Resource Availability and Implications for the Development of Plug-In Electric Vehicles
Abstract
:1. Introduction
2. Energy Storage for PEVs
2.1. Lead Acid Batteries
2.2. Nickel Metal Hydride Batteries
2.3. Lithium-Ion Batteries
2.4. Other Energy Storage Technologies
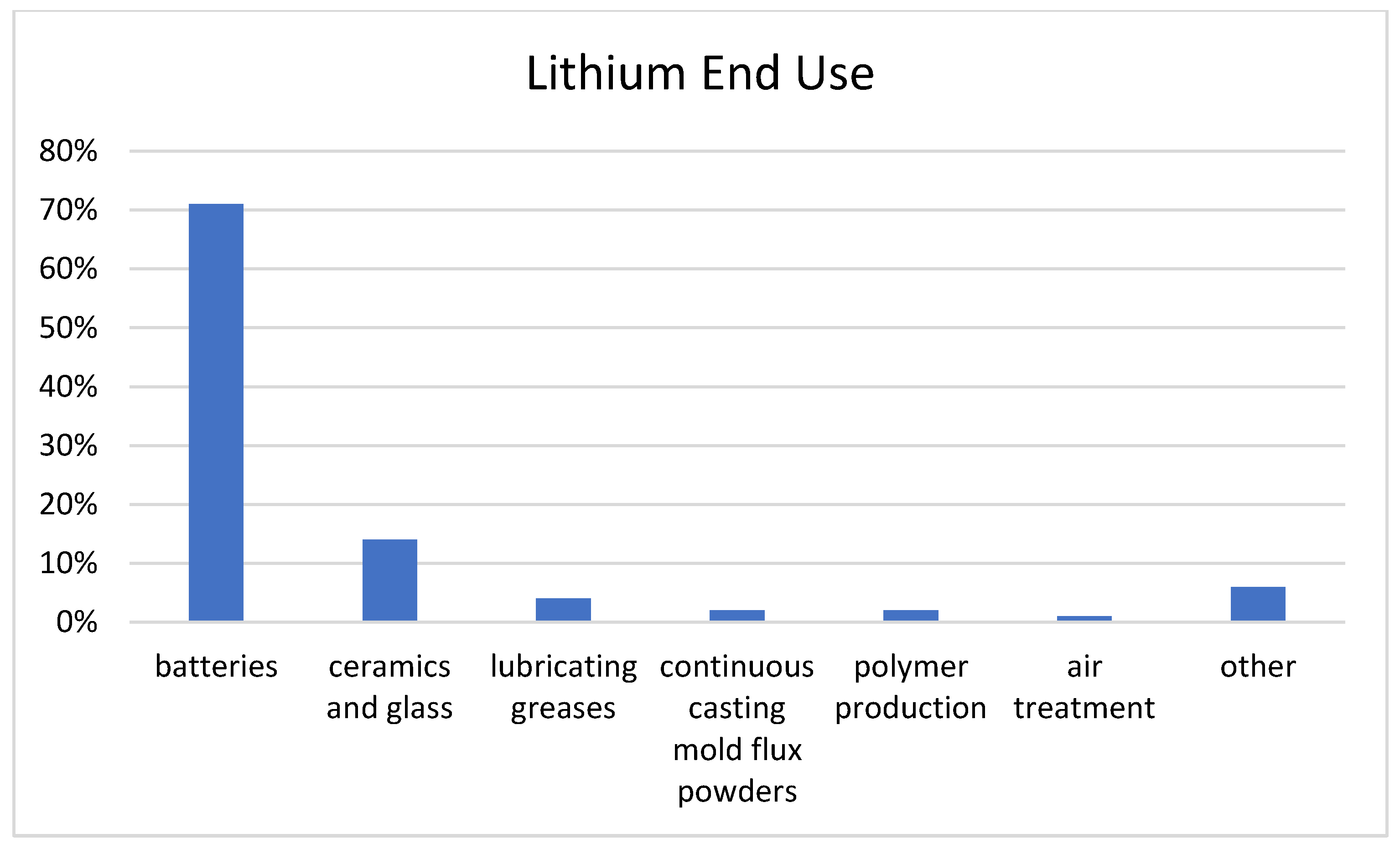
3. Lithium End Use
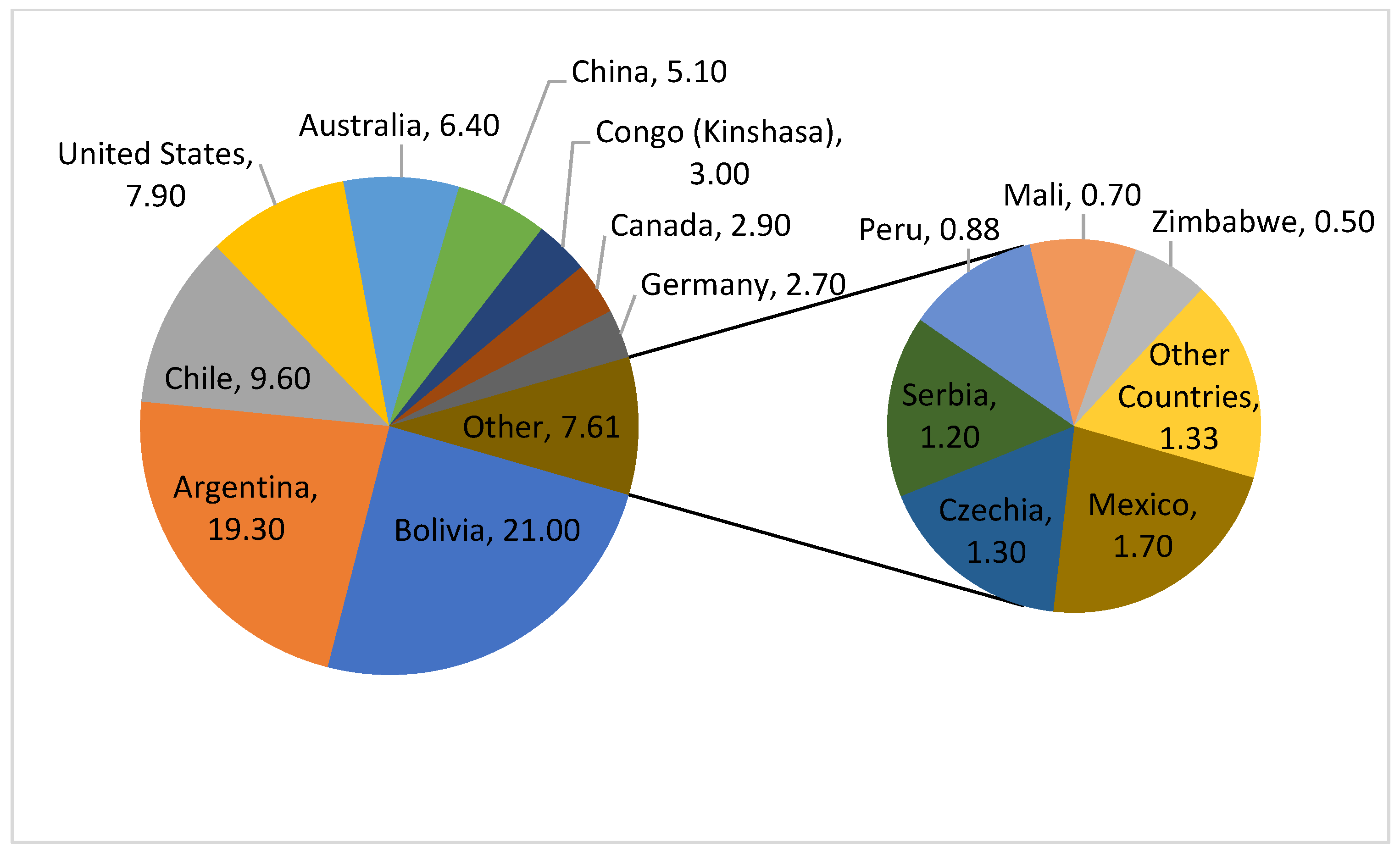
4. Occurrence and Availability of Lithium
4.1. Geological Overview
4.2. Lithium Resources and Reserves
4.3. Recycling
4.4. Future Supply
4.5. Other Factors That Affect Lithium Supply
5. Demand Forecast for Lithium
5.1. PEV Battery Size
5.2. Lithium Intensity
5.3. Future Demand
6. Conclusions and Future Perspectives
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andress, D.; Nguyen, T.D.; Das, S. Reducing GHG emissions in the United States’ transportation sector. Energy Sustain. Dev. 2011, 15, 117–136. [Google Scholar] [CrossRef]
- Elgowainy, A.; Rousseau, A.; Wang, M.; Ruth, M.; Andress, D.; Ward, J.; Joseck, F.; Nguyen, T.; Das, S. Cost of ownership and well-to-wheels carbon emissions/oil use of alternative fuels and advanced light-duty vehicle technologies. Energy Sustain. Dev. 2013, 17, 626–641. [Google Scholar] [CrossRef]
- Egbue, O.; Long, S.; A Samaranayake, V. Mass deployment of sustainable transportation: Evaluation of factors that influence electric vehicle adoption. Clean Technol. Environ. Policy 2017, 19, 1927–1939. [Google Scholar] [CrossRef]
