Abstract
The present study investigated the performance of Pitahaya (Hylocereus spp.) peel (PP) as a low-cost biosorbent in the removal of Co(II), Cd(II), Pb(II), and Ni(II) from single and multi-component solutions. The characterization of the samples was carried out by pHpzc, Fourier transform infrared spectroscopy (FTIR), and scanning electron microscopy/energy-dispersive X-ray spectroscopy (SEM/EDS). Biosorption was carried out by batch experimental procedure to examine the effects of contact time, solution pH, initial concentration of metal ions, and biosorbent dosage. The results indicate that the biosorption of Pb(II), Cd(II), Co(II), and Ni(II) Pitahaya peels followed pseudo-second-order kinetics, and equilibrium adsorption followed the Langmuir model. The maximum sorption capacities of PP for the metallic species were found to be as follows: Pb (82.64 mg g−1) > Cd (17.95 mg g−1) > Co (6.013 mg g−1) > Ni (5.322 mg g−1). However, the efficiency of the biosorption change when the metallic species are mixed. The re-generation of the PP after the adsorption of the metallic species was done using 0.1 M HNO3 solution, and the reusability of the biomass was carried out using two adsorption and desorption cycles.
1. Introduction
During the last three decades, adsorption utilizing biomass-derived materials as adsorbents (biosorbents) has become an alternative and effective technique for the removal from water as well as wastewater of a plethora of pollutants, namely dyes, heavy metals, or metalloid species [1,2,3,4,5,6]. There are several advantages to using biosorbent materials, such as low cost, effectiveness, availability, chemical stability, short regeneration cycle, and environmental friendliness [1,2,4,5]. In addition, there is a wide range of abundantly available biomass that can be used towards the synthesis of biosorbents, depending on the nature of the precursor and the synthetic process [6]. The physicochemical features of the final material, like the textural features and the surface chemistry heterogeneity, can be tuned accordingly to target specific pollutants [7].
Activated carbon is a traditional and efficient adsorbent used widely throughout the world to remove various pollutants; however, commercial activated carbon use is restricted due to high associated costs [8].
Agricultural waste has attracted interest because it is inexpensive, available in large quantities, non-toxic, and eco-friendly. Some examples are rice husks [9], banana and orange peels [10,11], pistachio (Pistacia vera) waste [12], sugarcane bagasse [13,14], coffee beans [15], tea leaves or stalks [16], Cassia fistula leaves [17], Moringa Oleifera [18], Aloe vera [19] and others [3,20,21]. The surface chemistry heterogeneity can be elevated containing carboxyl, hydroxyl, sulfhydryl, and amido functional groups [21,22], while the surface area and total pore volumes can be high. These interesting characteristics allow the agricultural waste to exhibit more adsorption capacity than living biomass [23]. It is important to mention that natural polysaccharides from different sources, among them agricultural waste, are a better alternative as adsorbents for wastewater treatment to remove heavy metals [24,25]. In this sense Phuengphai et al. [26] used fruit peels to remove copper (II) from an aqueous solution.
Pitahaya (Selenicereus undatus), also known as dragon fruit, belongs to the Cactaceae family and is the most cultivated species in the genus Hylocereus spp. [27]. This plant is native to Mexico and South America, but today is grown in different countries within Southeast Asia and northern Australia [27,28]. Vietnam, China, Indonesia, Malaysia, Nicaragua, Mexico, and Israel are the leading pitahaya producers globally, with total production reaching around 1.2 million tons in 2019 [28,29]. The high world production of this fruit and its availability in Mexico make the pitahaya peel (PP) a potential source of biosorbent among other agricultural wastes.
Previous studies show that the pitahaya peel is rich in polyphenols and antioxidants with high concentrations of soluble and insoluble fibers (69.3%), with the main constituents being lignin (37.18 ±1.02%), starch (11.07 ± 10.03%), pectins (10.79 ± 0.01%) and cellulose (9.25 ± 1.33%) [30]. In addition, recent studies reported the effectiveness of lignin and lignin-based materials in removing heavy metals from aqueous solutions due to their chemical structure, which contains hydroxyl, methoxyl, and phenolic groups [31].
Although the chemical composition and nutritional value of PP are aspects widely presented and discussed in the literature, the potentiality for PP to be used as a precursor for biosorbents and especially against Pb(II), Cd(II), Ni(II), and Co(II) metals from mono and multicomponent solutions has not been investigated as well as its regeneration and reusability. Hence, the objective of this study was to obtain PP derived biosorbents, determine the key physicochemical features, and evaluate their adsorptive efficiency by varying/optimizing the contact time, the initial pH of the dispersions, the initial metal ion concentration, and the mass of PP loading on sorption capacity, always considering each metallic species separately and in a mixture of them to know the affinity of this no-living biomass by each metallic species. The regeneration and reusability of the PP were part of the novelty of this investigation.
2. Materials and Methods
2.1. Materials and Chemicals
The pitahaya fruit was purchased from a local market in Ciudad del Carmen, Campeche, Mexico (20 units), washed, and manually separated in pulp and peel. The pitahaya peel (PP) was washed several times with distilled water to remove the adhering dirt and subsequently was dried in the sun for several days at a temperature between 30 °C and 34 °C. Then, it was crushed using a food grinder to obtain a powder form which passed through sieves of 100 mesh and was stored in glass bottles for further studies.
The synthetic stock solutions of Pb(II), Cd(II), Co(II), and Ni(II) at a concentration of 1000 mg/L were obtained by dissolving an appropriate quantity of PbCl2 (98%, Merck), CdCl2·2.5H2O (98%, Sigma-Aldrich), CoCl2·6H2O (99.7%, J.T. Baker), and NiCl2·6H2O (97%, Riedel de Haën) in deionized water (resistivity, 18.2 MΩ cm), respectively, and then diluted for the further necessary solution preparation.
2.2. Characterization Techniques
Scanning electron microscopy (Hitachi S4300N) coupled with energy-dispersive X-ray spectroscopy (EDS) were used to study PP surface morphology and chemical composition. The functional groups present on the PP surface were obtained by Fourier transform infrared spectroscopy (Nicolet Nexus 670 FT-IR) within the range of 4000–400 cm–1 using the KBr standard method.
