Sex Differences in the Expression of c-fos in a Rat Brain after Exposure to Environmental Noise
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Noise Exposure
2.3. c-Fos Immunohistochemistry
2.4. c-Fos+ Cell Analysis
2.5. Corticosterone Assays
2.6. Data Analysis
3. Results
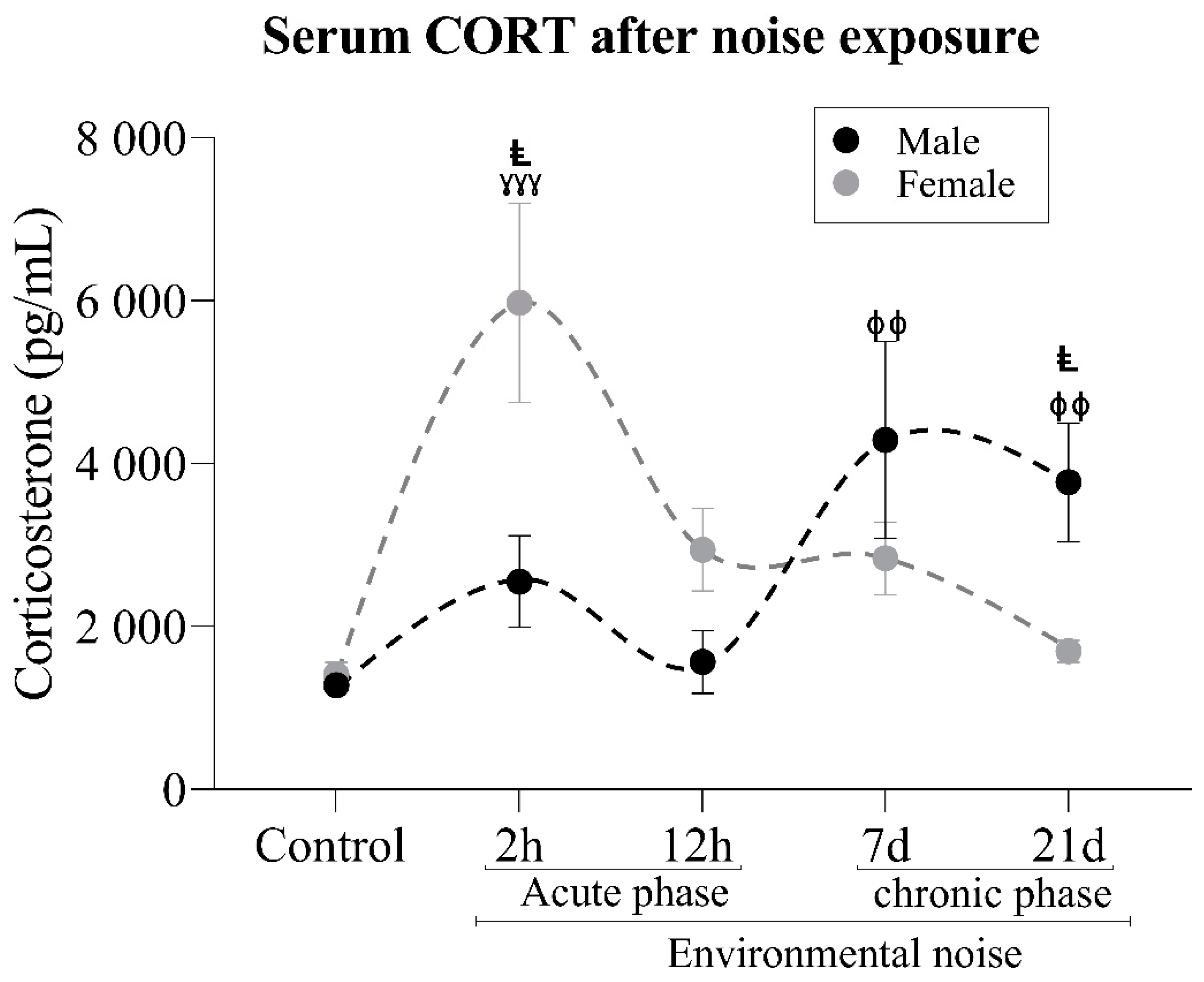
3.1. Corticosterone Analysis
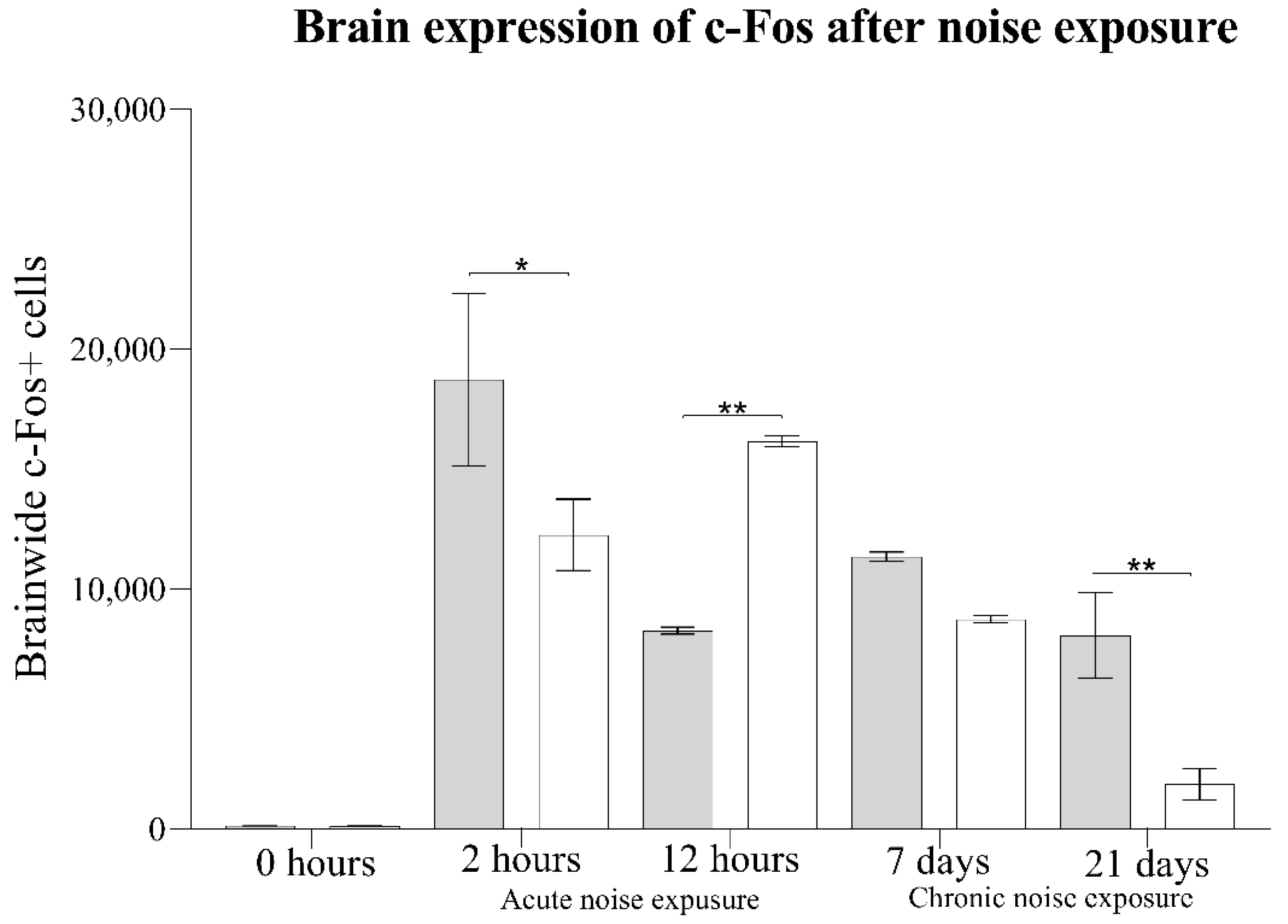
3.2. Brainwide Quantification of c-Fos+ Cells
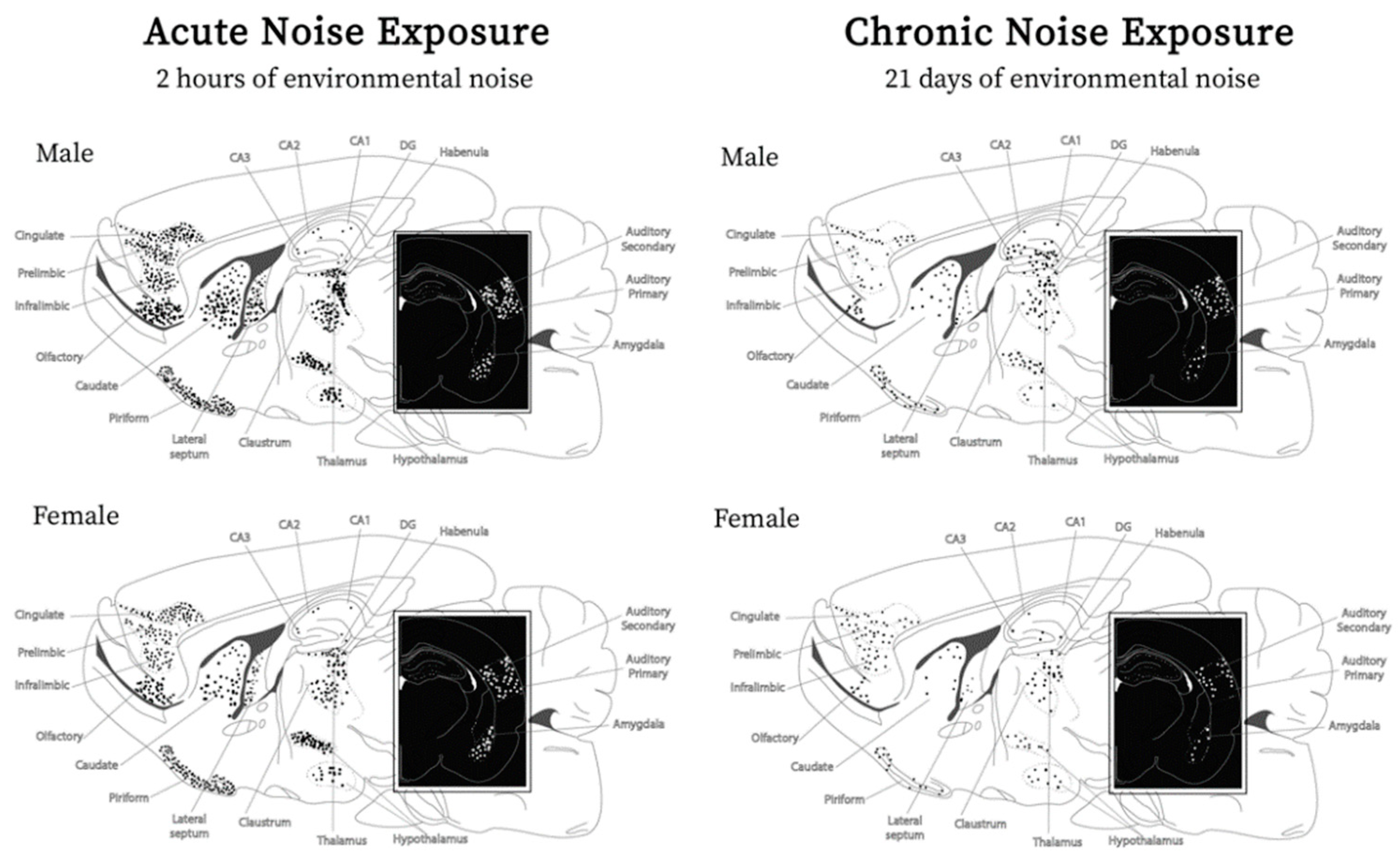
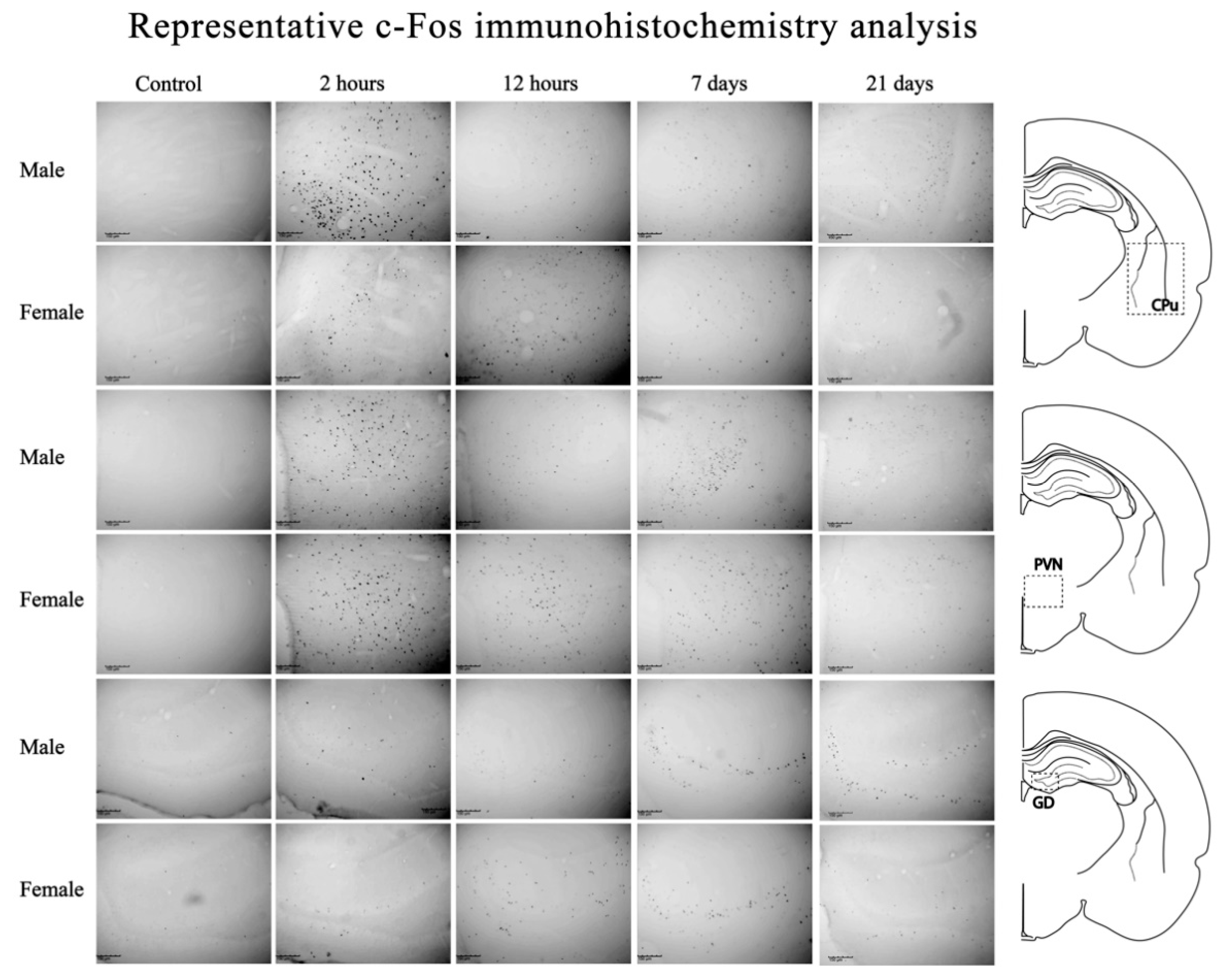
Brain Regions Affected by Noise
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chepesiuk, R. Decibel Hell: The Effects of Living in a Noisy World. Environ. Health Perspect. 2005, 113, A34–A41. [Google Scholar] [CrossRef] [PubMed]
