Application Progress of O3/UV Advanced Oxidation Technology in the Treatment of Organic Pollutants in Water
Abstract
:1. Introduction
2. Reaction Mechanism of O3/UV Process
3. Research Status of O3/UV Process
3.1. Treatment of Industrial Wastewater by O3/UV Process
3.2. Treatment of Trace Polluted Organic Matter by O3/UV Process
3.3. Treatment of Drinking Water by O3/UV Process
4. O3/UV Process Evaluation
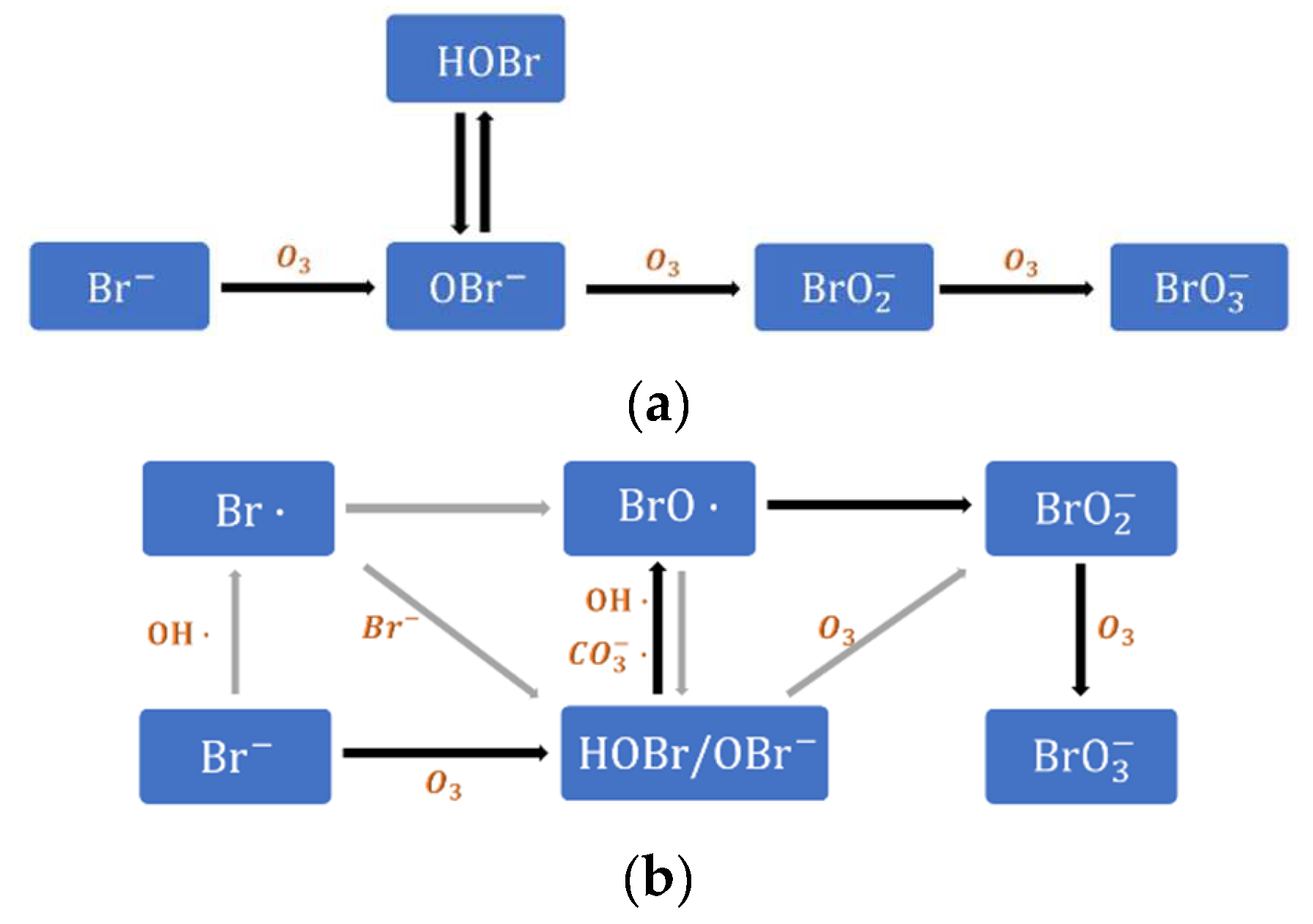
4.1. Evaluation of Bromate Formation Control
4.2. Economic Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COD | Chemical oxygen demand |
| BOD | Biochemical oxygen demand |
| VUV | Vacuum ultra violet |
| SBX | Sodium butyl xanthate |
| BAF | Biological aerated filter |
| BOD5 | Five-day biochemical oxygen demand |
| TN | Total nitrogen |
| TP | Total phosphorus |
| PNA | P-nitroaniline |
| TOrC | Refractory trace organic compounds |
| WWROC | Reverse osmosis concentrate |
| ATZ | Atrazine |
| TOC | Total organic carbon |
| SMX | Sulfamethoxazole |
| PPCPs | Pharmaceutical and personal care products |
| AOPs | Advanced oxidation process |
| TCNM | Trichloronitromethane |
| BHA | Butylated hydroxyanisole |
| EPA | Environmental Protection Agency |
| DOC | Dissolved organic carbon |
| NOM | Natural organic matter |
| HA | Humic acid |
| MTBE | Methyl tert-butyl ether |
| CODcr | Dichromate oxidizability |
| 2,4,6-TCP | 2,4,6-trichlorophenol |
| TNTs | TiO2 nanotubes |
| X3B | Brilliant red X-3B |
| CTX | Ceftriaxone |
| nano-ZnO | Nano-zinc oxide |
| Parabens | p-hydroxybenzoates |
References
- Garrison, R.L.; Prengle, H.W.; Mauk, C.E. Ozone-based system treats plating effluents. Metal. Progr. 1975, 108, 61–62. [Google Scholar]
- Fochtman, E.G.; Huff, J.E. Ozone-ultraviolet light treatment of TNT wastewaters. Water Pollut. 1975, 13, 211–223. [Google Scholar]
- Rice, R.G. Ozone treatment for industrial wastewater. Pollut. Technol. Rev. 1981, 1, 84–85. [Google Scholar]
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Gernjak, W. Consolidated vs. new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Sci. Total Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2019, 701, 135023. [Google Scholar] [CrossRef]
- Lee, Y.; Gerrity, D.; Lee, M.; Bogeat, A.E.; Salhi, E.; Gamage, S.; Trenholm, R.A.; Wert, E.C.; Snyder, S.A.; Gunten, U.V. Prediction of micropollutant elimination during ozonation of municpical wastewater effluents: Use of kinetic and water specific information. Environ. Sci. Technol. 2013, 47, 5872–5881. [Google Scholar] [CrossRef]
- Cerreta, G.; Roccamante, M.A.; Patricia, P.B.; Oller, I.; Aguera, A.; Malato, S.; Rizzo, L. Advanced treatment of urban wastewater by UV-C/free chlorine process: Micro-pollutants removal and effect of UV-C radiation on trihalomethanes formation. Water Res. 2019, 169, 115220. [Google Scholar] [CrossRef]
