A Systematic Review for Establishing Relevant Environmental Parameters for Urban Lighting: Translating Research into Practice
Abstract
1. Introduction
2. ALAN Lingua Franca
2.1. Concepts and Terminologies
2.2. Physical Properties of Artificial Lighting Considered by ULP and ALAN Research Domains
3. Scientific Question
Question 1. What are the most relevant parameters of artificial lighting for evaluating impacts on different organism groups that should be used by ALAN researchers in their research studies and by lighting professionals in their day-to-day practice in order to minimize the negative effects without having to turn off lighting?
Question 2. How do we translate ALAN research for lighting practice to address environmental concerns?
4. Materials and Methods
- Organism group was not identified.
- There was day-time exposure to artificial light.
- Horticultural studies were performed to enhance plant growth.
- Phased-out light sources were still often used for outdoor illumination, including incandescent lamps (Incandescent), tungsten-halogen lamps (THs), and high-pressure mercury vapor lamps (HPMs).
- Illuminance > 200 lx, as a maximal threshold to represent typical light-polluted scenarios in outdoor environments.
- Studies were conducted addressing the impact of ALAN on humans.
5. Results
5.1. Impact of Artificial Lighting on Organism Groups
5.1.1. Plants
5.1.2. Arthropods: Insects and Spiders
5.1.3. Fish
5.1.4. Amphibians
5.1.5. Reptiles
5.1.6. Birds
5.1.7. Non-Human Mammals: Bats, Primates, Rodents, and Marsupials
5.2. Translating ALAN Research into Applicable Lighting Practice
5.2.1. Application Value for Environmental Conservation
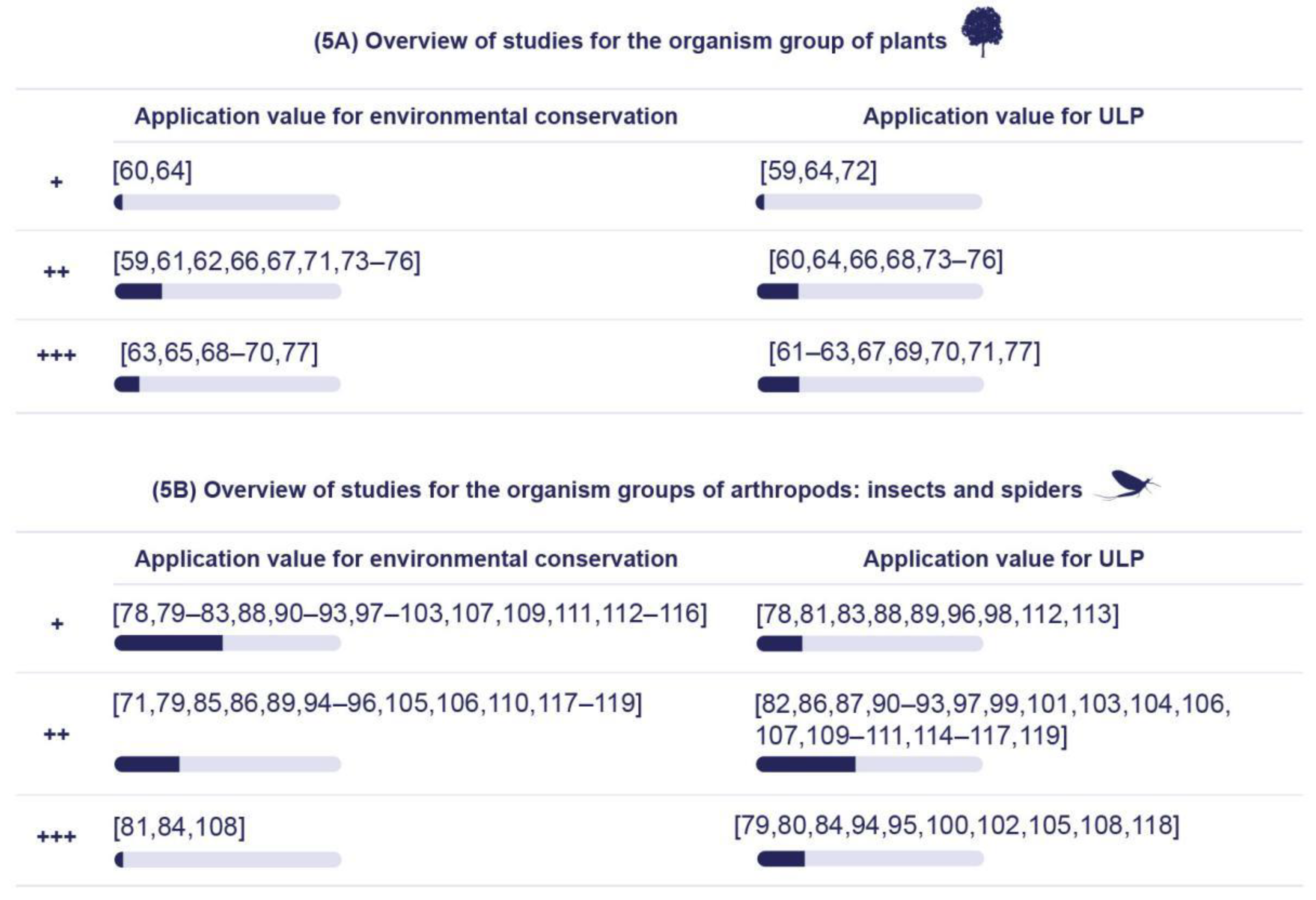

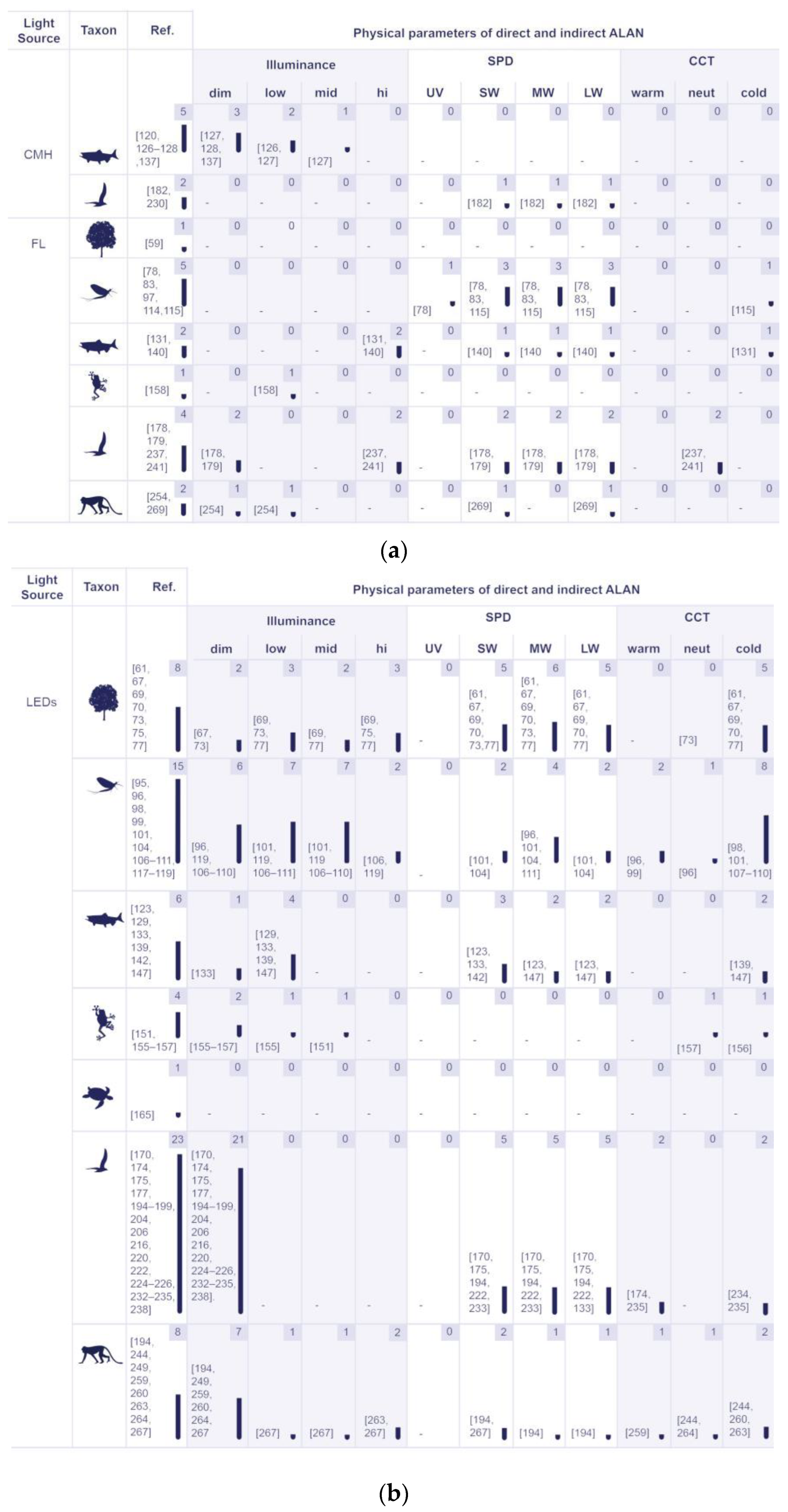
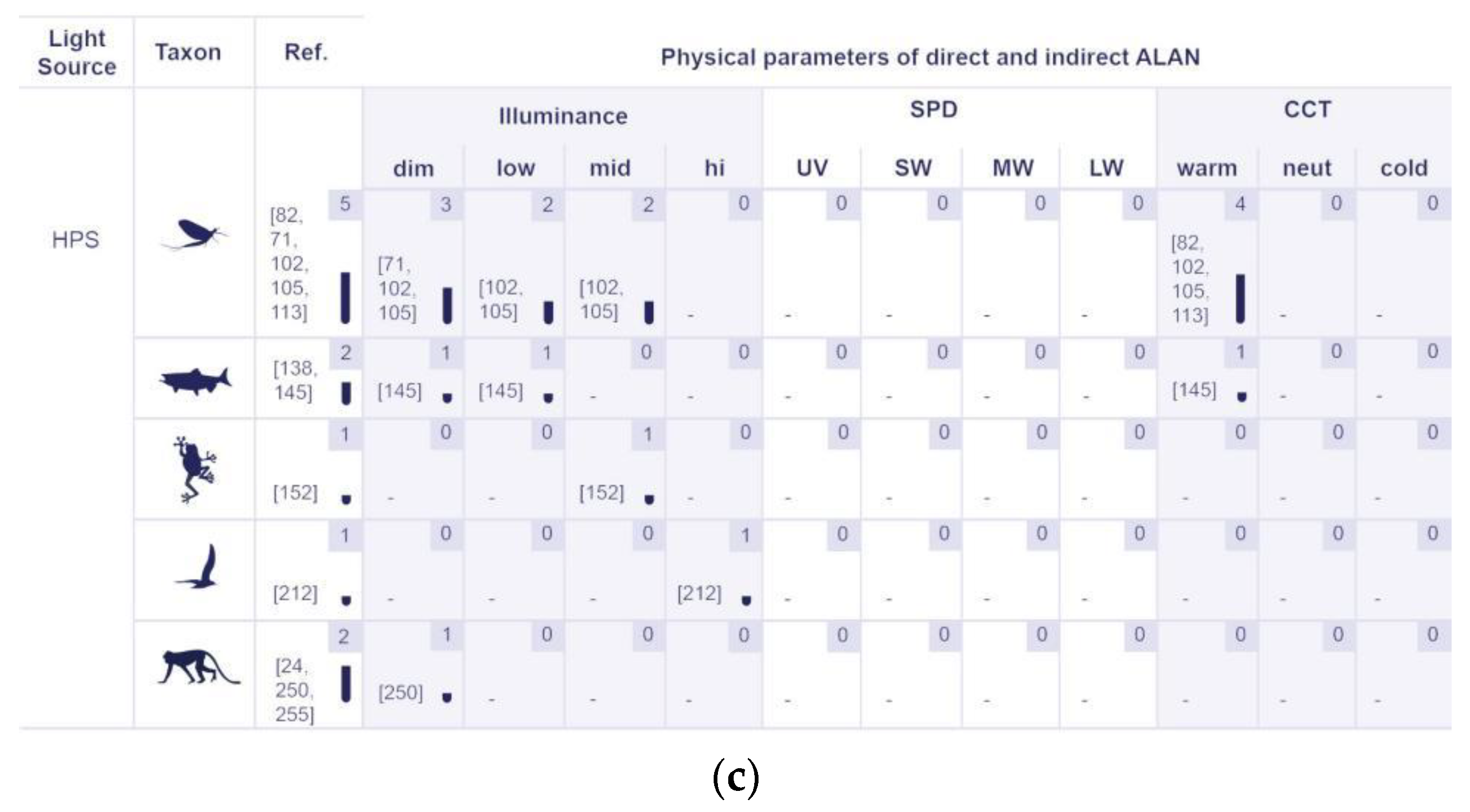
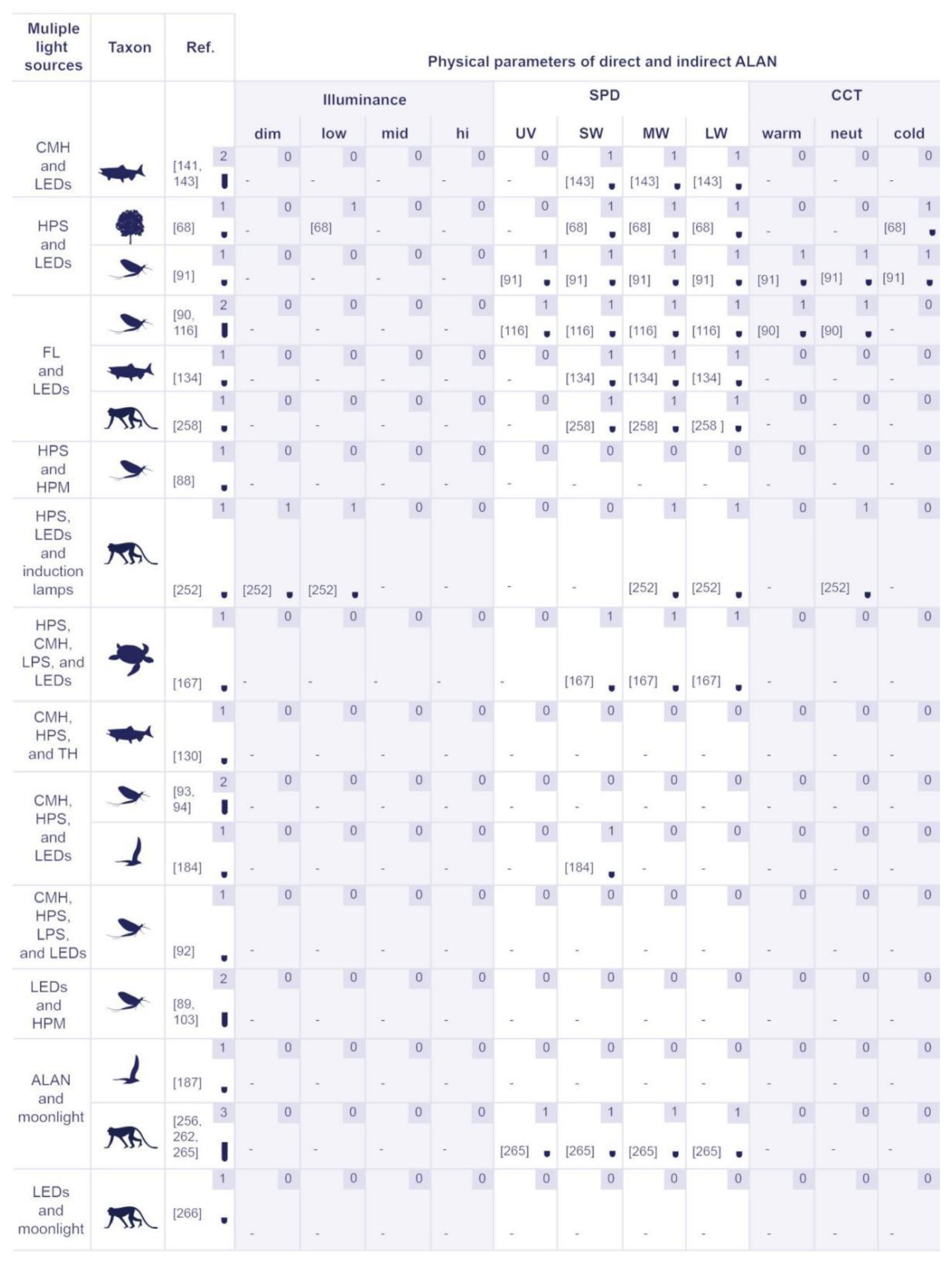
5.2.2. Physical Properties of Artificial Lighting Based on the Responses of Various Organism Groups

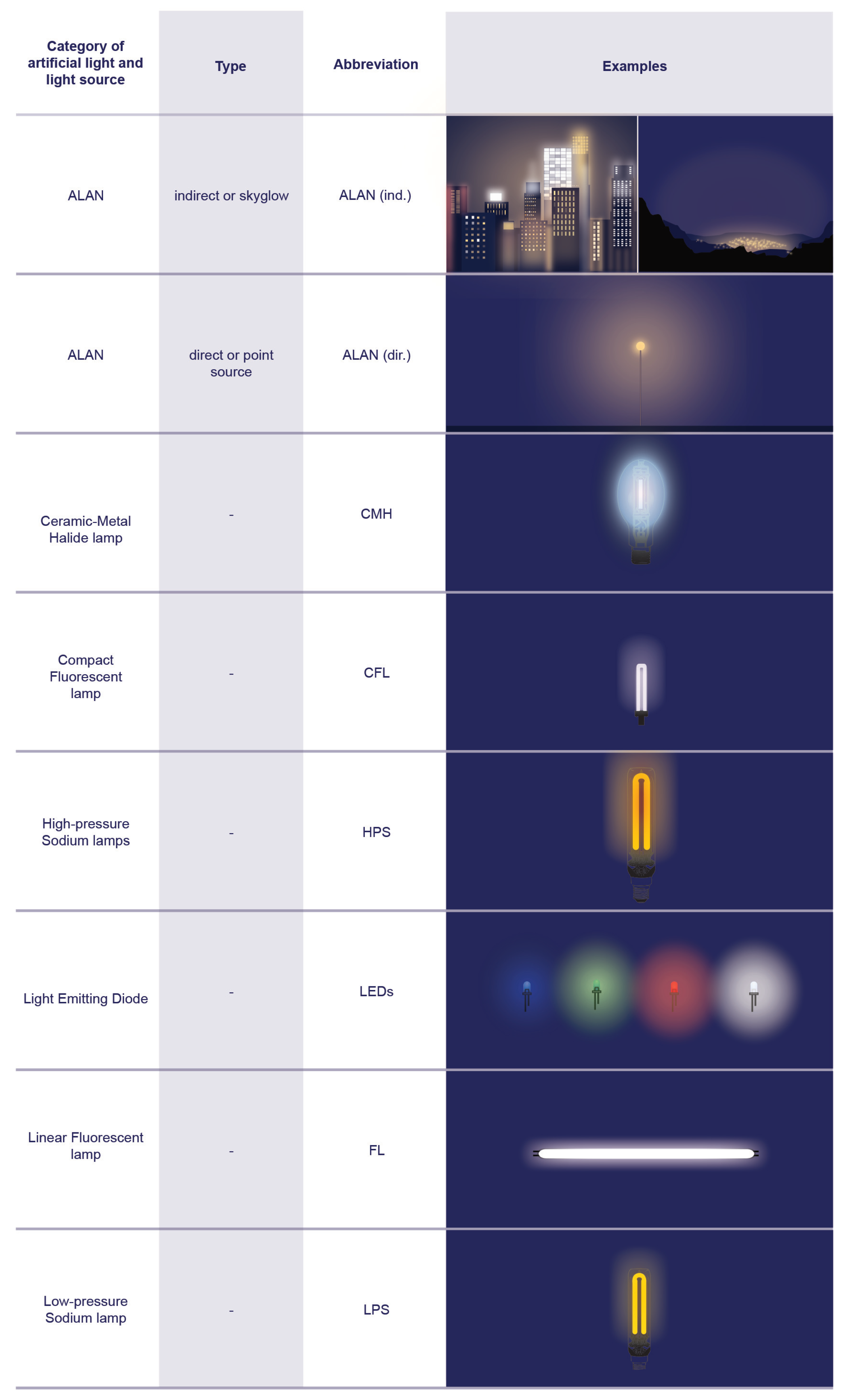
5.2.3. The Identified Light Sources

6. Limitations of the Study
6.1. Experimental Setting (Laboratory and Field Studies) versus Applied Outdoor Lighting
6.2. Research Methodology
6.3. Language of the Reviewed Studies
6.4. Sample Size (Number of Reviewed Research Studies and Publication Bias)
7. Discussion
Recommendations for Future ALAN Research and Urban Lighting
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Terminologies and Definitions Connected to Natural and Artificial Light

| Natural Energy Source | Terminology | Definitions |
|---|---|---|
| Sunlight | Sunrise/Sunset | The period when the Sun is at the horizon (at 0°); typical illuminance ca. 800 lx [280]. |
| Daylight | Solar radiation that reaches the Earth. This includes direct illumination and diffuse illumination from light scattered by air molecules, aerosol particles, cloud particles, or other particles in the atmosphere [281]. | |
| Twilight | The illumination of the lower atmosphere when the Sun is below the horizon [281,282]. | |
| Night | Civil twilight | The period when the Sun is between 0° and −6°; the lowest illuminance ca. 3.4 lx [21]. |
| Nautical twilight | The period when the Sun is between −6° and −12°; the lowest illuminance ca. 0.008 lx [21]. | |
| Astronomical twilight | The period when the Sun is between −12° and −18°; the lowest illuminance ca. 0.001 lx [21]. | |
| Astronomical night | The period when the Sun is more than −18° below the horizon. This is the period of absence of sunlight within the Earth’s atmosphere. | |
| Lunar cycle | Moonlight | Sunlight reflected on the surface of the moon, which due to changing phases and altitude during orbit, results in varied illuminance on the Earth; the maximum possible illuminance is ca. 0.3 lx for a full moon near zenith; typical peak full moon illuminances in mid latitudes are 0.1–0.2 lx [13,47]. |
| Stars, constellations and the Milky Way | Starlight and the Milky Way | The light emitted by stars of the Milky Way, as observed from the Earth during night time [283,284,285]. |
| Night sky brightness | Radiance/luminance of the night sky | The luminance of a typical clear night sky without light pollution and without airglow is around 200–250 mcd/m2. |
| Airglow | Nightglow | A natural dim light caused by the interaction of solar radiation and gases in the upper atmosphere of the Earth, which creates the natural glow of the night. The resulting glow means the night sky is never completely dark [285]. |
| Aurora (Borealis, Australis) | Polar lights | A natural source of light in the sky caused by the collision of charged particles from the Sun (the solar wind) with atmospheric constituents [286]. As shown in Figure A1j. |
| Living Organisms | Bioluminescence | The light emitted by a living organism caused by a chemical reaction [287]. As shown in Figure A1k–l. |
| Artificial Energy Source | Terminology | Definitions |
| Gas | Gas lighting | A structured system of underground pipes installed in cities to supply gaseous fuel combustion to produce artificial light for the night-time environment. Gas lighting has mainly been replaced with electric lighting, but a few remain as examples of industrial heritage in the urban landscape of cities such as Berlin and London [288,289,290]. |
| Electric | Electric lighting | A structured system powered by electrical current to produce artificial light at night [290]. |
| Responses | Terminology | Definition |
|---|---|---|
| Behavioral response | Diurnality | A behavior occurring during daylight or an organism active during the day and dormant during the night [291,292]. |
| Nocturnality (or crepuscular) | A behavior occurring during the night or an organism active around sunset, sunrise, or at night, when light conditions are lower compared to daylight [293]. | |
| Migration | The active movement of an organism or group of organisms from one location to a different one. The principle of migration could be periodic (seasonal, with a relative distance, due to changes in climate, temperature, or the availability of resources) or general (the active movement of an organism or group of organisms that may affect the distribution or range of a group of organisms across a landscape [291]. | |
| Navigation | The orientation of a living organism toward a destination (e.g., reaching a breeding area) regardless of its direction, by means other than the recognition of landmarks (e.g., the use of compass orientation) [291]. | |
| Orientation | The ability of an organism to head toward a particular direction without the reference of a landmark [291]. | |
| Phototaxis | The movement or direction of a living organism toward a light source as a behavioral response to changes in illuminance and color in light. This behavior is called positive phototaxis when the living organism is attracted or directed toward the light source, while avoidance or repulsion is called negative phototaxis [294]. | |
| Physiological response | Melatonin | A hormone secreted during the dark phase of the day responsible for regulating the sleep/active schedule and the modulation of circadian rhythms that varies between organisms [295]. |
| Metabolism | Chemical reactions that occur within the cells of an organism that provide energy for biological processes to occur, (e.g., breathing, repairing cells, growth, or digesting food) [291]. | |
| Characteristics and processes in plants | Short-day plants | Plants that require short periods of exposure to daylight and long periods of exposure to darkness to initiate the generative phase (less than 8 h/16 h light/dark rhythm) [291]. |
| Long-day plants | Plants that require long periods of exposure to daylight, with short periods of exposure to darkness, to initiate the generative phase (more than 16 h/8 h light/dark rhythm [296]. | |
| Photosynthesis | The complex process by which plants transform absorbed light (artificial or natural), carbon dioxide, water, certain inorganic salts, and chlorophyll into energy for growth and survival. An important by-product of this process is O2 and carbohydrates generated by the conversion of CO2 and water [297]. | |
| Visual response to light | Mesopic vision | The visual sensitivity of a vertebrate to see when exposed to conditions such as twilight at luminances between 0.01 and 3 cd/m2. Mesopic vision involves both photoreceptors (rods and cones) for scotopic and photopic vision [53,298]. |
| Photopic vision | The visual sensitivity of a vertebrate to see under lit conditions, such as daylight, which relies on the function of cones (a photoreceptors in the eye’s retina) and facilitates perception of colors [298,299]. | |
| Scotopic vision | The visual sensitivity of a vertebrate to perceive its surrounding environment under dim light conditions or during night time, which relies on the function of rods (a photoreceptors in the eye’s retina) and facilitates the perception of contrasts without colors [53]. |
| Definitions | |
|---|---|
