Skin Brightening Efficacy of Grammatophyllum speciosum: A Prospective, Split-Face, Randomized Placebo-Controlled Study
Abstract
1. Introduction
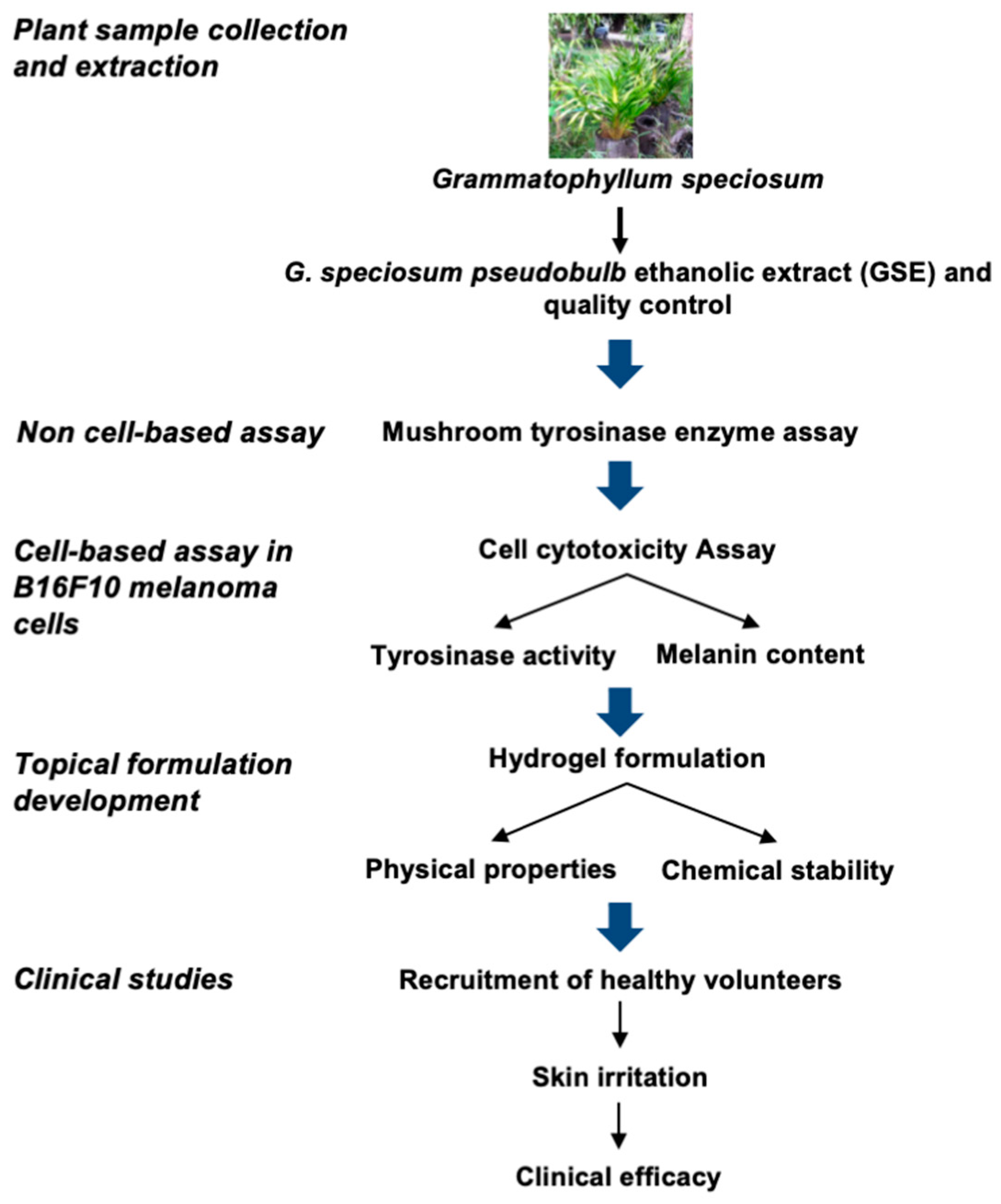
2. Materials and Methods
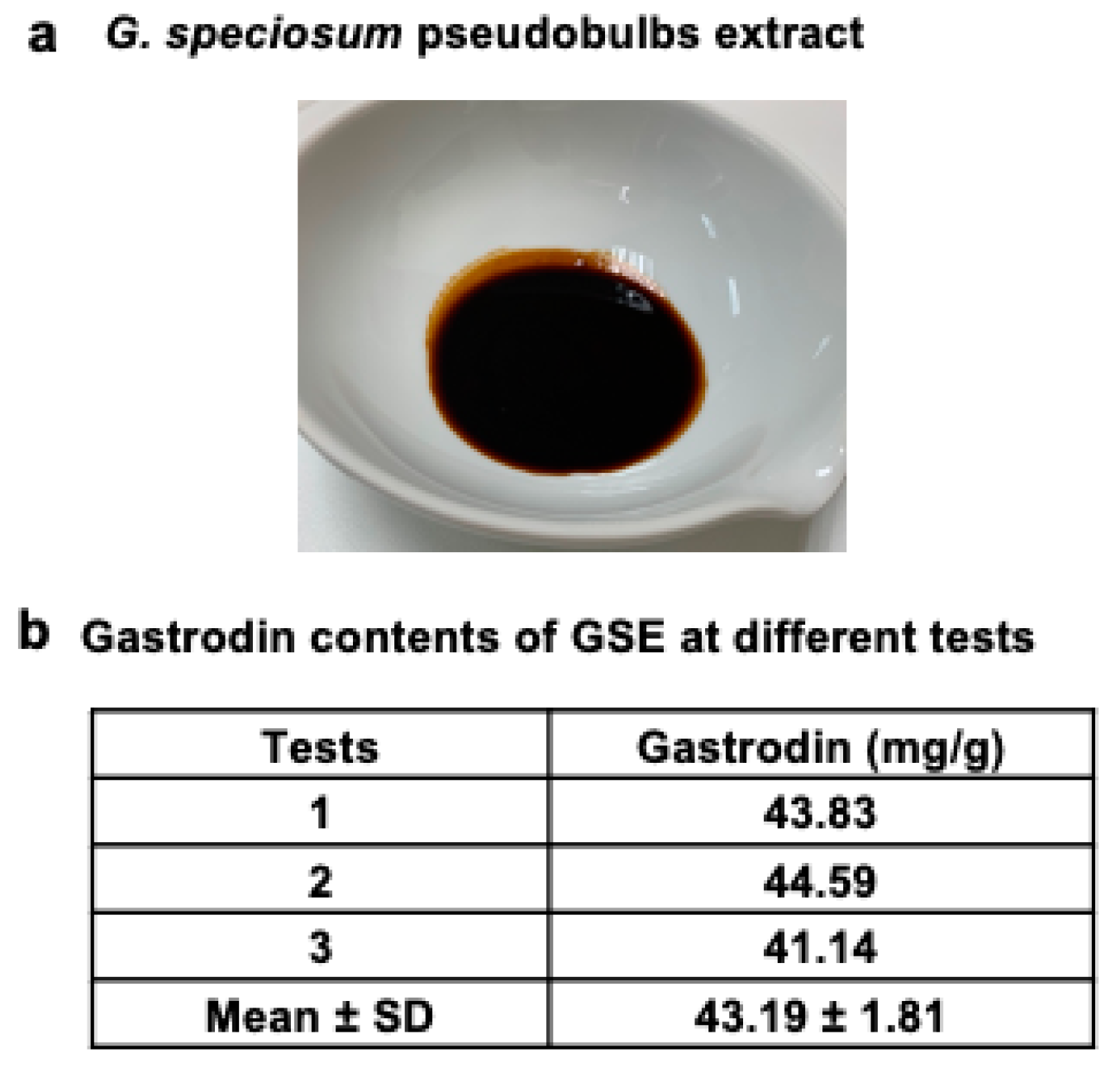
2.1. Plant Sample Preparation and Extraction
2.2. Quality Control of GSE
2.3. Mushroom Tyrosinase Enzyme Assay
- A: absorbance of blank solution;
- B: absorbance of blank solution, but without enzyme;
- C: absorbance of sample solution;
- D: absorbance of sample solution, but without enzyme.