- Guski, R.; Schreckenberg, D.; Schuemer, R. WHO Environmental Noise Guidelines for the European Region: A Systematic Review on Environmental Noise and Annoyance. Int. J. Environ. Res. Public Health 2017, 14, 1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stallen, P.J.M. A Theoretical Framework for Environmental Noise Annoyance. Noise Health 1999, 1, 69–80. [Google Scholar] [PubMed]
- Evans, E.F. Auditory Processing of Complex Sounds: An Overview. Philos. Trans. R Soc. Lond. B Biol. Sci. 1992, 336, 295–306. [Google Scholar] [CrossRef]
- Demanez, J.P.; Demanez, L. Anatomophysiology of the Central Auditory Nervous System: Basic Concepts. Acta Otorhinolaryngol. Belg. 2003, 57, 227–236. [Google Scholar]
- Flores, E.N.; Duggan, A.; Madathany, T.; Hogan, A.K.; Márquez, F.G.; Kumar, G.; Seal, R.P.; Edwards, R.H.; Liberman, M.C.; García-Añoveros, J. A Non-Canonical Pathway from Cochlea to Brain Signals Tissue-Damaging Noise. Curr. Biol. 2015, 25, 606–612. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.; Greferath, U.; Nag, N.; Nithianantharajah, J.; Wilson, Y.M. Tracing Functional Circuits Using C-Fos Regulated Expression of Marker Genes Targeted to Neuronal Projections. Front. Biosci. 2004, 9, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, K.J. C-Fos as a Transcription Factor: A Stressful (Re)View from a Functional Map. Neurochem. Int. 1998, 33, 287–297. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Zangenehpour, S.; Rahbar-Dehgan, F.; Ye, F. Molecular Maps of Neural Activity and Quiescence. Acta Neurobiol. Exp. 2000, 60, 403–410. [Google Scholar]
- Baker, M.A.; Weiler, E.M. Sex of Listener and Hormonal Correlates of Auditory Thresholds. Br. J. Audiol. 1977, 11, 65–68. [Google Scholar] [CrossRef]
- Luine, V.; Gomez, J.; Beck, K.; Bowman, R. Sex Differences in Chronic Stress Effects on Cognition in Rodents. Pharmacol. Biochem. Behav. 2017, 152, 13–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corso, J.F. Age and Sex Differences in Pure-Tone Thresholds. Survey of Hearing Levels from 18 to 65 Years. Arch. Otolaryngol. 1963, 77, 385–405. [Google Scholar] [CrossRef]
- Balogová, Z.; Popelář, J.; Chiumenti, F.; Chumak, T.; Burianová, J.S.; Rybalko, N.; Syka, J. Age-Related Differences in Hearing Function and Cochlear Morphology between Male and Female Fischer 344 Rats. Front. Aging Neurosci. 2017, 9, 428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabat, A. Extra-Auditory Effects of Noise in Laboratory Animals: The Relationship between Noise and Sleep. J. Am. Assoc. Lab. Anim. Sci. 2007, 46, 35–41. [Google Scholar] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2013; Available online: https://www.elsevier.com/books/the-rat-brain-in-stereotaxic-coordinates/paxinos/978-0-12-391949-6 (accessed on 26 October 2021).
