1. Introduction
The use of digital technology has great potential for medical research. Particularly, the COVID-19 pandemic has played a central role in accelerating the process of development and sophistication of digital technology in medical research [
1,
2]. Appropriately employing digital technology can significantly increase both efficiency and efficacy in clinical trials and medical education, which are key to the advancement of medical research [
3,
4,
5,
6]. More specifically, digital technology can contribute to the development of medical research by improving the efficiency of participant recruitment and retention, health data collection, and data analysis in clinical trials. It also increases the accuracy of the analysis by facilitating communication with the participants of an experiment and reducing the time for data collection. Thus, the development of digital technology is closely related to advances in medical research.
In a global perspective, the COVID-19 pandemic provides many countries with an opportunity to recognize the importance of employing digital technologies to respond to global challenge. Research collaboration for the development and distribution of vaccines, the prediction and tracking of confirmed cases, and the sharing of information have become globally critical issues. The role of digital technologies in medicine has received steady attention from scholars from diverse domains. In the field of medicine, researchers have mainly studied how digital technology contributes to the treatment or surgery of patients in specific fields. [
7,
8,
9]. Scholars from engineering fields have focused on data sharing, protection, and management including the interface, integration, and coordination of data [
10,
11]. Business researchers have predominantly investigated digital health ecosystem and interactions among various stakeholders from an ecosystem perspective [
12,
13,
14]. Although these existing studies have offered valuable and diverse insights, these studies have been conducted mainly in the context of specific types of organizations (e.g., hospital, biotech company, etc.) or industries (e.g., biotech industry, pharmaceutical industry, etc.), and national or global-level perspectives have been somehow neglected in this stream of research.
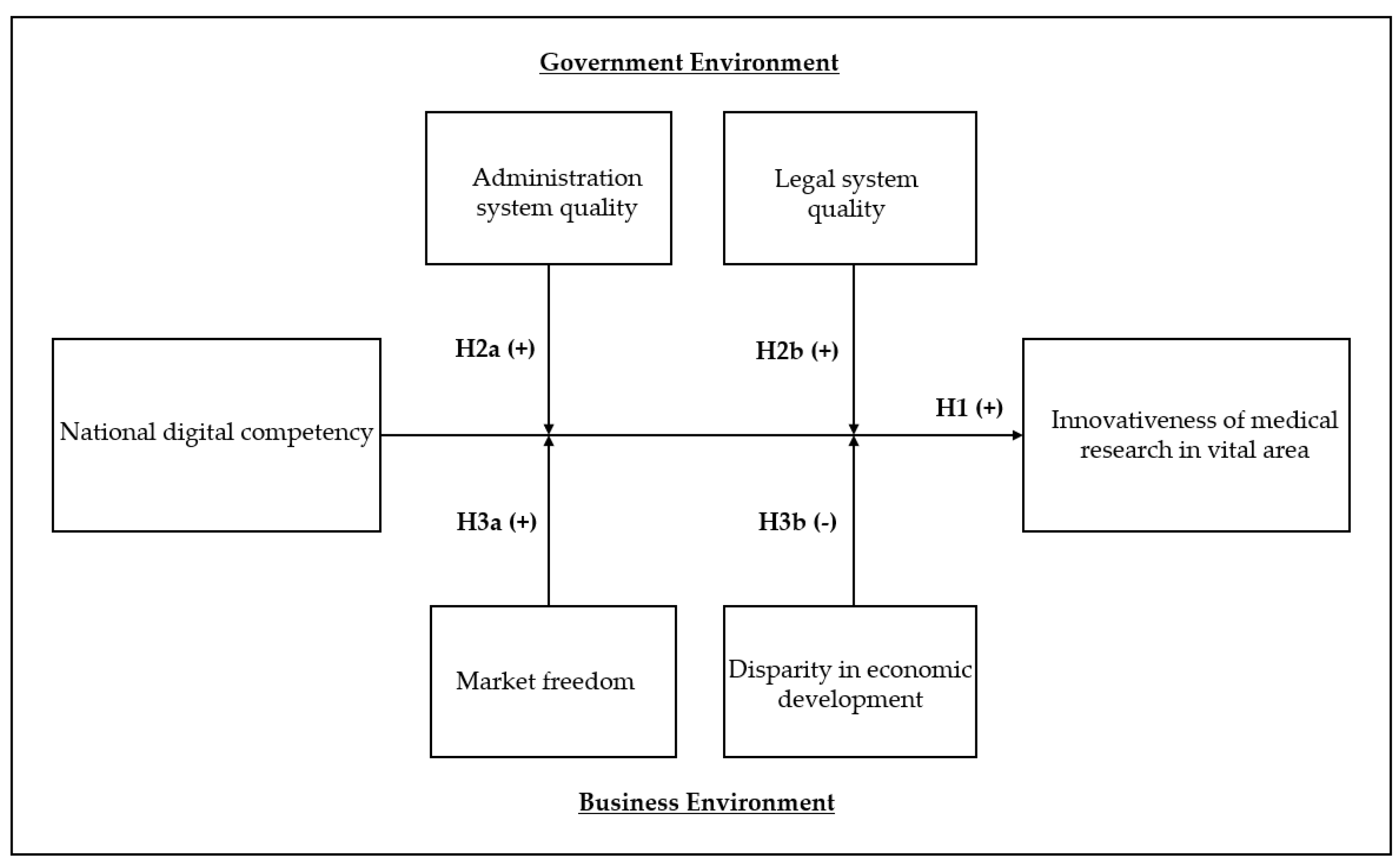
However, how digital competency contributes to the innovativeness of medical research has received scant attention and is barely supported by empirical evidence. Based on the awareness of this issue, this study examines how national digital competency impacts the innovativeness of medical research in a global context. As medical research is highly diversified, this study will pay special attention to the effects of national digital competency on the research performance of vital areas in medicine—a medical terminology—that comprehensively refers to four areas: (1) surgery; (2) internal medicine; (3) pediatrics, perinatology, and child health; and (4) obstetrics and gynecology. These areas are major disciplines in the field of medicine, encompassing various subfields. For