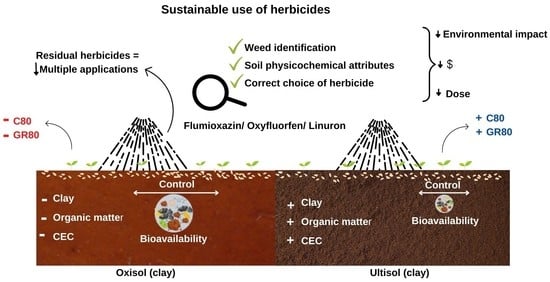
Sustainable Control of Galinsoga parviflora with Oxyfluorfen, Flumioxazin, and Linuron Application in Two Soils Cultivated with Garlic
Abstract
1. Introduction
2. Materials and Methods
3. Results
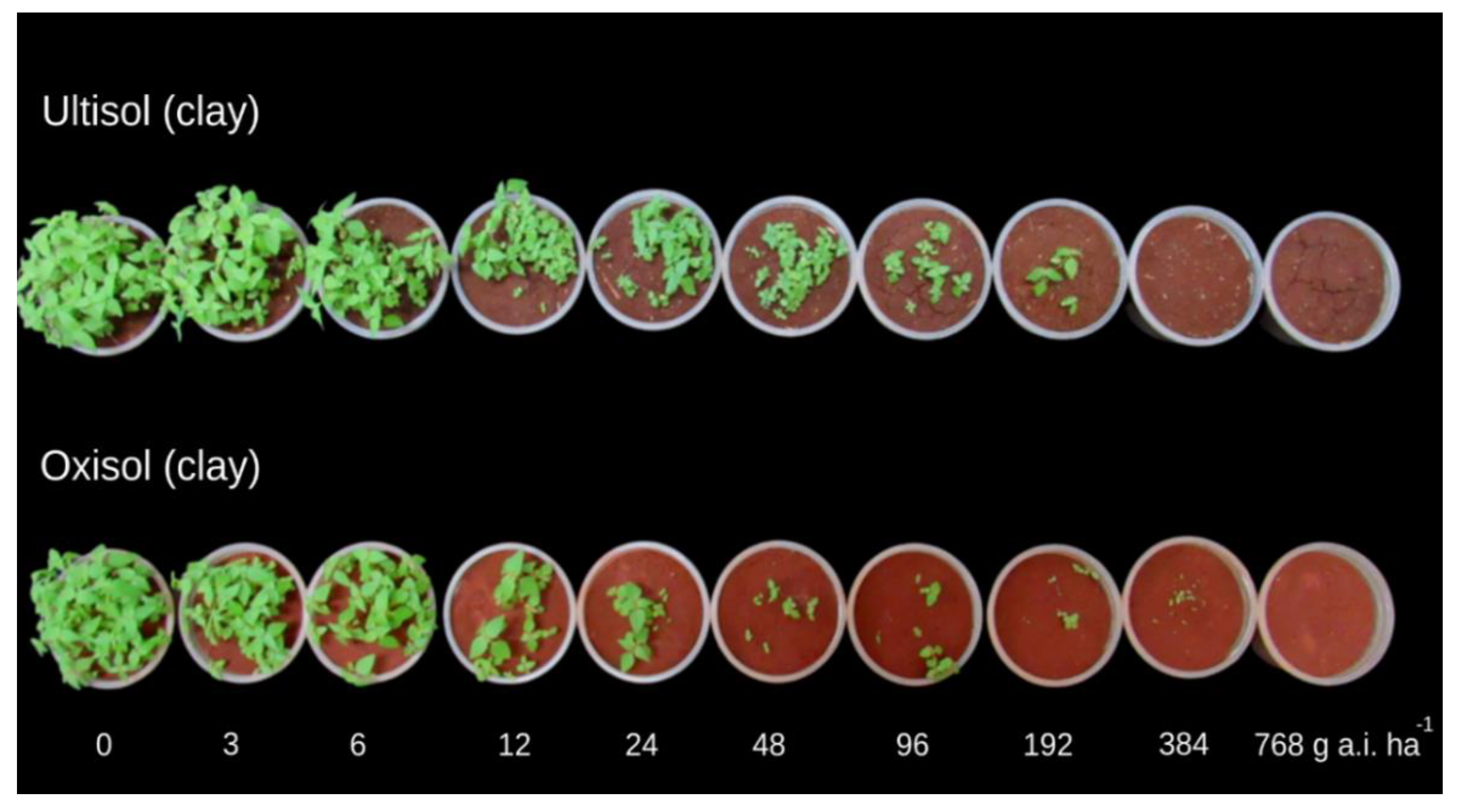
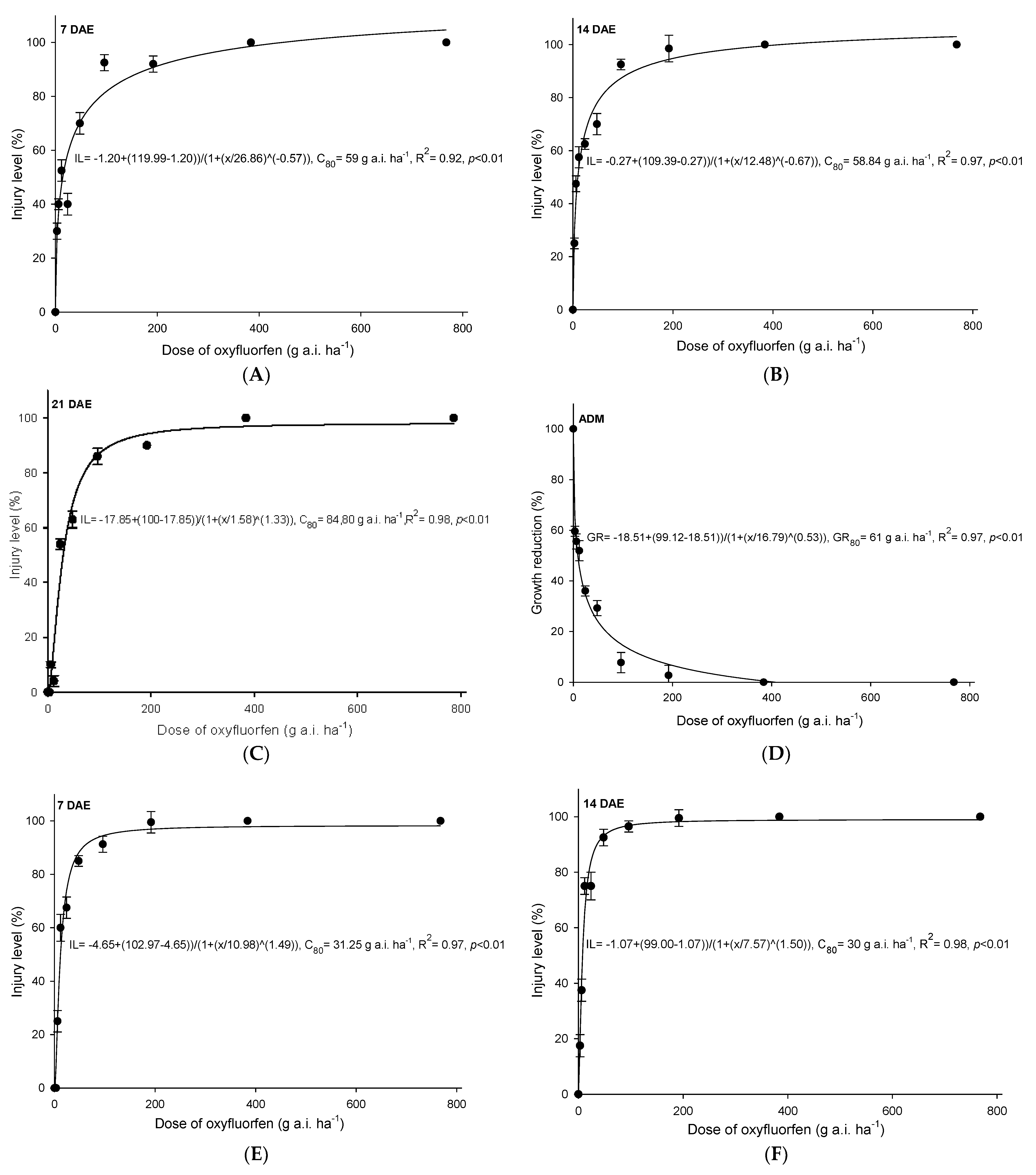
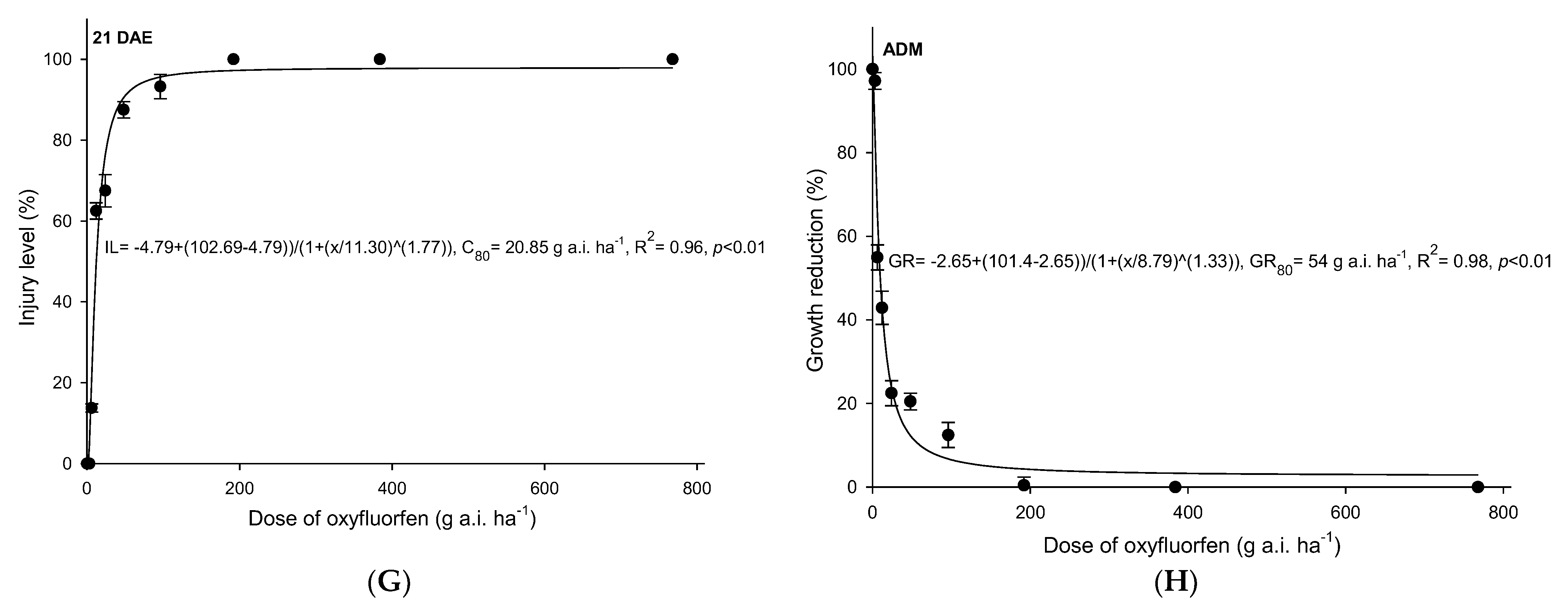
3.1. Oxyfluorfen
3.2. Linuron
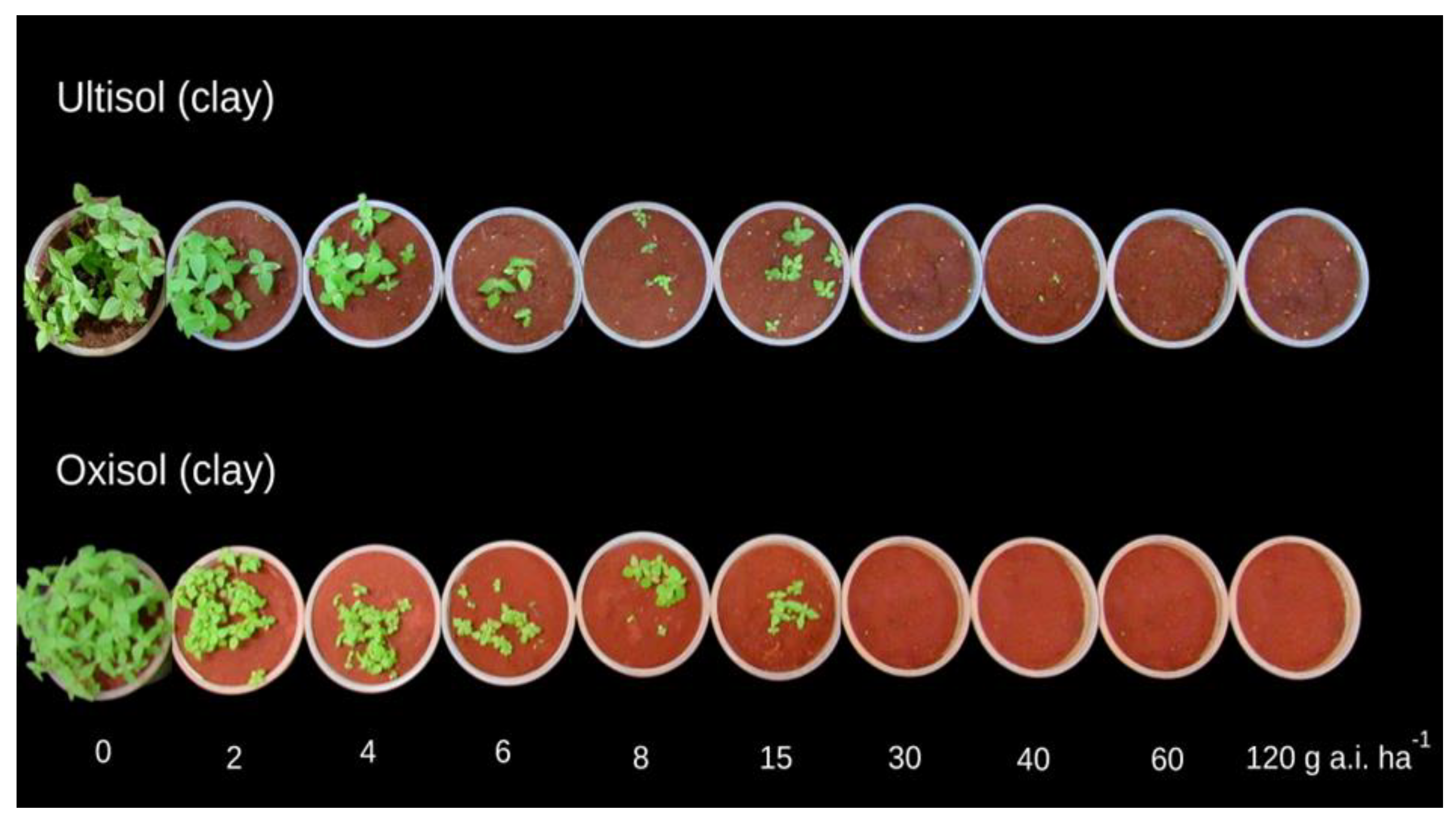
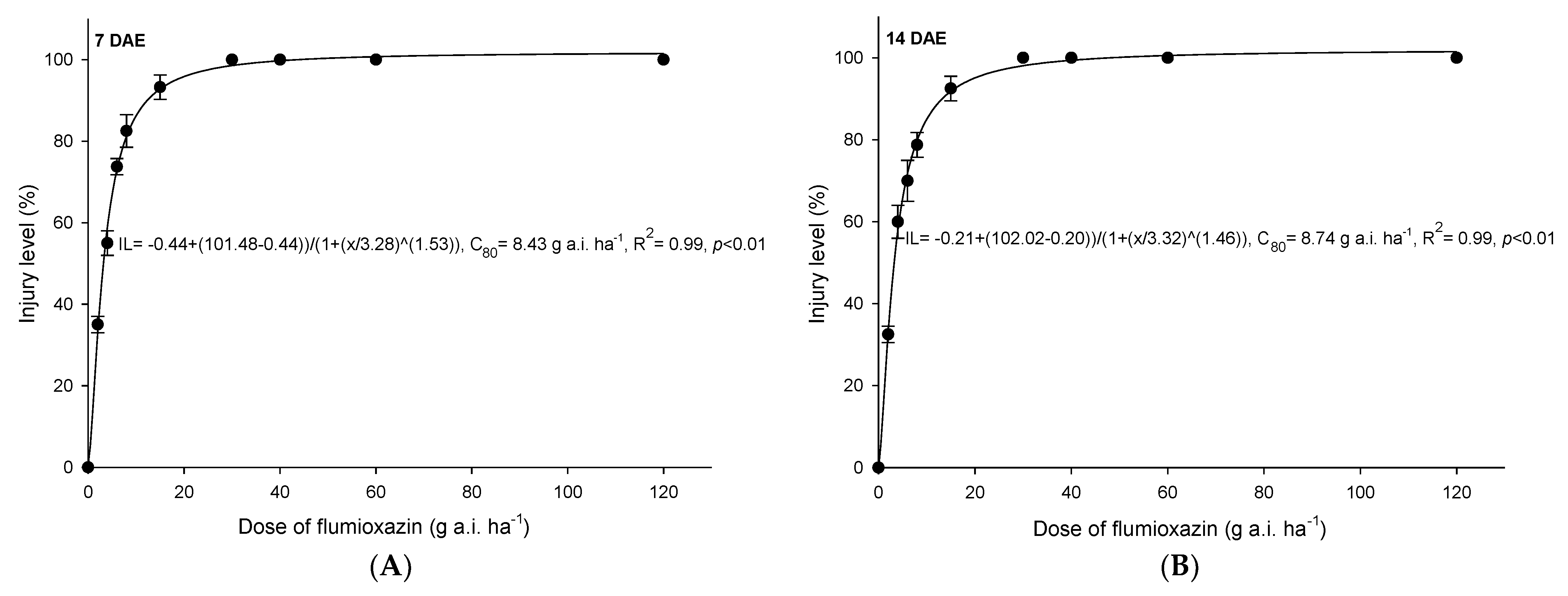
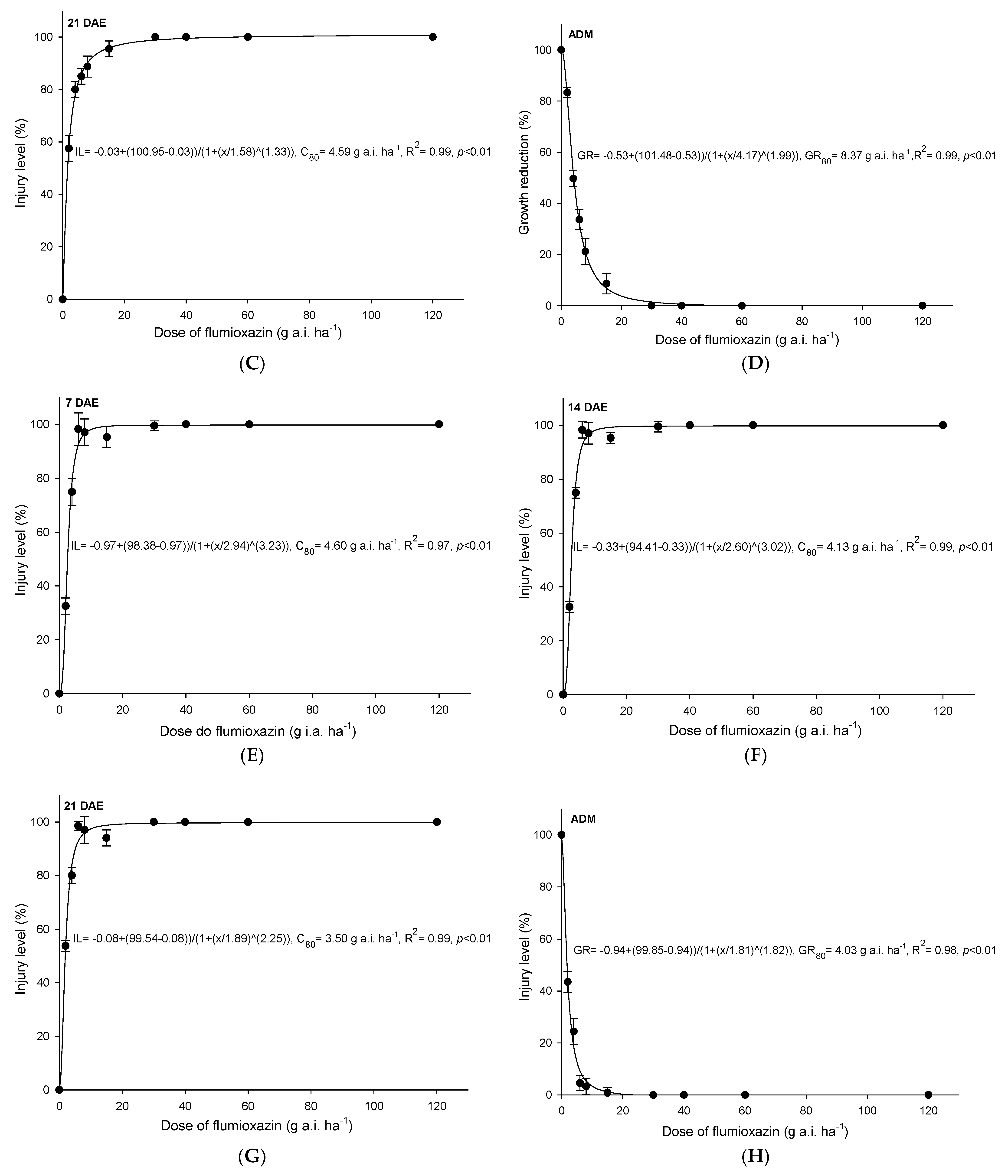
3.3. Flumioxazin
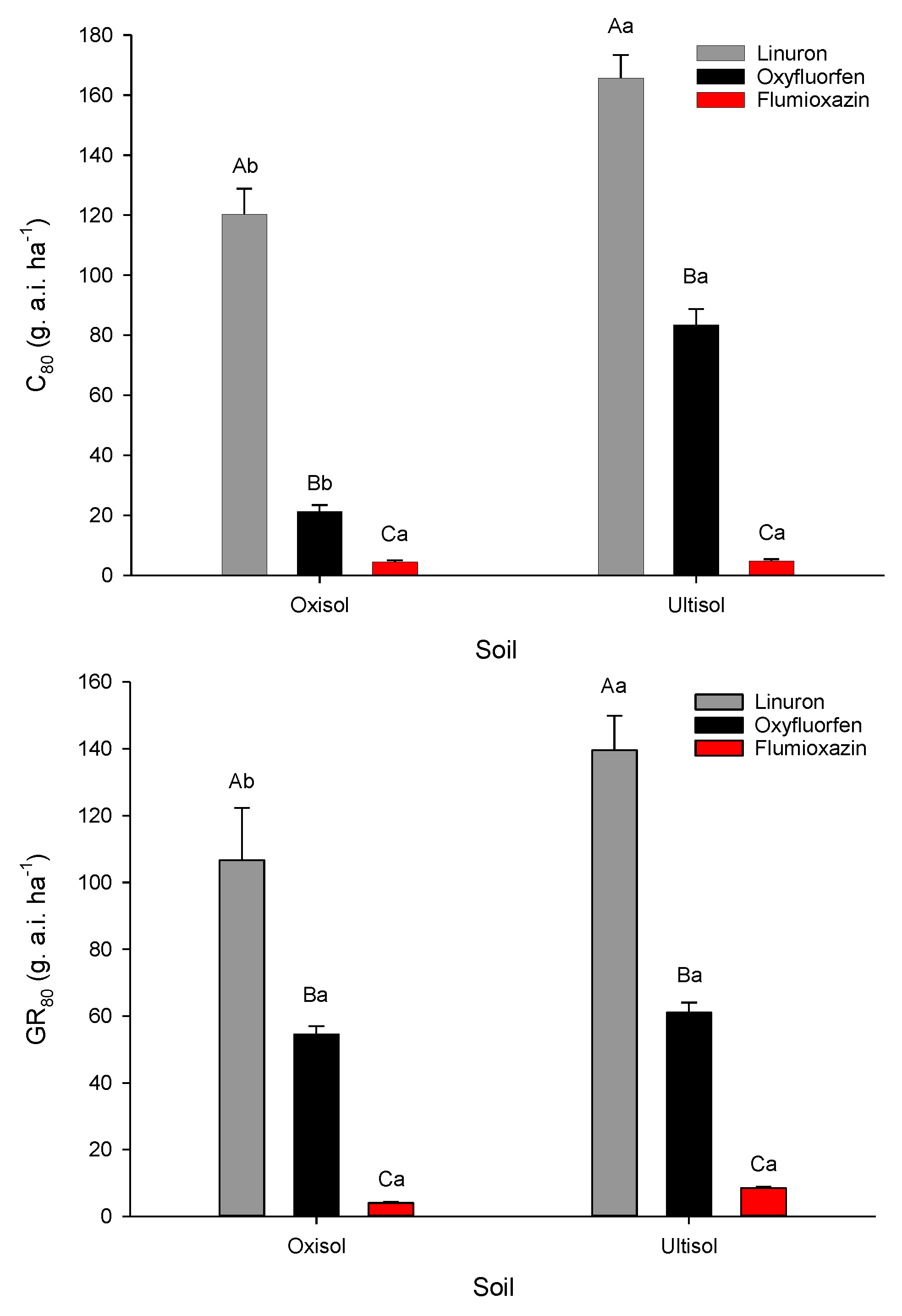