- Watson, S.J. Neuroendocrine and Behavioral Responses and Brain Pattern of C-Fos Induction Associated with Audiogenic Stress. J. Neuroendocrinol. 1997, 9, 577–588. [Google Scholar] [CrossRef] [Green Version]
- Henkin, R.I.; Knigge, K.M. Effect of Sound on the Hypothalamic-Pituitary-Adrenal Axis. Am. J. Physiol. 1963, 204, 701–704. [Google Scholar] [CrossRef] [Green Version]
- Palkovits, M.; Dobolyi, A.; Helfferich, F.; Usdin, T.B. Localization and Chemical Characterization of the Audiogenic Stress Pathway. Ann. N. Y. Acad. Sci. 2004, 1018, 16–24. [Google Scholar] [CrossRef]
- Campeau, S.; Dolan, D.; Akil, H.; Watson, S.J. c-fos MRNA Induction in Acute and Chronic Audiogenic Stress: Possible Role of the Orbitofrontal Cortex in Habituation. Stress 2002, 5, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Herbert, J. Regional Changes in c-fos Expression in the Basal Forebrain and Brainstem during Adaptation to Repeated Stress: Correlations with Cardiovascular, Hypothermic and Endocrine Responses. Neuroscience 1995, 64, 675–685. [Google Scholar] [CrossRef]
- Martinez, M.; Phillips, P.J.; Herbert, J. Adaptation in Patterns of C-Fos Expression in the Brain Associated with Exposure to Either Single or Repeated Social Stress in Male Rats. Eur. J. Neurosci. 1998, 10, 20–33. [Google Scholar] [CrossRef]
- Stamp, J.A.; Herbert, J. Multiple Immediate-Early Gene Expression during Physiological and Endocrine Adaptation to Repeated Stress. Neuroscience 1999, 94, 1313–1322. [Google Scholar] [CrossRef]
- Watanabe, Y.; Stone, E.; McEwen, B.S. Induction and Habituation of C-Fos and Zif/268 by Acute and Repeated Stressors. Neuroreport 1994, 5, 1321–1324. [Google Scholar] [PubMed]
- Alkadhi, K.A.; Tran, T.T. Chronic Psychosocial Stress Impairs Early LTP but Not Late LTP in the Dentate Gyrus of At-Risk Rat Model of Alzheimer’s Disease. Brain Res. 2014, 1588, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Quezada, D.; Moran-Torres, D.; Luquin, S.; Ruvalcaba-Delgadillo, Y.; García-Estrada, J.; Jáuregui-Huerta, F. Male/Female Differences in Radial Arm Water Maze Execution After Chronic Exposure to Noise. Noise Health 2019, 21, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Gerges, N.Z.; Aleisa, A.M.; Schwarz, L.A.; Alkadhi, K.A. Chronic Psychosocial Stress Decreases Calcineurin in the Dentate Gyrus: A Possible Mechanism for Preservation of Early Ltp. Neuroscience 2003, 117, 869–874. [Google Scholar] [CrossRef]
- Jáuregui-Huerta, F.; Garcia-Estrada, J.; Ruvalcaba-Delgadillo, Y.; Trujillo, X.; Huerta, M.; Feria-Velasco, A.; Gonzalez-Perez, O.; Luquin, S. Chronic Exposure of Juvenile Rats to Environmental Noise Impairs Hippocampal Cell Proliferation in Adulthood. Noise Health 2011, 13, 286–291. [Google Scholar] [CrossRef]
- Luczynski, P.; Moquin, L.; Gratton, A. Chronic Stress Alters the Dendritic Morphology of Callosal Neurons and the Acute Glutamate Stress Response in the Rat Medial Prefrontal Cortex. Stress 2015, 18, 654–667. [Google Scholar] [CrossRef]
- Ortiz, J.B.; Conrad, C.D. The Impact from the Aftermath of Chronic Stress on Hippocampal Structure and Function: Is There a Recovery? Front. Neuroendocr. 2018, 49, 114–123. [Google Scholar] [CrossRef]
- Burman, D.D.; Bitan, T.; Booth, J.R. Sex Differences in Neural Processing of Language among Children. Neuropsychologia 2008, 46, 1349–1362. [Google Scholar] [CrossRef] [Green Version]
- Krizman, J.; Skoe, E.; Kraus, N. Sex Differences in Auditory Subcortical Function. Clin. Neurophysiol. 2012, 123, 590–597. [Google Scholar] [CrossRef] [Green Version]
