Influences of Silica Fume on Compressive Strength and Chemical Resistances of High Calcium Fly Ash-Based Alkali-Activated Mortar
Abstract
1. Introduction
2. Materials and Methodology
2.1. Materials
2.2. Mix Proportions of Alkali-Activated Mortars
2.3. Testing Details
2.3.1. Compressive Strengths
2.3.2. Sulfuric Acid Resistance
2.3.3. Sulfate Resistance
3. Results and Discussion
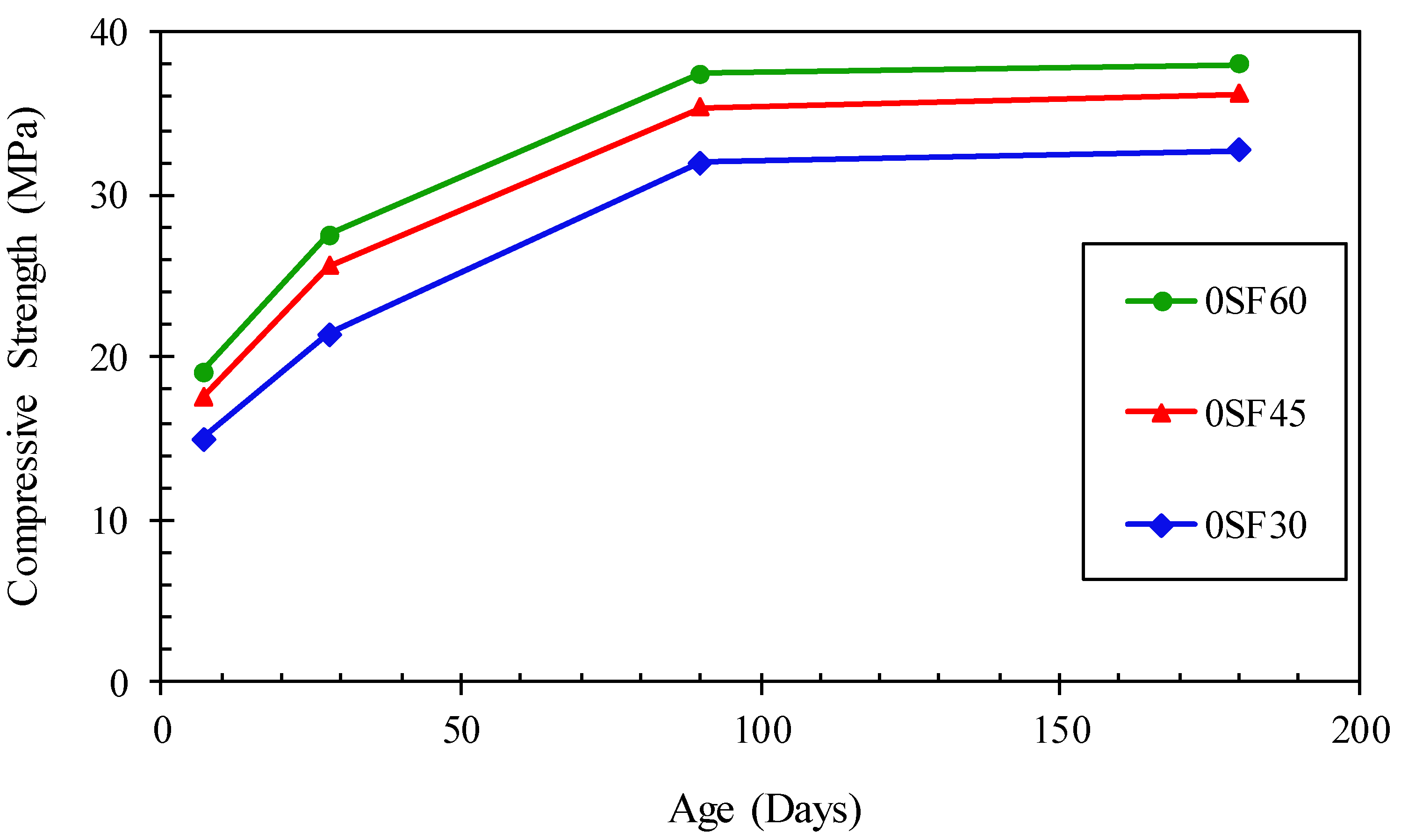
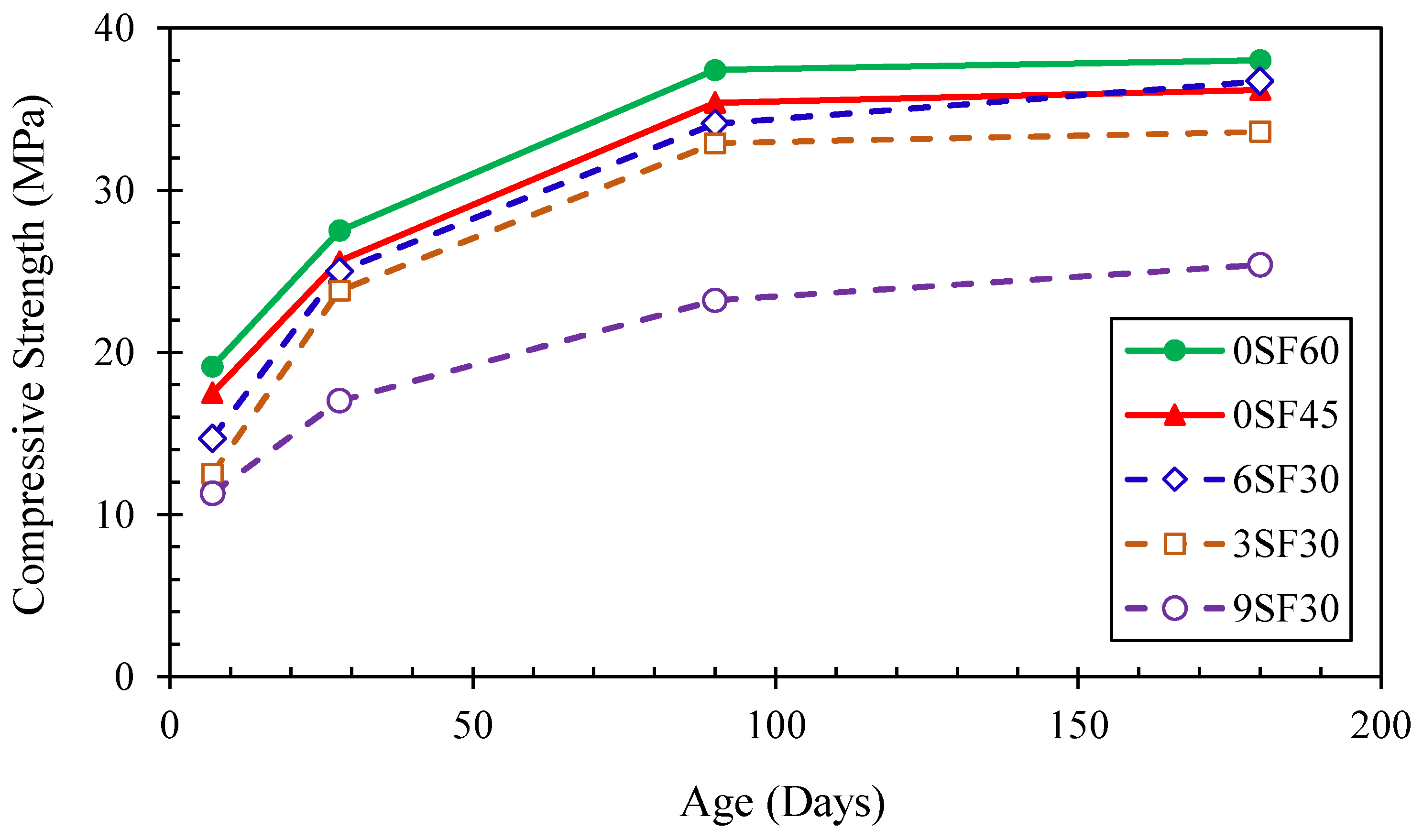
3.1. Compressive Strengths
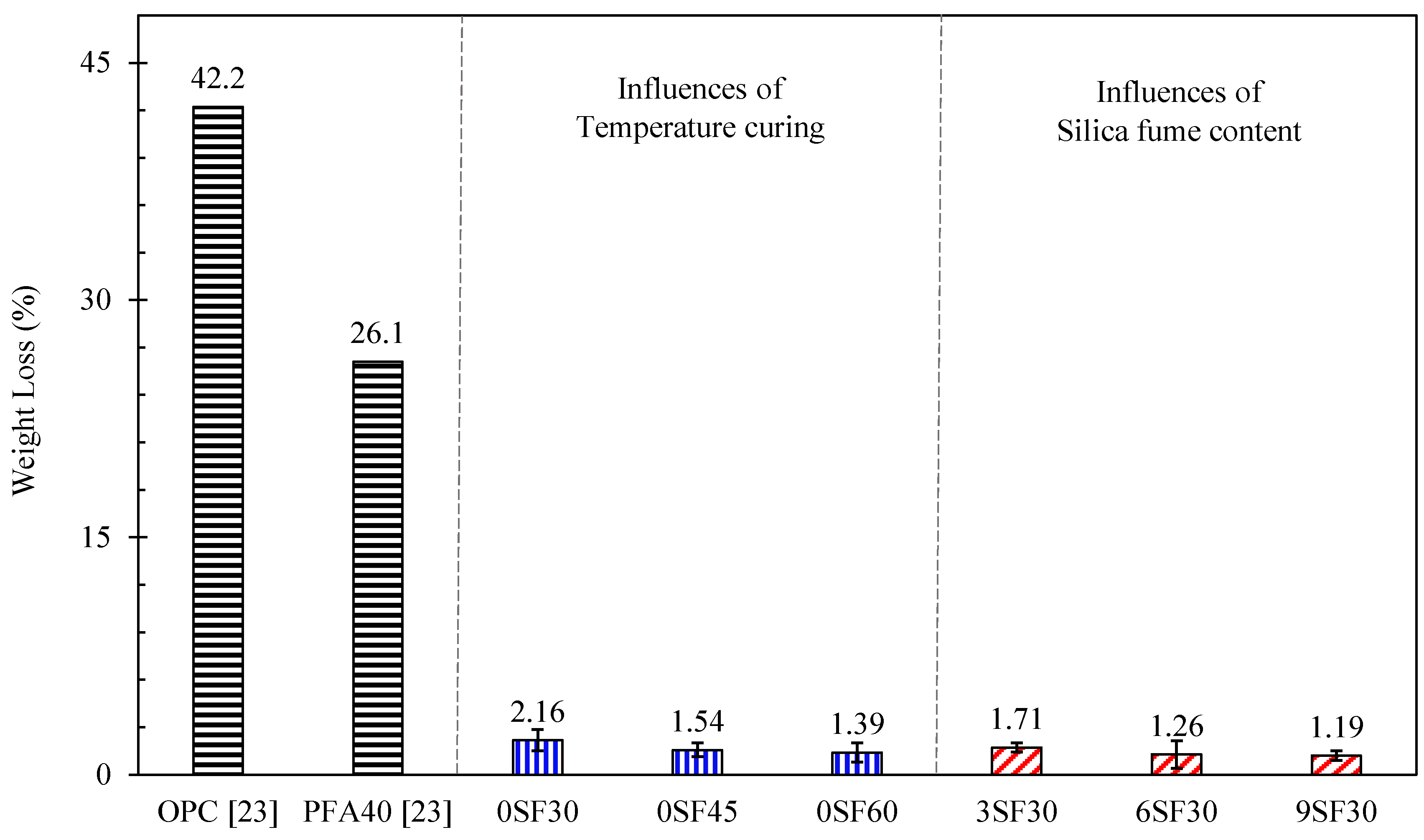
3.2. Resistance to Sulfuric Acid Attack
