Elevation-Dependent Fluctuations of the Soil Properties in a Subtropical Forest of Central China
Abstract
1. Introduction
2. Materials and Methods
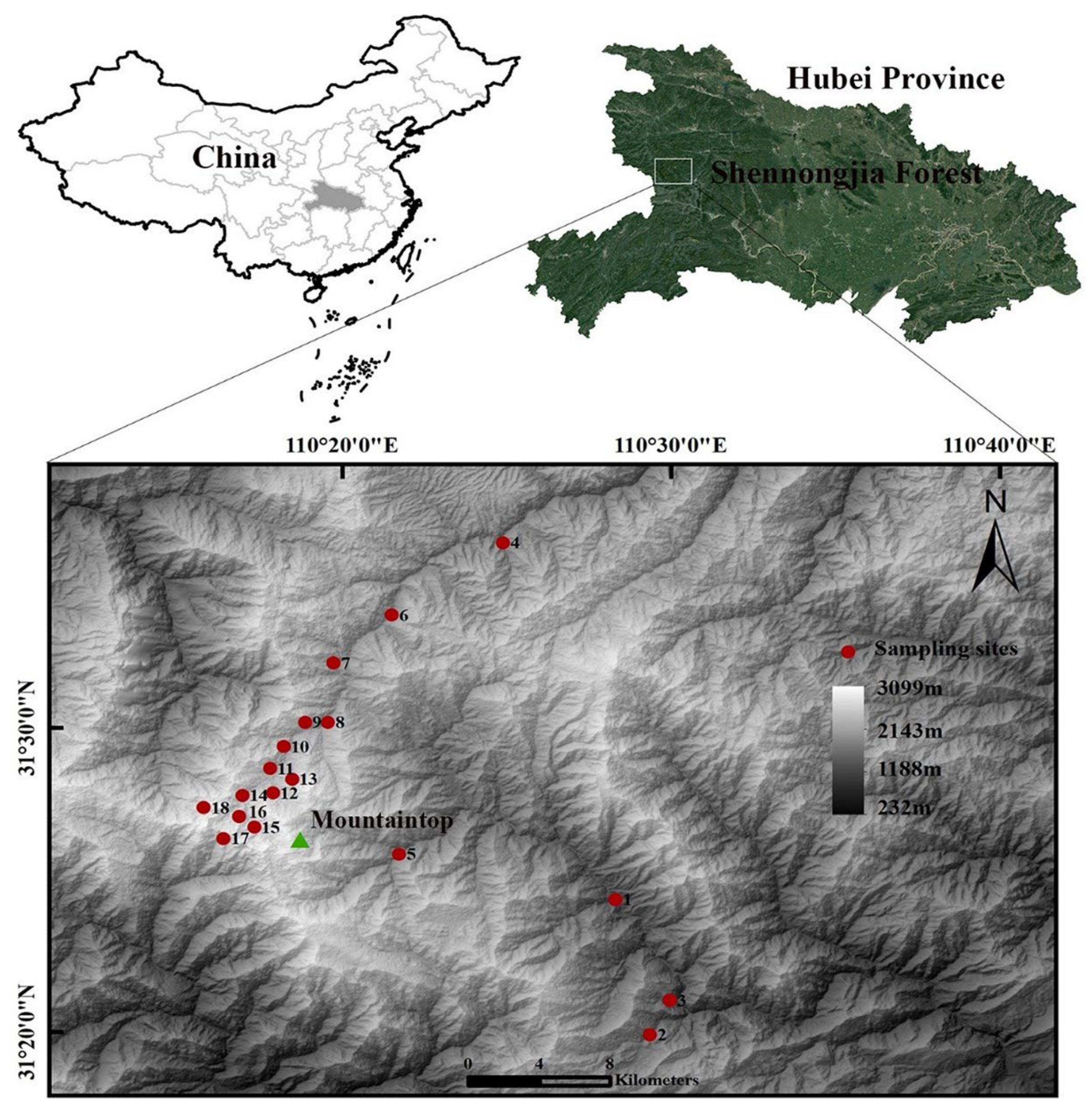
2.1. Study Area
2.2. Sample Collection
2.3. Sample Pretreatment and Analysis
2.4. Data Analysis
3. Results and Discussion
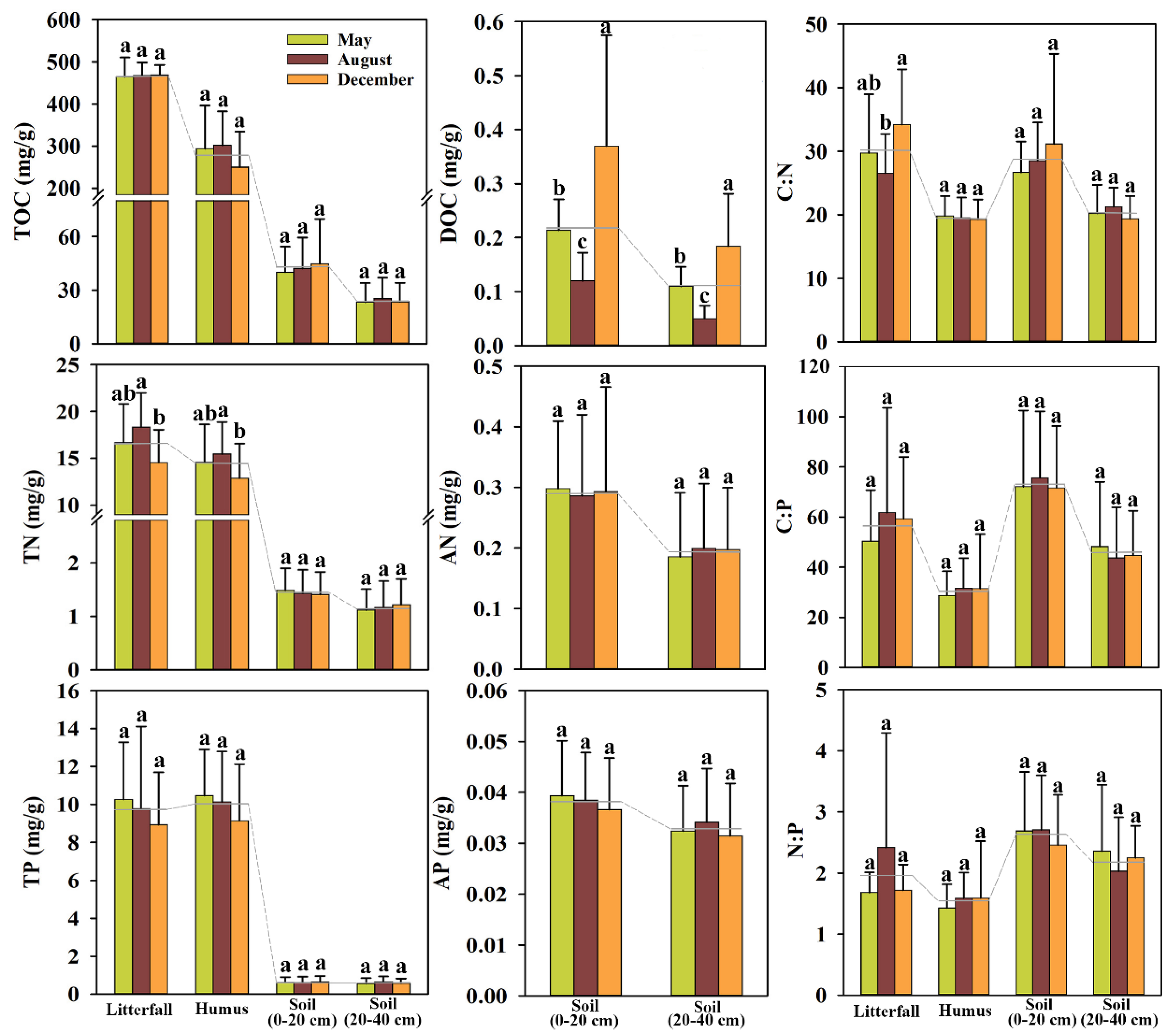
3.1. Comparison of Average Contents of C, N, and P and Their Stoichiometry in Various Layers
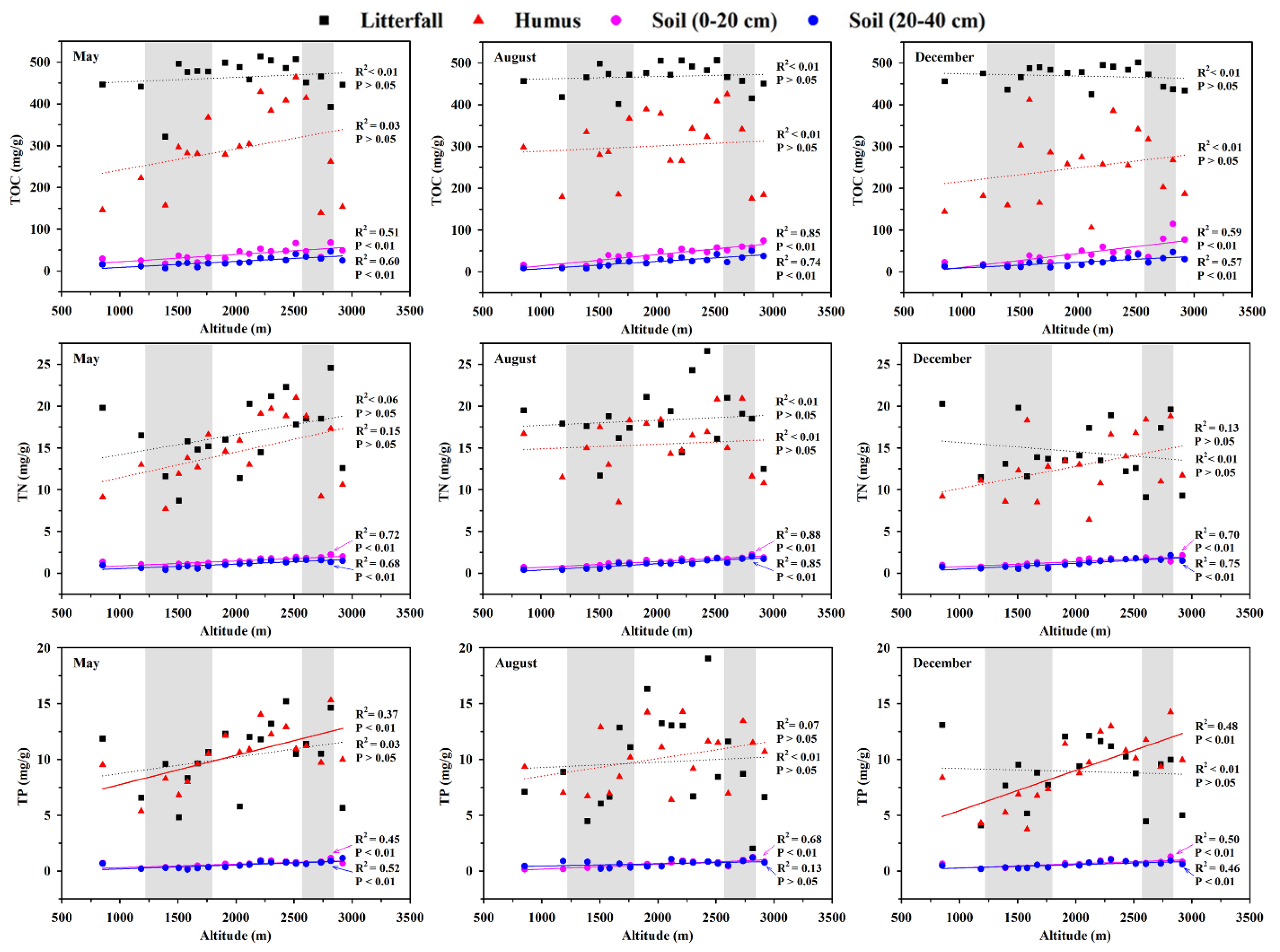
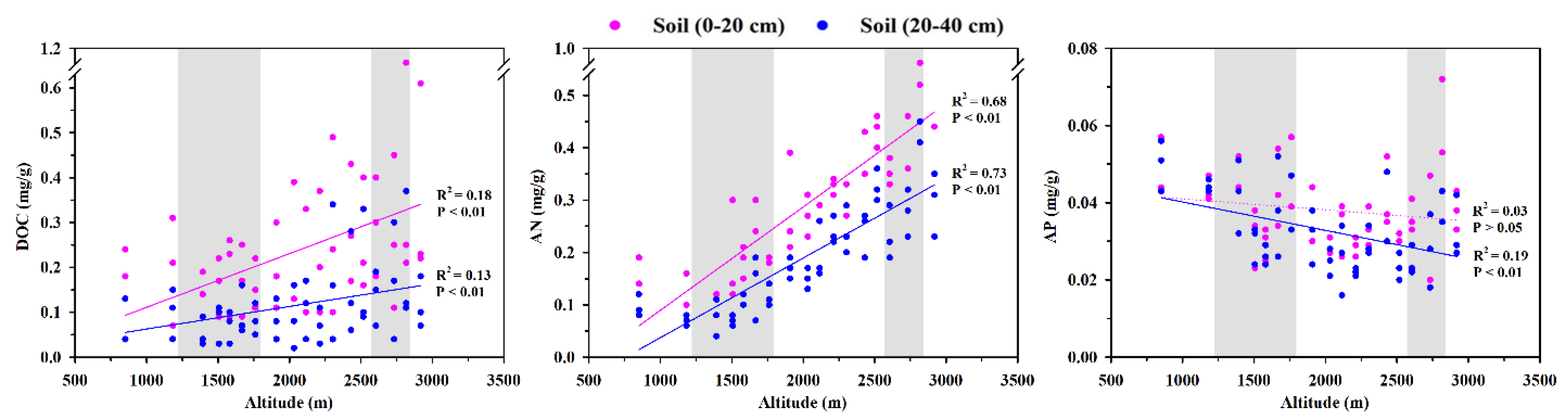
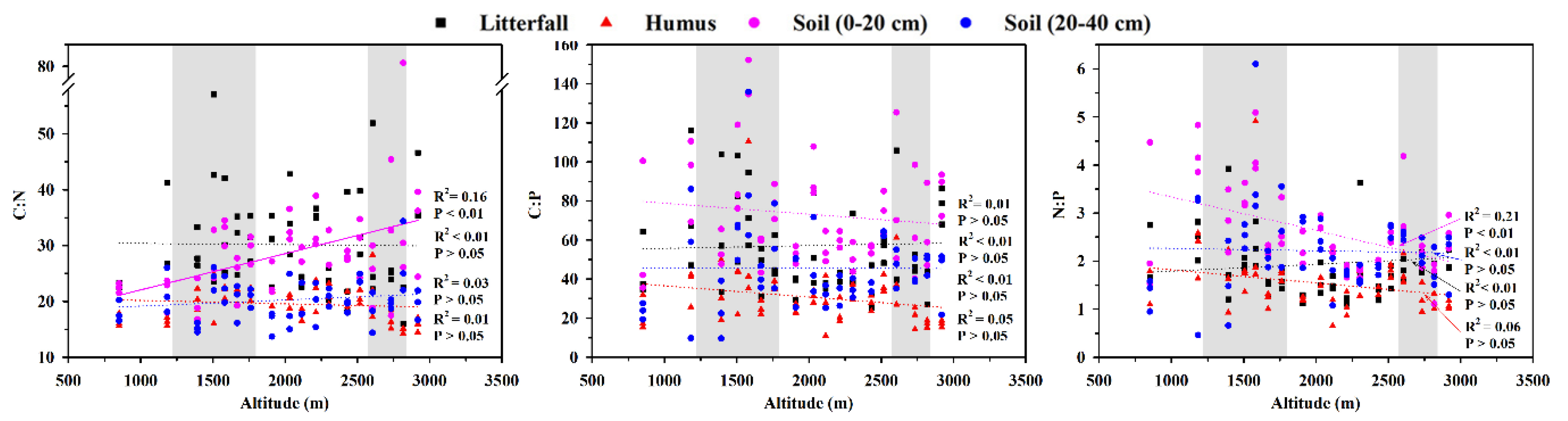
3.2. Response of C, N, and P Contents and Their Stoichiometry to Altitude
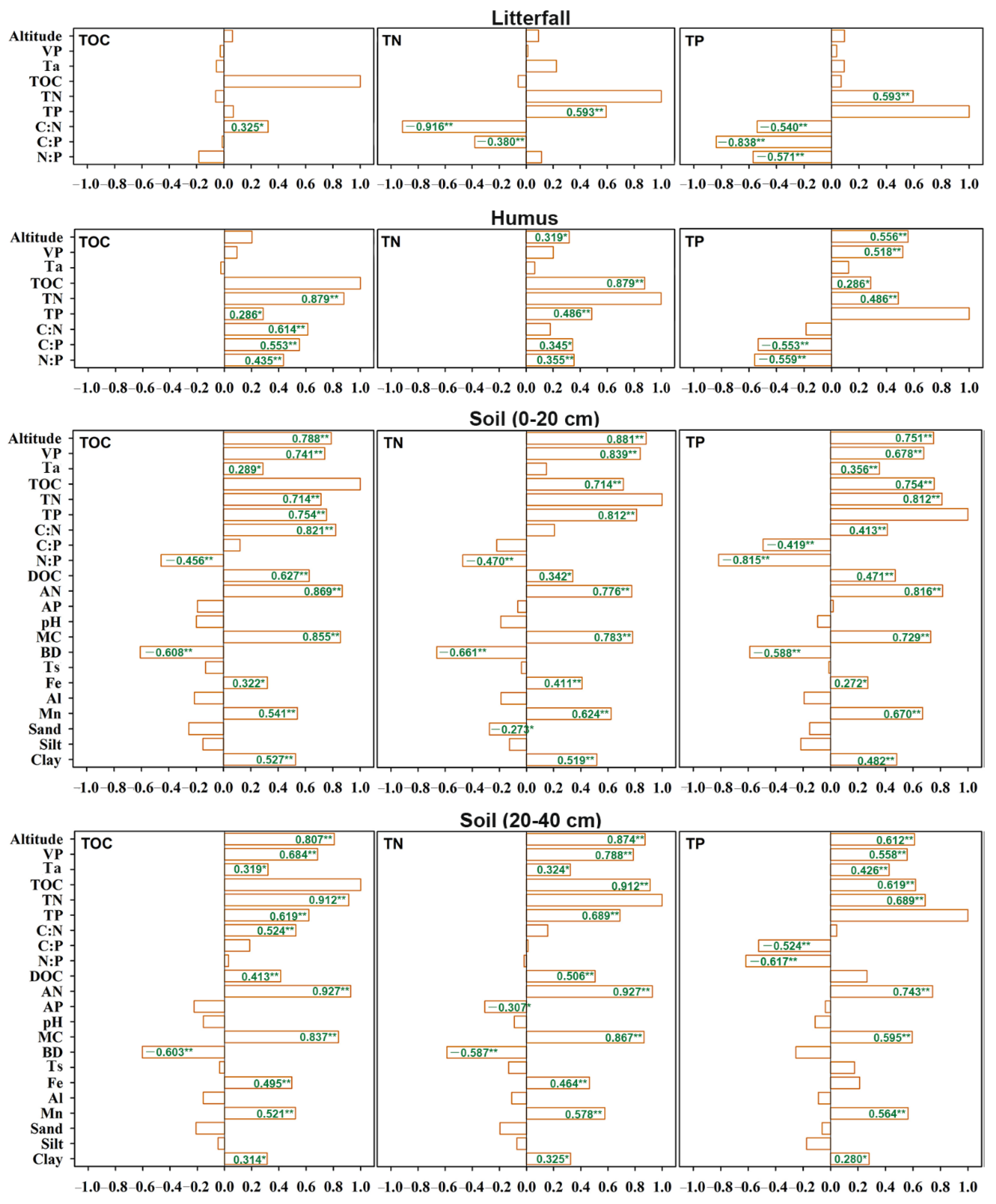
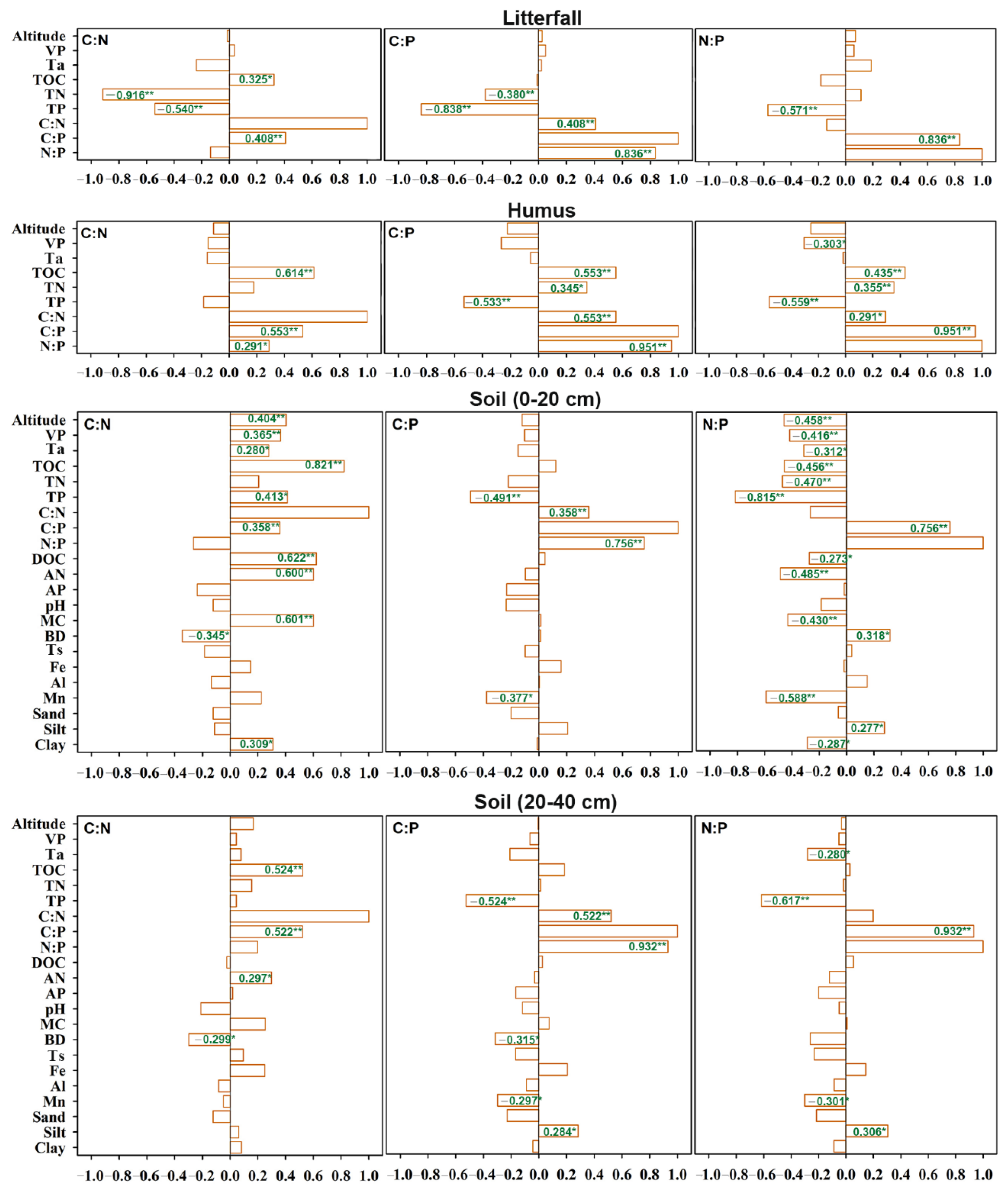
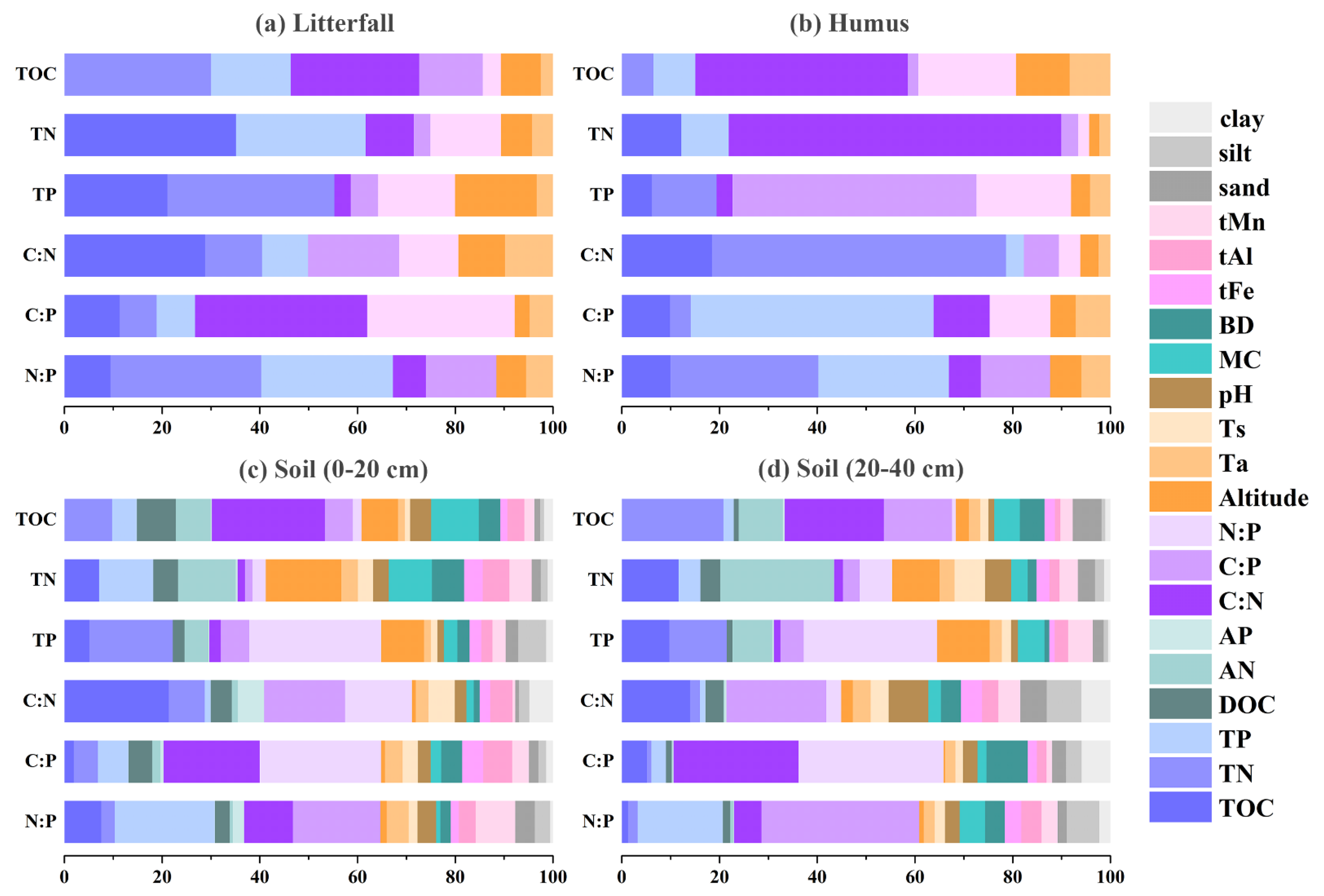
3.3. Driving Factors for C, N, and P Contents and Their Stoichiometric Patterns
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: London, UK, 2012. [Google Scholar]
- He, J.Z.; Zheng, Y.; Qu, J. Soil environmental micro-interfaces and pollution control. Huanjing Kexue Xuebao/Acta Sci. Circumstantiae 2009, 29, 21–27. [Google Scholar]
- Wang, M.; Gong, Y.; Lafleur, P.; Wu, Y. Patterns and drivers of carbon, nitrogen and phosphorus stoichiometry in Southern China’s grasslands. Sci. Total Environ. 2021, 785, 147201. [Google Scholar] [CrossRef]
- Buchkowski, R.; Schmitz, O.; Bradford, M. Microbial stoichiometry overrides biomass as a regulator of soil carbon and nitrogen cycling. Ecology 2015, 96, 1139–1149. [Google Scholar] [CrossRef]