- Quijano, G. Lithium Might Hold the Key to our Clean Energy Future, but Will this Star Metal Fully Deliver on its Green Potential? Bus. Hum. Rights J. 2020, 5, 276–281. [Google Scholar] [CrossRef]
- Kene, R.; Olwal, T.; van Wyk, B.J. Sustainable Electric Vehicle Transportation. Sustainability 2021, 13, 12379. [Google Scholar] [CrossRef]
- Straub, F.; Maier, O.; Göhlich, D. Car-Access Attractiveness of Urban Districts Regarding Shopping and Working Trips for Usage in E-Mobility Traffic Simulations. Sustainability 2021, 13, 11345. [Google Scholar] [CrossRef]
- Lieven, T.; Hügler, B. Did electric vehicle sales skyrocket due to increased environmental awareness while total vehicle sales declined during COVID-19? Sustainability 2021, 13, 13839. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, F.R.; Ning, B.; Tang, T. Optimal Charging Control for Plug-in Electric Vehicles in Smart Microgrids Fueled by Renewable Energy Sources. Int. J. Green Energy 2013, 10, 924–943. [Google Scholar] [CrossRef]
- Egbue, O. An Online Scheduling Mechanism for Vehicle-to-Grid Operation; American Society for Engineering Management: Indianapolis, IN, USA, 2015. [Google Scholar]
- Egbue, O.; Uko, C. Multi-agent approach to modeling and simulation of microgrid operation with vehicle-to-grid system. Electr. J. 2020, 33, 106714. [Google Scholar] [CrossRef]