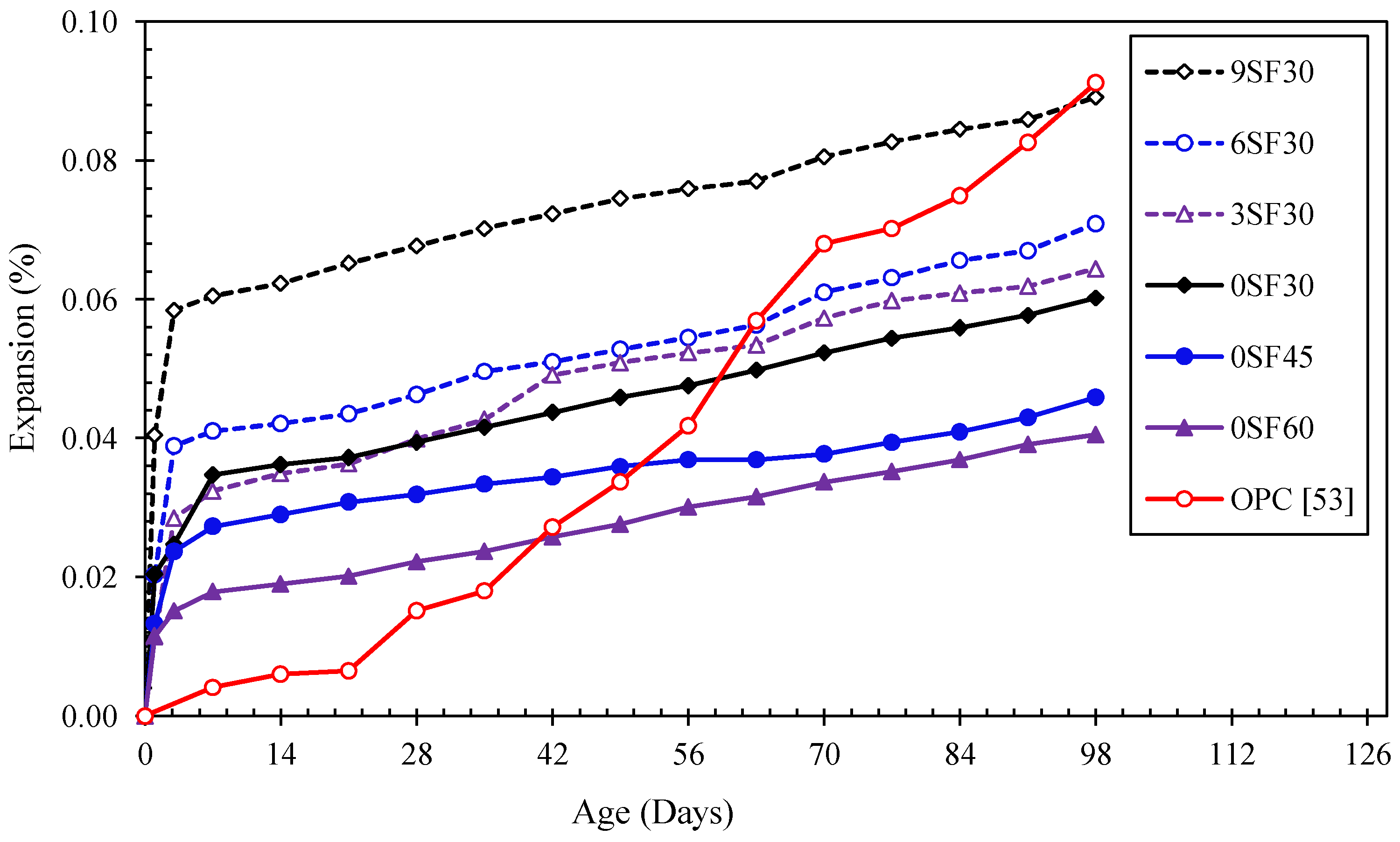
3.3. Resistance to Sulfate Attacks
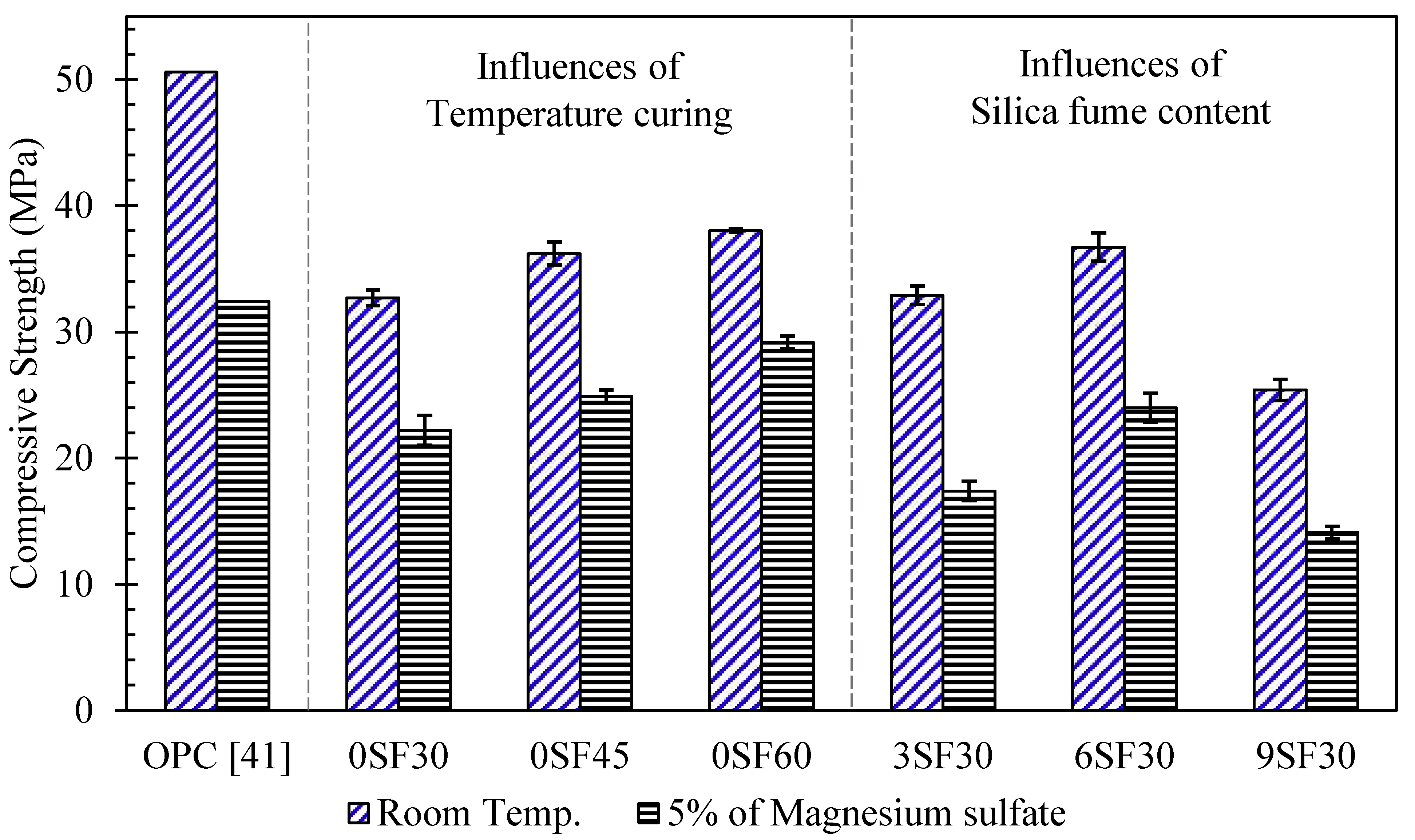
3.3.1. Magnesium Sulfate Resistance
3.3.2. Sodium Sulfate Resistance
4. Conclusions
- (1)
- The compressive strength of the alkali-activated mortar increased with extending the silica fume dosage. The optimum content of silica fume for use in alkali-activated mortars was 6%, which had a compressive strength as high as an alkali-activated mortar without silica fume and cured at room temperatures of 45 and 60 °C.