| Standard incandescent lamp | An artificial light source where the filament in the lamp is heated to increase the temperature of the filament so that it emits light. A black body radiator with a CCT. Not in common use anymore. |
| Tungsten-halogen lamp (TH) | An artificial light source with a tungsten filament that is sealed in a transparent housing and filled with a mixture of inert gas and a small amount of halogen to allow higher operating temperatures than standard incandescent lamps. A black body radiator with a CCT of ca. 3000 K. Not in common use anymore. |
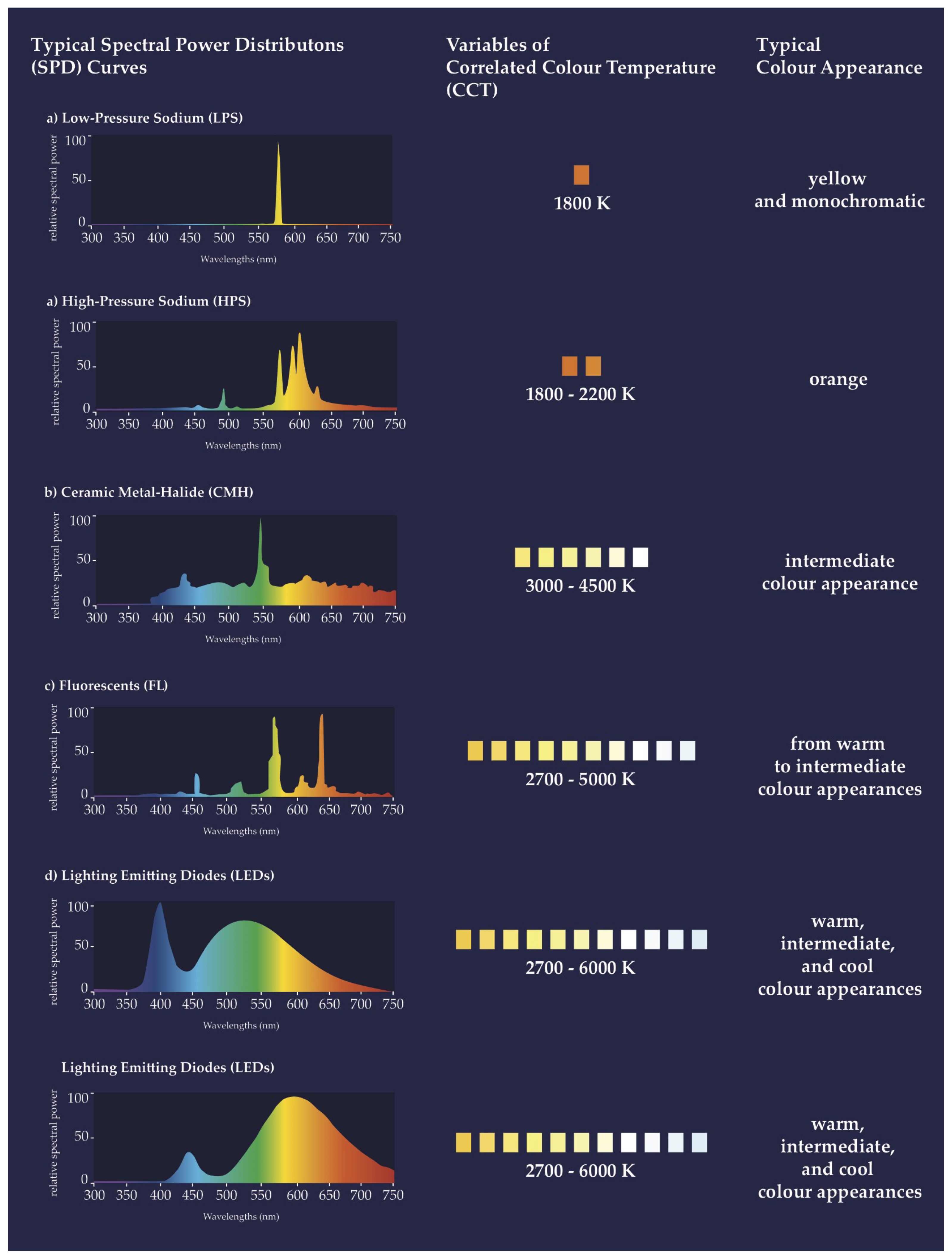
| Linear fluorescent lamp (FL) | An artificial low-pressure mercury gas discharge lamp that uses an electrical current to activate the phosphor coating on the interior of the lamp to glow. (The light that is emitted is rich in short and ultraviolet wavelengths.) FLs are commonly used. |
| Compact fluorescent lamp (CFL) | An artificial low-pressure mercury gas discharge lamp with a curved or folded tube (smaller in size when compared to fluorescents to fit household light sockets) and an integrated ballast. A CFL uses an electrical current that makes the phosphor coating on the lamp’s interior glow. (The light that is emitted is rich in short and ultraviolet wavelengths.) CFLs are commonly used. |
| Cold cathode (CC) and neon lamp | A gas-discharge source that produces artificial light via electricity emitted from a cathode to ignite mercury vapor through the scattering of kinetic energy. CC and neon lamps are commonly used. |
| High-pressure mercury vapor lamp (HPM) | An artificial light source in a double compartment bulb (a quartz discharge tube with mercury vapor at high pressure and an outer bulb coated with phosphor). The light that is emitted is rich in short ultraviolet wavelengths and visible light. This light source has been banned due to its negative impact on the environment. Not in common use anymore. |
| Ceramic metal halide lamp (CMH) | An artificial high-intensity discharge lamp (HID) that uses ceramic material to allow mercury, argon, and metal halide salts to heat at stable but high temperatures to produce light. This light source produces a color rendering index (CRI) and a correlated color temperature (CCT) close to daylight, dependent on the mixture of metal halide salts. CMHs are commonly used. |
| Low-pressure sodium lamp (LPS) | An artificial low-pressure gas discharge light source that uses sodium in the discharge arc tube to warm the lamp in order to produce light. The light that is emitted is from a narrow band of the spectrum (589 nm), a near-monochromatic amber color unique to sodium lamps. While highly energy efficient and favored around astronomical observatories, it is not in common use anymore and is being phased out in many countries. |
| High-pressure sodium lamp (HPS) | An artificial high-intensity gas discharge lamp (HID) that produces light through sodium vapor at high pressure. The light that is emitted is broader in spectrum compared to an LPS, with a peak at a wavelength of 586 nm. CCT ca. 2000 K. Still in use but is being phased out. |
| Light-emitting diode (LED) | An artificial light source that uses a chip of semiconductor material that emits light when an electric current pass through it. It is highly energy efficient, comparable to an LPS, but offers a full spectrum in the visible range and high CRI values. In use and rapidly replacing older light sources, such as LPSs, HPSs, and CMHs. The vast majority of LEDs currently in use for outdoor lighting around the world emit the whole visible spectrum, with peaks of blue wavelengths. |
| Terminology | Definitions |
|---|---|
| Radiant energy (Qe); luminous energy (Qv) | Energy of electromagnetic radiation, unit J (joule); example, energy of a photon. Luminous energy is the photometric equivalent (i.e., perceived energy referenced to luminosity function); unit (lm s). |
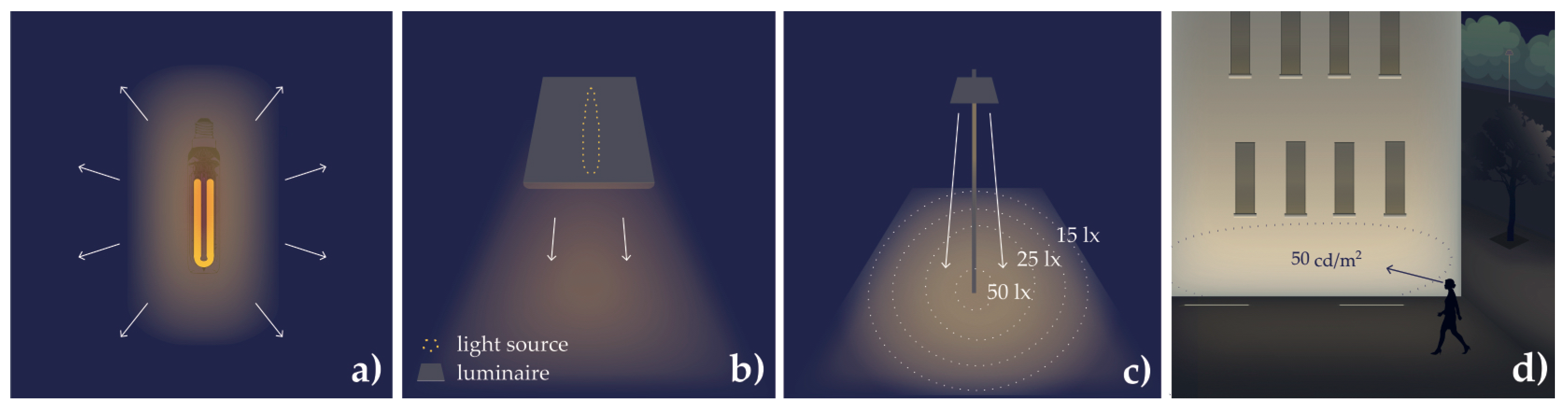
| Radiant flux (ϕe); luminous flux (ϕv) | Radiant energy per time, unit W (watt); example, all light (photons per second) emitted by a lamp in all directions (Figure A3). Luminous flux is the photometric equivalent; unit lm (lumen). |
| Radiant intensity (Ie); luminous intensity (Iv) | Radiant flux per solid angle; unit W/sr. Luminous intensity is the photometric equivalent; unit cd (candela, lm/sr). |
| Radiance (Ee); luminance (Ev) | Radiant flux emitted, reflected, transmitted, or received by a surface per solid angle per projected area; unit W/m2sr. Luminance is the photometric equivalent; unit cd/m2 (lm/m2sr) (Figure A3). |
| Irradiance (Ee); illuminance (Ev) | Radiant flux received by a surface per area; unit W/m2; example, horizontal irradiance (Figure A3). Illuminance is the photometric equivalent; unit lx (lm/m2). |
| Spectral irradiance (Ee, λ) | Irradiance per unit wavelength (also frequency); unit W/m2nm. |
| Luminous efficacy of a light source (Ksource) | Ratio of luminous flux to electrical power of a light source; unit lm/W; a measure of how well a light source produces visible light; not to be confused with the luminous efficacy of radiation. |
| Electrical power of a light source (Pi) | The rated electrical power consumed by a light source (including ballast, driver, control gear, etc., if applicable); unit W (watt). |
| Spectral power distribution (SPD) | Distribution of any radiant quantity (radiant energy, radiant flux, radiance, irradiance, etc.) as a function of wavelength, most commonly given in spectral irradiance, ideally provided in nm resolution. |
| Correlated color temperature (CCT) | Temperature of a Planckian radiator having the chromaticity nearest the chromaticity associated with the given spectral distribution; unit K (kelvin). The CCT can be roughly categorized as warm (<3300 K), neutral (3300–5300 K), or cool (>5300 K) in color appearance. |
| Color rendering index (CRI) | Measure of the degree to which the psychophysical color of an object illuminated by the test illuminant conforms to that of the same object illuminated by the reference illuminant. The CRI is given in 0–100, with 100 being the most accurate. |
| Reflectance (ρ) | The effectiveness of a material in reflecting radiant (or luminous) energy. |
| Flicker | Light source with a temporal change in radiant flux that can, e.g., be perceived as a stroboscopic effect. |
| Lamp survival factor (LSF) or lamp life | The estimated lifespan of a light source once installed and operating, measured in hours. Operating temperature, frequency of usage and switching, failure of electrical components, the supplied voltage, and vibrations are some factors that may shorten the estimated length of lamp life. |
| Operating time | The management and control of operating lighting systems for a determined geographical location to specify the hours a luminaire or a lighting system may operate and consume energy (for instance, hourly, daily, weekly, monthly, annually, or seasonal or for cultural events, religious events, or holidays). |
| Scene setting | The control of illuminance levels and/or color temperatures to set scenes at a determined time for a determined location. |
| Dimming | The manual or automatic control that manages light output with a programmable timer or sensor installed on-site to provide the gradual decrease of illuminance during a determined period at night or to perform a gradual increase of illuminance levels after dusk. |
| Terminology | Definitions |
|---|---|
| Artificial light at night (ALAN) | A phrase used as a broad term to summarize all forms of artificial lighting (e.g., electric and gas lighting sources), most commonly used to address the unintended consequence of artificial lighting when improperly managed as a form of anthropogenic pollution, which may affect a wide range of environmental processes. ALAN can be described as direct when the source of light is considered a point light source and the parameters of the mentioned source can be modified and as indirect when it addresses the skyglow effect, as it integrates multiple light sources that are reflected from other surfaces. |
| Astronomical light pollution (ALP) | Mismanaged artificial light that prevents the visibility of starlight and a naturally dark sky. |
| Ecological light pollution (ELP) | The adverse effect or negative impact of artificial lighting on living organisms and ecosystems. |
| Glare | The visual and physical discomfort caused by luminance levels that exceed the threshold our eyes can manage. |
| Light pollution (LP) | The widespread use of artificial illumination in the night-time environment and the unwanted consequence of improperly managed and poorly controlled lighting properties of artificial light sources and luminaires. Artificial light that is polluting the natural light. Light pollution occurs in four ways, skyglow, glare, light trespass, and clutter, all of which prevent visibility of the naturally dark sky and stars and is defined as astronomical light pollution. Light pollution also has an adverse effect on aquatic and terrestrial ecosystems and living organisms and is defined as ecological light pollution. |