- Gorito, A.M.; Pesqueira, J.F.J.R.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Nunes, O.C.; Almeida, C.M.R.; Silva, A.M.T. Ozone-based water treatment (O3, O3/UV, O3/H2O2) for removal of organic micropollutants, bacteria inactivation and regrowth prevention. J. Environ. Chem. Eng. 2021, 9, 105315. [Google Scholar] [CrossRef]
- Benotti, M.J.; Trenholm, R.A.; Vanderford, B.J.; Holady, J.C.; Stanford, B.D.; Snyder, S.A. Pharmaceuticals and endocrine disrupting compounds in U.S. drinking water. Environ. Sci. Technol. 2009, 43, 597–603. [Google Scholar] [CrossRef] [Green Version]
- Dickenson, E.R.V.; Snyder, S.A.; Sedlak, D.L.; Drewes, J.E. Indicator compounds for assessment of wastewater effluent contributions to flow and water quality. Water Res. 2011, 45, 1199–1212. [Google Scholar] [CrossRef]
- Focazio, M.J.; Kolpin, D.W.; Barnes, K.K.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Barber, L.B.; Thurman, M.E. A national reconnaissance for pharmaceuticals and other organic wastewater contaminants in the United States-Ⅱ) Untreated drinking water sources. Sci. Total Environ. 2008, 402, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Zoh, K.D. Occurrence and remvals of micropollutants in water environment. Environ. Eng. Res. 2016, 21, 319–332. [Google Scholar] [CrossRef] [Green Version]
- Reichert, G.; Hilgert, S.; Fuchs, S.; Azevedo, J.C.R. Emerging contaminants and antibiotic resistance in the different environmental matrices of Latin America. Environ. Pollut. 2019, 255, 113140. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.L.; Guo, W.S.; Ngo, H.H.; Nghiem, L.D.; Hai, F.L.; Zhang, J.; Liang, S.; Wang, X.C.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef] [Green Version]
- Sgroi, M.; Roccaro, P.; Korshin, G.V.; Greco, V.; Sciuto, S.; Anumol, T.; Snyder, S.A.; Vagliasindi, F.G.A. Use of fluorescence EEM to monitor the removal of emerging contaminants in full scale wastewater treatment plants. J. Hazard. Mater. 2016, 323, 367–376. [Google Scholar] [CrossRef]
- Schoenell, E.K.; Otto, N.; Rodrigues, M.A.S.; Metzger, J.W. Removal of organic micropollutants from treated municipal wastewater by O3/UV/H2O2 in a UVA-LED reactor. Ozone Sci. Eng. 2021, 43, 1900716. [Google Scholar] [CrossRef]
- Sgroi, M.; Anumol, T.; Vagliasindi, F.G.A.; Snyder, S.A.; Roccaro, P. Comparison of the new Cl2/O3/UV process with different ozone-and-UV-based AOPs for wastewater treatment at pilot scale: Removal of pharmaceuticals and changes in fluorescing organic matter. Sci. Total Environ. 2021, 765, 142720. [Google Scholar] [CrossRef]
- Guillossou, R.; Roux, L.J.; Brosillon, S.; Mailler, R.; Vulliet, E.; Morlay, C.; Nauleau, F.; Rocher, V.; Gasperi, J. Benefits of ozonation before activated carbon adsorption for the removal of organic micropollutants from wastewater effluents. Chemosphere 2020, 245, 125530. [Google Scholar] [CrossRef]
- Roccaro, P.; Sgroi, M.; Vagliasindi, F.G.A. Removal of xenobiotic compounds from wastewater for environment protection: Treatment process and costs. Chem. Eng. Trans. 2013, 32, 505–510. [Google Scholar]