example, geriatrics is included in internal medicine. (Pediatrics, etc., are included in this category not to distinguish a specific age group, but because babies and children require a fundamentally different medical approach than adults). The reason for focusing on those vital areas is because the research performance of those areas is straightly associated with fatalities through the whole lifespan of human. Knowledge in vital areas particularly becomes significant during pandemics, experiencing a new disease for which epidemiologic data have not been accumulated rapidly, and the death rate is determined within a short time. This is because knowledge about the effects of infectious diseases on body parts is directly related to human lives and forms the gist of coping with unprecedented pandemics. For example, at the beginning of the COVID-19 outbreak, many pediatric patients suffered due to a lack of adaptation period to changes in surgical environments and methods [
15]. In addition, many people have experienced unexpected pains due to a lack of knowledge about complications or organ damage caused by COVID-19.
In addition, as it takes time for the institution to embrace various effects of rapidly developed knowledge, drastic progress in digital technology in terms of both development and utilization could cause particular social interest in the process of taking advantage of such benefits. This is a social phenomenon that is often accompanied by radically innovative knowledge, and it is not uncommon. Therefore, researchers have recognized the importance of understanding how institutional environments influence the progress of medical research [
16,
17,
18,
19], but enough empirical evidence still has not been found. Hence, we examined how various institutional factors moderate this relationship. We support our arguments using digital competencies, publications, and diverse institutional data of 63 countries. Our conceptual framework is depicted in
Figure 1.
The remainder of this paper is organized as follows. First, we discuss how national digital competencies impact the degree of innovation research in medicine, and consequently, how this association is moderated by the government and business environments. Second, drawing on data from multiple databases, we provide the results of an empirical test. Third, we address the implications and contributions of this study.
3. Methods
Using 62 national-level panel data, we investigate the effect of national digital competency on the innovativeness of medical research and how government and business environmental factors moderate that relationship.
3.1. Data and Sample
For empirical analysis, we utilize multiple databases. Regarding national digital competency (NDC), we draw the data from the World Digital Competency data provided by the International Institute for Management Development (IMD), which is a top-tier global research institute in Switzerland. Since the late 1980s, the IMD’s annual report on national competency based on relevant proxies has been widely acknowledged by researchers in various disciplines [
44,
45,
46].
