Essential Oil Composition of Aerial Part of Pluchea ovalis (Pers.) DC., Silver Nanoparticles Synthesis, and Larvicidal Activities against Fall Armyworm
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material Collection, Authentication, and Preparation
2.2. Extraction of Essential Oil from P. ovalis
2.3. Physicochemical Properties
2.4. Instrument Analyses
2.5. Biosynthesis of Silver Nanoparticles
2.6. Characterization of POEO-AgNPs
2.7. Larvicidal Activity Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Extraction of Essential Oil of P. ovalis
3.2. Instrumental Analyses of Essential Oil of P. ovalis
3.3. Green Synthesis of AgNPs
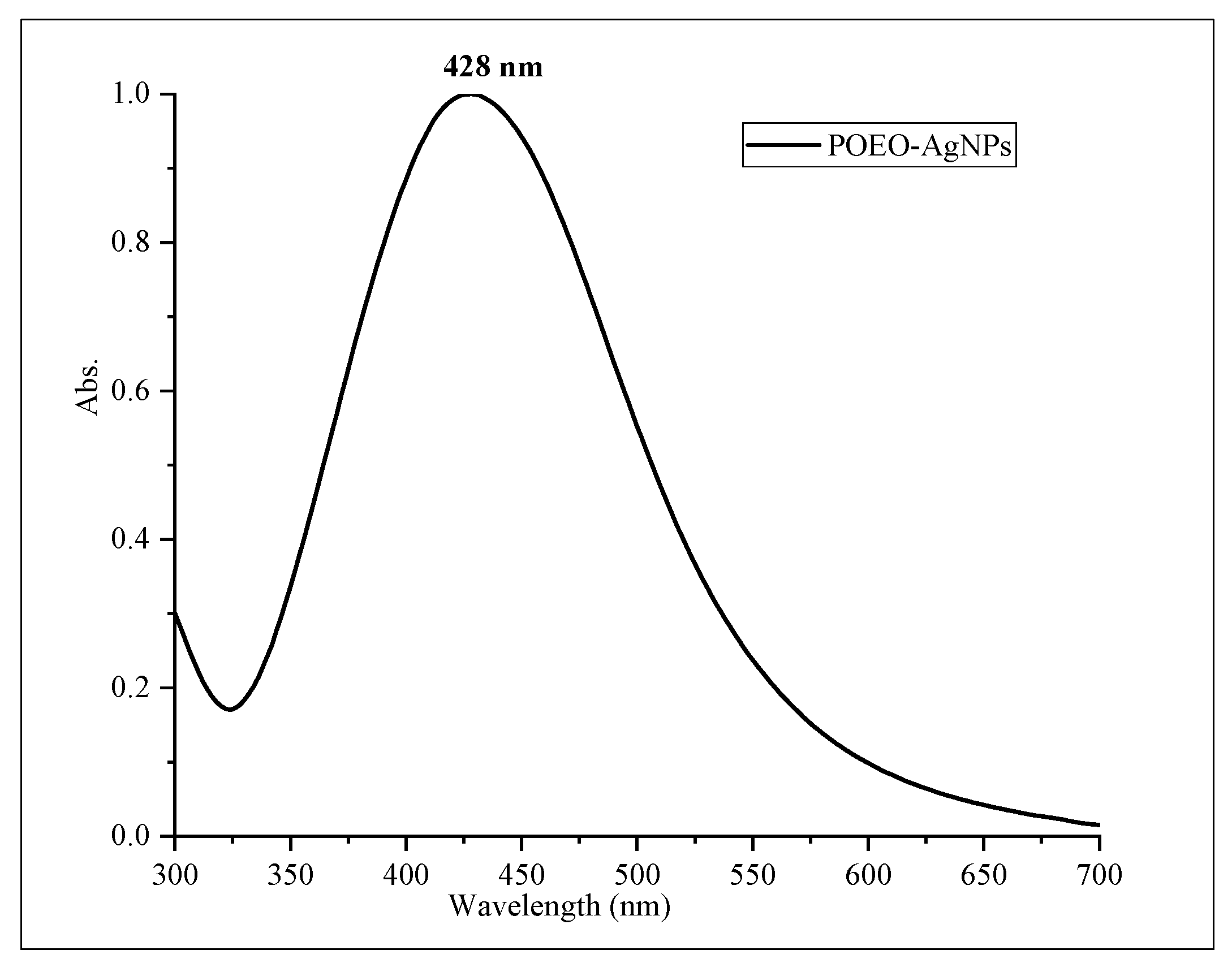
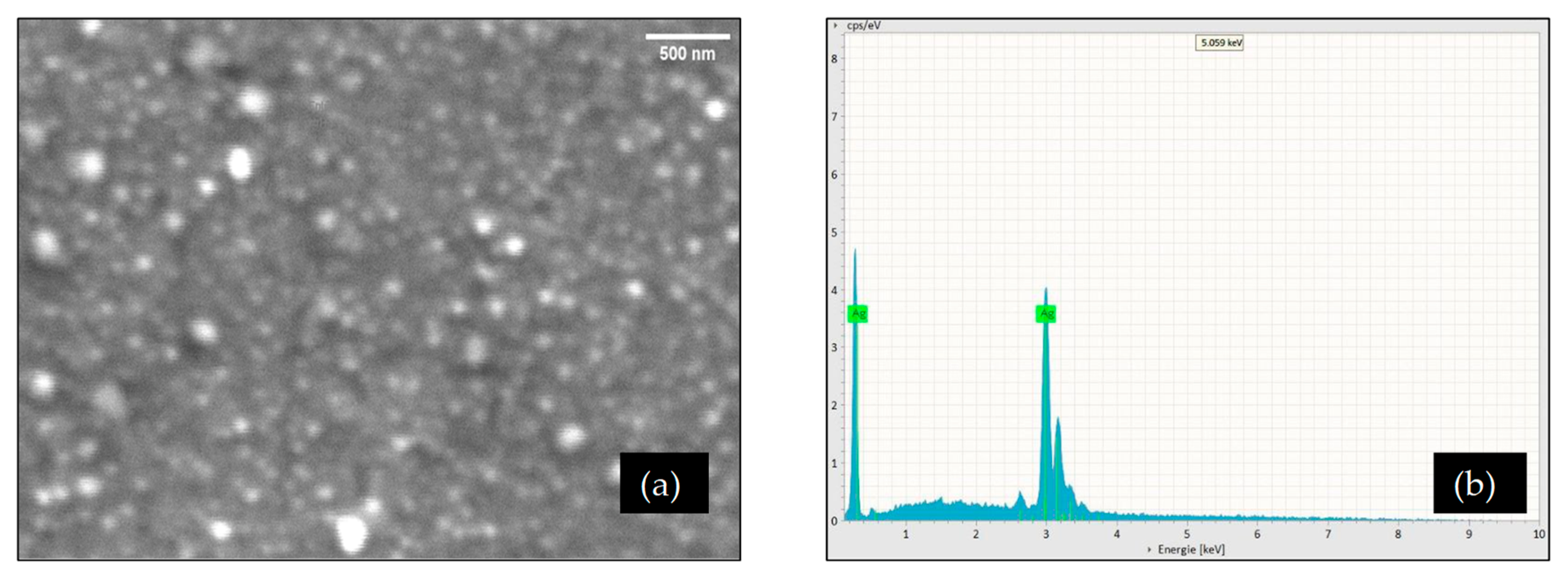
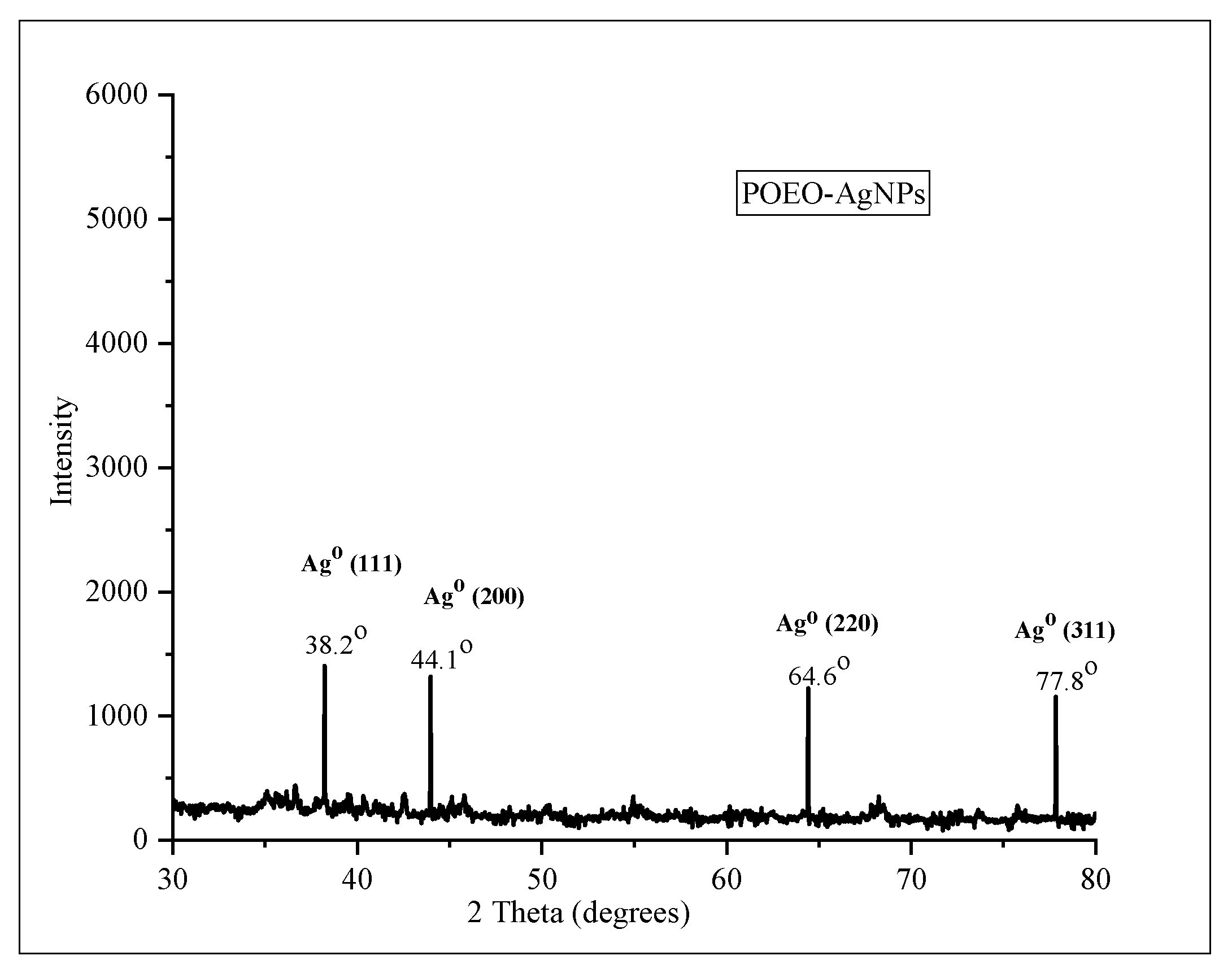
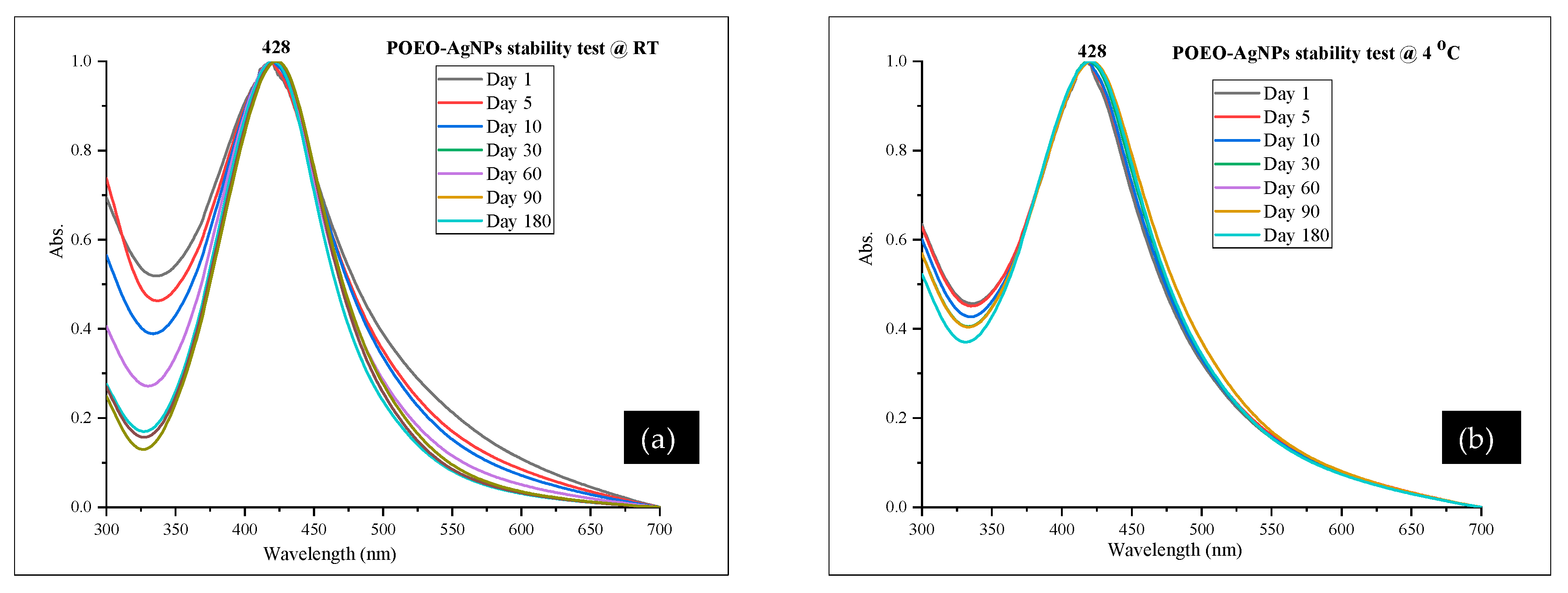
3.4. Characterization of POEO-AgNPs
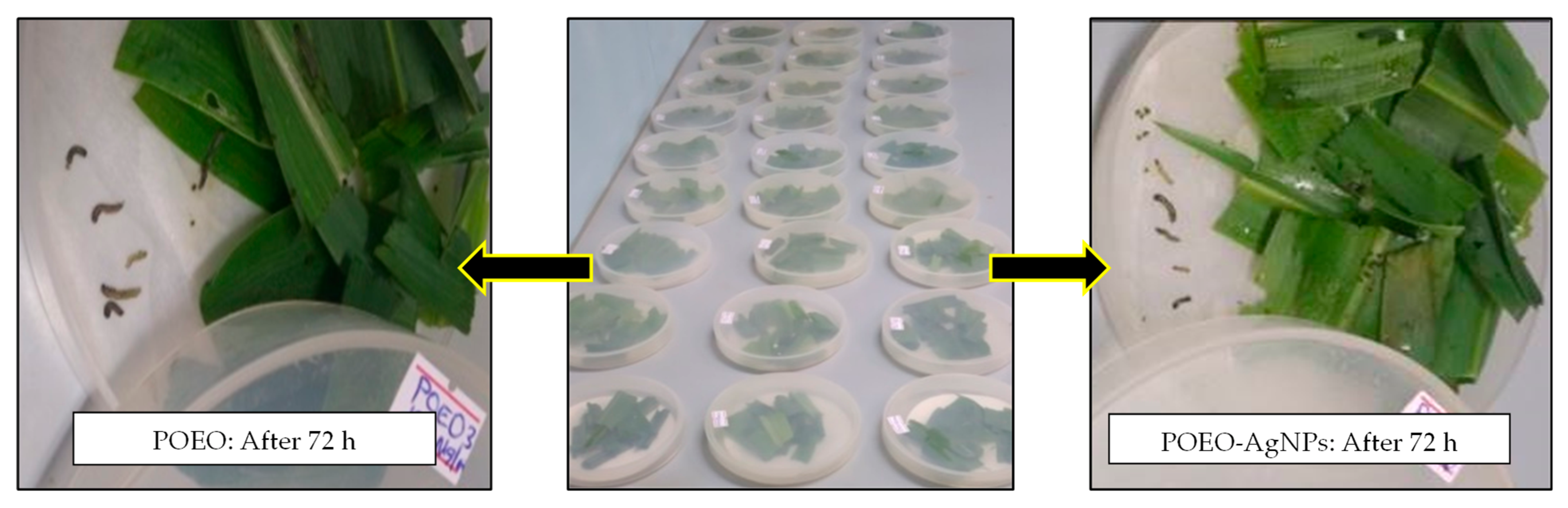
3.5. Larvicidal Activity Tests of POEO and Its AgNPs Solutions against Fall Armyworm Larvae
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddhartha, K.; Chinniah, C.; Shanthi, M. In vitro bioassay of certain botanical oils for their efficacy against maize fall armyworm (J.E. Smith) Spodoptera frugiperda (Noctuidae: Lepidoptera). J. Entomol. Zool. Stud. 2019, 7, 606–609. [Google Scholar]
- Tamboa, J.A.; Day, R.K.; Lamontagne-Godwin, J. Tackling fall armyworm (Spodoptera frugiperda) outbreak in Africa: An analysis of farmers’ control actions. Int. J. Pest Manag. 2020, 66, 298–310. [Google Scholar] [CrossRef]
- Sagar, G.C.; Aastha, B.; Laxman, K. An introduction of fall armyworm (Spodoptera frugiperda) with management strategies: A review paper. Nippon J. Environ. Sci. 2020, 1, 1010. [Google Scholar] [CrossRef]
- Ahmed, K.S.; Idrees, A.; Majeed, M.Z.; Majeed, M.I.; Shehzad, M.Z.; Ullah, M.I.; Afzal, A.; Li, J. Synergized toxicity of promising plant extracts and synthetic chemicals against fall armyworm Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) in Pakistan. Agronomy 2022, 12, 1289. [Google Scholar] [CrossRef]
- Phambala, K.; Tembo, Y.; Kasambala, T.; Kabambe, V.H.; Stevenson, P.C.; Belmain, S.C. Bioactivity of common pesticidal plants on fall armyworm larvae (Spodoptera frugiperda). Plants 2020, 9, 112. [Google Scholar] [CrossRef]
- Minh-Trong, T.; Linh-Phuong, N.; Dinh-Truong, N.; Le Cam-Huong, T.; Chi-Hien, D.; Kim, C.T.T.; Thanh-Danh, N. A novel approach using plant embryos for green synthesis of silver nanoparticles as antibacterial and catalytic agent. Res. Chem. Interm. 2021, 47, 4613–4633. [Google Scholar] [CrossRef]