2.4. Cell Culture
2.4.1. Cell Viability Assay
- A: absorbance of the sample;
- B: absorbance of the control.
2.4.2. Measurement of Intracellular Melanin Content
- Mt: ratio of melanin and the total amount of protein in the sample;
- Mc: ratio of melanin and the total amount of protein in the control.
2.4.3. Measurement of Cellular Tyrosinase Activity
- Tt: ratio of tyrosinase activity and the total amount of protein in the sample;
- Tc: ratio of tyrosinase activity and the total amount of protein in the control.
2.5. Stability and Physicochemical Properties of the GSE
2.6. Topical GSE Hydrogel Stability
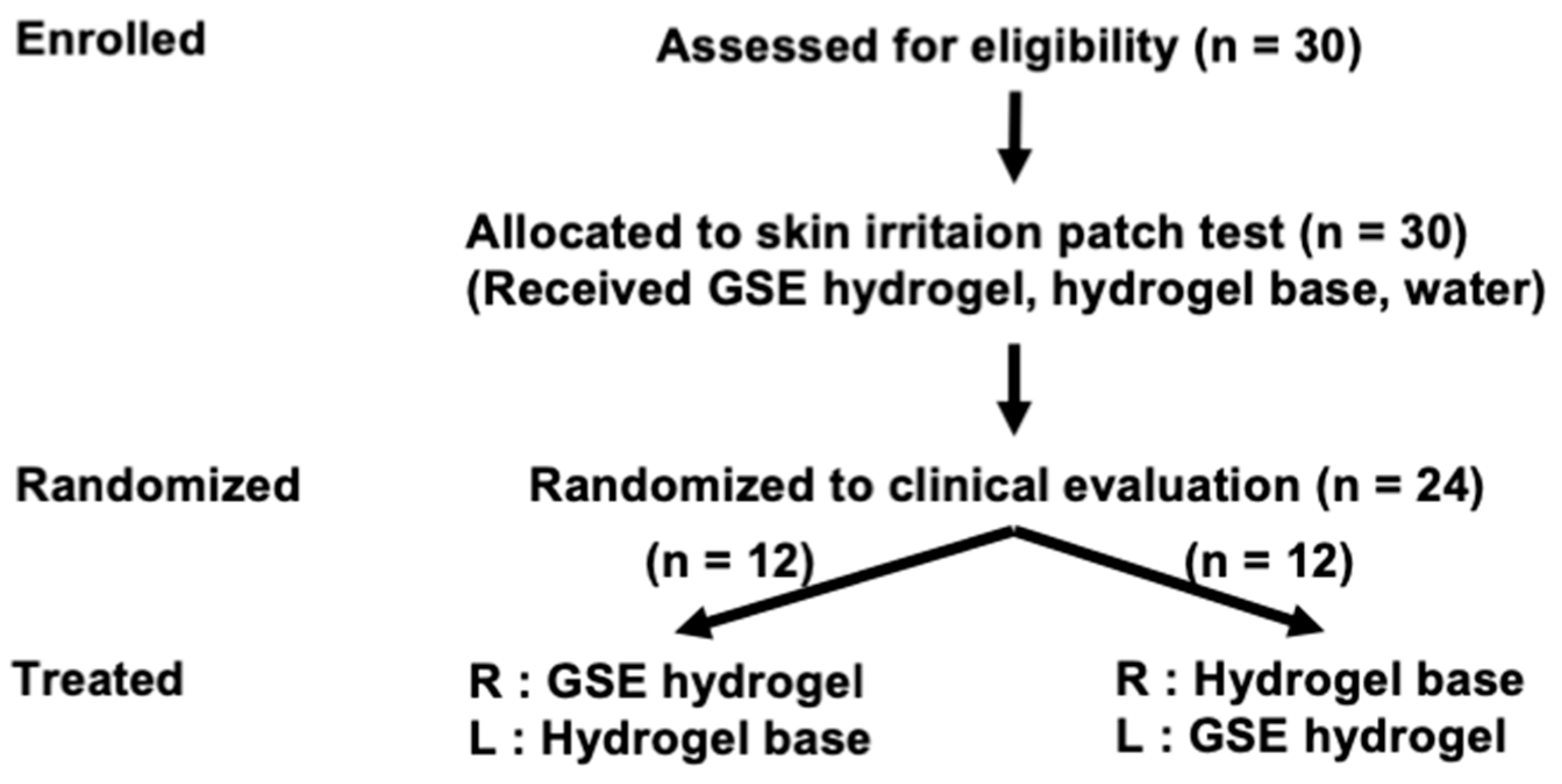
2.7. Approval of the Clinical Trial Protocol
2.8. Recruitment of Healthy Volunteers
2.9. Skin Irritation Evaluation
2.10. Clinical Efficacy Evaluation
- Md0: the melanin content on the baseline (D0);
- Mdm: the melanin content on D14, D28, D42, and D56 (measuring days).