To estimate the innovativeness of medical research, we use the data from the Journal and the Country Rank database offered by SCImago, which is an established data-mining and visualization group in Spain that provides a wide range of bibliometric data including journals and citations. The data of SCImago has demonstrated reliability in bibliometric research including top-tier medicine journals, such as
Nature and
Lancet [
47,
48]. We obtained the raw numerical values of published medical documents and citation data for each nation and constructed the dependent variable. For control variables, drawing on multiple databases, we collected nation-level data on innovation index health infrastructure, political rights index, globalization index, services sector value-adding, gross domestic product (GDP), government protectionism, science research legislation, and innovation index. We offer the details of these variables in the next section.
The final sample of our study comprises 63 countries with 341 nation-year observations between 2015 and 2020. The list of sample countries is shown in
Table 1. In total, there are 33 countries from Europe, 8 countries from South America, 2 countries from North America, 14 countries from Asia and the Pacific, 5 countries from Middle East, and 1 country from Africa. Our sample include a wide range of countries, including both advanced economies and catching-up economies. We used 2015 as the starting year because interest in digital health has drastically increased based on the emergence of digital transformation, as illustrated in
Figure 1. We use 2020 as the cutoff year as forward citation information generally suffer from the truncation issue [
49].
3.2. Variable Descriptions
Dependent variable. To estimate our dependent variable, the innovativeness of medical research, we use the number of forward citations per document published in the fields of Surgery, Pediatrics, Perinatology and Child Health, Obstetrics and Gynecology, and Internal Medicine based on the Journal and Country Rank from the Scimago database. Many researchers address that highly cited research is highly likely to be conducted based on combinations of a broad range of knowledge domains that provide an explorative perspective to researchers and enable them to avoids intellectual lock [
50]. Similarly, combinative knowledge from exploratory search can produce more innovative scientific research that ultimately becomes highly cited [
51,
52]. Therefore, the number of forward citations has been widely acknowledged and employed as a proxy for the innovativeness of research in prior studies [
53,
54,
55].
We first calculate the total number of published and citable documents in each of the vital areas in medicine and the total number of forward citations that those documents received. Both numbers are aggregated at the nation level. Then, consistent with previous literature, we estimate medical research performance as:
where citable document i,t,c represents the number of citable documents published by country i in medical field c at the time of year t. Total forward citations i,t,c represents the number of forward citations (the document receives after published) of the focal citable document.
Independent variable. National digital competency (NDC) is measured based on the digital competency ranking data from the IMD World Competency Yearbook, which offers a comprehensive estimation of the digital and technological level of each nation country by combining statistical and survey data.
Moderating Variables. We draw institutional data from the Global Economy database. The quality of the administration system is measured as Government effectiveness index from the Global Economy Database. This measure captures the quality of public services, the quality of the civil service and the degree of its independence from political pressures, the quality of policy formulation and implementation, and the credibility of the government’s commitment to such policies. Regarding the quality of the legal system, we employ the rule of law index from the same database. This indicator captures perceptions of the extent to which agents have confidence in and abide by the rules of society, the quality of contract enforcement, property rights, the police, and the courts, as well as the likelihood of crime and violence. To capture the quality of market freedom, we use the business freedom index from the Global Economy database constructing this measure based on the World Bank’s Doing Business study. Lastly, the disparity of economic development is measured as the uneven economic development index from the Global Economy database.
Control Variables. The research can be affected by the overall innovation environment. Therefore, we used the innovation index of a nation from the Global Economy database. The Global Economy database measured the innovation index (country level) using data from Cornell University, INSEAD, and the World Intellectual Property Organization, which provide an innovation index that comprehensively captures each country’s quality of institutions, human capital and research, infrastructure, and market and business sophistication. We use the World Bank’s gross domestic product (GDP) data as our control variable. Economic level has been cited as an indicator of digital technologies in healthcare [
42]. We also controlled for policy instruments that might have influenced the quality and application of the research. Based on the IMD National Competitiveness Data, we controlled for the nations’ government protectionism and scientific research legislation (laws relating to scientific research encourage innovation). The IMD also offers the measure of health infrastructure, the degree to which it meets the social needs of the focal society, of each nation. We also control the degree of globalization that may facilitate the innovative research in medicine and political rights index that can potentially influence the credibility of governmental policy. We also control for the portion of the services sector that can affect the business activities in the healthcare industry.