- Aouadi, G.; Soltani, A.; Grami, L.K.; Abada, M.B.; Haouel, S.; Boushih, E.; Chaanbi, M.; Elkahoui, S.; Hajlaoui, M.R.; Jemâa, J.M.B.; et al. Chemical investigations on Algerian Mentha rotundifolia and Myrtus communis essential oils and assessment of their insecticidal and antifungal activities. Int. J. Agric. Biol. 2021, 26, 667–680. [Google Scholar] [CrossRef]
- Isman, M.B. Plant essential oils for pest and disease management. J. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Kerdudo, A.; Gonnot, V.; Ellong, E.N.; Boyer, L.; Chandre, F.; Adenet, S.; Rochefort, K.; Michel, T.; Fernandez, X. Composition and bioactivity of Pluchea carolinensis (Jack.) G. essential oil from Martinique. Ind. Crops Prod. 2016, 89, 295–302. [Google Scholar] [CrossRef]
- Martins, E.; Poncelet, D.; Rodrigues, R.C.; Renard, D. Oil encapsulation techniques using alginate as encapsulating agent: Applications and drawbacks. J. Microencapsul. 2017, 34, 754–771. [Google Scholar] [CrossRef]
- Dias, C.N.; Alves, L.P.L.; Rodrigues, K.A.F.; Brito, M.C.A.; Rosa, C.S.; Amaral, F.M.M.; Monteiro, O.S.; Andrade, E.H.A.; Maia, J.G.S.; Moraes, D.F.C. Chemical composition and larvicidal activity of essential oils extracted from Brazilian legal Amazon plants against Aedes aegypti L. (Diptera: Culicidae). Evid.-Based Complement. Altern. Med. 2015, 490765. [Google Scholar] [CrossRef]
- Chantawee, A.; Soonwera, M. Efficacies of four plant essential oils as larvicide, pupicide and oviposition deterrent agents against dengue fever mosquito, Aedes aegypti Linn. (Diptera: Culicidae). Asian Pac. J. Trop. Biomed. 2018, 8, 217–225. [Google Scholar] [CrossRef]
- Alves, A.; Filho, P.; Pessoa, G.C.; Yamaguchi, L.F.; Kato, M.J. Larvicidal activity of essential oils from Piper Species against strains of Aedes aegypti (Diptera: Culicidae) resistant to Pyrethroids. Front. Plant Sci. 2021, 12, 685864. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, D. Essential oils: As potential larvicides. J. Drug Deliv. Ther. 2022, 12, 193–201. [Google Scholar] [CrossRef]
- Kale, S.K.; Parishwad, G.V.; Husainy, A.S.; Patil, A.S. Emerging agriculture applications of silver nanoparticles. ES Food Agrofor. 2021, 3, 17–22. [Google Scholar] [CrossRef]
- Montes-Garcia, V.; Squillaci, M.A.; Diez-Castellnou, M.; Ong, Q.K.; Stellacci, F.; Samorì, P. Chemical sensing with Au and Ag nanoparticles. Chem. Soc. Rev. 2021, 50, 1269–1304. [Google Scholar] [CrossRef]
