Recent Applications of the Electrocoagulation Process on Agro-Based Industrial Wastewater: A Review
Abstract
1. Introduction
2. Methods
2.1. Data Source and Strategy
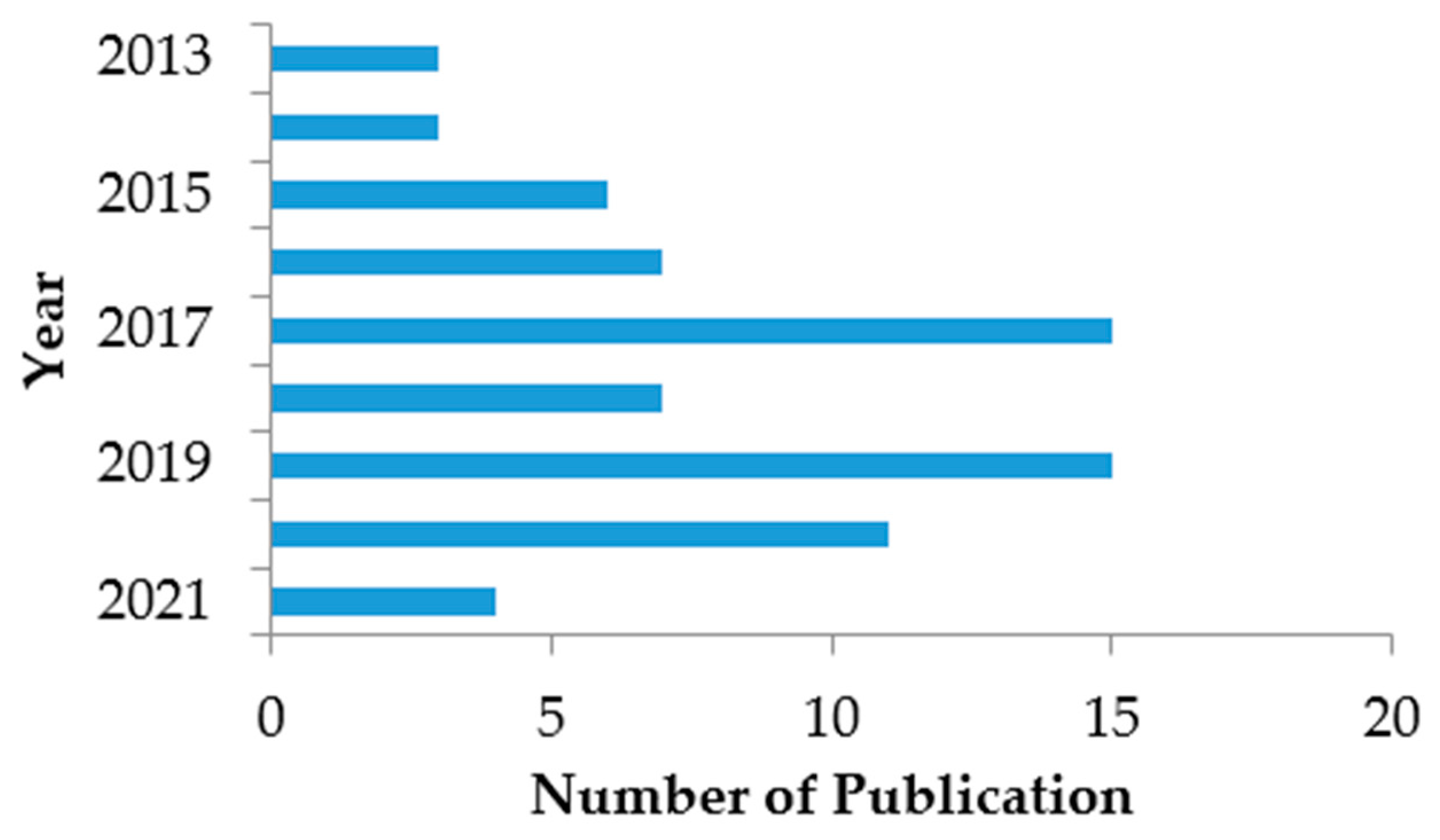
2.2. Bibliometric Analysis
3. Fundamental
3.1. Coagulation Principal
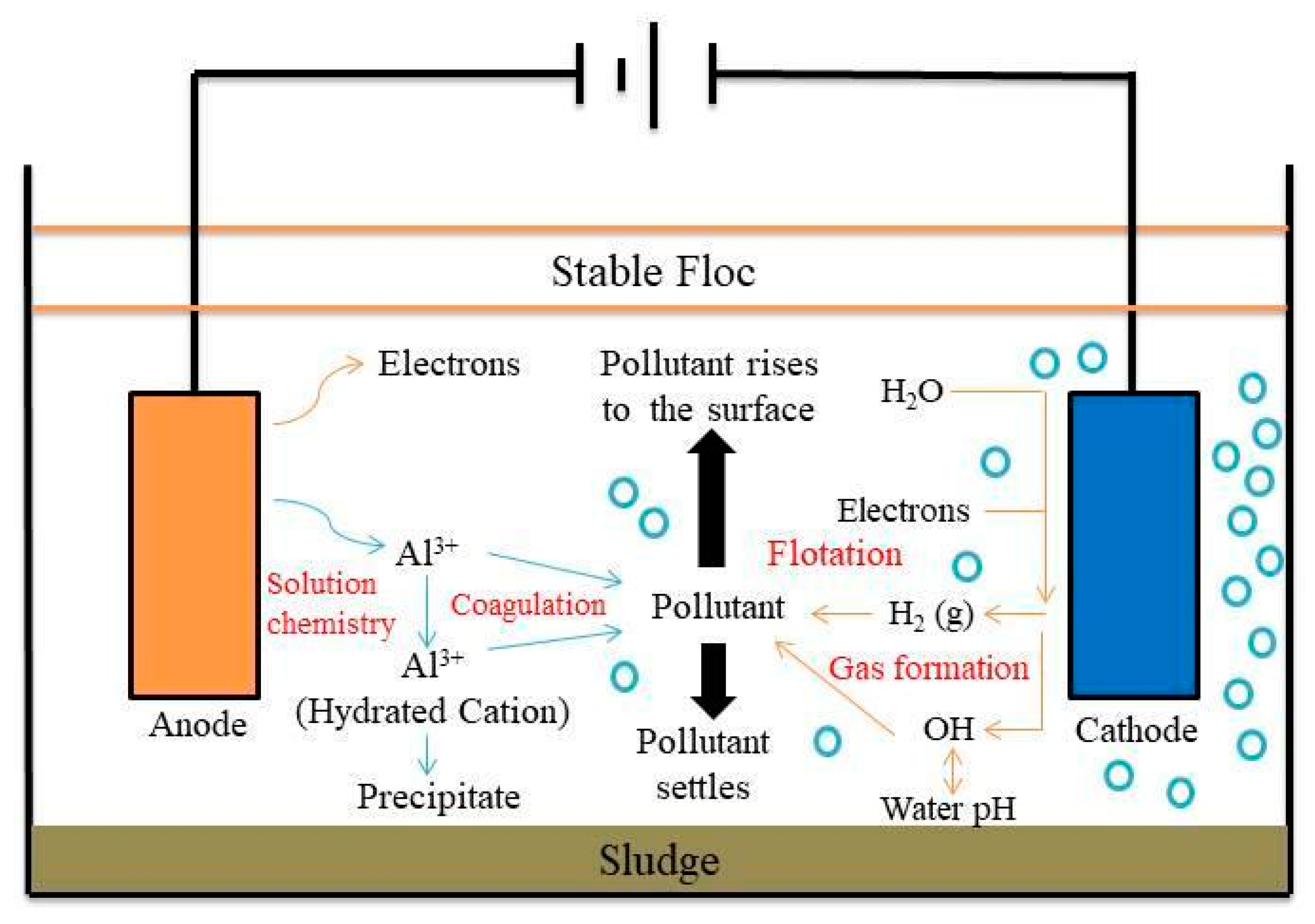
3.2. Electrocoagulation
- monomeric species such as Al(OH)2+, Al(OH)2+, Al2(OH)24+, and Al(OH)4−;
- polymeric species such as Al6(OH)153+, Al7(OH)174+, Al8(OH)204+, Al13O4(OH)247+ and Al13(OH)345+;
- amorphous species with very low solubility, such as Al(OH)3 and Al2O3 [55].