- Verma, R.; Balhara, Y.P.S.; Gupta, C.S. Gender Differences in Stress Response: Role of Developmental and Biological Determinants. Ind. Psychiatry J. 2011, 20, 4–10. [Google Scholar] [CrossRef]
- Heinsbroek, R.P.; Van Haaren, F.; Feenstra, M.G.; Endert, E.; Van de Poll, N.E. Sex- and Time-Dependent Changes in Neurochemical and Hormonal Variables Induced by Predictable and Unpredictable Footshock. Physiol. Behav. 1991, 49, 1251–1256. [Google Scholar] [CrossRef]
- Kitay, J.I. Sex Differences in Adrenal Cortical Secretion in the Rat. Endocrinology 1961, 68, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Garrett, J.E.; Wellman, C.L. Chronic Stress Effects on Dendritic Morphology in Medial Prefrontal Cortex: Sex Differences and Estrogen Dependence. Neuroscience 2009, 162, 195–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowman, R.E.; Beck, K.D.; Luine, V.N. Chronic Stress Effects on Memory: Sex Differences in Performance and Monoaminergic Activity. Horm. Behav. 2003, 43, 48–59. [Google Scholar] [CrossRef]
- Wei, J.; Yuen, E.Y.; Liu, W.; Li, X.; Zhong, P.; Karatsoreos, I.N.; McEwen, B.S.; Yan, Z. Estrogen Protects against the Detrimental Effects of Repeated Stress on Glutamatergic Transmission and Cognition. Mol. Psychiatry 2014, 19, 588–598. [Google Scholar] [CrossRef]
- Zavala, J.K.; Fernandez, A.A.; Gosselink, K.L. Female Responses to Acute and Repeated Restraint Stress Differ from Those in Males. Physiol. Behav. 2011, 104, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Moench, K.M.; Breach, M.R.; Wellman, C.L. Chronic Stress Produces Enduring Sex- and Region-Specific Alterations in Novel Stress-Induced c-Fos Expression. Neurobiol. Stress 2019, 10, 100147. [Google Scholar] [CrossRef]
- Panagiotaropoulos, T.; Papaioannou, A.; Pondiki, S.; Prokopiou, A.; Stylianopoulou, F.; Gerozissis, K. Effect of Neonatal Handling and Sex on Basal and Chronic Stress-Induced Corticosterone and Leptin Secretion. Neuroendocrinology 2004, 79, 109–118. [Google Scholar] [CrossRef]
- Seale, J.V.; Wood, S.A.; Atkinson, H.C.; Harbuz, M.S.; Lightman, S.L. Gonadal Steroid Replacement Reverses Gonadectomy-Induced Changes in the Corticosterone Pulse Profile and Stress-Induced Hypothalamic-Pituitary-Adrenal Axis Activity of Male and Female Rats. J. Neuroendocr. 2004, 16, 989–998. [Google Scholar] [CrossRef]
- Babb, J.A.; Masini, C.V.; Day, H.E.W.; Campeau, S. Stressor-Specific Effects of Sex on HPA Axis Hormones and Activation of Stress-Related Neurocircuitry. Stress 2013, 16, 664–677. [Google Scholar] [CrossRef] [PubMed]

| Summary of Immunohistochemical Quantification of Brain c-Fos Protein Expression in Male and Female Wistar Rats | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Brain Region | Gender | Control | Acute Noise Exposure | Chronic Noise Exposure | ||||||
| Experimental Group Means (± SEM) | ||||||||||
| 0 h | 2 h | 12 h | 7 days | 21 days | Factor | F | df | p | ||
| Forebrain | ||||||||||
| Caudate nucleus * | Male | 0.7 (0.08) | 72.47 (10.6) ᶲ | 28 (0.8) ᶲ | 32.93 (1.8) ᶲ | 31.43 (2.9) ᶲ | Sex | 43.11 | 4, 290 | 0.0001 |
| Female | 0.7 (0.08) | 53.47 (4.2) ˠ | 73.13 (1.8) ˠⱠ | 69.87 (2.6) ˠⱠ | 21.63 (4.7) ˠ | Time | 50.11 | 4, 290 | 0.0001 | |
| Interaction | 40.11 | 4, 290 | 0.0001 | |||||||
| Lateral septum * | Male | 1 (0.03) | 139.70 (15.3) ᶲⱠ | 61.03 (4.7) ᶲ | 58.53 (2.5) ᶲⱠ | 42.60 (7.0) ᶲ | Sex | 18.48 | 1, 290 | 0.0001 |