- Roccaro, P. Treatment processes for municipal wastewater reclamation: The challenges of emerging contaminants and direct potable reuse. Curr. Opin. Environ. Sci. Health 2018, 2, 46–54. [Google Scholar] [CrossRef]
- Villarin, M.C.; Merel, S. Assessment of current challenges and paradigm shifts in wastewater management. J. Hazard. Mater. 2020, 390, 122139. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.D.; Ternes, T.A. Water analysis: Emerging contaminants and current issues. Anal. Chem. 2018, 90, 398–428. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hosseinzadeh, S.; Wardenier, N.; Verheust, Y. Combining ozone with UV and H2O2 for the degradation of micropollutants from different origins: Lab-scale analysis and optimization. Environ. Technol. 2019, 40, 3773–3782. [Google Scholar] [CrossRef] [PubMed]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hubner, U. Evaluation of advanced oxidation processes for water and wastewater treatment. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Shao, Q.; Wang, Y.; Li, J.; Li, M.K.; Li, J.; Qiang, Z.M. Technical principle and research progress of UV/O3 process in water treatment. China Water Wastewater 2019, 35, 16–23. (In Chinese) [Google Scholar]
- Zhu, H.H.; Sun, S.H.; Feng, G.X.; Zhao, Q.H.; Zhou, A.R.; Jia, R.B. Research progress of ultraviolet combined advanced oxidation technology for drinking water treatment. Technol. Water Treat. 2019, 45, 1–7. (In Chinese) [Google Scholar]
- Zhang, T.; Li, Z.Y.; Dong, C.H.; Zhou, Z. Current situation of industrial wastewater treatment and countermeasures of pollution control in China. Water Wastewater Eng. 2020, 46, 1–3. (In Chinese) [Google Scholar]
- Glaze, W.H. Drinking-water treatment with ozone. Environ. Sci. Technol. 1987, 21, 224–230. [Google Scholar] [CrossRef]
- Wang, H.W.; Li, X.Y.; Hao, Z.P.; Sun, Y.J.; Wang, Y.N.; Li, W.H.; Tsang, Y.F. Transformation of dissolved organic matter in concentrated leachate from nanofiltration during ozone-based oxidation processes (O3, O3/H2O2 and O3/UV). J. Environ. Manag. 2017, 191, 244–251. [Google Scholar] [CrossRef]
- Zhang, J.C.; You, R.J.; Zhu, W.D.; Chen, Z.D.; Zhao, H.J.; Liu, T.T.; Zhao, X.G. Deep removal of organic matters in biochemical tailwater from chemical parks by UV/O3. Mod. Chem. Ind. 2020, 40, 166–169. (In Chinese) [Google Scholar]
- Fu, P.F.; Feng, J.; Yang, H.F.; Yang, T.W. Degradation of sodium n-butyl xanthate by vacuum UV-ozone (VUV/O3) in comparison with ozone and VUV photolysis. Process. Saf. Environ. 2016, 102, 64–70. [Google Scholar] [CrossRef]
- Song, Y.P.; Wang, J.B.; Nie, H.F.; Chang, F.M.; Dong, Q.Q.; Liu, X.; Liang, R.S.; Wang, K.J. Pilot study on treating printing and dyeing biochemical tail water with tubular O3/UV-BAF. Chinses J. Environ. Eng. 2019, 13, 264–271. (In Chinese) [Google Scholar]