- Wang, X.G.; Lu, X.T.; Han, X.G. Responses of nutrient concentrations and stoichiometry of senesced leaves in dominant plants to nitrogen addition and prescribed burning in a temperate steppe. Ecol. Eng. 2014, 70, 154–161. [Google Scholar] [CrossRef]
- Liu, L.; Gundersen, P.; Zhang, T.; Mo, J.M. Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biol. Biochem. 2012, 44, 31–38. [Google Scholar] [CrossRef]
- Sistla, S.A.; Schimel, J.P. Stoichiometric flexibility as a regulator of carbon and nutrient cycling in terrestrial ecosystems under change. New Phytol. 2021, 196, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, C.C.; Houlton, B.Z.; Smith, W.K.; Marklein, A.R.; Reed, S.C.; Parton, W.; Del Grosso, S.J.; Running, S.W. Patterns of new versus recycled primary production in the terrestrial biosphere. Proc. Natl. Acad. Sci. USA 2013, 110, 12733–12737. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, L.F.; Chen, Y.M.; Zhang, J.; Liu, Y. Litter stoichiometric traits have stronger impact on humification than environment conditions in an alpine treeline ecotone. Plant Soil 2020, 453, 545–560. [Google Scholar] [CrossRef]
- Chadwick, O.A.; Derry, L.A.; Vitousek, P.M.; Huebert, B.J.; Hedin, L.O. Changing sources of nutrients during four million years of ecosystem development. Nature 1999, 397, 491–497. [Google Scholar] [CrossRef]
