Decomposition Behavior of Biodegradable and Single-Use Tableware Items in the Warnow Estuary (Baltic Sea)
Abstract
1. Introduction
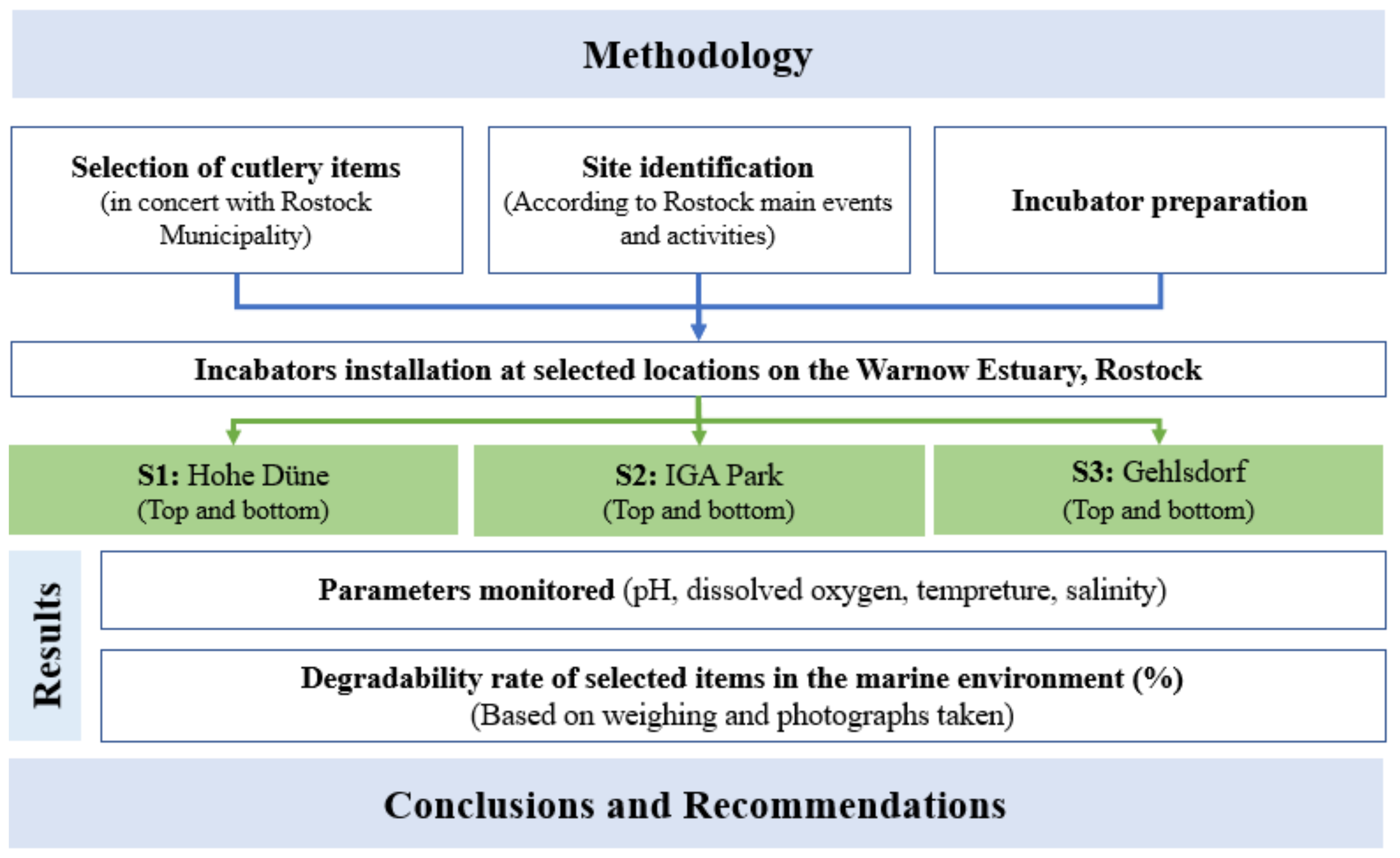
2. Study Site and Methods
2.1. Methods
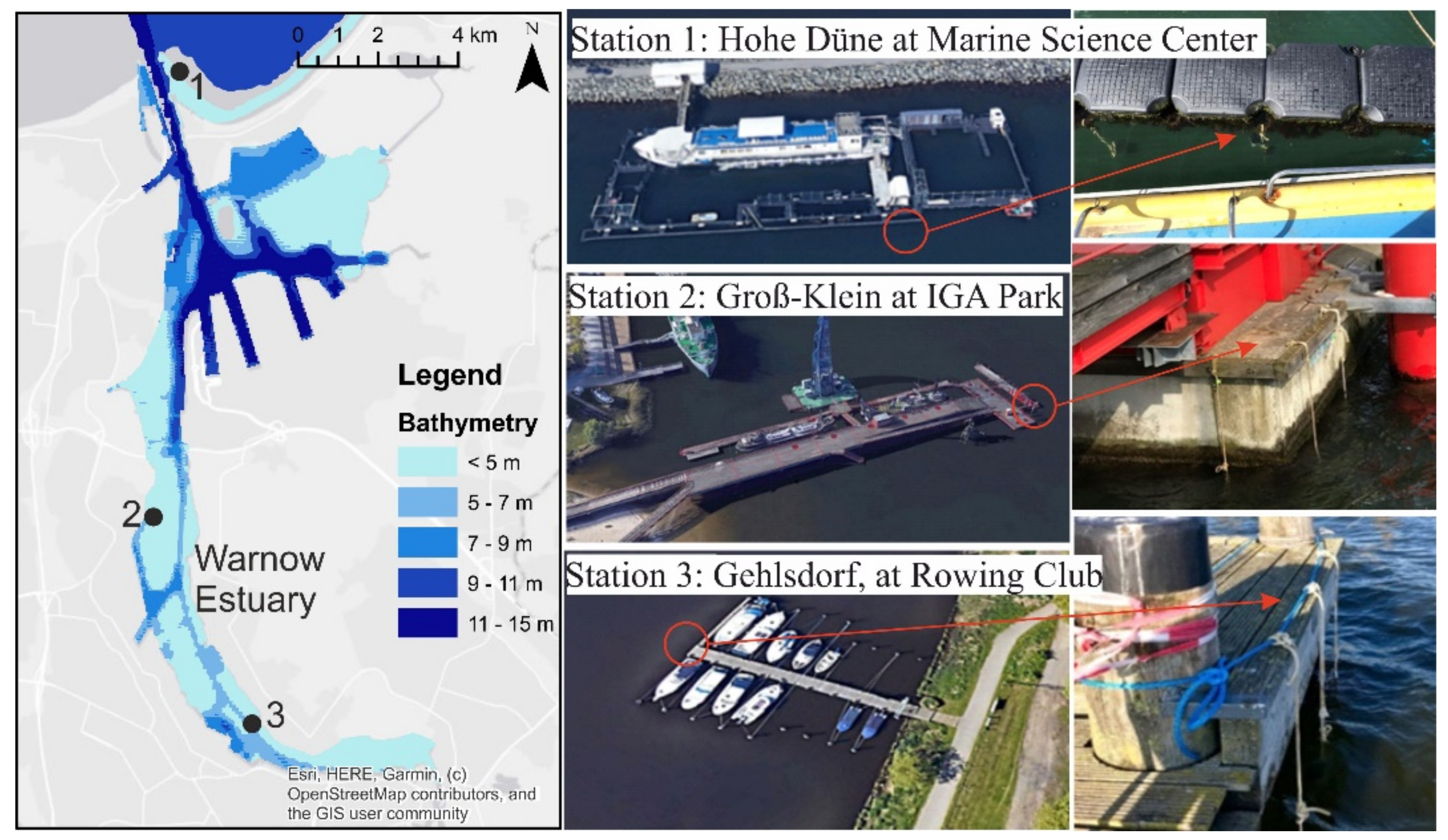
2.2. Study Site
2.3. Incubators Preparation
2.4. Incubators Installation
2.5. Used Tableware Items
- -
- The plates and bowls were made from discarded and pressed palm leaf from the ‘‘Adaka’’ nut palm. Depending on the source of the palm leaf used, its density is between 0.7–1.55 g/cm³ [36].
- -
- The sugar cane bagasse products used in these experiments are obtained from extracting sugar from the cane’s marrow leaf ‘‘bio-mass’’ (fiber), which is usually burned. The density of sugar cane is 1.20 g/m³ [37].
2.6. Parameter Monitoring Tools
3. Results
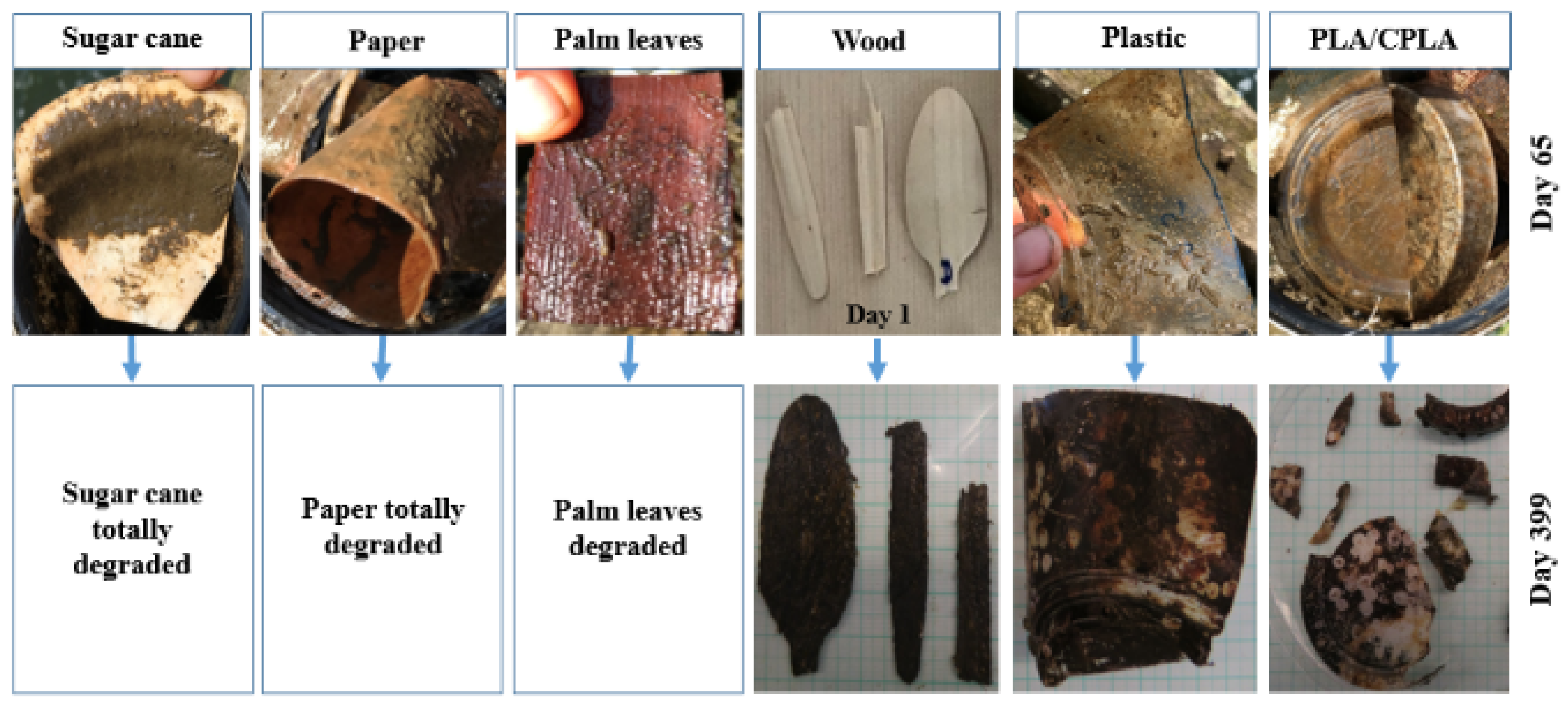
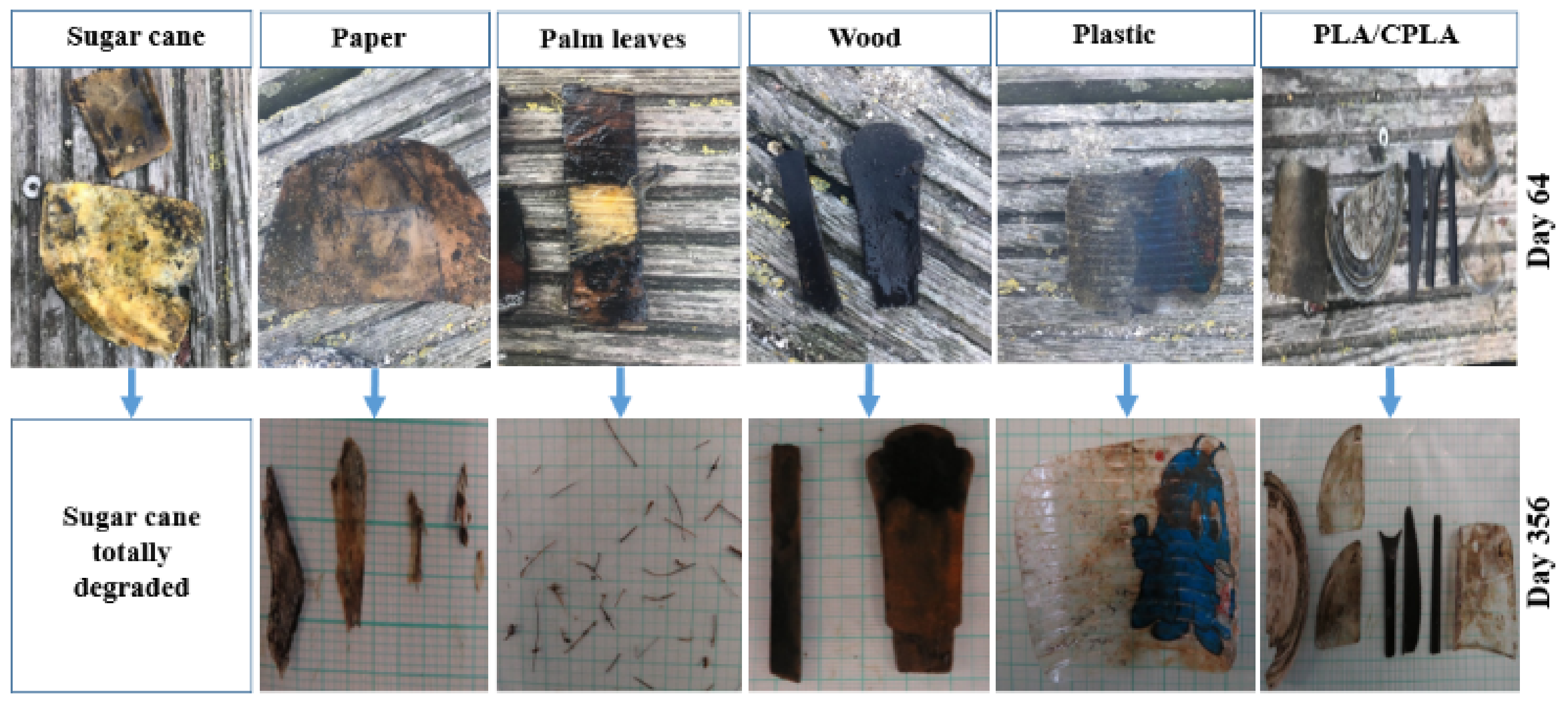
3.1. Degradation Rate Monitoring
3.2. Parameters and DR Monitoring
4. Discussion
4.1. Final Morphology of Tableware Items
4.2. Influence of Temperature on DR
5. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galgani, F.; Hanke, G.; Maes, T. Global distribution, composition and abundance of marine litter. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Berlin, Germany, 2015; pp. 29–56. [Google Scholar]
- Ryan, P.G. A brief history of marine litter research. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Berlin, Germany, 2015; pp. 1–25. [Google Scholar]
- Thompson, R.C. Microplastics in the marine environment: Sources, consequences and solutions. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Berlin, Germany, 2015; pp. 185–200. [Google Scholar]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Kühn, S.; Bravo Rebolledo, E.L.; van Franeker, J.A. Deleterious effects of litter on marine life. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Berlin, Germany, 2015; pp. 75–116. [Google Scholar]
- Veiga, J.M.; Fleet, D.; Kinsey, S.; Nilsson, P.; Vlachogianni, T.; Werner, S.; Galgani, F.; Thompson, R.C.; Dagevos, J.; Gago, J.; et al. Identifying Sources of Marine Litter. In MSFD GES TG Marine Litter Thematic Report; JRC Technical Report; European Union: Luxembourg, 2016. [Google Scholar] [CrossRef]
- Morritt, D.; Stefanoudis, P.V.; Pearce, D.; Crimmen, O.A.; Clark, P.F. Plastic in the Thames: A river runs through it. Mar. Pollut. Bull. 2014, 78, 196–200. [Google Scholar] [CrossRef]
- Free, C.M.; Jensen, O.P.; Mason, S.A.; Eriksen, M.; Williamson, N.J.; Boldgiv, B. High-levels of microplastic pollution in a large, remote, mountain lake. Mar. Pollut. Bull. 2014, 85, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Hoellein, T.; Rogas, M.; Pink, K.; Gasior, J.; Kelly, J. Anthropogenic litter in urban freshwater ecosystems: Distribution and microbial interactions. PLoS ONE 2014, 9, e98485. [Google Scholar] [CrossRef] [PubMed]
- Teuten, E.; Rowland, S.; Galloway, T.; Thompson, R. Potential for plastics to transport hydrophobic contaminants. J. Environ. Sci. Technol. 2007, 41, 7759–7764. [Google Scholar] [CrossRef] [PubMed]
- Mato, Y.; Isobe, T.; Takada, H.; Kanehiro, H.; Ohtake, C. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. J. Environ. Sci. Technol. 2001, 35, 318–324. [Google Scholar] [CrossRef]
- HELCOM. State of the Baltic Sea/Marine Litter 2018. Available online: http://stateofthebalticsea.helcom.fi/pressures-and-their-status/marine-litter/ (accessed on 15 August 2021).
