Morphological Characterization of Some Local Varieties of Fig (Ficus carica L.) Cultivated in Southern Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Leaf Sampling and Image Acquisition
2.3. Image Analysis
2.4. Leaf Size Measurements
2.5. Morphometric Data Analysis
3. Results
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stover, E.; Aradhya, M.; Fergunson, L.; Crisosto, C.H. The fig: Overview of an ancient fruit. Hortscience 2007, 42, 1083–1087. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Data Archives. FAOSTAT. 2022. Available online: https://www.fao.org/faostat/en/#data (accessed on 13 October 2022).
- Istituto Italiano di Statistica. StatBase, Coltivazioni. 2022. Available online: http://dati.istat.it/viewhtml.aspx?il=blank&vh=0000&vf=0&vcq=1100&graph=0&view-metadata=1&lang=it&QueryId=33654&metadata=DCSP_COLTIVAZIONI# (accessed on 13 October 2022).
- Flaishman, M.A.; Rodov, V.; Stover, E. Fig: Botany, horticulture, and breeding. Hortic. Rev. 2008, 34, 113–197. [Google Scholar]
- Richardson, A.D.; Keenana, T.F.; Migliavacca, M.; Ryua, Y.; Sonnentaga, O.; Toomey, M. Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agric. For. Meteorol. 2013, 169, 156–173. [Google Scholar] [CrossRef]
- Condit, I.J. Fig varieties: A monograph. Hilgardia 1955, 23, 323–538. [Google Scholar] [CrossRef]
- Condit, I.J. Fig characteristics useful in the identification of varieties. Hilgardia 1941, 14, 1–69. [Google Scholar] [CrossRef]
- Descriptors for Fig. International Plant Genetic Resources Institute, Rome, Italy, and International Centre for Advanced Mediterranean Agronomic Studies; IPGRI and CIHEAM: Paris, France, 2003.
- Saddoud, O.; Baraket, G.; Chatti, K.; Trifi, M.; Marrakchi, M.; Salhi-Hannachi, A.; Mars, M. Morphological variability of fig (Ficus carica L.) cultivars. Int. J. Fruit Sci. 2008, 8, 35–51. [Google Scholar] [CrossRef]
- Giraldo, E.; Lopez Corrales, M.; Hormaza, J.I. Selection of the most discriminating morphological qualitative variables for characterization of fig germplasm. J. Am. Soc. Hortic. Sci. 2010, 135, 240–249. [Google Scholar] [CrossRef]
- Gaaliche, B.; Saddoud, O.; Mars, M. Morphological and pomological diversity of Fig (Ficus carica L.) cultivars in Northwest of Tunisia. International Scholarly Research Network. Agronomy 2012, 2012, 326461. [Google Scholar]
- Khadivi-Khub, A.; Anjam, K. Characterization and evaluation of male fig (caprifig) accessions in Iran. Plant Syst. Evol. 2014, 300, 2177–2189. [Google Scholar] [CrossRef]
- Khadivi, A.; Mirheidari, F. Selection of the promising fig (Ficus carica L.) accessions using fruit-related characters. Food Sci. Nutr. 2021, 10, 2911–2921. [Google Scholar] [CrossRef]
- Mir, M.M.; Kumar, A.; Iqbal, U.; Mir, S.A.; Rehman, M.U.; Banday, S.A.; Rather, G.H.; Fayaz, S. Characterization of fig (Ficus carica L.) germplasm in central Kashmir of north western Himalayan region. Indian J. Plant Genet. Resour. 2018, 31, 57–63. [Google Scholar] [CrossRef]
- Hssaini, L.; Hanine, H.; Razouk, R.; Ennahli, S.; Mekaoui, A.; Ejjilani, A.; Charafi, J. Assessment of genetic diversity in Moroccan fig (Ficus carica L.) collection by combining morphological and physicochemical descriptors. Genet. Resour Crop Evol. 2019, 67, 457–474. [Google Scholar] [CrossRef]
- Baziar, G.; Jafari, M.; Noori, M.S.S.; Samarfard, S. Persian Fig Cultivars by Morphological Traits and RAPD Markers. Hortscience 2018, 53, 613–619. [Google Scholar] [CrossRef]
- Chessa, I.; Nieddu, G. Analysis of diversity in the fruit tree genetic resources from a Mediterranean island. Genet. Resour. Crop Evol. 2005, 52, 267–276. [Google Scholar] [CrossRef]
- Aradhya, M.K.; Stover, E.; Velasco, D.; Koehmstedt, A. Genetic structure and differentiation in cultivated fig (Ficus carica L.). Genetica 2010, 138, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Achtak, H.; Oukabli, A.; Ater, M.; Santoni, S.; Kjellberg, F.; Khadari, B. Microsatellite markers as reliable tools for fig cultivar identification. J. Am. Soc. Hortic. Sci. 2009, 134, 624–631. [Google Scholar] [CrossRef]