- Egbue, O.; Uko, C. A Multi-Objective Optimization Model for Vehicle-To-Grid Systems. In Proceedings of the Industrial and Systems Engineering Annual Conference and Expo, Orlando, FL, USA, 18–21 May 2019; Institute of Industrial Engineers: Norcross, GA, USA, 2019. [Google Scholar]
- US Department of Energy. US Department of Energy Critical Materials Strategy; US Department of Energy: Washington, DC, USA, 2010. Available online: http://energy.gov/sites/prod/files/DOE_CMS2011_FINAL_Full.pdf (accessed on 21 August 2021).
- Tahil, W. The Trouble with Lithium: Implications of Future PHEV Demand for Lithium Supply and Resources; Meridian International Research: Martainville, France, 2007; Available online: http://www.meridian-int-res.com/Projects/Lithium_Problem_2 (accessed on 2 May 2021).
- Tahil, W. The Trouble with Lithium 2: Under the Microscope; Meridian International Research: Martainville, France, 2008. [Google Scholar]
- Watari, T.; McLellan, B.C.; Ogata, S.; Tezuka, T. Analysis of Potential for Critical Metal Resource Constraints in the International Energy Agency’s Long-Term Low-Carbon Energy Scenarios. Minerals 2018, 8, 156. [Google Scholar] [CrossRef] [Green Version]
- Rosendahl, K.E.; Rubiano, D.R. How Effective is Lithium Recycling as a Remedy for Resource Scarcity? Environ. Resour. Econ. 2019, 74, 985–1010. [Google Scholar] [CrossRef] [Green Version]
- International Energy Agency. Global EV Outlook 2021; IEA International Energy Agency: Paris, France, 2021; Available online: https://www.iea.org/reports/global-ev-outlook-2021 (accessed on 12 July 2021).
