Investigation of the Phycoremediation Potential of Freshwater Green Algae Golenkinia radiata for Municipal Wastewater
Abstract
1. Introduction
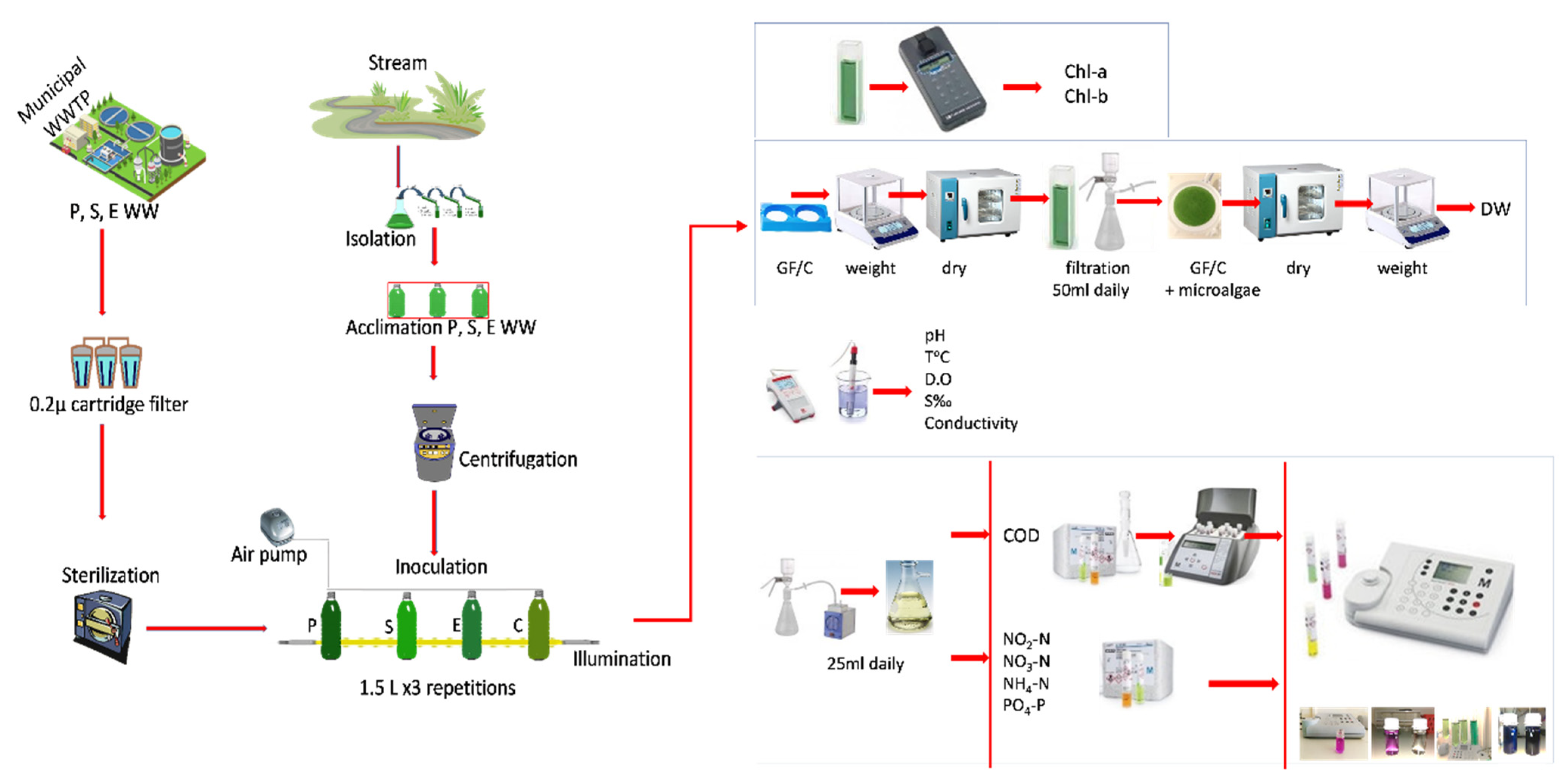
2. Materials and Methods
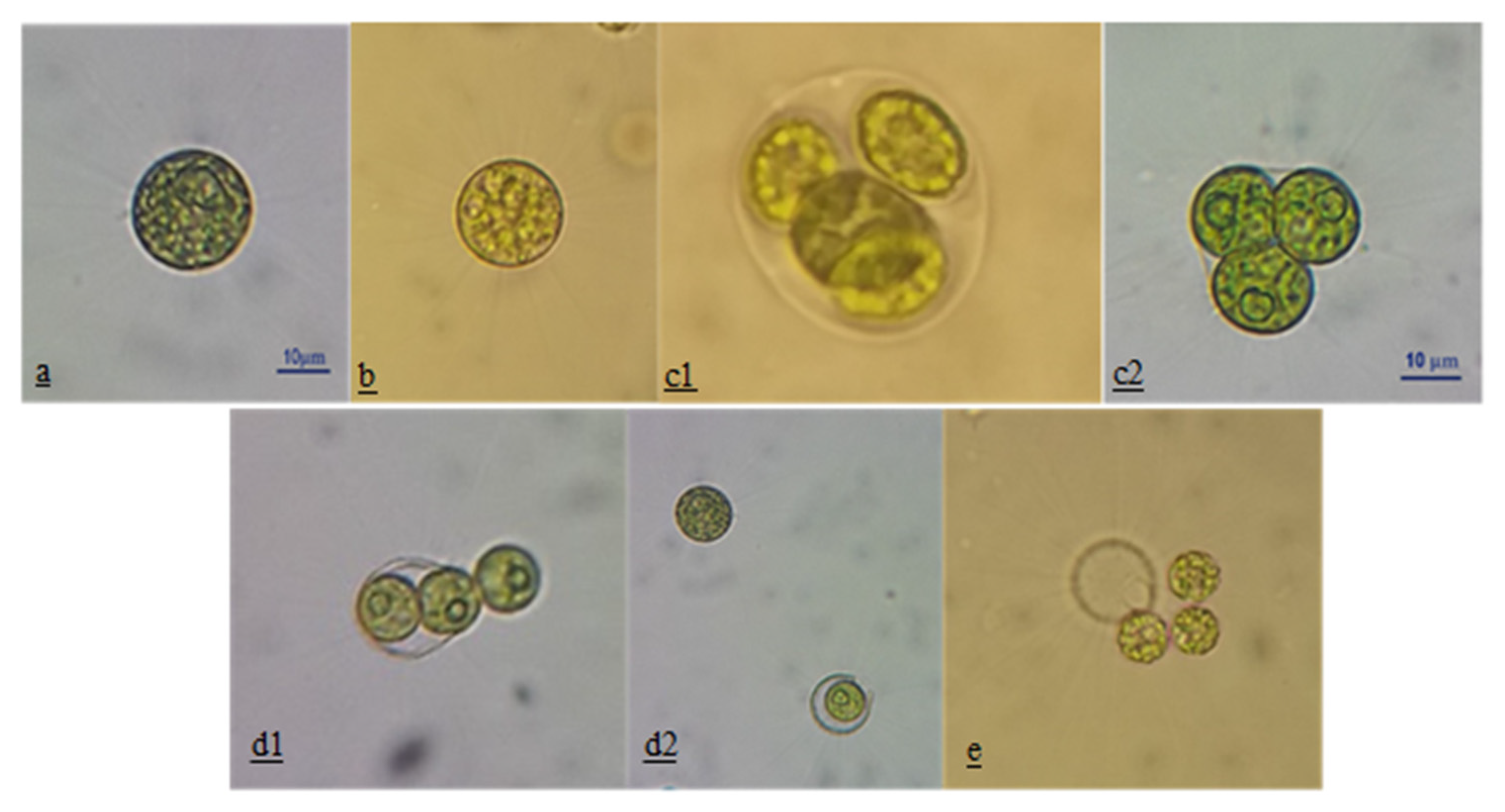
2.1. Microalgae Culture
2.2. Experimental Design
2.3. Equations and Statistics
3. Results and Discussions
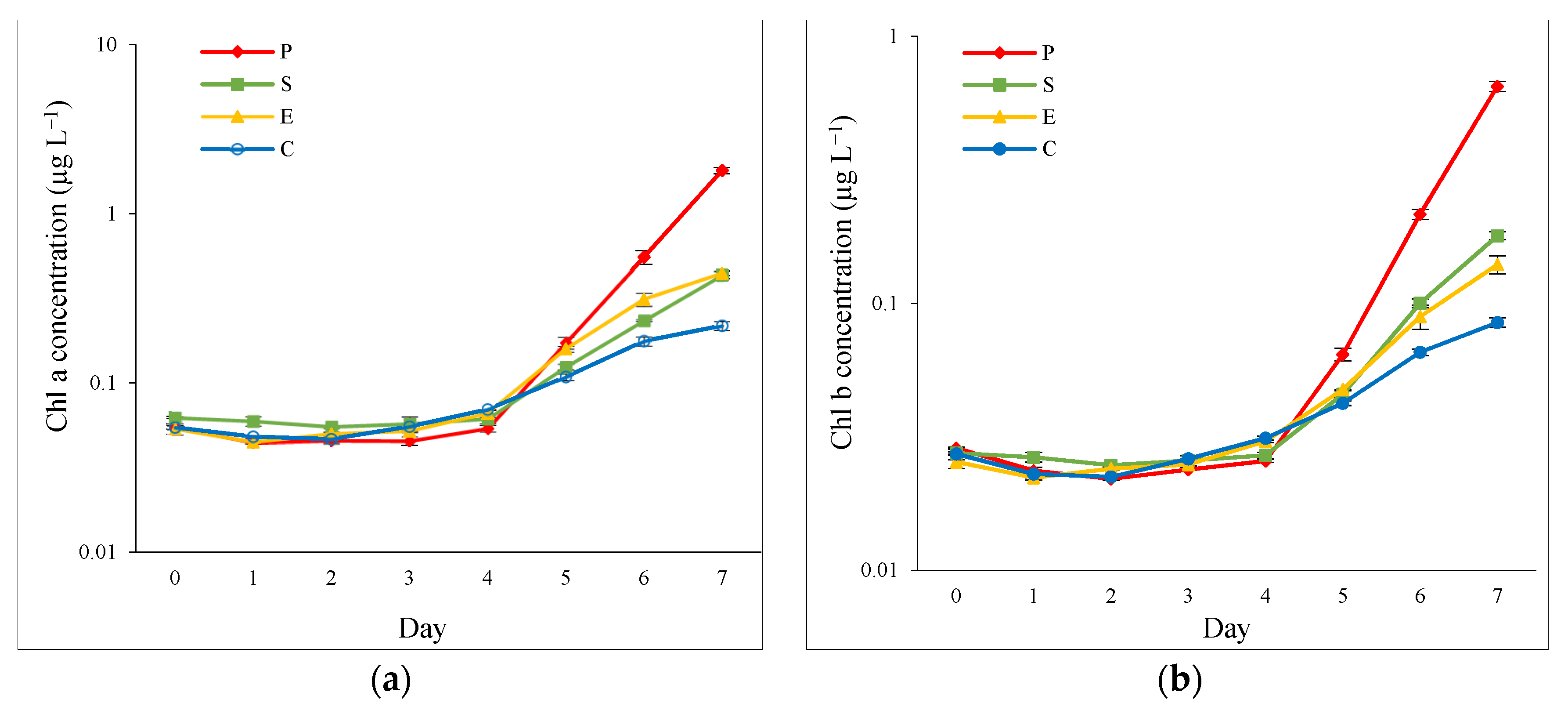
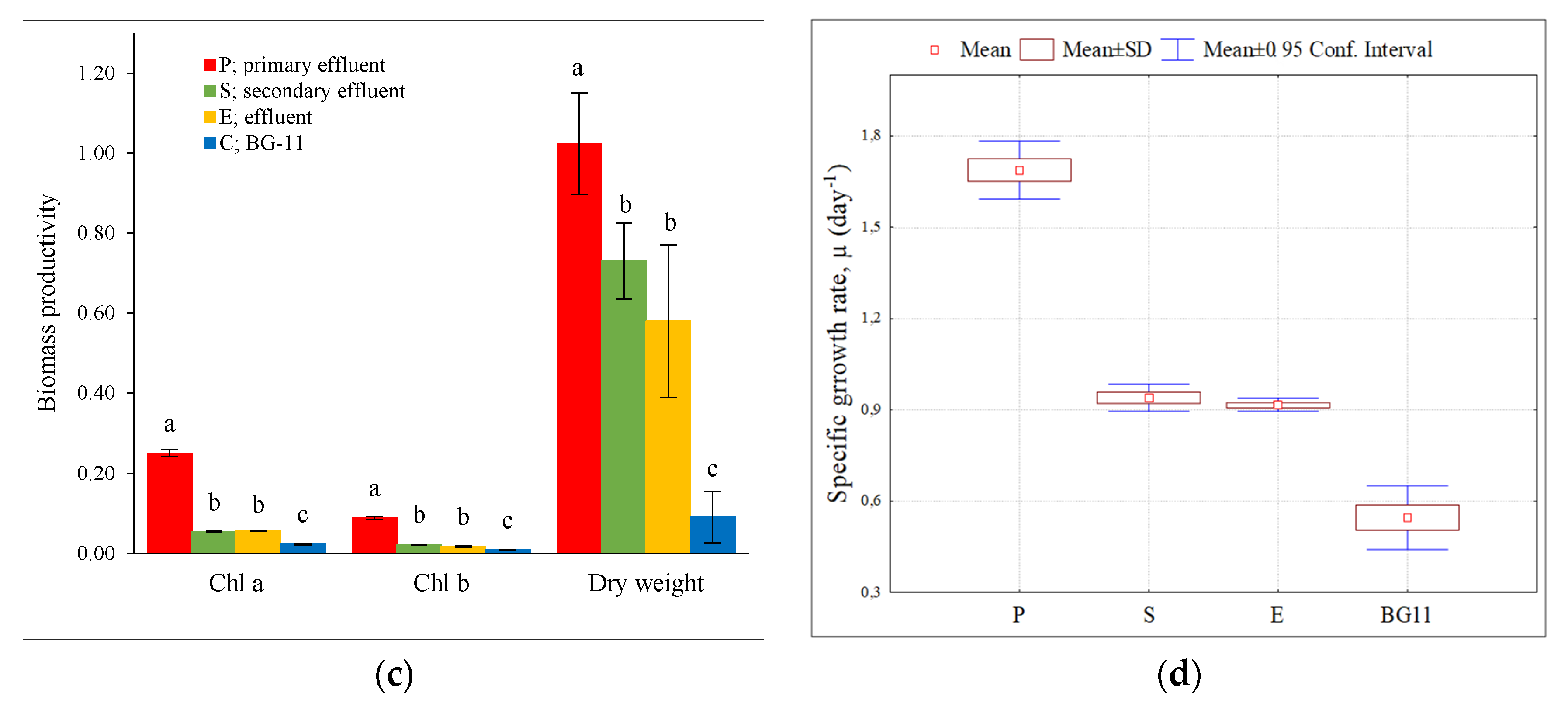
3.1. Growth Performance
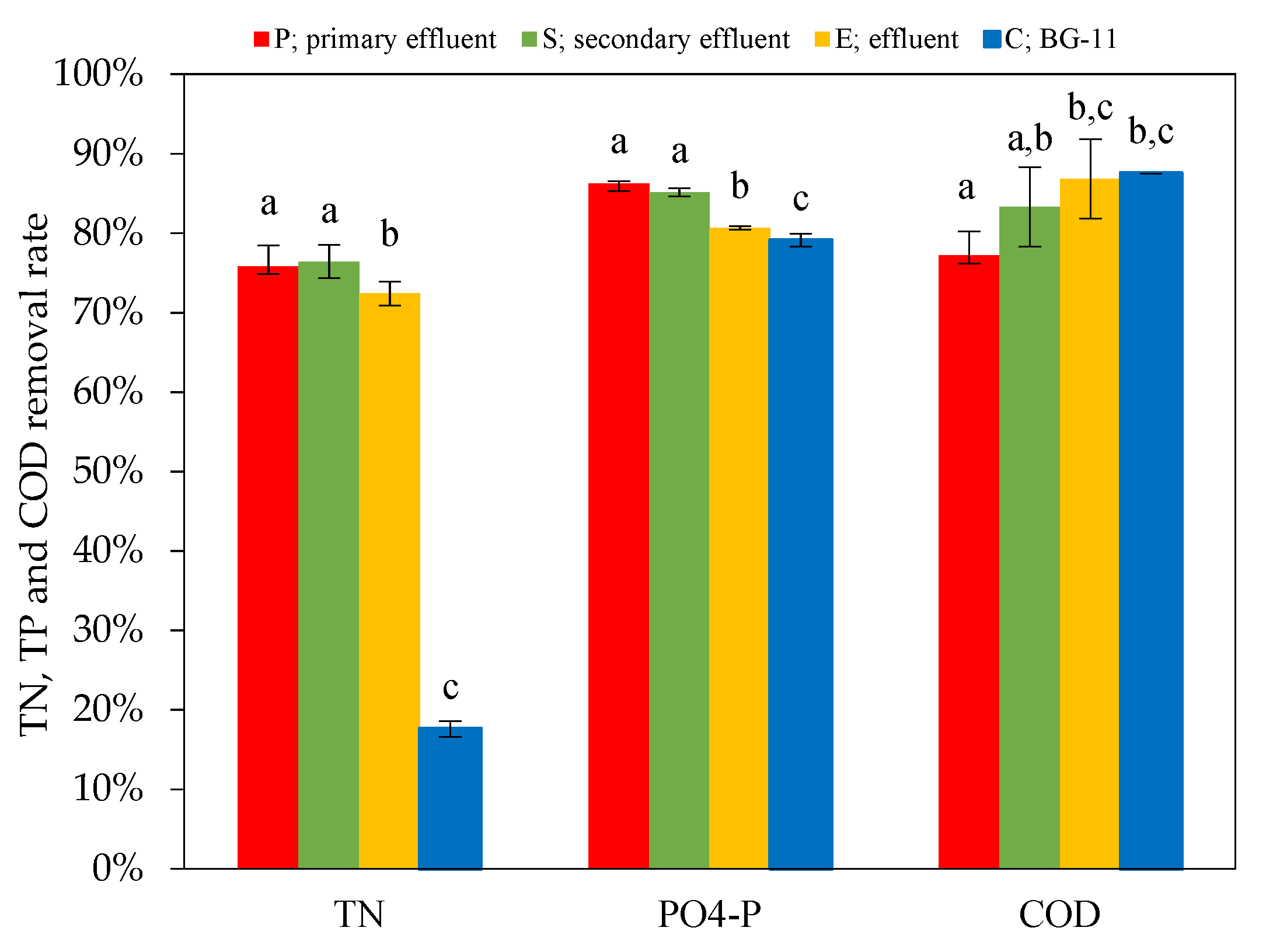
3.2. Nutrient Removal
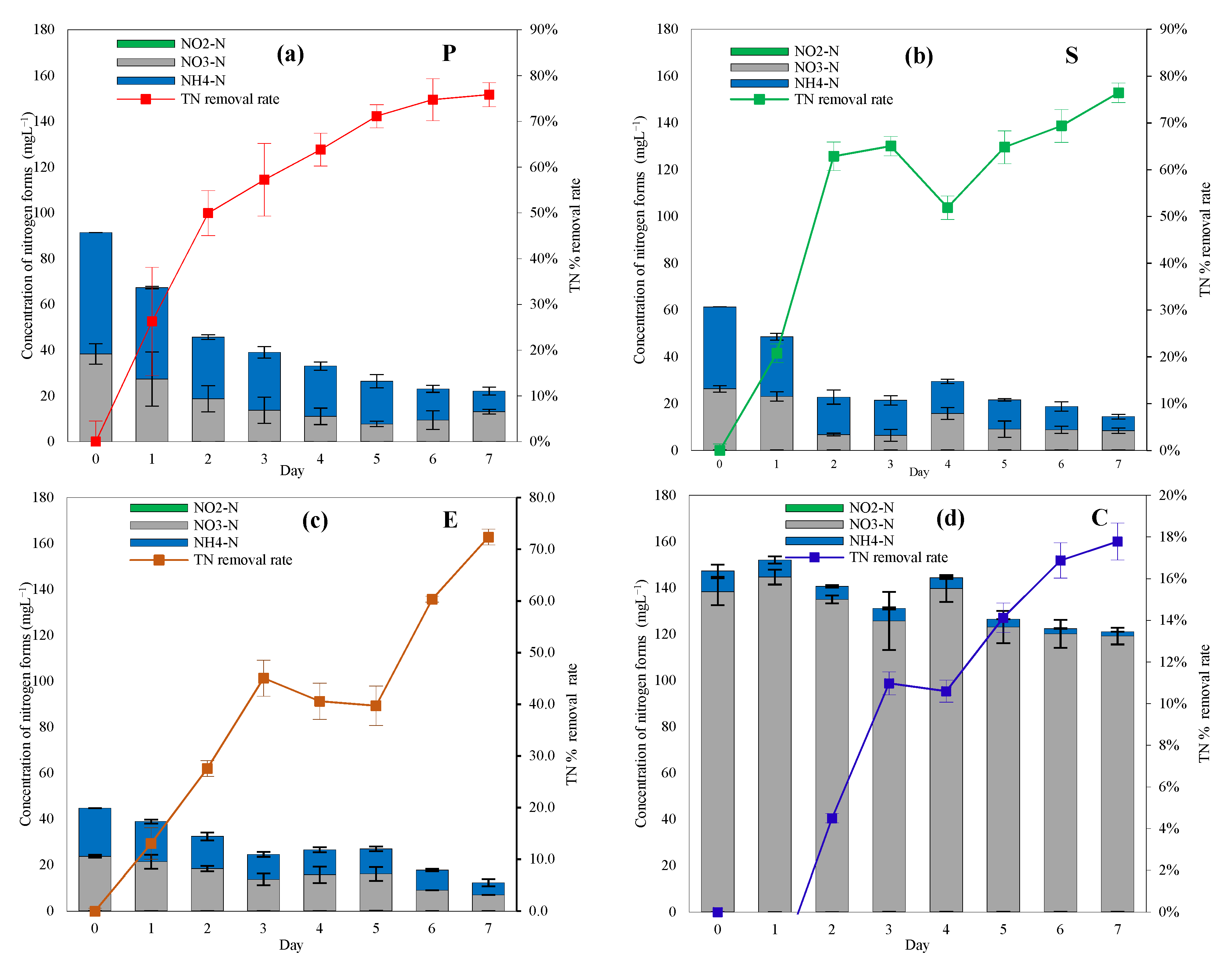
3.2.1. Nitrogen Removal
| Microalgae | Removal Efficiency (R%) | RT (d) | Type of Wastewater | Ref | |||||
|---|---|---|---|---|---|---|---|---|---|
| NH4-N | NO2-N | NO3-N | TN | PO4-P | COD | ||||
| Golenkinia radiata | 83.01 ± 3.26 | NR | 63.15 ± 3.45 | 75.80 ± 2.64 | 86.27 ± 2.71 | 77.22 ± 0.83 | 7 | Primary municipal wastewater | This study |
