Effect of Iron Application on Rice Plants in Improving Grain Nutritional Quality in Northeastern of Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design and Treatments
2.3. Rice Cultivation
2.4. Sampling and Measurement
2.4.1. Determination of Plant Growths and Yields
2.4.2. Grain Fe Concentration Measurement
2.4.3. Shoot Fe Concentration Measurement
2.4.4. Statistical Analysis
3. Results
3.1. Soil Characterizations
3.2. Rice Plant Growths
3.3. Yields and Yield Components
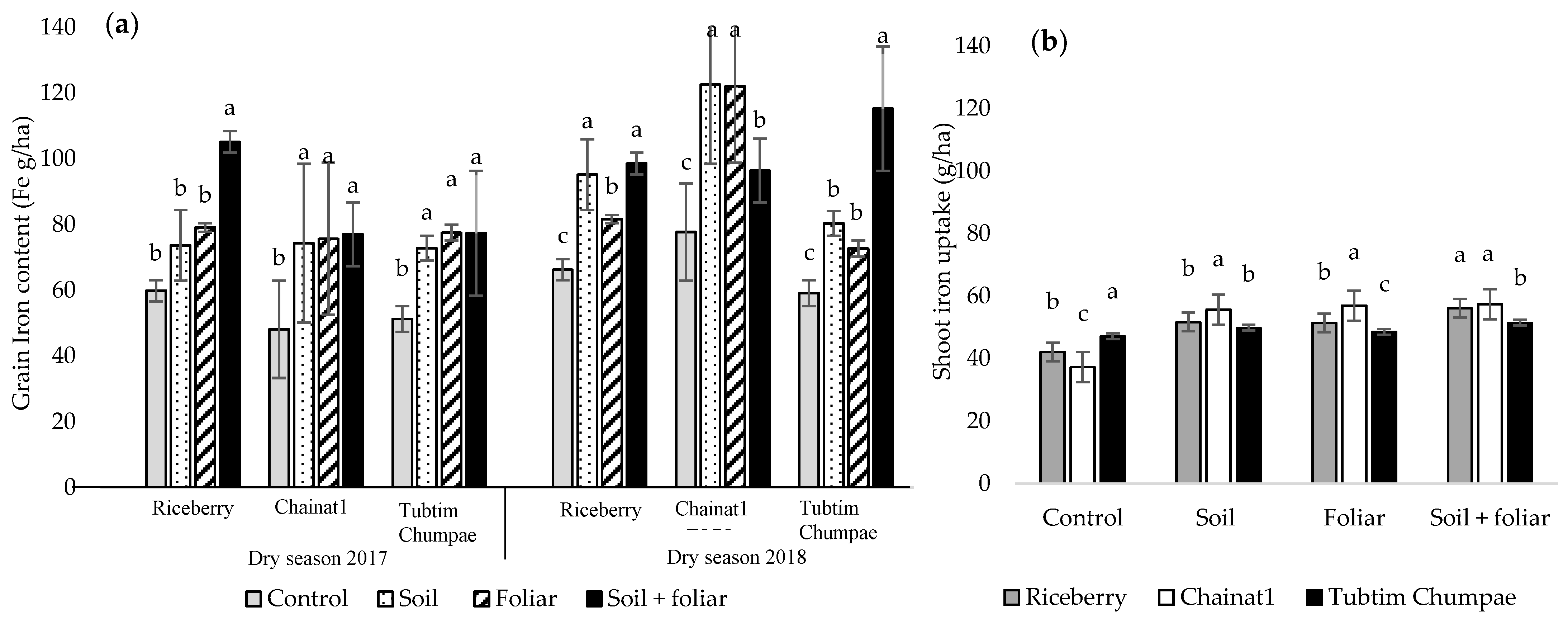
3.4. Iron Contents in Rice Grains
3.5. Iron Uptakes in Shoots
3.6. Relationship between Grain Iron Concentration and Yield
4. Discussion
4.1. Brown Rice Grain’s Iron Content
4.2. Grain Yields
4.3. Iron Uptake
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fitzgerald, M.A.; McCouch, S.R.; Hall, R.D. Not just a grain of rice: The quest for quality. Trends Plant Sci. 2009, 14, 133–139. [Google Scholar] [CrossRef]
- Welch, R.M.; Graham, R.D. Breeding for micronutrients in staple food crops from a human nutrition perspective. J. Exp. Bot. 2004, 55, 353–364. [Google Scholar] [CrossRef]
- Murray-Kolb, L.E.; Beard, J.L. Iron deficiency and child and maternal health. Am. J. Clin. Nutr. 2009, 89, 946S–950S. [Google Scholar] [CrossRef] [PubMed]
- Sperotto, R.A.; Vasconcelos, M.W.; Grusak, M.A.; Fett, J.P. Effects of different Fe supplies on mineral partitioning and remobilization during the reproductive development of rice (Oryza sativa L.). Rice 2012, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Corey, M.S. Groups: Process and Practice; Wadsworth Publishing Co Inc.: Wadsworth, OR, USA, 2006. [Google Scholar]
- Davidsson, L. Approaches to improve iron availability from complementary foods. J. Nutr. 2003, 133, 1560S–1562S. [Google Scholar] [CrossRef] [PubMed]
- Holm, P.B.; Kristiansen, K.N.; Pedersen, H.B. Transgenic approaches in commonly consumed cereals to improve iron and zinc content and bioavailability. J. Nutr. 2002, 132, 514S–516S. [Google Scholar] [CrossRef]
- Poletti, S.; Gruissem, W.; Sautter, C. The nutritional fortification of cereals. Curr. Opin. Biotechnol. 2004, 15, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Schachtman, D.P.; Barker, S.J. Molecular approaches for increasing the micronutrient density in edible portions of food crops. Field Crops Res. 1999, 60, 81–92. [Google Scholar] [CrossRef]
- Rengel, Z.; Batten, G.D.; Crowley, D.E. Agronomic approaches for improving the micronutrient density inedible portions of field crops. Field Crop Res. 1999, 60, 27–40. [Google Scholar] [CrossRef]