- Leff, J.W.; Wieder, W.R.; Taylor, P.G.; Townsend, A.R.; Nemergut, D.R.; Grandy, A.S.; Cleveland, C.C. Experimental litterfall manipulation drives large and rapid changes in soil carbon cycling in a wet tropical forest. Glob. Change Biol. 2012, 18, 2969–2979. [Google Scholar] [CrossRef] [PubMed]
- Santiago, L.S. Nutrient limitation of eco-physiological processes in tropical trees. Trees 2015, 29, 1291–1300. [Google Scholar] [CrossRef]
- Elser, J.J.; Andersen, T.; Baron, J.S.; Bergstroem, A.K.; Jansson, M.; Kyle, M.; Nydick, K.R.; Steger, L.; Hessen, D.O. Shifts in lake N:P stoichiometry and nutrient limitation driven by atmospheric nitrogen deposition. Science 2009, 326, 835–837. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Rivas-Ubach, A.; Peñuelas, J. The C:N:P stoichiometry of organisms and ecosystems in a changing world: A review and perspectives. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 33–47. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, J.; Ji, C.; Datta, A.; Li, P.; Ma, W.; Mohammat, A.; Shen, H.; Hu, H.; Knapp, B.O.; et al. Stoichiometric shifts in surface soils over broad geographical scales: Evidence from China’s grasslands. Glob. Ecol. Biogeogr. 2014, 23, 947–955. [Google Scholar] [CrossRef]
- Hui, D.F.; Yang, X.T.; Deng, Q.; Liu, Q.; Wang, X.; Yang, H.; Ren, H. Soil C:N:P stoichiometry in tropical forests on Hainan Island of China: Spatial and vertical variations. Catena 2021, 201, 105228. [Google Scholar] [CrossRef]