- Garcia-Muñoz, S.; Muñoz-Organero, G.; de Andrés, M.T.; Cabello, F. Ampelography—An old technique with future uses: The case of minor varieties of Vitis vinifera L. from the Balearic Islands. OENO One 2011, 45, 125–137. [Google Scholar] [CrossRef]
- Cope, J.S.; Corney, D.; Clark, J.Y.; Remagnino, P.; Wilkin, P. Plant species identification using digital morphometrics: A review. Expert Syst. Appl. 2012, 39, 7562–7573. [Google Scholar] [CrossRef]
- Rohlf, F.J.; Marcus, L.F. A revolutions in morphometrics. Tree 1993, 8, 129–132. [Google Scholar]
- Lo Bianco, R.; Mirabella, F. Use of leaf and fruit morphometric analysis to identify and classify white mulberry (Morus alba L.) genotypes. Agriculture 2018, 8, 157. [Google Scholar] [CrossRef]
- Klein, L.L.; Caito, M.; Chapnick, C.; Kitchen, C.; O’Hanlon, R.; Chitwood, D.H.; Miller, A.J. Digital morphometrics of two north american arapevines (Vitis: Vitaceae) quantifies leaf variation between species, within species, and among individuals. Front. Plant Sci. 2017, 8, 373. [Google Scholar] [CrossRef] [PubMed]
- Migicovsky, Z.; Li, M.; Chitwood, D.H.; Myles, S. Morphometrics Reveals Complex and Heritable Apple Leaf Shapes. Front. Plant Sci. 2018, 8, 2185. [Google Scholar] [CrossRef] [PubMed]
- Podgornik, M.; Vuk, I.; Vrhovnik, I.; Bandelj, D. A survey and morphological evaluation of fig (Ficus carica L.) genetic resources from Slovenia. Sci. Hortic. 2010, 125, 380–389. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Koppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, 1997–2018. Available online: https://imagej.nih.gov/ij/ (accessed on 13 October 2022).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Oso, O.A.; Jayeola, A.A. Digital Morphometrics: Application of Morpholeaf in shape visualization and species delimitation, using Cucurbitaceae leaves as a model. Appl. Plant Sci. 2021, 9, e11448. [Google Scholar] [CrossRef]
- Shi, P.; Niinemets, Ü.; Hui, C.; Niklas, K.J.; Yu, X.; Hölscher, D. Leaf bilateral symmetry and the scaling of the perimeter vs. the surface area in 15 vine species. Forests 2020, 11, 246. [Google Scholar] [CrossRef]
- Viscosi, V. Geometric morphometrics and leaf phenotypic plasticity: Assessing fluctuating asymmetry and allometry in European white oaks (Quercus). Bot. J. Linn. Soc. 2015, 179, 335–348. [Google Scholar] [CrossRef]
- Scoffoni, C.; Rawls, M.; McKown, A.; Cochard, H.; Sack, L. Decline of leaf hydraulic conductance with dehydration: Relationship to leaf size and venation architecture. Plant Physiol. 2011, 156, 832–843. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Paleontol. Electron. 2001, 4, 9. [Google Scholar]
- Iezzoni, A.F.; Pritts, N.P. Applications of principal component analysis to Horticultural Research. Hortscience 1991, 26, 334–338. [Google Scholar] [CrossRef]
- Fleming, A.J. The control of leaf development. New Phytol. 2005, 166, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Sack, L.; Scoffoni, C. Leaf venation: Structure, function, development, evolution, ecology and applications in the past, present and future. New Phytol. 2013, 198, 983–1000. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.K.; Vogelmann, T.C.; DeLucia, E.H.; Bell, D.T.; Shepherd, K.A. Leaf form and phtosynthesis. BioScience 1997, 47, 785–793. [Google Scholar] [CrossRef]
- Ren, T.; He, N.; Liu, Z.; Li, M.; Zhang, J.; Li, A.; Wei, C.; Lü, X.; Han, X. Environmental filtering rather than phylogeny determines plant leaf size in three floristically distinctive plateaus. Ecol. Indic. 2021, 130, 108049. [Google Scholar] [CrossRef]
- Moles, A.T. Being John Harper: Using evolutionary ideas to improve understanding of global patterns in plant traits. J. Ecol. 2018, 106, 1–18. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef]