4. Factors Affecting Electrocoagulation
4.1. Electrode Materials
4.2. Electrode Distance
4.3. Electrolysis Time
4.4. Current Density
4.5. Electrolyte Support
5. Type of Agro-Based Wastewater
5.1. Olive Oil Mill Wastewater
5.2. Sugar Industry Wastewater
5.3. Pulp and Paper Mill Wastewater
5.4. Palm Oil Mill Effluent
| Parameters | Units | POME |
|---|---|---|
| COD | mg/L | 75,000 |
| BOD | mg/L | 18,200 |
| pH | - | 4.6 |
| Oil and Grease | mg/L | 2000 |
| NH3-N | mg/L | 20 |
| TSS | mg/L | 50,000 |
| PO43− | mg/L | 15 |
| NO3− | mg/L | 500 |
5.5. Coffee Industry Wastewater
5.6. Vegetable Oil Refinery Wastewater
5.7. Nuts Processing Wastewater
6. Integration of Electrochemical Treatment with Other Methods
| Type of Agro-Based Wastewater | Electrode | Electrocoagulation with Other Methods | COD removal | Color removal | References |
|---|---|---|---|---|---|
| Wood-based industry wastewater | Al | Adsorption and filtration | 77% | - | [149] |
| Coffee wastewater | Al | Sequential batch reactor | 84% | 93% | [150] |
| Coffee pulp industry wastewater | Al | Anaerobic sequencing batch reactor | 96% | - | [151] |
| Olive oil mill effluents | Fe | Electro-oxidation and electro-fenton | 96% | - | [152] |
| Pulp and paper wastewater | Fe | UV-based sulfate radical | 61% | - | [153] |
| Sugar industry wastewater | Fe | Thermal method | 97.8% | 99.7% | [154] |
| Palm oil mill effluent | Al and Fe | H2O2 and coagulation | 95.08% | - | [143] |
| Pulp and paper wastewater | Fe | H2O2, Co3O4/UV/peroxymonosulfate and permanganate | 95% | 92% | [155] |
| Pulp and paper wastewater | Al | Sonication | 90% | 100% | [156] |
| Sugarcane industry wastewater | Al | Coagulation method | 98% | 99.5% | [157] |
| Olive oil mill effluents | Al | Coagulation | 92% | - | [158] |
| Olive oil mill effluents | Fe | Photo-catalytic degradation | 88% | 100% | [155] |
| Coffee industry wastewater | Al | Electro-oxidation | 74% | - | [159] |
| Coffee industry wastewater | Fe and stainless steel | PAC | 80% | 92% | [160] |
| Pistachio processing wastewater | Al and Fe | Anaerobic digestion | 43.7% | - | [161] |
| Sugar industry wastewater | Fe | Thermal | 97.8% | - | [124] |
| Soybean oil wastewater | Fe | H2O2 | 94% | - | [162] |
| Palm oil mill effluent | Fe | Coagulation | 68.84% | - | [163] |
| Olive oil mill wastewater | Al | Bio-augmentation | 63.9 | - | [164] |
| Sugar industry wastewater | Fe | Chemical method | 82% | 84% | [165] |
| Olive oil mill wastewater | Al | External loop airlift reactor | 79.24% | - | [166] |
| Olive oil mill wastewater | Al | Catalytic ozonation and bio-degradation | 98.4% | - | [167] |
| Sugar industry wastewater | Fe | Sequential batch reactor | 87% | - | [168] |
| Pistachio processing industry wastewater | Fe, stainless steel, Al and BDD | Fungal treatment | 90.1% | - | [169] |
| Palm oil mill effluent | Al | Adsorption method | 44% | 89% | [170] |
| Olive oil mill wastewater | Al | Impregnation method | 78% | - | [150] |
| Olive oil mill wastewater | Al | Peroxone process | 79.8% | - | [171] |
7. Energy Consumption and Cost
8. Conclusions
- It requires the understanding of economic feasibility and the effective power consumption method.
- Different types of wastewaters require different types of electrode materials. They should fulfil the criteria to enhance the performance of the treatment. Future studies of the development of electrode materials and the design are required.
- Ultra-stable electrolyte support may be required to achieve complete degradation and prevent the formation of unwanted by-products.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zaied, M.; Bellakhal, N. Electrocoagulation treatment of black liquor from paper industry. J. Hazard. Mater. 2009, 163, 995–1000. [Google Scholar] [CrossRef]
- Kamyab, H.; Chelliapan, S.; Lee, C.T.; Khademi, T.; Kumar, A.; Yadav, K.K.; Ebrahimi, S.S. Improved production of lipid contents by cultivating Chlorella pyrenoidosa in heterogeneous organic substrates. Clean Technol. Environ. Policy 2019, 21, 1969–1978. [Google Scholar] [CrossRef]
- Aljerf, L. Data of thematic analysis of farmer’s use behavior of Recycled Industrial Wastewater. Data Brief 2018, 21, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Labiadh, L.; Barbucci, A.; Cerisola, G.; Gadri, A.; Ammar, S.; Panizza, M. Role of anode material on the electrochemical oxidation of methyl oranges. J. Solid State Electrochem. 2015, 19, 3177–3183. [Google Scholar] [CrossRef]
- Lin, S.S.; Shen, S.L.; Zhou, A.; Lyu, H.M. Assessment and management of lake eutrophication: A case study in Lake Erhai, China. Sci. Total Environ. 2021, 751, 141618. [Google Scholar] [CrossRef]
- Breida, B.M.; Younssi, S.A.; Ouammou, M.; Bouhria, M.; Hafsi, M. Pollution of Water Sources from Agricultural and Industrial Effluents: Special Attention to NO3−, Cr(VI), and Cu(II); Water Chemistry; Intechopen: London, UK, 2019. [Google Scholar] [CrossRef]
- Sarkar, B.; Chakrabarti, P.P.; Vijaykumar, A.; Kale, V. Wastewater treatment in dairy industries-possibility of reuse. Desalination 2006, 195, 141–152. [Google Scholar] [CrossRef]
- Kamyab, H.; Din, M.F.M.; Tin, C.L.; Ponraj, M.; Soltani, M.; Mohamad, S.E.; Roudi, A.M. Micro-macro algal mixture as a promising agent for treating POME discharge and its potential use as animal feed stock enhancer. J. Teknol. 2014, 68, 1–4. [Google Scholar] [CrossRef]
- Kamyab, H.; Din, M.F.M.; Keyvanfar, A.; Majid, M.Z.A.; Talaiekhozani, A.; Shafaghat, A.; Ismail, H.H. Efficiency of microalgae Chlamydomonas on the removal of pollutants from palm oil mill effluent (POME). Energy Procedia 2015, 75, 2400–2408. [Google Scholar] [CrossRef]
- Keerio, H.A.; Bae, W. Experimental Investigation of Substrate Shock and Environmental Ammonium Concentration on the Stability of Ammonia-Oxidizing Bacteria (AOB). Water 2020, 12, 223. [Google Scholar] [CrossRef]
- Liang, Z.; Soranno, P.A.; Wagner, T. The role of phosphorus and nitrogen on chlorophyll a: Evidence from hundreds of lakes. Water Res. 2020, 185, 116236. [Google Scholar] [CrossRef]
- Pouran, S.R.; Aziz, A.R.A.; Daud, W.M.A.W. Review on the main advances in photo-fenton oxidation system for recalcitrant wastewaters. J. Ind. Eng. Chem. 2015, 21, 53–69. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of technique used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Oller, I.; Malato, S.; Perez, J.A.S. Combination of advanced oxidation processes and biological treatments for wastewater decontamination—A review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef] [PubMed]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Cox, M.; Negre, P.; Yurramendi, L. Industrial Liquid Effluent; INASMET Tecnalia: San Sebastian, Spain, 2007; p. 283. [Google Scholar]
- Kharayat, Y. Distillery wastewater: Bioremediation approaches. J. Integr. Environ. Sci. 2012, 9, 69–91. [Google Scholar] [CrossRef]
- Chowdhary, P.; Raj, A.; Bharagava, R.N. Environmental pollution and health hazards from distillery wastewater and treatment approaches to combat the environmental threats: A review. Chemosphere 2018, 194, 229–246. [Google Scholar] [CrossRef] [PubMed]