2.3. Zero-Point Charge (pHPZC)
The procedure to determine the pHPZC consisted of an addition 0.1 g of PP to 50 mL of 0.01 M NaCl at different pH values. The initial pH (pHinitial) values were adjusted from 2 to 11 (±0.1) by adding drops of 1.0 M HCl or 1.0 M NaOH. The mixtures were then agitated for 24 h at ambient temperature, and the final pH (pHfinal) value of each solution was determined using a pH meter (Thermo Scientific ORION 3-star pH Benchtop, Waltham, MA, USA). The pHPZC is obtained by the intersection of plotting the pHfinal vs. the pHinitial curve with the diagonal curve (pHinitial = pHinitial) [18].
2.4. Biosorption Experiments
Biosorption experiments were carried out by the batch technique. The effect of contact time was conducted at ambient temperature without variation of pH by the addition of 0.10 g of PP with 10 mL of metal ion solutions of 100 mg/L. The mixture was agitated in centrifuge tubes at 150 rpm using a rotary shaker for 5, 10, 15, 30, 60, 120, 180, 240, 360, 720, and 1440 min. After each contact time, the samples were centrifuged at 3500 rpm for 5 min, and the supernatant solutions were separated from PP for further analysis using the flame atomic absorption spectroscopy (FAAS, Thermo Scientific iCE 3000 Series).
The removal percentage (%R) and the amount of metallic ions sorbed per gram of PP at each contact time t, qt(mg/g), were calculated using the Equations (1) and (2):
where Ci (mg/L) and Cf (mg/L) are the initial and residual metal ion concentrations, respectively, V(L) is the volume of the metal ion solution, and m(g) is the PP mass.
The effect of pH solution on the sorption capacity was studied varying the pH solution from 2 to 8.5, fixing contact time, mass of PP, and initial concentration of metals at 24 h, 0.10 g and 100 mg/L, respectively. The PP mass’s effect on the sorption capacity was carried out in the range of 0.05–0.3 g. Each mass of biosorbent was mixed with 10 mL of metallic solutions at 100 mg/L. The samples were agitated during 24 h without varying the pH of solution. For isotherm study, the samples were agitated over 24 h in order to ensure that the equilibrium is attained, keeping invariant the solution pH (measured at pH 5.5). The mass of pithaya peel was the same as was used for the kinetic study (0.1 g).
The quaternary system of Pb(II), Cd(II), Co(II), and Ni(II) with an initial concentration of 100 mg/L was added to 0.1 g of pitahaya peel, and the mixture was agitated for 180 h at room temperature.
For the multi-component system, the sorption study was performed fixing contact time at 24 h, mass of biosorbent at 0.1 g, and initial metallic concentration at 100 mg/L without varying the pH of aqueous solution.
All experiments were performed by duplicate in order to verify the truthfulness of the experiment results.
2.5. Desorption and Regeneration Experiments
Desorption of metal ions and the possibility of PP regeneration were investigated. The following experimental procedure is similar to that described by Cima et al. [32]: 0.5 g of PP was added to 50 mL of each of the metal ions of 100 mg/L. The mixtures were shaken for 24 h at room temperature, then the PP biosorbent was separated by filtration, and the filtrates were analyzed for metal ions by FAAS. The dry PP biosorbent was weighed and placed in contact with 50 mL 0.01 M HCl. After 2 h of shaking, the samples were filtered, and the concentrations of desorbed metal ions were determined in the filtrates. The PP biosorbent was washed several times with deionized water to eliminate the acid excess before being reused in the next sorption/desorption cycle.
3. Results
3.1. Characterization
3.1.1. Zero-Point Charge (pHPZC)
The variation of the initial pH (pHinitial) versus the final pH (pHfinal) is shown in Figure 1. The point of intersection of this curve with the diagonal curve (pHinitial= pHfinal) corresponds to a zero-point charge (pHPZC). In this study, the pHPZC of Pitahaya peel was found to be 6.11. This value is similar to that of other various agricultural residues [6,22,33]. Therefore, when the pH of the solution is below 6.11, the surface of PP has a positive net charge, and thus the biosorption of cations can be unfavorable. In contrast, the surface of PP becomes negatively charged when the pH of the solution is above 6.11, and consequently, the cations can be favorably adsorbed [15].
We decided to perform the experiments at a pH slightly lower than the point of zero charge, so as to avoid the phenomena of precipitation in the case of all the studied metal cations and so that the initial pH would be the same for all the experiments. This does not mean that at this pH, the adsorption extent would be blocked. On the contrary, the recorded and reported removal efficiencies can be further enhanced by optimizing the pH (initial or final). A similar approach was followed previously [34], it was found that even at a pH close to and below the point of zero charge, the adsorption ability was almost the same and high when the experiments were performed at a pH slightly above the pHPZC.
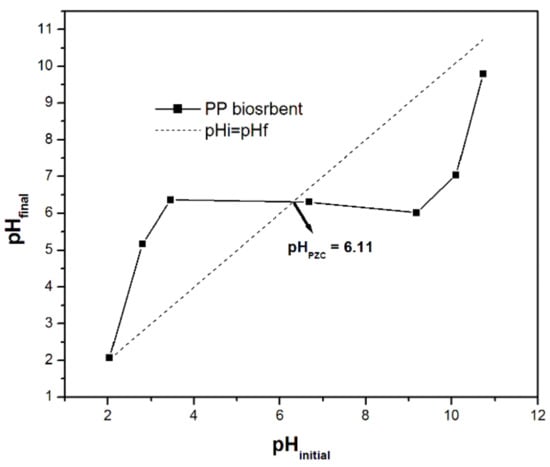
Figure 1.
pHPZC of PP biosorbent.
3.1.2. FT-IR Spectra of Pitahaya Peel
The FT-IR spectra of dried pitahaya peel before and after contact with Pb(II), Cd(II), Co(II), and Ni(II) are presented in Figure 2. The broadband located at 3315 cm−1 suggests the presence of hydroxyl groups on the surface of the pitahaya peel [35]. The band at 2916 cm−1 can be assigned to (–CH2) and (–CH3) vibrations of aliphatic groups [36]. The strong band observed at 1615 cm−1 is related to the C=O functional group, which can be linked to ester or/and carbonyl groups [37]. The bands found at 1322 cm−1 and 1027 cm−1 can be associated with C–O stretching vibrations in phenolic groups [35,36]. According to previous studies, carboxyl and hydroxyl functional groups are mainly responsible for heavy metal biosorption from aqueous solutions, since they provide active sites for surface complexation with metal ions, for an ion exchange process, or for a combination of both as a principal interaction between the functional groups and metal ions [10,38,39].