| Light trespass | Objectionable light from an artificial light source that intrudes into indoor settings and private property, illuminating spaces not meant to be illuminated. The term “light trespass” has been commonly used to describe the intrusion of light into settings where it is not meant to be, such as gardens or natural habitats. |
| Visual light clutter | Visual light clutter in the urban environment at night is defined as the state in which too many items lead to a degradation of the performance of a visual task at night. |
| Obtrusive light | The quantitative, directional, or spectral properties in light, which can cause visual discomfort and distraction and also hinder the perception of nearby environments. |
| Over-illumination | The presence of unnecessary artificial lighting caused by excessive brightness, too many luminaires, and the varied coloration of artificial light from different sources, which blends together and forms an unnatural gleam. |
| Skyglow | Glow from light radiated upward that is then scattered within the atmosphere and diverted back to the Earth. It often results in a diffuse light dome above densely populated areas and extends across landscapes. A consequence of luminaires improperly directing light upward toward the sky or from reflected light. Skyglow depends on the weather and atmospheric conditions. Skyglow used to have orange color from LPSs and HPSs, but today, skyglow can have a cool, white appearance from LEDs. |
References
- Mansfield, K.P. Architectural lighting design: A research review over 50 years. Lighting Res. Technol. 2017, 50, 80–97. [Google Scholar] [CrossRef]
- Brandi, U.; Geissmar, C. Light for Cities: Lighting Design for Urban Spaces. A Handbook; Birkhäuser: Basel, Switzerland, 2007. [Google Scholar]
- Kutlu, R.; Manav, B. Lighting scheme as a design tool in urban identity: A case study at Bosphorus Region in Istanbul. World Appl. Sci. J. 2013, 23, 81–87. [Google Scholar]
- Kelly, R. Lighting as an integral part of architecture. Coll. Art J. 1952, 12, 24–30. [Google Scholar] [CrossRef]
- Neumann, D. The Structure of Light: Richard Kelly and the Illumination of Modern Architecture; Yale University Press: London, UK, 2010. [Google Scholar]
- Zielinska-Dabkowska, K.; Xavia, K. Historic Urban Settings, LED Illumination and its Impact on Nighttime Perception, Visual Appearance, and Cultural Heritage Identity. In Proceedings of the 5th SGEM International Multidisciplinary Scientific Conferences on Social Sciences and Arts, SGEM2018, Florence, Italy, 23–36 October 2018; STEF92 Technology: Sofia, Bulgaria, 2008. ISBN 978-619-7408-69-0. [Google Scholar]
- Bará, S. Anthropogenic disruption of the night sky darkness in urban and rural areas. R. Soc. Open Sci. 2016, 3, 160541. [Google Scholar] [CrossRef] [PubMed]
- Riegel, K.W. Light Pollution: Outdoor lighting is a growing threat to astronomy. Science 1973, 179, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Longcore, T.; Rich, C. Ecological Light Pollution. Front. Ecol. Environ. 2004, 2, 191–198. [Google Scholar] [CrossRef]
- Jechow, A.; Kyba, C.C.; Hölker, F. Mapping the brightness and color of urban to rural skyglow with all-sky photometry. J. Quant. Spectrosc. Radiat. Transf. 2020, 250, 106988. [Google Scholar] [CrossRef]
- Ouyang, J.Q.; Davies, S.; Dominoni, D. Hormonally mediated effects of artificial light at night on behavior and fitness: Linking endocrine mechanisms with function. J. Exp. Biol. 2018, 221, jeb156893. [Google Scholar] [CrossRef]
- Falchi, F.; Cinzano, P.; Duriscoe, D.; Kyba, C.C.M.; Elvidge, C.D.; Baugh, K.; Portnov, B.A.; Rybnikova, N.A.; Furgoni, R. The new world atlas of artificial night sky brightness. Sci. Adv. 2016, 2, e1600377. [Google Scholar] [CrossRef] [PubMed]
- Kyba, C.C.; Kuester, T.; De Miguel, A.S.; Baugh, K.; Jechow, A.; Hölker, F.; Bennie, J.; Elvidge, C.D.; Gaston, K.J.; Guanter, L. Artificially Lit Surface of Earth at Night Increasing in Radiance and Extent. Sci. Adv. 2017, 3, e1701528. [Google Scholar] [CrossRef]
- Bennie, J.; Duffy, J.P.; Davies, T.W.; Correa-Cano, M.E.; Gaston, K.J. Global trends in exposure to light pollution in natural terrestrial ecosystems. Rem. Sens. 2015, 7, 2715–2730. [Google Scholar] [CrossRef]
- Hölker, F.; Wolter, C.; Perkin, E.K.; Tockner, K. Light pollution as a biodiversity threat. Trends Ecol. Evol. 2010, 25, 681–682. [Google Scholar] [CrossRef]
- Navara, K.J.; Nelson, R.J. The dark side of light at night: Physiological, epidemiological, and ecological consequences. J. Pineal Res. 2007, 43, 215–224. [Google Scholar] [CrossRef]
- Gaston, K.J.; Bennie, J.; Davies, T.W.; Hopkins, J. The ecological impacts of nighttime light pollution: A mechanistic appraisal. Biol. Rev. 2013, 88, 912–927. [Google Scholar] [CrossRef] [PubMed]
- Schroer, S.; Hölker, F. Impact of Lighting on Flora and Fauna; Springer: Cham, Switzerland, 2016; pp. 1–33. [Google Scholar]
- Zielinska-Dabkowska, K.M. Make lighting healthier. Nature 2018, 553, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Grubisic, M.; Haim, A.; Bhusal, P.; Dominoni, D.M.; Gabriel, K.M.A.; Jechow, A.; Kupprat, F.; Lerner, A.; Marchant, P.; Riley, W.; et al. Light Pollution, Circadian Photoreception, and Melatonin in Vertebrates. Sustainability 2019, 11, 6400. [Google Scholar] [CrossRef]
- Hänel, A.; Posch, T.; Ribas, S.J.; Aubé, M.; Duriscoe, D.; Jechow, A.; Kollath, Z.; Lolkema, D.E.; Moore, C.; Schmidt, N. Measuring Night Sky Brightness: Methods and Challenges. J. Quant. Spectrosc. Radiat. Transf. 2018, 205, 278–290. [Google Scholar] [CrossRef]
- Pérez Vega, C.; Zielinska-Dabkowska, K.M.; Hölker, F. Urban Lighting Research Transdisciplinary Framework—A Collaborative Process with Lighting Professionals. Int. J. Environ. Res. Public Health 2021, 18, 6. [Google Scholar] [CrossRef]
- Stone, E.L.; Jones, G.; Harris, S. Street Lighting Disturbs Commuting Bats. Curr. Biol. 2009, 19, 1123–1127. [Google Scholar] [CrossRef]
- Rodríguez, A.; Holmes, N.D.; Ryan, P.G.; Wilson, K.J.; Faulquier, L.; Murillo, Y.; Raine, A.F.; Penniman, J.F.; Neves, V.; Rodríguez, B.; et al. Seabird mortality induced by land-based artificial lights. Conserv. Biol. 2017, 31, 986–1001. [Google Scholar] [CrossRef]
- Frank, K. Impact of outdoor lighting on moths: An assessment. J. Lepid. Soc. 1987, 42, 63–93. [Google Scholar]
- Grubisic, M.; van Grunsven, R.H.; Kyba, C.C.; Manfrin, A.; Hölker, F. Insect declines and agroecosystems: Does light pollution matter? Ann. Appl. Biol. 2018, 173, 180–189. [Google Scholar] [CrossRef]
- Owens, A.C.; Cochard, P.; Durrant, J.; Farnworth, B.; Perkin, E.K.; Seymoure, B. Light pollution is a driver of insect declines. Biol. Conserv. 2020, 241, 108259. [Google Scholar] [CrossRef]
- Hölker, F.; Wurzbacher, C.; Weissenborn, C.; Monaghan, M.T.; Holzhauer, S.I.; Premke, K. Microbial diversity and community respiration in freshwater sediments influenced by artificial light at night. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2015, 370, 20140130. [Google Scholar] [CrossRef]
- Kupprat, F.; Hölker, F.; Kloas, W. Can skyglow reduce nocturnal melatonin concentrations in Eurasian perch? Environ. Pollut. 2020, 262, 114324. [Google Scholar] [CrossRef]
- Grubisic, M.; van Grunsven, R.H.A.; Manfrin, A.; Monaghan, M.T.; Hölker, F. A transition to white LED increases ecological impacts of nocturnal illumination on aquatic primary producers in a lowland agricultural drainage ditch. Environ. Pollut. 2018, 240, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Major, M. London: Light+ Dark= Legibility: An Approach to Urban Lighting. In Cities Lights Two Centuries of Urban Illumination; Isenstadt, S., Petty, M.M., Neumann, D., Eds.; Routledge: New York, NY, USA, 2015; pp. 152–158. [Google Scholar]
- Casciani, D. The Human and Social Dimension of Urban Lightscapes; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Zielinska-Dabkowska, K. Urban Lighting Masterplan—Definitions, Methodologies and Collaboration. In Urban Lighting for People: Evidence—Based Lighting Design for the Built Environment, 1st ed.; Davoudian, N., Ed.; RIBA Publishing: London, UK, 2019; pp. 18–41. [Google Scholar]
- Kyba, C.; Pritchard, S.B.; Ekirch, A.R.; Eldridge, A.; Jechow, A.; Preiser, C.; Kunz, D.; Henckel, D.; Hölker, F.; Barentine, J. Night Matters—Why the Interdisciplinary Field of “Night Studies” Is Needed. J—Multidiscip. Sci. J. 2020, 3, 1–6. [Google Scholar] [CrossRef]
- Schulte-Römer, N.; Meier, J.; Dannemann, E.; Söding, M. Lighting Professionals versus Light Pollution Experts? Investigating Views on an Emerging Environmental Concern. Sustainability 2019, 11, 1696. [Google Scholar] [CrossRef]
- International Association of Lighting Designers (IALD). Lighting Industry Resource Council (LIRC) Guidelines for Specification Integrity. 2017. Available online: https://www.iald.org/getmedia/94ff9ecf-8631-4153-980f-5fbef193c56e/IALD-LIRC-Spec-Integrity-2017-Interactive (accessed on 13 November 2020).