- Schernewski, G.; Radtke, H.; Robbe, E.; Haseler, M.; Hauk, R.; Meyer, L.; Piehl, S.; Riedel, J.; Labrenz, M. Emission, Transport, and Deposition of visible Plastics in an Estuary and the Baltic Sea—A Monitoring and Modeling Approach. J. Environ. Manag. 2021, 68, 860–881. [Google Scholar] [CrossRef]
- Official Journal of the European Union. DIRECTIVE (EU) 2019/904 of the European Parliament and of the Council on the Reduction of the Impact of Certain Plastic Products on the Environment, L 155/2. 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019L0904 (accessed on 14 January 2022).
- Ross, P.C. Plastic in the Environment 2019. Available online: https://www.researchgate.net/publication/335338250_Plastics_in_the_Environment (accessed on 22 August 2021).
- Rochman, C.; Browne, M.; Halpern, B.; Hentschel, B.; Hoh, E. Classify plastic waste as hazardous. Nature 2013, 494, 169–171. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Besseling, E.; Foekema, E.M. Leaching of plastic additives to marine organisms. Environ. Pollut. 2014, 187, 49–54. [Google Scholar] [CrossRef]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemicals from plastics to the environment and the wildlife. Phil. Trans. R. Soc. B 2009, 364, 2027–2045. [Google Scholar] [CrossRef]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environ. Pollut. 2014, 185, 16–23. [Google Scholar] [CrossRef]
- European Bioplastics. FACT SHEET: Bioplastics—Industry Standards and Labels. 2019. Available online: https://www.european-bioplastics.org/new-market-data-2019-bioplastics-industry-shows-dynamic-growth/ (accessed on 30 January 2021).
- Kržan, A. Biodegradable Polymers and Plastics 2012. Available online: https://docplayer.net/21617366-Biodegradable-polymers-and-plastics-andrej-krzan.html (accessed on 22 August 2021).
- ADEME. Biodegradable Plastics. European Standard EN NF 13432 Relating to the Requirements Relating to Packaging Recoverable by Composting and Biodegradation. ADEME Technical Sheets. 2012. Available online: https://librairie.ademe.fr/dechets-economie-circulaire/3064-plastiques-biodegradables.html (accessed on 27 July 2021).
- Tadahisa, I. Biodegradable and Bio-Based Polymers: Future Prospects of Eco-Friendly Plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef]
- Mudgal, S.; Muehmel, K.; Hoa, E.; Grémont, M.; Labouze, E. Options to Improve the Biodegradability Requirements in the Packaging Directive; Final Report; Prepared for DG Environment–European Commission; BIO Intelligence Service: Paris, France, 2012. [Google Scholar]
- Tokiwa, Y.; Calabia, B.P. Biodegradability and Biodegradation of Polyesters. J. Polym. Environ. 2007, 15, 259–267. [Google Scholar] [CrossRef]
- Song, J.H.; Murphy, R.J.; Narayan, R.; Davies, G.B.H. Biodegradable and compostable alternatives to conventional plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2127–2139. [Google Scholar] [CrossRef]
- UNEP. Biodegradable Plastics and Marine Litter. Misconceptions, Concerns and Impacts on Marine Environments. United Nations Environment Programme (UNEP), Nairobi. 2015. Available online: https://wedocs.unep.org/handle/20.500.11822/7468?show=full (accessed on 22 August 2021).