- Rajabizadeh, K.; Yazdanpanah, G.; Dowlatshahi, S.; Malakootian, M. Photooxidation process efficiency (UV/O3) for p-nitroaniline removal from aqueous solutions. Ozone-Sci. Eng. 2020, 42, 420–427. [Google Scholar] [CrossRef]
- Kaplan, A.; Mamane, H.; Lester, Y.; Avisar, D. Trace organic compound removal from wastewater reverse-osmosis concentrate by advanced oxidation processes with UV/O3/H2O2. Materials 2020, 13, 2785. [Google Scholar] [CrossRef]
- Jing, L.; Chen, B.; Wen, D.Y.; Zheng, J.S.; Zhang, B.Y. The removal of COD and NH3-N from atrazine production wastewater treatment using UV/O3: Experimental investigation and kinetic modeling. Environ. Sci. Pollut. Res. 2018, 25, 2691–2701. [Google Scholar] [CrossRef]
- Zhu, T.; Yang, S.P.; Tan, W.Q.; Wang, K.J. Degradation of 2,4,6-trichlorophenol by UV/O3/TiO2 coupling process. Environ. Eng. 2021, 39, 7–13. (In Chinese) [Google Scholar]
- Sun, J.; Yan, X.; Lv, K.L.; Sun, S.; Deng, K.J.; Du, D.Y. Photocatalytic degradation pathway for azo dye in TiO2 UV/O3 system: Hydroxyl radical versus hole. J. Mol. Catal. A Chem. 2013, 367, 31–37. [Google Scholar] [CrossRef]
- Malvestiti, J.A.; Dantas, R.F. Disinfection of secondary effluents by O3, O3/H2O2 and UV/H2O2: Influence of carbonate, nitrate, industrial contaminants and regrowth. J. Environ. Chem. Eng. 2018, 6, 560–567. [Google Scholar] [CrossRef]
- Rao, Y.F.; Chu, W. A new approach to quantify the degradation kinetics of linuron with UV, ozonation and UV/O3 processes. Chemosphere 2009, 74, 1444–1449. [Google Scholar] [CrossRef]
- Liu, X.W.; Garoma, T.; Chen, Z.L.; Wang, L.L.; Wu, Y.X. SMX degradation by ozonation and UV radiation: A kinetic study. Chemosphere 2012, 87, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Li, M.K.; Qiang, Z.M.; Pulgarin, C.; Kiwi, J. Accelerated methylene blue (MB) degradation by fenton reagent exposed to UV or VUV/UV light in an innovative micro photo-reactor. Appl. Catal. B Environ. 2016, 187, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.B.; Wang, S.P.; Wang, Z.; Zhou, R.F.; Chang, J. Degradation effect and kinetics of ibuprofen in water by UV/O3 advanced oxidation. Ind. Water Treat. 2020, 40, 40–43. (In Chinese) [Google Scholar]
- Paucar, N.E.; Kim, I.; Tanaka, H.; Sato, C. Effect of O3 dose on the O3/UV treatment process for the removal of pharmaceuticals and personal care products in secondary effluent. ChemEngineering 2019, 3, 53. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.J. Degradation of Typical Antibiotics in Effluent of Municipal Wastewater Treatment Plant by O3/UV. Master’s Thesis, Hebei University of Engineering, Handan, China, 2018. (In Chinese). [Google Scholar]
- Hashemi, S.Y.; Badi, M.Y.; Pasalari, H.; Azari, A.; Arfarinia, H.; Kiani, A. Degradation of ceftriaxone from aquatic solution using a heterogeneous and reusable O3/UV/Fe3O4@TiO2 systems: Operational factors, kinetics and mineralization. Int. J. Environ. Anal. Chem. 2020, 1, 1–17. [Google Scholar] [CrossRef]