- Liu, F.T.; Wang, X.Q.; Chi, Q.H.; Tian, M. Spatial variations in soil organic carbon, nitrogen, phosphorus contents and controlling factors across the “Three Rivers” regions of southwest China. Sci. Total Environ. 2021, 794, 148795. [Google Scholar] [CrossRef]
- Perez-Quezada, J.F.; Pérez, C.A.; Brito, C.E.; Fuentes, J.P.; Gaxiola, A.; Aguilera-Riquelme, D.; Lopatin, J. Biotic and abiotic drivers of carbon, nitrogen and phosphorus stocks in a temperate rainforest. For. Ecol. Manag. 2021, 494, 119341. [Google Scholar] [CrossRef]
- Soethe, N.; Lehmann, J.; Engels, C. Carbon and nutrient stocks in roots of forests at different altitudes in the Ecuadorian Andes. J. Trop. Ecol. 2007, 23, 319–328. [Google Scholar] [CrossRef]
- Sundqvist, M.K.; Wardle, D.A.; Vincent, A.; Giesler, R. Contrasting nitrogen and phosphorus dynamics across an elevational gradient for subarctic tundra heath and meadow vegetation. Plant Soil 2014, 383, 387–399. [Google Scholar] [CrossRef]
- Lu, S.J.; Si, J.H.; Qi, Y.; Wang, Z.Q.; Wu, X.C.; Hou, C.Y. Distribution characteristics of TOC, TN and TP in the wetland sediments of Longbao Lake in the San-Jiang head waters. Acta Geophys. 2016, 64, 2471–2486. [Google Scholar] [CrossRef]
- Wang, Z.C.; He, G.X.; Hou, Z.H.; Luo, Z.; Chen, S.X.; Lu, J.; Zhao, J. Soil C:N:P stoichiometry of typical coniferous (Cunninghamia lanceolata) and/or evergreen broadleaved (Phoebe bournei) plantations in south China. For. Ecol. Manag. 2021, 486, 118974. [Google Scholar] [CrossRef]