- Kroiss, H. What is the potential for utilizing the resources in sludge? Water Sci. Technol. 2004, 49, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.W.; Selvam, A. Speciation of heavy metals during co-composting of sewage sludge with lime. Chemosphere 2006, 63, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Farzadkia, M.; Bazrafshan, E. Lime Stabilization of Waste Activated Sludge. Health Scope 2014, 3, 16035. [Google Scholar] [CrossRef]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, X.; Zhou, J.; Li, R.; Yang, S.; Wang, B.; Deng, R. Treatment of oily sludge by two-stage wet air oxidation. J. Energy Inst. 2019, 92, 1451–1457. [Google Scholar] [CrossRef]
- Mailler, R.; Gasperi, J.; Chebbo, G.; Rocher, V. Priority and emerging pollutants in sewage sludge and fate during sludge treatment. Waste Manag. 2014, 34, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Kelessidis, A.; Stakinasis, A.S. Comparative study of the methods used for treatment and final disposal of sewage sludge in European countries. Waste Manag. 2012, 32, 1186–1195. [Google Scholar] [CrossRef]
- Sid, S.; Volant, A.; Lesage, G.; Heran, M. Cost minimization in a full-scale conventional wastewater treatment plant: Associated costs of biological energy consumption versus sludge production. Water Sci. Technol. 2017, 76, 2473–2481. [Google Scholar] [CrossRef]
- Mattos, L.F.A.; Bastos, R.G. COD and nitrogen removal from sugarcane vinasse by heterotrophic green algae Desmodesmus sp. Desalin. Water Treat. 2016, 57, 9465–9473. [Google Scholar] [CrossRef]
- Santana, H.; Cereijo, C.R.; Teles, V.C.; Nascimento, R.C.; Fernandes, M.S.; Brunale, P.; Campanha, R.C.; Soares, I.P.; Silva, F.C.P.; Sabaini, P.S.; et al. Microalgae cultivation in sugarcane vinasse: Selection, growth and biochemical characterization. Bioresour. Technol. 2017, 228, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Pawar, S.B.; Pandey, R.A. Current practices and challenges in using microalgae for treatment of nutrient rich wastewater from agro-based industries. Sci. Total Environ. 2019, 687, 1107–1126. [Google Scholar] [CrossRef]
- Bolognesi, S.; Cecconet, D.; Capodaglio, A.G. Agro-industrial wastewater treatment in microbial fuel cells. In Integrated Microbial Fuel Cells for Wastewater Treatment; Butterworth-Heinnman: Oxford, UK, 2020; pp. 93–133. [Google Scholar] [CrossRef]
- Mo, J.; Yang, Q.; Zhang, W.; Zheng, Y.; Zhang, Z. A review on agro-industrial waste (AIW) derived adsorbents for water and wastewater treatment. J. Environ. Manag. 2018, 227, 395–405. [Google Scholar] [CrossRef]
- Rabaey, K.; Verstraete, W. Microbial fuel cells: Novel biotechnology for energy generation. Trends Biotechnol. 2005, 23, 291–298. [Google Scholar] [CrossRef]
- Angenent, L.T.; Karim, K.; Al-Dahhan, M.H.; Wrenn, B.A.; Domíguez-Espinosa, R. Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol. 2004, 22, 477–485. [Google Scholar] [CrossRef]
- Sinha, S.; Yoon, Y.; Amy, G.; Yoon, J. Determining the effectiveness of conventional and alternative coagulants through effective characterization schemes. Chemosphere 2004, 57, 1115–1122. [Google Scholar] [CrossRef]
- Morin, N.; Crini, G. Eaux Industrielles Contaminees; PUFC: Besancon, France, 2017; Available online: https://www.researchgate.net/profile/Isabelle-Ghillebaert/publication/317013318_Eaux_industrielles_contaminees_Reglementation_parametres_chimiques_et_biologiques_procedes_d’epuration_innovants_Chapitre_6_Evaluation_du_risque_chimique_en_milieu_aquatique/links/591eefa90f7e9b64281df637/Eaux-industrielles-contaminees-Reglementation-parametres-chimiques-et-biologiques-procedes-depuration-innovants-Chapitre-6-Evaluation-du-risque-chimique-en-milieu-aquatique.pdf (accessed on 11 November 2021).
- Stoller, M.; Azizova, G.; Mammadova, A.; Vilardi, G.; Palma, L.D.; Chianese, A. Treatment of olive oil processing wastewater by ultrafiltration, nanofiltration, reverse osmosis and bio-filtration. Chem. Eng. Trans. 2016, 47, 409–414. [Google Scholar] [CrossRef]
- Wu, B. Membrane-based technology in greywater reclamation: A review. Sci. Total Environ. 2019, 656, 184–200. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Robinson, J.; Chong, M.F. A review on application of flocculants in wastewater treatment. Process Saf. Environ. Prot. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Sarkka, H.; Bhatnagar, A.; Sillanpaa, M. Recent developments of electro-oxidation in water treatment d a review. J. Electroanal. Chem. 2015, 754, 46–56. [Google Scholar] [CrossRef]
- Chellam, S.; Sari, M.A. Aluminum electrocoagulation as pretreatment during microfiltration of surface containing NOM: A review of fouling, NOM, DBP and virus control. J. Hazard. Mater. 2016, 304, 490–501. [Google Scholar] [CrossRef]
- Bashir, M.J.K.; Han, T.M.; Wei, L.J.; Aun, N.C.; Amr, S.S.A. Polishing of treated palm oil mill effluent (POME) from the ponding system by electrocoagulation process. Water Sci. Technol. 2016, 73, 2704–2712. [Google Scholar] [CrossRef]
- Markou, V.; Kontogianni, M.; Frontistis, Z.; Tekerlekopoulou, A.G.; Katsaounis, A.; Vayenas, D. Electrochemical treatment of biologically pre-treated dairy wastewater using dimensionally stable anodes. J. Environ. Manag. 2017, 202, 217–224. [Google Scholar] [CrossRef]
- Sahu, O.P.; Chaudhari, P.K. Electro-chemical treatment of sugar industry wastewater: COD and color removal. J. Electroanal. Chem. 2015, 739, 122–129. [Google Scholar] [CrossRef]
- Klidi, N.; Clematis, D.; Delucchi, M.; Gadria, A.; Ammara, A.; Panizza, M. Applicability of electrochemical methods to paper mill wastewater for reuse:Anodic oxidation with BDD and TiRuSnO2 anodes. J. Electroanal. Chem. 2018, 815, 16–23. [Google Scholar] [CrossRef]
- Ellouze, S.; Panizza, M.; Barbucci, A.; Cerisola, G.; Mhiria, T.; Elaouda, S.C. Ferulic acid treatment by electrochemical oxidation using a BDD anode. J. Taiwan Inst. Chem. Eng. 2016, 59, 132–137. [Google Scholar] [CrossRef]
- Brillas, E. A review on the photoelectro-Fenton process as efficient electrochemical advanced oxidation for wastewater remediation. Treatment with UV light, sunlight, and coupling with conventional and other photo-assisted advanced technologies. Chemosphere 2020, 250, 126–198. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, A.; Makula, M.M.; Macherzynski, B. Comparison of effectiveness of coagulation with aluminum sulfate and pre-hydrolyzed aluminum coagulants. Desalin. Water Treat. 2014, 52, 3843–3851. [Google Scholar] [CrossRef]
- Ghernaout, D.; Ghernaout, B.; Kellil, A. Natural organic matter removal and enhanced coagulation as a link between coagulation and electrocoagulation. Desalin. Water Treat. 2009, 2, 203–222. [Google Scholar] [CrossRef]
- Guminska, J.; Kłos, M. Analysis of post-coagulation properties of flocs in terms of coagulant choice. Environ. Prot. Eng. 2012, 38, 103–113. Available online: https://www.infona.pl/resource/bwmeta1.element.baztech-article-BPW8-0022-0035 (accessed on 14 November 2021).