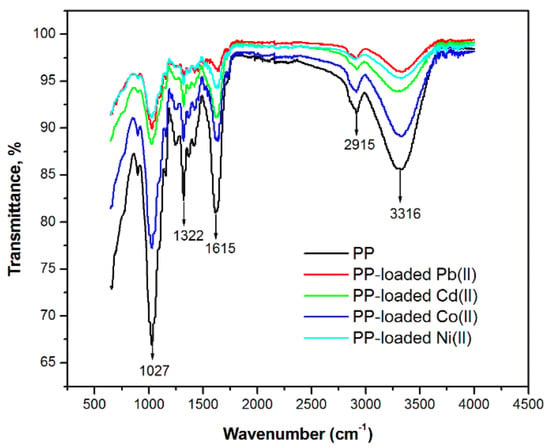
Figure 2.
FT-IR spectra of pitahaya peel before and after biosorption of Pb(II), Cd(II), Co(II), and Ni(II).
Table 1 presents a comparison between the FTIR spectra before and after metal ion biosorption. The results indicate that the position of the bands’ maxima related to the O-H and C=O functional groups were shifted by 15–19 cm−1 and 5–18 cm−1, respectively, confirming the participation of hydroxyl and carbonyl groups in the sorption of Pb(II), Cd(II), Co(II) and Ni(II). Similar results were reported in previous studies [35,37,39].

Table 1.
Main functional groups of Pitahaya peel and their loaded metal ions.
3.1.3. Scanning Electron Microscopy
The images in Figure 3a–f show the surface texture and morphology of pitahaya peel before and after uptake of metal ions with its respective EDS analysis. It can be seen that the surface of the pitahaya peel is irregular and present heterogeneously in size cages and cracks. Similar structural and morphological features were observed in other biosorbents, which played a key role in retaining metal ions [17,40,41]. In general, the presence of the observed cages and surface cracks can have a positive impact on the removal efficiency by providing pathways towards the reactive adsorption sites and helping mass transfer phenomena within the body of the material.
The EDS spectra of PP (Figure 3a) show the predominant peaks of carbon and oxygen, with other minor peaks of calcium, potassium, nitrogen, magnesium, chloride, sulfur, and aluminum. These elements were also detected on the surface of PP before the biosorption process. The uptake of metal ions on PP’s surface after the biosorption process was confirmed by the presence of additional peaks characteristic to each metal ion (Figure 3b–f).
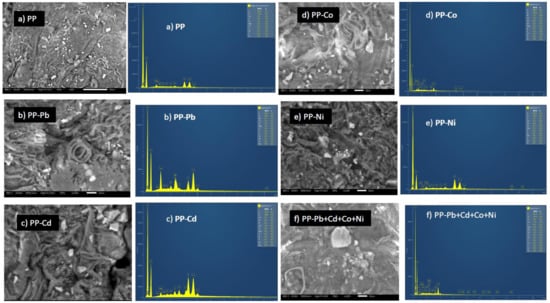
Figure 3.
SEM images and EDS spectra of pitahaya peel: (a) before biosorption and (b–e) after biosorption of Pb(II), Cd(II), Co(II), and Ni(II), respectively, as well as after adsorption of Pb(II), Cd(II), Co(II), and Ni(II) simultaneously (f).
3.2. Sorption of the Metallic Chemical Species from Aqueous Solutions
3.2.1. pH Effect
Figure 4 shows the percent of heavy metals removed by pitahaya peel at different values of pH solutions. As can be seen in Figure 4, the biosorption percent of Pb(II), Cd(II), Cd(II) and Ni(II) increases from 66%, 54%,19%, and 5% to 77%, 63%, 30% and 11%, respectively, with increasing pH values in the range 2–8.5. The lower biosorption capacity of the PP at lower acidic pH can be attributed to both (a) the competition between the metal ions charged positively and the protons for the biosorption site of the biosorbent [37], and (b) the electrostatic repulsion between the positive surface charge below the pHpzc and the cationic metallic species. As the pH of the solution is increased up to the pHpzc(6.11), the surface of the Pitahaya peel becomes negatively charged, thus the biosorption of metal ions is increased due to the attraction of electrostatic forces [42]. It is important to consider that when the pH increased between 0 to 6.5, the degree of positive charge on the surface was decreasing [34], and for this reason the amount of heavy metals adsorbed did not vary drastically from pH 5.5 to 6.5. In addition, at a high pH value, the metal ions in their hydroxide forms are precipitated on the surface of the pitahaya peel [26]. Therefore, the proposal mechanisms for the metallic species removal by PP could be ion exchange, chemisorption, and precipitation.
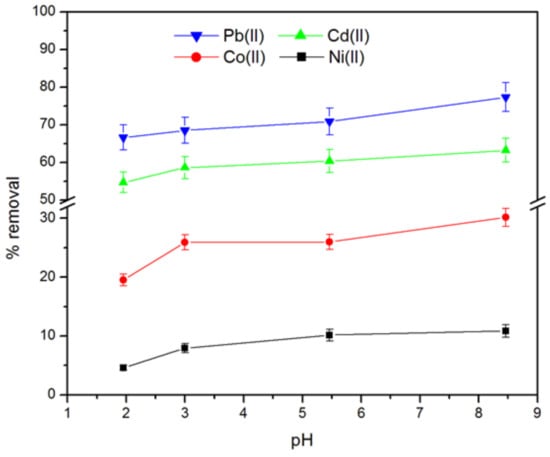
Figure 4.
Effect of initial pH on removal of Pb(II), Cd(II), Co(II) and Ni(II) (mass of biosorbent = 0.1 g, ambient temperature, initial concentration of metallic ions = 100 mg L−1, and contact time = 24 h).
3.2.2. Kinetics
Figure 5 shows the amount of metallic ions sorbed per gram of pitahaya peel from aqueous solutions as a function of contact time. It can be observed from this figure that more than 80% of Pb(II), Cd(II), Co(II), and Ni(II) are removed within the first 30 min, revealing fast kinetics at the initial stage. As time increases further, the removal extent increases but to a lesser extent. The high uptake of heavy metal ions in a short time after contact with pitahaya peel is considered one of the most critical advantages of the biosorption technique from the economic wastewater treatment point of view. Similar kinetic behavior has been reported in other studies, in which it was found that the equilibrium time depends on the metal ions and agricultural waste types [17,43].