- Garrett, D.E. Handbook of Lithium and Natural Calcium Chloride; Academic Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Wäger, P.; Hischier, R.; Eugster, M. Environmental impacts of the Swiss collection and recovery systems for Waste Electrical and Electronic Equipment (WEEE): A follow-up. Sci. Total Environ. 2011, 409, 1746–1756. [Google Scholar] [CrossRef]
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Iclodean, C.; Varga, B.; Burnete, N.; Cimerdean, D.; Jurchiş, B. Comparison of different battery types for electric vehicles. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Busan, Korea, 25–27 August 2017; IOP Publishing: Bristol, UK, 2017. [Google Scholar]
- Fuhs, A. Hybrid Vehicles: And the Future of Personal Transportation; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Shukla, A.; Kumar, T.P. Lithium economy: Will it get the electric traction? J. Phys. Chem. Lett. 2013, 4, 551–555. [Google Scholar] [CrossRef]
- Verma, S.; Mishra, S.; Gaur, A.; Chowdhury, S.; Mohapatra, S.; Dwivedi, G.; Verma, P. A comprehensive review on energy storage in hybrid electric vehicle. J. Traffic Transp. Eng. (Engl. Ed.) 2021, 8, 621–637. [Google Scholar] [CrossRef]
- Duvall, M.; Alexander, M. Batteries for Electric Drive Vehicles—Status 2005: Performance, Durability, and Cost of Advanced Batteries for Electric, Hybrid Electric, and Plug-In Hybrid Electric Vehicles; Technical Report 1010201; Electric Power Research Institute: Palo Alto, CA, USA, 2005. [Google Scholar]
- Argyrou, M.C.; Christodoulides, P.; Kalogirou, S.A. Energy storage for electricity generation and related processes: Technologies appraisal and grid scale applications. Renew. Sustain. Energy Rev. 2018, 94, 804–821. [Google Scholar] [CrossRef]
- Li, M.M.; Yang, C.C.; Wang, C.C.; Wen, Z.; Zhu, Y.F.; Zhao, M.; Li, J.C.; Zheng, W.T.; Lian, J.S.; Jiang, Q. Design of Hydrogen Storage Alloys/Nanoporous Metals Hybrid Electrodes for Nickel-Metal Hydride Batteries. Sci. Rep. 2016, 6, 27601. [Google Scholar] [CrossRef] [Green Version]
- Bini, M.; Capsoni, D.; Ferrari, S.; Quartarone, E.; Mustarelli, P. 1—Rechargeable lithium batteries: Key scientific and technological challenges. In Rechargeable Lithium Batteries; Franco, A.A., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 1–17. [Google Scholar] [CrossRef]
- Kim, J.G.; Son, B.; Mukherjee, S.; Schuppert, N.; Bates, A.; Kwon, O.; Choi, M.J.; Chung, H.Y.; Park, S. A review of lithium and non-lithium based solid state batteries. J. Power Sources 2015, 282, 299–322. [Google Scholar] [CrossRef]
- Xie, J.; Lu, Y.-C. A retrospective on lithium-ion batteries. Nat. Commun. 2020, 11, 1–4. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Tian, G.; Zhang, Q.; Knapp, M.; Ehrenberg, H.; Chen, G.; Shen, Z.; Yang, G.; Gu, L.; et al. Lithium lanthanum titanate perovskite as an anode for lithium ion batteries. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef]
- Agubra, V.; Fergus, J. Lithium ion battery anode aging mechanisms. Materials 2013, 6, 1310–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roselin, L.S.; Juang, R.-S.; Hsieh, C.-T.; Sagadevan, S.; Umar, A.; Selvin, R.; Hegazy, H.H. Recent Advances and Perspectives of Carbon-Based Nanostructures as Anode Materials for Li-ion Batteries. Materials 2019, 12, 1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, Z.; Wang, S. A review of power battery thermal energy management. Renew. Sustain. Energy Rev. 2011, 15, 4554–4571. [Google Scholar] [CrossRef]
- Amirault, J.; Chien, J.; Garg, S.; Gibbons, D.; Ross, B.; Tang, M.; Xing, J.; Sidhu, I.; Kaminsky, P.; Tenderich, B. The Electric Vehicle Battery Landscape: Opportunities and Challenges; Technical Brief (2009.9); Center for Entrepreneurship & Technology (CET) University of California: Berkeley, CA, USA, 2009. [Google Scholar]
- Wanger, T.C. The lithium future—Resources, recycling, and the environment. Conserv. Lett. 2011, 4, 202–206. [Google Scholar] [CrossRef]
- Zelinsky, M.A.; Koch, J.M.; Young, K.-H. Performance Comparison of Rechargeable Batteries for Stationary Applications (Ni/MH vs. Ni–Cd and VRLA). Batteries 2018, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Lee, K.T.; Jeong, S.; Cho, J. Roles of Surface Chemistry on Safety and Electrochemistry in Lithium Ion Batteries. Acc. Chem. Res. 2013, 46, 1161–1170. Available online: https://login.libpdb.d.umn.edu:2443/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=aph&AN=87704104&site=ehost-live (accessed on 11 January 2022). [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Ramesh, R.; Kumar, P. Safety mechanisms in lithium-ion batteries. J. Power Sources 2006, 155, 401–414. [Google Scholar] [CrossRef]