- (2)
- The weight loss of the alkali-activated mortar due to a sulfuric acid attack decreased with increasing silica fume content and curing temperature. All alkali-activated mortars lost 2.16% or less of their weight 28 days after the acid attack, which was much less than for OPC mortars and mortars containing 40% of FA.
- (3)
- The loss of compressive strength of the alkali-activated mortars immersed in magnesium sulfate solutions decreased with an increasing curing temperature.
- (4)
- The expansion of the alkali-activated mortar due to the sodium sulfate attack increased with an increasing silica fume content and decreased with an increasing curing temperature. Compared with OPC mortars, all alkali-activated mortars were more vulnerable to attack by 5% sodium sulfate at 1–35 days. After 98 days of immersion, all alkali-activated mortars had a better performance than the OPC mortars.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bosoaga, A.; Mašek, O.; Oakey, J.E. CO2 Capture Technologies for Cement Industry. Energy Procedia 2009, 1, 133–140. [Google Scholar] [CrossRef]
- Zhang, M.; Lastra, R.; Malhotra, V. Rice-husk ash paste and concrete: Some aspects of hydration and the microstructure of the interfacial zone between the aggregate and paste. Cem. Concr. Res. 1996, 26, 963–977. [Google Scholar] [CrossRef]
- Kiattikomol, K.; Jaturapitakkul, C.; Songpiriyakij, S.; Chutubtim, S. A study of ground coarse fly ashes with different finenesses from various sources as pozzolanic materials. Cem. Concr. Compos. 2001, 23, 335–343. [Google Scholar] [CrossRef]
- Sata, V.; Jaturapitakkul, C.; Rattanashotinunt, C. Compressive Strength and Heat Evolution of Concretes Containing Palm Oil Fuel Ash. J. Mater. Civ. Eng. 2010, 22, 1033–1038. [Google Scholar] [CrossRef]
- Wongkvanklom, A.; Posi, P.; Kampala, A.; Kaewngao, T.; Chindaprasirt, P. Beneficial utilization of recycled asphaltic concrete aggregate in high calcium fly ash geopolymer concrete. Case Stud. Constr. Mater. 2021, 15, e00615. [Google Scholar] [CrossRef]
- Ibrahim, K. Recycled waste glass powder as a partial replacement of cement in concrete containing silica fume and fly ash. Case Stud. Constr. Mater. 2021, 15, e00630. [Google Scholar] [CrossRef]
- Podolsky, Z.; Liu, J.; Dinh, H.; Doh, J.; Guerrieri, M.; Fragomeni, S. State of the art on the application of waste materials in geopolymer concrete. Case Stud. Constr. Mater. 2021, 15, e00637. [Google Scholar] [CrossRef]
- Palomo, A.; Grutzeck, M.W.; Blanco, M.T. Alkali-activated fly ashes: A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rattanasak, U.; Jaturapitakkul, C. Utilization of fly ash blends from pulverized coal and fluidized bed combustions in geopolymeric materials. Cem. Concr. Compos. 2011, 33, 55–60. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer: Inorganic polymeric new materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Bakharev, T. Thermal behaviour of geopolymers prepared using class F fly ash and elevated temperature curing. Cem. Concr. Res. 2006, 36, 1134–1147. [Google Scholar] [CrossRef]
- Kong, D.; Sanjayan, J. Damage behavior of geopolymer composites exposed to elevated temperatures. Cem. Concr. Compos. 2008, 30, 986–991. [Google Scholar] [CrossRef]
- Kong, D.L.; Sanjayan, J.G. Effect of elevated temperatures on geopolymer paste, mortar and concrete. Cem. Concr. Res. 2010, 40, 334–339. [Google Scholar] [CrossRef]
- Rickard, W.D.; van Riessen, A.; Walls, P. Thermal character of geopolymers synthesized from Class F fly ash containing high concentrations of iron and aquartz. Int. J. Appl. Ceram. Technol. 2010, 7, 81–88. [Google Scholar] [CrossRef]