- Moles, A.T.; Perkins, S.E.; Laffan, S.W.; Flores-Moreno, H.; Awasthy, M.; Tindall, M.L.; Sack, L.; Pitman, A.; Kattge, J.; Aarssen, L.W.; et al. Which is a better predictor of plant traits: Temperature or precipitation? J. Veg. Sci. 2014, 25, 1167–1180. [Google Scholar] [CrossRef]
- Rodrigues, M.G.F.; dos Santos, T.P.; Ferreira, A.F.A.; Monteiro, L.N.H.; Nakanishi, E.S.S.; Boliani, A.C. Morphological characterization of active germoplasm bank fig tree accessions. Rev. Bras. Frutic. Jaboticabal 2019, 41, e-074. [Google Scholar] [CrossRef]
- Yu, X.; Shi, P.; Hui, C.; Miao, L.; Liu, C.; Zhang, Q.; Feng, C. Effects of salt stress on the leaf shape and scaling of Pyrus betulifolia Bunge. Symmetry 2019, 11, 991. [Google Scholar] [CrossRef]
- Viscosi, V.; Lepais, O.; Gerber, S.; Fortini, P. Leaf morphological analyses in four European oak species (Quercus) and their hybrids: A comparison of traditional and geometric morphometric method. Plant Biosys. 2009, 143, 564–574. [Google Scholar] [CrossRef]
- Sack, L.; Scoffoni, C.; John, G.P.; Poorter, H.; Mason, C.M.; Mendez-Alonzo, R.; Donovan, L.A. How do leaf veins influence the worldwide leaf economic spectrum? Review and synthesis. J. Exp. Bot. 2013, 64, 4053–4080. [Google Scholar] [CrossRef] [PubMed]
- Viala, P.; Vermorel, V. Ampelographié: Traité General de Viticulture; Tome I. Masson and Cie: Paris, France, 1909. [Google Scholar]
- Galet, P. Précis d’Ampélographie Pratique; Impr. P. Déhan: Montpellier, France, 1952. [Google Scholar]
- Chitwood, D.H.; Ranjan, A.; Martinez, C.C.; Headland, L.R.; Thiem, T.; Kumar, R.; Covington, M.F.; Hatcher, T.; Taylor, D.T.; Zimmerman, S.; et al. A modern ampelography: A genetic basis for leaf shape and venation patterning in Grape. Plant Physiol. 2014, 164, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Bookstein, F.L. “size and shape”: A comment on semantic. Syst. Zool. 1989, 38, 173–180. [Google Scholar] [CrossRef]
- Abdelsalam, N.R.; Awad, R.M.; Ali, H.M.; Salem, M.Z.M.; Abdellatif, K.F.; Elshikh, M.S. Morphological, pomological, and specific molecular marker resources for genetic diversity analyses in Fig (Ficus carica L.). Hortscience 2019, 54, 1299–1309. [Google Scholar] [CrossRef]
- Chitwood, D.H.; Rundell, S.M.; Li, D.Y.; Woodford, Q.L.; Yu, T.T.; Lopez, J.R.; Greenblatt, D.; Kang, J.; Londo, J.P. Climate and developmental plasticity: Interannual variability in grapevine leaf morphology. Plant Physiol. 2016, 170, 1480–1491. [Google Scholar] [CrossRef]
- Viscosi, V.; Cardini, A. Leaf Morphology, Taxonomy and Geometric Morphometrics: A Simplified Protocol for Beginners. PLoS ONE 2011, 6, e25630. [Google Scholar] [CrossRef]
- Milijković, D.; Stefanović, M.; Orlović, S.; Neđić, M.S.; Kesić, L.; Stojnić, S. Wild cherry (Prunus avium (L.) leaf shape and size variations in natural populations at different elevations. Alp. Bot. 2019, 129, 163–174. [Google Scholar] [CrossRef]
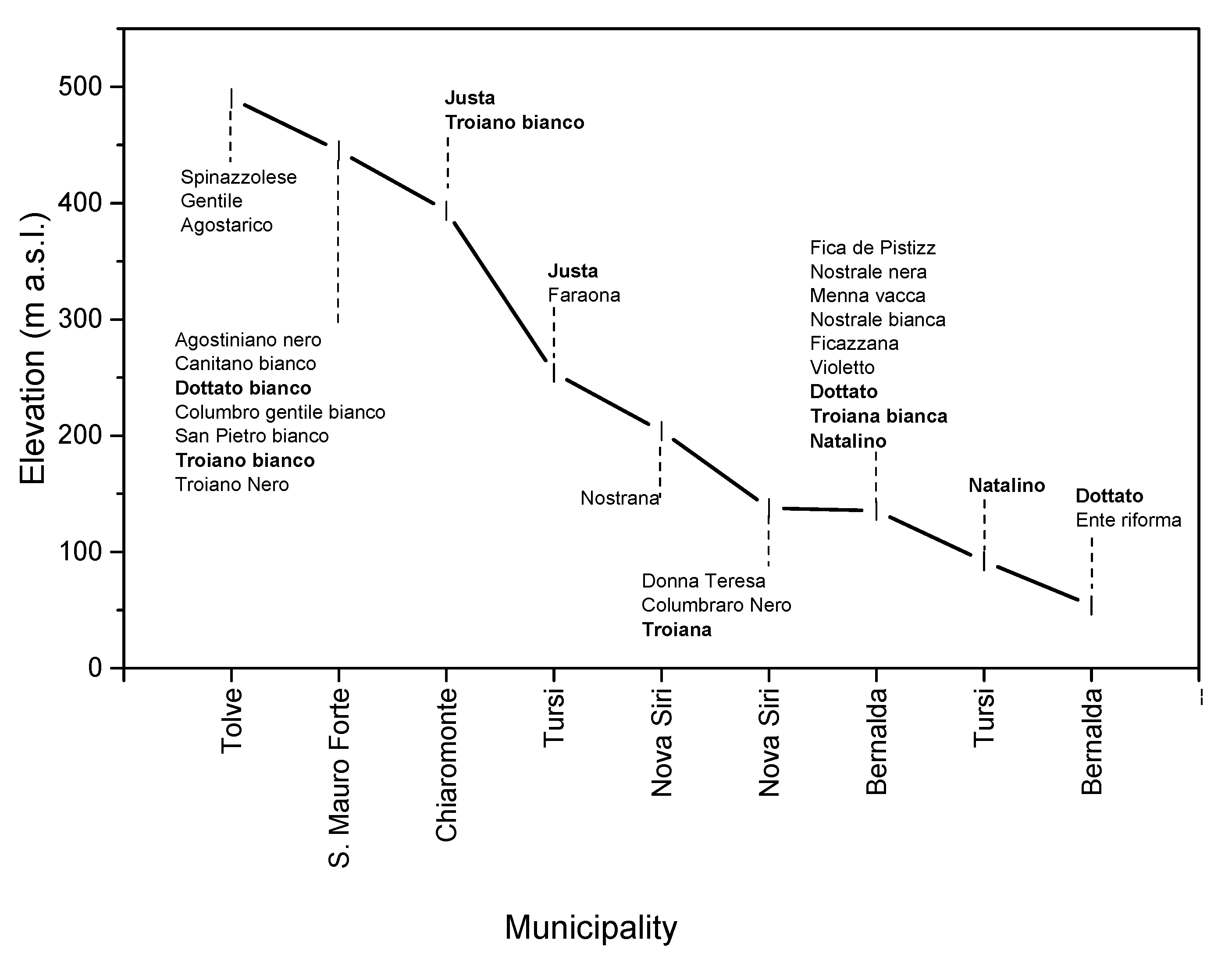

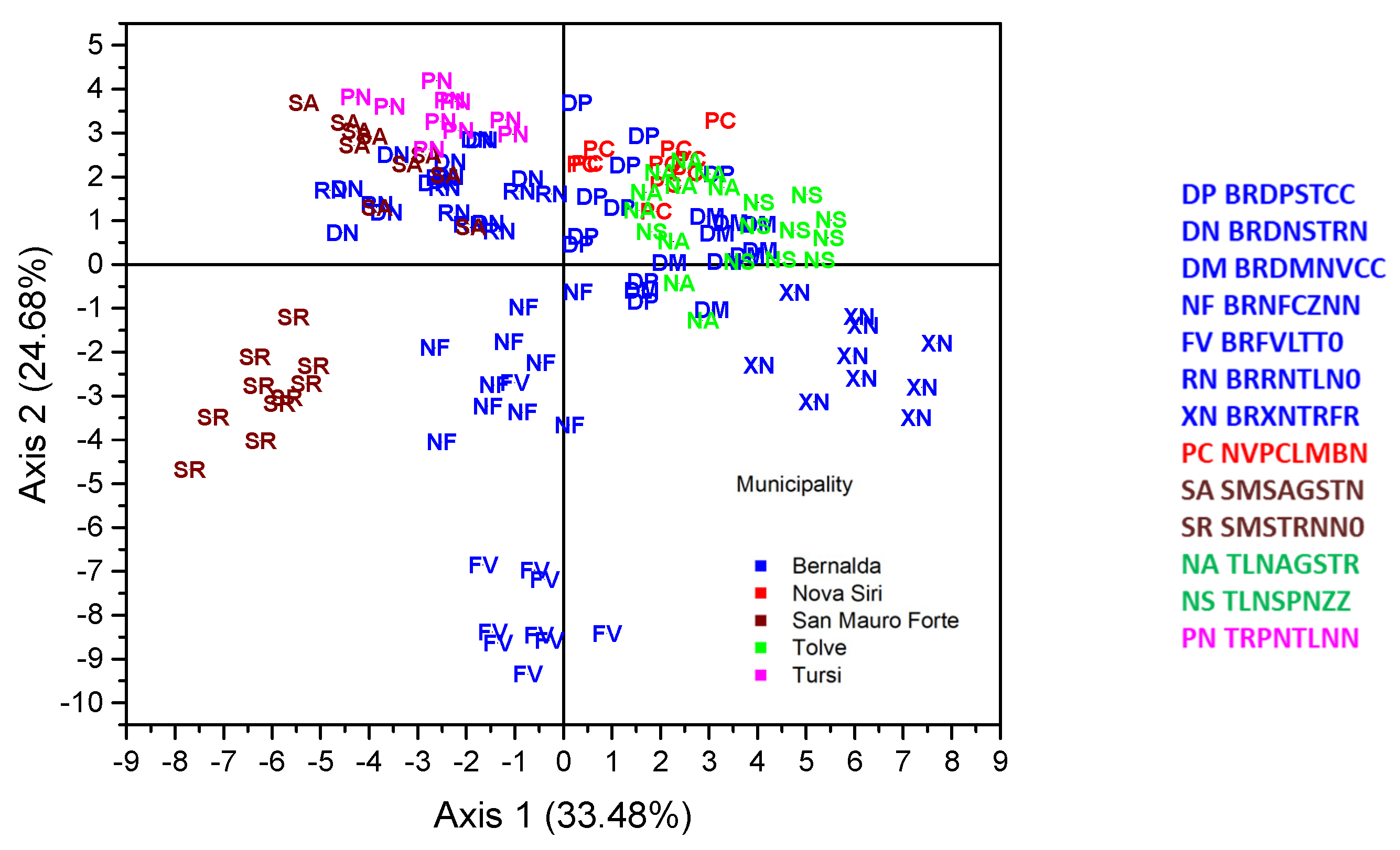
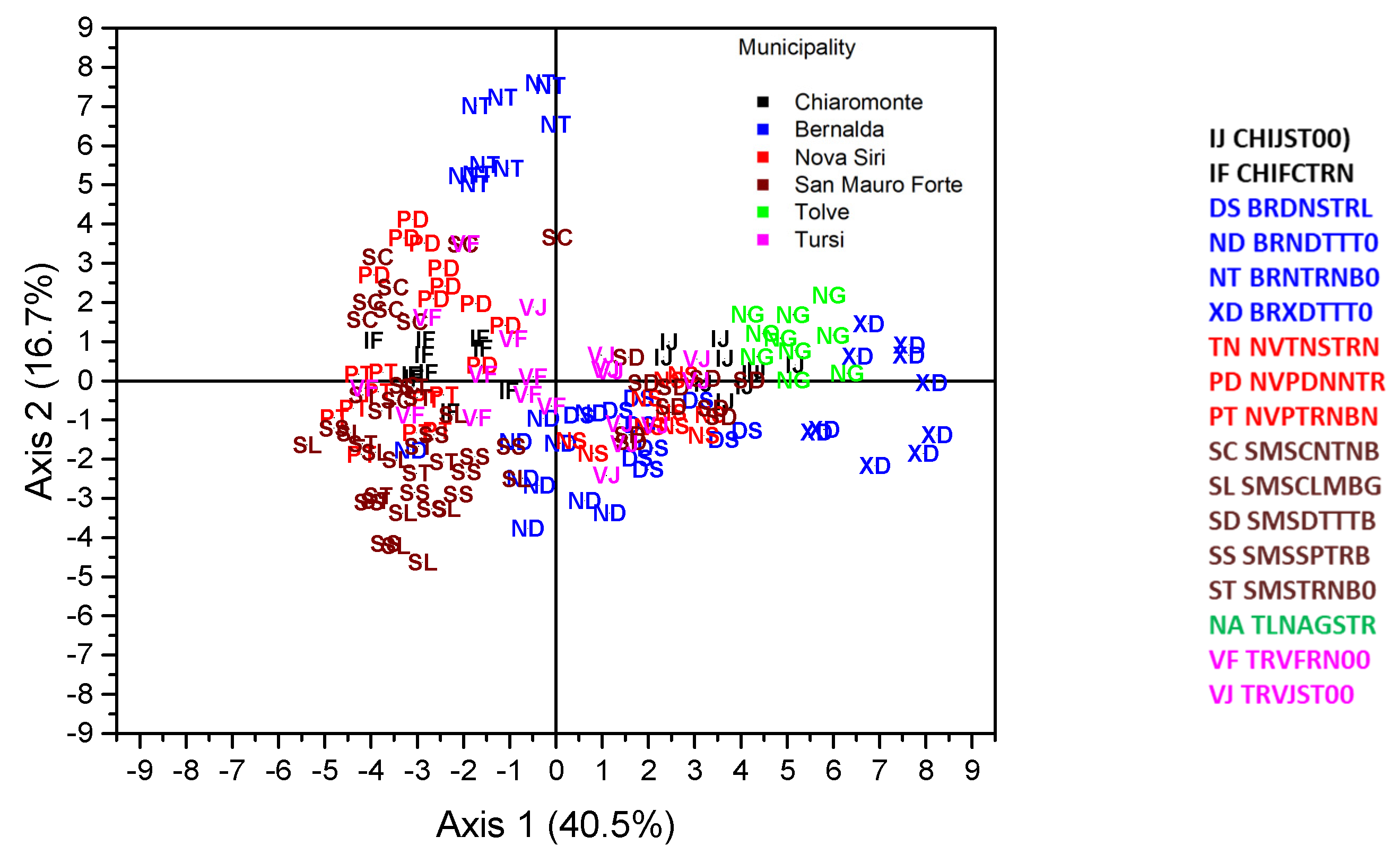
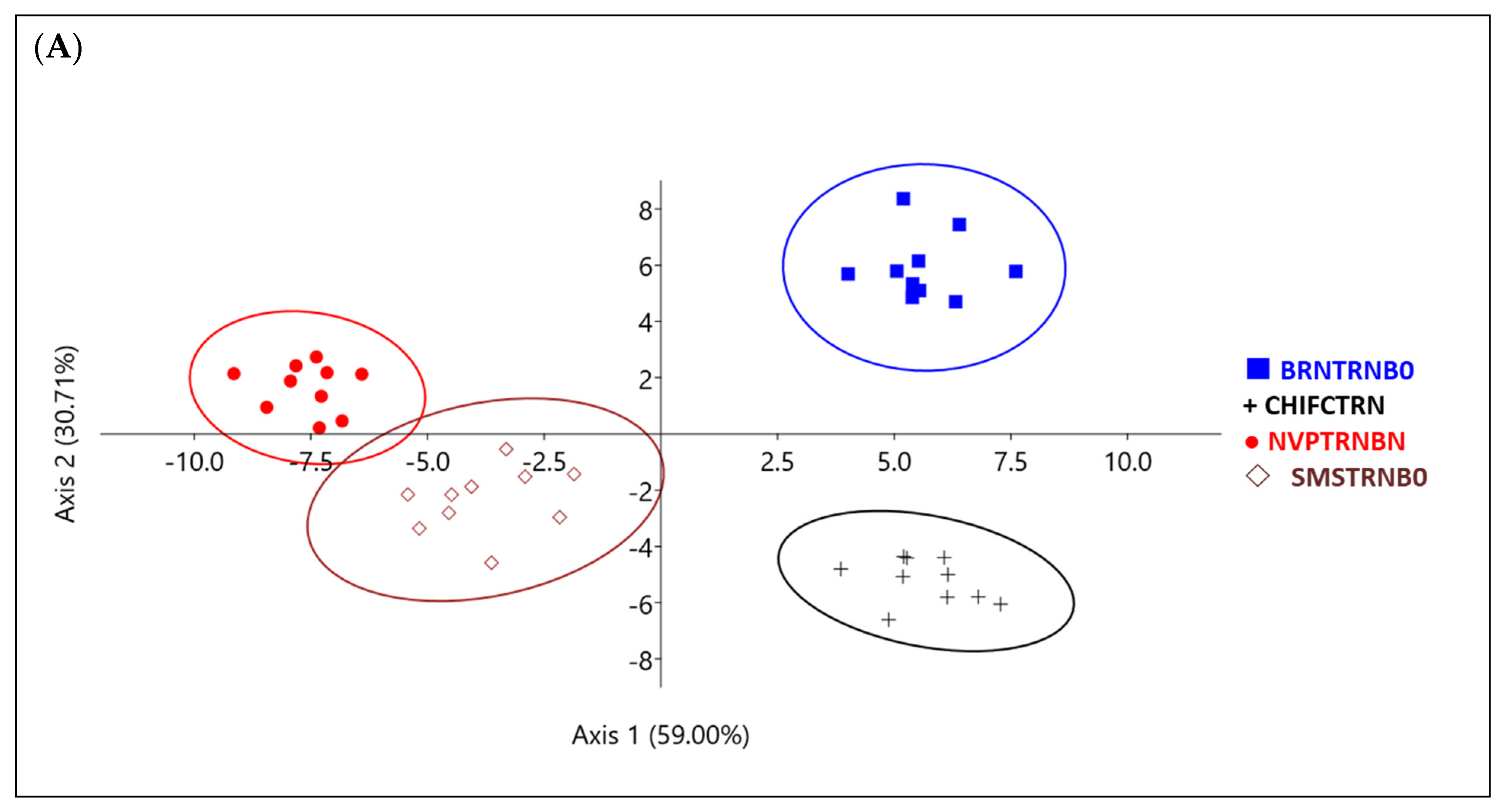
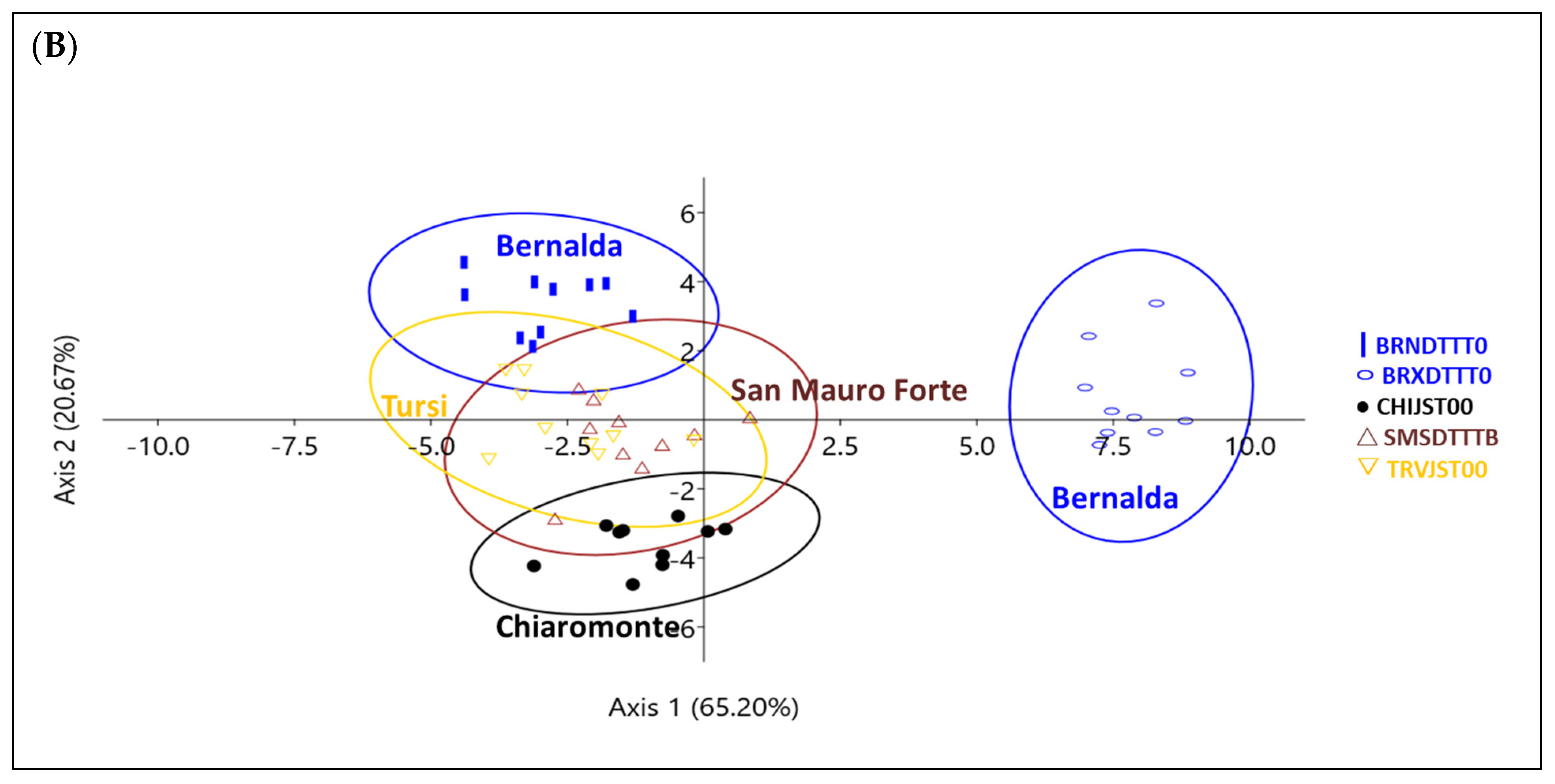
| Place | Name | Accession Code (a) | Skin Color (b) | Shape of Leaf Base (c) |
|---|---|---|---|---|
| Bernalda | Fica de Pistizz | BRDPSTCC | Greyed-Purple | Cordate |
| Nostrale nera | BRDNSTRN | Black | Cordate | |
| Menna vacca | BRDMNNVC | Greyed-Purple | Cordate | |
| Nostrale bianca | BRDNSTRL | Yellow-Green | Cordate | |
| Ficazzana | BRNFCZZN | Black | Decurrent | |
| Violetto | BRFVLTT0 | Greyed-Purple | Calcarate | |
| Dottato | BRNDTTT0 | Green | Cordate | |
| Troiana bianca | BRNTRNB0 | Yellow-Green | Calcarate | |
| Natalino | BRRNTLN0 | Black | Cordate | |
| Dottato | BRXDTTT0 | Green | Cordate | |
| Ente Riforma | BRXNTRFR | Greyed-Purple | Cordate | |
| Chiaromonte | Justa | CHIJST00 | Green | Cordate |
| Troiano bianco | CHIFCTRN | Yellow-Green | Cordate | |
| Nova Siri | Nostrana | NVTNSTRN | Green | Cordate |
| Donna Teresa | NVPDNNTR | Green | Cordate | |
| Columbraro nero | NVPCLMBN | Black | Truncate | |