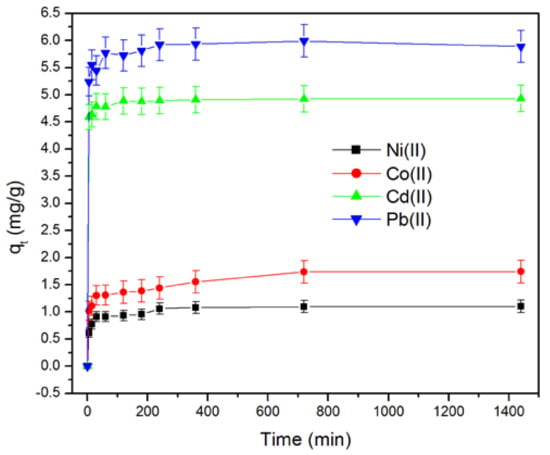
Figure 5.
Effect of contact time on heavy metal ion removal by PP (mass of PP = 0.1 g, initial concentration of metallic ions = 100 mg.L−1, room temperature, and pH = 5.5).
Several studies have suggested that the high uptake of heavy metal ions in the first stage is due to the large number of free binding sites on biosorbent surfaces, which are gradually occupied with increasing contact time until equilibrium was reached [17,41,43,44,45].
This work analyzes experimental kinetic data of metal ions on pitahaya peel biosorbent by pseudo-first-order, pseudo-second-order, Elovich, and intraparticle diffusion kinetic models. Table 2 presents the non-linear and linear expressions of these models.

Table 2.
Non-linear and linear equations of pseudo-first-order, pseudo-second-order, Elovich, and intraparticle diffusion kinetic models.
Where qe (mg/g) and qt (mg/g) are amounts of the metal ions sorbed at equilibrium and at time t, respectively, k1(min−1) and k2(g.mg−1·min−1) are the pseudo-first and pseudo-second-order rate constants, respectively, α represents the initial adsorption rate (mg. g−1.min−1), β is the desorption constant (g.mg−1), kid (mg. g−1.min−0.5) represents the constant of the intraparticle diffusion model.
The values of the constants of pseudo-first-order (k1, qe) and pseudo-second-order (k2, qe), (α, β) and (kid, C) for Pb(II), Cd(II), Co(II), and Ni(II) with their corresponding determination coefficients (R2) were calculated using a linear equation and the estimated parameters are tabulated on Table 2. From Table 3, it can be observed that the pseudo-second-order equation better fitted the experimental kinetic data on Pb(II), Cd(II), Co(II), and Ni(II). Furthermore, the values of the determination coefficients for the pseudo-second order kinetic model are near 1 (R2 ≥ 0.999) compared to those obtained from the fitting by the pseudo-first-order kinetic model (R2 0.551–0.914). Moreover, the values of the calculated amounts of metals ions (qe,cal) obtained from the pseudo-second order model agree with the experimental values (qe,exp). These results confirmed that the pseudo-second-order model could satisfactorily describe the kinetic data and the values of the pseudo-second-order sorption rate constants (k2) presented the following order: Pb(II) > Cd(II) > Co(II) > Ni(II). According to numerous reports, the pseudo-second-order kinetic model provided a better correlation for the experimental data [6,8,15,17,33,43,45].

Table 3.
Kinetic parameters of the Pb(II), Cd(II), Co(II), and Ni(II) sorption onto pitahaya peel.
3.2.3. Mass Effect
Figure 6 shows the effect of PP mass on the removal of Pb(II), Cd(II), Co(II), and Ni(II). It can be observed that the percent removal of metals ions increases with increasing biosorbent mass. As shown in Figure 6, when the PP mass increases from 5.0 to 30.0 mg/L, the percent removal increases from 63–79%, 53–75%, 16–26% and 13–24% for Pb(II), Cd(II), Co(II) and Ni(II), respectively. Similar behavior was reported by other researchers, which can be attributed to the increase in surface area available for sorption [33,46].
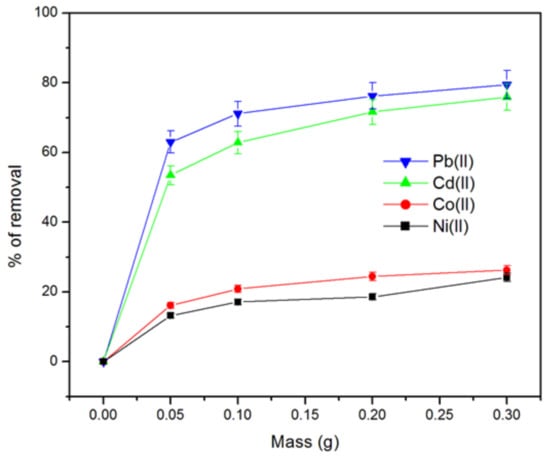
Figure 6.
Influence of PP biosorbent dose on the removal of Pb(II), Cd(II), Co(II) and Ni(II) (initial concentration of metallic ions = 100 mg.L−1, ambient temperature, pH = 5.5, and contact time = 24 h).
3.2.4. Isotherms
Sorption isotherm fitting is usually used to evaluate the maximum biosorption capacity of sorbent [47]. In this study, experimental isotherms for Pb(II), Cd(II), Co(II), and Ni(II) onto pitahaya peel were conducted at ambient temperature without adjusting pH (pH of the solutions was around 6) and with a mass of biosorbent of 0.1 g.
Figure 7 depicts the relationship between the amount of metal ions adsorbed on pitahaya peel (qe) and its equilibrium concentration in aqueous solutions (Ce). It can be seen that the amount of Pb(II), Cd(II), Co(II), and Ni(II) sorbed on PP increases with increasing concentration of the metal ion solution at equilibrium.
In this study, Langmuir and Freundlich’s models were used to analyze the experimental data. The non-linear and linear expressions for these models are presented in Table 4.

Table 4.
Non-linear and linear equations of Langmuir and Freundlich isotherms.
Where qe (mg/g) is the amount of metal ions adsorbed at equilibrium, Ce (mg/L) is the concentration of metal ion solution at equilibrium, qm (mg/g) is the theoretical maximum monolayer adsorption capacity, KL(L/mg) is the affinity coefficient of the sorbent for the solute, KF (mg/g(L/mg)1/n) is the Freundlich constant associated with the adsorption capacity, and n is the heterogeneity factor.
Table 5 lists the Langmuir and Freundlich biosorption constants with their corresponding determination coefficients (R2). As can be seen in Figure 8a and Figure 8b, the Langmuir model provides a better fit to the experimental data for adsorption of Pb(II), Cd(II), Co(II), and Ni(II) onto pitahaya peel compared to the Freundlich model [48]. As indicated in Table 5, in all the cases, the values of the correlation coefficients of the Langmuir plots were found in the range of 0.994 to 0.998, which were higher than those obtained from the Freundlich model (R2 = 0.969–0.989). The Langmuir sorption model is commonly used to describe the biosorption equilibrium, a fundamental assumption considered on monolayer coverage of adsorbate on the adsorbent surface [47]. According to the results presented in Table 5, the maximum uptake capacity (qm) for Pb(II), Cd(II), Co(II), and Ni(II) were 82.64, 17.95, 6.01, and 5.32 mg/g, respectively, which are higher than some other biosorbents reported in previous studies (Table 6).