Appendix A provides detailed information for variable descriptions regarding measurement and source.
3.3. Models
Using unbalanced panel data, we employed a fixed-effect regression model to investigate the effect of national digital competency on the innovativeness of medical research in vital areas and moderating effects delivered by various environmental factors. To control unobserved heterogeneity, we employed a fixed-effects regression model instead of a random-effects model based on the Hausman test [
56]. We considered the time lag (two years) between the dependent and independent variables with consideration because the bibliometric information (documents and citations) includes the past two years.
- (1)
IMR1 (S, P, O, I) i,t+2 = α0i + α1Natioanl digital competency (NDC) i,t +.
α2Controls i,t + ei,t
- (2)
IMR 1 (S, P, O, I) i,t+3 = β0i + β1Natioanl digital competency (NDC) i,t +. β2Natioanl digital competency (NDC) x Institutional environment factors
Β3Controls i,t + ei,t
where α0i represents country fixed effects and ei,t is the random error. IMR (S), IMR (P), IMR (O), and IMR (I) refer to the innovativeness of medical research in Surgery, Pediatrics, Perinatology and Child Health, Obstetrics and Gynecology, and Internal Medicine, respectively.
4. Results
Table 2 and
Table 3 present the descriptive statistics and correlation matrices, respectively. Considering space limitations, we used an abbreviated name of each variable for the correlation matrix. The summary statistics indicated that national digital competency (NDC) was positively correlated with forward citations per document in all vital fields, including Surgery (ρ = 0.30,
p < 0.05), Pediatrics, Perinatology, Child Health (ρ = 0.21,
p < 0.05), Obstetrics and Gynecology (ρ = 0.20,