3.4. Comparison of Galinsoga parviflora Control with Linuron, Oxyfluorfen, and Flumiozaxin in the Two Soils
4. Discussion
4.1. Oxyfluofen
4.2. Linuron
4.3. Flumioxazin
4.4. Comparison of Galinsoga parviflora Control with Linuron, Oxyfluorfen, and Flumiozaxin in the Two Soils
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, C.T.; Ferreira, E.A.; Pereira, G.A.M.; Fialho, C.T.; Ribeiro, V.H.V.; do Santos, J.B. Phytosociology in organic system of lettuce production. Hortic. Argent. 2018, 37, 42–60. [Google Scholar]
- Das, M.; Acharya, B.D.; Saquib, M.; Chettri, M.K. Seed germination and seedling growth of some crops and weed seeds under different environmental conditions. J. Res. Weed Sci. 2020, 3, 363–381. [Google Scholar]
- Lorenzi, H.; Souza, H.M. Ornamental Plants of Brazil: Shrubs, Herbs and Vines, 3rd ed.; Plantarum: Nova Odessa, Brazil, 2014; 558p. [Google Scholar]
- Coutinho, P.W.R.; de Moraes Echer, M.; Kestring, K.; da Silva, R.H.; do Nascimento, A.S. Agronomic performance of leeks in different population densities. Res. Soc. Dev. 2021, 10, e13310212258. [Google Scholar] [CrossRef]
- Büll, L.T.; Bôas, R.L.V.; Fernandes, D.M.; Bertani, R.M.A. Fertilização potássica na cultura do alho vernalizado. Sci. Agric. 2001, 58, 157–163. [Google Scholar] [CrossRef]