- Chen, W.; Liang, J.; Yang, Z.; Li, G. A Review of Lithium-Ion Battery for Electric Vehicle Applications and Beyond. Energy Procedia 2019, 158, 4363–4368. [Google Scholar] [CrossRef]
- Catenacci, M.; Verdolini, E.; Bosetti, V.; Fiorese, G. Going electric: Expert survey on the future of battery technologies for electric vehicles. Energy Policy 2013, 61, 403–413. [Google Scholar] [CrossRef] [Green Version]
- Cluzel, C.; Douglas, C. Cost and Performance of EV Batteries; Final Report for the Committee on Climate Change; Element Energy: Cambridge, UK, 2012. [Google Scholar]
- Christensen, J.; Albertus, P.; Sanchez-Carrera, R.S.; Lohmann, T.; Kozinsky, B.; Liedtke, R.; Ahmed, J.; Kojic, A. A critical review of Li/air batteries. J. Electrochem. Soc. 2011, 159, R1–R30. [Google Scholar] [CrossRef]
- Girishkumar, G.; McCloskey, B.; Luntz, A.; Swanson, S.; Wilcke, W. Lithium—Air battery: Promise and challenges. J. Phys. Chem. Lett. 2010, 1, 2193–2203. [Google Scholar] [CrossRef]
- Ebensperger, A.; Maxwell, P.; Moscoso, C. The lithium industry: Its recent evolution and future prospects. Resour. Policy 2005, 30, 218–231. [Google Scholar] [CrossRef]
- Prior, T.; Wäger, P.A.; Stamp, A.; Widmer, R.; Giurco, D. Sustainable governance of scarce metals: The case of lithium. Sci. Total Environ. 2013, 461–462, 785–791. [Google Scholar] [CrossRef]
- Wadia, C.; Albertus, P.; Srinivasan, V. Resource constraints on the battery energy storage potential for grid and transportation applications. J. Power Sources 2011, 196, 1593–1598. [Google Scholar] [CrossRef] [Green Version]
- Miao, Y.; Liu, L.; Zhang, Y.; Tan, Q.; Li, J. An overview of global power lithium-ion batteries and associated critical metal recycling. J. Hazard. Mater. 2021, 425, 127900. [Google Scholar] [CrossRef]
- Dorn, F.M.; Peyré, F.R. Lithium as a Strategic Resource: Geopolitics, Industrialization, and Mining in Argentina. J. Lat. Am. Geogr. 2020, 19, 68–90. [Google Scholar] [CrossRef]
- United States Geological Survey. Mineral Commodity Summaries 2021; United States Geological Survey: Reston, VI, USA, 2021. Available online: https://pubs.usgs.gov/periodicals/mcs2021/mcs2021.pdf (accessed on 11 July 2021).
- Vikström, H.; Davidsson, S.; Höök, M. Lithium availability and future production outlooks. Appl. Energy 2013, 110, 252–266. [Google Scholar] [CrossRef] [Green Version]
- Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Kesler, S.E.; Everson, M.P.; Wallington, T.J. Global Lithium Availability. J. Ind. Ecol. 2011, 15, 760–775. [Google Scholar] [CrossRef]
- Yaksic, A.; Tilton, J.E. Using the cumulative availability curve to assess the threat of mineral depletion: The case of lithium. Resour. Policy 2009, 34, 185–194. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Dallas, J.; Casanova, S.; Pelech, T.; Bournival, G.; Saydam, S.; Canbulat, I. Towards a low-carbon society: A review of lithium resource availability, challenges and innovations in mining, extraction and recycling, and future perspectives. Miner. Eng. 2021, 163, 106743. [Google Scholar] [CrossRef]
- Speirs, J.; Contestabile, M.; Houari, Y.; Gross, R. The future of lithium availability for electric vehicle batteries. Renew. Sustain. Energy Rev. 2014, 35, 183–193. [Google Scholar] [CrossRef]
- United States Geological Survey. Mineral Commodity Summaries; United States Geological Survey: Reston, VI, USA, 2016; Available online: https://s3-us-west-2.amazonaws.com/prd-wret/assets/palladium/production/mineral-pubs/mcs/mcs2016.pdf (accessed on 21 August 2021).
- Otto, J. Resources and reserves: Thoughts on their evolution. Miner. Econ. 2020, 33, 253–255. [Google Scholar] [CrossRef]
- Dessemond, C.; Lajoie-Leroux, F.; Soucy, G.; Laroche, N.; Magnan, J.-F. Spodumene: The Lithium Market, Resources and Processes. Minerals 2019, 9, 334. [Google Scholar] [CrossRef] [Green Version]
- Ambrose, H.; Kendall, A. Understanding the future of lithium: Part 1, resource model. J. Ind. Ecol. 2020, 24, 80–89. [Google Scholar] [CrossRef]
- Clarke, G.; Harben, P. Lithium Availability Wall Map; Gerry Clarke: London, UK, 2009. [Google Scholar]
- Evans, K.R. An Abundance of Lithium: Part Two. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.363.1242&rep=rep1&type=pdf (accessed on 12 July 2021).