| 82.85 ± 2.85 | NR | 68.29 ± 3.37 | 76.40 ± 2.08 | 81.01 ± 1.26 | 83.33 ± 1.99 | Secondary municipal wastewater | |||
| 74.60 ± 7.27 | 47.22 ± 8.33 | 70.41 ± 0.73 | 72.40 ± 1.52 | 80.67 ± 4.16 | 86.84 ± 2.51 | Effluent municipal wastewater | |||
| 77.99 ± 5.74 | NR | 15.35 ± 2.85 | 17.80 ± 1.24 | 79.13 ± 4.66 | 87.53 ± 0.02 | BG-11 | |||
| Golenkinia sp. SDEC-16 | >99 | 96.7 | 48.87 | 8 | Campus sewage | [21] | |||
| 22.4 | >99 | BG-11 | |||||||
| Chlorella vulgaris | 84.0 | 95.0 | 33.79 | 13 | Urban wastewater | [48] | |||
| Scenedesmus obliquus | 95.0 | 92 | 6.86 | ||||||
| Chlorella ellipsoidea SDEC11 | 36.0 | >99.0 | 8 | Campus sewage | [49] | ||||
| Chlorella sp. | 94 | 100 | 14 | [50] | |||||
| Chlorella sp.277 | 92 | 86 | 9 | MWW | [51] | ||||
| Chlorella sp. | 89 | 80.9 | 90.08 | 14 | Concentrated MWW | [52] | |||