- Asgari, E.; Esrafili, A.; Rostami, R.; Farzadkia, M. O3, O3/UV and O3/UV/ZnO for abatement of Parabens in aqueous solutions; effect of operational parameters and mineralization/biodegradability improvement. Process Saf. Environ. 2019, 125, 238–250. [Google Scholar] [CrossRef]
- Peyton, G.R.; Huang, F.Y.; Burleson, J.L.; Glaze, W.H. Destruction of pollutants in water with ozone in combination with ultraviolet radiation. 1. general principles and oxidation of tetrachloroethylene. Environ. Sci. Technol. 1982, 16, 448–453. [Google Scholar] [CrossRef]
- Yang, Z.Z. Performance and Mechanism for Degradation of Sulfadiazine in Water by UV/O3 Process. Master’s Thesis, Harbin Institute of Technology, Harbin, China, 2016. (In Chinese). [Google Scholar]
- Ding, C.S.; Xu, Y.Y.; Wang, W.W.; Fu, Y.P. Degradation of trichloronitromethane in drinking water by UV/H2O2/O3 process. China Water Wastewater 2014, 30, 51–54. (In Chinese) [Google Scholar]
- Lau, T.K.; Chu, W.; Graham, N. Reaction pathways and kinetics of butylated hydroxyanisole with UV, ozonation, and UV/O3 processes. Water Res. 2007, 41, 765–774. [Google Scholar] [CrossRef]
- Dutschke, M.; Schnabel, T.; Schutz, F.; Springer, C. Degradation of chlorinated volatile organic compounds from contaminated ground water using a carrier-bound TiO2/UV/O3-system. J. Environ. Manag. 2022, 304, 114236. [Google Scholar] [CrossRef]
- Lin, L.; Xie, M.N.; Liang, Y.M.; He, Y.Q.; Chan, G.Y.S.; Luan, T.G. Degradation of cypermethrin, malathion and dichlorovos in water and on tealeaves with O3/UV/TiO2 treatment. Food Control 2012, 28, 374–379. [Google Scholar] [CrossRef]
- Ratpukdi, T.; Casey, F.; DeSutter, T.; Khan, E. Bromate formation by ozone-VUV in comparison with ozone and ozone-UV: Effects of pH, ozone dose, and VUV power. J. Environ. Eng. 2011, 137, 187–195. [Google Scholar] [CrossRef]
- Zhao, G.Y. Research on Emerging Contaminants Removal from Drinking Water by UV-Micro O3 Process. Ph.D. Thesis, Dongnan University, Nanjing, China, 2015. (In Chinese). [Google Scholar]
- Ratpukdi, T.; Siripattanakul, S.; Khan, E. Mineralization and biodegradability enhancement of natural organic matter by ozone–VUV in comparison with ozone, VUV, ozone–UV, and UV: Effects of pH and ozone dose. Water Res. 2010, 44, 3531–3543. [Google Scholar] [CrossRef]
- Zhao, G.Y.; Lu, X.W.; Zhou, Y.; Gu, Q. Simultaneous humic acid removal and bromate control by O3 and UV/O3 processes. Chem. Eng. J. 2013, 232, 74–80. [Google Scholar] [CrossRef]
- Kim, I.H.; Yamashita, N.; Kato, Y.; Tanaka, H. Discussion on the application of UV/H2O2, O3 and O3/UV processes as technologies for sewage reuse considering the removal of pharmaceuticals and personal care products. Water Sci. Technol. 2009, 59, 945–955. [Google Scholar] [CrossRef]
- Mascolo, G.; Ciannarella, R.; Balest, L.; Lopez, A. Effectiveness of UV-based advanced oxidation processes for the remediation of hydrocarbon pollution in the groundwater: A laboratory investigation. J. Hazard. Mater. 2008, 152, 1138–1145. [Google Scholar] [CrossRef]