- Hu, Q.J.; Sheng, M.Y.; Bai, Y.X.; Jie, Y.; Xiao, H.L. Response of C, N, and P stoichiometry characteristics of Broussonetia papyrifera to altitude gradients and soil nutrients in the karst rocky ecosystem, SW China. Plant Soil 2020, 475, 123–136. [Google Scholar] [CrossRef]
- Bangroo, S.A.; Najar, G.R.; Rasool, A. Effect of altitude and aspect on soil organic carbon and nitrogen stocks in the Himalayan Mawer Forest Range. Catena 2017, 158, 63–68. [Google Scholar] [CrossRef]
- Svensson, T.; Sandén, P.; Bastviken, D.; Öberg, G. Chlorine transport in a small catchment in southeast Sweden during two years. Biogeochemistry 2007, 82, 181–199. [Google Scholar] [CrossRef]
- Zhao, C.M.; Chen, W.L.; Tian, Z.Q.; Xie, Z.Q. Altitudinal pattern of plant species diversity in Shennongjia mountains, central China. J. Integr. Plant Biol. 2005, 47, 1431–1449. [Google Scholar] [CrossRef]
- Wang, M.; Wang, C.H.; Yang, L.; Guo, H. Impacts of short-term nitrogen addition on the thallus nitrogen and phosphorus balance of the dominant epiphytic lichens in the Shennongjia mountains, China. J. Plant Ecol. 2019, 12, 751–758. [Google Scholar] [CrossRef]
- Dang, H.S.; Zhang, Y.J.; Zhang, K.R.; Jiang, M.X.; Zhang, Q.F. Climate-growth relationships of subalpine fir (Abies fargesii) across the altitudinal range in the Shennongjia Mountains, central China. Clim. Chang. 2013, 117, 903–917. [Google Scholar] [CrossRef]