p < 0. 5), and Internal Medicine (ρ = 0.30,
p < 0.05). The relatively high correlation among dependent variables could be attributed to their academic relatedness. However, no dependent variable is used in the same regression equation, and hence, multicollinearity was not a major concern in analyses.
Table 4 demonstrate the results of the main effect. Hypothesis 1 predicts that NDC will have a positive impact on the innovativeness of medical research in the field of the vital area. In
Table 4, there are positive coefficients of Model 1 (β = 3.664,
p < 0.001), Model 3 (β = 3.826,
p < 0.01), Model 5 (β = 3.403,
p < 0.05), and Model 7 (β = 5.148,
p < 0.01), providing support for Hypothesis 1 with the baseline regression model. These results indicate that a national digital capability positively influences research performance in vital areas. These results are held after employing full model regression in Model 2 (β = 2.449,
p < 0.05), Model 4 (β = 2.606,
p < 0.05), Model 6 (β = 1.841,
p < 0.05), and Model 8 (β = 3.251,
p < 0.01).
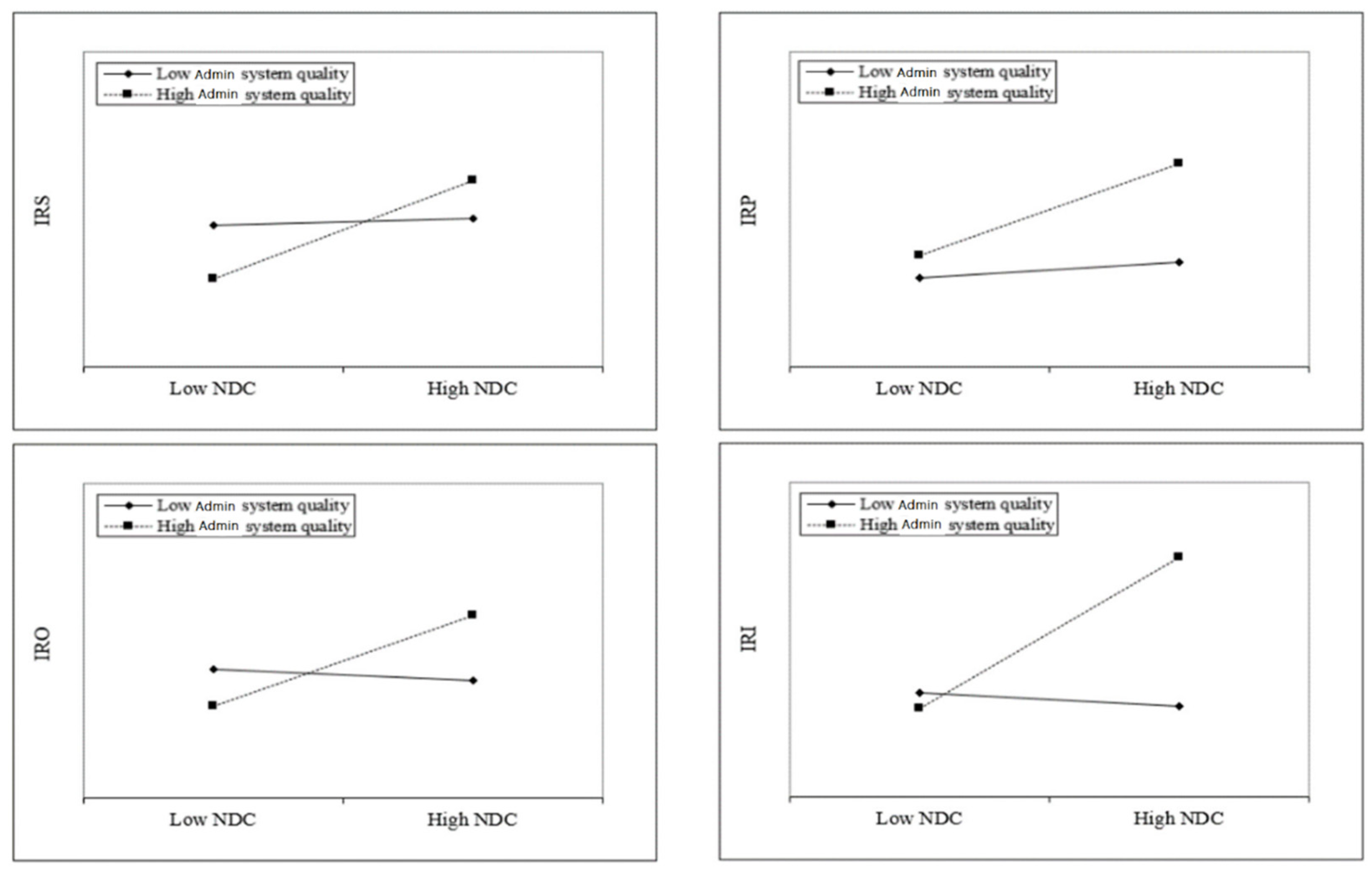
Next, we shift our attention to investigate how the main effect is moderated by various institutional variables. Hypothesis 2a posits that the quality of administration will enhance the positive impact of NDC on innovativeness of medical research. In
Table 5, the positive coefficients of Model 2 (β = 3.052,
p < 0.001), Model 4 (β = 2.531,
p < 0.01), Model 6 (β = 3.359,
p < 0.01), and Model 8 (β = 5.482,
p < 0.001) provide support for Hypothesis 2a.
Figure 2 provides a plot to understand these results. The plot indicates that the impact of national digital competency (NDC) on the innovativeness of medical research is contingent on the quality of the administration system.