- Yang, S.P.; Song, Y.P.; Chang, F.M.; Wang, K.J. Evaluation of chemistry and key reactor parameters for industrial water treatment applications of the UV/O3 process. Environ. Res. 2020, 188, 109660. [Google Scholar] [CrossRef]
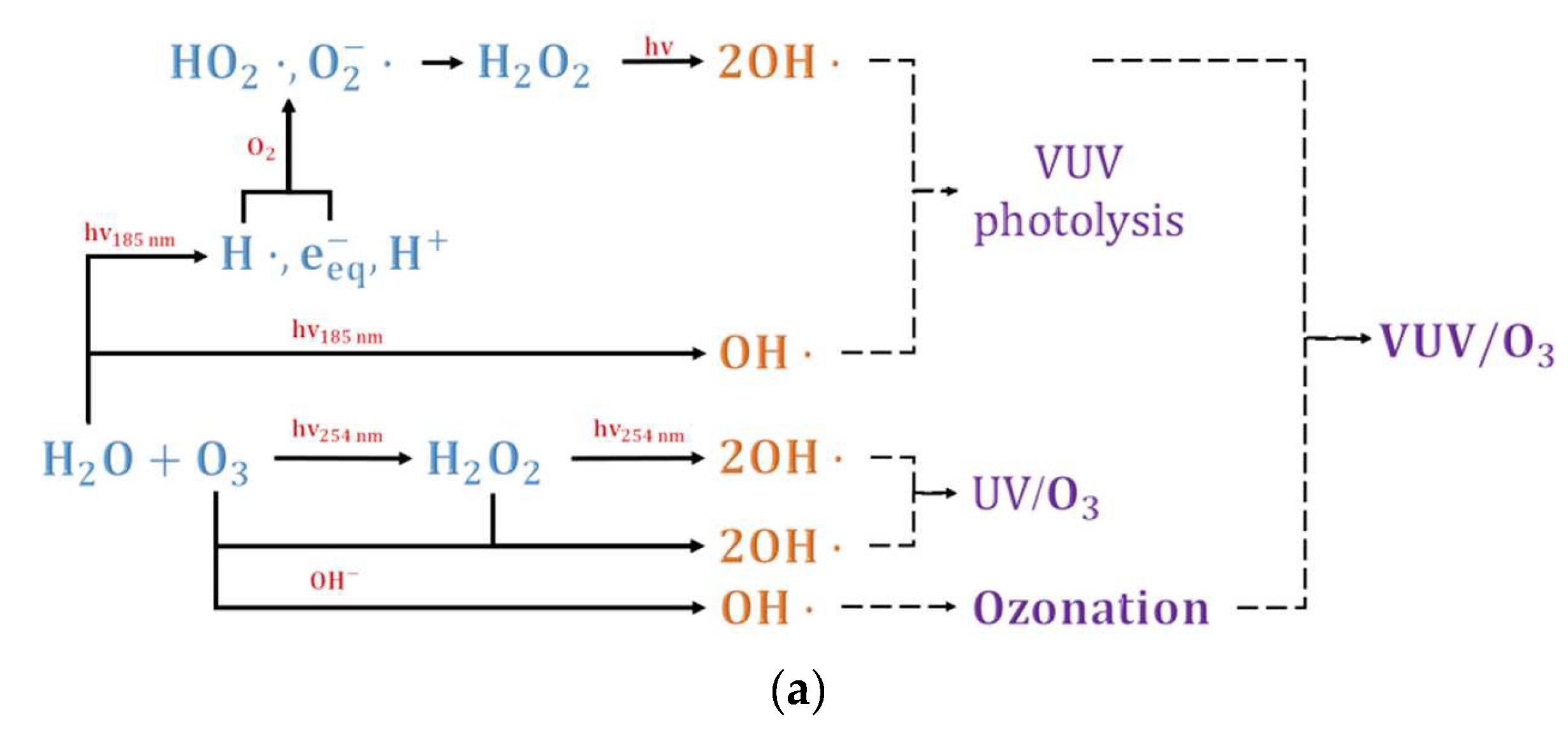
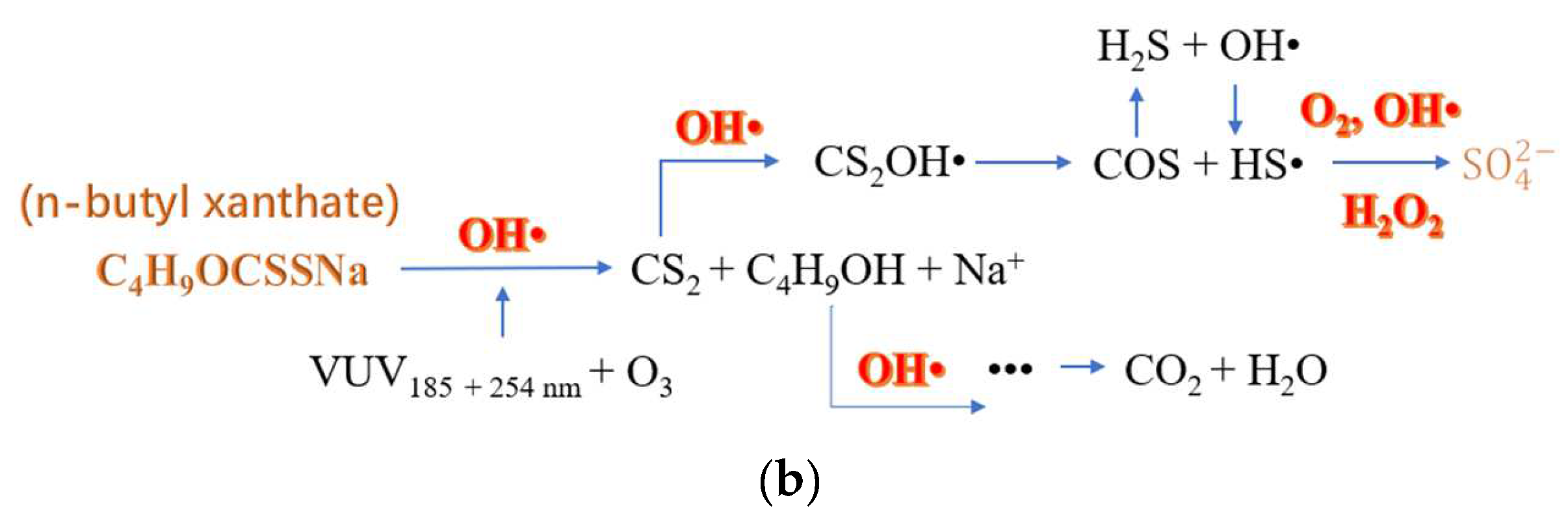
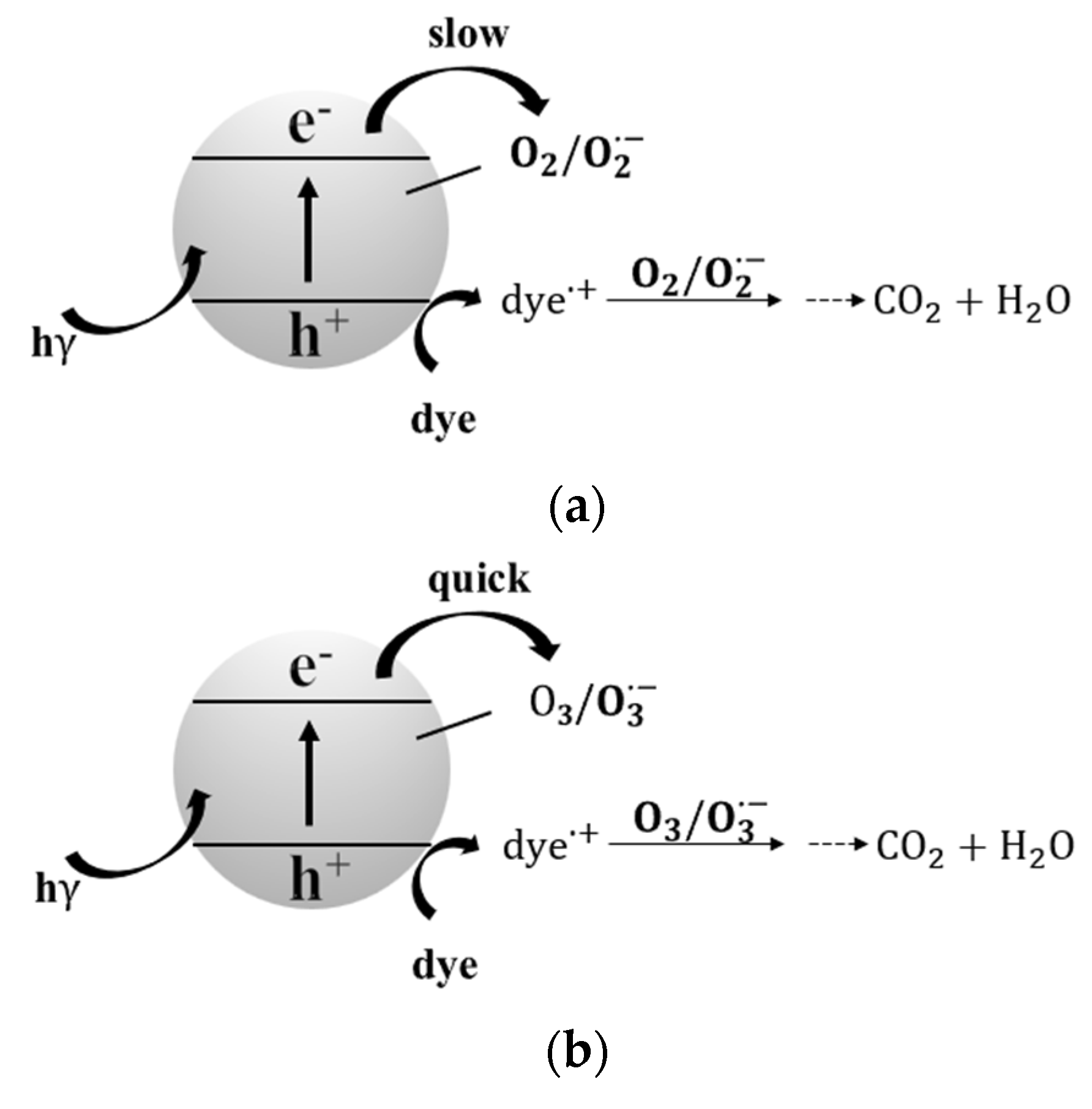
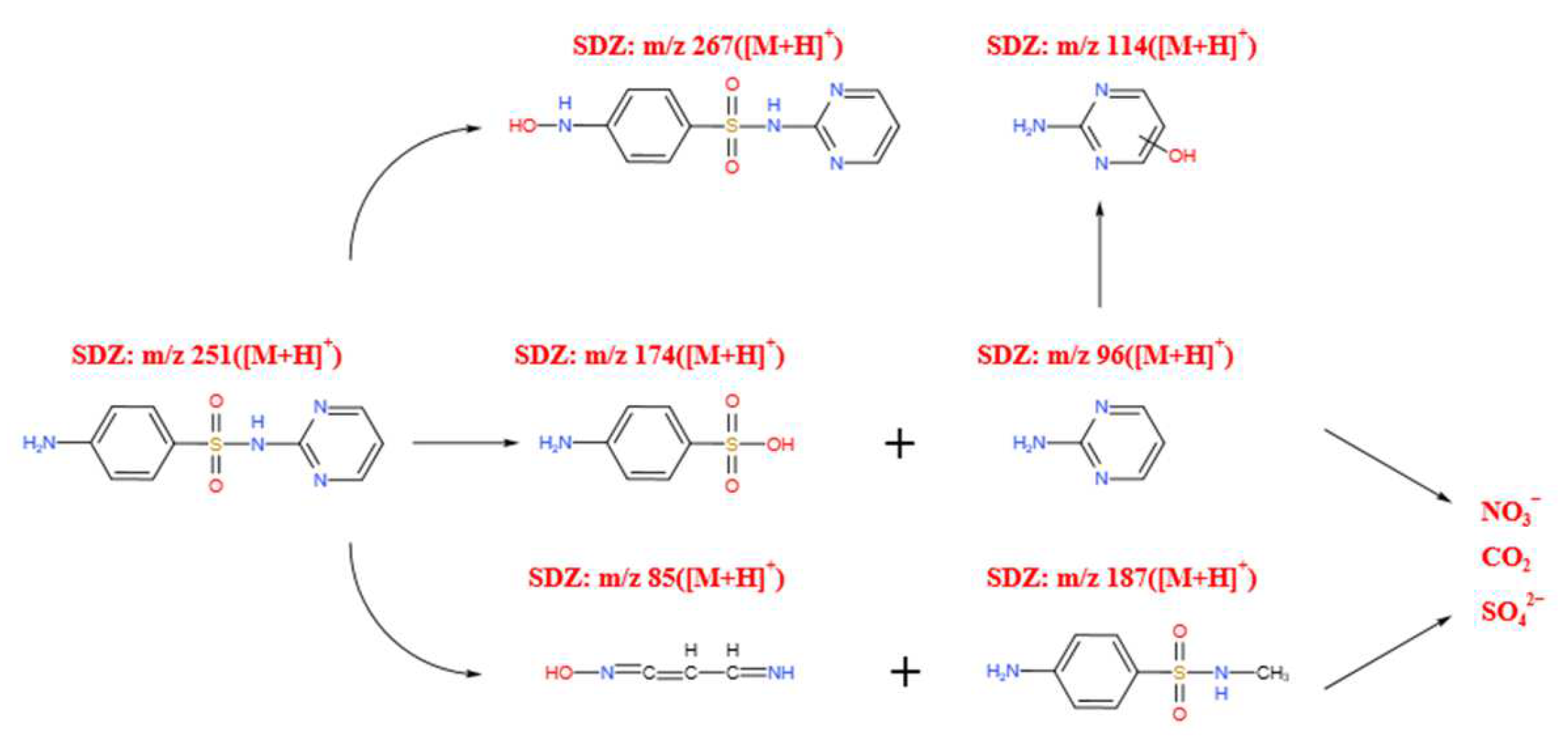
| No. | Reaction | Reaction Rate Constant (L·mol−1·s−1) |
|---|---|---|
| 1 | 1.8 × 1010 | |
| 2 | 2.8 × 106 | |
| 3 | 1.6 × 109 | |
| 4 | 5.2 × 1010 | |
| 5 | 2.7 × 107 | |
| 6 | 7.5 × 109 | |
| 7 | 3.0 × 109 |
| Application Occasions | Process Types | Research Results | Data Sources |
|---|---|---|---|
| Industrial wastewater | O3/UV process | The best treatment conditions of O3/UV were a UV lamp power of 16 W, O3 dosage of 0.6 mL/min, pH = 7.1 and irradiation time of 120 min, at which the chemical oxygen demand could be reduced from 82.3 mg/L to 49.5 mg/L, reaching the first-class A standard. | [31] |
| Under an initial PNA concentration of 10 mg/L, ozone input and supply rate of 0.9 g/h, contact time of 40 min and pH value of 9.0, the PNA removal rates of synthetic solution and real sample reach 94% and 81%, respectively. | [34] | ||
| UV photolysis made a significant contribution to the degradation of ATZ. The removal rate of ATZ exceeded 95% after a 180-min treatment. The removal of COD and ammonia nitrogen was mainly guided by ·OH, especially in an alkaline environment, and the optimal pH value was 12. | [36] | ||
| O3/VUV process | Degraded by O3 alone, vacuum ultraviolet alone and VUV/O3, they found that both O3 alone and VUV/O3 could completely degrade SBX within 5 min. After 40 min, the removal rates of COD by the three processes were 38.3%, 40.5% and 64.9%, respectively. | [32] | |
| O3/UV-BAF process | The COD removal rate of the single BAF treatment was only 22.5%, while the COD removal rate of the O3/UV-BAF collaborative process was 46.93%. In addition, the removal rate of UV254 was 39.73%, and the removal rates of chromaticity, total nitrogen and total phosphorus were 54%, 34.52% and 53.81%, respectively. | [33] | |
| UV/O3/H2O2 process | The UV/O3/H2O2 combined process to treat the reverse osmosis concentrate rich in metal ions and refractory trace organic compounds. After adding H2O2, the O3/UV process produced a more robust oxidation, and more ·OH was generated, making the reaction more active, and the TOrC removal rate was higher than 90%. | [35] | |