According to the qm value, the selectivity order is Pb(II) > Cd(II) > Co(II) > Ni(II), which follows the adsorption of the same metals by Moringa oleifera biomass [18].
In the case of lead, the experimental qm was found to be 30 mg/g, lower than qm calculated from the Langmuir model (82.64 mg/g), indicating that biomass was not fully covered by the metal [48].
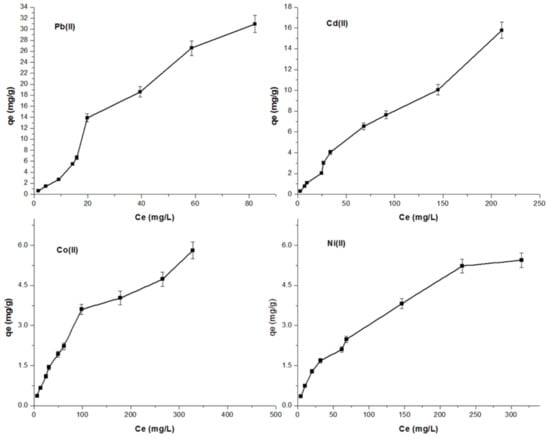
Figure 7.
Sorption isotherms of Pb(II), Cd(II), Co(II) and Ni(II) (mass of biosorbent = 0.1 g, room temperature, pH = 5.5, and contact time = 24 h) using PP as adsorbent.
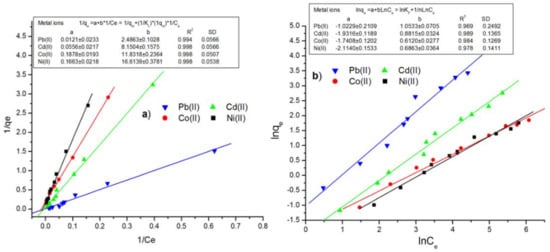
Figure 8.
Linear fitting of (a) Langmuir and (b) Freundlich adsorption isotherm for Pb(II), Cd(II), Co(II), and Ni(II) using PP.

Table 5.
Langmuir and Freundlich isotherm parameters for Pb(II), Cd(II), Co(II) and Ni(II) using PP.
Table 5.
Langmuir and Freundlich isotherm parameters for Pb(II), Cd(II), Co(II) and Ni(II) using PP.
| Isotherm Models | Metal Ions | |||
|---|---|---|---|---|
| Pb(II) | Cd(II) | Co(II) | Ni(II) | |
| Langmuir | ||||
| qm(mg g−1) | 82.64 | 17.95 | 6.013 | 5.322 |
| KL (L mg−1) | 0.487 | 0.683 | 1.001 | 1.588 |
| R2 | 0.994 | 0.998 | 0.998 | 0.998 |
| Freundlich | ||||
| n | 0.949 | 1.134 | 1.147 | 1.634 |
| KF(mg(n−1)/n L1/ng−1) | 0.359 | 0.145 | 0.121 | 0.175 |
| R2 | 0.969 | 0.989 | 0.978 | 0.984 |

Table 6.
Comparison of the maximum metal uptake capacities of different peel fruits Pb(II), Cd(II), Co(II), and Ni(II) using PP.
Table 6.
Comparison of the maximum metal uptake capacities of different peel fruits Pb(II), Cd(II), Co(II), and Ni(II) using PP.
| Peel Fruits | Maximum Metal Uptake Capacity (mg g−1) | Refs | |||
|---|---|---|---|---|---|
| Pb(II) | Cd(II) | Co(II) | Ni(II) | ||
| Modified orange | 476.1 | 293.3 | ----- | 162.6 | [10] |
| Banana | 2.18–41.44 | 5.71–98.4 | 4.75–20.97 | 6.88–8.91 | [11,49] |
| Kiwi | ----- | 15.87 | ----- | ----- | [50] |
| Tangerine | ----- | 17.54 | ----- | ----- | [50] |
| Orange | 7.97 | ---- | 1.82 | 6.88 | [51] |
| Pummelo | ----- | 21.83 | ----- | ----- | [49] |
| Durian | ----- | 18.55 | ----- | ----- | [49] |
| Mango | 99.05 | 68.92 | ----- | 39.75 | [52] |
| Pomegranate | ----- | ----- | ----- | 52.00 | [52] |
| Pitahaya (In this study) | 82.64 | 17.95 | 6.01 | 5.32 | |
3.3. Single and Multi-Component Removal
The sorption capacity of the biosorbent in the multi-component system is highly interesting because, generally, the contaminated waste streams contain multiple heavy metals with different concentrations. In the present work, the sorption of metallic species by PP was also investigated. As seen in Figure 9, the amount of Pb(II), Cd(II), Co(II), and Ni(II) in mg/g biosorbed from aqueous solution on pitahaya peel is diminished from 5.985, 4.482, 1.728, 1.455 to 1.378, 1.235, 0.441 and 0.378, respectively, when all metal ions are simultaneously present in aqueous solution. The reduction of the biosorption capacity of pitahaya peel in the multi-component system is due to the possible competition between metal ions on the adsorption sites of the adsorbent (PP). This result indicates that the presence of more than one metal ion in an aqueous solution affects the sorption capacity of the biosorbent besides the biomass surface properties and other physicochemical factors of the solution (pH and temperature and concentration of solute) [53,54].
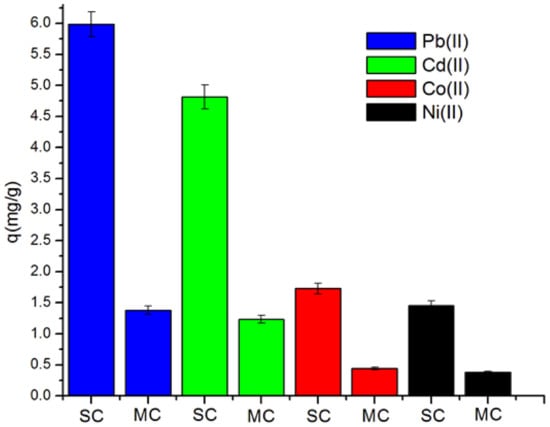
Figure 9.