- Aciksoz, S.B.; Yazici, A.; Ozturk, L.; Cakmak, I. Biofortification of wheat with iron through soil and foliar application of nitrogen and iron fertilizers. Plant Soil 2011, 349, 215–225. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, L.; Xin, Z.; Zhao, L.; An, X.; Hu, Q. Effect of foliar application of zinc, selenium, and iron fertilizers on nutrient concentration and yield of rice grain in China. J. Agric. Food Chem. 2008, 56, 2079–2084. [Google Scholar] [CrossRef]
- Yuan, L.; Wu, L.; Yang, C.; Lv, Q. Effects of iron and zinc foliar applications on rice plants and their grain accumulation and grain nutritional quality. J. Sci. Food Agric. 2012, 93, 254–261. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of cerial grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Wei, Y.; Shohag, M.J.I.; Xiaoe, Y.; Zhang, Y. Effects of foliar iron application on iron concentration in polished rice grain and its bioavailability. J. Agric. Food Chem. 2012, 60, 11433–11439. [Google Scholar] [CrossRef]
- Peleg, Z.; Saranga, Y.; YaZici, A.; Fahima, T.; Ozturk, L.; Cakmak, I. Grain zinc, iron and protein concentrations and zinc-efficiency in wild emmer wheat under contrasting irrigation regimes. Plant Soil 2007, 306, 57–67. [Google Scholar] [CrossRef]
- Li, M.; Wang, S.; Tian, X.; Li, S.; Chen, Y.; Jia, Z.; Liu, K.; Zhao, A. Zinc and iron concentrations in grain milling fractions through combined folia applications of Zn and micronutrients. Field Crops Res. 2016, 187, 135–141. [Google Scholar] [CrossRef]
- Pataco, I.M.; Lidon, F.C.; Ramos, I.; Oliveira, K.; Guerra, M.; Pessoa, M.F.; Carvalho, M.L.; Ramalho, J.C.; Leitao, A.E.; Santos, J.P. Biofortification of durum eheat grains with nutrients. J. Plant Int. 2017, 12, 39–50. [Google Scholar]
- Zhao, D.S.; Li, Q.F.; Zhang, C.Q.; Zhang, C.; Yang, Q.Q.; Pan, L.X.; Ren, X.Y.; Lu, J.; Gu, M.H.; Liu, Q.Q. GS9 acts as a transcriptional activator to regulate rice grain shape and appearance quality. Nat. Commun. 2018, 9, 1240. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ashraf, U.; Tian, H.; Mo, Z.; Pan, S.; Anjum, S.A.; Duan, M.; Tang, X. Manganese-induced regulations in growth, yield formation, quality characters, rice aroma and enzyme involved in 2-acetyl-1-pyrroline biosynthesis in fragrant rice. Plant Physiol. Biochem. 2016, 103, 167–175. [Google Scholar] [CrossRef]
- Dou, Z.; Tang, S.; Li, G.H.; Liu, Z.H.; Ding, C.Q.; Chen, L.; Wang, S.H.; Ding, Y.F. Application of nitrogen fertilizer at heading stage improves rice quality under elevated temperature during grain-filling stage. Crop Sci. 2017, 57, 2183–2192. [Google Scholar] [CrossRef]