- Tayeh, B.A.; Zeyad, A.M.; Agwa, I.S.; Amin, M. Effect of elevated temperatures on mechanical properties of lightweight geo-polymer concrete. Case Stud. Constr. Mater. 2021, 15, e00673. [Google Scholar]
- Mohapatra, A.K.; Bera, D.K.; Rath, A.K. Effect of Silica Fume on Strength Enhancement of Geo-Polymer Mortar in Ambient Curing. In Lecture Notes in Civil Engineering; Springer: Singapore, 2021; Volume 75, pp. 819–830. [Google Scholar]
- Janardhanan, T.; Venkatasubramani, V. Feasibility studies on compressive strength of ground coal ash geopolymer mortar. Period. Polytech. Civ. Eng. 2015, 59, 373. [Google Scholar]
- Mehta, P.K. Sulfate attack on concrete: A critical review. In Concrete Durability; Villarreal, R.R., Ed.; University Autonoma de Nuevo Leon: Leon, Mexico, 1993; pp. 107–132. [Google Scholar]
- Wakely, L.D.; Poole, T.S.; Ernzen, J.J.; Neeley, D.B. Salt saturated mass concrete under chemical attack. In High Performance Concrete in Severe Environments; Zia, P., Ed.; American Concrete Institute: Farmington Hills, MI, USA, 1993; Volume SP-140, pp. 239–267. [Google Scholar]
- Irassar, E.; Di Maio, A.; Batic, O. Sulfate attack on concrete with mineral admixtures. Cem. Concr. Res. 1996, 26, 113–123. [Google Scholar] [CrossRef]
- Ferraris, C.F.; Clifton, J.R.; Stutzman, P.E.; Garbocsi, E.J. Mechanisms of degradation of Portland cement-based systems by sul-fate attack. In Mechanisms of Chemical Degradation of Cement-Based Systems; Scrivener, K.L., Young, J.F., Eds.; E and FN Spon: London, UK, 1997; pp. 185–192. [Google Scholar]
- Santhanam, M.; Cohen, M.D.; Olek, J. Mechanism of sulfate attack: A fresh look: Part 2. Proposed mechanisms. Cem. Concr. Res. 2003, 33, 341–346. [Google Scholar] [CrossRef]
- Taylor, H.F.W.; Gollop, R.S. Some chemical and microstructural aspects of concrete durability. In Mechanisms of Chemical Degradation of Cement-Based Systems; Scrivener, K.L., Young, J.F., Eds.; E and FN Spon: London, UK, 1997; pp. 177–184. [Google Scholar]
- Bonen, D.; Cohen, M. Magnesium sulfate attack on Portland cement paste: I. Microstructural analysis. Cem. Concr. Res. 1992, 22, 169–180. [Google Scholar] [CrossRef]
- Bonen, D.; Cohen, M. Magnesium sulfate attack on Portland cement paste: II. Chemical and mineralogical analyses. Cem. Concr. Res. 1992, 22, 707–718. [Google Scholar] [CrossRef]
- Davidovits, J. Properties of geopolymer cements. In Proceedings of first International Conference on Alkaline Cements and Concretes; Kiev, Ukraine, 11–14 October 1994, SRIBM: Kiev, Ukraine, 1994; Volume 1, pp. 131–149. [Google Scholar]
- Temuujin, J.; Minjigmaa, A.; Lee, M.; Chen-Tan, N.; van Riessen, A. Characterisation of class F fly ash geopolymer pastes immersed in acid and alkaline solutions. Cem. Concr. Compos. 2011, 33, 1086–1091. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Chareerat, T.; Sirivivatnanon, V. Workability and strength of coarse high calcium fly ash geopolymer. Cem. Concr. Compos. 2007, 29, 224–229. [Google Scholar] [CrossRef]
- Bakharev, T. Durability of geopolymer materials in sodium and magnesium sulfate solutions. Cem. Concr. Res. 2005, 35, 1233–1246. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.; Van Deventer, J.; Lorenzen, L. The potential use of geopolymeric materials to immobilise toxic metals: Part, I. Theory and applications. Miner. Eng. 1997, 10, 659–669. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer, green chemistry and sustainable development solutions. In Proceedings of the World Congress Ge-opolymer 2005; Geopolymer Institute: Aisne, France, 2005. [Google Scholar]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Sata, V.; Sathonsaowaphak, A.; Chindaprasirt, P. Resistance of lignite bottom ash geopolymer mortar to sulfate and sulfuric acid attack. Cem. Concr. Compos. 2012, 34, 700–708. [Google Scholar] [CrossRef]