- Kyba, C.C.; Ruhtz, T.; Fischer, J.; Hölker, F. Cloud coverage acts as an amplifier for ecological light pollution in urban ecosystems. PLoS ONE 2011, 6, e17307. [Google Scholar] [CrossRef]
- Kolláth, Z. Measuring and modelling light pollution at the Zselic Starry Sky Park. J. Phys. Conf. Ser. 2010, 218, 012001. [Google Scholar] [CrossRef]
- Vaidya, H.; Chatterji, T. SDG 11 Sustainable Cities and Communities. In Actioning the Global Goals for Local Impact; Springer: Singapore, 2020; pp. 173–185. [Google Scholar]
- Wood, D.J.; Gray, B. Toward a comprehensive theory of collaboration. J. Appl. Behav. Sci. 1991, 27, 139–162. [Google Scholar] [CrossRef]
- Thomson, A.M.; Perry, J.L.; Miller, T.K. Conceptualizing and measuring collaboration. J. Public Adm. Res. Theory 2009, 19, 23–56. [Google Scholar] [CrossRef]
- Lang, D.J.; Wiek, A.; Bergmann, M.; Stauffacher, M.; Martens, P.; Moll, P.; Swilling, M.; Thomas, C.J. Transdisciplinary Research in Sustainability Science: Practice, Principles, and Challenges. Sustain. Sci. 2012, 7, 25–43. [Google Scholar] [CrossRef]
- Zielinska-Dabkowska, K. Human Centric Lighting. The New X Factor? ARC Lighting Archit. 2019, 108, 81–86. [Google Scholar]
- Houser, K.; Boyce, P.; Zeitzer, J.; Herf, M. Human-Centric Lighting: Myth, Magic or Metaphor? Light. Res. Technol. 2021, 53, 97–118. [Google Scholar] [CrossRef]
- Houser, K.W. Ethics and Fallacies of Human-Centric Lighting and Artificial Light at Night. Leukos 2021, 17, 319–320. [Google Scholar] [CrossRef]
- Cinzano, P.; Falchi, F.; Elvidge, C.D. Moonlight without the moon. In Earth-Moon Relationships; Springer: Dordrecht, The Netherlands, 2001; pp. 517–522. [Google Scholar]
- Jechow, A.; Hölker, F. Snowglow—The Amplification of Skyglow by Snow and Clouds Can Exceed Full Moon Illuminance in Suburban Areas. J. Imaging 2019, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Jechow, A.; Hölker, F. How dark is a river? Artificial light at night in aquatic systems and the need for comprehensive night-time light measurements. Wiley Interdiscip. Rev. Water 2019, 6, e1388. [Google Scholar] [CrossRef]
- Sliney, D.H. How Light Reaches the Eye and Its Components. Int. J. Toxicol. 2002, 21, 501–509. [Google Scholar] [CrossRef]
- Wiltschko, R.; Nießner, C.; Wiltschko, W. The Magnetic Compass of Birds: The Role of Cryptochrome. Front. Physiol. 2021, 12, 584. [Google Scholar] [CrossRef]
- Schweikert, L.E.; Fitak, R.R.; Caves, E.M.; Sutton, T.T.; Johnsen, S. Spectral Sensitivity in Ray-Finned Fishes: Diversity, Ecology and Shared Descent. J. Exp. Biol. 2018, 221, jeb189761. [Google Scholar] [CrossRef] [PubMed]
- Barghini, A.; Souza de Medeiros, B.A. UV Radiation as an Attractor for Insects. Leukos 2012, 9, 47–56. [Google Scholar] [CrossRef]
- Galadí-Enríquez, D. Beyond CCT: The spectral index system as a tool for the objective, quantitative characterization of lamps. J. Quant. Spectrosc. Radiat. Transf. 2018, 206, 399–408. [Google Scholar] [CrossRef]
- Kalinkat, G.; Grubisic, M.; Jechow, A.; van Grunsven, R.H.A.; Schroer, S.; Hölker, F. Assessing Long-Term Effects of Artificial Light at Night on Insects: What Is Missing and How to Get There. Insect Conserv. Divers. 2021, 14, 260–270. [Google Scholar] [CrossRef]
- Pullin, A.; Frampton, G.; Jongman, R.; Kohl, C.; Livoreil, B.; Lux, A.; Pataki, G.; Petrokofsky, G.; Podhora, A.; Saarikoski, H.; et al. Selecting appropriate methods of knowledge synthesis to inform biodiversity policy. Biodivers. Conserv. 2016, 25, 1285–1300. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Meline, T. Selecting studies for systematic review: Inclusion and exclusion criteria. Contemp. Issues Commun. Sci. Disord. 2006, 33, 21–27. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Futsaether, C.M.; Vollsnes, A.V.; Kruse, O.M.O.; Otterholt, E.; Kvaal, K.; Eriksen, A.B. Effects of the Nordic photoperiod on ozone sensitivity and repair in different clover species studied using infrared imaging. AMBIO A J. Hum. Environ. 2009, 38, 437–444. [Google Scholar] [CrossRef]
- Whitman, C.M.; Heins, R.D.; Cameron, A.C.; Carlson, W.H. Lamp type and irradiance level for daylength extensions influence flowering of Campanula carpatica ‘Blue Clips’, Coreopsis grandiflora ‘Early Sunrise’, and Coreopsis verticillata ‘Moonbeam’. J. Am. Soc. Hortic. Sci. 1998, 123, 802–807. [Google Scholar] [CrossRef]
- Bennie, J.; Davies, T.W.; Cruse, D.; Inger, R.; Gaston, K.J. Artificial light at night causes top-down and bottom-up trophic effects on invertebrate populations. J. Appl. Ecol. 2018, 55, 2698–2706. [Google Scholar] [CrossRef]
- Kwak, M.J.; Lee, S.H.; Khaine, I.; Je, S.M.; Lee, T.Y.; You, H.N.; Lee, H.K.; Jang, J.H.; Kim, I.; Woo, S.Y. Stomatal movements depend on interactions between external night light cue and internal signals activated by rhythmic starch turnover and abscisic acid (ABA) levels at dawn and dusk. Acta Physiol. Plant. 2017, 39, 162. [Google Scholar] [CrossRef]
- Ffrench-Constant, R.H.; Somers-Yeates, R.; Bennie, J.; Economou, T.; Hodgson, D.; Spalding, A.; McGregor, P.K. Light pollution is associated with earlier tree budburst across the United Kingdom. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160813. [Google Scholar] [CrossRef]
- Blanchard, M.G.; Runkle, E.S. Intermittent Light from a Rotating High-pressure Sodium Lamp Promotes Flowering of Long-day Plants. HortScience 2010, 45, 236. [Google Scholar] [CrossRef]
- Viera-Pérez, M.; Hernandez-Calvento, L.; Hesp, P.A.; Santana-Del Pino, A. Effects of artificial light on flowering of foredune vegetation. Ecology 2019, 100, e02678. [Google Scholar] [CrossRef]
- Cathey, H.M.; Campbell, L.E. Effectiveness of five vision-lighting sources on photo-regulation of 22 species of ornamental plants. J. Am. Soc. Hortic. Sci. 1975, 100, 65–71. [Google Scholar]
- Bennie, J.; Davies, T.W.; Cruse, D.; Inger, R.; Gaston, K.J. Cascading effects of artificial light at night: Resource-mediated control of herbivores in a grassland ecosystem. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140131. [Google Scholar] [CrossRef] [PubMed]
- Macgregor, C.J.; Pocock, M.J.O.; Fox, R.; Evans, D.M. Effects of street lighting technologies on the success and quality of pollination in a nocturnally pollinated plant. Ecosphere 2019, 10, e02550. [Google Scholar] [CrossRef]
- Pu, G.Z.; Zeng, D.J.; Mo, L.; Liao, J.X.; Chen, X.X. Artificial Light at Night Alleviates the Negative Effect of Pb on Freshwater Ecosystems. Int. J. Mol. Sci. 2019, 20, 1343. [Google Scholar] [CrossRef]
- Liu, Z.; Lv, Y.; Ding, R.; Chen, X.; Pu, G. Light Pollution Changes the Toxicological Effects of Cadmium on Microbial Community Structure and Function Associated with Leaf Litter Decomposition. Int. J. Mol. Sci. 2020, 21, 422. [Google Scholar] [CrossRef]
- Grenis, K.; Murphy, S.M. Direct and indirect effects of light pollution on the performance of an herbivorous insect. Insect Sci. 2019, 26, 770–776. [Google Scholar] [CrossRef]
- Škvareninová, J.; Tuhárska, M.; Škvarenina, J.; Babálová, D.; Slobodníková, L.; Slobodník, B.; Středová, H.; Minďaš, J. Effects of light pollution on tree phenology in the urban environment. Morav. Geogr. Rep. 2017, 25, 282–290. [Google Scholar] [CrossRef]
- Bennie, J.; Davies, T.W.; Cruse, D.; Bell, F.; Gaston, K.J. Artificial light at night alters grassland vegetation species composition and phenology. J. Appl. Ecol. 2018, 55, 442–450. [Google Scholar] [CrossRef]
- Schroer, S.; Häffner, E.; Hölker, F. Impact of artificial illumination on the development of a leaf mining moth in urban trees. Int. J. Sustain. Lighting 2019, 21, 1–10. [Google Scholar] [CrossRef]
- McMunn, M.S.; Yang, L.H.; Ansalmo, A.; Bucknam, K.; Claret, M.; Clay, C.; Cox, K.; Dungey, D.R.; Jones, A.; Kim, A.Y.; et al. Artificial Light Increases Local Predator Abundance, Predation Rates, and Herbivory. Environ. Entomol. 2019, 48, 1331–1339. [Google Scholar] [CrossRef]
- Massetti, L. Assessing the impact of street lighting on Platanus × acerifolia phenology. Urban For. Urban Green. 2018, 34, 71–77. [Google Scholar] [CrossRef]
- Pu, G.Z.; Zeng, D.J.; Mo, L.; He, W.; Zhou, L.W.; Huang, K.C.; Liao, J.X.; Qiu, S.; Chai, S.F. Does artificial light at night change the impact of silver nanoparticles on microbial decomposers and leaf litter decomposition in streams? Environ. Sci. Nano 2019, 6, 1728–1739. [Google Scholar] [CrossRef]
- Heiling, A.M. Why do nocturnal orb-web spiders (Araneidae) search for light? Behav. Ecol. Sociobiol. 1999, 46, 43–49. [Google Scholar] [CrossRef]
- Graham, M.R.; Pinto, M.B.; Cushing, P.E. A test of the light attraction hypothesis in camel spiders of the Mojave Desert (Arachnida: Solifugae). J. Arachnol. 2019, 47, 293–296. [Google Scholar] [CrossRef]
- Goretti, E.; Coletti, A.; Di Veroli, A.; Di Giulio, A.M.; Gaino, E. Artificial light device for attracting pestiferous chironomids (Diptera): A case study at Lake Trasimeno (Central Italy). Ital. J. Zool. 2011, 78, 336–342. [Google Scholar] [CrossRef]
- Van Langevelde, F.; Braamburg-Annegarn, M.; Huigens, M.E.; Groendijk, R.; Poitevin, O.; van Deijk, J.R.; Ellis, W.N.; van Grunsven, R.H.A.; de Vos, R.; Vos, R.A.; et al. Declines in moth populations stress the need for conserving dark nights. Glob. Chang. Biol. 2018, 24, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Degen, T.; Mitesser, O.; Perkin, E.K.; Weiß, N.-S.; Oehlert, M.; Mattig, E.; Hölker, F. Street lighting: Sex-independent impacts on moth movement. J. Anim. Ecol. 2016, 85, 1352–1360. [Google Scholar] [CrossRef]
- Van Langevelde, F.; Ettema, J.A.; Donners, M.; Wallisdevries, M.; Groenendijk, D. Effect of spectral composition of artificial light on the attraction of moths. Biol. Conserv. 2011, 144, 2274–2281. [Google Scholar] [CrossRef]
- Van Grunsven, R.H.; van Deijk, J.R.; Donners, M.; Berendse, F.; Visser, M.E.; Veenendaal, E.; Spoelstra, K. Experimental Light at Night Has a Negative Long-Term Impact on Macro-Moth Populations. Curr. Biol. 2020, 30, R694–R695. [Google Scholar] [CrossRef]
- Meyer, L.A.; Sullivan, S.M.P. Bright lights, big city: Influences of ecological light pollution on reciprocal stream–riparian invertebrate fluxes. Ecol. Appl. 2013, 23, 1322–1330. [Google Scholar] [CrossRef]
- Sullivan, S.M.P.; Hossler, K.; Meyer, L.A. Artificial lighting at night alters aquatic-riparian invertebrate food webs. Ecol. Appl. 2019, 29, e01821. [Google Scholar] [CrossRef]
- Nankoo, S.; Raymond, S.; Galvez-Cloutier, R. The impact of the Jacques Cartier bridge illumination on the food chain: From insects to predators. Community Ecol. 2019, 20, 172–180. [Google Scholar] [CrossRef]
- Eisenbeis, G. Artificial night lighting and insects: Attraction of insects to streetlamps in a rural setting in Germany. Ecol. Conseq. Artif. Night Lighting 2006, 2, 191–198. [Google Scholar]
- Van Grunsven, R.H.A.; Becker, J.; Peter, S.; Heller, S.; Holker, F. Long-Term Comparison of Attraction of Flying Insects to Streetlights after the Transition from Traditional Light Sources to Light-Emitting Diodes in Urban and Peri-Urban Settings. Sustainability 2019, 11, 6198. [Google Scholar] [CrossRef]
- Longcore, T.; Aldern Hannah, L.; Eggers John, F.; Flores, S.; Franco, L.; Hirshfield-Yamanishi, E.; Petrinec Laina, N.; Yan Wilson, A.; Barroso André, M. Tuning the white light spectrum of light emitting diode lamps to reduce attraction of nocturnal arthropods. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140125. [Google Scholar] [CrossRef]
- Pawson, S.; Bader, M. LED lighting increases the ecological impact of light pollution irrespective of color temperature. Ecol. Appl. 2014, 24, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Van Grunsven, R.; Donners, M.; Boekee, K.; Tichelaar, I.; van Geffen, K.G.; Groenendijk, D.; Berendse, F.; Veenendaal, E. Spectral composition of light sources and insect phototaxis, with an evaluation of existing spectral response models. J. Insect Conserv. 2014, 18, 225–231. [Google Scholar] [CrossRef]
- Wakefield, A.; Broyles, M.; Stone, E.; Harris, S.; Jones, G. Quantifying the attractiveness of broad-spectrum street lights to aerial nocturnal insects. J. Appl. Ecol. 2017, 55, 714–722. [Google Scholar] [CrossRef]
- Robertson, B.A.; Horvath, G. Color polarization vision mediates the strength of an evolutionary trap. Evol. Appl. 2019, 12, 175–186. [Google Scholar] [CrossRef]
- Szaz, D.; Horvath, G.; Barta, A.; Robertson, B.A.; Farkas, A.; Egri, A.; Tarjanyi, N.; Racz, G.; Kriska, G. Lamp-Lit Bridges as Dual Light-Traps for the Night-Swarming Mayfly, Ephoron virgo: Interaction of Polarized and Unpolarized Light Pollution. PLoS ONE 2015, 10, e0121194. [Google Scholar] [CrossRef] [PubMed]
- Van Geffen, K.G.; van Eck, E.; de Boer, R.A.; van Grunsven, R.H.A.; Salis, L.; Berendse, F.; Veenendaal, E.M. Artificial light at night inhibits mating in a Geometrid moth. Insect Conserv. Divers. 2015, 8, 282–287. [Google Scholar] [CrossRef]
- Altermatt, F.; Ebert, D. Reduced flight-to-light behaviour of moth populations exposed to long-term urban light pollution. Biol. Lett. 2016, 12, 20160111. [Google Scholar] [CrossRef]
- Farnworth, B.; Innes, J.; Kelly, C.; Littler, R.; Waas, J.R. Photons and foraging: Artificial light at night generates avoidance behaviour in male, but not female, New Zealand weta. Environ. Pollut. 2018, 236, 82–90. [Google Scholar] [CrossRef]
- Czaczkes, T.J.; Bastidas-Urrutia, A.M.; Ghislandi, P.; Tuni, C. Reduced light avoidance in spiders from populations in light-polluted urban environments. Sci. Nat. 2018, 105, 11–12. [Google Scholar] [CrossRef]
- Sanders, D.; Kehoe, R.; Cruse, D.; van Veen, F.J.F.; Gaston, K.J. Low Levels of Artificial Light at Night Strengthen Top-Down Control in Insect Food Web. Curr. Biol. 2018, 28, 2474–2478. [Google Scholar] [CrossRef]
- Willmott, N.J.; Henneken, J.; Elgar, M.A.; Jones, T.M. Guiding lights: Foraging responses of juvenile nocturnal orb-web spiders to the presence of artificial light at night. Ethology 2019, 125, 289–297. [Google Scholar] [CrossRef]
- Holzhauer, S.I.J.; Franke, S.; Kyba, C.C.M.; Manfrin, A.; Klenke, R.; Voigt, C.C.; Lewanzik, D.; Oehlert, M.; Monaghan, M.T.; Schneider, S.; et al. Out of the Dark: Establishing a Large-Scale Field Experiment to Assess the Effects of Artificial Light at Night on Species and Food Webs. Sustainability 2015, 7, 15593–15616. [Google Scholar] [CrossRef]