- Lange, X. Numerical Simulations of Estuarine Circulation in a Non-Tidal Estuary. Master’s Thesis, Dissertation, Leibniz Institute for Baltic Sea Research Warnemünde Partner Institute, Mathematical-Scientific Faculty, University of Rostock, Rostock, Germany, 2015. [Google Scholar]
- Oberbeckmann, S.; Kreikemeyer, B.; Labrenz, M. Environmental Factors Support the Formation of Specific Bacterial Assemblages on Microplastics. Front. Microbiol. 2018, 8, 2709. [Google Scholar] [CrossRef]
- Drumright, R.E.; Gruber, P.R.; Henton, D.E. Polylactic acid technology. J. Adv. Mater. 2009, 12, 841–1846. [Google Scholar] [CrossRef]
- Fukushima, K.; Sogo, K.; Miura, S.; Kimura, Y. Production of D-lactic acid by bacterial fermentation of rice starch. Macromol. Biosci. 2004, 4, 1021–1027. [Google Scholar] [CrossRef]
- Timbuktu, W.; Sriroth, K.; Tokiwa, Y. Lactic acid production from sugar-cane juice by a newly isolated Lactobacillus sp. Biotechnol. Lett. 2006, 28, 811–814. [Google Scholar]
- Alaerts, L.; Augustinus, M.; Van Acker, K. Impact of Bio-Based Plastics on Current Recycling of Plastics. Sustainability 2018, 10, 1487. [Google Scholar] [CrossRef]
- Ohkita, T.; Lee, S.H. Thermal degradation and biodegradability of poly (lactic acid)/corn starch biocomposites. J. Appl. Polym. Sci. 2006, 100, 3009–3017. [Google Scholar] [CrossRef]
- Popa, M.E.; Mitelut, A.; Niculita, P.; Geicu, M.; Ghidurus, M.; Turtoi, M. Biodegradable Materials for Food Packaging Applications. J. Environ. Prot. Ecol. 2011, 12, 1825–1834. [Google Scholar]
- Pradeep, P.; Edwin Raja Dhas, J. Characterization of chemical and physical properties of palm fibers. MSEJ 2015, 2, 01–06. [Google Scholar] [CrossRef]
- Wahid, M.K.; Ahmad, M.N.; Osman, M.H.; Maidin, N.A.; Rahman, M.H.A.; Firdaus, H.M.S.; Kasno, M.A. Development of Biodegradable Plastics for Packaging using wastes from oil palm and sugar cane. IJRTE 2019, 8. [Google Scholar]
- Sonntag, R.E.; Borgnakke, C.; Van Wylen, G.J. Fundamentals of Thermodynamics, 5th ed.; Wiley & Sons Inc.: Hoboken, NJ, USA, 1998; p. 649. [Google Scholar]
- Beer, F.P.; Johnston, E.R. Mechanics of Materials. In J Scientific Research, 2nd ed.; McGraw-Hill: New York, NY, USA, 1992. [Google Scholar]
- Dobrowolska, E.; Wroniszewska, P.; Jankowska, A. Density Distribution in Wood of European Birch (Betula pendula Roth.). Forests 2020, 11, 445. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology. 2020. Available online: https://www.nist.gov/ (accessed on 17 July 2021).