- Marcuzzo, L.L.; Santos, L. Survival of Pseudomonas marginalis pv. marginalis from garlic in weedy plants. Rev. Agron. Bras. 2021, 5, 498–501. [Google Scholar] [CrossRef]
- Imaizumi, V.M.; Laurindo, L.F.; Manzan, B.; Guiguer, E.L.; Oshiiwa, M.; Otoboni, A.M.M.B.; Barbalho, S.M. Garlic: A systematic review of the effects on cardiovascular diseases. Crit. Rev. Food Sci. Nutr. 2022, 1, 1–23. [Google Scholar] [CrossRef]
- Takagi, H. Garlic Allium sativum L. In Onions and Allied Crops; CRC Press: Boca Raton, FL, USA, 2020; pp. 109–146. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAOSTAT). 2022. Available online: https://www.fao.org/faostat/en/#home (accessed on 3 December 2022).
- Companhia Nacional de Abastecimento (Conab). Custos de produção. Available online: https://www.conab.gov.br/info-agro/custos-de-producao/planilhas-de-custo-de-producao/itemlist/category/789-alho (accessed on 1 December 2022).
- Rahman, U.H.; Khattak, A.M.; Sadiq, M.; Ullah, K.; Javeria, S.; Ullah, I. Influence of different weed management practices on yield of garlic crop. Sarhad J. Agric. 2012, 28, 213–218. [Google Scholar]
- Štefanić, E.; Maletić, Đ.; Zima, D.; Štefanić, I. Economic evaluation of different strategy for weed management in garlic production. Zb. Veleučilišta u Rijeci 2020, 8, 445–454. [Google Scholar] [CrossRef]
- Zhao, N.; Zuo, L.; Li, W.; Guo, W.; Liu, W.; Wang, J. Greenhouse and field evaluation of isoxaflutole for weed control in maize in China. Sci. Rep. 2017, 7, 12690. [Google Scholar] [CrossRef]
- Ministério da Agricultura, Pecuária e Abastecimento. Consulta de Produtos Formulados. Available online: http://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons (accessed on 2 June 2022).