- Rice Science Center. Riceberry. 2016. Available online: http://www.news-articles-rice-rsc-rgdu-knowledge/rice-breedinglab/riceberry-variety (accessed on 16 February 2017).
- Chumpae Rice Research Center. Tubtim Chumpae Rice. 2015. Available online: http://www.thairice.org/html/doc-dl/brochure-tuptimpare.pdf (accessed on 18 February 2017).
- Suwannawong, S. Plant Nutrition Analysis; Kasetsart University: Bangkok, Thailand, 2001; p. 62. [Google Scholar]
- Zarcinas, B.A.; Cartwright, B.; Spouncer, L.R. Nitric acid digestion and multi-element analysis of plant material by inductively coupled plasma spectrometry. Commun. Soil Sci. Plant Anal. 1987, 181, 31–146. [Google Scholar] [CrossRef]
- Piepho, H.P.; Büchse, A.; Richter, C. A mixed modelling approach for randomized experiments with repeated measures. J. Agron. Crop Sci. 2004, 190, 230–247. [Google Scholar] [CrossRef]
- Khoshgoftarmanesh, A.H.; Schulin, R.; Chaney, R.L.; Daneshbakhsh, B.; Afyuni, M. Micronutrient efficient genotypes for crop yield and nutritional quality in sustainable agriculture. A review. Agron. Sustain. Dev. 2009, 30, 83–107. [Google Scholar] [CrossRef]
- Fageria, N.K. The Use of Nutrients in Crop Plants; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Borg, M.; Brownfield, L.; Twell, D. Male gametophyte development: A molecular perspective. J. Exp. Bot. 2009, 60, 1465–1478. [Google Scholar] [CrossRef]
- Kobayashi, H.; Sakurai, T.; Imai, M.; Takahashi, N.; Fukuda, A.; Yayoi, O. Contribution of intragenic DNA methylation in mouse gametic DNA methylomes to establish oocyte-specific heritable marks. PLoS Genet. 2012, 8, e1002440. [Google Scholar] [CrossRef]
- Kamali, B.; Chandra Sekaran, N.; Kalaiselvi, T.; Chitdeshwari, T. Exogenouse foliar application of FeSO4 on enrichment of iron in rice grain and yield. J. Pharmacogn. Phytochem. 2020, 9, 3344–3348. [Google Scholar]
- Impa, S.M.; Morete, M.J.; Ismail, A.M.; Schulin, R.; Johnson-Beebout, S.E. Zn uptake, translocation, and grain Zn loading in rice (Oryza sativa L.) genotypes selected for Zn deficiency tolerance and high grain Zn. J. Exp. Bot. 2013, 64, 2739–2751. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Beebout, S.E.; Lauren, J.G.; Duxbury, J.M. Immobilization of zinc fertilizer in flooded soils monitored by adapted DTPA soil test. Commun. Soil Sci. Plant Anal. 2009, 40, 1842–1861. [Google Scholar] [CrossRef]