- Haddock, J.K.; Threlfall, C.G.; Law, B.; Hochuli, D.F. Responses of insectivorous bats and nocturnal insects to local changes in street light technology. Austral Ecol. 2019, 44, 1052–1064. [Google Scholar] [CrossRef]
- Cochard, P.; Galstian, T.; Cloutier, C. Light Environments Differently Affect Parasitoid Wasps and their Hosts’ Locomotor Activity. J. Insect Behav. 2017, 30, 595–611. [Google Scholar] [CrossRef]
- Manfrin, A.; Lehmann, D.; van Grunsven, R.H.A.; Larsen, S.; Syvaranta, J.; Wharton, G.; Voigt, C.C.; Monaghan, M.T.; Holker, F. Dietary changes in predators and scavengers in a nocturnally illuminated riparian ecosystem. Oikos 2018, 127, 960–969. [Google Scholar] [CrossRef]
- Firebaugh, A.; Haynes, K.J. Light pollution may create demographic traps for nocturnal insects. Basic Appl. Ecol. 2019, 34, 118–125. [Google Scholar] [CrossRef]
- Botha, L.M.; Jones, T.M.; Hopkins, G.R. Effects of lifetime exposure to artificial light at night on cricket (Teleogryllus commodus) courtship and mating behaviour. Anim. Behav. 2017, 129, 181–188. [Google Scholar] [CrossRef]
- Thompson, E.K.; Cullinan, N.L.; Jones, T.M.; Hopkins, G.R. Effects of artificial light at night and male calling on movement patterns and mate location in field crickets. Anim. Behav. 2019, 158, 183–191. [Google Scholar] [CrossRef]
- McLay, L.K.; Green, M.P.; Jones, T.M. Chronic exposure to dim artificial light at night decreases fecundity and adult survival in Drosophila melanogaster. J. Insect Physiol. 2017, 100, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Willmott, N.J.; Henneken, J.; Selleck, C.J.; Jones, T.M. Artificial light at night alters life history in a nocturnal orb-web spider. PeerJ 2018, 6, e5599. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; Jo, Y.C.; Huang, Z.J.; Song, H.S.; Ryu, K.H.; Huang, Q.Y.; Lei, C.L. Influence of green light illumination at night on biological characteristics of the oriental armyworm, Mythimna separata (Lepidoptera: Noctuidae). Bull. Entomol. Res. 2020, 110, 136–143. [Google Scholar] [CrossRef]
- Davies, T.W.; Bennie, J.; Cruse, D.; Blumgart, D.; Inger, R.; Gaston, K.J. Multiple night-time light-emitting diode lighting strategies impact grassland invertebrate assemblages. Glob. Chang. Biol. 2017, 23, 2641–2648. [Google Scholar] [CrossRef] [PubMed]
- Perkin, E.K.; Holker, F.; Tockner, K. The effects of artificial lighting on adult aquatic and terrestrial insects. Freshw. Biol. 2014, 59, 368–377. [Google Scholar] [CrossRef]
- Maryam, S.; Fadzly, N.; Zuharah, W.F. The effects of light and height of building in attracting Paederus fuscipes Curtis to disperse towards human residential areas. Trop. Life Sci. Res. 2016, 27, 95. [Google Scholar] [CrossRef]
- Fadzly, N.; Burns, K.C. What weta want: Colour preferences of a frugivorous insect. Arthropod-Plant Interact. 2010, 4, 267–276. [Google Scholar] [CrossRef]
- Allema, A.; Rossing, W.; Van der Werf, W.; Heusinkveld, B.; Bukovinszky, T.; Steingröver, E.; Van Lenteren, J. Effect of light quality on movement of Pterostichus melanarius (Coleoptera: Carabidae). J. Appl. Entomol. 2012, 136, 793–800. [Google Scholar] [CrossRef]
- Egri, Á.; Száz, D.; Farkas, A.; Pereszlényi, Á.; Horváth, G.; Kriska, G. Method to improve the survival of night-swarming mayflies near bridges in areas of distracting light pollution. R. Soc. Open Sci. 2017, 4, 171166. [Google Scholar] [CrossRef] [PubMed]
- Eccard, J.A.; Scheffler, I.; Franke, S.; Hoffmann, J. Off-grid: Solar powered LED illumination impacts epigeal arthropods. Insect Conserv. Divers. 2018, 11, 600–607. [Google Scholar] [CrossRef]
- Duarte, C.; Quintanilla-Ahumada, D.; Anguita, C.; Manriquez, P.H.; Widdicombe, S.; Pulgar, J.; Silva-Rodriguez, E.A.; Miranda, C.; Manriquez, K.; Quijon, P.A. Artificial light pollution at night (ALAN) disrupts the distribution and circadian rhythm of a sandy beach isopod. Environ. Pollut. 2019, 248, 565–573. [Google Scholar] [CrossRef]
- McConnell, A.; Routledge, R.; Connors, B. Effect of artificial light on marine invertebrate and fish abundance in an area of salmon farming. Mar. Ecol. Prog. Ser. 2010, 419, 147–156. [Google Scholar] [CrossRef]
- Lowe, R.H. The Influence of Light and Other Factors on the Seaward Migration of the Silver Eel (Anguilla anguilla L.). J. Anim. Ecol. 1952, 21, 275–309. [Google Scholar] [CrossRef]
- Cullen, P.; McCarthy, T. The effects of artificial light on the distribution of catches of silver eel, Anguilla anguilla (L.), across the Killaloe eel weir in the Lower River Shannon. Biol. Environ. Proc. R. Ir. Acad. 2000, 100B, 165–169. [Google Scholar]
- Elvidge, C.; Ford, M.; Pratt, T.; Smokorowski, K.E.; Sills, M.; Patrick, P.; Cooke, S. Behavioural guidance of yellow-stage American eel Anguillla rostrata with a light-emitting diode (LED) device. Endanger. Species 2018, 35, 159–168. [Google Scholar] [CrossRef]
- Schmidt, M.B.; Balk, H.; Gassner, H. Testing in situ avoidance reaction of vendace, Coregonus albula, in relation to continuous artificial light from stationary vertical split-beam echosounding. Fish. Manag. Ecol. 2009, 16, 376–385. [Google Scholar] [CrossRef]
- Mavraki, N.; Georgiadis, M.; Koutsikopoulos, C.; Tzanatos, E. Unravelling the nocturnal appearance of bogue Boops boops shoals in the anthropogenically modified shallow littoral. J. Fish Biol. 2016, 88, 2060–2066. [Google Scholar] [CrossRef] [PubMed]
- Riley, W.D.; Bendall, B.; Ives, M.J.; Edmonds, N.J.; Maxwell, D.L. Street lighting disrupts the diel migratory pattern of wild Atlantic salmon, Salmo salar L., smolts leaving their natal stream. Aquaculture 2012, 330, 74–81. [Google Scholar] [CrossRef]
- Riley, W.D.; Davison, P.I.; Maxwell, D.L.; Bendall, B. Street lighting delays and disrupts the dispersal of Atlantic salmon (Salmo salar) fry. Biol. Conserv. 2013, 158, 140–146. [Google Scholar] [CrossRef]
- Riley, W.D.; Davison, P.I.; Maxwell, D.L.; Newman, R.C.; Ives, M.J. A laboratory experiment to determine the dispersal response of Atlantic salmon (Salmo salar) fry to street light intensity. Freshw. Biol. 2015, 60, 1016–1028. [Google Scholar] [CrossRef]
- Bolton, D.; Mayer-Pinto, M.; Clark, G.F.; Dafforn, K.A.; Brassil, W.A.; Becker, A.; Johnston, E.L. Coastal urban lighting has ecological consequences for multiple trophic levels under the sea. Sci. Total Environ. 2017, 576, 1–9. [Google Scholar] [CrossRef]
- Tałanda, J.; Maszczyk, P.; Babkiewicz, E. The reaction distance of a planktivorous fish (Scardinius erythrophthalmus) and the evasiveness of its prey (Daphnia pulex × pulicaria) under different artificial light spectra. Limnology 2018, 19, 311–319. [Google Scholar] [CrossRef]
- Franke, S.; Brüning, A.; Hölker, F.; Kloas, W. Study of biological action of light on fish. J. Light Vis. Environ. 2013, 37, 194–204. [Google Scholar] [CrossRef]
- Brüning, A.; Holker, F.; Franke, S.; Preuer, T.; Kloas, W. Spotlight on fish: Light pollution affects circadian rhythms of European perch but does not cause stress. Sci. Total Environ. 2015, 511, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Brüning, A.; Hölker, F.; Franke, S.; Kleiner, W.; Kloas, W. Impact of different colours of artificial light at night on melatonin rhythm and gene expression of gonadotropins in European perch. Sci. Total Environ. 2016, 543, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Brüning, A.; Holker, F.; Franke, S.; Kleiner, W.; Kloas, W. Influence of light intensity and spectral composition of artificial light at night on melatonin rhythm and mRNA expression of gonadotropins in roach Rutilus rutilus. Fish Physiol. Biochem. 2018, 44, 1–12. [Google Scholar] [CrossRef]
- Takemura, A.; Ueda, S.; Hiyakawa, N.; Nikaido, Y. A direct influence of moonlight intensity on changes in melatonin production by cultured pineal glands of the golden rabbitfish, Siganus guttatus. J. Pineal Res. 2006, 40, 236–241. [Google Scholar] [CrossRef]
- Khan, Z.A.; Labala, R.K.; Yumnamcha, T.; Devi, S.D.; Mondal, G.; Devi, H.S.; Rajiv, C.; Bharali, R.; Chattoraj, A. Artificial Light at Night (ALAN), an alarm to ovarian physiology: A study of possible chronodisruption on zebrafish (Danio rerio). Sci. Total Environ. 2018, 628–629, 1407–1421. [Google Scholar] [CrossRef]
- Newman, R.C.; Ellis, T.; Davison, P.I.; Ives, M.J.; Thomas, R.J.; Griffiths, S.W.; Riley, W.D. Using novel methodologies to examine the impact of artificial light at night on the cortisol stress response in dispersing Atlantic salmon (Salmo salar L.) fry. Conserv. Physiol. 2015, 3, cov051. [Google Scholar] [CrossRef][Green Version]
- Szekeres, P.; Wilson, A.D.; Haak, C.R.; Danylchuk, A.J.; Brownscombe, J.W.; Elvidge, C.K.; Shultz, A.D.; Birnie-Gauvin, K.; Cooke, S.J. Does coastal light pollution alter the nocturnal behavior and blood physiology of juvenile bonefish (Albula vulpes)? Bull. Mar. Sci. 2017, 93, 491–505. [Google Scholar] [CrossRef]
- O’Connor, J.J.; Fobert, E.K.; Besson, M.; Jacob, H.; Lecchini, D. Live fast, die young: Behavioural and physiological impacts of light pollution on a marine fish during larval recruitment. Mar. Pollut. Bull. 2019, 146, 908–914. [Google Scholar] [CrossRef]
- Wang, H.; Shi, W.J.; Wang, L.; Zhu, C.K.; Pan, Z.J.; Chang, G.L.; Wu, N.; Ding, H.Y. Can larval growth be manipulated by artificial light regimes in Nile tilapia (Oreochromis niloticus)? Aquaculture 2019, 506, 161–167. [Google Scholar]
- Hansen, T.J.; Fjelldal, P.G.; Folkedal, O.; Vågseth, T.; Oppedal, F. Effects of light source and intensity on sexual maturation, growth and swimming behaviour of Atlantic salmon in sea cages. Aquac. Environ. Interact. 2017, 9, 193–204. [Google Scholar] [CrossRef]
- Barki, A.; Zion, B.; Shapira, L.; Karplus, I. The effects of illumination and daily number of collections on fry yields in guppy breeding tanks. Aquac. Eng. 2013, 57, 108–113. [Google Scholar] [CrossRef]
- Migaud, H.; Cowan, M.; Taylor, J.; Ferguson, H.W. The effect of spectral composition and light intensity on melatonin, stress and retinal damage in post-smolt Atlantic salmon, Salmo salar. Aquaculture 2007, 270, 390–404. [Google Scholar] [CrossRef]
- Porter, M.J.R.; Woolcott, H.M.; Pankhurst, N.W. The use of additional lighting and artificial photoperiods to recondition early maturing Atlantic salmon (Salmo salar) in Tasmania. Fish Physiol. Biochem. 2003, 28, 391–393. [Google Scholar] [CrossRef]
- Brüning, A.; Kloas, W.; Preuer, T.; Holker, F. Influence of artificially induced light pollution on the hormone system of two common fish species, perch and roach, in a rural habitat. Conserv. Physiol. 2018, 6, coy016. [Google Scholar] [CrossRef]
- Pulgar, J.; Zeballos, D.; Vargas, J.; Aldana, M.; Manriquez, P.H.; Manriquez, K.; Quijón, P.A.; Widdicombe, S.; Anguita, C.; Quintanilla, D.; et al. Endogenous cycles, activity patterns and energy expenditure of an intertidal fish is modified by artificial light pollution at night (ALAN). Environ. Pollut. 2019, 244, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Fobert, E.K.; Silva, K.B.D.; Swearer, S.E. Artificial light at night causes reproductive failure in clownfish. Biol. Lett. 2019, 15, 20190272. [Google Scholar] [CrossRef]
- Feuka, A. Effects of Light Pollution on Habitat Selection in Post-Metamorphic Wood Frogs and Unisexual Blue-Spotted Salamanders (Ambystoma laterale × jeffersonianum). Herpetol. Conserv. Biol. 2016, 12, 470–476. [Google Scholar]
- Bliss-Ketchum, L.L.; de Rivera, C.E.; Turner, B.C.; Weisbaum, D.M. The effect of artificial light on wildlife use of a passage structure. Biol. Conserv. 2016, 199, 25–28. [Google Scholar] [CrossRef]
- Coelho, I.P.; Teixeira, F.Z.; Colombo, P.; Coelho, A.V.P.; Kindel, A. Anuran road-kills neighboring a pen-urban reserve in the Atlantic Forest, Brazil. J. Environ. Manag. 2012, 112, 17–26. [Google Scholar] [CrossRef]
- Baker, B.J.; Richardson, J. The effect of artificial light on male breeding-season behaviour in green frogs, Rana clamitans melanota. Can. J. Zool. 2006, 84, 1528–1532. [Google Scholar] [CrossRef]
- Dias, K.S.; Dosso, E.S.; Hall, A.S.; Schuch, A.P.; Tozetti, A.M. Ecological light pollution affects anuran calling season, daily calling period, and sensitivity to light in natural Brazilian wetlands. Sci. Nat. 2019, 106, 46. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, B.W. Effects of enhanced lighting on the behaviour of nocturnal frogs. Anim. Behav. 1993, 45, 893–899. [Google Scholar] [CrossRef]
- Buchanan, B.W. Low-Illumination Prey Detection by Squirrel Treefrogs. J. Herpetol. 1998, 32, 270–274. [Google Scholar] [CrossRef]
- Touzot, M.; Teulier, L.; Lengagne, T.; Secondi, J.; Théry, M.; Libourel, P.-A.; Guillard, L.; Mondy, N. Artificial light at night disturbs the activity and energy allocation of the common toad during the breeding period. Conserv. Physiol. 2019, 7, coz002. [Google Scholar] [CrossRef]
- Touzot, M.; Lengagne, T.; Secondi, J.; Desouhant, E.; Thery, M.; Dumet, A.; Duchamp, C.; Mondy, N. Artificial light at night alters the sexual behaviour and fertilisation success of the common toad. Environ. Pollut. 2020, 259, 113883. [Google Scholar] [CrossRef]
- Dananay, K.L.; Benard, M.F. Artificial light at night decreases metamorphic duration and juvenile growth in a widespread amphibian. Proc. R. Soc. B Biol. Sci. 2018, 285, 20180367. [Google Scholar] [CrossRef]
- Gastón, M.S.; Pereyra, L.C.; Vaira, M. Artificial light at night and captivity induces differential effects on leukocyte profile, body condition, and erythrocyte size of a diurnal toad. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2019, 331, 93–102. [Google Scholar] [CrossRef]
- Underhill, V.A.; Hobel, G. Mate choice behavior of female Eastern Gray Treefrogs (Hyla versicolor) is robust to anthropogenic light pollution. Ethology 2018, 124, 537–548. [Google Scholar] [CrossRef]
- Koen, E.L.; Minnaar, C.; Roever, C.L.; Boyles, J.G. Emerging threat of the 21st century lightscape to global biodiversity. Glob. Chang. Biol. 2018, 24, 2315–2324. [Google Scholar] [CrossRef]
- Borchard, P.; Eldridge, D.J. Does artificial light influence the activity of vertebrates beneath rural buildings? Aust. J. Zool. 2013, 61, 424–429. [Google Scholar] [CrossRef]
- Martín, B.; Pérez, H.; Ferrer, M. Effects of natural and artificial light on the nocturnal behaviour of the wall gecko. Anim. Biodivers. Conserv. 2018, 41, 209–215. [Google Scholar] [CrossRef]