- Siddhu, G.M.; Patil, B.T.; Bachkar, C.B.; Handal, S.B. Weed management in garlic (Allium sativum L.). J. Pharmac. Phytochem. 2018, 7, 1440–1444. [Google Scholar]
- Guerra, N.; Haramoto, R.; Schmitt, J.; Costa, G.D.; Schiessel, J.J.; Neto, A.M.O. Weed control and selectivity herbicides pre emerging in garlic cultivars. Planta Daninha 2020, 38, 1–8. [Google Scholar] [CrossRef]
- Laforest, M.; Simard, M.J.; Meloche, S.; Maheux, L.; Tardif, F.; Page, E. Cross-resistance to photosystem II inhibitors observed in target site–resistant but not in non–target site resistant common ragweed (Ambrosia artemisiifolia). Weed Sci. 2022, 70, 144–150. [Google Scholar] [CrossRef]
- Galvin, L.B.; Becerra-Alvarez, A.; Al-Khatib, K. Assessment of oxyfluorfen-tolerant rice systems and implications for rice-weed management in California. Pest Manag. Sci. 2022, 78, 4905–4912. [Google Scholar] [CrossRef]
- Camacho, M.E.; Gannon, T.W.; Ahmed, K.A.; Mulvaney, M.J.; Heitman, J.L.; Amoozegar, A.; Leon, R.G. Evaluation of imazapic and flumioxazin carryover risk for carinata (Brassica carinata) Establishment. Weed Sci. 2022, 70, 503–513. [Google Scholar] [CrossRef]
- Vitta, J.I.; Faccini, D.E.; Nisensohn, L.A. Control of Amaranthus quitensis in Soybean Crops in Argentina: An Alternative to Reduce Herbicide Use. Crop Prot. 2000, 19, 511–513. [Google Scholar] [CrossRef]
- Walker, S.R.; Medd, R.W.; Robinson, G.R.; Cullis, B.R. Improved management of Avena ludoviciana and Phalaris paradoxa with more densely sown wheat and less herbicide. Weed Res. 2002, 42, 257–270. [Google Scholar] [CrossRef]
- Barros, J.F.C.; Basch, G.; de Carvalho, M. Effect of Reduced Doses of a Post-Emergence Herbicide to Control Grass and Broad-Leaved Weeds in No-till Wheat under Mediterranean Conditions. Crop Prot. 2007, 26, 1538–1545. [Google Scholar] [CrossRef]
- Ernst, M. Critical Time of Palmer Amaranth Removal in Soybean Affected by Residual Herbicides. Crops Soils 2022, 55, 48–51. [Google Scholar] [CrossRef]
- Carvalho, S.J.P.; Lombardi, B.P.; Nicolai, M.; López-Ovejero, R.F.; Christoffoleti, P.J.; Medeiros, D. Dose-response curves for evaluating weed growth control fluxes by imazapic herbicide. Planta Daninha 2005, 23, 535–542. [Google Scholar] [CrossRef][Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 8 June 2022).
- EFSA—European Food Security Authority. Conclusion on the Peer Review of the Pesticide Risk Assessment of the Active Substance Oxyfluorfen. EFSA J. 2010, 8, 1–78. [Google Scholar] [CrossRef]
- Mantzos, N.; Karakitsou, A.; Hela, D.; Patakioutas, G.; Leneti, E.; Konstantinou, I. Persistence of oxyfluorfen in soil, runoff water, sediment and plants of a sunflower cultivation. Sci. Total Environ. 2014, 472, 767–777. [Google Scholar] [CrossRef]
- Netto, A.G.; Presoto, J.C.; Resende, L.S.; Malardo, M.R.; de Fátima Andrade, J.; Nicolai, M.; Carvalho, S.J.P.; Rodrigues, M.R.; Marçal, M.B.T. Effectiveness and selectivity of herbicides applied under pre-emergence conditions in weed management for coffee crops. Coffee Sci. 2021, 16, e161963. [Google Scholar] [CrossRef]
- Mendes, K.F.; Inoue, M.H.; Tornisielo, V.L. Herbicidas No Ambiente: Comportamento e Destino, 1st ed.; Editora UFV: Viçosa, Brazil, 2022; 304p. [Google Scholar]
- Oliveira, R.S., Jr.; Ferreira, L.R.; Silva, J.F. Tolerância do alho (Allium sativum cv. Gigante roxão) ao oxyfluorfen. Rev. Ceres. 1994, 41, 81–87. [Google Scholar]
- PPDB—Pesticide Properties Database. Footprint: Creating Tools for Pesticide Risk Assessment and Management in Europe. Developed by the Agriculture & Environment Research Unit (AERU), University of Hertfordshire, Funded by UK National Sources and the EU-funded FOOTPRINT Project (FP6-SSP-022704). Available online: http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/1663.htm#none (accessed on 26 June 2022).