| 82 | 68.0 | 83. | 10 | MWW | [44] | ||||
| 68.4 | 83.2 | 50.9 | Raw sewage | ||||||
| 68.5 | 90.6 | 56.5 | Primary MWW | ||||||
| 82.8 | 85.6 | 83 | Centrate MWW | ||||||
| Chlorella vulgaris | 60.7 | 9.9 | 60.2 | 34.8 | 40.1 | 28 | Primary MWW | [33] | |
| 70.9 | 22.2 | 55.9 | 11.5 | 49.1 | Secondary MWW | ||||
| 64.1 | 23.1 | 33.6 | 25.8 | 61.1 | Centrate MWW | ||||
| Chlorella vulgaris and Chlorella protothecoides | 73.1 | 74.4 | 60 | 29 | MWW | [53] | |||
| Chlorella sorokiniana UTEX 1230 and Lemna minor | 88.0 | 91.0 | 99.0 | 7 | MWW | [54] | |||
| Scenedesmus sp | 79.0 | 57.0 | 84.0 | MWW | [55] | ||||
| Scenedesmus sp. | 80.0 | 99.0 | 86.0 | 66.0 | 20 | Domestic wastewater | [56] | ||
| Chlorella sp. YG01 | 84.1 | 84.1 | 82.3 | 14 | Secondary MWW | [57] | |||
| Chlorella sp. YG02 | 68.2 | 99.0 | |||||||
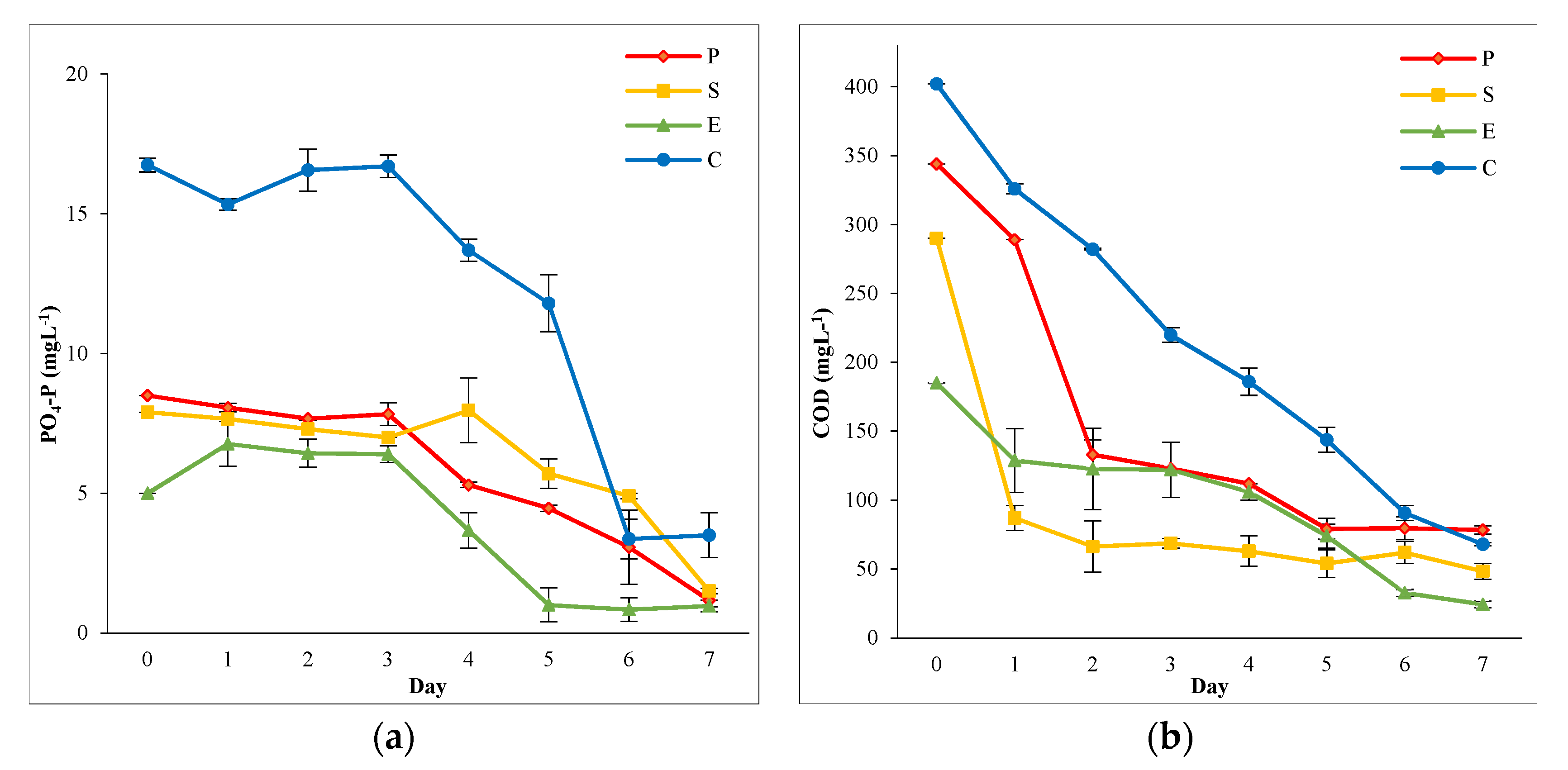
3.2.2. Phosphorus Removal
3.2.3. COD Removal