- Naje, A.S.; Chelliapan, S.; Zakaria, Z.; Ajeel, M.A.; Alaba, P.A. A review of electrocoagulation technology for the treatment of textile wastewater. Rev. Chem. Eng. 2017, 33, 263–292. [Google Scholar] [CrossRef]
- Hakizimana, J.N.; Gourich, B.; Chafi, M.; Stiriba, Y.; Vial, C.; Drogui, P.; Naja, J. Electrocoagulation process in water treatment: A review of electrocoagulation modeling approaches. Desalination 2017, 404, 1–21. [Google Scholar] [CrossRef]
- Verma, A.K.; Dash, R.R.; Bhunia, P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manag. 2012, 93, 154–168. [Google Scholar] [CrossRef]
- Dura, A.; Breslin, C. Electrocoagulation for the Effective Removal of Pollutants. ECS Meet. Abstr. 2012, 1417. Available online: https://mural.maynoothuniversity.ie/6744/1/adelaide-dura.pdf (accessed on 15 November 2021). [CrossRef]
- Aoudj, S.; Khelifa, A.; Drouiche, N.; Hecini, M.; Hamitouche, H. Electrocoagulation process applied to wastewater containing dyes from textile industry. Chem. Eng. Processing Process Intensif. 2010, 49, 1176–1182. [Google Scholar] [CrossRef]
- Emamjomeh, M.M.; Sivakumar, M. Review of pollutants removed by electrocoagulation and electrocoagulation/flotation processes. J. Environ. Manag. 2009, 90, 1663. [Google Scholar] [CrossRef] [PubMed]
- Yassine, W.; Akazdam, S.; Zyade, S.; Gourich, B. Treatment of olive mill wastewater using electrocoagulation process. J. Appl. Surf. Interfaces 2019, 4, 24–30. Available online: https://revues.imist.ma/index.php/jasi/article/view/14006 (accessed on 10 November 2021).
- Rakhmania; Kamyab, H.; Yuzir, M.A.; Al-Qaim, F.F.; Purba, L.D.A.; Riyadi, F.A. Application of Box-Behnken design to mineralization and color removal of palm oil mill effluent by electrocoagulation process. Environ. Sci. Pollut. Res. 2021, 1–13. [Google Scholar] [CrossRef]
- Gurses, A.; Yalcin, M.; Dogan, C. Electrocoagulation of some reactive dyes: A statistical investigation of some electrochemical variable. Waste Manag. 2002, 22, 491–499. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Mahvi, A.H.; Nasseri, S.; Mesdaghinia, A.R.; Vaezi, F.; Nazmara, S.H. Removal of cadmium from industrial effluents by electrocoagulation process using iron electrodes. Iran. J. Environ. Health Sci. Eng. 2006, 3, 261–266. Available online: https://www.hindawi.com/journals/jchem/2013/640139/ (accessed on 10 November 2021).
- Ridruejo, C.; Centellas, F.; Cabot, P.L.; Sires, I.; Brillas, E. Electrochemical Fenton-based treatment of tetracaine in synthetic and urban wastewater using active and non-active anodes. Water Res. 2018, 128, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Mussa, Z.H.; Al-Qaim, F.F.; Othman, M.R.; Abdullah, M.P. Removal of simvastatin from aqueous solution by electrochemical process using graphite-PVC as anode: A case study of evaluation of the toxicity, kinetics and chlorinated by-products. J. Environ. Chem. Eng. 2016, 4, 3338–3347. [Google Scholar] [CrossRef]
- Aljerf, L. High-efficiency extraction of bromocresol purple dye and heavy metals as chromium from industrial effluent by adsorption onto a modified surface of zeolite: Kinetics and equilibrium study. J. Environ. Manag. 2018, 225, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Sahu, O. Treatment of food-agro (sugar) industry wastewater with copper metal and salt: Chemical oxidation and electro-oxidation combined study in batch mode. Water Resour. Ind. 2017, 17, 19–25. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, Q.; Xue, J.; Guan, R.; Li, Q.; Liu, X.; Jia, H.; Wu, Y. 3D hierarchically porous NiO/NF electrode for the removal of chromium(VI) from wastewater by electrocoagulation. Chem. Eng. J. 2020, 402, 126151. [Google Scholar] [CrossRef]
- Goren, A.Y.; Kobya, M. Arsenic removal from groundwater using an aerated electrocoagulation reactor with 3D Al electrodes in the presence of anions. Chemosphere 2021, 263, 128253. [Google Scholar] [CrossRef]
- Msindo, Z.S.; Sibanda, V.; Potgieter, J.H. Electrochemical and physical characterisation of lead-based anodes in comparison to Ti–(70%) IrO2/(30%) Ta2O5 dimensionally stable anodes for use in copper electrowinning. J. Appl. Electrochem. 2010, 40, 691–699. [Google Scholar] [CrossRef]
- Moats, M.; Hardee, K.; Brown, C. Mesh-on-lead anodes for copper electrowinning. J. Miner. Met. Mater. Soc. 2003, 55, 46–48. [Google Scholar] [CrossRef]
- Khandegar, V.; Saroha, A.K. Electrochemical treatment of distillery spent wash using aluminium and iron electrodes. Chin. J. Chem. Eng. 2012, 20, 439–443. [Google Scholar] [CrossRef]
- Bouhezila, F.; Hariti, M.; Lounici, H.; Mameri, N. Treatment of the OUED SMAR town landfill leachate by an electrochemical reactor. Desalination 2011, 280, 347–353. [Google Scholar] [CrossRef]
- Phalakornkule, C.; Polgumhang, S.; Tongdaung, W. Performance of an electrocoagulation process in treating direct dye: Batch and continuous up flow processes. World Acad. Sci. Eng. Technol. 2009, 57, 277–282. [Google Scholar] [CrossRef]
- Nasrullah, M.; Zularisam, A.W.; Krishnan, S.; Sakinah, M.; Singh, L.; Fen, Y.W. High performance electrocoagulation process in treating palm oil mill effluent using high current intensity application. Chin. J. Chem. Eng. 2019, 27, 208–217. [Google Scholar] [CrossRef]
- Holt, P.K.; Barton, G.W.; Mitchell, C.A. The future for electrocoagulation as a localised water treatment technology. Chemosphere 2005, 59, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, M.C.; Santos, E.V.; Martínez-Huitlea, C.A.; Fajardo, A.S.; Castro, S.S.L. Obtaining high-added value products from the technical cashew-nut shell liquid using electrochemical oxidation with BDD anodes. Sep. Purif. Technol. 2020, 250, 117099. [Google Scholar] [CrossRef]
- Byoud, F.; Wakrim, A.; Benhsinat, C.; Zaroual, Z.; El-Ghachtouli, S.; Tazi, A.; Chaair, H.; Assabbane, A.; Azzi, M. Electrocoagulation treatment of the food dye waste industry: Theoretical and experimental study. J. Mater. Environ. Sci. 2017, 12, 4301–4312. [Google Scholar] [CrossRef]
- Hamdi, M.; Khadir, A.; Garcia, J.L. The use of Aspergillus niger for the bioconversion of olive mill waste-waters. Appl. Microbiol. Biotechnol. 1991, 34, 828–831. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Yue, P.L. Investigation on the electrolysis voltage ofelectrocoagulation. Chem. Eng. Sci. 2002, 57, 2449–2455. [Google Scholar] [CrossRef]
- Parama, K.S.K.; Balasubramanian, N.; Srinivasakannan, C. Decolorization and COD reduction of paper industrial effluent using electro-coagulation. Chem. Eng. J. 2009, 151, 97–104. [Google Scholar] [CrossRef]
- Chiang, L.C.; Chang, J.E.; Tseng, S.C. Electrochemical oxidation pretreatment of refractory organic pollutants. Water Sci. Technol. 1997, 36, 123–130. [Google Scholar] [CrossRef]
- Niaounakis, M.; Halvadakis, C.P. Olive-Mill Waste Management, 2nd ed.; Typothito: Athens, Greece, 2006; Available online: https://www.sciencedirect.com/bookseries/waste-management-series/vol/5/suppl/C (accessed on 11 November 2021).