- Fasel, D.; Tran, M. Availability of lithium in the context of future D–T fusion reactors. Fusion Eng. Des. 2005, 75-79, 1163–1168. [Google Scholar] [CrossRef]
- Grosjean, C.; Miranda, P.H.; Perrin, M.; Poggi, P. Assessment of world lithium resources and consequences of their geographic distribution on the expected development of the electric vehicle industry. Renew. Sustain. Energy Rev. 2012, 16, 1735–1744. [Google Scholar] [CrossRef]
- Kesler, S.E.; Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Everson, M.P.; Wallington, T.J. Global lithium resources: Relative importance of pegmatite, brine and other deposits. Ore Geol. Rev. 2012, 48, 55–69. [Google Scholar] [CrossRef]
- Kushnir, D.; Sandén, B. The time dimension and lithium resource constraints for electric vehicles. Resour. Policy 2012, 37, 93–103. [Google Scholar] [CrossRef]
- Mohr, S.H.; Mudd, G.; Giurco, D. Lithium Resources and Production: Critical Assessment and Global Projections. Minerals 2012, 2, 65–84. [Google Scholar] [CrossRef]
- Jiao, N.; Evans, S. Business Models for Sustainability: The Case of Second-life Electric Vehicle Batteries. Procedia CIRP 2016, 40, 250–255. [Google Scholar] [CrossRef] [Green Version]
- UNEP. Recycling Rates of Metals—A Status Report; A Report of the Working Group on the Global Metal Flows to the International Resource Panel; United Nations Environment Programme: Nairobi, Kenya, 2011. [Google Scholar]
- Zhang, X.; Xie, Y.; Lin, X.; Li, H.; Cao, H. An overview on the processes and technologies for recycling cathodic active materials from spent lithium-ion batteries. J. Mater. Cycles Waste Manag. 2013, 15, 420–430. [Google Scholar] [CrossRef]
- Silwamba, M.; Nyambe, I.; Chirwa, M.; Banda, K.; Nakata, H.; Nakayama, S.; Ishizuka, M.; Ito, M.; Hiroyoshi, N.; Tabelin, C.B.; et al. Detoxification of lead-bearing zinc plant leach residues from Kabwe, Zambia by coupled extraction-cementation method. J. Environ. Chem. Eng. 2020, 8, 104197. [Google Scholar] [CrossRef]
- Silwamba, M.; Ito, M.; Hiroyoshi, N.; Tabelin, C.B.; Hashizume, R.; Fukushima, T.; Park, I.; Jeon, S.; Igarashi, T.; Sato, T.; et al. Recovery of Lead and Zinc from Zinc Plant Leach Residues by Concurrent Dissolution-Cementation Using Zero-Valent Aluminum in Chloride Medium. Metals 2020, 10, 531. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Deshmane, V.G.; Paranthaman, M.P.; Bhave, R.; Moyer, B.A.; Harrison, S. Lithium Recovery from Aqueous Resources and Batteries: A Brief Review. Johns. Matthey Technol. Rev. 2018, 62, 161–176. [Google Scholar] [CrossRef]
- Xu, J.; Thomas, H.R.; Francis, R.W.; Lum, K.R.; Wang, J.; Liang, B. A review of processes and technologies for the recycling of lithium-ion secondary batteries. J. Power Sources 2008, 177, 512–527. [Google Scholar] [CrossRef]
- Dutta, D.; Kumari, A.; Panda, R.; Jha, S.; Gupta, D.; Goel, S.; Jha, M.K. Close loop separation process for the recovery of Co, Cu, Mn, Fe and Li from spent lithium-ion batteries. Sep. Purif. Technol. 2018, 200, 327–334. [Google Scholar] [CrossRef]
- Huang, T.; Liu, L.; Zhang, S. Recovery of cobalt, lithium, and manganese from the cathode active materials of spent lithium-ion batteries in a bio-electro-hydrometallurgical process. Hydrometallurgy 2019, 188, 101–111. [Google Scholar] [CrossRef]
- Zhuang, L.; Sun, C.; Zhou, T.; Li, H.; Dai, A. Recovery of valuable metals from LiNi0.5Co0.2Mn0.3O2 cathode materials of spent Li-ion batteries using mild mixed acid as leachant. Waste Manag. 2019, 85, 175–185. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, M.; Zhao, Z.; Tong, B.; Fan, Y.; Hua, Z. Hydrometallurgical Processes for Recycling Spent Lithium-Ion Batteries: A Critical Review. ACS Sustain. Chem. Eng. 2018, 6, 13611–13627. [Google Scholar] [CrossRef]