2.11. Statistical Analysis
3. Results
3.1. Plant Sample Preparation, Extraction, and Quality Control of G. speciosum
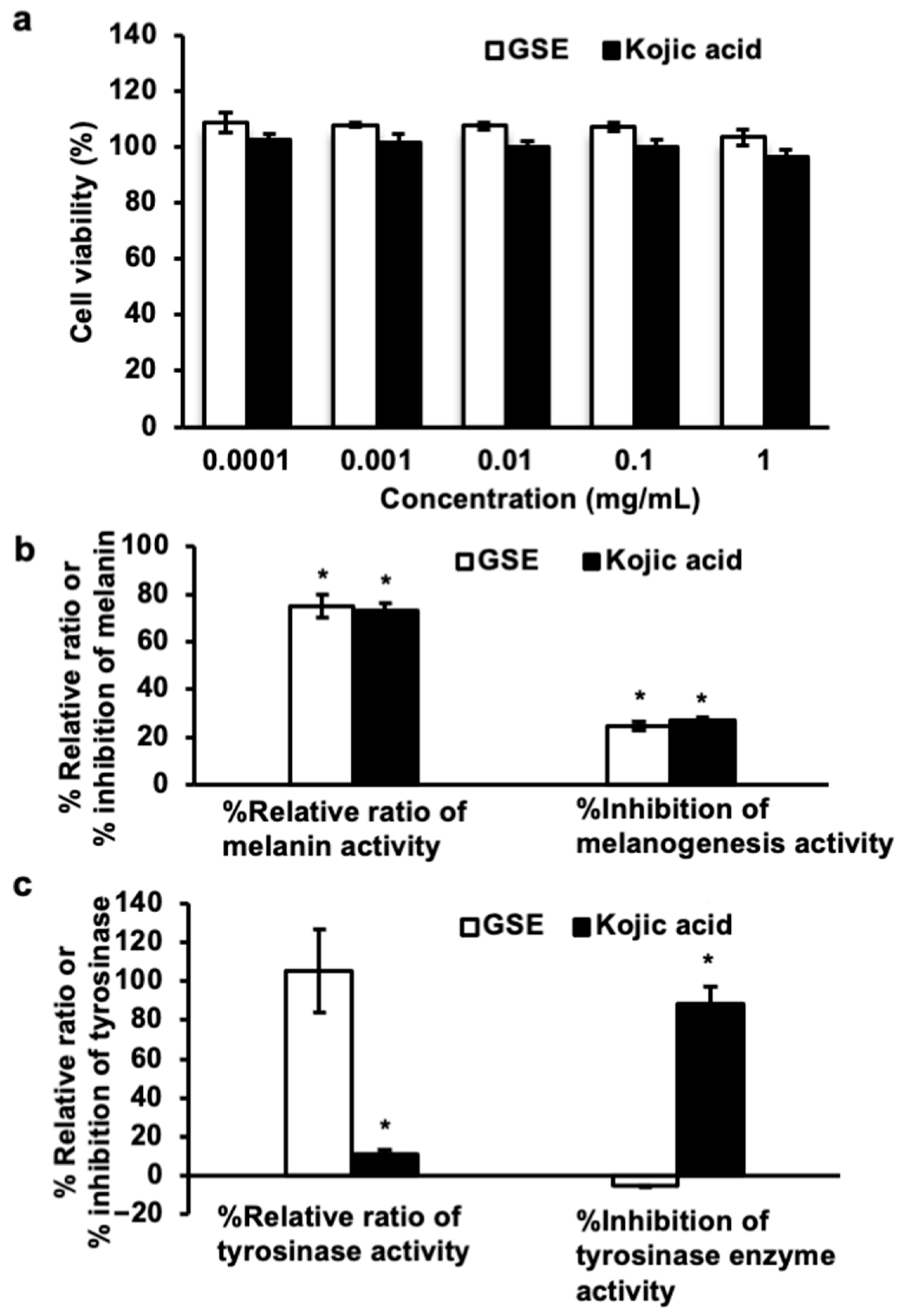
3.2. Effect of GSE on In Vitro Mushroom Tyrosinase Activity
3.3. Effect of GSE on the Viability of B16F10 Cells
3.4. Effect of GSE on Melanin Content in B16F10 Cells
3.5. Effect of GSE on Tyrosinase Enzyme Activity in B16F10 Cells
3.6. Stability and Physicochemical Characteristics of the GSE
3.7. The Formulation Development and Physical and Chemical Stability of GSE-Containing Hydrogels
3.8. Skin Irritation Testing
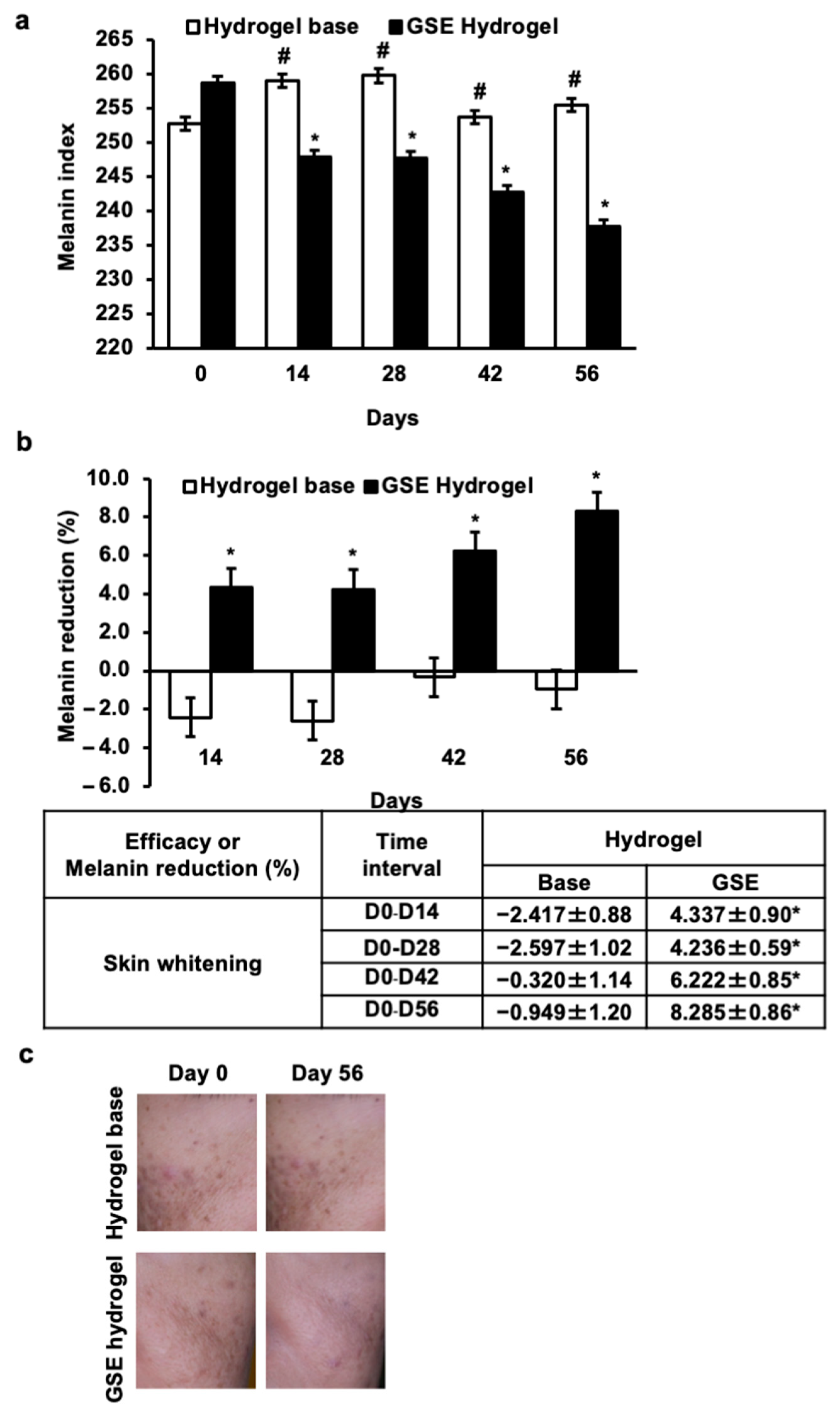
3.9. Skin Whitening Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumari, S.; Tien Guan Thng, S.; Kumar Verma, N.; Gautam, H.K. Melanogenesis Inhibitors. Acta Derm. Venereol. 2018, 98, 924–931. [Google Scholar] [CrossRef]
- D’Mello, S.A.N.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. Available online: https://www.mdpi.com/1422-0067/17/7/1144 (accessed on 1 November 2022). [CrossRef]
- Solano, F. Photoprotection and Skin Pigmentation: Melanin-Related Molecules and Some Other New Agents Obtained from Natural Sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef]
- Hu, Y.; Zeng, H.; Huang, J.; Jiang, L.; Chen, J.; Zeng, Q. Traditional Asian Herbs in Skin Whitening: The Current Development and Limitations. Front. Pharmacol. 2020, 11, 982. Available online: https://www.frontiersin.org/articles/10.3389/fphar.2020.00982 (accessed on 1 November 2022). [CrossRef]
- Nontachaiyapoom, S.; Sasirat, S.; Manoch, L. Symbiotic seed germination of Grammatophyllum speciosum Blume and Dendrobium draconis Rchb. f., native orchids of Thailand. Sci. Hortic. 2011, 130, 303–308. Available online: http://www.sciencedirect.com/science/article/pii/S030442381100330X (accessed on 1 November 2022). [CrossRef]