- Tokiwa, Y.; Ugwu, U.S.; Calabia, B.P. Biodegradability of Plastics. IJMS 2009, 10, 3722–3742. [Google Scholar] [CrossRef] [PubMed]
- Carlton, J.T. The Light and Smith Manual, Intertidal Invertebrates from Central California to Oregon, 4th ed.; University of California Press: Berkeley, CA, USA, 2007. [Google Scholar] [CrossRef][Green Version]
- Rutkowska, M.; Heimowska, A.; Krasowska, K.; Janik, H. Biodegradability of polyethylene starch blends in sea water. Pol. J. Environ. Stud. 2002, 11, 267–274. [Google Scholar]
- Rutkowska, M.; Krasowska, K.; Heimowska, A.; Steinka, L.; Janik, H.; Haponiuk, J.; Karlsson, S. Biodegradation of modified poly(ε-caprolactone) in different environments. Pol. J. Environ. Stud. 2002, 11, 413–420. [Google Scholar]
- Rutkowska, M.; Jastrzebska, M.; Janik, H. Biodegradation of polycaprolactone in sea water. React. Funct. Polym. 1998, 38, 27–30. [Google Scholar] [CrossRef]
- Law, K.L.; Morét-Ferguson, S.E.; Goodwin, D.S.; Zettler, E.R.; DeForce, E.; Kukulka, T.; Proskurowski, G. Distribution of Surface Plastic Debris in the Eastern Pacific Ocean from an 11-Year Data Set. J. Environ. Sci. Technol. 2014, 48, 4732–4738. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Huang, D.; Hu, Z.D.; Liu, T.Y.; Lu, B.; Zhen, Z.C.; Wang, G.X.; Ji, J.H. Seawater degradation of PLA accelerated by water-soluble PVA. e-Polymers 2020, 20, 759–772. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Laforsch, C.; Greiner, A.; Agarwal, S. Fate of so-called biodegradable polymers and freshwater. Glob. Chall. 2017, 1, 1700048. [Google Scholar] [CrossRef]
- Ter Halle, A.; Ladirat, L.; Martignac, M.; Mingotaud, A.F.; Boyron, O.; Perez, E. To what extent are microplastics from the open ocean weathered? J. Environ. Pollut. 2017, 227, 167–174. [Google Scholar] [CrossRef]
- Chubarenko, I.P.; Esiukova, E.E.; Bagaev, A.V.; Bagaeva, M.A.; Grave, A.N. Three-dimensional distribution of anthropogenic microparticles in the body of sandy beaches. Sci. Total Environ. 2018, 628, 1340–1351. [Google Scholar] [CrossRef]




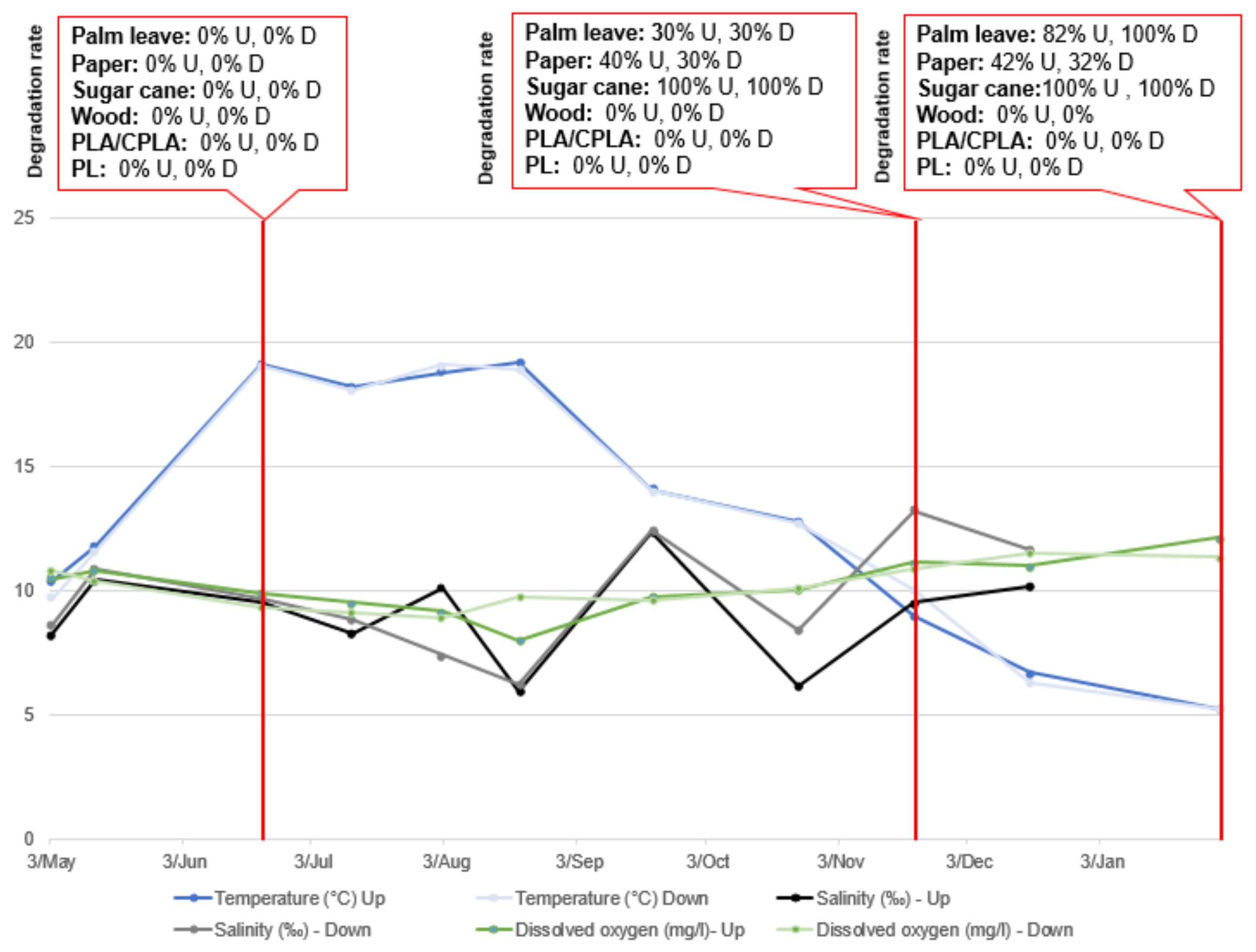
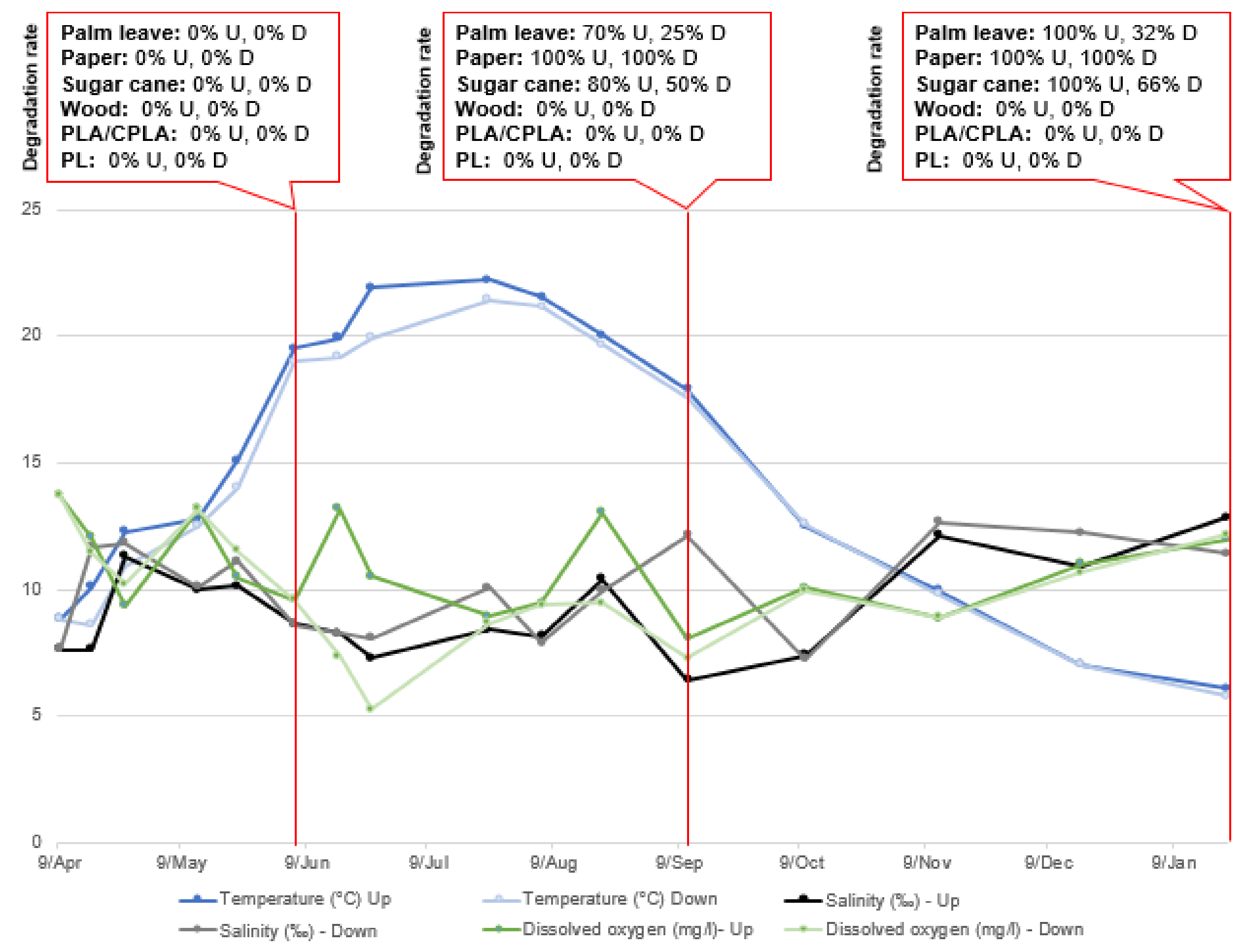
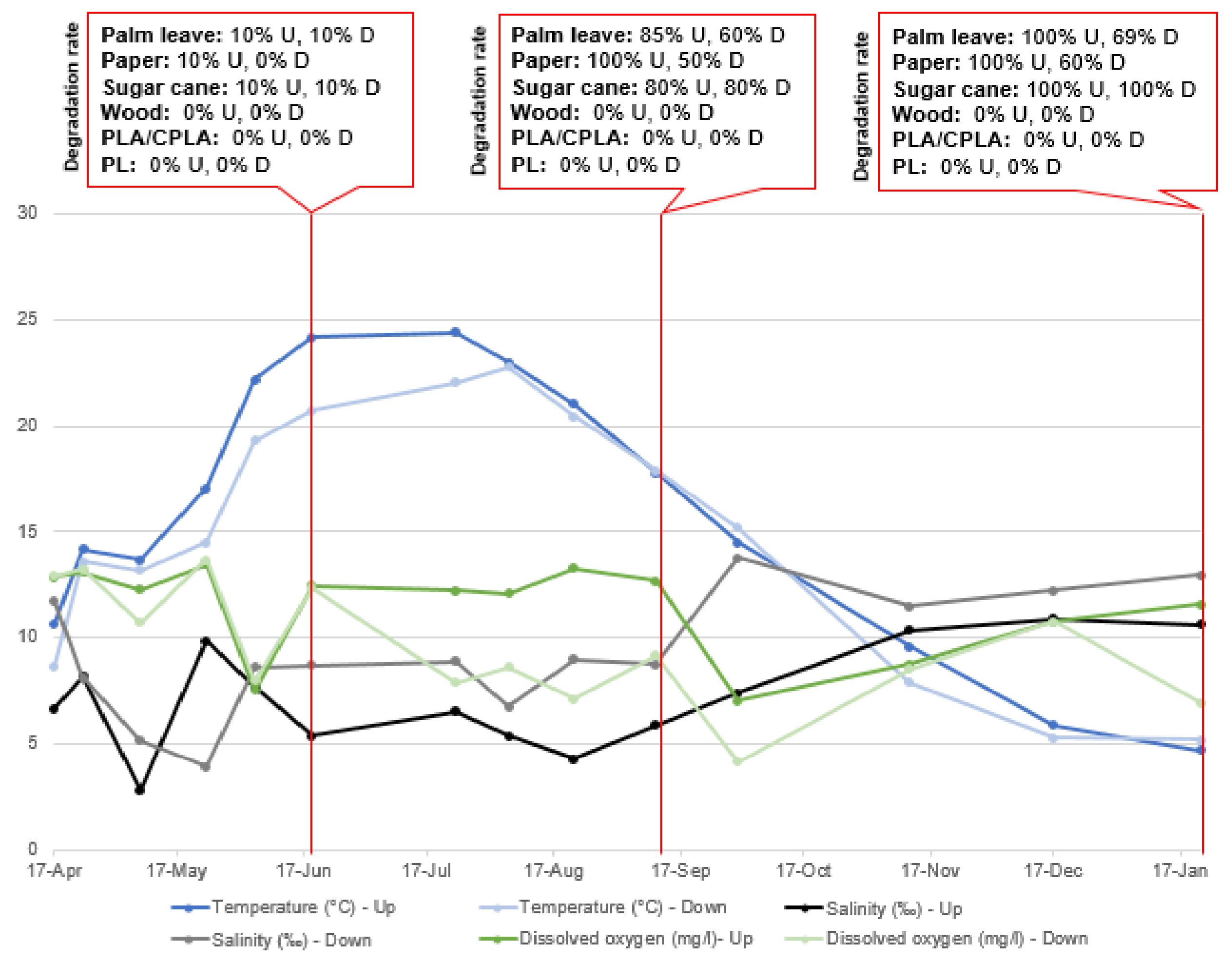
| Incubators and Content | Initial Weights of Samples | Final Total Weight | Degradation Rate (DR) (%) | Final Status of the Item |