3.3. Reuse Potential of Final Effluent
| Parameters (mg L−1) | Final Concentration | WW Discharge Criteria | WW Reuse for Irrigation | |||||
|---|---|---|---|---|---|---|---|---|
| P | S | E | EU Directive 91/271 [69] | Turkey 2004/25687 [70] | Turkey 2010/27527 b [71] | EU COM (2018) 337 [72] | USEPA (2012) [73] | |
| COD | 78.3 ± 2.88 | 48.33 ± 5.7 | 24.33 ± 2.5 | 125 | <100 | ˂30 c (B) <20 d (A) | 25 (B, C, D) c 10 (A) d | 30 c 10 d |
| TP | 1.16 ± 0.0 | 1.50 ± 0.0 | 0.96 ± 0.0 | 1 or 2 a | <1 | - | - | |
| TN | 22.1 ± 2.64 | 14.47 ± 2.08 | 11.38 ± 0.52 | 10-15 a | <12 | - | - | |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koul, B.; Sharma, K.; Shah, M.P. Phycoremediation: A sustainable alternative in wastewater treatment (WWT) regime. Environ. Technol. Innov. 2022, 25, 102040. [Google Scholar] [CrossRef]
- Qadir, M.; Drechsel, P.; Jiménez Cisneros, B.; Kim, Y.; Pramanik, A.; Mehta, P.; Olaniyan, O. Global and regional potential of wastewater as a water, nutrient and energy source. In Natural Resources Forum Oxford, UK; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2020; Volume 44, pp. 40–51. [Google Scholar] [CrossRef]
- Jones, E.R.; Michelle, T.H.; van Vliet, M.Q.; Marc, F.P. Bierkens. Country-level and gridded estimates of wastewater production, collection, treatment and reuse. Earth Syst. Sci. Data 2021, 13, 237. [Google Scholar] [CrossRef]
- UN Habitat and WHO, Progress on Wastewater Treatment—Global Status and Acceleration Needs for SDG Indicator 6.3.1. United Nations Human Settlements Programme (UN-Habitat) and World Health Organization (WHO), Geneva. Available online: https://www.unwater.org/app/uploads/2021/09/SDG6_Indicator_Report_631_Progress-on-Wastewater-Treatment_2021_EN.pdf. (accessed on 5 November 2022).