| Troiana | NVPTRNBN | Yellow-Green | Decurrent | |
| San Mauro Forte | Agostiniano Nero | SMSAGSTN | Brown | Decurrent |
| Canitano bianco | SMSCNTNB | Green | Truncate | |
| Dottato bianco | SMSDTTTB | Green | Cordate | |
| Columbro bianco gentile | SMSCLMBG | Green | Decurrent | |
| San Pietro bianco | SMSSPTRB | Green | Decurrent | |
| Troiano bianco | SMSTRNB0 | Yellow-Green | Decurrent | |
| Troiano nero | SMSTRNN0 | Black | Decurrent | |
| Tolve | Spinazzolese | TLNSPNZZ | Black | Cordate |
| Gentile | TLNGNTLB | Yellow-Green | Cordate | |
| Agostarico | TLNAGSTR | Black | Cordate | |
| Tursi | Justa | TRVJST00 | Green | Decurrent |
| Natalino | TRPNTLNN | Black | Truncate | |
| Faraona | TRVFRN00 | Green | Decurrent |
| Leaf Trait | Abbreviation | Units |
|---|---|---|
| Petiole length | PL | mm |
| Main vein length | MVL | mm |
| Upper vein length (a) | UVL | mm |
| Lower vein length (a) | LVL | mm |
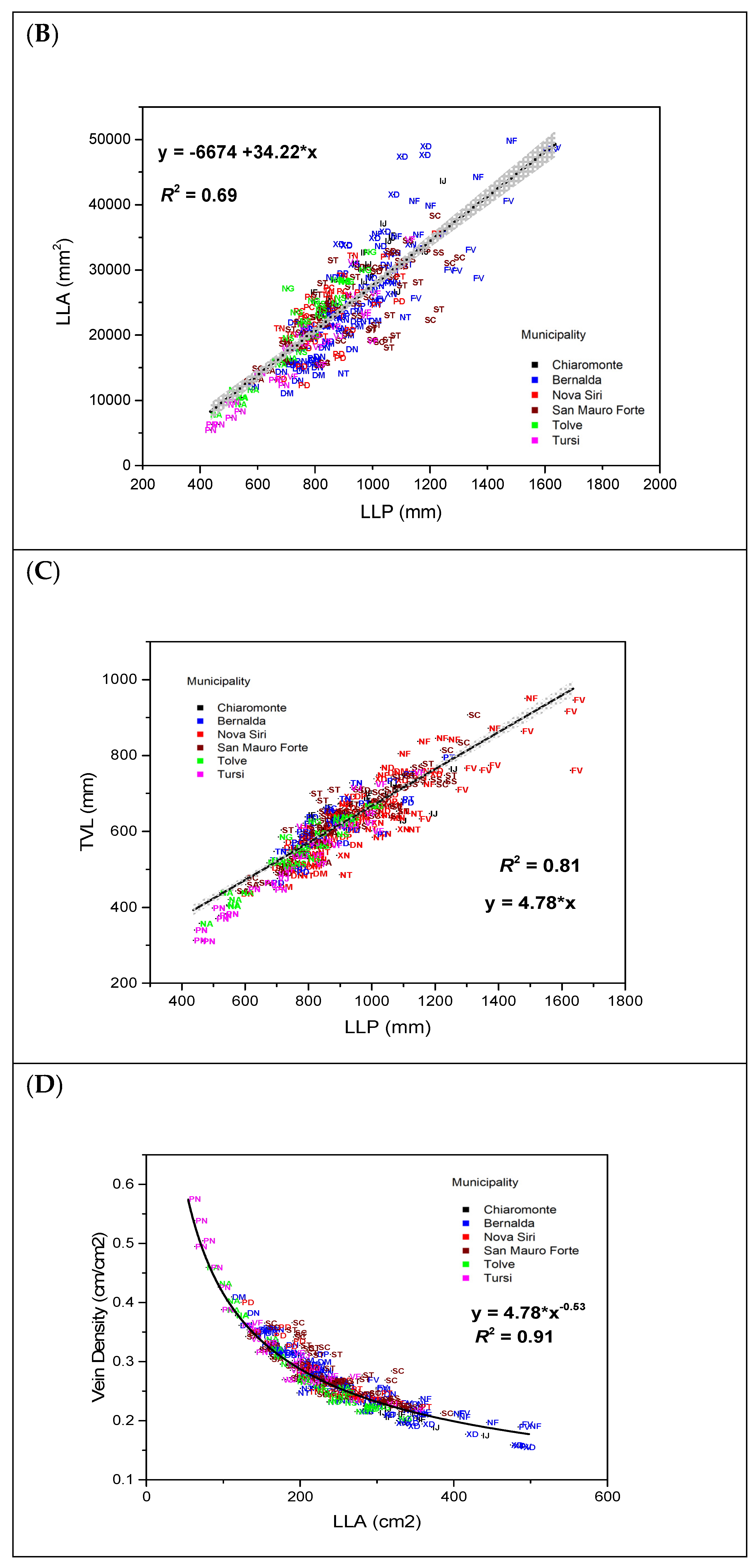
| Total veins length | TVL | mm |
| Leaf lamina length (a) | LLL | mm |
| Leaf lamina width | LLW | mm |
| Leaf lamina area | LLA | mm2 |
| Leaf lamina perimeter | LLP | mm |
| Veins density | VD | mm mm−2 |
| Central lobe length | CLL | mm |
| Central lobe width | CLW | mm |
| Petiole to upper sinus distance (a) | PUS | mm |
| Petiole to lower sinus distance (a) | PLS | mm |
| Petiole sinus depth | PSD = LLL − MVL | mm |
| Leaf lamina area to leaf lamina perimeter ratio | LLA/LLP | mm |
| Leaf lamina length to leaf lamina width ratio | LLL/LLW | |
| Central lobe length to leaf lamina length | CLL/LLL | |
| Central lobe length to central lobe width | CLL/CLW | |
| Circularity | CRC | |
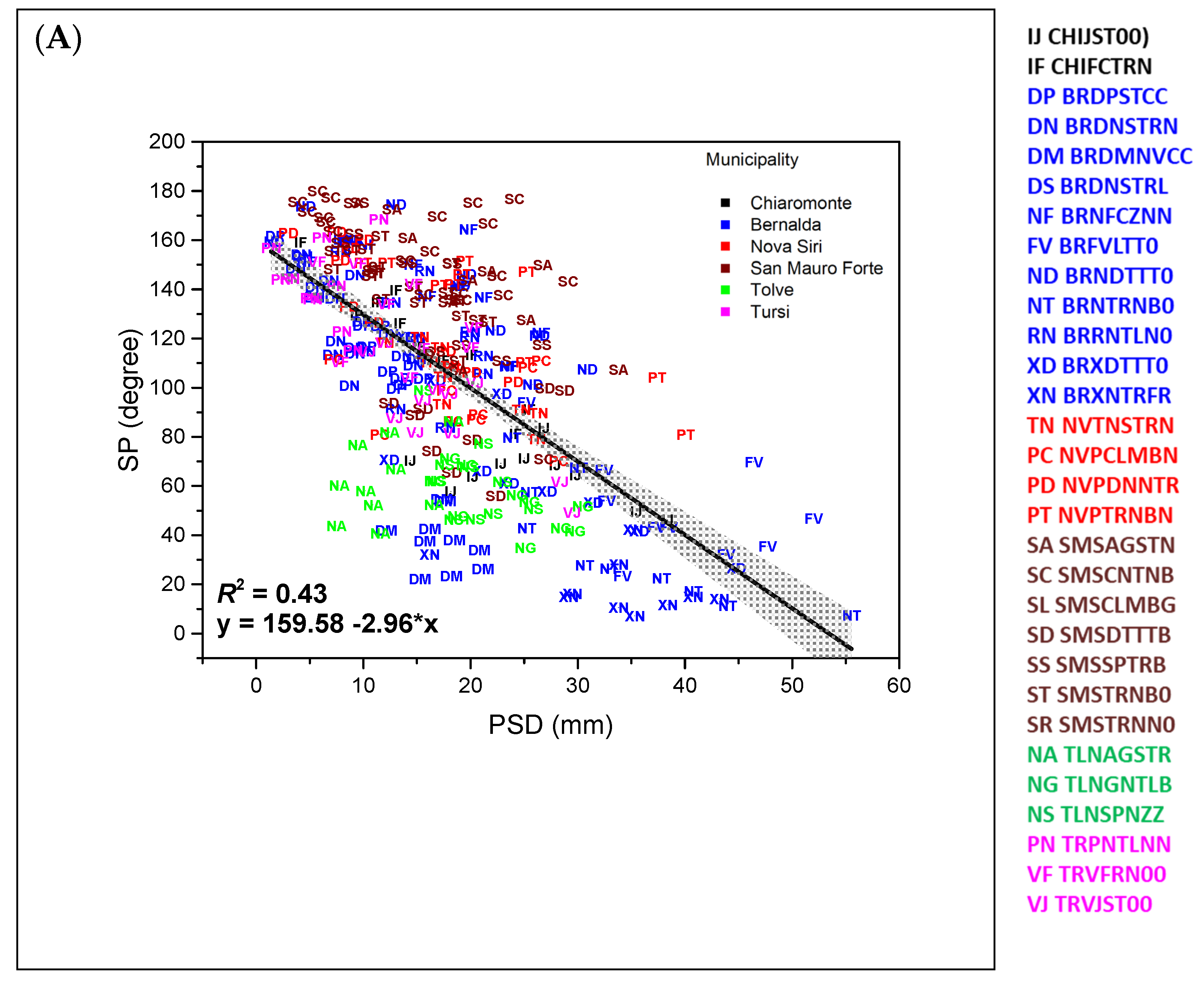
| Petiole sinus angle (angle between the left and right lines that connect LM 1 to LM 3 and LM 1 to LM 16) | SP | degree |
| Main vein to lower vein angle (a) | MV^LV | degree |
| Main vein to lower sinus angle (a) | MV^LS | degree |
| Main vein to upper vein angle (a) | MV^UV | degree |
| Main vein to upper sinus angle (a) | MV^US | degree |
| Accession Code | PL (mm) | MVL (mm) | UVL (mm) | LVL (mm) | TLV (mm) | LLL (mm) | LLW (mm) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BNDTTT0 | 80.43 | bcd | 193.79 | bcdef | 147.02 | cdefg | 73.28 | defghi | 805.57 | a | 210.65 | defgh | 184.59 | cdefg |
| BXDTTT0 | 97.78 | ab | 243.53 | a | 174.09 | ab | 58.47 | hijk | 777.38 | ab | 268.51 | a | 222.33 | a |
| BNFCZZN | 102.72 | a | 241.84 | a | 186.90 | a | 94.96 | abcd | 708.66 | abc | 259.78 | ab | 213.28 | abc |
| BDMNNVC | 62.43 | defgh | 173.00 | efghi | 134.89 | efghi | 70.67 | efghi | 707.61 | abc | 190.28 | fghi | 150.81 | hij |
| BDNSTRL | 66.91 | def | 183.84 | defgh | 140.16 | cdefgh | 51.99 | ijk | 702.70 | abcd | 190.93 | fghi | 154.95 | ghij |
| BRDNSTRN | 65.24 | defg | 154.96 | hi | 124.25 | ghi | 65.21 | efghij | 690.21 | bcde | 165.20 | ij | 140.42 | jk |
| BRXNTRFR | 75.18 | cdef | 203.20 | bcd | 153.00 | bcdef | 62.27 | fghijk | 677.22 | bcdef | 236.72 | bcde | 190.11 | bcdef |
| BRRNTLN0 | 59.57 | fgh | 171.71 | efghi | 135.63 | efghi | 74.62 | defgh | 666.20 | bcdefg | 187.86 | ghij | 155.64 | ghij |
| BRDPSTCC | 76.70 | cdef | 195.82 | bcdef | 149.96 | bcdefg | 66.99 | efghij | 663.42 | bcdefg | 206.87 | efgh | 173.80 | efghi |
| BRNTRNB0 | 80.14 | bcd | 167.02 | fghi | 129.63 | fghi | 72.29 | efghi | 652.61 | cdefg | 201.66 | fgh | 172.07 | efghi |
| BRFVLTT0 | 102.89 | a | 214.59 | abc | 174.65 | ab | 106.74 | a | 634.40 | cdefgh | 253.60 | abc | 215.48 | ab |
| CHIFCTRN | 90.21 | abc | 197.17 | bcdef | 153.32 | bcdef | 79.80 | cdefgh | 633.74 | cdefgh | 212.56 | defgh | 195.16 | abcdef |
| CHIJST00 | 75.34 | cdef | 218.86 | ab | 162.25 | abcd | 66.93 | efghij | 629.72 | cdefgh | 244.76 | abcd | 201.54 | abcde |
| NVPCLMBN | 62.14 | defgh | 191.58 | bcdef | 142.99 | cdefg | 62.00 | fghijk | 616.91 | cdefgh | 211.03 | defgh | 174.16 | efghi |
| NVPDNNTR | 65.00 | defg | 168.78 | fghi | 132.92 | efghi | 68.66 | efghij | 611.88 | cdefgh | 180.64 | hij | 154.58 | ghij |
