022).




| Chemical | Recipe A-1 | Recipe A-2 (2× Catalyst) 1 | Recipe B-1 | Recipe B-2 (1.5× Catalyst) 2 | Recipe B-3 (2× Catalyst) 2 |
|---|---|---|---|---|---|
| TMOS (g) | 26.4 | 26.4 | 26.4 | 26.4 | 26.4 |
| Methanol (g) | 66.1 | 66.1 | 57.1 | 57.1 | 57.1 |
| DI water (g) | 10.9 | 10.1 | 11.7 | 10.9 | 10.2 |
| 1.5 M NH3 (µL) | 816 | 1630 | 1500 | 2250 | 3000 |
| Dye | Fluorescein | Rhodamine 6G | Rhodamine B |
|---|---|---|---|
| Melting point (°C) | 315 | 290 | 210 |
| Color in solution | Yellow | Orange | Pink |
| Mass % in methanol stock | 0.05% | 0.16% | 0.075% |
| Chemical structure | 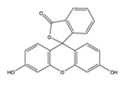 | 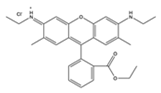 | 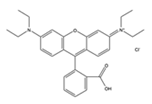 |
| Step | T (°C) | Heat/Cool Rate (°C/min) | Force (kN) | Force Rate (kN/min) | Dwell Time (min) |
|---|---|---|---|---|---|
| Seal | 37 | -- | 200–267 | -- | 30 |
| Heat | 288 | 1–2.2 | 200–267 | -- | 30–55 |
| Release | 288 | -- | 1 | 4.4–13.2 | 5–15 |
| Cool | 32 | 1–2.2 | 1 | -- | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carroll, M.K.; Anderson, A.M.; Mangu, S.T.; Hajjaj, Z.; Capron, M. Aesthetic Aerogel Window Design for Sustainable Buildings. Sustainability 2022, 14, 2887. https://doi.org/10.3390/su14052887
Carroll MK, Anderson AM, Mangu ST, Hajjaj Z, Capron M. Aesthetic Aerogel Window Design for Sustainable Buildings. Sustainability. 2022; 14(5):2887. https://doi.org/10.3390/su14052887
Chicago/Turabian StyleCarroll, Mary K., Ann M. Anderson, Sri Teja Mangu, Zineb Hajjaj, and Margeaux Capron. 2022. "Aesthetic Aerogel Window Design for Sustainable Buildings" Sustainability 14, no. 5: 2887. https://doi.org/10.3390/su14052887
APA StyleCarroll, M. K., Anderson, A. M., Mangu, S. T., Hajjaj, Z., & Capron, M. (2022). Aesthetic Aerogel Window Design for Sustainable Buildings. Sustainability, 14(5), 2887. https://doi.org/10.3390/su14052887