|---|---|---|---|---|
| HD.1.1.U Paper | Plate (1.6 g), small cup (0.3 g) | 1.1 g (−0.8 g) | 42.1% | Soft to the touch |
| HD.1.2.U Plastics | Cup (1 g), box (2.1 g) | 4.3 g (+1.2 g) | 0% | Not degraded |
| HD.1.3.D Paper | Plate (1.8 g), small cup (0.4 g) | 1.5 g (−0.7 g) | 31.8% | Soft to the touch |
| HD.1.4.D Plastics | Cup (1 g), box (1.8 g) | 3.5 g (+0.7 g) | 0% | Not degraded |
| HD.2.1.U Wood | Spoon (2.5 g), fork (2 g) | 4.8 g (+0.3 g) | 0% | Soft to the touch |
| HD.2.2.U Palm leaf | Plate (3.3 g) | 0.6 g (−2.7 g) | 81.8% | Small fibers |
| HD.2.3.D Wood | Spoon (1.6 g), fork (2 g) | 5.8 g (+2.2 g) | 0% | Soft |
| HD.2.4.D Palm leaf | Plate (2.9 g) | 0 g (−2.9 g) | 100% | Totally degraded |
| HD.3.1.U PLA/CPLA | PLA (lid 0.9 g, cup 1.2 g)/CPLA (lid 1.9 g, spoon 2.4 g) | 6.6 g (+0.2 g) | 0% | Not degraded |
| HD.3.2.U Sugar cane | Bleached (1.1 g)/unbleached (1.2 g) | 0 g (−2.3 g) | 100% | Totally degraded |
| HD.3.3.D PLA/CPLA | PLA (lid 1 g, cup 1.1 g)/CPLA (lid 1.7 g, spoon 2 g) | 6 g (+0.2 g) | 0% | Not degraded |
| HD.3.4.D Sugar cane | Bleached (1 g)/unbleached (1.3 g) | 0 g (−2.3 g) | 100% | Totally degraded |
| Incubators and Content | Initial Weights of Samples | Final Total Weight | Degradation Rate (%) | Final Status of the Item |
|---|---|---|---|---|
| I.1.1.U Plastics/CPLA | Cup (0.6 g), box (1.5 g), knife (1.6 g) | 4 g (+0.3 g) | 0% | Not degraded |
| I.1.2.U Sugar cane | Bleached (2.8 g)/unbleached (1.3 g) | 0 g (−4.1 g) | 100% | Totally degraded |
| I.1.3.D Plastics/CPLA | Cup (0.6 g), box (1.5 g), knife (1.8 g) | 4.5 g (+0.6 g) | 0% | Not degraded |
| I.1.4.D Sugar cane | Bleached (2.8 g)/unbleached (1.3 g) | 1.4 g (−2.7 g) | 65.8% | Soft to the touch |
| I.2.1.U Wood | Tableware (1.3 g)/(2.2 g) | 7.1 g (+3.6 g) | 0% | Soft to the touch |
| I.2.2.U Palm leaf | Rectangular plate (1.5 g) | 0 g (−1.5 g) | 100% | Totally degraded |
| I.2.3.D Wood | Tableware (1.8 g)/(1.7 g) | 6.8 g (+3.3 g) | 0% | Soft to the touch |
| I.2.4.D Palm leaf | Plate (1.6 g) | 1.1 g (−0.5 g) | 31.2% | Small fibers |
| I.3.1.U Paper | Plate (1.7 g), cup (2 g) | 0 g (−3.7 g) | 100% | Totally degraded |
| I.3.2.U PLA/CPLA | PLA (cup 2.5 g)/CPLA (knife 2.3 g, lid 1.7 g) | 18.2 g (+11.7 g) | 0% | Not degraded |
| I.3.3.D Paper | Plate (1.7 g), cup (1.9 g) | 0 g (−3.6 g) | 100% | Soft to the touch |
| I.3.4.D PLA/CPLA | PLA (cup 2.5 g)/CPLA (knife 2.3 g, lid 1.7 g) | 9.1 g (+2.6 g) | 0% | Not degraded |