- Wang, H.; Hou, P.; Jiang, J.B.; Xiao, R.L.; Zhai, J.; Fu, Z.; Hou, J. Ecosystem health assessment of Shennongjia National Park, China. Sustainability 2020, 12, 7672. [Google Scholar] [CrossRef]
- Zhong, Z.L.; Bing, H.J.; Xiang, Z.X.; Wu, Y.H.; Zhou, J.; Ding, S.M. Terrain-modulated deposition of atmospheric lead in the soils of alpine forest, central China. Sci. Total Environ. 2021, 790, 148106. [Google Scholar] [CrossRef] [PubMed]
- Borovec, J.; Sirová, D.; Mošnerová, P.; Rejmánková, E.; Vrba, J. Spatial and temporal changes in phosphorus partitioning within a freshwater cyanobacterial mat community. Biogeochemistry 2010, 101, 323–333. [Google Scholar] [CrossRef]
- Kaal, J.; Costa-Casais, M.; Ferro-Vázquez, C.; Pontevedra-Pombal, X.; Martínez-Cortizas, A. Soil formation of “Atlantic Rankers” from NW Spain—A high resolution aluminium and iron fractionation study. Pedosphere 2008, 18, 441–453. [Google Scholar] [CrossRef]
- Vereecken, H.; Maes, J.; Feyen, J.; Darius, P. Estimating the soil moisture retention characteristic from texture, bulk density, and carbon content. Soil Sci. 1989, 148, 389–403. [Google Scholar] [CrossRef]
- Chen, D.V.; Ding, J. Study on Influencing Factors of Measurement of Total Nitrogen by Digestion with UV-Alkaline Potassium Persulfate and Reduction with Hydrazine Sulphate Spectrophotometric Method and Application. 2008. Available online: https://ieeexplore.ieee.org/stamp/stamp.jsp?tp=&arnumber=4536020&tag=1 (accessed on 14 September 2022).