| NVTNSTRN | 72.11 | cdef | 199.18 | bcde | 144.10 | cdefg | 62.25 | fghijk | 610.89 | cdefgh | 218.23 | defg | 176.66 | defgh |
| NVPTRNBN | 61.86 | defgh | 187.40 | cdefg | 146.28 | cdefg | 86.32 | abcde | 603.94 | cdefgh | 209.80 | efgh | 184.18 | cdefg |
| SMSAGSTN | 71.86 | cdef | 160.30 | ghi | 116.24 | hi | 66.19 | efghij | 601.57 | cdefgh | 180.26 | hij | 156.12 | ghij |
| SMSCLMBG | 73.65 | cdef | 184.94 | cdefgh | 132.45 | efghi | 80.52 | cdefg | 599.82 | cdefgh | 206.12 | efgh | 176.61 | defgh |
| SMSCNTNB | 97.97 | ab | 196.72 | bcdef | 157.57 | bcde | 97.87 | abc | 592.23 | defgh | 206.48 | efgh | 165.48 | fghij |
| SMSDTTTB | 67.03 | def | 191.03 | bcdef | 143.07 | cdefg | 61.33 | ghijk | 584.11 | efgh | 210.14 | efgh | 171.25 | fghi |
| SMSSPTRB | 76.92 | cdef | 207.11 | bcd | 164.47 | abc | 83.33 | bcdef | 571.95 | fghi | 223.41 | cdef | 204.77 | abcd |
| SMSTRNB0 | 78.50 | cde | 190.33 | bcdefg | 154.68 | bcdef | 83.26 | bcdef | 570.87 | fghi | 205.71 | efgh | 189.72 | bcdef |
| SMTRNN0 | 72.66 | cdef | 181.86 | defgh | 151.16 | bcdef | 103.01 | ab | 568.13 | fghi | 194.16 | fghi | 171.92 | efghi |
| TLNAGSTR | 44.23 | h | 143.07 | Ij | 109.39 | ij | 51.60 | ijk | 561.76 | ghi | 154.85 | jk | 144.50 | ij |
| TLNGNTLB | 76.49 | cdef | 214.40 | abc | 141.85 | cdefgh | 59.41 | ghijk | 560.31 | ghi | 238.08 | bcde | 183.80 | cdefg |
| TLNSPNZZ | 71.30 | def | 189.04 | bcdefg | 137.25 | defgh | 48.38 | jk | 533.87 | hi | 209.16 | efgh | 175.98 | defgh |
| TRFRN00 | 62.10 | defgh | 185.44 | cdefg | 141.79 | cdefgh | 67.46 | efghij | 525.16 | hi | 199.12 | fghi | 172.12 | efghi |
| TRPJST00 | 60.92 | efgh | 177.78 | defgh | 133.94 | efghi | 58.05 | hijk | 465.06 | ij | 195.78 | Fghi | 166.37 | fghij |
| TRPNTLNN | 47.60 | gh | 123.33 | J | 88.932 | j | 42.05 | k | 385.29 | j | 129.24 | K | 113.65 | k |
| Accession Code | LLA (mm2) | LLP (mm) | VD (mm/mm2) | CLL (mm) | CLW (mm) | PUS (mm) | PLS (mm) | |||||||
| BNDTTT0 | 24,896.90 | cdefghi | 894.11 | cdefg | 0.0258 | fghijk | 105.03 | cdefghi | 75.78 | efghij | 95.098 | bcdefg | 72.30 | cdefghi |
| BXDTTT0 | 38,869.51 | a | 1022.62 | bcde | 0.0186 | l | 134.73 | ab | 104.89 | a | 121.30 | a | 71.01 | cdefghi |
| BNFCZZN | 37,850.28 | a | 1175.20 | b | 0.0215 | jkl | 144.53 | a | 90.80 | bcde | 106.02 | bc | 94.50 | a |
| BDMNNVC | 19,075.72 | hijk | 879.04 | cdefg | 0.0318 | bcde | 96.23 | fghij | 67.60 | hijkl | 82.13 | efghij | 59.61 | ghijk |
| BDNSTRL | 19,840.07 | ghijk | 782.42 | fgh | 0.0294 | cdefgh | 95.13 | ghij | 71.43 | fghijk | 97.22 | bcdef | 56.06 | ijk |
| BRDNSTRN | 15,718.37 | jkl | 779.85 | fgh | 0.0341 | bc | 86.27 | ijk | 62.88 | jkl | 76.33 | hijk | 55.99 | ijk |
| BRXNTRFR | 28,175.07 | bcdef | 1037.90 | bcd | 0.0227 | ijkl | 123.21 | abc | 81.40 | cdefghi | 85.06 | defghij | 65.87 | efghij |
| BRRNTLN0 | 20,013.26 | fghijk | 825.27 | efg | 0.0299 | cdefg | 100.77 | defghij | 75.64 | efghij | 81.42 | fghij | 63.61 | efghijk |
| BRDPSTCC | 23,653.82 | defghij | 881.20 | cdefg | 0.0269 | efghij | 104.01 | cdefghi | 77.08 | defghij | 102.76 | bcd | 69.66 | defghij |
| BRNTRNB0 | 22,565.61 | defghij | 925.65 | cdef | 0.0259 | fghijk | 117.88 | bcdef | 87.66 | bcdef | 55.07 | l | 60.07 | ghijk |
| BRFVLTT0 | 35,938.39 | ab | 1379.74 | a | 0.0224 | ijkl | 145.00 | a | 76.54 | defghij | 76.32 | hijk | 75.34 | bcdefg |
| CHIFCTRN | 28,346.79 | bcde | 914.79 | cdefg | 0.0237 | hijkl | 108.78 | cdefgh | 103.71 | ab | 104.41 | bc | 84.16 | abcd |
| CHIJST00 | 32,754.14 | abc | 1045.23 | bcd | 0.0209 | kl | 122.10 | bcd | 94.74 | abc | 107.62 | bc | 70.73 | defghij |
| NVPCLMBN | 23,846.44 | defghij | 800.92 | fgh | 0.0253 | ghijk | 101.29 | defghij | 81.89 | cdefgh | 102.19 | bcd | 67.62 | efghij |
| NVPDNNTR | 17,740.28 | ijk | 832.48 | efg | 0.0329 | bcd | 107.12 | cdefghi | 68.68 | ghijkl | 67.67 | jkl | 60.48 | ghijk |
| NVTNSTRN | 24,803.63 | cdefghi | 809.60 | efg | 0.0249 | ghijk | 105.79 | cdefghi | 84.52 | cdefg | 108.23 | abc | 67.42 | efghij |