- Shehabeldine, A.M.; Elbahnasawy, M.A.; Hasaballah, A.I. Green photosynthesis of silver nanoparticles using Echinochloa stagnina extract with reference to their antibacterial, cytotoxic, and larvicidal activities. Bionanoscience 2021, 11, 526–538. [Google Scholar] [CrossRef]
- Allam, R.O.; Badawy, A.M.; Ali, M.A. Green synthesized silver nanoparticles for controlling subterranean termites, Psammotermes hypostoma (Desn.). SVU-Int. J. Agric. Sci. 2022, 4, 135–143. [Google Scholar] [CrossRef]
- Poudel, D.K.; Niraula, P.; Aryal, H.; Budhathoki, B.; Phuyal, S.; Marahatha, R.; Subedi, K. Plant-mediated green synthesis of AgNPs and their possible applications: A critical review. J. Nanotechnol. 2022, 2779237. [Google Scholar] [CrossRef]
- Sahayaraj, K.; Madasamy, M.; Radhika, S.A. Insecticidal activity of bio-silver and gold nanoparticles against Pericallia ricini Fab. (Lepidoptera: Archidae). J. Biopestic. 2016, 9, 63–72. [Google Scholar]
- Rehman, H.; Majeed, B.; Farooqi, M.A.; Rasul, A.; Sagheer, M.; Ali, Q.; Akhtar, Z.R. Green synthesis of silver nitrate nanoparticles from Camelina sativa (L.) and its effect to control insect pests of stored grains. Int. J. Trop. Insect Sci. 2021, 41, 3031–3039. [Google Scholar] [CrossRef]
- Benelli, C.G.; Losic, D. Nanoparticles for pest control: Current status and future perspectives. J. Pest Sci. 2017, 91, 1–15. [Google Scholar] [CrossRef]
- Huq, M.A.; Ashrafudoulla, M.; Rahman, M.M.; Balusamy, S.R.; Akter, S. Green synthesis and potential antibacterial applications of bioactive silver nanoparticles: A review. Polymers 2022, 14, 742. [Google Scholar] [CrossRef] [PubMed]
- Perera, W.; Kevers, C.; Michiels, J.A.; Spengler, S.I.; Dommes, J. Variation in phenolic constituents and antioxidant capacities of plant organs of three Cuban species of Pluchea Cass. (Asteraceae) under ex vitro and in vitro growth conditions. J. Med. Plant Res. 2013, 7, 2527–2536. [Google Scholar] [CrossRef]
- Hussain, H.; Al-Harrasi, A.; Abbas, G.; Ur Rehman, N.; Mabood, F.; Ahmed, I.; Saleem, M.; van Ree, T.; Green, I.R.; Anwarg, S.; et al. The genus Pluchea: Phytochemistry, traditional uses, and biological activities. Chem. Biodivers. 2013, 10, 1944–1971. [Google Scholar] [CrossRef]
- Srivastava, P.; Shanker, K. Pluchea lanceolata (Rasana): Chemical and biological potential of Rasayana herb used in traditional system of medicine. Fitoterapia 2012, 83, 1371–1385. [Google Scholar] [CrossRef]