| O3/UV/TiO2 process | The O3/UV/TiO2 process increased the ozone utilization rate by 11.7% compared with the O3/UV process. In the coupled process, the mineralization rate of 2,4,6-TCP increased first and then decreased with the increase of the initial concentration. The mineralization rate still reached 83.31% even at the 2,4,6-TCP concentration of 30 mg/L. Moreover, this coupling process had a strong adaptability to pH. Sun et al. found that the maximum adsorption capacity of Brilliant Red X-3B(X3B) on the surface of TiO2 nanotubes (TNTs) was three times that of P25TiO2 when TNTs and P25TiO2 were used as photocatalysts to degrade X3B, respectively, in the O3/UV/TiO2 process, and the photocatalytic activity of TNTs was better than P25TiO2 under the same conditions. In the O2/UV/TiO2 system, X3B was degraded only by photocatalytic oxidation but not by chemical oxidation (ozonation). | [37,38] | |
| Trace polluted organic matter | O3/UV process | The rates of flaxseed pyrimidine treated by O3/UV were about 3.5 times and 2.5 times faster than those treated by UV alone and O3 alone, respectively. No removal of total organic carbon was observed during the process of UV reaction alone. TOC mineralization reached about 15% after 100 min in the process of O3 reaction alone, and the effect was not obvious. However, nearly 80% mineralization can be achieved after 100 min using O3/UV. | [40] |
| The pseudo-first-order reaction rate constants were 3.8 × 10−3 s−1, 2.9 × 10−3 s−1 and 1.9 × 10−3 s−1 when the SMX concentration was 0.5 mg/L, 1.0 mg/L and 4.5 mg/L, respectively. The experimental results show that the degradation rate of pollutants decreases with the increase of the initial concentration. | [41,42] | ||
| The O3/UV process had a nearly 82% mineralization effect on ibuprofen when the reaction lasted 130 min. The mineralization process was per pseudo-zero reaction kinetics model, which was helpful to reduce the secondary pollution of intermediate products to the environment. | [43] | ||
| When the treatment time was 10 min and the O3 concentration was 6 mg/L, among the 38 PPCPs detected in secondary sewage, 31 degraded to or below its detection limit. | [44] | ||
| Using the response surface optimization method, it was obtained that the optimal reaction conditions of the O3/UV process were ozone dosage of 5 mg/L, UV intensity of 4.16 W/L, initial pH of 9.41, a reaction time of 154 min, and the average removal rate of fluoroquinolone antibiotics could reach 68.81%. | [45] | ||
| O3/UV/Fe3O4 & TiO2 process | Under a reaction time of 30 min, photocatalyst dosage 2 g/L, pH 9, initial CTX concentration 10 mg/L and ozone dosage 0.2 g/h, the removal rate of CTX and TOC were 92.4% and 72.5%, respectively. At the same time, after six continuous uses of the catalyst, the TOC removal rate was still high, proving that Fe3O4 & TiO2 has a good reutilization potential. | [46] | |
| O3/UV/ZnO process | The O3/UV and O3/UV/ZnO can degrade methyl-, ethyl-, propyl-, buthyl and benzyparabens in a group of common p-hydroxybenzoates. The highest degradation rate of O3/UV was 60% at 60 min, while the highest degradation rate of O3/UV/ZnO was 93% at 20 min. The results showed that the value of BOD5/COD after photocatalytic ozonation increased, which improved the biodegradability and can be used as the pretreatment of bioreactor. | [47] | |