| NVPTRNBN | 25,918.93 | cdefghi | 968.02 | cdef | 0.0257 | fghijk | 108.24 | cdefgh | 86.67 | cdef | 86.24 | defghi | 75.05 | bcdefg |
| SMSAGSTN | 18,240.32 | hijk | 730.39 | ghi | 0.0296 | cdefg | 89.24 | hijk | 83.30 | cdefgh | 83.70 | efghij | 59.45 | ghijk |
| SMSCLMBG | 23,719.15 | defghij | 858.09 | defg | 0.0262 | efghijk | 104.14 | cdefghi | 82.40 | cdefgh | 90.55 | cdefgh | 78.66 | abcde |
| SMSCNTNB | 23,238.54 | defghij | 1046.52 | bcd | 0.0319 | bcde | 119.13 | bcde | 69.91 | ghijk | 80.73 | fghij | 77.30 | bcdef |
| SMSDTTTB | 23,376.00 | defghij | 836.65 | efg | 0.0262 | efghijk | 100.79 | defghij | 75.77 | efghij | 99.34 | bcde | 67.65 | efghij |
| SMSSPTRB | 29,427.05 | bcd | 1061.29 | bc | 0.0243 | ghijkl | 119.83 | bcde | 88.03 | bcde | 93.82 | bcdefgh | 88.73 | abc |
| SMSTRNB0 | 26,302.39 | cdefgh | 855.36 | defg | 0.0256 | fghijk | 98.92 | efghij | 93.40 | bc | 106.66 | bc | 91.30 | ab |
| SMTRNN0 | 22,348.32 | defghij | 1073.94 | bc | 0.0312 | bcdef | 117.29 | cdef | 65.48 | ijkl | 69.04 | ijkl | 69.52 | defghij |
| TLNAGSTR | 13,416.85 | kl | 613.64 | hi | 0.0366 | b | 79.85 | jk | 55.60 | kl | 70.01 | ijkl | 56.68 | hijk |
| TLNGNTLB | 27,493.85 | cdefg | 861.82 | defg | 0.0225 | ijkl | 119.86 | bcde | 92.27 | bcd | 108.54 | ab | 64.56 | efghijk |
| TLNSPNZZ | 22,321.37 | defghij | 797.21 | fgh | 0.0254 | ghijk | 106.79 | cdefghi | 80.33 | cdefghi | 95.04 | bcdefg | 55.68 | jk |
| TRFRN00 | 21,436.81 | defghijk | 916.02 | cdefg | 0.0291 | cdefgh | 111.76 | cdefg | 75.38 | efghij | 78.19 | ghij | 69.55 | defghij |
| TRPJST00 | 20,663.83 | efghijk | 788.76 | fgh | 0.0278 | defghi | 93.57 | ghij | 77.01 | defghij | 91.01 | bcdefgh | 61.44 | fghijk |
| TRPNTLNN | 9249.23 | l | 535.99 | i | 0.0442 | a | 70.65 | k | 52.92 | l | 59.55 | kl | 49.05 | k |
| Accession Code | PSD (mm) | LLA/LLP (mm2 mm−1) | LLL/LLW | CLL/LLL | CLL/CLW | CRC | ||||||||
| BNDTTT0 | 16.86 | efghij | 27.77 | bcdefgh | 1.14 | cde | 0.50 | gh | 1.39 | cdef | 0.485 | a | ||
| BXDTTT0 | 24.98 | bcde | 37.83 | a | 1.21 | abc | 0.50 | fgh | 1.28 | efgh | 0.474 | ab | ||
| BNFCZZN | 17.94 | defghij | 32.23 | b | 1.22 | abc | 0.56 | abcdefg | 1.59 | bc | 0.469 | abc | ||
| BDMNNVC | 17.28 | efghij | 21.32 | jklm | 1.27 | ab | 0.50 | fgh | 1.43 | cdef | 0.468 | abc | ||
| BDNSTRL | 7.09 | jk | 25.03 | ghijkl | 1.23 | abc | 0.50 | gh | 1.33 | cdefg | 0.456 | abcd | ||
| BRDNSTRN | 10.24 | hijk | 20.18 | lm | 1.18 | abcde | 0.52 | defgh | 1.38 | cdef | 0.441 | abcde | ||
| BRXNTRFR | 33.52 | abc | 27.06 | cdefgh | 1.25 | abc | 0.52 | defgh | 1.52 | bcde | 0.440 | abcde | ||
| BRRNTLN0 | 16.15 | efghijk | 24.19 | hijkl | 1.21 | abcde | 0.54 | bcdefgh | 1.34 | cdefg | 0.431 | abcdef | ||
| BRDPSTCC | 11.06 | hijk | 26.89 | defghi | 1.19 | abcde | 0.50 | fgh | 1.36 | cdef | 0.428 | abcdef | ||
| BRNTRNB0 | 34.63 | ab | 24.45 | hijkl | 1.18 | abcde | 0.59 | abc | 1.36 | cdef | 0.419 | abcdefg | ||
| BRFVLTT0 | 39.01 | a | 25.69 | fghijk | 1.19 | abcde | 0.57 | abcde | 1.93 | a | 0.415 | abcdefg | ||
| CHIFCTRN | 15.39 | efghijk | 30.94 | bcde | 1.09 | de | 0.51 | fgh | 1.05 | h | 0.411 | abcdefgh | ||
| CHIJST00 | 25.89 | bcde | 31.35 | bcd | 1.22 | abcd | 0.50 | gh | 1.29 | efgh | 0.405 | abcdefghi | ||
| NVPCLMBN | 19.44 | defghi | 29.78 | bcdefg | 1.21 | abcde | 0.48 | h | 1.24 | fgh | 0.398 | abcdefghij | ||
| NVPDNNTR | 11.86 | ghijk | 21.23 | jklm | 1.17 | bcde | 0.59 | ab | 1.57 | bcd | 0.393 | bcdefghij | ||
| NVTNSTRN | 19.05 | defghi | 30.50 | bcdef | 1.24 | abc | 0.48 | h | 1.25 | efgh | 0.388 | cdefghijk | ||
| NVPTRNBN | 22.40 | defg | 26.55 | defghi | 1.14 | cde | 0.52 | efgh | 1.25 | efgh | 0.381 | defghijk | ||
| SMSAGSTN | 19.96 | defghi | 24.78 | ghijkl | 1.16 | bcde | 0.50 | gh | 1.08 | gh | 0.369 | efghijk | ||
| SMSCLMBG | 21.17 | defgh | 27.52 | bcdefgh | 1.17 | bcde | 0.50 | fgh | 1.27 | efgh | 0.352 | fghijk | ||