- Mandeel, Q.; Taha, A. Assessment of in vitro antifungal activities of various extracts of indigenous Bahraini medicinal plants. Pharm. Biol. 2005, 43, 340–348. [Google Scholar] [CrossRef]
- Saba, N.; Khatoon, R.; Ahmad, V.U.; Khan, S.S. A new eudesmane sesquiterpene from Pluchea arguta. Nat. Prod. Comm. 2011, 6, 1–2. [Google Scholar] [CrossRef]
- Ruan, J.; Li, Z.; Yan, J.; Huang, P.; Yu, H.; Han, L.; Zhang, Y.; Wang, Y. Bioactive constituents from the aerial parts of Pluchea indica less. Molecules 2018, 23, 2104. [Google Scholar] [CrossRef]
- Goyal, S.K. Biological studies of the plants from genus Pluchea. Ann. Biol. Res. 2011, 2, 25–34. [Google Scholar]
- Shahin, S.M.; Jaleel, A.; Alyafei, M.A. The essential oil-bearing plants in the United Arab Emirates (UAE): An overview. Molecules 2021, 26, 6486. [Google Scholar] [CrossRef]
- Alghamdi, S.B. Identification of volatile constituents of Albaha Pluchea ovalis. Int. Mod. Pharm. Res. 2020, 4, 60–69. [Google Scholar]
- Magalhães, L.A.M.; Lima, M.P.; Marques, M.O.M.; Facanali, R.; Pinto, A.C.S.; Tadei, W.P. Chemical composition and larvicidal activity against Aedes aegypti larvae of essential oils from four Guarea species. Molecules 2010, 15, 5734–5741. [Google Scholar] [CrossRef]
- Akintayo, G.O.; Lateef, A.; Azeez, M.A.; Asafa, T.B.; Oladipo, I.C.; Badmus, J.A.; Ojo, S.A.; Elegbede, J.A.; Gueguim-Kana, E.B.; Beukes, L.S.; et al. Synthesis, bioactivities, and cytogenotoxicity of animal fur-mediated silver nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2020, 805, 1–24. [Google Scholar] [CrossRef]
- Paula, A.; Melo, Z.D.; Vinicius, M.; Brisola, D.O.; Sganzerla, W.G. Antibacterial activity, morphology, and physicochemical stability of biosynthesized silver nanoparticles using thyme (Thymus vulgaris) essential oil. Mater. Res. Express 2020, 7, 015087. [Google Scholar] [CrossRef]
- Singh, D.; Rathod, V.; Ninganagouda, S.; Hiremath, J.; Singh, A.K.; Mathew, J. Optimization and characterization of silver nanoparticle by endophytic fungi Penicillium sp. isolated from Curcuma longa (Turmeric) and application studies against MDR E. coli and S. aureus. Bioinorg. Chem. Appl. 2014, 408021. [Google Scholar] [CrossRef]
- Maciel, M.V.B.; Almeida, A.R.; Machado, M.H.; Elias, W.C.; da Rosa, C.G.; Teixeira, G.L.; Noronha, C.M.; Bertoldi, F.C.; Nunes, M.R.; de Armas, R.D.; et al. Green synthesis, characteristics, and antimicrobial activity of silver nanoparticles mediated by essential oils as reducing agents. Biocatal. Agric. Biotechnol. 2020, 28, 101746. [Google Scholar] [CrossRef]