- Prom-U.-Thai, C.; Rashid, A.; Ram, H.; Zou, C.; Guilherme, L.R.G.; Corguinha, A.P.B.; Guo, S.; Kaur, C.; Naeem, A.; Yamuangmorn, S.; et al. Simultaneous biofortification of rice with zinc, iodine, iron and selenium through foliar treatment of a micronutrient cocktail in five countries. Front. Plant Sci. 2020, 11, 589835. [Google Scholar]
- Rout, G.R.; Sahoo, S. Role of iron in plant growth and metabolism. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef]
- Babaeian, M. Efficacy of different iron, zinc and magnesium fertilizers on yield and yield components of barley. Afr. J. Microbiol. Res. 2012, 6, 5754–5756. [Google Scholar]
- Saenchai, C.; Prom-u-thai, C.; Jamjod, S.; Dell, B.; Rerkasem, B. Genotypic variation in milling depression of iron and zinc concentration in rice grain. Plant Soil 2012, 361, 271–278. [Google Scholar] [CrossRef]
- Ponnamperuma, F.N.; Castro, R.U. Varietal Differences in Resistance to Adverse Soil Conditions; International Rice Research Institute, Rice Breeding: Los Baños, Philippines, 1972; pp. 677–684. [Google Scholar]
- Annual Report for 1970; International Rice Research Institute (IRRI): Laguna, Philippines, 1971; p. 265.
- De Datta, S.K. Principles and Practices of Rice Production; John Wiley and Sons, Inc.: New York, NY, USA, 1981; p. 353. [Google Scholar]
- Dobermann, A.; Fairhurst, T.H. Rice Nutrient Disorders and Nutrient Management; Oxford Graphic Printers Pte Ltd.: Singapore, 2000; p. 191. [Google Scholar]
- Saenchai, C.; Prom-u-thai, C.T.; Jamjod, S.; Dell, B.; Rerkasem, B. Iron and zinc partitioning in rice plant during grain filling. Khon Kaen Agr. J. 2015, 43, 67–78. (in Thai). [Google Scholar]
- Yadav, G.S.; Shivay, Y.S.; Kumar, D.; Babu, S. Enhancing iron density and uptake in grain and straw of aerobic rice through mulching and rhizo-foliar fertilization of iron. Glob. Afr. J. Agric. Res. 2013, 8, 5447–5454. [Google Scholar]
- Wang, Q.; Chen, M.; Hao, Q.; Zeng, H.; He, Y. Research and progress on the mechanism of iron transfer and accumulation in rice grains. Plants 2021, 10, 2610. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shankhdhar, D.; Shankhdhar, S.C. Enhanching grain iron content of rice by the application of plant growth promoting rhizobacteria. Plant Soil Environ. 2013, 59, 89–94. [Google Scholar] [CrossRef]


| Month | Rainfall | Temperature (°C) | Relative Humidity | Sunlight | ||
|---|---|---|---|---|---|---|
| (mm) | Minimum | Maximum | Average | (%) | (h day−1) | |
| 2017 | ||||||
| January | 1.2 | 20.3 | 30.8 | 25.6 | 63 | 5.9 |
| February | 4.6 | 19.1 | 32.3 | 25.7 | 56 | 8.3 |