| SMSCNTNB | 9.77 | ijk | 21.83 | ijklm | 1.25 | abc | 0.58 | abcd | 1.73 | ab | 0.350 | fghijkl | ||
| SMSDTTTB | 19.12 | defghi | 27.63 | bcdefgh | 1.25 | abc | 0.48 | h | 1.34 | cdefg | 0.339 | ghijkl | ||
| SMSSPTRB | 16.30 | efghijk | 27.60 | bcdefgh | 1.09 | de | 0.53 | cdefgh | 1.36 | cdef | 0.330 | hijkl | ||
| SMSTRNB0 | 15.39 | efghijk | 30.75 | bcdef | 1.09 | e | 0.48 | h | 1.06 | h | 0.328 | hijklm | ||
| SMTRNN0 | 12.30 | ghijk | 20.79 | klm | 1.13 | cde | 0.60 | a | 1.87 | a | 0.328 | hijklm | ||
| TLNAGSTR | 11.78 | ghijk | 21.30 | jklm | 1.09 | de | 0.52 | efgh | 1.44 | cdef | 0.325 | ijklm | ||
| TLNGNTLB | 23.68 | cdef | 32.01 | bc | 1.30 | a | 0.50 | fgh | 1.30 | defgh | 0.318 | jklmn | ||
| TLNSPNZZ | 20.07 | defghi | 27.90 | bcdefgh | 1.19 | abcde | 0.51 | fgh | 1.33 | cdefg | 0.304 | klmn | ||
| TRFRN00 | 13.68 | fghijk | 23.08 | hijkl | 1.16 | bcde | 0.56 | abcdef | 1.49 | bcdef | 0.265 | lmn | ||
| TRPJST00 | 18.00 | defghij | 25.97 | efghij | 1.18 | abcde | 0.49 | h | 1.22 | fgh | 0.244 | mn | ||
| TRPNTLNN | 5.91 | k | 16.91 | m | 1.14 | cde | 0.55 | abcdefg | 1.37 | cdef | 0.236 | n | ||
| Accession Code | SP (Degree) | MV^LV (Degree) | MV^LS (Degree) | MV^UV (Degree) | MV^US (Degree) | |||||||||
| BNDTTT0 | 140.96 | ab | 79.71 | efg | 72.63 | efghijk | 32.22 | abcd | 16.22 | cdef | ||||
| BXDTTT0 | 69.40 | ghij | 105.08 | a | 92.93 | a | 30.46 | bcd | 18.46 | abcdef | ||||
| BNFCZZN | 138.02 | ab | 77.11 | efgh | 64.14 | ijkl | 28.37 | d | 15.11 | ef | ||||
| BDMNNVC | 37.53 | kl | 75.95 | efghi | 61.82 | jkl | 31.66 | abcd | 14.53 | f | ||||
| BDNSTRL | 128.67 | abcd | 81.79 | efg | 72.99 | efghij | 29.83 | cd | 16.73 | bcdef | ||||
| BRDNSTRN | 135.91 | abc | 78.02 | efgh | 63.50 | ijkl | 30.08 | cd | 19.16 | abcde | ||||
| BRXNTRFR | 19.27 | l | 104.37 | a | 89.46 | abc | 31.32 | bcd | 14.92 | ef | ||||
| BRRNTLN0 | 117.61 | bcdef | 78.85 | efg | 64.38 | ijkl | 29.68 | cd | 19.87 | abcd | ||||
| BRDPSTCC | 116.06 | bcdef | 79.21 | efg | 71.78 | efghijk | 29.87 | cd | 15.86 | def | ||||
| BRNTRNB0 | 36.08 | kl | 96.00 | abc | 80.40 | bcdef | 37.10 | a | 20.70 | abc | ||||
| BRFVLTT0 | 50.96 | jkl | 85.31 | cde | 65.49 | hijkl | 33.00 | abcd | 19.10 | abcde | ||||
| CHIFCTRN | 120.39 | bcde | 78.59 | efgh | 74.12 | defghij | 37.26 | a | 21.10 | ab | ||||
| CHIJST00 | 64.57 | hijk | 98.13 | ab | 91.79 | ab | 31.867 | abcd | 18.44 | abcdef | ||||
| NVPCLMBN | 95.26 | defgh | 84.95 | cdef | 78.37 | cdefg | 32.24 | abcd | 18.51 | abcdef | ||||
| NVPDNNTR | 137.88 | ab | 72.73 | fghi | 59.94 | kl | 32.10 | abcd | 16.60 | bcdef | ||||
| NVTNSTRN | 103.30 | cdefg | 85.01 | cdef | 78.71 | cdefg | 32.93 | abcd | 19.14 | abcde | ||||
| NVPTRNBN | 132.66 | abc | 79.31 | efg | 66.82 | ghijk | 33.84 | abcd | 17.47 | abcdef | ||||
| SMSAGSTN | 142.68 | ab | 81.57 | efg | 70.48 | fghijk | 36.20 | ab | 21.80 | a | ||||
| SMSCLMBG | 154.55 | a | 82.82 | defg | 71.80 | efghijk | 36.32 | ab | 17.68 | abcdef | ||||
| SMSCNTNB | 156.66 | a | 63.75 | i | 52.72 | l | 31.66 | abcd | 14.49 | f | ||||
| SMSDTTTB | 86.22 | fghi | 86.36 | bcde | 77.50 | cdefgh | 31.33 | bcd | 17.13 | bcdef | ||||
| SMSSPTRB | 139.55 | ab | 78.15 | efgh | 68.97 | fghijk | 35.08 | abc | 16.61 | bcdef | ||||
| SMSTRNB0 | 142.56 | ab | 72.26 | ghi | 66.28 | ghijk | 34.08 | abc | 19.17 | abcde | ||||
| SMTRNN0 | 142.12 | ab | 66.223 | hi | 53.34 | l | 30.71 | bcd | 16.03 | def | ||||
| TLNAGSTR | 61.98 | hijk | 80.83 | efg | 71.65 | fghijk | 32.84 | abcd | 17.55 | abcdef | ||||
| TLNGNTLB | 53.15 | ijkl | 94.35 | abcd | 84.53 | abcde | 33.27 | abcd | 18.63 | abcdef | ||||
| TLNSPNZZ | 62.97 | hijk | 96.54 | abc | 85.99 | abcd | 32.23 | abcd | 18.38 | abcdef | ||||
| TRFRN00 | 124.98 | abcd | 80.68 | efg | 70.73 | fghijk | 34.50 | abc | 15.85 | def | ||||
| TRPJST00 | 89.03 | efgh | 85.76 | bcde | 75.40 | defghi | 32.80 | abcd | 17.43 | abcdef | ||||
| TRPNTLNN | 142.76 | ab | 75.31 | efghi | 68.17 | fghijk | 34.31 | abc | 18.39 | abcdef | ||||