- Alsubki, R.; Tabassum, H.; Abudawood, M.; Rabaan, A.A.; Alsobaie, S.F.; Ansar, S. Green synthesis, characterization, enhanced functionality, and biological evaluation of silver nanoparticles based on Coriander sativum. Saudi J. Biol. Sci. 2021, 28, 2102–2108. [Google Scholar] [CrossRef]
- Mahajan, P.G.; Dige, N.C.; Vanjare, B.D.; Phull, A.R.; Kim, S.J.; Lee, K.H. Gallotannin mediated silver colloidal nanoparticles as multifunctional nano platform: Rapid colorimetric and turn-on fluorescent sensor for Hg2+, catalytic and in vitro anticancer activities. J. Lumin. 2019, 206, 624–633. [Google Scholar] [CrossRef]
- Ramdath, S.; Mellem, J.; Mbatha, L.S. Anticancer and antimicrobial activity evaluation of Cowpea-Porous-Starch-Formulated silver nanoparticles. J. Nanotechnol. 2021, 5525690. [Google Scholar] [CrossRef]
- Barman, K.; Chowdhury, D.; Baruah, P.K. Bio-synthesized silver nanoparticles using Zingiber officinale rhizome extract as efficient catalyst for the degradation of environmental pollutants. Inorg. Nano-Met. Chem. 2020, 50, 57–65. [Google Scholar] [CrossRef]
- Elgamal, A.M.; Ahmed, R.F.; Abd-elgawad, A.M.; Gendy, A.E.; Elshamy, A.I.; Nassar, M.I. Chemical profiles, anti-cancer, and anti-aging activities of essential oils of Pluchea dioscoridis (L.) DC. and Erigeron bonariensis L. Plants 2021, 10, 667. [Google Scholar] [CrossRef] [PubMed]
- Kibrom, G.; Dibaba, A.; Nigist, A. Cuathemone sesquiterpenes and flavones from Laggera tomentosa endemic to Ethiopia. Bull. Chem. Soc. Ethiop. 2010, 24, 267–271. [Google Scholar]
- Gao, C.X.; Lin, F.X.; Luo, Y.X.; Zhou, X.M. Eudesmane-type sesquiterpenes and aporphine-type alkaloids from Fissistigma maclurei. Biochem. Syst. Ecol. 2021, 97, 104298. [Google Scholar] [CrossRef]
- Kavi, G.; Raghunandan, S.N.; Deepika, A. GC-MS analysis and identification of daidzein by high performance thin layer chromatography (HPTLC) of Pluchea lanceolata: A bone healing plant of semi-arid land. J. Pharm. Res. 2012, 5, 257–260. [Google Scholar]
- Karthik, R.; Chen, S.M.; Elangovan, A.; Muthukrishnan, P.; Shanmugam, R.; Lou, B.S. Phyto mediated biogenic synthesis of gold nanoparticles using Cerasus serrulata and its utility in detecting hydrazine, microbial activity, and DFT studies. J. Colloid Interface Sci. 2016, 468, 163–175. [Google Scholar] [CrossRef]