- Dellagreca, M.; Previtera, L.; Temussi, F.; Zarrelli, A. Low-molecular-weight components of olive oil mill waste-waters. Phytochem. Anal. 2004, 15, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Pulido, J.M.O.; Ortega, M.D.V.; Hodaifa, G.; Ferez, A.M. Physicochemical analysis and adequation of olive oil mill wastewater after advanced oxidation process for reclamation by pressure-driven membrane technology. Sci. Total Environ. 2014, 503–504, 113–121. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Olive mill wastewater treatment by anodic oxidation with parallel plate electrodes. Water Res. 2006, 40, 1179–1184. [Google Scholar] [CrossRef]
- Rehim, S.S.A.E.; Mohamed, N.F. Passivity breakdown of lead anode in alkaline nitrate solutions. Corros. Sci. 1998, 40, 1883–1896. [Google Scholar] [CrossRef]
- Sounni, F.; Aissam, H.; Ghomari, O.; Merzouki, M.; Benlemlih, M. Electrocoagulation of olive mill wastewaters to enhance biogas production. Biotechnol. Lett. 2018, 40, 297–301. [Google Scholar] [CrossRef]
- Ghahrchi, M.; Rezaee, A.; Adibzadeh, A. Study of kinetic models of olive oil mill wastewater treatment using electrocoagulation process. Desalin. Water Treat. 2021, 211, 123–130. [Google Scholar] [CrossRef]
- Razali, N.A.M.; Salleh, W.N.W.; Rosman, N.; Ismail, N.H.; Ahmad, S.Z.N.; Aziz, F.; Jye, L.W.; Ismail, A.F. Palm oil mill effluent treatment using tungsten trioxide: Adsorption and photocatalytic degradation. Mater. Today Proc. 2021, 42, 22–27. [Google Scholar] [CrossRef]
- Longhi, P.; Vodopivec, B.; Fiori, G. Electrochemical treatment of olive oil mill wastewater. Ann. Chim. 2001, 91, 169–174. [Google Scholar]
- Tezcan-Un, U.; Ugur, S.; Koparal, A.S.; Bakir-Ogutveren, U. Electrocoagulation of olive mill wastewaters. Sep. Purif. Technol. 2006, 52, 136–141. [Google Scholar] [CrossRef]
- Syaichurrozi, I.; Sarto, S.; Sediawan, W.B.; Hidayat, M. Effect of Current and Initial pH on Electrocoagulation in Treating the Distillery Spent Wash with Very High Pollutant Content. Water 2020, 13, 11. [Google Scholar] [CrossRef]
- Benekos, A.K.; Zampeta, C.; Argyriou, R.; Economou, C.N.; Triantaphyllidou, I.E.; Tatoulis, T.I.; Tekerlekopoulou, A.G.; Vayenas, D.G. Treatment of table olive processing wastewaters using electrocoagulation in laboratory and pilot-scale reactors. Process Saf. Environ. Prot. 2019, 131, 38–47. [Google Scholar] [CrossRef]
- Ntaikou, I.; Antonopoulou, G.; Vayenasa, D.; Lyberatosa, G. Assessment of electrocoagulation as a pretreatment method of olive mill wastewater towards alternative processes for biofuels production. Renew. Energy 2020, 154, 1252–1262. [Google Scholar] [CrossRef]
- Sahu, O.P.; Gupta, V.; Chaudhari, P.K.; Srivastava, V.V. Electrochemical treatment of actual sugar industry wastewater using aluminum electrodes. Int. J. Environ. Sci. Technol. 2015, 12, 3519–3530. [Google Scholar] [CrossRef][Green Version]
- Asaithambhi, P.; Matheswaran, M. Electrochemical treatment of simulated sugar industrial effluent: Optimization and modeling using a response surface methodology. Arab. J. Chem. 2016, 9, 981–987. [Google Scholar] [CrossRef]
- Kolhe, A.S.; Sarode, A.G.; Ingale, S.R. Study of effluent from the sugar cane industry. Sodh Samiksha Aur Mulyankan 2009, 2, 303–306. Available online: https://www.researchgate.net/publication/351249954_Quality_and_Treatment_of_Sugar_Industry_Effluent_-A_Study (accessed on 12 November 2021).
- Guven, G.; Perendeci, A.; Tanylac, A. Electrochemical treatment of simulated beet sugar factory wastewater. Chem. Eng. J. 2009, 151, 149–159. [Google Scholar] [CrossRef]
- Aljerf, L. Green technique development for promoting the efficiency of pulp slurry reprocess. Sci. J. King Faisal Univ. 2016, 17, 1–10. [Google Scholar] [CrossRef]
- Brillas, E.; Sauleda, R.; Casado, J. Degradation of4-chlorophenol by anodic oxida- tion, electro-Fenton, photoelectro-Fenton and peroxi-coagulation processes. J. Electrochem. Soc. 1998, 145, 759–765. [Google Scholar] [CrossRef]
- Chindaphan, K.; Thaveesangsakulthai, I.; Naranaruemol, S.; Nhujak, T.; Panchompoo, J.; Chailapakol, O.; Kulsing, C. Miniaturized electrocoagulation approach for removal of polymeric pigments and selective analysis of non- and mono-hydroxylated phenolic acids in wine with HPLC-UV. RSC Adv. 2021, 11, 5885–5893. [Google Scholar] [CrossRef]
- Ksibi, M.; Amor, S.B.; Cherif, S.; Elaloui, S. Photodegradation of lignin from black liquor using a UV/TiO2 system. J. Photochem. Photobiol. A 2003, 154, 211–218. [Google Scholar] [CrossRef]
- Ali, M.; Sreekrishnan, T.R. Aquatic toxicity from pulp and paper mill effluents: A review. Adv. Environ. Res. 2001, 5, 175–196. [Google Scholar] [CrossRef]
- Kamyab, H.; Chelliapan, S.; Din, M.F.M.; Rezania, S.; Khademi, T.; Kumar, A. Palm oil mill effluent as an environmental pollutant. Palm Oil 2018, 13, 13–28. Available online: https://www.intechopen.com/chapters/60482 (accessed on 13 November 2021).