- Uawonggul, N.; Chaveerach, A.; Thammasirirak, S.; Arkaravichien, T.; Chuachan, C.; Daduang, S. Screening of plants acting against Heterometrus laoticus scorpion venom activity on fibroblast cell lysis. J. Ethnopharmacol. 2006, 103, 201–207. [Google Scholar] [CrossRef]
- Sahakitpichan, P.; Mahidol, C.; Disadee, W.; Chimnoi, N.; Ruchirawat, S.; Kanchanapoom, T. Glucopyranosyloxybenzyl derivatives of (R)-2-benzylmalic acid and (R)-eucomic acid, and an aromatic glucoside from the pseudobulbs of Grammatophyllum speciosum. Tetrahedron 2013, 69, 1031–1037. Available online: http://www.sciencedirect.com/science/article/pii/S0040402012018005 (accessed on 1 November 2022). [CrossRef]
- Chowjarean, V.; Sucontphunt, A.; Vchirawongkwin, S.; Charoonratana, T.; Songsak, T.; Harikarnpakdee, S.; Tengamnuay, P. Validated RP-HPLC method for quantification of gastrodin in ethanolic extract from the pseudobulbs of Grammatophyllum speciosum blume. Malays. J. Anal. Sci. 2018, 22, 219–226. [Google Scholar]
- Harikarnpakdee, S.; Chowjarean, V. Grammatophyllum speciosum Ethanolic Extract Promotes Wound Healing in Human Primary Fibroblast Cells. Int. J. Cell Biol. 2018, 2018, 7836869. [Google Scholar] [CrossRef]
- Chowjarean, V.; Phiboonchaiyanan, P.P.; Harikarnpakdee, S.; Tengamnuay, P. A natural skin anti-ageing serum containing pseudobulb ethanolic extract of Grammatophyllum speciosum: A randomized double-blind, placebo-controlled trial. Int. J. Cosmet. Sci. 2019, 41, 548–557. [Google Scholar] [CrossRef]
- Chowjarean, V.; Nimmannit, U.; Chaotham, C.; Eaknai, W.; Sucontphunt, A.; Plaimee, P.P.; Tengamnuay, P.; Chanvorachote, P. Grammatophyllum speciosum Extract Potentiates Stemness in Keratinocyte Cells. Chiang Mai J. Sci. 2018, 45, 237–248. [Google Scholar]