| Female | 1 (0.03) | 64.2 (4.4) ˠ | 95.30 (5.4) ˠⱠ | 19.63 (1.9) | 33.27 (8.0) ˠ | Time | 70.97 | 4, 290 | 0.0001 | |
| Interaction | 19.84 | 4, 290 | 0.0001 | |||||||
| Claustrum * | Male | 0.5 (0.09) | 79.40 (6.1) ᶲⱠ | 19.40 (1.6) ᶲ | 56.70 (1.7) ᶲⱠ | 46.23 (3.3) ᶲ | Sex | 0.3096 | 1, 290 | 0.5783 |
| Female | 0.5 (0.09) | 58.6 (3.4) ˠ | 71.93 (1.4) ˠⱠ | 29.43 (1.2) ˠ | 36.60 (4.0) ˠ | Time | 141.6 | 4, 290 | 0.0001 | |
| Interaction | 58.33 | 4, 290 | 0.0001 | |||||||
| Olfactory bulb * | Male | 1.1 (0.06) | 201 (26) ᶲⱠ | 74.97 (3.0) ᶲ | 107.90 (8.5) ᶲⱠ | 45.80 (2.7) ᶲ | Sex | 7.771 | 1, 290 | 0.0057 |
| Female | 1.1 (0.06) | 109.6 (13.4) ˠ | 154.67 (6.2) ˠⱠ | 51.60 (2.1) ˠ | 24.77 (4.2) ˠ | Time | 73.84 | 4, 290 | 0.0001 | |
| Interaction | 20.51 | 4, 290 | 0.0001 | |||||||
| Amygdala complex * | Male | 0.6 (0.08) | 52.27 (3.7) ᶲ | 35.50 (1.8) ᶲ | 44.13 (1.7) ᶲ | 36.40 (3.6) ᶲⱠ | Sex | 39.77 | 1, 290 | 0.0001 |
| Female | 0.6 (0.08) | 60.53 (3.5) ˠ | 55.77 (1.9) ˠⱠ | 77.87 (2.3) ˠⱠ | 23.40 (2.3) ˠ | Time | 195.4 | 4, 290 | 0.0001 | |
| Interaction | 26.64 | 4, 290 | 0.0001 | |||||||
| Habenular complex * | Male | 0.5 (0.09) | 35.27 (4.9) ᶲ | 15 (1.0) ᶲ | 21.60 (1.5) ᶲ | 14.23 (1.6) Ⱡ | Sex | 0.2757 | 1, 290 | 0.5999 |
| Female | 0.5 (0.09) | 28.4 (2) ˠ | 30.77 (1.3) ˠⱠ | 27.50 (1.0) ˠ | 2.60 (0.4) ˠ | Time | 86.58 | 4, 290 | 0.0001 | |
| Interaction | 15.26 | 4, 290 | 0.0001 | |||||||
| Hippocampus | ||||||||||
| Dentate gyrus * | Male | 0.2 (0.07) | 12.77 (1.2) ᶲ | 18.53 (0.8) ᶲ | 38.87 (1.0) ᶲⱠ | 42 (3.5) ᶲⱠ | Sex | 19.14 | 4, 290 | 0.0001 |
| Female | 0.2 (0.07) | 13.4 (0.6) ˠ | 28.60 (1.2) ˠⱠ | 31.60 (1.6) ˠ | 16.97 (1.7) ˠ | Time | 160.4 | 4, 290 | 0.0001 | |
| Interaction | 35.78 | 4, 290 | 0.0001 | |||||||
| CA3 * | Male | 0.2 (0.07) | 15.87 (0.8) ᶲⱠ | 09.57 (0.8) ᶲ | 22.17 (0.6) ᶲ | 27.20 (2.2) ᶲⱠ | Sex | 31.19 | 4, 290 | 0.0001 |
| Female | 0.2 (0.07) | 8.8 (0.6) ˠ | 19.93 (0.9) ˠⱠ | 16.53 (0.9) ˠ | 7.60 (2.4) ˠ | Time | 73.71 | 4, 290 | 0.0001 | |
| Interaction | 38.95 | 4, 290 | 0.0001 | |||||||
| CA2 | Male | 0.1 (0.06) | 17.8 (0.8) ᶲⱠ | 6.47 (0.4) ᶲ | 14.50 (0.4) ᶲⱠ | 5.70 (0.7) | Sex | 54.82 | 4, 290 | 0.0001 |
| Female | 0.1 (0.06) | 6.8 (0.6) ˠ | 11.23 (0.5) ˠⱠ | 6.87 (0.4) ˠ | 2.53 (0.4) | Time | 94.84 | 4, 290 | 0.0001 | |
| Interaction | 36.39 | 4, 290 | 0.0001 | |||||||
| CA1 * | Male | 0.1 (0.09) | 24.03 (4.4) ᶲⱠ | 2.23 (0.2) | 10.63 (0.5) ᶲ | 9.30 (1.04) ᶲⱠ | Sex | 6.362 | 1, 290 | 0.0001 |
| Female | 0.1 (0.09) | 4.5 (0.7) ˠ | 7.07 (0.7) | 6 (0.6) ˠ | 1.67 (0.2) ˠ | Time | 19.02 | 4, 290 | 0.0001 | |
| Interaction | 14.83 | 4, 290 | 0.0001 | |||||||
| Cortex | ||||||||||
| Prelimbic * | Male | 0.8 (0.6) | 113.9 (13.3) ᶲ | 20.17 (1.0) | 75.30 (5.3) ᶲ | 38.60 (5.6) ᶲ | Sex | 0.2748 | 1, 290 | 0.6005 |
| Female | 0.8 (0.6) | 88.8 (8.4) ˠ | 92.67 (1.7) | 20.47 (1.1) ˠ | 57.80 (13.5) ˠ | Time | 51.18 | 4, 290 | 0.0001 | |
| Interaction | 23.18 | 4, 290 | 0.0001 | |||||||
| Infralimbic * | Male | 0.7 (0.07) | 115.1 (11.7) ᶲ | 30.57 (1.3) ᶲ | 58.50 (5.1) ᶲ | 32.87 (4.5) ᶲ | Sex | 0.1482 | 1, 290 | 0.7005 |
| Female | 0.7 (0.07) | 81 (6.4) ˠ | 93.30 (2.0) ˠ | 18.57 (0.8) | 51.10 (9.4) ˠ | Time | 78.01 | 4, 290 | 0.0001 | |
| Interaction | 27.04 | 4, 290 | 0.0001 | |||||||
| Cingulate * | Male | 1.1 (0.05) | 154.3 (19.1) ᶲ | 41.57 (1.2) | 153.57 (6.6) ᶲⱠ | 92.90 (15.0) ᶲ | Sex | 58.64 | 1, 290 | 0.4445 |
| Female | 1.1 (0.05) | 132.7 (14.8) ˠ | 146.73 (5.1) ˠⱠ | 59.33 (2.8) ˠ | 76.13 (19.6) ˠ | Time | 42.87 | 4, 290 | 0.0001 | |