- Roy, J.J.; Cao, B.; Madhavi, S. A review on the recycling of spent lithium-ion batteries (LIBs) by the bioleaching approach. Chemosphere 2021, 282, 130944. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, Z.; Lin, X.; Zhang, Y.; He, Y.; Cao, H.; Sun, Z. A Mini-Review on Metal Recycling from Spent Lithium Ion Batteries. Engineering 2018, 4, 361–370. [Google Scholar] [CrossRef]
- Ordoñez, J.; Gago, E.; Girard, A. Processes and technologies for the recycling and recovery of spent lithium-ion batteries. Renew. Sustain. Energy Rev. 2016, 60, 195–205. [Google Scholar] [CrossRef]
- Barik, S.; Prabaharan, G.; Kumar, B. An innovative approach to recover the metal values from spent lithium-ion batteries. Waste Manag. 2016, 51, 222–226. [Google Scholar] [CrossRef]
- Chen, X.; Ma, H.; Luo, C.; Zhou, T. Recovery of valuable metals from waste cathode materials of spent lithium-ion batteries using mild phosphoric acid. J. Hazard. Mater. 2017, 326, 77–86. [Google Scholar] [CrossRef]
- He, L.-P.; Sun, S.-Y.; Song, X.-F.; Yu, J.-G. Leaching process for recovering valuable metals from the LiNi1/3Co1/3Mn1/3O2 cathode of lithium-ion batteries. Waste Manag. 2017, 64, 171–181. [Google Scholar] [CrossRef]
- Li, L.; Fan, E.; Guan, Y.; Zhang, X.; Xue, Q.; Wei, L.; Wu, F.; Chen, R. Sustainable Recovery of Cathode Materials from Spent Lithium-Ion Batteries Using Lactic Acid Leaching System. ACS Sustain. Chem. Eng. 2017, 5, 5224–5233. [Google Scholar] [CrossRef]
- Purnomo, C.W.; Kesuma, E.P.; Perdana, I.; Aziz, M. Lithium recovery from spent Li-ion batteries using coconut shell activated carbon. Waste Manag. 2018, 79, 454–461. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, M.; Yao, Y.; Wang, H.; Tong, B.; Zhao, Z. A novel approach for the selective extraction of Li+ from the leaching solution of spent lithium-ion batteries using benzo-15-crown-5 ether as extractant. Sep. Purif. Technol. 2019, 237, 116325. [Google Scholar] [CrossRef]
- Barrios, O.C.; González, Y.C.; Barbosa, L.I.; Orosco, P. Chlorination roasting of the cathode material contained in spent lithium-ion batteries to recover lithium, manganese, nickel and cobalt. Miner. Eng. 2021, 176, 107321. [Google Scholar] [CrossRef]
- Gaines, L.; Nelson, P. Lithium-Ion Batteries: Examining Material Demand and Recycling Issues, The Minerals. In Proceedings of the Metals and Materials Society 2010 Annual Meeting, Seattle, WA, USA, 14–18 February 2010. [Google Scholar]
- DeRousseau, M.; Gully, B.; Taylor, C.; Apelian, D.; Wang, Y. Repurposing Used Electric Car Batteries: A Review of Options. Jom 2017, 69, 1575–1582. [Google Scholar] [CrossRef]
- Marcos, J.; Scheller, C.; Godina, R.; Spengler, T.; Carvalho, H. Sources of uncertainty in the closed-loop supply chain of lithium-ion batteries for electric vehicles. Clean. Logist. Supply Chain 2021, 1, 100006. [Google Scholar] [CrossRef]
- Egbue, O.; Long, S. Critical Issues in the Supply Chain of Lithium for Electric Vehicle Batteries. Eng. Manag. J. 2012, 24, 52–62. Available online: https://login.libpdb.d.umn.edu:2443/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=aph&AN=84608288&site=ehost-live (accessed on 1 September 2021). [CrossRef]
- Shao, L.; Hu, J.; Zhang, H. Evolution of global lithium competition network pattern and its influence factors. Resour. Policy 2021, 74, 102353. [Google Scholar] [CrossRef]
- Tian, X.; Geng, Y.; Sarkis, J.; Gao, C.; Sun, X.; Micic, T.; Hao, H.; Wang, X. Features of critical resource trade networks of lithium-ion batteries. Resour. Policy 2021, 73, 102177. [Google Scholar] [CrossRef]
- Egbue, O.; Long, S. Barriers to widespread adoption of electric vehicles: An analysis of consumer attitudes and perceptions. Energy Policy 2012, 48, 717–729. [Google Scholar] [CrossRef]
- Statistica. Range of Selected MY 2019 and MY 2020 Electric Vehicle Models in the U.S.; Statistica: New York, NY, USA, 2021; Available online: https://www.statista.com/statistics/797331/electric-vehicle-battery-range/ (accessed on 8 August 2021).