| Incubators and Content | Initial Weights of Samples | Final Total Weight | Degradation Rate (%) | Final Status of the Item |
|---|---|---|---|---|
| G.1.1.U Sugar cane | Bleached (1.6 g)/unbleached (1.9 g) | 0 g (−3.5 g) | 100% | Totally degraded |
| G.1.2.U PLA/CPLA | PLA (cup 1 g, box 1.8 g)/CPLA (lid 1.5 g, spoon 3.1 g, knife 2.5 g) | 11.6 g (+1++6.7 g) | 0% | Not degraded |
| G.1.3.D Sugar cane | Bleached (1.6 g), unbleached (1.8 g) | 0 g (−3.4 g) | 100% | Totally degraded |
| G.1.4.D PLA/CPLA | PLA (cup 1 g, box 1.8 g)/CPLA (lid 1.6 g, spoon 2.7 g, knife 1.9 g) | 10.6 g (+1.6 g) | 0% | Not degraded |
| G.2.1.U Palm leaf | Plate (4 g) | 0 g (−4 g) | 100% | Totally degraded |
| G.2.2.U Wood | Spoon (2.3 g), stick (0.7 g) | 6.7 g (+3.7 g) | 0% | Soft to the touch |
| G.2.3.D Palm leaf | Plate (4.2 g) | 1.3 g (−2.9 g) | 69% | Small fibers |
| G.2.4.D Wood | Spoon (1.5 g), stick (0.8 g) | 4.6 g (+2.3 g) | 0% | Soft to the touch |
| G.3.1.U Paper | Plate (1.6 g), cup (1.7 g) | 0 g (−3.3 g) | 100% | Soft to the touch |
| G.3.2.U Plastics | Cup (0.8 g), box (1.6 g) | 3.3 g (+0.9 g) | 0% | Not degraded |
| G.3.3.D Paper | Plate (1.7 g), cup (1.7 g) | 1.3 g (−2.1 g) | 61.7% | Soft to the touch |
| G.3.4.D Plastics | Cup (0.9 g), box (1.7 g) | 3.2 g (+0.6 g) | 0% | Not degraded |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baccar Chaabane, A.; Robbe, E.; Schernewski, G.; Schubert, H. Decomposition Behavior of Biodegradable and Single-Use Tableware Items in the Warnow Estuary (Baltic Sea). Sustainability 2022, 14, 2544. https://doi.org/10.3390/su14052544
Baccar Chaabane A, Robbe E, Schernewski G, Schubert H. Decomposition Behavior of Biodegradable and Single-Use Tableware Items in the Warnow Estuary (Baltic Sea). Sustainability. 2022; 14(5):2544. https://doi.org/10.3390/su14052544
Chicago/Turabian StyleBaccar Chaabane, Amina, Esther Robbe, Gerald Schernewski, and Hendrik Schubert. 2022. "Decomposition Behavior of Biodegradable and Single-Use Tableware Items in the Warnow Estuary (Baltic Sea)" Sustainability 14, no. 5: 2544. https://doi.org/10.3390/su14052544
APA StyleBaccar Chaabane, A., Robbe, E., Schernewski, G., & Schubert, H. (2022). Decomposition Behavior of Biodegradable and Single-Use Tableware Items in the Warnow Estuary (Baltic Sea). Sustainability, 14(5), 2544. https://doi.org/10.3390/su14052544