| Interaction | 20.00 | 4, 290 | 0.0001 | |||||||
| Insular * | Male | 0.5 (0.09) | 40.1 (2.7) ᶲ | 27.57 (1.0) ᶲ | 49.40 (2.5) ᶲⱠ | 18.83 (3.0) ᶲ | Sex | 5.034 | 1, 290 | 0.0256 |
| Female | 0.5 (0.09) | 41.6 (4.2) ˠ | 59.97 (3.0) ˠⱠ | 23.50 (0.9) ˠ | 30.47 (4.8) ˠ | Time | 81.16 | 4, 290 | 0.0001 | |
| Interaction | 29.07 | 4, 290 | 0.0001 | |||||||
| Piriform * | Male | 1 (0.1) | 159.5 (23.2) ᶲ | 90.10 (2.4) ᶲ | 82.13 (2.4) ᶲ | 64.63 (5.8) ᶲ | Sex | 2.027 | 1, 290 | 0.1556 |
| Female | 1 (0.1) | 139.3 (16.1) ˠ | 225 (5.5) ˠⱠ | 42.30 (1.1) | 32.53 (4.8) ˠ | Time | 101.4 | 4, 290 | 0.0001 | |
| Interaction | 28.90 | 4, 290 | 0.0001 | |||||||
| Temporal auditory * (secondary) | Male | 0.6 (0.08) | 122.33 (10) ᶲⱠ | 128.73 (5.5) ᶲ | 77.03 (4.0) ᶲ | 69.00 (9.9) ᶲⱠ | Sex | 0.2242 | 1, 290 | 0.6362 |
| Female | 0.6 (0.08) | 75.3 (6.9) ˠ | 153.67 (3.0) ˠ | 129.37 (6.1) ˠⱠ | 29.70 (4.2) ˠ | Time | 164.2 | 4, 290 | 0.0001 | |
| Interaction | 24.55 | 4, 290 | 0.0001 | |||||||
| Temporal auditory * (primary) | Male | 0.7 (0.08) | 129.8 (10.8) ᶲⱠ | 132.33 (4.7) ᶲⱠ | 61.73 (1.8) ᶲ | 61.83 (9.2) ᶲⱠ | Sex | 16.62 | 1, 290 | 0.0001 |
| Female | 0.7 (0.08) | 85.13 (6.2) ˠ | 88.33 (3.1) ˠ | 115.60 (5.8) ˠⱠ | 24.00 (3.6) | Time | 137.4 | 4, 290 | 0.0001 | |
| Interaction | 27.91 | 4, 290 | 0.0001 | |||||||
| Hypothalamus | ||||||||||
| Paraventricular nucleus * | Male | 1.2 (0.1) | 118.3 (14.7) ᶲ | 33.73 (1.3) ᶲ | 91.47 (2.4) ᶲ | 63.17 (5.4) ᶲⱠ | Sex | 0.7501 | 4, 290 | 0.3872 |
| Female | 1.2 (0.1) | 127.6 (10.9) ˠⱠ | 100.03 (2.2) ˠ | 63.67 (2.9) ˠ | 30.07 (5.6) ˠ | Time | 94.12 | 4, 290 | 0.0001 | |
| Interaction | 19.89 | 4, 290 | 0.0001 | |||||||
| Ventromedial nucleus * | Male | 0.5 (0.09) | 114.2 (14.6) ᶲⱠ | 22.67 (1.4) | 45.23 (1.5) ᶲ | 22.70 (3.0) | Sex | 1.198 | 1, 290 | 0.2745 |
| Female | 0.5 (0.09) | 78.4 (5.1) ˠ | 74.43 (2.9) ˠⱠ | 44.23 (2.3) ˠ | 27.13 (6.3) ˠ | Time | 80.88 | 4, 290 | 0.0001 | |
| Interaction | 15.74 | 4, 290 | 0.0001 | |||||||
| Thalamus | ||||||||||
| Paraventricular nucleus * | Male | 0.7 (0.1) | 104.2 (10.8) ᶲⱠ | 55.77 (2.8) ᶲ | 85.60 (5.5) ᶲ | 57.73 (6.5) ᶲ | Sex | 12.38 | 1, 140 | 0.0006 |
| Female | 0.7 (0.1) | 59 (5.2) ˠ | 71.67 (1.6) ˠ | 66.27 (5.9) ˠ | 44.27 (6.7) ˠ | Time | 67.75 | 4, 140 | 0.0001 | |
| Interaction | 8.409 | 4, 140 | 0.0001 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Quezada, D.; Luquín, S.; Ruvalcaba-Delgadillo, Y.; García-Estrada, J.; Jauregui-Huerta, F. Sex Differences in the Expression of c-fos in a Rat Brain after Exposure to Environmental Noise. Sustainability 2022, 14, 2798. https://doi.org/10.3390/su14052798
Fernández-Quezada D, Luquín S, Ruvalcaba-Delgadillo Y, García-Estrada J, Jauregui-Huerta F. Sex Differences in the Expression of c-fos in a Rat Brain after Exposure to Environmental Noise. Sustainability. 2022; 14(5):2798. https://doi.org/10.3390/su14052798
Chicago/Turabian StyleFernández-Quezada, David, Sonia Luquín, Yaveth Ruvalcaba-Delgadillo, Joaquin García-Estrada, and Fernando Jauregui-Huerta. 2022. "Sex Differences in the Expression of c-fos in a Rat Brain after Exposure to Environmental Noise" Sustainability 14, no. 5: 2798. https://doi.org/10.3390/su14052798
APA StyleFernández-Quezada, D., Luquín, S., Ruvalcaba-Delgadillo, Y., García-Estrada, J., & Jauregui-Huerta, F. (2022). Sex Differences in the Expression of c-fos in a Rat Brain after Exposure to Environmental Noise. Sustainability, 14(5), 2798. https://doi.org/10.3390/su14052798