- Hadas, A.; Kautsky, L.; Goek, M.; Erman Kara, E. Rates of decomposition of plant residues and available nitrogen in soil, related to residue composition through simulation of carbon and nitrogen turnover. Soil Biol. Biochem. 2004, 36, 255–266. [Google Scholar] [CrossRef]
- Wilson, S.D.; Kleb, H.R. The influence of prairie and forest vegetation on soil moisture and available nitrogen. Am. Midl. Nat. 1996, 136, 222. [Google Scholar] [CrossRef]
- Zibilske, L.M.; Bradford, J.M.; Smart, J.R. Conservation tillage induced changes in organic carbon, total nitrogen and available phosphorus in a semi-arid alkaline subtropical soil. Soil Tillage Res. 2002, 66, 153–163. [Google Scholar] [CrossRef]
- Tüzen, M. Determination of heavy metals in soil, mushroom and plant samples by atomic absorption spectrometry. Microchem. J. 2003, 74, 289–297. [Google Scholar] [CrossRef]
- De’Ath, G. Boosted trees for ecological modeling and prediction. Ecology 2007, 88, 243–251. [Google Scholar] [CrossRef]
- Frey, S.J.K.; Hadley, A.S.; Johnson, S.L.; Schulze, M.; Jones, J.A.; Betts, M.G. Spatial models reveal the microclimatic buffering capacity of old-growth forests. Sci. Adv. 2016, 2, e1501392. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.Z.; Chen, A.P.; Piao, S.L.; Rabin, S.; Shen, Z.H. Environmental determinants of tropical forest and savanna distribution: A quantitative model evaluation and its implication. J. Geophys. Res. Biogeosci. 2014, 119, 1432–1445. [Google Scholar] [CrossRef]
- Liu, R.; Ma, T.; Qiu, W.K.; Peng, Z.Q.; Shi, C.X. The environmental functions and ecological effects of organic carbon in silt. J. Earth Sci. 2020, 31, 834–844. [Google Scholar] [CrossRef]
- Hu, L.; Ade, L.J.; Wu, X.W.; Zi, H.B.; Luo, X.P.; Wang, C.T. Changes in soil C:N:P stoichiometry and microbial structure along soil depth in two forest soils. Forests 2019, 10, 113. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Gu, H.Y.; Pan, J.P.; Chen, X.W. Soil phosphorus fractions and their availability over natural succession from clear-cut of a mixed broadleaved and Korean pine forest in northeast China. J. For. Res. 2022, 33, 253–260. [Google Scholar] [CrossRef]
- Smeck, N.E. Phosphorus dynamics in soils and landscapes. Geoderma 1985, 36, 185–199. [Google Scholar] [CrossRef]
- Chen, X.; Feng, J.G.; Ding, Z.J.; Tang, M.; Zhu, B. Changes in soil total, microbial and enzymatic C-N-P contents and stoichiometry with depth and latitude in forest ecosystems. Sci. Total Environ. 2022, 816, 151583. [Google Scholar] [CrossRef]
- Wei, X.Y.; Yang, Y.L.; Shen, Y.; Chen, Z.H.; Dong, Y.L.; Wu, F.Z.; Zhang, L. Effects of Litterfall on the Accumulation of Extracted Soil Humic Substances in Subalpine Forests. Front. Plant Sci. 2020, 11, 254. [Google Scholar] [CrossRef] [PubMed]
- Norby, R.J.; Cotrufo, M.F. Global change: A question of litter quality. Nature 1998, 396, 17–18. [Google Scholar] [CrossRef]
- Bing, H.J.; Wu, Y.H.; Zhou, J.; Sun, H.Y.; Luo, J.; Wang, J.P.; Yu, D. Stoichiometric variation of carbon, nitrogen, and phosphorus in soils and its implication for nutrient limitation in alpine ecosystem of Eastern Tibetan Plateau. J. Soils Sediments 2016, 16, 405–416. [Google Scholar] [CrossRef]
- Boutton, T.W.; Liao, J.D. Changes in soil nitrogen storage and δ15N with woody plant encroachment in a subtropical savanna parkland landscape. J. Geophys. Res. 2010, 115, G03019. [Google Scholar] [CrossRef]
- Tessier, J.T.; Raynal, D.J. Vernal nitrogen and phosphorus retention by forest understory vegetation and soil microbes. Plant Soil 2003, 256, 443–453. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef]
- Han, W.X.; Fang, J.Y.; Guo, D.L.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Öberg, G.; Holm, M.; Sandén, P.; Svensson, T.; Parikka, M. The role of organic-matter-bound chlorine in the chlorine cycle: A case study of the Stubbetorp Catchment, Sweden. Biogeochemistry 2005, 75, 241–269. [Google Scholar] [CrossRef]
- Kaiser, K.; Kaupenjohann, M.; Zech, W. Sorption of dissolved organic carbon in soils: Effects of soil sample storage, soil-to-solution ratio, and temperature. Geoderma 2001, 99, 317–328. [Google Scholar] [CrossRef]
- Weintraub, M.N.; Scott-Denton, L.E.; Schmidt, S.K.; Monson, R.K. The effects of tree rhizodeposition on soil exoenzyme activity, dissolved organic carbon, and nutrient availability in a subalpine forest ecosystem. Oecologia 2007, 154, 327–338. [Google Scholar] [CrossRef]
- Kopáček, J.; Evans, C.D.; Hejzlar, J.; Kaňa, J.; Porcal, P.; Šantrůčková, H. Factors affecting the leaching of dissolved organic carbon after tree dieback in an Unmanaged European Mountain Forest. Environ. Sci. Technol. 2018, 52, 6291–6299. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.G.; Zhao, P.; Shen, W.J.; Rao, X.Q.; Hu, Y.T. Physiological homeostasis and morphological plasticity of two tree species subjected to precipitation seasonal distribution changes. Perspect. Plant Ecol. Evol. Syst. 2017, 25, 1–19. [Google Scholar] [CrossRef]
- Li, W.B.; Jin, C.J.; Guan, D.X.; Wang, Q.K.; Wang, A.Z.; Yuan, F.H.; Wu, J.B. The effects of simulated nitrogen deposition on plant root traits: A meta-analysis. Soil Biol. Biochem. 2015, 82, 112–118. [Google Scholar] [CrossRef]