- Zazouli, M.A.; Ahmadi, M. Pretreatment of paper recycling plant wastewater by electrocoagulation using aluminum and iron electrodes. J. Mater. Environ. Sci. 2017, 8, 2140–2146. [Google Scholar]
- Ugurlu, M.; Gurses, A.; Dogar, C.; Yalcin, M. The removal of lignin and phenol from paper mill effluents by electrocoagulation. J. Environ. Manag. 2007, 87, 420–428. [Google Scholar] [CrossRef]
- Valenzuela, L.S.T.; Jimenez, J.A.S.; Martinez, K. Coffee By-Products: Nowadays and Perspectives. In Coffee—Production and Research; Intechopen: London, UK, 2020. [Google Scholar] [CrossRef]
- Chin, M.J.; Eong, P.P.; Ti, T.B.; Seng, C.E.; Ling, C.K. Biogas from palm oil mill effluent (POME): Opportunities and challenges from Malaysia’s perspective. Renew. Sustain. Energy Rev. 2013, 26, 717–726. [Google Scholar] [CrossRef]
- Kamyab, H.; Chelliapan, S.; Din, M.F.M.; Shahbazian, R.Y.; Rezania, S.; Khademi, T.; Azimi, M. Evaluation of Lemna minor and Chlamydomonas to treat palm oil mill effluent and fertilizer production. J. Water Process Eng. 2017, 17, 229–236. [Google Scholar] [CrossRef]
- Tan, Y.H.; Goha, P.S.; Lai, G.S.; Lau, W.J.; Ismail, A.F. Treatment of aerobic treated palm oil mill effluent (AT-POME) by using TiO2 photocatalytic process. J. Technol. Sci. Eng. 2014, 70, 61–63. [Google Scholar] [CrossRef]
- Kamyab, H.; Chelliapan, S.; Din, M.F.M.; Lee, C.T.; Rezania, S.; Khademi, T.; Bong, C.P.C. Isolate new microalgal strain for biodiesel production and using FTIR spectroscopy for assessment of pollutant removal from palm oil mill effluent (POME). Chem. Eng. Trans. 2018, 63, 91–96. [Google Scholar] [CrossRef]
- Baranitharan, E.; Khan, R.M.; Prasad, D.M.R. Treatment of palm oil mill effluent in microbial fuel cell using polyacrylonitrile carbon felt electrodes. J. Med. Bioeng. 2013, 2, 252–256. [Google Scholar] [CrossRef]
- Agustin, M.B.; Sengpracha, W.P.; Phutdhawong, W. Electrocoagulation of Palm Oil Mill Effluent. Int. J. Environ. Res. Public Health 2008, 5, 177–180. [Google Scholar] [CrossRef]
- Phalakornkule, C.; Mangmeemak, J.; Intrachod, K.; Nuntakumjorn, B. Pretreatment of palm oil mill effluent by electrocoagulation and coagulation. ScienceAsia 2010, 36, 142–149. [Google Scholar] [CrossRef]
- Bouknana, D.; Hammoutia, B.; Salghid, R.; Jodehe, S.; Zarrouka, A.; Warade, I.; Aouniti, A.; Sbaa, M. Physicochemical Characterization of Olive Oil Mill Wastewaters in the eastern region of Morocco. J. Mater. Environ. Sci. 2014, 5, 1039–1058. [Google Scholar]
- Phan, H.Q.H.; Hoan, N.X.; Huy, N.N.; Duc, N.D.D.; Anh, N.T.N.; Que, N.T.; Thuy, N.T. Pre-treatment potential of electrocoagulation process using aluminum and titanium electrodes for instant coffee processing wastewater. J. Environ. Sustain. 2019, 3, 170–185. [Google Scholar] [CrossRef]
- Rigueto, C.V.T.; Nazari, M.T.; De Souza, C.F.; Cadore, J.S.; Brião, V.B.; Piccin, J.S. Alternative techniques for caffeine removal from wastewater: An overview of opportunities and challenges. J. Water Proc. Eng. 2020, 35, 101231. [Google Scholar] [CrossRef]
- Gomez, I.D.; Garcia, M.A.G. Integration of environmental and economic performance of Electro-Coagulation-Anodic Oxidation sequential process for the treatment of soluble coffee industrial effluent. Sci. Total Environ. 2021, 764, 142818. [Google Scholar] [CrossRef]
- Sontaya, K.; Pitiyont, B.; Punsuvon, P. Decolorization and COD Removal of Palm Oil Mill Wastewater by Electrocoagulation. Int. J. Environ. Chem. Ecol. Geol. Geophys. Eng. 2013, 7, 606–609. [Google Scholar] [CrossRef]
- Nasrullah, M.; Singh, L.; Krishnan, S.; Sakinah, M.; Zularism, A.W. Electrode design for electrochemical cell to treat palm oil mill effluent by electrocoagulation process. Environ. Technol. Innov. 2018, 9, 323–341. [Google Scholar] [CrossRef]
- Murthy, P.S.; Naidu, M.M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Rodríguez, P.S.; Pérez, S.R.M.; Fern¢ndez, B.M. Studies of anaerobic biodegradability of the wastewater of the humid benefit the coffee. J. Interciencia 2000, 25, 386–390. Available online: https://www.researchgate.net/publication/297388993_Study_of_the_anaerobic_biodegradability_of_the_wastewaters_of_the_humid_benefit_of_the_coffee (accessed on 11 November 2021).
- Mussatto, S.I.; Carneiro, L.M.; Silva, J.P.A.; Roberto, I.C.; Teixeira, J.A. A study on chemical constituents and sugars extraction from spent coffee grounds. Carbohydr. Polym. 2011, 83, 368–374. [Google Scholar] [CrossRef]
- Pujol, D.; Liu, C.; Gominho, J.; Olivella, M.À.; Fiol, N.; Villaescusa, I.; Pereira, H. The chemical composition of exhausted coffee waste. Ind. Crops Prod. 2013, 50, 423–429. [Google Scholar] [CrossRef]
- Gengec, E.; Kobya, M.; Demirbas, E.; Akyol, A.; Oktor, K. Optimization of baker’s yeast wastewater using response surface methodology by electrocoagulation. Desalination 2012, 286, 200–209. [Google Scholar] [CrossRef]
- Khansorthong, S.; Hunsom, M. Remediation of wastewater from pulp and paper mill industry by the electrochemical technique. J. Chem. Eng. 2009, 151, 228–234. [Google Scholar] [CrossRef]
- Sahana, M.; Srikantha, H.; Mahesh, H.; Swamy, M.H. Coffee processing industrial wastewater treatment using batch electrochemical coagulation with stainless steel and Fe electrodes and their combinations, and recovery and reuse of sludge. Water Sci. Technol. 2018, 78, 279–289. [Google Scholar] [CrossRef]
- Asha, G.; Kumar, B.M. Comparison of Aluminum and Iron Electrodes for COD Reduction from Coffee Processing Wastewater by Electrocoagulation Process. J. Sci. Res. Rep. 2016, 9, 22815. [Google Scholar] [CrossRef]
- Waldron, K.W.; Moates, G.K.; Faulds, C.B. Prebiotic Potential and Antimicrobial Effect from a By-Product of the Almond Processing Industry. In Total Food; RSC Publishing: Cambridge, UK, 2009; pp. 86–89. [Google Scholar] [CrossRef]
- Pandey, R.A.; Sanyal, P.B.; Chattopadhyay, N.; Kaul, S.N. Treatment and reuse of wastes of a vegetable oil refinery. Resour. Conserv. Recycl. 2003, 37, 101–117. [Google Scholar] [CrossRef]
- Bucas, G.; Saliot, A. Sea transport of animal and vegetable oils and its environmental consequences. Mar. Pollut. Bull. 2002, 44, 1388–1396. [Google Scholar] [CrossRef]