- Şişman-Aydin, G.; Oral, R. Investigation of the hormesis/toxicity potential of Manisa (Turkey) urban wastewater treatment plant by using Selenastrum capricornutum printz. Fresenius Environ. Bull. 2014, 23, 1183–1189. [Google Scholar]
- Rao, P.H.; Kumar, R.R.; Mohan, N. Phycoremediation: Role of algae in waste management. In Environmental Contaminants: Ecological Implications and Management. Microorganisms for Sustainability; Bharagava, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; Volume 14. [Google Scholar] [CrossRef]
- Sisman-Aydin, G. Comparative study on phycoremediation performance of three native microalgae for primary-treated municipal wastewater. Environ. Technol. Innov. 2022, 28, 102932. [Google Scholar] [CrossRef]
- Sisman-Aydin, G. Microalgae technology and environmental use. Harran Univ. J. Eng. 2019, 4, 81–92. [Google Scholar]
- Dehghani, M.H.; Changani, F. The effect of acoustic cavitation on Chlorophyceae from effluent of wastewater treatment plant. Environ. Technol. 2006, 27, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.V.; Pastrana, L.M.; Fuciños, P. Microalgae encapsulation systems for food, pharmaceutical and cosmetics applications. Mar. Drugs 2020, 18, 644. [Google Scholar] [CrossRef]
- Zhou, G.J.; Ying, G.G.; Liu, S.; Zhou, L.J.; Chen, Z.F.; Peng, F.Q. Simultaneous removal of inorganic and organic compounds in wastewater by freshwater green microalgae. Environ. Sci. Process Impacts 2014, 16, 2018–2027. [Google Scholar] [CrossRef]
- Wollmann, F.; Dietze, S.; Ackermann, J.U.; Bley, T.; Walther, T.; Steingroewer, J.; Krujatz, F. Microalgae wastewater treatment: Biological and technological approaches. Eng. Life Sci. 2019, 19, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J.; Machlis, L. Nutrition of the green alga Golenkinia. Am. J. Bot. 1968, 55, 590–599. [Google Scholar] [CrossRef]
- Ellis, R.; Spooner, T.; Yakulis, R. Regulation of chlorophyll synthesis in the green alga Golenkinia. Plant Physiol. 1975, 55, 791–795. [Google Scholar] [CrossRef][Green Version]
- Usmani, M.A.; Suseela, M.R.; Toppo, K.; Sheikh, S.; Nayaka, S. Biomass nutrient profile of the green alga Golenkinia radiata Chodat. Int. J. Recent Adv. Multidiscip. Res. 2015, 2, 755–761. [Google Scholar] [CrossRef]
- Moestrup, Ø. Observations on the fine structure of spermatozoids and vegetative cells of the green alga Golenkinia. J. Phycol. 1972, 7, 169–183. [Google Scholar] [CrossRef][Green Version]
- Choi, S.A.; Choi, W.I.; Lee, J.S.; Kim, S.W.; Lee, G.A.; Yun, J.; Park, J.Y. Hydrothermal acid treatment for sugar extraction from Golenkinia sp. Bioresour. Technol. 2015, 190, 408–411. [Google Scholar] [CrossRef]
- Yoo, G.; Park, M.S.; Yang, J.W.; Choi, M. Lipid content in microalgae determines the quality of biocrude and Energy Return on Investment of hydrothermal liquefaction. Appl. Energy 2015, 156, 354–361. [Google Scholar] [CrossRef]
- Rearte, T.A.; Vélez, C.G.; Beligni, M.V.; Figueroa, F.L.; Gómez, P.I.; Flaig, D.; de Iorio, A.F. Biological characterization of a strain of Golenkinia (Chlorophyceae) with high oil and carotenoid content induced by increased salinity. Algal Res. 2018, 33, 218–230. [Google Scholar] [CrossRef]
- Simsek, K.; Sisman-Aydin, G. Cultivation of green microalgae Golenkinia radiata (Chodat, 1894) in textile wastewater without sterilization. Int. J. Res. Sci. Innov. Appl. 2018, 5, 124–130. [Google Scholar]
- Nie, C.; Pei, H.; Jiang, L.; Cheng, J.; Han, F. Growth of large-cell and easily-sedimentation microalgae Golenkinia SDEC-16 for biofuel production and campus sewage treatment. Renew. Energy 2018, 122, 517–525. [Google Scholar] [CrossRef]
- Throndsen, J. The dilution-culture method. In Phytoplankton Manual; Sournia, A., Ed.; UNESCO: Paris, France, 1978; pp. 218–224. [Google Scholar]
- Akinci, G.; Guven, D.E.; Ugurlu, S.K. Assessing pollution in Izmir Bay from rivers in western Turkey: Heavy metals. Environ. Sci. Process Impacts 2013, 15, 2252–2262. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (Order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef]
- Zhu, L.Z.; Wang, Q.; Shu, J.; Takala, E.; Hiltunen, P.; Feng, Z.Y. Nutrient removal and biodiesel production by integration of freshwater algae cultivation with piggery wastewater treatment. Water Res. 2013, 47, 4294–4302. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hu, Z.; Qi, Y.; Song, C.; Chen, G. The interactions of algae-activated sludge symbiotic system and its effects on wastewater treatment and lipid accumulation. Bioresour. Technol. 2019, 292, 122017. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J. Effects of acetate on the growth and chlorophyll content of Golenkinia. J. Phycol. 1970, 6, 364–368. [Google Scholar] [CrossRef]
- Ellis, R.J. Heterotrophic nutrition and its effects on chlorophyll synthesis in Golenkinia (Chlorophyceae). J. Phycol. 1977, 13, 304–306. [Google Scholar] [CrossRef]
- Rahimian, H.; Rayburn, W.R. The effect of calcium on growth and seta formation in Golenkinia minutissima. Bot. Gaz. 1979, 140, 5–10. [Google Scholar] [CrossRef]
- Ellis, R.J.; Timson, C. The absence of protochlorophyll(ide) accumulation in algae with inhibited chlorophyll synthesis. Plant Physiol. 1980, 65, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo-Mirquez, L.; Lopes, F.; Taidi, B.; Pareau, D. Nitrogen and phosphate removal from wastewater with a mixed microalgae and bacteria culture. Biotechnol. Rep. 2016, 11, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Schagerl, M.; Siedler, R.; Konopáčová, E.; Ali, S.S. Estimating biomass and vitality of microalgae for monitoring cultures: A Roadmap for Reliable Measurements. Cells 2022, 11, 2455. [Google Scholar] [CrossRef]