| March | 81.4 | 23.1 | 34.7 | 28.9 | 64 | 7.4 |
| April | 37.0 | 24.7 | 35.6 | 30.2 | 64 | 7.7 |
| May | 188.0 | 24.8 | 34.1 | 29.5 | 76 | 5.5 |
| Mean | 62.4 | 22.4 | 33.5 | 28.0 | 64.6 | 7.0 |
| 2018 | ||||||
| January | 1.0 | 19.3 | 30.5 | 24.9 | 67 | 6.2 |
| February | 5.4 | 19.3 | 30.7 | 25 | 63 | 7.1 |
| March | 28.0 | 22.5 | 33.6 | 28.05 | 62 | 7.8 |
| April | 206.2 | 23.7 | 34.1 | 28.9 | 66 | 7.3 |
| May | 163.1 | 24.4 | 34.1 | 29.25 | 77 | 6.9 |
| Mean | 80.7 | 21.8 | 32.6 | 27.2 | 67.0 | 7.1 |
| Parameters | 2017 | 2018 | Analysis Method |
|---|---|---|---|
| pH (1:1 soil:water) | 7.31 | 7.47 | pH meter |
| Total N (%) | 0.035 | 0.031 | Kjeldahl nitrogen method |
| Available P (mg/kg) | 5.28 | 8.83 | Bray II |
| Exchangeable K (mg/kg) | 92.78 | 83.01 | Flame photometer |
| Exchangeable Ca (mg/kg) | 475 | 544 | Flame photometer |
| Available Fe (mg/kg) | 29.39 | 63.14 | Atomic absorption spectrophotometer |
| Organic matter (%) | 0.629 | 0.578 | Wet oxidation |
| CEC (c mol (+)/kg) | 4.62 | 4.82 | Ammonium acetate extract |
| Textural class | Sandy loam | Sandy loam | Hydrometer method |
| Sand (%) | 73.57 | 73.64 | Hydrometer method |
| Silt (%) | 15.14 | 16.14 | Hydrometer method |
| Clay (%) | 11.29 | 10.22 | Hydrometer method |
| Treatment | 30 DAT | 60 DAT | 90 DAT | |||
|---|---|---|---|---|---|---|
| Plant Height (cm) | Tiller (No./Hill) | Plant Height (cm) | Tiller (No./Hill) | Plant height (cm) | Tiller (No./Hill) | |
| Year (Y) | ||||||
| 2017 | 45.47 b | 10.42 | 69.41 b | 14.92 | 90.59 | 16.95 |
| 2018 | 47.62 a | 11.27 | 71.08 a | 14.61 | 90.00 | 16.35 |
| Method of application (M) | ||||||
| Control | 45.92 | 11.10 | 70.02 | 15.05 | 90.68 | 17.42 |
| Soil | 46.99 | 10.67 | 70.42 | 14.78 | 92.21 | 16.98 |
| Foliar | 46.76 | 11.00 | 70.19 | 14.08 | 88.47 | 16.19 |
| Soil + foliar | 46.52 | 10.63 | 70.35 | 15.16 | 89.83 | 17.20 |
| Cultivar (C) | ||||||
| Chainat1 | 49.67 a | 12.28 a | 74.82 a | 12.96 a | 96.38 a | 19.81 a |
| Riceberry | 42.86 c | 10.85 b | 63.10 c | 10.56 b | 88.64 b | 16.78 b |
| Tubtim Chumpae | 47.12 b | 9.42 c | 72.82 b | 8.78 c | 85.87 b | 16.87 b |
| F-test | ||||||
| Y | ** | ns | ** | ns | ns | ns |
| M | ns | ns | ns | ns | ns | ns |
| C | ** | ** | ** | ** | ** | * |
| Y × M | ns | ns | ns | ns | ns | ns |
| Y × C | ns | ns | ns | ns | ns | ns |
| M × C | ns | ns | ns | ns | ns | ns |
| Y × M × C | ns | ns | ns | ns | ns | ns |
| Treatment | Panicle No. (No./Hill) | Grain (No./Panicle) | 1000 Grain Weight (g) | Filled Grain (%) | Grain Yield (kg/ha) |