- AOAC. Guideline for Dietary Supplements and Botanicals. 2013. Available online: http://www.eoma.aoac.org/app_k.pdf (accessed on 1 November 2022).
- Kanlayavattanakul, M.; Lourith, N.; Chaikul, P. Jasmine rice panicle: A safe and efficient natural ingredient for skin aging treatments. J. Ethnopharmacol. 2016, 193, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Manosroi, A.; Chaikul, P.; Abe, M.; Manosroi, W.; Manosroi, J. Melanogenesis of methyl myristate loaded niosomes in B16F10 melanoma cells. J. Biomed. Nanotechnol. 2013, 9, 626–638. [Google Scholar] [CrossRef]
- Estanqueiro, M.; Conceição, J.; Amaral, M.H.; Santos, D.; Silva, J.B.; Lobo, J.M.S. Characterization and stability studies of emulsion systems containing pumice. Braz. J. Pharm. Sci. 2014, 50, 361–369. [Google Scholar] [CrossRef]
- Ivens, U.; Serup, J.; O’goshi, K. Allergy patch test reading from photographic images: Disagreement on ICDRG grading but agreement on simplified tripartite reading. Skin Res. Technol. 2007, 13, 110–113. [Google Scholar] [CrossRef]
- Whangsomnuek, N.; Mungmai, L.; Mengamphan, K.; Amornlerdpison, D. Efficiency of Skin Whitening Cream Containing Etlingera elatior Flower and Leaf Extracts in Volunteers. Cosmetics 2019, 6, 39. Available online: https://www.mdpi.com/2079-9284/6/3/39 (accessed on 1 November 2022). [CrossRef]
- Zheng, Y.; Lee, E.H.; Lee, S.Y.; Lee, Y.; Shin, K.O.; Park, K.; Kang, I.J. Morus alba L. root decreases melanin synthesis via sphingosine-1-phosphate signaling in B16F10 cells. J. Ethnopharmacol. 2023, 301, 115848. [Google Scholar] [CrossRef]
- Saeedi, M.; Eslamifar, M.; Khezri, K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed. Pharmacother. 2019, 110, 582–593. Available online: https://www.sciencedirect.com/science/article/pii/S0753332218367477 (accessed on 1 November 2022). [CrossRef]