- Al-Momani, F.A.; Örmeci, B. Performance of Chlorella vulgaris, Neochloris oleoabundans, and mixed indigenous microalgae for treatment of primary effluent, secondary effluent, and centrate. Ecol. Eng. 2016, 95, 280–289. [Google Scholar] [CrossRef]
- Li, Y.; Horsman, M.; Wang, B.; Wu, N.; Lan, C.Q. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 2008, 81, 629–636. [Google Scholar] [CrossRef]
- Ferreira, V.S.; Pinto, R.F.; Sant’Anna, C. Low light intensity and nitrogen starvation modulate the chlorophyll content of Scenedesmus dimorphus. J. Appl. Microbiol. 2016, 120, 661–670. [Google Scholar] [CrossRef]
- Tanaka, A.; Tanaka, R. The biochemistry, physiology, and evolution of the chlorophyll cycle. Adv. Bot. Res. 2019, 90, 183–212. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, S.M. Effect of the N/P ratio on biomass productivity and nutrient removal from municipal wastewater. Bioprocess Biosyst. Eng. 2015, 38, 761–766. [Google Scholar] [CrossRef]
- Wang, L.; Min, M.; Li, Y.; Chen, P.; Chen, Y.; Liu, Y.; Ruan, R. Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl. Microbiol. Biotechnol. 2010, 162, 1174–1186. [Google Scholar] [CrossRef]
- Rearte, T.A.; Figueroa, F.L.; Gómez-Serrano, C.; Vélez, C.G.; Marsili, S.; Iorio, A.D.F.; Acién-Fernández, F.G. Optimization of the production of lipids and carotenoids in the microalga Golenkinia aff. brevispicula. Algal Res. 2020, 51, 102004. [Google Scholar] [CrossRef]
- Wang, B.; Lan, C.Q. Biomass production and nitrogen and phosphorus removal by the green alga Neochloris oleoabundans in simulated wastewater and secondary municipal wastewater effluent. Bioresour. Technol. 2011, 102, 5639–5644. [Google Scholar] [CrossRef]
- Reyimu, Z.; Özçimen, D. Batch cultivation of marine microalgae Nannochloropsis oculata and Tetraselmis suecica in treated municipal wastewater toward bioethanol production. J. Clean. Prod. 2017, 150, 40–46. [Google Scholar] [CrossRef]
- Su, Y. Revisiting carbon, nitrogen, and phosphorus metabolisms in microalgae for wastewater treatment. Sci. Total Environ. 2021, 762, 144590. [Google Scholar] [CrossRef]
- Beuckels, A.; Smolders, E.; Muylaert, K. Nitrogen availability influences phosphorus removal in microalgae-based wastewater treatment. Water Res. 2015, 77, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Chen, P. Min, M.; Chen, Y.; Zhu, J.; Roger, R.R. Anaerobic digested dairy manure as a nutrient supplement for cultivation of oil-rich green microalgae Chlorella sp. Bioresour. Technol. 2010, 101, 2623–2628. [Google Scholar] [CrossRef] [PubMed]
- Aydın, G.Ş.; Buyukisik, B.; Kocatas, A. Effects of different nitrogen (NO3-N ve NH4-N) sources on the growth of harmful marine diatom: Thalassiosira allenii Takano (Bacillariophyceae). J. Tekirdag Agric. Fac. 2013, 10, 90–96. [Google Scholar]
- Glibert, P.M.; Wilkerson, F.P.; Dugdale, R.C.; Raven, J.A.; Dupont, C.L.; Leavitt, P.R.; Kana, T.M. Pluses and minuses of ammonium and nitrate uptake and assimilation by phytoplankton and implications for productivity and community composition, with emphasis on nitrogen-enriched conditions. Limnol. Oceanogr. 2016, 61, 165–197. [Google Scholar] [CrossRef]
- Sanz-Luque, E.; Chamizo-Ampudia, A.; Llamas, A.; Galvan, A.; Fernandez, E. Understanding nitrate assimilation and its regulation in microalgae. Front. Plant Sci. 2015, 6, 899. [Google Scholar] [CrossRef]
- Gouveia, L.; Graça, S.; Sousa, C.; Ambrosano, L.; Ribeiro, B.; Botrel, E.P.; Neto, P.C.; Ferreira, A.F.; Silva, C.M. Microalgae biomass production using wastewater: Treatment and costs. Algal Res. 2016, 16, 167–176. [Google Scholar] [CrossRef]
- Han, L.; Pei, H.Y.; Hu, W.R.; Han, F.; Song, M.M.; Zhang, S. Nutrient removal and lipid accumulation properties of newly isolated microalgal strains. Bioresour. Technol. 2014, 165, 38–41. [Google Scholar] [CrossRef]
- Abdelaziz, A.E.; Leite, G.B.; Belhaj, M.A.; Hallenbeck, P.C. Screening microalgae native to Quebec for wastewater treatment and biodiesel production. Bioresour. Technol. 2014, 157, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Luong, T.T.; Lee, D.; Oh, Y.K.; Lee, T. Reuse of effluent water from a municipal wastewater treatment plant in microalgae cultivation for biofuel production. Bioresour. Technol. 2011, 102, 8639–8645. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.F.; Chen, P. Characterization of a microalga Chlorella sp. well adapted to highly concentratedmunicipal wastewater for nutrient removal and biodiesel production. Bioresour. Technol. 2011, 102, 5138–5144. [Google Scholar] [CrossRef]
- Oberholster, P.J.; Steyn, M.; Botha, A.M. A Comparative study of improvement of phycoremediation using a consortium of microalgae in municipal wastewater treatment pond systems as an alternative solution to Africa’s sanitation challenges. Processes 2021, 9, 1677. [Google Scholar] [CrossRef]
- Kotoula, D.; Iliopoulou, A.; Irakleous-Palaiologou, E.; Gatidou, G.; Aloupi, M.; Antonopoulou, P.; Stasinakis, A.S. Municipal wastewater treatment by combining in series microalgae Chlorella sorokiniana and macrophyte Lemna minör. Preliminary results. J. Clean. Prod. 2020, 271, 122704. [Google Scholar] [CrossRef]
- Ting, H.; Haifeng, L.; Shanshan, M.; Zhang, Y.; Zhidan, L.; Na, D. Progress in microalgae cultivation photobioreactors and applications in wastewater treatment a review. Int. J. Agric. Biol. Eng. 2017, 10, 1–29. [Google Scholar] [CrossRef]