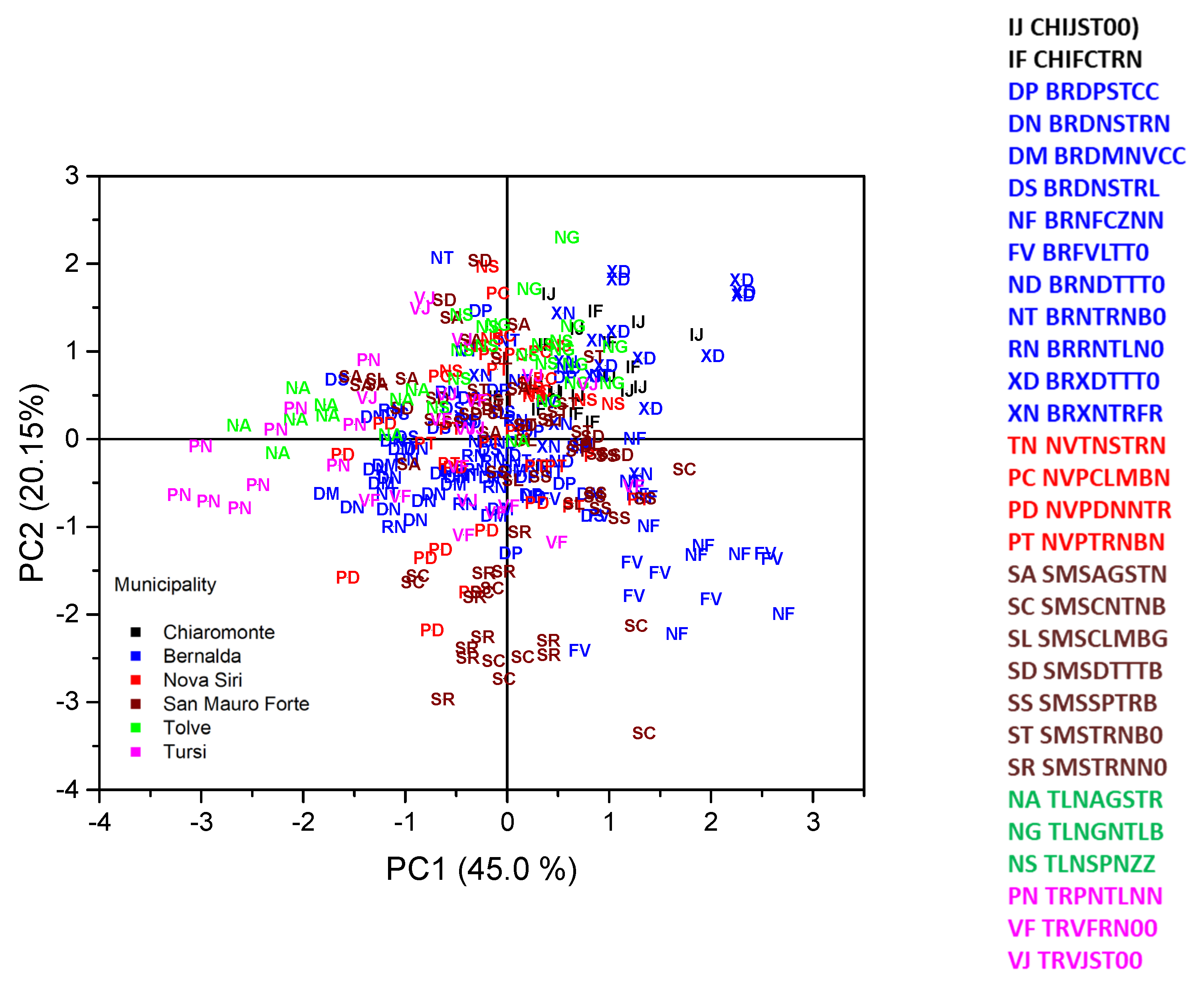
| Traits | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| LLP (mm) | 0.251 | −0.182 | 0.160 | 0.036 |
| Vein Density (mm/mm−2) | −0.268 | −0.151 | −0.026 | −0.020 |
| MVL + LVL + UVL | 0.276 | −0.150 | −0.067 | 0.024 |
| LLL (mm) | 0.292 | 0.036 | 0.051 | −0.084 |
| LLW (mm) | 0.273 | 0.050 | 0.039 | 0.174 |
| PL (mm) | 0.226 | −0.096 | −0.008 | 0.056 |
| MVL (mm) | 0.286 | 0.003 | −0.056 | −0.118 |
| UVL (mm) | 0.277 | −0.105 | −0.068 | −0.049 |
| LVL (mm) | 0.175 | −0.273 | −0.052 | 0.216 |
| LLA/LLP (mm) | 0.237 | 0.222 | −0.161 | −0.024 |
| PSD (mm) | 0.163 | 0.122 | 0.361 | 0.064 |
| SP (degree) | −0.060 | −0.178 | −0.391 | 0.218 |
| MV^LV (degree) | 0.069 | 0.322 | 0.340 | −0.114 |
| MV^LS (degree) | 0.050 | 0.370 | 0.220 | −0.100 |
| MV^UV (degree) | −0.022 | 0.140 | 0.176 | 0.516 |
| MV^US (degree) | −0.026 | 0.246 | 0.126 | 0.374 |
| PL + MVL (mm) | 0.287 | −0.036 | −0.042 | −0.059 |
| CLL (mm) | 0.259 | −0.106 | 0.190 | −0.025 |
| CLW (mm) | 0.232 | 0.194 | −0.057 | 0.154 |
| PUS (mm) | 0.197 | 0.174 | −0.334 | −0.129 |
| PLS (mm) | 0.218 | −0.010 | −0.222 | 0.207 |
| LLL/LLW | 0.062 | −0.027 | 0.031 | −0.537 |
| Circularity | −0.024 | 0.352 | −0.275 | −0.053 |
| CLL/CLW | 0.024 | −0.335 | 0.264 | −0.150 |
| CLL/LLL | −0.014 | −0.282 | 0.294 | 0.107 |
| Eigenvalue | 11.25 | 5.04 | 2.66 | 2.02 |
| Contribution to total variance (%) | 45.00 | 20.15 | 10.63 | 8.08 |
| Given Group | Number of Leaves | Correct Classification (n) | Correct Classification (%) | Predicted Group(s) | ||
|---|---|---|---|---|---|---|
| BRDPSTCC | 10 | 8 | 80 | 1 BRNFCZZN | 1 NVPCLMBN | |
| BRDNSTRN | 10 | 10 | 100 | |||
| BRDMNNVC | 10 | 10 | 100 | |||
| BRNFCZZN | 10 | 6 | 60 | 1 BRNFCZZN | 2 TLNAGSTR | 1 TRPNTLNN |
| BRFVLTT0 | 10 | 9 | 90 | 1 BRNFCZZN | ||
| BRRNTLN0 | 10 | 10 | 100 | |||
| BRXNTRFR | 10 | 9 | 90 | 1 TLNSPNZZ | ||
| NVPCLMBN | 10 | 9 | 90 | 1 BRDPSTCC | ||
| SMSAGSTN | 10 | 10 | 100 | |||
| SMSTRNN0 | 10 | 10 | 100 | |||
| TLNAGSTR | 10 | 10 | 100 | |||
| TLNSPNZZ | 10 | 10 | 100 | |||
| TRPNTLNN | 10 | 9 | 90 | 1 BRRNTLN0 | ||
| Total Average | 130 | 120 | 92.31 | |||
| Given Group | Number of Leaves | Correct Classification (n) | Correct Classification (%) | Predicted Groups | |
|---|---|---|---|---|---|
| CHIJST00 | 10 | 10 | 100 | ||
| CHIFCTRN | 10 | 10 | 100 | ||
| BRDNSTRL | 10 | 10 | 100 | ||
| BRNDTTT0 | 10 | 10 | 100 | ||
| BRNTRNB0 | 10 | 10 | 100 | ||
| BRXDTTT0 | 10 | 10 | 100 | ||
| NVTNSTRN | 10 | 9 | 90 | 1 SMSDTTTB | |
| NVPDNNTR | 10 | 8 | 80 | 2 TRVFRN00 | |
| NVPTRNBN | 10 | 10 | 100 | ||
| SMSCNTNB | 10 | 10 | 100 | ||
| SMSCLMBG | 10 | 9 | 90 | 1 NVPTRNBN | |
| SMSDTTTB | 10 | 6 | 60 | 2 NVTNSTRN | 2 TRVJST00 |
| SMSSPTRB | 10 | 10 | 100 | ||
| SMSTRNB0 | 10 | 10 | 100 | ||
| TLNGNTLB | 10 | 9 | 90 | 1 CHIJST00 | |
| TRVFRN00 | 10 | 7 | 70 | 1 NVPDNNTR | 2 SMSSPTRB |
| TRVJST00 | 10 | 7 | 70 | 2 BRDNSTRL | 1 NVTNSTRN |
| Total Average | 170 | 155 | 91.18 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuzzo, V.; Gatto, A.; Montanaro, G. Morphological Characterization of Some Local Varieties of Fig (Ficus carica L.) Cultivated in Southern Italy. Sustainability 2022, 14, 15970. https://doi.org/10.3390/su142315970
Nuzzo V, Gatto A, Montanaro G. Morphological Characterization of Some Local Varieties of Fig (Ficus carica L.) Cultivated in Southern Italy. Sustainability. 2022; 14(23):15970. https://doi.org/10.3390/su142315970
Chicago/Turabian StyleNuzzo, Vitale, Antonio Gatto, and Giuseppe Montanaro. 2022. "Morphological Characterization of Some Local Varieties of Fig (Ficus carica L.) Cultivated in Southern Italy" Sustainability 14, no. 23: 15970. https://doi.org/10.3390/su142315970
APA StyleNuzzo, V., Gatto, A., & Montanaro, G. (2022). Morphological Characterization of Some Local Varieties of Fig (Ficus carica L.) Cultivated in Southern Italy. Sustainability, 14(23), 15970. https://doi.org/10.3390/su142315970