- García-Gavín, J.; González-Vilas, D.; Fernández-Redondo, V.; Toribio, J. Pigmented contact dermatitis due to kojic acid. A paradoxical side effect of a skin lightener. Contact Dermat. 2010, 62, 63–64. [Google Scholar] [CrossRef]
- Matos, P.; Paranhos, A.; Batista, M.T.; Figueirinha, A. Synergistic Effect of DIBOA and Verbascoside from Acanthus mollis Leaf on Tyrosinase Inhibition. Int. J. Mol. Sci. 2022, 23, 13536. [Google Scholar] [CrossRef]
- Briganti, S.; Camera, E.; Picardo, M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment. Cell Res. 2003, 16, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Ebanks, J.P.; Wickett, R.R.; Boissy, R.E. Mechanisms regulating skin pigmentation: The rise and fall of complexion coloration. Int. J. Mol. Sci. 2009, 10, 4066–4087. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Arora, P.; Garg, K.V. Cosmeceuticals for Hyperpigmentation: What is Available? J. Cutan. Aesthetic Surg. 2013, 6, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zou, Y.; Xu, H.; Fan, L.; Guo, H.; Li, X.; Li, G.; Zhang, X.; Dong, M. Gastrodin protect primary cultured rat hippocampal neurons against amyloid-beta peptide-induced neurotoxicity via ERK1/2-Nrf2 pathway. Brain Res. 2012, 1482, 13–21. [Google Scholar] [CrossRef]
- Huang, Q.; Shi, J.; Gao, B.; Zhang, H.Y.; Fan, J.; Li, X.J.; Fan, J.Z.; Han, Y.H.; Zhang, J.K.; Yang, L.; et al. Gastrodin: An ancient Chinese herbal medicine as a source for anti-osteoporosis agents via reducing reactive oxygen species. Bone 2015, 73, 132–144. [Google Scholar] [CrossRef] [PubMed]
| Parameters | GSE | |
|---|---|---|
| Before HC 1 | After HC 1 | |
| Gastrodin content (μg/g) | 21.53 ± 0.55 | 22.06 ± 0.80 |
| Color | Brownish yellow | Brownish yellow |
| pH | 5.3 | 5.3 |
| % (w/w) | |||||