|---|---|---|---|---|---|
| Year (Y) | |||||
| 2017 | 13.02 | 129.73 | 25.82 b | 92.66 b | 3104.0 b |
| 2018 | 13.07 | 120.44 | 30.27 a | 93.61 a | 3869.1 a |
| Method of application (M) | |||||
| Control | 12.34 | 120.85 | 27.56 | 93.14 | 3335.1 |
| Soil | 13.09 | 127.52 | 28.62 | 92.58 | 3619.5 |
| Foliar | 13.45 | 125.24 | 28.08 | 94.62 | 3436.5 |
| Soil + foliar | 13.30 | 126.73 | 27.90 | 93.92 | 3555.2 |
| Cultivar (C) | |||||
| Chainat1 | 13.79 a | 127.62 a | 31.20 a | 92.20 b | 3923.5 a |
| Riceberry | 12.65 b | 134.84 a | 28.11 b | 95.44 a | 3370.2 b |
| Tubtim Chumpae | 12.69 ab | 112.79 b | 24.81 c | 91.77 b | 3165.9 b |
| F-test | |||||
| Y | ns | ns | ** | * | ** |
| M | ns | ns | ns | ns | ns |
| C | ** | ** | ** | ** | ** |
| Y × M | ns | ns | ns | ns | ns |
| Y × C | ns | ns | ns | ns | ns |
| M × C | ns | ns | ns | ns | ns |
| Y × M × C | ns | ns | ns | ns | ns |
| Treatment | Grain Iron Content (Fe g/ha) | Shoot Iron Uptake (Fe g/ha) |
|---|---|---|
| Year (Y) | ||
| 2017 | 71.86 b | 32.28 b |
| 2018 | 90.27 a | 68.67 a |
| Method of application (M) | ||
| Control | 60.20 c | 42.18 c |
| Soil | 86.52 ab | 52.42 b |
| Foliar | 82.54 b | 52.31 b |
| Soil + foliar | 94.99 a | 54.99 a |
| Cultivar (C) | ||
| Riceberry | 77.49 b | 50.34 b |
| Chainat1 | 91.75 a | 51.81 a |
| Tubtim Chumpae | 73.95 b | 49.27 b |
| F-test | ||
| Y | ** | ** |
| M | ** | ** |
| C | ** | ** |
| Y × M | ns | ns |
| Y × C | ns | ns |
| M × C | ns | ** |
| Y × M × C | ** | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butsai, W.; Kaewpradit, W.; Harrell, D.L.; Polthanee, A. Effect of Iron Application on Rice Plants in Improving Grain Nutritional Quality in Northeastern of Thailand. Sustainability 2022, 14, 15756. https://doi.org/10.3390/su142315756
Butsai W, Kaewpradit W, Harrell DL, Polthanee A. Effect of Iron Application on Rice Plants in Improving Grain Nutritional Quality in Northeastern of Thailand. Sustainability. 2022; 14(23):15756. https://doi.org/10.3390/su142315756
Chicago/Turabian StyleButsai, Wipada, Wanwipa Kaewpradit, Dustin L. Harrell, and Anan Polthanee. 2022. "Effect of Iron Application on Rice Plants in Improving Grain Nutritional Quality in Northeastern of Thailand" Sustainability 14, no. 23: 15756. https://doi.org/10.3390/su142315756
APA StyleButsai, W., Kaewpradit, W., Harrell, D. L., & Polthanee, A. (2022). Effect of Iron Application on Rice Plants in Improving Grain Nutritional Quality in Northeastern of Thailand. Sustainability, 14(23), 15756. https://doi.org/10.3390/su142315756