Comparison of sorption capacity of heavy metal ions from single- and multi-component solutions. (SC and MC, respectively). Mass of biosorbent = 0.1 g, ambient temperature, initial concentration of metallic ions = 100 mg L−1, pH = 5.5, and contact time = 24 h.
3.4. Sorption–Desorption Cycles
The reuse of biosorbent materials is useful to evaluate the cost-effectiveness of these materials for the treatment of industrial wastewater [47,55,56]. Two consecutive adsorption–desorption experiments in batches were carried out in this work. As shown in Figure 10, the sorption of metal ions decreases from the first to the second cycle. The amounts of Pb(II), Cd(II), Co(II), and Ni(II) sorbed in the first and second cycles were 6.56–0.71 mg/g, 6.107–4.102 mg/g, 1.630–1.462 mg/g, and 1.131–0.813 mg/g, respectively. Similar results were reported in previous studies, in which the decrease of the sorption capacity of the biosorbent was attributed to the irreversibility of metal ions previously adsorbed in the prior cycle [56]. Desorption efficiencies of Pb(II), Cd(II), Co(II), and Ni(II) in the first and second cycle were 58.60–8.95%, 69.40–61.98%, and 77.50–85.84%, respectively. This result confirms the efficiency of HCl (1 M) as a metal desorbing agent by removing bound metal ions from active sites on the surface of the biosorbent [57].
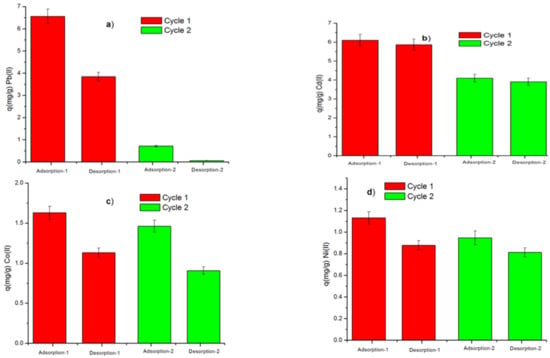
Figure 10.
Sorption–desorption cycles of (a) Pb(II), (b) Cd(II), (c) Co(II) and (d) Ni(II). (Mass of biosorbent = 0.1 g, ambient temperature, initial concentration of metallic ions = 100 mg L−1, pH = 5.5, and contact time = 24 h).
4. Conclusions
The uptake of metal ions was evidenced utilizing FT-IR analysis, where it is suggested that there was an interaction between the carboxyl and hydroxyl functional groups on the surface of pitahaya peel and metal ions. SEM analysis revealed a heterogeous and amorphous surface with cracks, while the openings of cages were also visible. Their presence had a positive impact by elavating mass transfer phenomena and hence the movement of the metal cations towards the active adsorption sites.
The sorption capacity of metal ions is dependent on contact time, solution pH, the mass of pitahaya peel, and initial metal concentration. Therefore, the increase in these parameters leads to higher uptake capacities.
The pseudo-second-order kinetic equation is the most appropriate model to describe the sorption process of Pb(II), Cd(II), Co(II), and Ni(II) by the pitahaya peel. Furthermore, the sorption isotherm data fitted well with the Langmuir isotherm, suggesting that the biosorption of heavy metals was dominated by the formation of a monolayer, with maximum uptake capacities of Pb(II), Cd(II), Co(II), and Ni(II) being 83 mg/g, 18 mg/g, 6 mg/g, and 5 mg/g, respectively.
The pitahaya peel diminishes its biosorption capacity for metallic chemical species from the multi-component aqueous system in 77%, 72%, 74%, and 74% for Pb(II), Cd(II), Co(II), and Ni(II), respectively.
Pitahaya peel is regenerated using HCl solution (0.1 N) and is reused for two sorption/desorption cycles. Desorption efficiency of Pb(II), Cd(II), Co(II), and Ni(II) in the first cycle was 58.60%, 69.40%, 61.98%, and 78%, respectively, whereas in the second cycle, the metallic ions desorbed were 8.95%, 62%, and 85.84%, respectively.
According to these results, it can be concluded that the pitahaya peel could be considered a promising agricultural waste and environmentally friendly material for the removal of Pb(II), Cd(II), Co(II), and Ni(II) from aqueous solution.
Author Contributions
M.A. and J.V. conceived and planned the experiments; J.V. and M.T.O. contributed to the characterization of the samples. M.A. wrote the manuscript with input from all authors. D.A.G., E.C.L., M.T.O., J.V., I.A. (Ioannis Anastopoulos) and I.A. (Ismeli Alfonso) have contributed in the interpretation of the results. All authors provided critical feedback and helped shape the research, analysis and manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by National Institute of Nuclear Research “Nuevos sorbentes a base de zeolitas naturales y sargazo entre otros, para la disminución de contaminantes radiactivos, no-radiactivos y microbiológicos del agua, que representen un problema de salud pública”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Elgarahy, A.M.; Elwakeel, K.Z.; Mohammad, S.H.; Elshoubaky, G.A. A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Clean. Eng. Technol. 2021, 4, 100209. [Google Scholar] [CrossRef]
- Jain, C.K.; Malik, D.S.; Yadav, A.K. Applicability of plant based biosorbents in the removal of heavy metals: A review. Environ. Process. 2016, 3, 495–523. [Google Scholar] [CrossRef]
- Yadav, S.; Yadav, A.; Bagotia, N.; Sharma, A.K.; Kumar, S. Adsorptive potential of modified plant-based adsorbents for sequestration of dyes and heavy metals from wastewater—A review. J. Water Process Eng. 2021, 42, 102148. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef] [PubMed]
- Asgher, M. Biosorption of Reactive Dyes: A Review. Water Air Soil Pollut. 2012, 223, 2417–2435. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Pashalidis, I.; Hosseini-bandegharaei, A.; Giannakoudakis, D.A.; Robalds, A.; Usman, M.; Escudero, L.B.; Zhou, Y.; Colmenares, J.C.; Nunez-Delago, A.; et al. Agricultural biomass/waste as adsorbents for toxic metal decontamination of aqueous solutions. J. Mol. Liq. 2019, 295, 111684. [Google Scholar] [CrossRef]
- Gunarathne, V.; Ashiq, A.; Ginige, M.P. Green Adsorbents for Pollutant Removal. Environ. Nanotechnol. 2018, 18, 53. Available online: http://link.springer.com/10.1007/978-3-319-92111-2 (accessed on 9 January 2022).