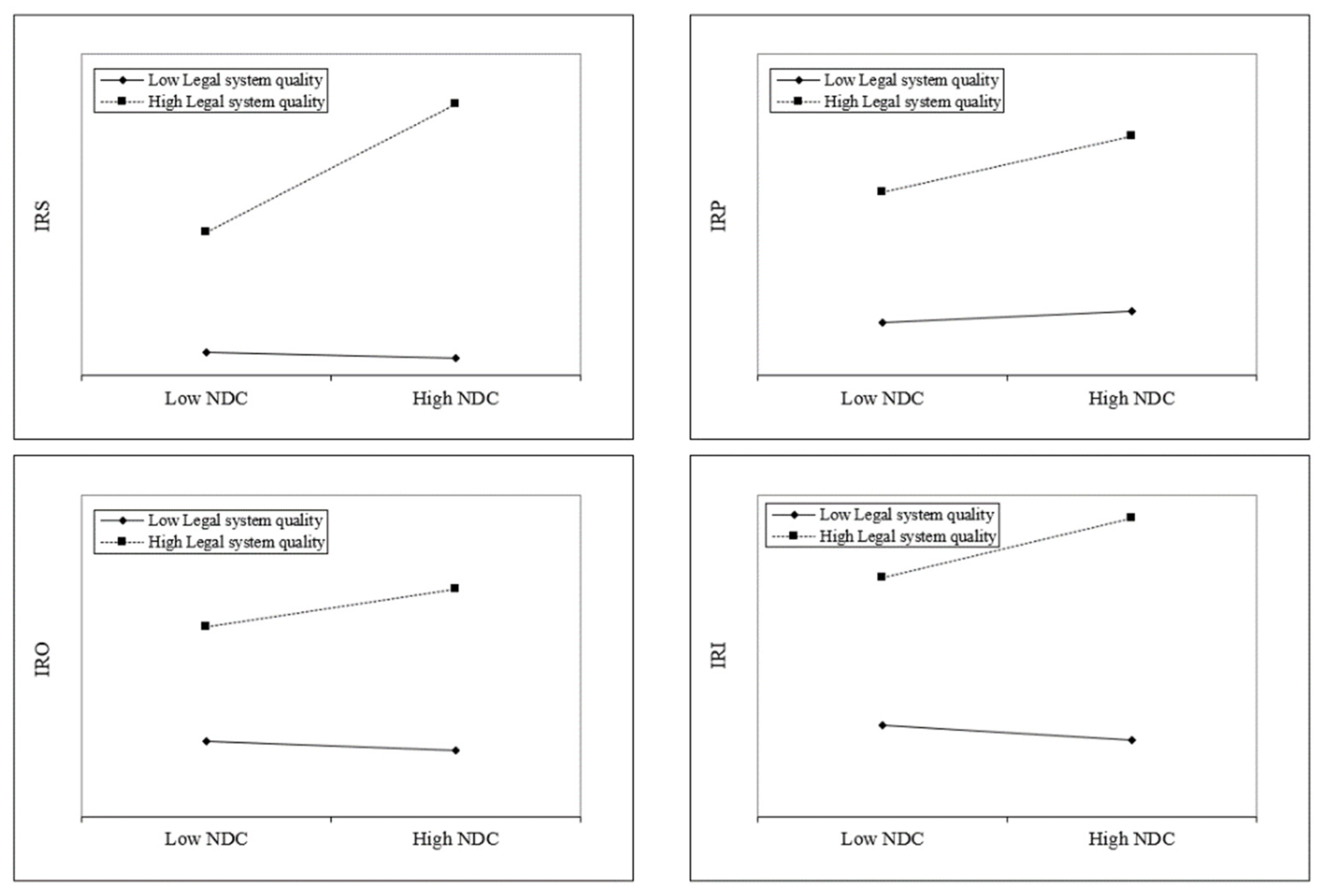
Hypothesis 2b predicts that quality of the legal system will strengthen the positive effect of NDC on the innovativeness of medical research. In
Table 6, the positive coefficients of Model 2 (β = 2.661,
p < 0.001), Model 4 (β = 1.668,
p < 0.05), Model 6 (β = 2.641,
p < 0.00), and Model 8 (β = 4.720,
p < 0.001) offer support for Hypothesis 2b. To aid in understanding these results, we plotted the interaction effects in
Figure 3. The slope of the high administration system quality line changes steeply over the high vs. low NDC in the areas of Surgery (IRS) and Obstetrics and Gynecology (IRO), which are more strongly moderated than the others.