- Kamrowski, R.L.; Limpus, C.; Moloney, J.; Hamann, M. Coastal light pollution and marine turtles: Assessing the magnitude of the problem. Endanger. Species Res. 2012, 19, 85–98. [Google Scholar] [CrossRef]
- Wilson, P.; Thums, M.; Pattiaratchi, C.; Meekan, M.; Pendoley, K.; Fisher, R.; Whiting, S. Artificial light disrupts the nearshore dispersal of neonate flatback turtles Natator depressus. Mar. Ecol. Prog. Ser. 2018, 600, 179–192. [Google Scholar] [CrossRef]
- Lorne, J.K.; Salmon, M. Effects of exposure to artificial lighting on orientation of hatchling sea turtles on the beach and in the ocean. Endanger. Species Res. 2007, 3, 23–30. [Google Scholar] [CrossRef]
- Thums, M.; Whiting, S.D.; Reisser, J.; Pendoley, K.L.; Pattiaratchi, C.B.; Proietti, M.; Hetzel, Y.; Fisher, R.; Meekan, M.G. Artificial light on water attracts turtle hatchlings during their near shore transit. R. Soc. Open Sci. 2016, 3, 12. [Google Scholar] [CrossRef]
- Davies, T.W.; Bennie, J.; Inger, R.; De Ibarra, N.H.; Gaston, K.J. Artificial light pollution: Are shifting spectral signatures changing the balance of species interactions? Glob. Chang. Biol. 2013, 19, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Holveck, M.J.; Gregoire, A.; Doutrelant, C.; Lambrechts, M.M. Nest height is affected by lamppost lighting proximity in addition to nestbox size in urban great tits. J. Avian Biol. 2019, 50. [Google Scholar] [CrossRef]
- Sprau, P.; Mouchet, A.; Dingemanse, N.J. Multidimensional environmental predictors of variation in avian forest and city life histories. Behav. Ecol. 2017, 28, 59–68. [Google Scholar] [CrossRef]
- De Jong, M.; Ouyang, J.Q.; da Silva, A.; van Grunsven, R.H.A.; Kempenaers, B.; Visser, M.E.; Spoelstra, K. Effects of nocturnal illumination on life-history decisions and fitness in two wild songbird species. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140128. [Google Scholar] [CrossRef]
- Gryz, J.; Krauze-Gryz, D. Influence of habitat urbanisation on time of breeding and productivity of tawny owl (Strix aluco). Pol. J. Ecol. 2018, 66, 153–161. [Google Scholar] [CrossRef]
- Dominoni, D.M.; Partecke, J. Does light pollution alter daylength? A test using light loggers on free-ranging European blackbirds (Turdus merula). Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140118. [Google Scholar] [CrossRef] [PubMed]
- Partecke, J.; Van’t Hof, T.J.; Gwinner, E. Underlying physiological control of reproduction in urban and forest-dwelling European blackbirds Turdus merula. J. Avian Biol. 2005, 36, 295–305. [Google Scholar] [CrossRef]
- Dominoni, D.M.; de Jong, M.; Bellingham, M.; O’Shaughnessy, P.; van Oers, K.; Robinson, J.; Smith, B.; Visser, M.E.; Helm, B. Dose-response effects of light at night on the reproductive physiology of great tits (Parus major): Integrating morphological analyses with candidate gene expression. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2018, 329, 473–487. [Google Scholar] [CrossRef]
- De Jong, M.; Lamers, K.P.; Eugster, M.; Ouyang, J.Q.; Da Silva, A.; Mateman, A.C.; van Grunsven, R.H.A.; Visser, M.E.; Spoelstra, K. Effects of experimental light at night on extra-pair paternity in a songbird. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2018, 329, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Schoech, S.J.; Bowman, R.; Hahn, T.P.; Goymann, W.; Schwabl, I.; Bridge, E.S. The effects of low levels of light at night upon the endocrine physiology of western scrub-jays (Aphelocoma californica). J. Exp. Zool. Part A Ecol. Genet. Physiol. 2013, 319, 527–538. [Google Scholar] [CrossRef]
- De Jong, M.; Jeninga, L.; Ouyang, J.Q.; van Oers, K.; Spoelstra, K.; Visser, M.E. Dose-dependent responses of avian daily rhythms to artificial light at night. Physiol. Behav. 2016, 155, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Moaraf, S.; Vistoropsky, Y.; Pozner, T.; Heiblum, R.; Okuliarova, M.; Zeman, M.; Barnea, A. Artificial light at night affects brain plasticity and melatonin in birds. Neurosci. Lett. 2020, 716, 134639. [Google Scholar] [CrossRef]
- Jiang, J.X.; He, Y.; Kou, H.H.; Ju, Z.Q.; Gao, X.B.; Zhao, H.F. The effects of artificial light at night on Eurasian tree sparrow (Passer montanus): Behavioral rhythm disruption, melatonin suppression and intestinal microbiota alterations. Ecol. Indic. 2020, 108, 105702. [Google Scholar] [CrossRef]
- McLaren, J.D.; Buler, J.J.; Schreckengost, T.; Smolinsky, J.A.; Boone, M.; van Loon, E.E.; Dawson, D.K.; Walters, E.L. Artificial light at night confounds broad-scale habitat use by migrating birds. Ecol. Lett. 2018, 21, 356–364. [Google Scholar] [CrossRef]
- Van Doren, B.M.; Horton, K.G.; Dokter, A.M.; Klinck, H.; Elbin, S.B.; Farnsworth, A. High-intensity urban light installation dramatically alters nocturnal bird migration. Proc. Natl. Acad. Sci. USA 2017, 114, 11175–11180. [Google Scholar] [CrossRef]
- Poot, H.; Ens, B.J.; de Vries, H.; Donners, M.A.H.; Wernand, M.R.; Marquenie, J.M. Green Light for Nocturnally Migrating Birds. Ecol. Soc. 2008, 13, 47. [Google Scholar] [CrossRef]
- Horton, K.G.; Nilsson, C.; Van Doren, B.M.; La Sorte, F.A.; Dokter, A.M.; Farnsworth, A. Bright lights in the big cities: Migratory birds’ exposure to artificial light. Front. Ecol. Environ. 2019, 17, 209–214. [Google Scholar] [CrossRef]
- Rodriguez, A.; Dann, P.; Chiaradia, A. Reducing light-induced mortality of seabirds: High pressure sodium lights decrease the fatal attraction of shearwaters. J. Nat. Conserv. 2017, 39, 68–72. [Google Scholar] [CrossRef]
- Winger, B.M.; Weeks, B.C.; Farnsworth, A.; Jones, A.W.; Hennen, M.; Willard, D.E. Nocturnal flight-calling behaviour predicts vulnerability to artificial light in migratory birds. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190364. [Google Scholar] [CrossRef]
- Jones, J.; Francis, C.M. The effects of light characteristics on avian mortality at lighthouses. J. Avian Biol. 2003, 34, 328–333. [Google Scholar] [CrossRef]
- Miles, W.; Money, S.; Luxmoore, R.; Furness, R.W. Effects of artificial lights and moonlight on petrels at St Kilda. Bird Study 2010, 57, 244–251. [Google Scholar] [CrossRef]
- Rodriguez, A.; Burgan, G.; Dann, P.; Jessop, R.; Negro, J.J.; Chiaradia, A. Fatal Attraction of Short-Tailed Shearwaters to Artificial Lights. PLoS ONE 2014, 9, e110114. [Google Scholar]
- Cabrera-Cruz, S.A.; Smolinsky, J.A.; Buler, J.J. Light pollution is greatest within migration passage areas for nocturnally-migrating birds around the world. Sci. Rep. 2018, 8, 3261. [Google Scholar] [CrossRef]
- Cabrera-Cruz, S.A.; Smolinsky, J.A.; McCarthy, K.P.; Buler, J.J. Urban areas affect flight altitudes of nocturnally migrating birds. J. Anim. Ecol. 2019, 88, 1873–1887. [Google Scholar] [CrossRef] [PubMed]
- Deppe, L.; Rowley, O.; Rowe, L.K.; Shi, N.; McArthur, N.; Gooday, O.; Goldstien, S.J. Investigation of fallout events in Hutton’s shearwaters (Puffinus huttoni) associated with artificial lighting. Notornis 2017, 64, 181–191. [Google Scholar]
- Troy, J.R.; Holmes, N.D.; Veech, J.A.; Green, M.C. Using observed seabird fallout records to infer patterns of attraction to artificial light. Endanger. Species Res. 2013, 22, 225–234. [Google Scholar] [CrossRef]
- La Sorte, F.A.; Fink, D.; Buler, J.J.; Farnsworth, A.; Cabrera-Cruz, S.A. Seasonal associations with urban light pollution for nocturnally migrating bird populations. Glob. Chang. Biol. 2017, 23, 4609–4619. [Google Scholar] [CrossRef] [PubMed]
- Spoelstra, K.; van Grunsven, R.H.A.; Donners, M.; Gienapp, P.; Huigens, M.E.; Slaterus, R.; Berendse, F.; Visser, M.E.; Veenendaal, E. Experimental illumination of natural habitat-an experimental set-up to assess the direct and indirect ecological consequences of artificial light of different spectral composition. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140129. [Google Scholar] [CrossRef] [PubMed]
- Raap, T.; Pinxten, R.; Eens, M. Light pollution disrupts sleep in free-living animals. Sci. Rep. 2015, 5, 13557. [Google Scholar] [CrossRef]
- Raap, T.; Pinxten, R.; Eens, M. Artificial light at night disrupts sleep in female great tits (Parus major) during the nestling period, and is followed by a sleep rebound. Environ. Pollut. 2016, 215, 125–134. [Google Scholar] [CrossRef]
- Ulgezen, Z.N.; Kapyla, T.; Meerlo, P.; Spoelstra, K.; Visser, M.E.; Dominoni, D.M. The preference and costs of sleeping under light at night in forest and urban great tits. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190872. [Google Scholar] [CrossRef] [PubMed]
- Welbers, A.; van Dis, N.E.; Kolvoort, A.M.; Ouyang, J.; Visser, M.E.; Spoelstra, K.; Dominoni, D.M. Artificial Light at Night Reduces Daily Energy Expenditure in Breeding Great Tits (Parus major). Front. Ecol. Evol. 2017, 5, 55. [Google Scholar] [CrossRef]
- De Jong, M.; Caro, S.P.; Gienapp, P.; Spoelstra, K.; Visser, M.E. Early Birds by Light at Night: Effects of Light Color and Intensity on Daily Activity Patterns in Blue Tits. J. Biol. Rhythm. 2017, 32, 323–333. [Google Scholar] [CrossRef]
- Byrkjedal, I.; Lislevand, T.; Vogler, S. Do passerine birds utilise artificial light to prolong their diurnal activity during winter at northern latitudes? Ornis Nor. 2012, 35, 37–42. [Google Scholar] [CrossRef]
- Dominoni, D.M.; Carmona-Wagner, E.O.; Hofmann, M.; Kranstauber, B.; Partecke, J. Individual-based measurements of light intensity provide new insights into the effects of artificial light at night on daily rhythms of urban-dwelling songbirds. J. Anim. Ecol. 2014, 83, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Buij, R.; Gschweng, M. Nocturnal hunting by Eleonora’s Falcons Falco eleonorae on their breeding and non-breeding grounds. Acta Ornithol. 2017, 52, 35–49. [Google Scholar] [CrossRef]
- Amichai, E.; Kronfeld-Schor, N. Artificial Light at Night Promotes Activity throughout the Night in Nesting Common Swifts (Apus apus). Sci. Rep. 2019, 9, 11052. [Google Scholar] [CrossRef]
- Spoelstra, K.; Verhagen, I.; Meijer, D.; Visser, M.E. Artificial light at night shifts daily activity patterns but not the internal clock in the great tit (Parus major). Proc. R. Soc. B Biol. Sci. 2018, 285, 20172751. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.; Valcu, M.; Kempenaers, B. Light pollution alters the phenology of dawn and dusk singing in common European songbirds. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140126. [Google Scholar] [CrossRef] [PubMed]
- Titulaer, M.; Spoelstra, K.; Lange, C.Y.; Visser, M.E. Activity patterns during food provisioning are affected by artificial light in free living great tits (Parus major). PLoS ONE 2012, 7, e37377. [Google Scholar] [CrossRef] [PubMed]
- Stracey, C.M.; Wynn, B.; Robinson, S.K. Light Pollution Allows the Northern Mockingbird (Mimus polyglottos) to Feed Nestlings after Dark. Wilson J. Ornithol. 2014, 126, 366–369. [Google Scholar] [CrossRef]
- Dwyer, R.G.; Bearhop, S.; Campbell, H.A.; Bryant, D.M. Shedding light on light: Benefits of anthropogenic illumination to a nocturnally foraging shorebird. J. Anim. Ecol. 2013, 82, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Foley, G.J.; Wszola, L.S. Observation of Common Nighthawks (Chordeiles minor) and Bats (Chiroptera) Feeding Concurrently. Northeast. Nat. 2017, 24. [Google Scholar] [CrossRef]
- Russ, A.; Ruger, A.; Klenke, R. Seize the night: European Blackbirds (Turdus merula) extend their foraging activity under artificial illumination. J. Ornithol. 2015, 156, 123–131. [Google Scholar] [CrossRef]
- Da Silva, A.; Valcu, M.; Kempenaers, B. Behavioural plasticity in the onset of dawn song under intermittent experimental night lighting. Anim. Behav. 2016, 117, 155–165. [Google Scholar] [CrossRef]
- Kempenaers, B.; Borgström, P.; Loës, P.; Schlicht, E.; Valcu, M. Artificial Night Lighting Affects Dawn Song, Extra-Pair Siring Success, and Lay Date in Songbirds. Curr. Biol. 2010, 20, 1735–1739. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.; Kempenaers, B. Singing from North to South: Latitudinal variation in timing of dawn singing under natural and artificial light conditions. J. Anim. Ecol. 2017, 86, 1286–1297. [Google Scholar] [CrossRef]
- Miller, M.W. Apparent effects of light pollution on singing behavior of American robins. Condor 2006, 108, 130–139. [Google Scholar] [CrossRef]
- Nordt, A.; Klenke, R. Sleepless in town–drivers of the temporal shift in dawn song in urban European blackbirds. PLoS ONE 2013, 8, e71476. [Google Scholar]
- Da Silva, A.; de Jong, M.; van Grunsven, R.H.A.; Visser, M.E.; Kempenaers, B.; Spoelstra, K. Experimental illumination of a forest: No effects of lights of different colours on the onset of the dawn chorus in songbirds. R. Soc. Open Sci. 2017, 4, 160638. [Google Scholar] [CrossRef] [PubMed]
- Dorado-Correa, A.M.; Rodriguez-Rocha, M.; Brumm, H. Anthropogenic noise, but not artificial light level predicts song behaviour in an equatorial bird. R. Soc. Open Sci. 2016, 3, 160231. [Google Scholar] [CrossRef]
- Stuart, C.J.; Grabarczyk, E.E.; Vonhof, M.J.; Gill, S.A. Social factors, not anthropogenic noise or artificial light, influence onset of dawn singing in a common songbird. Auk 2019, 136, ukz045. [Google Scholar] [CrossRef]
- Bohm, F.; Bruckner, J.; Eichhorn, D.; Geiger, R.; Johl, B.; Kahl, S.; Kleudgen, I.; Kohler, K.; Kreifelts, V.; Metschke, K.; et al. Cloud cover but not artificial light pollution affects the morning activity of Wood Pigeons. Ornis Fenn. 2016, 93, 246–252. [Google Scholar]
- Raap, T.; Sun, J.C.; Pinxten, R.; Eens, M. Disruptive effects of light pollution on sleep in free-living birds: Season and/or light intensity-dependent? Behav. Process. 2017, 144, 13–19. [Google Scholar] [CrossRef]
- Raap, T.; Pinxten, R.; Eens, M. Artificial light at night causes an unexpected increase in oxalate in developing male songbirds. Conserv. Physiol. 2018, 6, coy005. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Peng, X.T.; Ren, Z.F.; Liu, M.; Dang, R.; Chen, Y.Q.; Liu, F.B. The effect of artificial light with different SPDs and intensities on the sleep onset of silvereyes. Biol. Rhythm Res. 2019, 50, 787–804. [Google Scholar] [CrossRef]
- Caorsi, V.; Sprau, P.; Zollinger, S.A.; Brumm, H. Nocturnal resting behaviour in urban great tits and its relation to anthropogenic disturbance and microclimate. Behav. Ecol. Sociobiol. 2019, 73, 19. [Google Scholar] [CrossRef]