|---|---|---|---|---|---|
| GSE | PEG 400 | HPMC | Microcare PHC® 1 | DI Water | |
| Base | - | 12 | 2.5 | 1 | 84.5 |
| GSE hydrogel | 0.5 | 12 | 2.5 | 1 | 84.0 |
| 0.5% (w/w) GSE Hydrogel | Condition 40 °C 75% RH | ||
|---|---|---|---|
| Gastrodin Content (μg/g) | pH | Viscosity 1 (cps) | |
| 0 months (D0) | 22.86 ± 0.98 | 5.31 | 5537.53 ± 20.49 |
| 3 months (D90) | 22.93 ± 0.53 | 5.35 | 5585.58 ± 30.35 |
| 6 months (D180) | 22.57 ± 0.72 | 5.28 | 5507.18 ± 80.73 |
| Test Substances | ICDRG Value | |||
|---|---|---|---|---|
| First Time | Second Time | |||
| 24 h | 72 h | 24 h | 72 h | |
| Hydrogel base | 0 | 0 | 0 | 0 |
| GSE hydrogel | 0 | 0 | 0 | 0 |
| DI water | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowjarean, V.; Phiboonchaiyanan, P.P.; Harikarnpakdee, S. Skin Brightening Efficacy of Grammatophyllum speciosum: A Prospective, Split-Face, Randomized Placebo-Controlled Study. Sustainability 2022, 14, 16829. https://doi.org/10.3390/su142416829
Chowjarean V, Phiboonchaiyanan PP, Harikarnpakdee S. Skin Brightening Efficacy of Grammatophyllum speciosum: A Prospective, Split-Face, Randomized Placebo-Controlled Study. Sustainability. 2022; 14(24):16829. https://doi.org/10.3390/su142416829
Chicago/Turabian StyleChowjarean, Verisa, Preeyaporn Plaimee Phiboonchaiyanan, and Saraporn Harikarnpakdee. 2022. "Skin Brightening Efficacy of Grammatophyllum speciosum: A Prospective, Split-Face, Randomized Placebo-Controlled Study" Sustainability 14, no. 24: 16829. https://doi.org/10.3390/su142416829
APA StyleChowjarean, V., Phiboonchaiyanan, P. P., & Harikarnpakdee, S. (2022). Skin Brightening Efficacy of Grammatophyllum speciosum: A Prospective, Split-Face, Randomized Placebo-Controlled Study. Sustainability, 14(24), 16829. https://doi.org/10.3390/su142416829