- Oleksyn, J.; Reich, P.B.; Zytkowiak, R.; Karolewski, P.; Tjoelker, M.G. Nutrient conservation increases with latitude of origin in European Pinus sylvestris populations. Oecologia 2003, 136, 220–235. [Google Scholar] [CrossRef]
- Zhou, T.; Geng, Y.J.; Ji, C.; Xu, X.R.; Wang, H.; Pan, J.J.; Bumberger, J.; Haase, D.; Lausch, A. Prediction of soil organic carbon and the C:N ratio on a national scale using machine learning and satellite data: A comparison between Sentinel-2, Sentinel-3 and Landsat-8 images. Sci. Total Environ. 2021, 755, 142661. [Google Scholar] [CrossRef] [PubMed]
- Gerdol, R.; Iacumin, P.; Brancaleoni, L. Differential effects of soil chemistry on the foliar resorption of nitrogen and phosphorus across altitudinal gradients. Funct. Ecol. 2019, 33, 1351–1361. [Google Scholar] [CrossRef]
- Xu, G.C.; Li, Z.B.; Li, P.; Zhang, T.G.; Cheng, S.D. Spatial variability of soil available phosphorus in a typical watershed in the source area of the middle Dan River, China. Environ. Earth Sci. 2014, 71, 3953–3962. [Google Scholar] [CrossRef]
- McGroddy, M.E.; Daufresne, T.; Hedin, L.O. Scaling of C:N:P stoichiometry in forests worldwide: Implications of terrestrial redfield-type ratios. Ecology 2004, 85, 2390–2401. [Google Scholar] [CrossRef]
- Cao, Y.M.; Chen, Y. Ecosystem C:N:P stoichiometry and carbon storage in plantations and a secondary forest on the Loess Plateau, China. Ecol. Eng. 2017, 105, 125–132. [Google Scholar] [CrossRef]
- Qualls, R.G.; Richardson, C.J. Phosphorus enrichment affects litter decomposition, immobilization, and soil microbial phosphorus in Wetland Mesocosms. Soil Sci. Soc. Am. J. 2020, 64, 799–808. [Google Scholar] [CrossRef]
- Huggett, R.J. Soil chronosequences, soil development, and soil evolution: A critical review. Catena 1998, 32, 155–172. [Google Scholar] [CrossRef]
- Torn, M.S.; Trumbore, S.E.; Chadwick, O.A.; Vitousek, P.M.; Hendricks, D.M. Mineral control of soil organic carbon storage and turnover. Nature 1997, 389, 170–173. [Google Scholar] [CrossRef]
- Morford, S.L.; Houlton, B.Z.; Dahlgren, R.A. Direct quantification of long-term rock nitrogen inputs to temperate forest ecosystems. Ecology 2016, 97, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Houlton, B.Z.; Morford, S.L.; Dahlgren, R.A. Convergent evidence for widespread rock nitrogen sources in Earth’s surface environment. Science 2018, 360, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Kabala, C.; Chachulski, A.; Gądek, B.; Korabiewski, B.; Mętrak, M.; Suska-Malawska, M. Soil development and spatial differentiation in a glacial river valley under cold and extremely arid climate of East Pamir Mountains. Sci. Total Environ. 2021, 758, 144308. [Google Scholar] [CrossRef]
- Abera, T.; Bekele, L. Soil bulk density, soil moisture content and yield of Tef (Eragrostis tef) influenced by Acacia seyal Del canopy in Parkland agro-forestry system. J. Soil Sci. Environ. Manag. 2019, 10, 124–129. [Google Scholar] [CrossRef]
- Quijano, L.; Kuhn, N.J.; Navas, A. Effects of interrill erosion on the distribution of soil organic and inorganic carbon in different sized particles of Mediterranean Calcisols. Soil Tillage Res. 2020, 196, 104461. [Google Scholar] [CrossRef]
- Zhou, W.X.; Han, G.L.; Liu, M.; Zeng, J.; Liang, B.; Liu, J.K.; Qu, R. Determining the distribution and interaction of soil organic carbon, nitrogen, pH and texture in soil profiles: A Case Study in the Lancangjiang River Basin, Southwest China. Forests 2020, 11, 532. [Google Scholar] [CrossRef]
- Zhang, K.; Su, Y.Z.; Yang, R. Variation of soil organic carbon, nitrogen, and phosphorus stoichiometry and biogeographic factors across the desert ecosystem of Hexi Corridor, northwestern China. J. Soils Sediments 2019, 19, 49–57. [Google Scholar] [CrossRef]
- Olander, L.P.; Vitousek, P.M. Biological and geochemical sinks for phosphorus in soil from a Wet Tropical Forest. Ecosystems 2004, 7, 404–419. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen-phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Palumbo, G.; He, J.; Pinton, R.; Cesco, S. Review on iron availability in soil: Interaction of Fe minerals, plants, and microbes. J. Soils Sediments 2014, 14, 538–548. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, F.; Chen, L.; Zheng, J.; Chen, Z.; Wang, X.; Xia, X. Elevation-Dependent Fluctuations of the Soil Properties in a Subtropical Forest of Central China. Sustainability 2022, 14, 15855. https://doi.org/10.3390/su142315855
Ju F, Chen L, Zheng J, Chen Z, Wang X, Xia X. Elevation-Dependent Fluctuations of the Soil Properties in a Subtropical Forest of Central China. Sustainability. 2022; 14(23):15855. https://doi.org/10.3390/su142315855
Chicago/Turabian StyleJu, Fanfan, Liuzhu Chen, Jiejun Zheng, Zhanqiang Chen, Xiaoli Wang, and Xinxing Xia. 2022. "Elevation-Dependent Fluctuations of the Soil Properties in a Subtropical Forest of Central China" Sustainability 14, no. 23: 15855. https://doi.org/10.3390/su142315855
APA StyleJu, F., Chen, L., Zheng, J., Chen, Z., Wang, X., & Xia, X. (2022). Elevation-Dependent Fluctuations of the Soil Properties in a Subtropical Forest of Central China. Sustainability, 14(23), 15855. https://doi.org/10.3390/su142315855