- Anastopoulos, I.; Pashalidis, I. Environmental applications of Luffa cylindrica-based adsorbents. J. Mol. Liq. 2020, 319, 114127. [Google Scholar] [CrossRef]
- Abbas, M.N.; Abass, S. Rice Husks as a Biosorbent Agent for Pb 2 + Ions From Contaminated Aqueous Solutions: A Review. Biochem. Cell Arch. 2020, 20, 1813–1820. [Google Scholar]
- Feng, N.; Guo, X.; Liang, S.; Zhu, Y.; Liu, J. Biosorption of heavy metals from aqueous solutions by chemically modified orange peel. J. Hazard. Mater. 2011, 185, 49–54. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Conradie, J. Banana peel as a biosorbent for the decontamination of water pollutants. A review. Environ. Chem. Lett. 2020, 18, 1085–1112. [Google Scholar] [CrossRef]
- Adaobi, C.; Ighalo, J.O.; Ahmadi, S.; Engineering, E.H.; Ugonabo, V.I. Pistachio (Pistacia vera) waste as adsorbent for wastewater treatment: A review. Biomass Convers. Biorefnery 2021, 42, 1–19. [Google Scholar]
- Ngah, W.W.; Hanafiah, M.M. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: A review. Bioresour Technol. 2008, 99, 3935–3948. [Google Scholar] [CrossRef] [PubMed]
- Bagotia, N.; Sharma, A.K.; Kumar, S. A review on modified sugarcane bagasse biosorbent for removal of dyes. Chemosphere 2021, 268, 129309. [Google Scholar]
- Anastopoulos, I.; Karamesouti, M.; Mitropoulos, A.; Kyzas, G. A review for coffee adsorbents. J. Mol. Liq. 2017, 229, 555–565. [Google Scholar] [CrossRef]
- Liakos, E.V.; Rekos, K.; Giannakoudakis, D.A.; Mitropoulos, A.C.; Fu, J.; Kyzas, G.Z. Activated Porous Carbon Derived from Tea and Plane Tree Leaves Biomass for the Removal of Pharmaceutical Compounds from Wastewaters. Antibiotics 2021, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Suddique, M.; Shah, G.M.; Ahmad, I.; Murtaza, B.; Shah, N.S.; Mubeen, M.; Ahmad, S.; Zakir, A.; Schotting, R.J. Kinetic and equilibrium studies for cadmium biosorption from contaminated water using Cassia fistula biomass. Int. J. Environ. Sci. Technol. 2018, 16, 3099–3108. [Google Scholar] [CrossRef]
- Abatal, M.; Olguin, M.; Anastopoulos, I.; Giannakoudakis, D.; Lima, E.; Vargas, J.; Aguilar, C. Comparison of Heavy Metals Removal from Aqueous Solution by Moringa oleifera Leaves and Seeds. Coatings 2021, 11, 508. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Hosseini-Bandegharaei, A.; Tsafrakidou, P.; Triantafyllidis, K.S.; Kornaros, M.; Anastopoulos, I. Aloe vera waste biomass-based adsorbents for the removal of aquatic pollutants: A review. J. Environ. Manag. 2018, 227, 354–364. [Google Scholar] [CrossRef]
- Bayuo, J. An extensive review on chromium (vi) removal using natural and agricultural wastes materials as alternative biosorbents. J. Environ. Health Sci. Eng. 2021, 19, 1193–1207. [Google Scholar] [CrossRef]
- Mussa, M.; Mateso, S.; Chebude, Y. Potentials of agricultural wastes as the ultimate alternative adsorbent for cadmium removal from wastewater. A review. Sci. Afr. 2021, 13, e00934. [Google Scholar]
- Pathak, P.; Mandavgane, S.; Kulkarni, B.D. Fruit Peel Waste: Characterization and its Potential Uses. Curr. Sci. 2017, 113, 444–454. [Google Scholar] [CrossRef]
- Gadd, G.M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, Z.; Yang, Y.; Fang, G.; Yao, J.; Shao, Z.; Chen, X. Robust Protein Hydrogels from Silkworm Silk. ACS Sustain. Chem. Eng. 2016, 4, 1500–1506. [Google Scholar] [CrossRef]
- Maity, S.; Biswas, R.; Verma, S.K.; Sarkar, A. Natural polysaccharides as potential biosorbents for heavy metal removal. Food Med. Environ. Appl. Polysacch. 2021, 627–665. [Google Scholar]
- Phuengphai, P.; Singjanusong, T.; Kheangkhun, N.; Wattanakornsiri, A. Removal of copper(II) from aqueous solution using chemically modified fruit peels as efficient low-cost biosorbents. Water Sci. Eng. 2021, 14, 286–294. [Google Scholar] [CrossRef]
- De Ello, F.R.; Bernardo, C.L.; Dias, C.O.; Zuge, L.C.B.; Silveira, J.L.M.; Amante, E.R.; Canido, L.M.B. Evaluation of the chemical characteristics and rheological behavior of pitaya (Hylocereus undatus) peel. Exot. Fruits 2014, 69, 381–390. [Google Scholar] [CrossRef]
- Mercado-Silva, E.M. Pitaya—Hylocereus undatus (Haw). In Exotic Fruits Reference Guide; Elsevier: Amsterdam, The Netherlands, 2018; pp. 333–338. [Google Scholar]
- Antonio, P. Mercado y Consumo de Fruta del Dragón en 2020. 2020, pp. 1–14. Available online: https://avogoconsulting.com (accessed on 26 September 2021).