- Dorado, J.; Almendros, G. Organo-mineral interactions involved in herbicide sorption on soil amended with peats of different maturity degree. Agronomy 2021, 11, 869. [Google Scholar] [CrossRef]
- Vouzounis, N.A.; Americanos, P.G. Residual Activity of Linuron and Pendimethalin Determined by Bioassays in Field Trials; Ministry of Agriculture, Natural Resources and Residual Activity of Linuron and Pendimethalin: Nicosia, Cyprus, 1995. [Google Scholar]
- Dos Santos, H.G.; Jacomine, P.T.; Dos Anjos, L.H.C.; De Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; De Almeida, J.A.; de Araujo Filho, J.C.; De Oliveira, J.B.; Cunha, T.J.F. Brazilian Soil Classification System, 5th ed.; Embrapa: Brasília, Brazil, 2018; 355p. [Google Scholar]
- Barbosa, A.R. Prospecting for Post-Emergence Herbicides in Garlic Crop. Master’s Dissertation, Federal University of Viçosa, Rio Paranaíba, Brazil, 2021; 32p. [Google Scholar]
- Glaspie, C.F.; Jones, E.A.; Penner, D.; Pawlak, J.A.; Everman, W.J. Effect of clay, soil organic matter, and soil pH on initial and residual weed control with flumioxazin. Agronomy 2021, 11, 1326. [Google Scholar] [CrossRef]
- Melo, C.A.D.; Reis, M.R.D.; Barbosa, A.R.; Dias, R.D.C.; Silva, G.S.D. Effectiveness of reduced doses of flumioxazin herbicide at weed control in direct sow onions. Rev. Colomb. Cienc. Hortíc. 2019, 13, 71–80. [Google Scholar] [CrossRef]
- Rodrigues, B.N.; Almeida, F.S. Herbicide Guide, 7th ed.; IAPAR: Londrina, Brazil, 2018; 764p. [Google Scholar]
- Dan, Y.; Ji, M.; Tao, S.; Luo, G.; Shen, Z.; Zhang, Y.; Sang, W. Impact of rice straw biochar addition on the sorption and leaching of phenylurea herbicides in saturated sand column. Sci. Total Environ. 2021, 769, 144536. [Google Scholar] [CrossRef]
- Yamashita, O.M.; Tieppo, R.C.; Carvalho, R.V.; de Carvalho, M.A.C.; Dallacort, R.; Peres, W.M.; David, G.Q.; Rabelo, H.D.O.; da Rocha, A.M.; de Oliveira, L.C.A. Mobility of flumioxazin herbicide in a Dystrophic Red Yellow Latosol at Brazilian Southern Amazon. Aust. J. Crop Sci. 2020, 14, 775–781. [Google Scholar] [CrossRef]
- Correia, N.M.; Carvalho, A.D.F. Selectivity of herbicides to sweet potato. Weed Contr. J. 2021, 20, e202100740. [Google Scholar] [CrossRef]
- Hess, F.D. Herbicide absorption and translocation and their relationship to plant tolerances and susceptibility. In Weed Physiology; Duke, S.O., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 192–214. [Google Scholar]
- Smith, T.P.; Marble, C.; Steed, S.; Boyd, N. Biology and Management of Galinsoga (Galinsoga quadriradiata) in Ornamental Crop Production: ENH1329/EP593, 10/2020. EDIS 2020, 1, 1–5. [Google Scholar]
- Reis, M.R.; Melo, C.A.D.; Raposo, T.P.; Aquino, R.F.B.A.; Aquino, L.A. Selectivity of herbicides to cabbage (Brassica oleracea var. capitata). Planta Daninha 2017, 35, e017163938. [Google Scholar] [CrossRef]
- Auskalnis, A.; Kadzys, A. Effect of timing and dosage in herbicide application on weed biomass in spring wheat. Agron. Resear. 2006, 4, 133–136. [Google Scholar]



| Soil Classification 1 (Textural Class) | OM | pH | K+ | Ca2+ | Mg2+ | Al3+ | H + Al | CEC | Sand | Silt | Clay | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | (H2O) | mg dm−3 | cmolc dm−3 | % | ||||||||
| Ultisol (clay) | 4.61 | 6.20 | 9.3 | 203 | 6.70 | 2.98 | 0.00 | 14.10 | 3.4 | 17.3 | 79.3 | |
| Oxisol (clay) | 3.76 | 6.71 | 217.6 | 68.0 | 5.16 | 2.46 | 0.00 | 8.49 | 9.0 | 38.5 | 52.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paula, D.F.d.; Silva, E.M.G.d.; Silva, L.B.X.d.; Lima, A.d.C.; Billu, P.B.; Reis, M.R.d.; Mendes, K.F. Sustainable Control of Galinsoga parviflora with Oxyfluorfen, Flumioxazin, and Linuron Application in Two Soils Cultivated with Garlic. Sustainability 2022, 14, 16637. https://doi.org/10.3390/su142416637
Paula DFd, Silva EMGd, Silva LBXd, Lima AdC, Billu PB, Reis MRd, Mendes KF. Sustainable Control of Galinsoga parviflora with Oxyfluorfen, Flumioxazin, and Linuron Application in Two Soils Cultivated with Garlic. Sustainability. 2022; 14(24):16637. https://doi.org/10.3390/su142416637
Chicago/Turabian StylePaula, Dilma F. de, Elisa Maria G. da Silva, Laryssa B. X. da Silva, Alessandro da C. Lima, Patrick B. Billu, Marcelo R. dos Reis, and Kassio F. Mendes. 2022. "Sustainable Control of Galinsoga parviflora with Oxyfluorfen, Flumioxazin, and Linuron Application in Two Soils Cultivated with Garlic" Sustainability 14, no. 24: 16637. https://doi.org/10.3390/su142416637
APA StylePaula, D. F. d., Silva, E. M. G. d., Silva, L. B. X. d., Lima, A. d. C., Billu, P. B., Reis, M. R. d., & Mendes, K. F. (2022). Sustainable Control of Galinsoga parviflora with Oxyfluorfen, Flumioxazin, and Linuron Application in Two Soils Cultivated with Garlic. Sustainability, 14(24), 16637. https://doi.org/10.3390/su142416637