- Sridhar, S.; Kale, A.; Khan, A.A. Reverse osmosis of edible vegetable oil industry effluent. J. Membr. Sci. 2002, 205, 83–90. [Google Scholar] [CrossRef]
- Tezcan-Un, U. Treatment of vegetable oil refinery wastewater by electrocoagulation. Fresenius Environ. Bull. 2007, 16, 1056–1060. [Google Scholar]
- Tezcan-Un, U.; Koparal, A.S.; Ogutveren, U.B. Electrocoagulation of vegetable oil refinery wastewater using aluminum electrodes. J. Environ. Manag. 2009, 90, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Preeti, V.; Ramesh, S.T.; Gandhimathi, R.; Nidhessh, P.V. Optimization of batch electrocoagulation process using Box-Behnken experimental design for the treatment of crude vegetable oil refinery wastewater. J. Dispers. Sci. Technol. 2019, 41, 592–599. [Google Scholar] [CrossRef]
- Sharma, S.; Aygun, A.; Simsek, H. Electrochemical treatment of sunflower oil refinery wastewater and optimization of the parameters using response surface methodology. Chemosphere 2020, 249, 126511. [Google Scholar] [CrossRef] [PubMed]
- Bayar, S.; Boncukcouglu, R.; Yilmaz, A.E.; Fil, B.A. Pre-Treatment of Pistachio Processing Industry Wastewaters (PPIW) by Electrocoagulation using Al Plate Electrode. J. Sep. Sci. Technol. 2014, 49, 1008–1018. [Google Scholar] [CrossRef]
- Celik, I.; Demirer, G.N. Biogas production from pistachio (pistacia vera L.) processing waste. Biocatal. Agric. Biotechnol. 2015, 4, 767–772. [Google Scholar] [CrossRef]
- Pasqualone, A.; Laddomada, B.; Spina, A.; Todaro, A.; Guzman, C.; Summo, C.; Mita, G.; Giannone, V. Almond by-products: Extraction and characterization of phenolic compounds and evaluation of their potential use in composite dough with wheat flour. LWT 2018, 89, 299–306. [Google Scholar] [CrossRef]
- Lipan, L.; Moriana, A.; Lluch, D.B.L.; Lamadrid, M.C.; Sendra, E.; Hernandez, F.; Araujo, L.V.; Corell, M.; Barrachina, A.A.C. Nutrition Quality Parameters of Almonds as Affected by Deficit Irrigation Strategies. Molecules 2019, 24, 2646. [Google Scholar] [CrossRef] [PubMed]
- Bodoira, R.; MAestri, D. Phenolic Compounds from Nuts: Extraction, Chemical Profiles, and Bioactivity. J. Agric. Food Chem. 2020, 68, 927–948. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.; Vidal, L.; Beltran, A.; Canals, A.; Garrigos, M.C. Microwave-Assisted Extraction of Phenolic Compounds from Almond Skin Byproducts (Prunus amygdalus): A Multivariate Analysis Approach. J. Agric. Food Chem. 2015, 63, 5395–5402. [Google Scholar] [CrossRef]
- Tabib, M.; Tao, Y.; Ginies, C.; Bornard, I.; Rakotomanomana, N.; Remmal, A.; Chemat, F. A One-Pot Ultrasound-Assisted Almond Skin Separation/Polyphenols Extraction and its Effects on Structure, Polyphenols, Lipids, and Proteins Quality. Appl. Sci. 2020, 10, 3628. [Google Scholar] [CrossRef]
- Valero, D.; Ortiz, J.M.; García, V.; Expósito, E.; Montiel, V.; Grupo, A.A. Electrocoagulation of wastewater from the almond industry. Chemosphere 2011, 84, 1290–1295. [Google Scholar] [CrossRef]
- Costa, P.R.F.D.; Costa, E.C.T.D.A.; Castro, S.S.L.; Fajardo, A.S.; Huitle, C.A.M. A sequential process to treat a cashew-nut effluent: Electrocoagulation plus electrochemical oxidation. J. Electroanal. Chem. 2019, 834, 79–85. [Google Scholar] [CrossRef]
- Jaafarzadeh, N.; Omidinasab, M.; Ghanbari, F. Combined electrocoagulation and UV-based sulfate radical oxidation processes for treatment of pulp and paper wastewater. Process Saf. Environ. Prot. 2016, 102, 462–472. [Google Scholar] [CrossRef]
- Fil, B.A.; Boncukcouglu, R.; Yilmaz, A.E.; Bayar, S. Electro-Oxidation of Pistachio Processing Industry Wastewater Using Graphite Anode. Soil Air Water 2014, 42, 1232–1238. [Google Scholar] [CrossRef]
- Guclu, D. Optimization of electrocoagulation of pistachio processing wastewaters using the response surface methodology. Desalin. Water Treat. 2015, 54, 3338–3347. [Google Scholar] [CrossRef]
- Bayar, S.; Yilmaz, A.E.; Koksal, Z.; Boncukcouglu, R.; Fil, B.A.; Yilmaz, M.T. The Effect of Initial pH on Pistachio Processing Industrial Wastewater Pre-treatment by Electrocoagulation Method. Adv. Res. Eng. 2015, 37, 151–154. [Google Scholar] [CrossRef][Green Version]
- Sharma, S.; Simsek, H. Treatment of canola-oil refinery effluent using electrochemical methods: A comparison between combined electrocoagulation and electrooxidation and electrochemical peroxidation methods. Chemosphere 2019, 221, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Chanworrawoot, K.; Hunsom, M. Treatment of wastewater from pulp and paper mill industry by electrochemical methods in membrane reactor. J. Environ. Manag. 2012, 113, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Can, O.T.; Gengec, E.; Kobya, M. TOC and COD removal from instant coffee and coffee products production wastewater by chemical coagulation assisted electrooxidation. J. Water Process Eng. 2019, 28, 28–35. [Google Scholar] [CrossRef]
- Isik, Z.; Arikan, E.B.; Ozay, Y.; Bouras, H.D.; Dizge, N. Electrocoagulation and electrooxidation pre-treatment effect on fungal treatment of pistachio processing wastewater. Chemosphere 2020, 244, 125383. [Google Scholar] [CrossRef]
- Hansson, H.; Marques, M.; Laohaprapanon, S.; Hogland, W. Electrocoagulation coupled to activated carbon sorption/filtration for treatment of cleaning wastewaters from wood-based industry. Desalin. Water Treat. 2013, 52, 5243–5251. [Google Scholar] [CrossRef]
- Mahesh, S.; Srikantha, H.; Lobo, A.L. Performance Evaluation of two Batch Operations using Electrochemical Coagulation followed by Sequential Batch Reactor in treating Coffee wastewater. Int. J. Chem. Tech. Res. 2014, 6, 339–346. Available online: https://www.researchgate.net/publication/286442411_Performance_Evaluation_of_two_Batch_Operations_using_Electrochemical_Coagulation_followed_by_Sequential_Batch_Reactor_in_treating_Coffee_wastewater (accessed on 9 November 2021).
- Asha, G.; Kumar, B.M. Coffee Pulping Wastewater Treatment by Electrochemical Treatment followed Anaerobic Sequencing Batch Reactor. Int. J. Sci. Eng. Res. 2015, 6, 1447–1456. Available online: https://www.semanticscholar.org/paper/Coffee-Pulping-Wastewater-Treatment-by-Treatment-Kumar/2e595d676258898e41e943e80015865a86ca8175 (accessed on 13 November 2021).