- Keijok, W.J.; Pereira, R.H.A.; Alvarez, L.A.C.; Prado, A.R.; da Silva, A.R.; Ribeiro, J.; de Oliveira, J.P.; Guimarães, M.C. Controlled biosynthesis of gold nanoparticles with Coffea arabica using factorial design. Sci. Rep. 2019, 9, 16019. [Google Scholar] [CrossRef]
- Khan, S.; Singh, S.; Gaikwad, S.; Nawani, N.; Junnarkar, M.; Pawar, S.V. Optimization of process parameters for the synthesis of silver nanoparticles from Piper betle leaf aqueous extract, and evaluation of their antiphytofungal activity. Environ. Sci. Pollut. Res. 2020, 27, 27221–27233. [Google Scholar] [CrossRef]
- Chokshi, K.; Pancha, I.; Ghosh, T.; Paliwal, C.; Maurya, R.; Ghosh, A.; Mishra, S. Green synthesis, characterization, and antioxidant potential of silver nanoparticles biosynthesized from de-oiled biomass of thermotolerant oleaginous microalgae: Acutodesmus dimorphus. RSC Adv. 2016, 6, 72269–72274. [Google Scholar] [CrossRef]
- Suwannakul, S.; Wacharanad, S.; Chaibenjawong, P. Rapid green synthesis of silver nanoparticles and evaluation of their properties for oral disease therapy. Songklanakarin J. Sci. Technol. 2018, 40, 831–839. [Google Scholar] [CrossRef]
- Khodadadi, S.; Mahdinezhad, N.; Fazeli-Nasab, B.; Heidari, M.J.; Fakheri, B.; Miri, A. Investigating the possibility of green synthesis of silver nanoparticles using Vaccinium Arctostaphylos extract and evaluating its antibacterial properties. Biomed. Res. Int. 2021, 5572252. [Google Scholar] [CrossRef]
- Vinayagam, R.; Varadavenkatesan, T.; Selvaraj, R. Evaluation of the anticoagulant and catalytic activities of the Bridelia retusa fruit extract-functionalized silver nanoparticles. J. Clust. Sci. 2017, 28, 2919–2932. [Google Scholar] [CrossRef]
- Mikac, L.; Ivanda, M.; Gotić, M.; Mihelj, T.; Horvat, L. Synthesis and characterization of silver colloidal nanoparticles with different coatings for SERS application. J. Nanoparticle Res. 2014, 16, 1–13. [Google Scholar] [CrossRef]
- Sutthanont, N.; Attrapadung, S.; Nuchprayoon, S. Larvicidal activity of synthesized silver nanoparticles from Curcuma zedoaria essential oil against Culex quinquefasciatus. Insects 2019, 10, 27. [Google Scholar] [CrossRef]
- Mondal, N.K.; Chowdhury, A.; Dey, U.; Mukhopadhya, P.; Chatterjee, S.; Das, K.; Datta, J.K. Green synthesis of silver nanoparticles and its application for mosquito control. Asian Pac. J. Trop. Dis. 2014, 4, 204–210. [Google Scholar] [CrossRef]
- Rónavári, A.; Kovács, D.; Igaz, N.; Vágvölgyi, C.; Boros, I.M.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Biological activity of green-synthesized silver nanoparticles depends on the applied natural extracts: A comprehensive study. Int. J. Nanomed. 2017, 12, 871–883. [Google Scholar] [CrossRef]