| Drinking water | O3/UV process | The degradation rate of the O3/UV process was higher than the sum of O3 and UV rates alone. By studying the comparative experiment of TOC removal, it was found that ·OH played an essential role in making the degradation process more thorough and reducing the formation of intermediate products. | [49] |
| BHA can form precipitation and be easily removed during ozonation. When the reaction time was 180 min, O3/UV could achieve a 90% mineralization effect. The intermediate products such as hydroquinone and 1, 4-benzoquinone detected in the experiment were entirely removed after the reaction. | [51] | ||
| UV/O3/H2O2 process | When the initial concentration of TCNM was 20 μg/L, the UV intensity was 31 μW/cm2, the H2O2 dosage was 15 mg/L and the ozone dosage was 10 mg/L, and the removal rate of the process reached 97.82% after 150 min. | [50] | |
| O3/UV/TiO2 process | The degradation rate of trichloromethane was higher than 85% and the degradation rate of cis-dichloroethylene, trichloroethylene and tetrachloroethylene were higher than 98%. No oxidation by-product or toxic conversion product increase was observed, so it was considered that the pollutants were fully mineralized. The removal effect on malathion and dichlorvos in water under alkaline conditions reached 100%, while under acidic and neutral conditions the removal rate of cypermethrin in water was close to 100%. The degradation of three pollutants in tea by O3/UV/TiO2 was not affected by pH. Simple water flushing was effective for dichlorvos, but not for insoluble cypermethrin and malathion. The removal rates of the above two pollutants in 30 min were 80% and 78%, respectively. | [52,53] |
| No. | Reaction | Reaction Rate Constant (L·mol−1·s−1) |
|---|---|---|
| 1 | 160 | |
| 2 | 100 | |
| 3 | ≤0.013 | |
| 4 | >105 | |
| 5 | 4.5 × 109 | |
| 6 | 2.0 × 109 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Li, Q.; Feng, W. Application Progress of O3/UV Advanced Oxidation Technology in the Treatment of Organic Pollutants in Water. Sustainability 2022, 14, 1556. https://doi.org/10.3390/su14031556
Lu H, Li Q, Feng W. Application Progress of O3/UV Advanced Oxidation Technology in the Treatment of Organic Pollutants in Water. Sustainability. 2022; 14(3):1556. https://doi.org/10.3390/su14031556
Chicago/Turabian StyleLu, Hai, Qingpo Li, and Weihao Feng. 2022. "Application Progress of O3/UV Advanced Oxidation Technology in the Treatment of Organic Pollutants in Water" Sustainability 14, no. 3: 1556. https://doi.org/10.3390/su14031556
APA StyleLu, H., Li, Q., & Feng, W. (2022). Application Progress of O3/UV Advanced Oxidation Technology in the Treatment of Organic Pollutants in Water. Sustainability, 14(3), 1556. https://doi.org/10.3390/su14031556