- Esfandyari, Y.; Mahdavi, Y.; Seyedsalehi, M.; Hoseini, M.; Safari, G.H.; Ghozikali, M.G.; Kamani, H.; Jaafari, J. Degradation and biodegradability improvement of the olive mill wastewater by peroxi-electrocoagulation/electrooxidation-electroflotation process with bipolar aluminum electrodes. Environ. Sci. Pollut. Res. 2015, 22, 6288–6297. [Google Scholar] [CrossRef]
- Sahu, O.; Rao, D.G.; Gopal, R.; Tiwari, A.; Pal, D. Treatment of wastewater from sugarcane process industry by electrochemical and chemical process: Aluminum (metal and salt). J. Water Process Eng. 2017, 17, 50–62. [Google Scholar] [CrossRef]
- Nasrullah, M.; Singh, L.; Mohamad, Z.; Norsita, S.; Krishnan, S.; Wahida, N.; Zularisam, A.W. Treatment of palm oil mill effluent by electrocoagulation with presence of hydrogen peroxide as oxidizing agent and polialuminum chloride as coagulant-aid. Water Resour. Ind. 2017, 17, 7–10. [Google Scholar] [CrossRef]
- Jaafarzadeh, N.; Ghanbari, F.; Ahmadi, M.; Omidinasab, M. Efficient integrated processes for pulp and paper wastewater treatment and phytotoxicity reduction: Permanganate, electro-Fenton and Co3O4/UV/peroxymonosulfate. Chem. Eng. J. 2017, 308, 142–150. [Google Scholar] [CrossRef]
- Asaithambi, P.; Aziz, A.R.A.; Sajjadi, B.; Daud, W.M.A.W. Sono assisted electrocoagulation process for the removal of pollutant from pulp and paper industry effluent. Environ. Sci. Pollut. Res. 2017, 24, 5168–5178. [Google Scholar] [CrossRef]
- Hmidi, K.; Ksentini, I.; Mansour, L.B. Treatment of olive-pomace oil refinery wastewater using combined coagulation-electroflotation process. J. Water Chem. Technol. 2017, 39, 275–280. [Google Scholar] [CrossRef]
- Ates, H.; Dizge, N.; Yatmaz, H.C. Combined process of electrocoagulation and photocatalytic degradation for the treatment of olive washing wastewater. Water Sci. Technol. 2017, 75, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Taquez, H.N.I.; Pavas, E.G.; Blatchley, E.R.; García, M.A.G.; Gómez, I.D. Integrated electrocoagulation-electrooxidation process for the treatment of soluble coffee effluent: Optimization of COD degradation and operation time analysis. J. Environ. Manag. 2017, 200, 530–538. [Google Scholar] [CrossRef]
- Ozay, Y.; Ünsar, E.K.; Isık, Z.; Yılmaz, F.; Dizge, N.; Perendeci, N.A.; Mazmanci, M.A.; Yalvac, M. Optimization of electrocoagulation process and combination of anaerobic digestion for the treatment of pistachio processing wastewater. J. Clean. Prod. 2018, 196, 42–50. [Google Scholar] [CrossRef]
- Sahu, O.; Rao, D.G.; Thangavel, A.; Ponnappan, S. Treatment of sugar industry wastewater using a combination of thermal and electrocoagulation processes. Int. J. Sustain. Eng. 2018, 11, 16–25. [Google Scholar] [CrossRef]
- Davarnejad, R.; Sabzehei, M.; Parvizi, F.; Heidari, S.; Rashidi, A. Study on Soybean Oil Plant Wastewater Treatment Using the Electro-Fenton Technique. Chem. Eng. Technol. 2019, 42, 2717–2725. [Google Scholar] [CrossRef]
- Mujeli, M.; Hussain, S.A.; Ismail, M.H.; Biak, D.R.A.; Jami, M.S. Screening of electrocoagulation process parameters for treated palm oil mill effluent using minimum-runs resolution IV design. Int. J. Environ. Sci. Technol. 2019, 16, 811–820. [Google Scholar] [CrossRef]
- Abdullah, H.M.; El-Shatoury, S.A.; El-Shahawy, A.A.; Ghorab, S.A.; Nasr, M.; Trujillo, M.E. An integrated bioaugmentation/electrocoagulation concept for olive mill wastewater management and the reuse in irrigation of biofuel plants: A pilot study. Environ. Sci. Pollut. Res. 2019, 26, 15803–15815. [Google Scholar] [CrossRef]
- Sahu, O. Suitability of chemical and electrocoagulation process on sugar industry wastewater treatment. Int. J. Energy Water Resour. 2019, 3, 117–125. [Google Scholar] [CrossRef]
- Elkacmi, R.; Boudouch, O.; Hasib, A.; Bouzaid, M.; Bennajah, M. Photovoltaic electrocoagulation treatment of olive mill wastewater using an external-loop airlift reactor. Sustain. Chem. Pharm. 2020, 17, 100274. [Google Scholar] [CrossRef]
- Khani, M.R.; Mahdizadeh, H.; Kannan, K.; Kalankesh, L.R.; Kamarehei, B.; Baneshi, M.M.; Shahamat, Y.D. Olive mill wastewater (OMW) Treatment by hybrid processes of electrocoagulation/catalytic ozonation and biodegradation. Environ. Eng. Manag. J. 2020, 19, 1401–1410. [Google Scholar]
- Gondudey, S.; Chaudhari, P.K. Treatment of Sugar Industry Effluent Through SBR Followed by Electrocoagulation. Sugar Technol. 2020, 22, 303–310. [Google Scholar] [CrossRef]
- Sia, Y.Y.; Tan, I.A.W.; Abdullah, M.O. Palm Oil Mill Effluent Treatment Using Electrocoagulation-Adsorption Hybrid Process. Mater. Sci. Forum 2020, 997, 139–149. [Google Scholar] [CrossRef]
- Bargaoui, M.; Jellali, S.; Azzaz, A.A.; Jeguirim, M.; Akrout, H. Optimization of hybrid treatment of olive mill wastewaters through impregnation onto raw cypress sawdust and electrocoagulation. Environ. Sci. Pollut. Res. 2021, 28, 24470–24485. [Google Scholar] [CrossRef] [PubMed]
- Shahamat, Y.D.; Hamidi, F.; Mohammadi, H.; Ghahrchi, M. Optimisation of COD removal from the olive oil mill wastewater by combined electrocoagulation and peroxone process: Modelling and determination of kinetic coefficients. Int. J. Environ. Anal. Chem. 2021, 1–14. [Google Scholar] [CrossRef]
- Hanafi, F.; Belaoufi, A.; Mountadar, M.; Assobhei, O. Augmentation of biodegradability of olive mill wastewater by electrochemical pre-treatment: Effect on phytotoxicity and operating cost. J. Hazard. Mater. 2011, 190, 94–99. [Google Scholar] [CrossRef]
- Hanafi, F.; Assobhei, O.; Mountadar, M. Detoxification and discoloration of Moroccan olive mill wastewater by electrocoagulation. J. Hazard. Mater. 2010, 174, 807–812. [Google Scholar] [CrossRef]
- Sridhar, R.; Sivakumarb, V.; Immanuel, V.P.; Maran, J.P. Treatment of pulp and paper industry bleaching effluent by electrocoagulant process. J. Hazard. Mater. 2011, 186, 1495–1502. [Google Scholar] [CrossRef]
- Gonder, Z.B.; Balcioglu, G.; Vergili, I.; Kaya, Y. Electrochemical treatment of carwash wastewater using Fe and Al electrode: Techno-economic analysis and sludge characterization. J. Environ. Manag. 2017, 200, 380–390. [Google Scholar] [CrossRef]
| Parameters | Units | OOMW |
|---|---|---|
| COD | mg/L | 175,000 |
| BOD | mg/L | 25,000 |
| pH | - | 4 |
| Oil and Grease | mg/L | 13,000 |
| NH3-N | mg/L | 37 |
| TSS | mg/L | 99,000 |
| PO43− | mg/L | 57.5 |
| NO3− | mg/L | 395 |
| Type of Nuts | Electrode Material | Electrode Type | COD removal | Phenolic removal | References |
|---|---|---|---|---|---|
| Pistachio processing industry wastewater | Al | Unipolar | 60.1% | 77.3% | [143] |
| Pistachio processing industry wastewater | Graphite | Unipolar | 99.79% | 100% | [144] |
| Pistachio processing industry wastewater | Al | Unipolar | 57.4% | - | [134] |
| Pistachio processing industry wastewater | Al and stainless steel | Unipolar | 60% | 95% | [144] |
| Cashew nut processing industry wastewater | Fe, BDD (Boron Doped-Diamond) and stainless steel | Multipolar | 80% | - | [145] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakhmania; Kamyab, H.; Yuzir, M.A.; Abdullah, N.; Quan, L.M.; Riyadi, F.A.; Marzouki, R. Recent Applications of the Electrocoagulation Process on Agro-Based Industrial Wastewater: A Review. Sustainability 2022, 14, 1985. https://doi.org/10.3390/su14041985
Rakhmania, Kamyab H, Yuzir MA, Abdullah N, Quan LM, Riyadi FA, Marzouki R. Recent Applications of the Electrocoagulation Process on Agro-Based Industrial Wastewater: A Review. Sustainability. 2022; 14(4):1985. https://doi.org/10.3390/su14041985
Chicago/Turabian StyleRakhmania, Hesam Kamyab, Muhammad Ali Yuzir, Norhayati Abdullah, Le Minh Quan, Fatimah Azizah Riyadi, and Riadh Marzouki. 2022. "Recent Applications of the Electrocoagulation Process on Agro-Based Industrial Wastewater: A Review" Sustainability 14, no. 4: 1985. https://doi.org/10.3390/su14041985
APA StyleRakhmania, Kamyab, H., Yuzir, M. A., Abdullah, N., Quan, L. M., Riyadi, F. A., & Marzouki, R. (2022). Recent Applications of the Electrocoagulation Process on Agro-Based Industrial Wastewater: A Review. Sustainability, 14(4), 1985. https://doi.org/10.3390/su14041985