- Perveen, R.; Shujaat, S.; Naz, M.; Qureshi, M.Z.; Nawa, S.; Shahzad, K.; Ikram, M. Green synthesis of antimicrobial silver nanoparticles with Brassicaceae seeds. Mater. Res. Express 2021, 8, 055007. [Google Scholar] [CrossRef]
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed. Nanomed-Nanotechnol. 2016, 12, 789–799. [Google Scholar] [CrossRef]

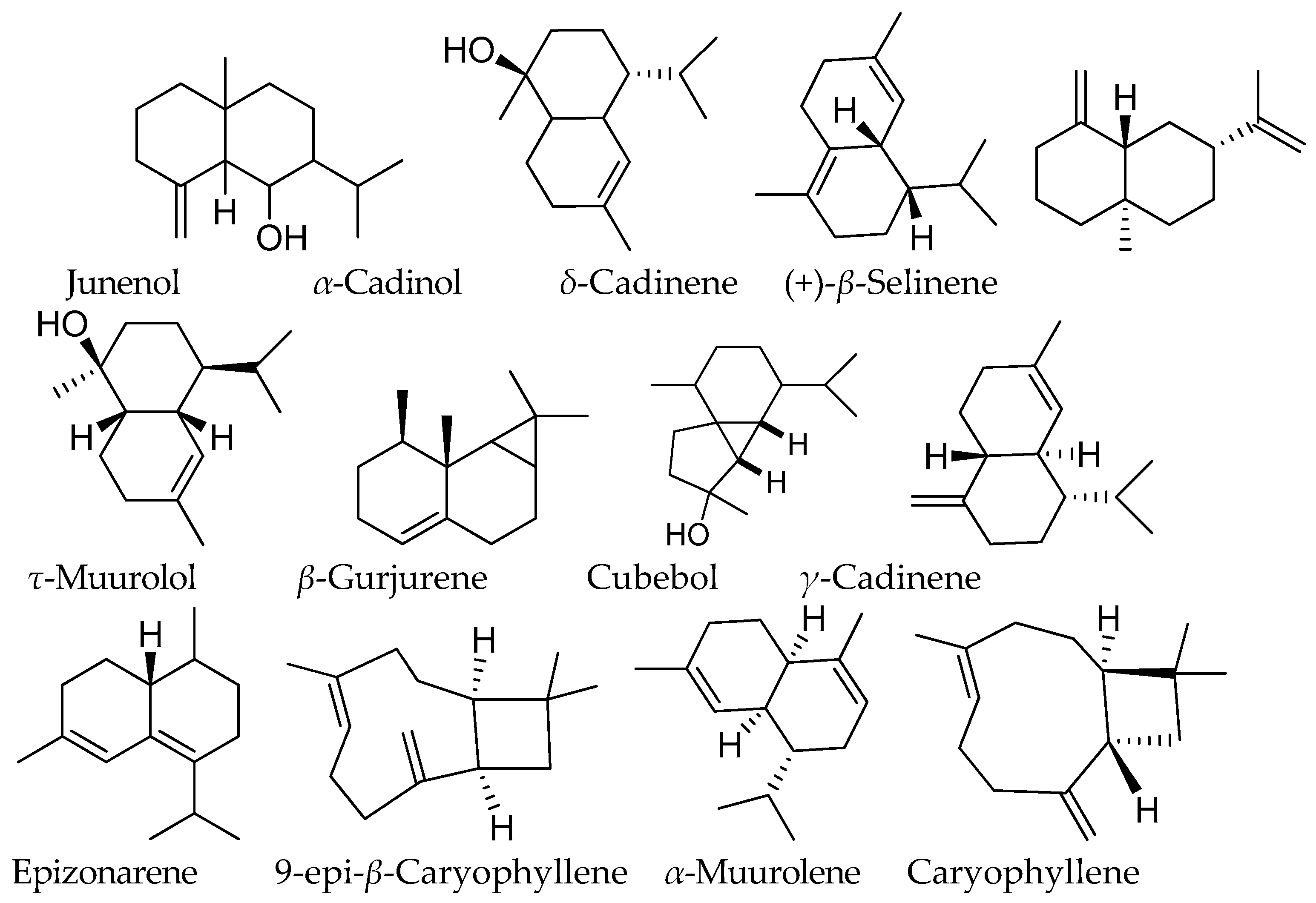


| RT (min) | KIexp. | KIlit | Compound Name | Area% |
|---|---|---|---|---|
| 26.835 | 1342 | 1338 | Presilphiperfol-7-ene | 0.25 |
| 28.016 | 1377 | 1376 | Modephene | 1.42 |
| 28.225 | 1383 | 1382 | α-Isocomene | 1.06 |
| 28.978 | 1405 | 1412 | β-Isocomene | 0.34 |
| 29.235 | 1414 | 1413 | Caryophyllene | 1.45 |
| 30.506 | 1455 | 1451 | 9-epi-β-Caryophyllene | 1.73 |
| 30.973 | 1469 | 1468 | γ-Muurolene | 0.49 |
| 31.430 | 1483 | 1484 | (+)-β-Selinene | 5.86 |
| 31.625 | 1489 | 1489 | β-Guaiene | 0.62 |
| 31.706 | 1492 | 1491 | α-Muurolene | 1.67 |
| 32.154 | 1506 | 1506 | γ-Cadinene | 2.99 |
| 32.220 | 1509 | 1510 | 2-Isopropyl-5-methyl-9-methylene [4.4.0]dec-1-ene | 0.28 |
| 32.325 | 1512 | 1512 | δ-Cadinene | 12.93 |
| 32.506 | 1518 | 1518 | Cubebol | 3.00 |
| 32.659 | 1524 | 1523 | (+)-δ-Cadinene | 0.49 |
| 32.992 | 1530 | 1534 | α-Calacorene | 0.35 |
| 33.259 | 1535 | 1545 | β-Pinone | 0.88 |
| 33.597 | 1544 | 1554 | β-Calacorene | 0.43 |
| 33.935 | 1556 | 1597 | Ziza-6(13)-ene | 0.21 |
| 34.821 | 1567 | 1600 | β-Oplopenone | 0.32 |
| 35.173 | 1570 | 1608 | β-Epicubenol | 1.43 |
| 35.492 | 1576 | 1636 | Muurola-4,10(14)-dien-1β-ol | 0.31 |
| 35.544 | 1596 | 1621 | α-Epicubenol | 0.79 |
| 35.730 | 1608 | 1646 | 11,11-Dimethyl-4,8-bis (methylene)bicyclo [7.2.0] undecan-3-ol | 0.37 |
| 35.868 | 1619 | 1629 | Junenol | 20.73 |
| 35.944 | 1621 | 1622 | β-Gurjurene | 4.17 |
| 36.002 | 1628 | 1637 | τ-Muurolol | 4.70 |
| 36.073 | 1633 | 1672 | Epizonarene | 2.69 |
| 36.349 | 1636 | 1649 | α-Cadinol | 15.54 |
| 36.563 | 1638 | 1676 | 1,2,3,4-Tetrahydro-1,6-dimethyl-4-(1-methylethyl)-1-naphthalenol | 0.62 |
| 37.392 | 1640 | 1687 | Schyobunol | 1.09 |
| 37.735 | 1650 | 1694 | Eudesma-4(15),7-dien-1β-ol | 0.41 |
| 38.030 | 1658 | 1752 | (+)-γ-Costol | 0.22 |
| 38.440 | 1687 | 1730 | 10-Hydroxyoplopanone | 0.46 |
| 41.463 | 1696 | 1810 | 14-Hydroxy-δ-cadinene | 1.07 |
| 42.340 | 1698 | 1867 | Platambin | 0.36 |