- Jamilah, B.; Shu, C.E.; Kharidah, M.; Dzulkifly, M.A.; Noranizan, A. Physico-chemical characteristics of red pitaya (Hylocereus polyrhizus) peel. Int. Food Res. J. 2011, 286, 279–285. [Google Scholar]
- Naseer, A.; Jamshaid, A.; Hamid, A.; Muhammad, N.; Ghauri, M.; Iqbal, J.; Rafiq, S.; Khuram, S.; Shah, N.S. Lignin and Lignin Based Materials for the Removal of Heavy Metals from Waste Water-An Overview. Z. Phys. Chem. 2019, 233, 315–345. [Google Scholar] [CrossRef]
- Cimá-Mukul, C.A.; Olguín, M.T.; Abatal, M.; Vargas, J.; Barrón-Zambrano, J.A.; Ávila-Ortega, A. Assessment of leucaena leucocephala as bio-based adsorbent for the removal of pb2+, cd2+ and ni2+ from water. Desalin Water Treat. 2020, 173, 331–342. [Google Scholar] [CrossRef]
- Rauf, A.; Mahmud, T.; Ashraf, M.; Rehman, R.; Basharat, S. Sorption studies on removal of Cd2+ from the aqueous solution using fruit-peels of Litchi chinensis Sonn. Desalination Water Treat. 2020, 187, 277–286. [Google Scholar] [CrossRef]
- Erdem, M.; Ucar, S.; Karagöz, S.; Tay, T. Removal of Lead (II) Ions from Aqueous Solutions onto Activated Carbon Derived from Waste Biomass. Sci. World J. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaghetti, J.C.P.; Lima, E.C.; Royer, B.; Cunha, B.M.; Cardoso, N.F.; Brasil, J.L.; Dias, S.L.P. Pecan nutshell as biosorbent to remove Cu (II), Mn (II) and Pb (II) from aqueous solutions. J. Hazard Mater. 2009, 162, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Pehlivan, E.; Arslan, G. Removal of metal ions using lignite in aqueous solution—Low cost biosorbents. Fuel Process Technol. 2007, 88, 609–615. [Google Scholar] [CrossRef]
- Villen-Guzman, M.; Gutierrez-Pinilla, D.; Gomez-Lahoz, C.; Vereda-Alonso, C.; Rodriguez-Maroto, J.; Arhoun, B. Optimization of Ni (II) biosorption from aqueous solution on modified lemon peel. Environ. Res. 2019, 179, 108849. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Sillanpää, M.; Witek-krowiak, A. Agricultural waste peels as versatile biomass for water purification—A review. Chem. Eng. J. 2015, 270, 244–271. [Google Scholar] [CrossRef]
- Kayranli, B.; Gok, O.; Yilmaz, T.; Gok, G.; Celebi, H.; Seckin, I.Y.; Kalat, D. Zinc Removal Mechanisms with Recycled Lignocellulose: From Fruit Residual to Biosorbent then Soil Conditioner. Water Air Soil Pollut. 2021, 232, 1–15. [Google Scholar] [CrossRef]
- Morosanu, I.; Teodosiu, C.; Paduraru, C.; Ibanescu, D.; Tofan, L. Biosorption of lead ions from aqueous ef fl uents by rapeseed biomass. New Biotechnol. 2017, 39, 110–124. [Google Scholar] [CrossRef]
- Singh, R.J.; Martin, C.E.; Barr, D.; Rosengren, R.J.; Jasmine, R.; Martin, C.E.; Bar, D.; Rosengren, R.J. Immobilised apple peel bead biosorbent for the cocktail solution. Cogent. Environ. Sci. 2019, 5, 1673116. [Google Scholar] [CrossRef]
- Prasanna, L.; Reddy, J.; Roh, H.; Choi, Y.; Chang, Y.; Yang, J. Hydrometallurgy Adsorption removal of Co (II) from waste-water using graphene oxide. Hydrometallurgy 2016, 165, 90–96. [Google Scholar]
- Parab, H.; Joshi, S.; Shenoy, N.; Lali, A. Determination of kinetic and equilibrium parameters of the batch adsorption of Co (II), Cr (III) and Ni (II) onto coir pith. Process Biochem. 2006, 41, 609–615. [Google Scholar] [CrossRef]
- Olu-Owolabi, B.I.; Oputu, O.U.; Adebowale, K.O.; Ogunsolu, O.; Olujimi, O.O. Biosorption of Cd 2 + and Pb 2 + ions onto mango stone and cocoa pod waste: Kinetic and equilibrium studies. Sci. Res. Essays 2012, 7, 1614–1629. [Google Scholar] [CrossRef]
- Qaiser, S.; Saleemi, A.R.; Umar, M. Biosorption of lead from aqueous solution by Ficus religiosa leaves: Batch and column study. J. Hazard. Mater. 2009, 166, 998–1005. [Google Scholar] [CrossRef]
- Bayomie, O.S.; Kandeel, H.; Shoeib, T.; Yang, H.; Youssef, N.; El-Sayed, M.M.H. Novel approach for effective removal of methylene blue dye from water using fava bean peel waste. Sci. Rep. 2020, 10, 7824. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Raja, F.D. Experimental characterisation and evaluation of perlite as a sorbent for heavy metal ions in single and quaternary solutions. J. Water Process Eng. 2014, 4, 179–184. [Google Scholar] [CrossRef]
- Jnr, M.H.; Spiff, A.I. Equilibrium Sorption Study of Al3+, Co2+ and Ag+ in Aqueous Solutions by Fluted Pumpkin (Telfairia Occidentalis HOOK f) Waste Biomass. Acta Chim Slov. 2005, 52, 174–181. [Google Scholar]
- Saikaew, W.; Kaewsarn, P. Cadmium ion removal using biosorbents derived from fruit peel wastes. Songklanakarin J. Sci. Technol. 2009, 31, 547–554. [Google Scholar]
- Al-Qahtani, K.M. Water purification using different waste fruit cortexes for the removal of heavy metals. J. Taibah Univ. Sci. 2016, 10, 700–708. [Google Scholar] [CrossRef] [Green Version]
- Dlamini, M. Adsorption of heavy metals from water using banana and orange peels. Indian J Sci Technol. 2017, 10, 1–14. [Google Scholar]
- Gupta, V.K.; Nayak, A.; Agarwal, S. Bioadsorbents for remediation of heavy metals: Current status and their future prospects. Environ. Eng. Res. 2015, 20, 1–18. [Google Scholar] [CrossRef]
- Masood, F.; Malik, A.; Masood, F.; Malik, A. Single and Multi-Component Adsorption of Metal Ions by Acinetobacter sp. FM4. Sep. Sci. Technol. 2015, 50, 892–900. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Balasubramanian, R. Is biosorption suitable for decontamination of metal-bearing wastewaters ? A critical review on the state-of-the-art of biosorption processes and future directions. J. Environ. Manag. 2015, 160, 283–296. [Google Scholar] [CrossRef]
- Ronda, A.; Calero, M.; Blázquez, G.; Pérez, A.; Martín-Lara, M. Optimization of the use of a biosorbent to remove heavy metals: Regeneration and reuse of exhausted biosorbent. J. Taiwan Inst. Chem. Eng. 2015, 51, 109–118. [Google Scholar] [CrossRef]
- Kyzas, G.Z. Commercial Coffee Wastes as Materials for Adsorption of Heavy Metals from Aqueous Solutions. Materials 2012, 5, 1826–1840. [Google Scholar] [CrossRef]
- Costley, S.C.; Wallis, F.M. Bioremediation of heavy metals in a synthetic wastewater using a rotating biological contactor. Water Res. 2001, 35, 3715–3723. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).