- Ariffin, M.; Bhutta, M.; Hussin, M.; Tahir, M.M.; Aziah, N. Sulfuric acid resistance of blended ash geopolymer concrete. Constr. Build. Mater. 2013, 43, 80–86. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Zhou, W.; Luo, W.; Shen, C. An investigation of the microstructure and durability of a fluidized bed fly ash–metakaolin geopolymer after heat and acid exposure. Mater. Des. 2015, 74, 125–137. [Google Scholar] [CrossRef]
- Pereira, A.; Akasaki, J.L.; Melges, J.L.P.; Tashima, M.M.; Soriano, L.; Borrachero, M.V.; Monzó, J.; Payá, J. Mechanical and durability properties of alkali-activated mortar based on sugarcane bagasse ash and blast furnace slag. Ceram. Int. 2015, 41, 13012–13024. [Google Scholar] [CrossRef]
- Rao, G.M.; Kireety, C.H. Durability Studies on Alkali Activated Fly Ash and GGBS-Based Geopolymer Mortars. In Sustainable Construction and Building Materials; (Lecture Notes in Civil Engineering); Das, B.B., Neithalath, N., Eds.; Springer: Singapore, 2019; Volume 25, pp. 85–97. [Google Scholar]
- Li, Z.; Peethamparan, S. Leaching resistance of alkali-activated slag and fly ash mortars exposed to organic acid. Green Mater. 2018, 6, 117–130. [Google Scholar] [CrossRef]
- ElYamany, H.E.; Elmoaty, A.E.M.A.; Diab, A.R.A. Sulphuric Acid Resistance of Slag Geopolymer Concrete Modified with Fly Ash and Silica Fume. Iran. J. Sci. Technol. Trans. Civ. Eng. 2020, 45, 2297–2315. [Google Scholar] [CrossRef]
- Allahverdi, A.; Skvara, F. Sulfuric acid attack on hardened paste of geopolymer cements-Part 1. Mechanism of corrosion at relatively high concentrations. Ceram. Silik. 2005, 49, 225. [Google Scholar]
- Allahverdi, A.; Skvara, F. Sulfuric acid attack on hardened paste of geopolymer cements-part 2. Corrosion mechanism at mild and relatively low concentrations. Ceram. Silik. 2006, 50, 1. [Google Scholar]
- Thokchom, S.; Ghosh, P.; Ghosh, S. Resistance of fly ash based geopolymer mortars in sulfuric acid. ArPN J. Eng. Appl. Sci. 2009, 4, 65–70. [Google Scholar]
- SWallah, E.; Rangan, B.V. Low-calcium fly ash-based geopolymer concrete: Long-term properties. In Res Rep.-GC2; Curtin University Australia: Bentley, Australia, 2006; pp. 76–80. [Google Scholar]
- Valencia-Saavedra, W.G.; de Gutiérrez, R.M.; Puertas, F. Performance of FA-based geopolymer concretes exposed to acetic and sulfuric acids. Constr. Build. Mater. 2020, 257, 119503. [Google Scholar] [CrossRef]
- American Society for Testing and Materials (ASTM) C230; Standard Specification for Flow Table for Use in Tests of Hydraulic Cement. ASTM International: West Conshohocken, PY, USA, 2014.
- American Society for Testing and Materials (ASTM) C109-16; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-In. Or [50 mm] Cube Specimens). ASTM International: West Conshohocken, PY, USA, 2016.
- American Society for Testing and Materials (ASTM) C267; Standard Test Methods for Chemical Resistance of Mortars, Grouts, and Monolithic Surfacings and Polymer Concretes. ASTM International: West Conshohocken, PY, USA, 2003.
- American Society for Testing and Materials (ASTM) C1012; Standard Test Method for Length Change of Hydraulic-Cement Mortars Exposed to a Sulfate Solution. ASTM International: West Conshohocken, PY, USA, 2018.