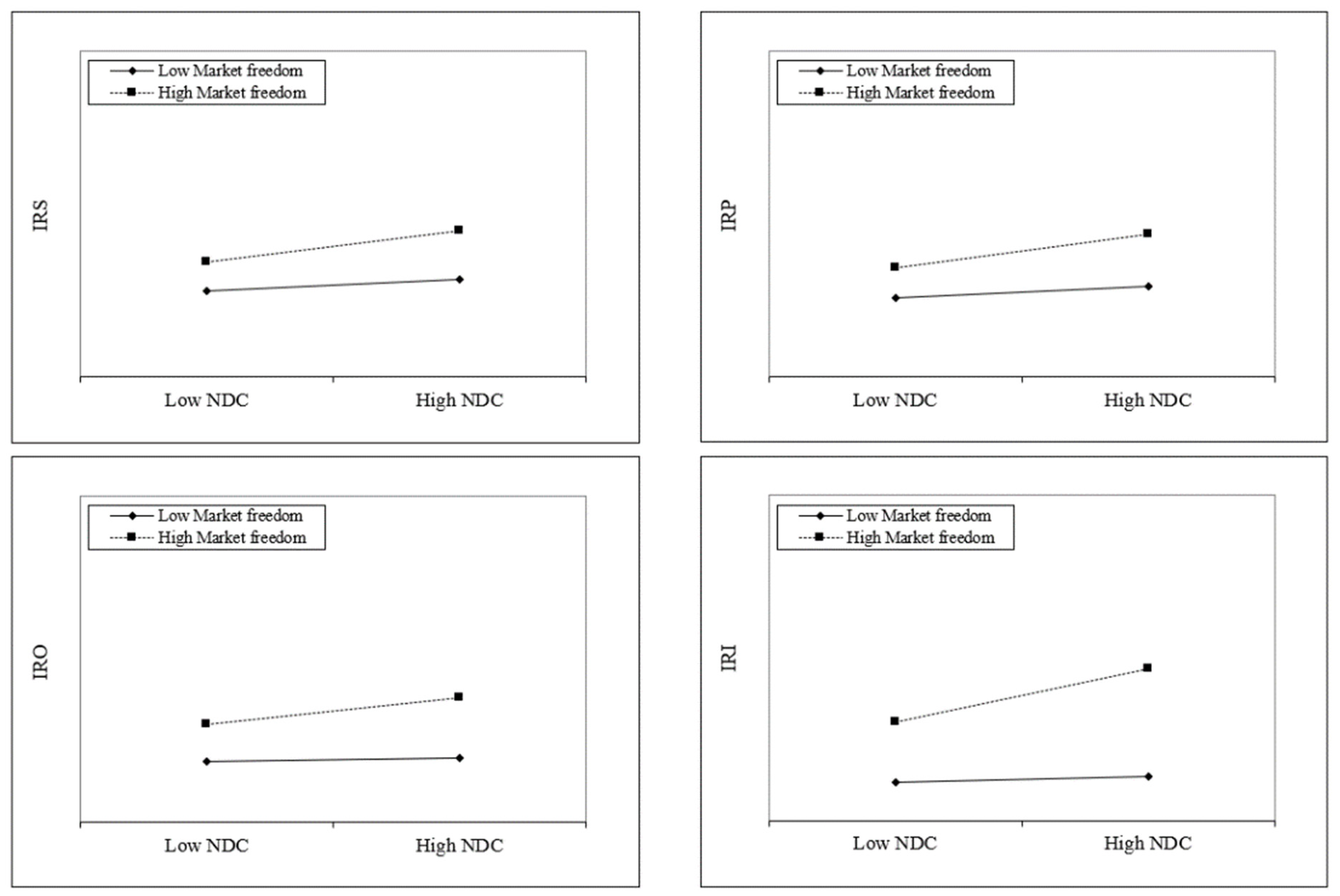
Hypothesis 3a postulates that market freedom will augment the positive impact of NDC on the innovativeness of medical research. In
Table 7, the positive coefficients of Model 2 (β = 0.089,
p < 0.05), Model 4 (β = 0.095,
p < 0.05), Model 6 (β = 0.101,
p < 0.05), and Model 8 (β = 0.218,
p < 0.01) provide support for Hypothesis 3a.
Figure 4 offers a plot to understand these results, and the slope of the high market freedom line increases steeply over the high vs. low NDC in the area of Internal Medicine (IRI) compared with the others.
Hypothesis 3b anticipates that disparity in the economic development system will diminish the positive effect of NDC on the innovativeness of medical research. In
Table 8, the negative coefficients of Model 2 (β = −0.702,
p < 0.05) and Model 8 (β = −1.274,
p < 0.01) offer partial support for Hypothesis 3b. To help in understanding these results, we plotted the interaction effects in
Figure 5.