| 44.225 | 1710 | 1983 | β-Cyclocostunolide | 0.42 |
| Sesquiterpenes | 91.27 | |||
| Oxygenated sesquiterpene hydrocarbons | 51.84 | |||
| Non-oxygenated sesquiterpene hydrocarbons | 39.43 | |||
| Non-terpene hydrocarbons | 0.88 | |||
| Total composition% | 92.15 | |||
| Test Sample | Concentration | Larvae * | ||||
|---|---|---|---|---|---|---|
| Total | Dead (Mean + SD) | Mortality% | LC50 | LC90 | ||
| POEO solution (µg/mL) | 500 | 10 | 6.33 + 1.15 a | 63.30 | 154.88 | 11,749.00 |
| 250 | 10 | 5.67 + 0.58 a | 56.70 | |||
| 125 | 10 | 4.67 + 1.53 a | 46.70 | |||
| POEO-AgNPs solution | 100% | 10 | 5.33 + 1.15 b | 53.30 | 69.18 | 1318.26 |
| 50% | 10 | 4.33 + 0.58 b | 43.30 | |||
| 25% | 10 | 3.00 + 0.00 b | 30.0 | |||
| Dursban (+ve control) | - | 10 | 9.67 + 0.58 | 96.70 | ||
| Tween-80 (-ve control) | - | 10 | 0.00 + 0.00 | 0.00 | ||
| DH2O (-ve control) | - | 10 | 0.00 + 0.00 | 0.00 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonfa, Y.H.; Tessema, F.B.; Bachheti, A.; Tadesse, M.G.; Eid, E.M.; Abou Fayssal, S.; Adelodun, B.; Choi, K.S.; Širić, I.; Kumar, P.; et al. Essential Oil Composition of Aerial Part of Pluchea ovalis (Pers.) DC., Silver Nanoparticles Synthesis, and Larvicidal Activities against Fall Armyworm. Sustainability 2022, 14, 15785. https://doi.org/10.3390/su142315785
Gonfa YH, Tessema FB, Bachheti A, Tadesse MG, Eid EM, Abou Fayssal S, Adelodun B, Choi KS, Širić I, Kumar P, et al. Essential Oil Composition of Aerial Part of Pluchea ovalis (Pers.) DC., Silver Nanoparticles Synthesis, and Larvicidal Activities against Fall Armyworm. Sustainability. 2022; 14(23):15785. https://doi.org/10.3390/su142315785
Chicago/Turabian StyleGonfa, Yilma Hunde, Fekade Beshah Tessema, Archana Bachheti, Mesfin Getachew Tadesse, Ebrahem M. Eid, Sami Abou Fayssal, Bashir Adelodun, Kyung Sook Choi, Ivan Širić, Pankaj Kumar, and et al. 2022. "Essential Oil Composition of Aerial Part of Pluchea ovalis (Pers.) DC., Silver Nanoparticles Synthesis, and Larvicidal Activities against Fall Armyworm" Sustainability 14, no. 23: 15785. https://doi.org/10.3390/su142315785
APA StyleGonfa, Y. H., Tessema, F. B., Bachheti, A., Tadesse, M. G., Eid, E. M., Abou Fayssal, S., Adelodun, B., Choi, K. S., Širić, I., Kumar, P., & Bachheti, R. K. (2022). Essential Oil Composition of Aerial Part of Pluchea ovalis (Pers.) DC., Silver Nanoparticles Synthesis, and Larvicidal Activities against Fall Armyworm. Sustainability, 14(23), 15785. https://doi.org/10.3390/su142315785