- Prabha, V.; Revathi, V. Geopolymer Mortar Incorporating High Calcium Fly Ash and Silica Fume. Arch. Civ. Eng. 2019, 65, 3–16. [Google Scholar] [CrossRef]
- Das, S.K.; Mustakim, S.M.; Adesina, A.; Mishra, J.; Alomayri, T.S.; Assaedi, H.S.; Kaze, C.R. Fresh, strength and microstructure properties of geopolymer concrete incorporating lime and silica fume as replacement of fly ash. J. Build. Eng. 2020, 32, 101780. [Google Scholar] [CrossRef]
- Aydın, S.; Yazici, H.; Yiğiter, H.; Baradan, B. Sulfuric acid resistance of high-volume fly ash concrete. Build. Environ. 2007, 42, 717–721. [Google Scholar] [CrossRef]
- Izquierdo, S.; Diaz, J.; Mejía De Gutiérrez, R.; Torres, J. Durability of FCC-blended mortar exposed to sodium and magnesium sulfate. Rev. Ing. Constr. 2016, 31, 183–190. [Google Scholar] [CrossRef][Green Version]
- Rodanan, A. Durability of Cement Mortar Containing Black Rice Husk Ash under Sodium Sulfate and Magnesium Sulfate Attack. Master’s Thesis, Thammasat University, Bangkok, Thailland, 2004. [Google Scholar]
- Chindaprasirt, P.; Paisitsrisawat, P.; Rattanasak, U. Strength and resistance to sulfate and sulfuric acid of ground fluidized bed combustion fly ash–silica fume alkali-activated composite. Adv. Powder Technol. 2014, 25, 1087–1093. [Google Scholar] [CrossRef]
- Clark, B.A.; Brown, P.W. Formation of Ettringite from Monosubstituted Calcium Sulfoaluminate Hydrate and Gypsum. J. Am. Ceram. Soc. 2004, 82, 2900–2905. [Google Scholar] [CrossRef]

| Materials | Specific Gravity | Retained in a No. 325 Sieve (%) |
|---|---|---|
| Fly Ash | 2.26 | 28.70 |
| Silica Fume | 2.32 | 1.46 |
| Chemical Composition (%) | Fly Ash | Silica Fume |
|---|---|---|
| Silicon dioxide (SiO2) | 27.9 | 98.2 |
| Aluminum oxide (Al2O3) | 14.4 | - |
| Ferric oxide (Fe2O3) | 15.6 | - |
| Calcium oxide (CaO) | 27.9 | - |
| Magnesium oxide (MgO) | 2.2 | 0.7 |
| Sodium oxide (Na2O) | 1.9 | 0.5 |
| Potassium oxide (K2O) | 2.8 | 0.6 |
| Sulfur oxide (SO3) | 7.1 | - |
| Loss on ignition (LOI) | 0.2 | - |
| Sample | Mix Proportion by Weight | SP (%) | Flow (%) | ||||
|---|---|---|---|---|---|---|---|
| FA | SF | Sand | NaOH | Na2SiO3 | |||
| 0SF30 | 100 | - | 275 | 40 | 20 | 6 | 108 |
| 3SF30 | 97 | 3 | 275 | 40 | 20 | 7 | 110 |
| 6SF30 | 94 | 6 | 275 | 40 | 20 | 8 | 113 |
| 9SF30 | 91 | 9 | 275 | 40 | 20 | 9 | 114 |
| 0SF45 | 100 | - | 275 | 40 | 20 | 6 | 108 |
| 0SF60 | 100 | - | 275 | 40 | 20 | 6 | 108 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sothornchaiwit, K.; Dokduea, W.; Tangchirapat, W.; Keawsawasvong, S.; Thongchom, C.; Jaturapitakkul, C. Influences of Silica Fume on Compressive Strength and Chemical Resistances of High Calcium Fly Ash-Based Alkali-Activated Mortar. Sustainability 2022, 14, 2652. https://doi.org/10.3390/su14052652
Sothornchaiwit K, Dokduea W, Tangchirapat W, Keawsawasvong S, Thongchom C, Jaturapitakkul C. Influences of Silica Fume on Compressive Strength and Chemical Resistances of High Calcium Fly Ash-Based Alkali-Activated Mortar. Sustainability. 2022; 14(5):2652. https://doi.org/10.3390/su14052652
Chicago/Turabian StyleSothornchaiwit, Kantiya, Warayut Dokduea, Weerachart Tangchirapat, Suraparb Keawsawasvong, Chanachai Thongchom, and Chai Jaturapitakkul. 2022. "Influences of Silica Fume on Compressive Strength and Chemical Resistances of High Calcium Fly Ash-Based Alkali-Activated Mortar" Sustainability 14, no. 5: 2652. https://doi.org/10.3390/su14052652
APA StyleSothornchaiwit, K., Dokduea, W., Tangchirapat, W., Keawsawasvong, S., Thongchom, C., & Jaturapitakkul, C. (2022). Influences of Silica Fume on Compressive Strength and Chemical Resistances of High Calcium Fly Ash-Based Alkali-Activated Mortar. Sustainability, 14(5), 2652. https://doi.org/10.3390/su14052652