5. Discussion and Conclusions
5.1. Summary and Implications
As seen in the COVID-19 crisis, the development and utilization of digital technologies in medicine is becoming a global issue, not just a matter of individual hospitals or companies. However, while the usage of digital technologies for medical purposes has been studied in various fields including medicine, engineering, and business, the predominant interests of existing studies have been the quality of medical services, data management, and interests of individual institutions in the ecosystem, with micro-level perspectives. The main purpose of our study is to expand interest in digital competency and medical research to the national-level perspective.
Accelerated by the COVID-19 pandemic, the development of digital technology has provided significant benefits. Our study used data from 63 nations to demonstrate that digital competency positively impacts the innovativeness of medical research in vital areas: Surgery, Pediatrics, Perinatology, Child Health, Obstetrics and Gynecology, and Internal Medicine, and we discuss how this association varies in different institutional environments. This is an important finding for medical research because producing innovative results is critical to the sustainable progress of the field. In addition, our analysis provides several insights into the use of digital technologies for medical research, which is meaningful to researchers, practitioners, and policymakers.
First, while we find a positive association between digital competency and innovativeness in medical research, the benefit of digital competency is notably contingent upon different types of institutional environments. The institutional environment likely modulates the positive impact of digital competency on the innovativeness of medical research. While the quality of administration, legal system, and market liberalization strengthen the main effect, the disparity in economic development alleviates the positive impact of digital competency. When a new technology brings about a macro-level socioeconomic change, how much and in what direction it is affected by institutional influence is a significant concern for innovation and institutional perspectives. As innovative medical research is an important criterion for determining digital health systems, it has received many institutional benefits or constraints. Our study provides a more detailed picture of the important institutional factors in medical research by suggesting two dimensions of the institutional environment—the government’s executive capacity and the economic system—and their impact.
Second, despite explicit benefits, the rapid adoption of new technologies may result in unintended or unexpected side effects. For example, economic inequality within the same country significantly reduces the positive impact of digital capabilities. This is because it implies the development and a distribution gap. In other words, although digital technology can facilitate the development of medical research, the benefits are not evenly distributed across people or regions. Consequently, many researchers focus on health inequality arising from the increased use of digital technology in medicine [
3,
39]. In other words, although the medical benefits significantly increase with digital technology for individuals (or groups) with the accessibility and ability to utilize such technology, for the remaining individuals (or groups), this change may leave them far behind where they are less likely to leverage these benefits. Hence, future studies can recognize this health inequality and develop digital health technologies to solve social problems.
The third important implication is for policymakers and institutions. Our findings support the most vital areas of medicine, but different results were obtained for some regions. For example, Pediatrics, Perinatology, Child Health, Obstetrics and Gynecology are unaffected by economic disparities. This could mean the system weakly affects children’s health or important health issues such as cancer. Therefore, these blind spots should be carefully considered when designing a system.
As seen during the COVID-19 pandemic, if a global infectious disease such as a pandemic re-emerges, ultimately, the collective intelligence through research collaboration can make an effective global response the key to cope with the crisis. Countries that adhere to closed systems may be able to keep their distance from some issues that can be potentially problematic, but they will be excluded from many of the great benefits that those collaborations will bring. To make those collaborations effective and efficient, in-depth understanding of the digital competency and institutional environment of individual countries must be a precedent. Our study empirically demonstrates that digital competency is conducive to the innovativeness of medical research at the country level. However, sophisticated design for the institutional environment must be concurrently considered to maximize its positive impact.
5.2. Limitations and Suggestions for Future Research
Our study has several limitations. Although it measured the innovativeness of medical research using established measures, due to the intrinsic characteristics of medicine, experimentations, services, and practices are critical to realizing the innovativeness of new knowledge. There can be limitations in accurately reflecting the degree of innovativeness using a document-based measure. Future research could conduct an in-depth analysis to investigate how innovative publications are utilized in digital health systems.
The scope of the medical research used in this study can be extended. Although this is important because of its direct linkage to mortality, recent medical services and practices require extensive cooperation within medicine, such as anesthesiology or radiology, and across other fields, such as material and biomedical engineering. Hence, further studies should be conducted using more comprehensive data. In addition, while we use the simple slope analysis to depict our moderating effects due to the methodological limitation, future study can employ more sophisticate analysis techniques to investigate the detailed mechanisms of moderating effects.