- Sun, J.C.; Raap, T.; Pinxten, R.; Eens, M. Artificial light at night affects sleep behaviour differently in two closely related songbird species. Environ. Pollut. 2017, 231, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.Q.; de Jong, M.; van Grunsven, R.H.; Matson, K.D.; Haussmann, M.F.; Meerlo, P.; Visser, M.E.; Spoelstra, K. Restless roosts: Light pollution affects behavior, sleep, and physiology in a free-living songbird. Glob. Chang. Biol. 2017, 23, 4987–4994. [Google Scholar] [CrossRef]
- Raap, T.; Pinxten, R.; Eens, M. Cavities shield birds from effects of artificial light at night on sleep. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2018, 329, 449–456. [Google Scholar] [CrossRef]
- Russ, A.; Lucenicova, T.; Klenke, R. Altered breeding biology of the European blackbird under artificial light at night. J. Avian Biol. 2017, 48, 1114–1125. [Google Scholar] [CrossRef]
- Ciach, M.; Frohlich, A. Habitat type, food resources, noise and light pollution explain the species composition, abundance and stability of a winter bird assemblage in an urban environment. Urban Ecosyst. 2017, 20, 547–559. [Google Scholar] [CrossRef]
- Dominoni, D.; Smit, J.A.H.; Visser, M.E.; Halfwerk, W. Multisensory pollution: Artificial light at night and anthropogenic noise have interactive effects on activity patterns of great tits (Parus major). Environ. Pollut. 2020, 256, 113314. [Google Scholar] [CrossRef]
- Lebbin, D.J.; Harvey, M.G.; Lenz, T.C.; Andersen, M.J.; Ellis, J.M. Nocturnal migrants foraging at night by artificial light. Wilson J. Ornithol. 2007, 119, 506–508. [Google Scholar] [CrossRef]
- Russ, A.; Reitemeier, S.; Weissmann, A.; Gottschalk, J.; Einspanier, A.; Klenke, R. Seasonal and urban effects on the endocrinology of a wild passerine. Ecol. Evol. 2015, 5, 5698–5710. [Google Scholar] [CrossRef]
- Raap, T.; Casasole, G.; Costantini, D.; AbdElgawad, H.; Asard, H.; Pinxten, R.; Eens, M. Artificial light at night affects body mass but not oxidative status in free-living nestling songbirds: An experimental study. Sci. Rep. 2016, 6, 35626. [Google Scholar] [CrossRef] [PubMed]
- Dominoni, D.M.; Jensen, J.K.; de Jong, M.; Visser, M.E.; Spoelstra, K. Artificial light at night, in interaction with spring temperature, modulates timing of reproduction in a passerine bird. Ecol. Appl. 2019, 30, 02062. [Google Scholar] [CrossRef] [PubMed]
- Grunst, M.L.; Raap, T.; Grunst, A.S.; Pinxten, R.; Parenteau, C.; Angelier, F.; Eens, M. Early-life exposure to artificial light at night elevates physiological stress in free-living songbirds☆. Environ. Pollut. 2019, 259, 113895. [Google Scholar] [CrossRef]
- Alaasam, V.J.; Duncan, R.; Casagrande, S.; Davies, S.; Sidher, A.; Seymoure, B.; Shen, Y.T.; Zhang, Y.; Ouyang, J.Q. Light at night disrupts nocturnal rest and elevates glucocorticoids at cool color temperatures. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2018, 329, 465–472. [Google Scholar] [CrossRef]
- Zhang, S.P.; Chen, X.Y.; Zhang, J.R.; Li, H.C. Differences in the reproductive hormone rhythm of tree sparrows (Passer montanus) from urban and rural sites in Beijing: The effect of anthropogenic light sources. Gen. Comp. Endocrinol. 2014, 206, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Malek, I.; Haim, A. Bright artificial light at night is associated with increased body mass, poor reproductive success and compromised disease tolerance in Australian budgerigars (Melopsittacus undulatus). Integr. Zool. 2019, 14, 589–603. [Google Scholar] [CrossRef]
- Raap, T.; Thys, B.; Grunst, A.S.; Grunst, M.L.; Pinxten, R.; Eens, M. Personality and artificial light at night in a semi-urban songbird population: No evidence for personality-dependent sampling bias, avoidance or disruptive effects on sleep behaviour. Environ. Pollut. 2018, 243, 1317–1324. [Google Scholar] [CrossRef]
- Clewley, G.D.; Plummer, K.E.; Robinson, R.A.; Simm, C.H.; Toms, M.P. The effect of artificial lighting on the arrival time of birds using garden feeding stations in winter: A missed opportunity? Urban Ecosyst. 2016, 19, 535–546. [Google Scholar] [CrossRef]
- Cianchetti-Benedetti, M.; Becciu, P.; Massa, B.; Dell’Omo, G. Conflicts between touristic recreational activities and breeding shearwaters: Short-term effect of artificial light and sound on chick weight. Eur. J. Wildl. Res. 2018, 64, 19. [Google Scholar] [CrossRef]
- Malek, I.; Haim, A.; Izhaki, I. Melatonin mends adverse temporal effects of bright light at night partially independent of its effect on stress responses in captive birds. Chronobiol. Int. 2020, 37, 189–208. [Google Scholar] [CrossRef]
- Zhang, X.J.; Yang, W.Y.; Liang, W.; Wang, Y.; Zhang, S.P. Intensity dependent disruptive effects of light at night on activation of the HPG axis of tree sparrows (Passer montanus). Environ. Pollut. 2019, 249, 904–909. [Google Scholar] [CrossRef]
- Saini, C.; Hutton, P.; Gao, S.S.; Simpson, R.K.; Giraudeau, M.; Sepp, T.; Webb, E.; McGraw, K.J. Exposure to artificial light at night increases innate immune activity during development in a precocial bird. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 233, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Dimovski, A.M.; Robert, K.A. Artificial light pollution: Shifting spectral wavelengths to mitigate physiological and health consequences in a nocturnal marsupial mammal. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2018, 329, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.P.; Bennie, J.; Duran, A.P.; Gaston, K.J. Mammalian ranges are experiencing erosion of natural darkness. Sci. Rep. 2015, 5, 12042. [Google Scholar] [CrossRef] [PubMed]
- Voigt, C.C.; Roeleke, M.; Marggraf, L.; Pētersons, G.; Voigt-Heucke, S.L. Migratory bats respond to artificial green light with positive phototaxis. PLoS ONE 2017, 12, e0177748. [Google Scholar]
- Mathews, F.; Roche, N.; Aughney, T.; Jones, N.; Day, J.; Baker, J.; Langton, S. Barriers and benefits: Implications of artificial night-lighting for the distribution of common bats in Britain and Ireland. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 30140124. [Google Scholar] [CrossRef] [PubMed]
- Voigt, C.C.; Scholl, J.M.; Bauer, J.; Teige, T.; Yovel, Y.; Kramer-Schadt, S.; Gras, P. Movement responses of common noctule bats to the illuminated urban landscape. Landsc. Ecol. 2020, 35, 189–201. [Google Scholar] [CrossRef]
- Stone, E.L.; Jones, G.; Harris, S. Conserving energy at a cost to biodiversity? Impacts of LED lighting on bats. Glob. Chang. Biol. 2012, 18, 2458–2465. [Google Scholar] [CrossRef]
- Lewanzik, D.; Voigt, C.C. Artificial light puts ecosystem services of frugivorous bats at risk. J. Appl. Ecol. 2014, 51, 388–394. [Google Scholar] [CrossRef]
- Kuijper, D.P.; Schut, J.; van Dullemen, D.; Toorman, H.; Goossens, N.; Ouwehand, J.; Limpens, H. Experimental evidence of light disturbance along the commuting routes of pond bats (Myotis dasycneme). Lutra 2008, 51, 37. [Google Scholar]
- Zeale, M.R.K.; Stone, E.L.; Zeale, E.; Browne, W.J.; Harris, S.; Jones, G. Experimentally manipulating light spectra reveals the importance of dark corridors for commuting bats. Glob. Chang. Biol. 2018, 24, 5909–5918. [Google Scholar] [CrossRef]
- Polak, T.; Korine, C.; Yair, S.; Holderied, M. Differential effects of artificial lighting on flight and foraging behaviour of two sympatric bat species in a desert. J. Zool. 2011, 285, 21–27. [Google Scholar] [CrossRef]
- Nozaki, M.; Tsushima, M.; Mori, Y. Diurnal changes in serum melatonin concentrations under indoor and outdoor environments and light suppression of nighttime melatonin secretion in the female Japanese monkey. J. Pineal Res. 1990, 9, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Le Tallec, T.; Perret, M.; Théry, M. Light pollution modifies the expression of daily rhythms and behavior patterns in a nocturnal primate. PLoS ONE 2013, 8, e79250. [Google Scholar] [CrossRef]
- Le Tallec, T.; Théry, M.; Perret, M. Melatonin concentrations and timing of seasonal reproduction in male mouse lemurs (Microcebus murinus) exposed to light pollution. J. Mammal. 2016, 97, 753–760. [Google Scholar] [CrossRef]
- Klante, G.; Steinlechner, S. A short red-light pulse during dark phase of LD-Cycle perturbs the hamster circadian clock. J. Comp. Physiol. A Sens. Neural Behav. Physiol. 1995, 177, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Alves-Simoes, M.; Coleman, G.; Canal, M.M. Effects of type of light on mouse circadian behaviour and stress levels. Lab. Anim. 2016, 50, 21–29. [Google Scholar] [CrossRef]
- Hoffmann, J.; Schirmer, A.; Eccard, J.A. Light pollution affects space use and interaction of two small mammal species irrespective of personality. BMC Ecol. 2019, 19, 26. [Google Scholar] [CrossRef]
- Hoffmann, J.; Palme, R.; Eccard, J.A. Long-term dim light during nighttime changes activity patterns and space use in experimental small mammal populations. Environ. Pollut. 2018, 238, 844–851. [Google Scholar] [CrossRef]
- Pilorz, V.; Tam, S.K.E.; Hughes, S.; Pothecary, C.A.; Jagannath, A.; Hankins, M.W.; Bannerman, D.M.; Lightman, S.L.; Vyazovskiy, V.V.; Nolan, P.M.; et al. Melanopsin Regulates Both Sleep-Promoting and Arousal-Promoting Responses to Light. PLoS Biol. 2016, 14, e1002482. [Google Scholar] [CrossRef]
- Le Tallec, T.; Théry, M.; Perret, M. Effects of light pollution on seasonal estrus and daily rhythms in a nocturnal primate. J. Mammal. 2015, 96, 438–445. [Google Scholar] [CrossRef]
- Benedetto, M.M.; Guido, M.E.; Contin, M.A. Nov-Visual Photopigments Effects of Constant Light-Emitting Diode Light Exposure on the Inner Retina of Wistar Rats. Front. Neurol. 2017, 8, 417. [Google Scholar] [CrossRef]
- Walker, W.H.; Melendez-Fernandez, O.H.; Nelson, R.J. Prior exposure to dim light at night impairs dermal wound healing in female C57BL/6 mice. Arch. Dermatol. Res. 2019, 311, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Biebouw, K.; Blumstein, D.T. Tammar wallabies (Macropus eugenii) associate safety with higher levels of nocturnal illumination. Ethol. Ecol. Evol. 2003, 15, 159–172. [Google Scholar] [CrossRef]
- Robert, K.A.; Lesku, J.A.; Partecke, J.; Chambers, B. Artificial light at night desynchronizes strictly seasonal reproduction in a wild mammal. Proc. R. Soc. B Biol. Sci. 2015, 282, 20151745. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.M.; Prendergast, R.L.; Sheridan, J.T.; Murphy, B.A. Blue light from light-emitting diodes directed at a single eye elicits a dose-dependent suppression of melatonin in horses. Vet. J. 2013, 196, 231–235. [Google Scholar] [CrossRef]
- Ikeno, T.; Weil, Z.M.; Nelson, R.J. Dim light at night disrupts the short-day response in Siberian hamsters. Gen. Comp. Endocrinol. 2014, 197, 56–64. [Google Scholar] [CrossRef]
- Fuller, G.; Raghanti, M.A.; Dennis, P.M.; Kuhar, C.W.; Willis, M.A.; Schook, M.W.; Lukas, K.E. A comparison of nocturnal primate behavior in exhibits illuminated with red and blue light. Appl. Anim. Behav. Sci. 2016, 184, 126–134. [Google Scholar] [CrossRef]
- Gaston, K.J.; Visser, M.E.; Hölker, F. The biological impacts of artificial lightat night: The research challenge. Phil. Trans. R. Soc. B 2015, 370, 20140133. [Google Scholar] [CrossRef]
- Rich, C.; Longcore, T. Ecological Consequences of Artificial Night Lighting; Island Press: Washington, DC, USA, 2006. [Google Scholar]
- Sanders, D.; Frago, E.; Kehoe, R.; Patterson, C.; Gaston, K.J. A meta-analysis of biological impacts of artificial light at night. Nat. Ecol. Evol. 2021, 5, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Hölker, F.; Bolliger, J.; Davies, T.W.; Giavi, S.; Jechow, A.; Kalinkat, G.; Longcore, T.; Spoelstra, K.; Tidau, S.; Visser, M.E.; et al. 11 pressing research questions on how light pollution affects biodiversity. Front. Ecol. Evol. 2021, 9, 767177. [Google Scholar] [CrossRef]
- Briolat, E.S.; Gaston, K.J.; Bennie, J.; Rosenfeld, E.J.; Troscianko, J. Artificial nighttime lighting impacts visual ecology links between flowers, pollinators and predators. Nat. Commun. 2021, 12, 4163. [Google Scholar] [CrossRef]
- Inger, R.; Bennie, J.; Davies, T.W.; Gaston, K.J. Potential biological and ecological effects of flickering artificial light. PLoS ONE 2014, 9, e98631. [Google Scholar]
- Gaston, K.J. Nighttime ecology: The “nocturnal problem” revisited. Am. Nat. 2019, 193, 481–502. [Google Scholar] [CrossRef]
- Zielinska-Dabkowska, K.M.; Xavia, K. Looking up to the stars. A call for action to save New Zealand’s dark skies for future generations to come. Sustainability 2021, 13, 13472. [Google Scholar] [CrossRef]
- Raworth, K. Doughnut Economics: Seven Ways to Think Like a 21st-Century Economist; Chelsea Green Publishing: Hartford, VT, USA, 2017. [Google Scholar]
- Zielinska-Dabkowska, K.M.; Bobkowska, K.; Szlachetko, K. An Impact Analysis of Artificial Light at Night (ALAN) on Bats. A Case Study of the Historic Monument and Natura 2000 Wisłoujście Fortress in Gdansk, Poland. Int. J. Environ. Res. Public Health 2021, 18, 11327. [Google Scholar] [CrossRef]
- Encyclopaedia Britannica. Sunlight. Encyclopædia Britannica: 2019. Available online: https://www.britannica.com/science/sunlight-solar-radiation (accessed on 8 September 2019).
- Naylor, J. Out of the Blue: A 24-Hour Skywatcher’s Guide; Cambridge University Press: Cambridge, UK, 2002; pp. 64–73. [Google Scholar]
- Encyclopaedia Britannica. Twilight. Encyclopædia Britannica: 2019. Available online: https://www.britannica.com/science/twilight-glow (accessed on 8 September 2019).
- Futura Sciences. Twilight. 2021. Available online: http://www.futura-sciences.us/dico/d/universe-twilight-50005416/ (accessed on 11 November 2021).
- Robinson, K. Starlight: An Introduction to Stellar Physics for Amateurs; Springer Science & Business Media: Cham, Switzerland, 2009. [Google Scholar]
- European Southern Observatory. Airglow. In ESOcast 78: Airglow; ESO: Garching bei München, Germany, 2015; Available online: https://www.eso.org/public/announcements/ann15084/ (accessed on 8 September 2019).
- Encyclopaedia Britannica. Aurora (Atmospheric Phenomenon). Encyclopædia Britannica. 2019. Available online: https://www.britannica.com/science/aurora-atmospheric-phenomenon (accessed on 8 September 2019).
- Encyclopaedia Britannica. Bioluminescence. Encyclopædia Britannica. 2018. Available online: https://www.britannica.com/science/bioluminescence (accessed on 8 September 2019).