- Ziemann, S.; Müller, D.B.; Schebek, L.; Weil, M. Modeling the potential impact of lithium recycling from EV batteries on lithium demand: A dynamic MFA approach. Resour. Conserv. Recycl. 2018, 133, 76–85. [Google Scholar] [CrossRef]
- Maxwell, P.; Mora, M. Lithium and Chile: Looking back and looking forward. Miner. Econ. 2019, 33, 57–71. [Google Scholar] [CrossRef]
- International Energy Agency. Technology Roadmap: Electric and Plug-In Hybrid Electric Vehicles; T.R. International Energy Agency: Paris, France, 2011. [Google Scholar]
- International Energy Agency. Energy Technology Perspectives 2020; IEA International Energy Agency: Paris, France, 2020; Available online: https://www.iea.org/reports/energy-technology-perspectives-2020 (accessed on 8 August 2021).


| Reference | Lithium Resources | Lithium Reserves |
|---|---|---|
| (Million Tons) | ||
| Ambrose and Kendall [61] | 99.5 | 37.5 |
| Clarke and Harben [62] | 39.4 | _ |
| Evans [63] | 29.9 | _ |
| Fasel and Tran [64] | 9.4–21 | 4–6 |
| Grosjean et al. [65] | 37.1–43.6 | _ |
| Gruber et al. [54] | 38.7 | 19.3 |
| Kesler et al. [66] | 30.9 | _ |
| Kushnir and Sandén [67] | _ | 30.0 |
| Mohr et al. [68] | 71.3 | 23.1 |
| Speirs et al. [57] | 65 | 15.0 |
| Tahil [14] | _ | 3.9 |
| Yaksic and Tilton [55] | 64 | 29.4 |
| USGS [52] | 86 | 21.0 |
| Vehicle | Battery Type | Battery Size (kWh) | All Electric Range (Miles) |
|---|---|---|---|
| 2019 Tesla Model 3 AWD | Li-ion | 75 | 322 |
| 2020 Tesla Model Y AWD | Li-ion | 75 | 316 |
| 2020 Chevrolet Bolt EV | Li-ion | 66 | 259 |
| 2020 Hyundai Kona EV | Lithium polymer | 64 | 258 |
| 2020 Nissan Leaf SL+ | Li-ion | 62 | 215 |
| 2019 Audi e-tron | Li-ion | 95 | 204 |
| 2020 Porsche Taycan 4S | Li-ion | 79.2 | 203 |
| 2020 Hyundai Ioniq EV | Lithium polymer | 38.3 | 170 |
| 2019 BMW i3 EV | Li-ion | 42.2 | 153 |
| 2010 | 2015 | 2020 | 2025 | 2030 | 2035 | 2040 | 2045 | 2050 | |
|---|---|---|---|---|---|---|---|---|---|
| PHEV | 0 | 0.7 | 4.9 | 13.1 | 24.6 | 35.6 | 47.7 | 56.3 | 59.7 |
| BEV | 0 | 0.3 | 2 | 4.5 | 8.7 | 13.9 | 23.2 | 33.9 | 46.6 |
| Total | 0 | 1 | 6.9 | 17.6 | 33.3 | 49.5 | 70.9 | 90.2 | 106.3 |
| STEP | SDS | |||||
|---|---|---|---|---|---|---|
| 2020 | 2025 | 2030 | 2020 | 2025 | 2030 | |
| PHEV | 0.969034 | 4.226493 | 7.761233 | 0.969034 | 6.060351 | 10.984190 |
| BEV | 2.008024 | 7.114262 | 14.370678 | 2.008024 | 11.939460 | 28.687724 |
| Total | 2.977058 | 11.340755 | 22.131911 | 2.977058 | 17.999811 | 39.671914 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egbue, O.; Long, S.; Kim, S.D. Resource Availability and Implications for the Development of Plug-In Electric Vehicles. Sustainability 2022, 14, 1665. https://doi.org/10.3390/su14031665
Egbue O, Long S, Kim SD. Resource Availability and Implications for the Development of Plug-In Electric Vehicles. Sustainability. 2022; 14(3):1665. https://doi.org/10.3390/su14031665
Chicago/Turabian StyleEgbue, Ona, Suzanna Long, and Seong Dae Kim. 2022. "Resource Availability and Implications for the Development of Plug-In Electric Vehicles" Sustainability 14, no. 3: 1665. https://doi.org/10.3390/su14031665
APA StyleEgbue, O., Long, S., & Kim, S. D. (2022). Resource Availability and Implications for the Development of Plug-In Electric Vehicles. Sustainability, 14(3), 1665. https://doi.org/10.3390/su14031665