- Giles & Steve. British Gas Lamplight Content Creation. Available online: https://www.gilesandsteve.com/799904201174 (accessed on 8 September 2019).
- Gaslicht-Kultur, E.V.; Kujath, B. Gaslicht Berlin: N.d. Available online: http://gaslicht-kultur.de/ (accessed on 8 September 2019).
- Encyclopaedia Britannica. Lighting; Encyclopædia Britannica. 2017. Available online: https://www.britannica.com/technology/lighting (accessed on 29 April 2021).
- Allaby, M. A Dictionary of Ecology; Oxford University Press: Oxford, UK, 2010; p. 85, 103, 116, 228, 257, 292. [Google Scholar]
- Allaby, M. A Dictionary of Zoology, 5th ed.; Oxford University Press: Oxford, UK, 2020. [Google Scholar]
- Barrows, E.M. Animal Behavior Desk Reference: A Dictionary of Animal Behavior, Ecology, and Evolution; CRC Press: Boca Raton, FL, USA, 2000; p. 438. [Google Scholar]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, H. Metabolism; Encyclopædia Britannica. 2020. Available online: https://www.britannica.com/science/metabolism (accessed on 29 April 2021).
- Lambers, H.; Bassham, J.A. Photosynthesis; Encyclopædia Britannica. 2021. Available online: https://www.britannica.com/science/photosynthesis (accessed on 29 April 2021).
- Kelber, A. Vision: Rods See in Bright Light. Curr. Biol. 2018, 28, R364–R366. [Google Scholar] [CrossRef]
- Pothukuchi, K. City Light or Star Bright: A Review of Urban Light Pollution, Impacts, and Planning Implications. J. Plan. Lit. 2021, 36, 155–169. [Google Scholar] [CrossRef]
- Raynham, P.J. The SLL Code for Lighting; The Society of Light and Lighting: London, UK, 2012. [Google Scholar]
- Bass, M. Handbook of Optics; Van Stryland, E.W., Williams, D.R., Wolfe, W.L., Eds.; Radiometry and Photometry; McGraw-Hill: New York, NY, USA, 1995; Chapter 7; Volume 2, pp. 33–66. [Google Scholar]
- Fördergemeinschaft Gutes Licht. Licht Wissen 01—Lighting with Artificial Light; Fördergemeinschaft Gutes Licht. 2016. Available online: https://www.licht.de/fileadmin/Publications/licht-wissen/1608_lw01_E_Artificial_Light_web.pdf (accessed on 13 March 2020).
- Fördergemeinschaft Gutes Licht. Licht Wissen 03—Roads, Paths and Squares; Fördergemeinschaft Gutes Licht. 2014. Available online: https://www.licht.de/fileadmin/Publications/licht-wissen/1409_LW03_E_Roads-Paths-Squares_web.pdf (accessed on 13 March 2020).
- Fördergemeinschaft Gutes Licht. Licht Wissen 13—Outdoor Workplaces. Fördergemeinschaft Gutes Licht. 2007. Available online: https://www.licht.de/fileadmin/Publications/licht-wissen/0712_lw13_E_Outdoor_Workplaces_web.pdf (accessed on 13 March 2020).
- British Standard Institute (BSI). Light and Lighting. Basic Terms and Criteria for Specifying Lighting Requirements; British Standard Institution: London, UK, 2011. [Google Scholar]
- Schanda, J. Colorimetry: Understanding the CIE System; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 207–215. [Google Scholar]
- McColgan, M. Light pollution. Natl. Lighting Prod. Inf. Program (NLPIP) Lighting Answ. 2007, 7, 1–20. Available online: https://www.lrc.rpi.edu/programs/nlpip/lightinganswers/pdf/print/LightPollution.pdf (accessed on 13 March 2020).
- International Commission of Illumination (CIE). Guide on the Limitation of the Effects of Obtrusive Light from Outdoor Lighting Installations; Technical Report: CIE 150; Commission International de l’Éclairage (CIE): Vienna, Austria, 2003. [Google Scholar]


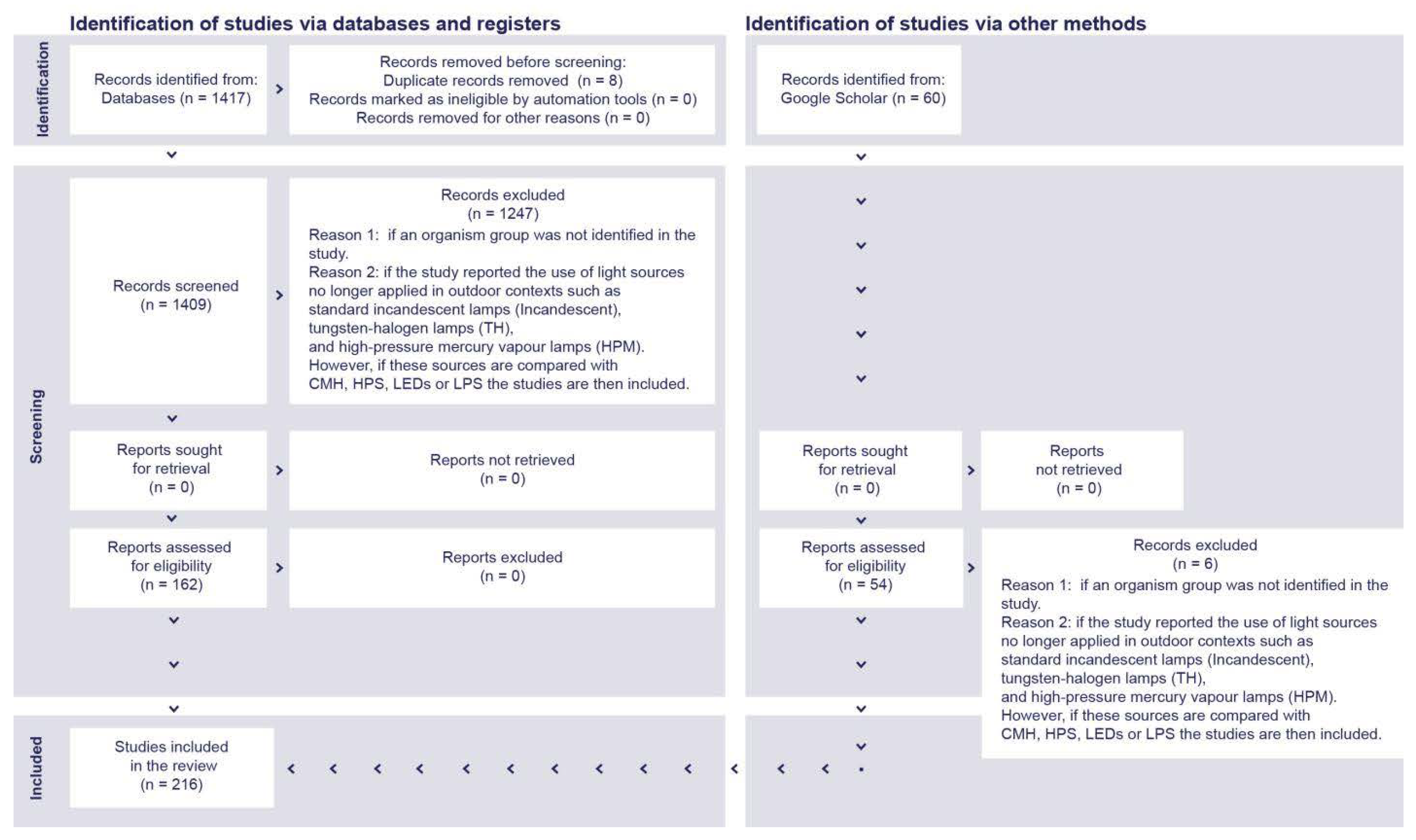
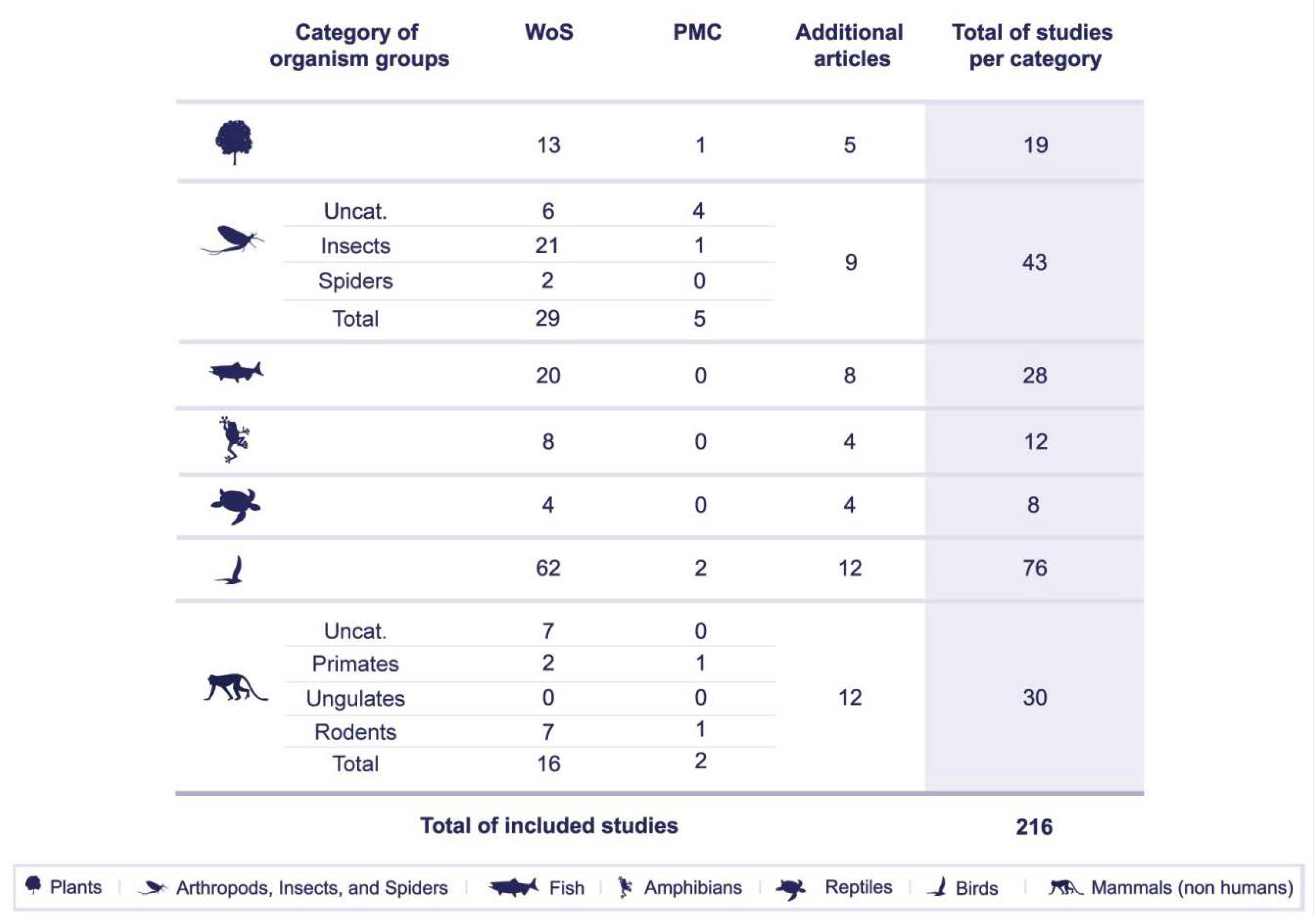

| Table No. | Description |
|---|---|
| A1 | Overview of terminologies and definitions of natural and artificial light sources relevant for ALAN research and ULP |
| A2 | Overview of terminologies and definitions of responses to light in living organisms and ecosystems |
| A3 | Overview of various types of electric light sources commonly used in urban settings in the past and the present |
| A4 | Overview of radiometric and photometric quantities of light and units |
| A5 | Overview of terminologies and definitions of ALAN as a pollutant |
| Physical Quantity | Domains | |
|---|---|---|
| ULP | ALAN Research | |
| Irradiance, Ee (W/m2) | rare | common |
| Illuminance, Ev (lx) | common | rare |
| PAR * photon flux density (PPFD) EPAR (µmol photons/m2) | not used | rare |
| Radiance, Le (W/m2·sr) | not used | rare |
| Luminance, Lv (cd/m2) | common | not used |
| Sky radiance (astronomy) ** Lsky,SQM (mags/arcsec2) | not used | rare |
| Radiant flux, Φe (W) | not used | rare |
| Luminous flux, Φv (lm) | common | rare |
| PAR* photon flux (PPF) ΦPAR (µmol photons/s) | not used | rare |
| Spectral power distribution (SPD; e.g., spectral irradiance in W/m2∙nm) | rare (increasing) | rare (increasing) |
| Correlated color temperature (CCT; K) | common | rare |
| Color rendering index (CRI; Ra) | common | not used |
| Flicker frequency (Hz) | rare (increasing) | not used |
| Flicker % | rare (increasing) | not used |
| (Degree of) Polarization | not used | just emerging |
| Category of Organism Groups | Keyword #1 | Keyword #2 | Keyword #3 | Keyword #4 | Keyword #5 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Plants | “artificial + light” | AND | plant * | - | - | - | - | - | - |
| Arthropods | “artificial + light” | AND | arthropod * | OR | insect * | OR | spider * | - | - |
| Fish | “artificial + light” | AND | fish * | - | - | - | - | - | - |
| Amphibians | “artificial + light” | AND | amphibian * | - | - | - | - | - | - |
| Reptiles | “artificial + light” | AND | reptile * | - | - | - | - | - | - |
| Birds | “artificial + light” | AND | bird * | - | - | - | - | - | - |
| Non-human mammals | “artificial + light” | AND | mammal * | OR | ungulate * | OR | primate * | OR | rodent * |
| Category of Artificial Light and Light Sources | Category of Organism Groups | ||||||
|---|---|---|---|---|---|---|---|
| Plants | Arthropods: Insects and Spiders | Fish | Amphibians | Reptiles | Birds | Non-Human Mammals: Bats, Primates, Rodents, and Marsupials | |
| ALAN (dir.) | 10 | 6 | 4 | 2 | 4 | 16 | 8 |
| ALAN (ind.) | - | 3 | 5 | 4 | 2 | 28 | 4 |
| CMH | - | - | 5 | - | - | 2 | - |
| FL | 1 | 5 | 2 | 1 | - | 4 | 2 |
| HPS | - | 5 | 2 | 1 | - | 1 | 3 |
| LED | 7 | 15 | 6 | 4 | 1 | 23 | 8 |
| LPS | - | - | - | - | - | - | - |
| CMH and LED | - | - | 2 | - | - | - | - |
| CMH, HPS, and LED | - | 2 | - | - | - | 1 | - |
| CMH, HPS, and TH | - | - | 1 | - | - | - | - |
| CMH, HPM, LED, and LPS | - | 1 | - | - | - | - | - |
| FL and LED | - | 2 | 1 | - | - | - | 1 |
| HPS and HPM | - | 1 | - | - | - | - | - |
| HPS and LED | 1 | 1 | - | - | - | - | |
| HPS, LED, and induction lamp | - | - | - | - | - | - | 1 |
| HPS, MH, LPS, and LED | - | - | - | - | 1 | - | - |
| LED and HPM | - | 2 | - | - | - | - | - |
| ALAN and moonlight | - | - | - | - | - | 1 | 2 |
| LED and moonlight | - | - | - | - | - | - | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez Vega, C.; Zielinska-Dabkowska, K.M.; Schroer, S.; Jechow, A.; Hölker, F. A Systematic Review for Establishing Relevant Environmental Parameters for Urban Lighting: Translating Research into Practice. Sustainability 2022, 14, 1107. https://doi.org/10.3390/su14031107
Pérez Vega C, Zielinska-Dabkowska KM, Schroer S, Jechow A, Hölker F. A Systematic Review for Establishing Relevant Environmental Parameters for Urban Lighting: Translating Research into Practice. Sustainability. 2022; 14(3):1107. https://doi.org/10.3390/su14031107
Chicago/Turabian StylePérez Vega, Catherine, Karolina M. Zielinska-Dabkowska, Sibylle Schroer, Andreas Jechow, and Franz Hölker. 2022. "A Systematic Review for Establishing Relevant Environmental Parameters for Urban Lighting: Translating Research into Practice" Sustainability 14, no. 3: 1107. https://doi.org/10.3390/su14031107
APA StylePérez Vega, C., Zielinska-Dabkowska, K. M., Schroer, S., Jechow, A., & Hölker, F. (2022). A Systematic Review for Establishing Relevant Environmental Parameters for Urban Lighting: Translating Research into Practice. Sustainability, 14(3), 1107. https://doi.org/10.3390/su14031107