- Baldev, E.; Mubarak Ali, D.; Pugazhendhi, A.; Thajuddin, N. Wastewater as an economical and ecofriendly green medium for microalgal biofuel production. Fuel 2021, 294, 120484. [Google Scholar] [CrossRef]
- Rasoul-Amini, S.; Montazeri-Najafabady, N.; Shaker, S.; Safari, A.; Kazemi, A.; Mousavi, P. Mobashera, M.A.; Ghasemiab, Y. Removal of nitrogen and phosphorus from wastewater using microalgae free cells in bath culture system. Biocatal. Agric. Biotechnol. 2014, 3, 126–131. [Google Scholar] [CrossRef]
- Aydın, G.Ş.; Buyukisik, B.; Kocatas, A. Effect of phosphate and silicate on the growth of harmful marine diatom: Thalassiosira allenii Takano (Bacillariophyceae). J. Tekirdag Agric. Fac. 2014, 11, 44–52. [Google Scholar]
- Zhou, Y.; Nguyen, B.T.; Zhou, C.; Straka, L.; Lai, Y.S.; Xia, S.; Rittmann, B.E. The distribution of phosphorus and its transformations during batch growth of Synechocystis. Water Res. 2017, 122, 355–362. [Google Scholar] [CrossRef]
- Wang, T.; Ni, Z.; Kuang, B.; Zhou, L.; Chen, X.; Lin, Z.; Jia, J. Two-stage hybrid microalgal electroactive wetland-coupled anaerobic digestion for swine wastewater treatment in South China: Full-scale verification. Sci. Total Environ. 2022, 153312. [Google Scholar] [CrossRef]
- Diaz, J.M.; Björkman, K.M.; Haley, S.T.; Ingall, E.D.; Karl, D.M.; Longo, A.F.; Dyhrman, S.T. Polyphosphate dynamics at Station ALOHA, North Pacific subtropical gyre. Limnol. Oceanogr. 2016, 61, 227–239. [Google Scholar] [CrossRef]
- Dyhrman, S.T. Nutrients and their acquisition: Phosphorus physiology in microalgae. Physiol. Microalgae 2016, 155–183. [Google Scholar] [CrossRef]
- Marella, T.K.; Parine, N.R.; Tiwari, A. Potential of diatom consortium developed by nutrient enrichment for biodiesel production and simultaneous nutrient removal from wastewater. Saudi J. Biol. Sci. 2018, 25, 704–709. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhang, T.Y.; Dao, G.H.; Hu, H.Y. Microalgae-based advanced municipal wastewater treatment for reuse in water bodies. Appl. Microbiol. Biotechnol. 2017, 101, 2659–2675. [Google Scholar] [CrossRef]
- Venkata, S.G.; Rajvanshi, M.; Navish, K.B.; Govindachary, S.; Prasad, V.; Dasgupta, S. Carbon streaming in microalgae: Extraction and analysis methods for high value compounds. Bioresour. Technol. 2017, 244, 1304–1306. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, J.E.; Cho, Y.B.; Hwang, S.J. Growth rate, organic carbon, and nutrient removal rates of Chlorella sorokiniana in autotrophic, heterotrophic, and mixotrophic conditions. Bioresour. Technol. 2013, 144, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, W.; Yang, H.; Wang, F.; Ruan, R. Trophic mode conversion and nitrogen deprivation of microalgae for high ammonium removal from synthetic wastewater. Bioresour. Technol. 2015, 196, 668–676. [Google Scholar] [CrossRef] [PubMed]
- EU. EU-Level Instruments on Water Reuse Final Report to Support the Commission’s Impact Assessment. Amec Foster Wheeler Environment & Infrastructure UK Ltd., IEEP, ACTeon, IMDEA and NTUA. 3 October 2016. Available online: https://ec.europa.eu/environment/water/blueprint/pdf/EU_level_instruments_on_water-2nd-IA_support-study_AMEC.pdf (accessed on 13 August 2022).
- EU. Wastewater Discharge Standards to the Aquatic Environment Council Directive 91/271/EEC Urban Waste Water Directive. 1991. Available online: https://ec.europa.eu/environment/water/water-urbanwaste/legislation/directive_en.htm (accessed on 13 August 2022).
- LG. Water Pollution and Control Regulation 31.12.2004/25687 (Resettlement LG-13/2/2008-26786) Legal Gazette of The Public of Turkey. 2004. Available online: https://www.mevzuat.gov.tr/mevzuat?MevzuatNo=7221&MevzuatTur=7&MevzuatTertip=5 (accessed on 13 August 2022).
- LG. The Public of Turkey’s Legislation for Water Reuse in Irrigation. Wastewater Treatment Plants Technical Procedures Communiqué, LG-20.03.2010/resettlement LG-27527, 2018. Legal Gazette of The Public of Turkey. 2010. Available online: https://www.resmigazete.gov.tr/eskiler/2010/03/20100320-7.htm (accessed on 13 August 2022).
- EU. Water Reuse-Legislative Framework in EU Regions. 2018. Available online: https://cor.europa.eu/en/engage/studies/Documents/Water-reuse.pdf (accessed on 11 November 2022).
- USEPA. Guidelines for Water Reuse. Available online: https://www.epa.gov/sites/default/files/2019-08/documents/2012-guidelines-water-reuse.pdf (accessed on 11 November 2022).
- Bi, L.J.; Hu, Z.Y. Flora algarum sinicarum Aquae Dulcis Tomus VIII. Chlorophyta Chlorococcales (I); Science Press: Beijing, China, 2004. [Google Scholar]
- Day, S.A.; Wickham, R.P.; Entwisle, T.J.; Tyler, P.A. Bibliographic check-list of non-marine algae in Australia. In Flora of Australia Supplementary Series 4; Taylor & Francis: Melbourne, Australia, 1995. [Google Scholar]
- Hirose, H.; Yamagishi, T.; Akiyama, M. Illustrations of the Japanese Fresh-Water Algae; Uchida Rokakuho Publishing Co., Ltd.: Tokyo, Japan, 1977. (In Japanese) [Google Scholar]
- Hu, H.; Wei, Y. The Freshwater Algae of China. Systematics, Taxonomy and Ecology; China Science Publishing & Media Ltd.: Beijing, China, 2006. [Google Scholar]
- John, D.M.; Tsarenko, P.M. Order Chlorococcales. In The Freshwater Algal Flora of the British Isles. An Identification Guide to Freshwater and Terrestrial Algae; John, D.M., Whitton, B.A., Brook, A.J., Eds.; Cambridge University Press: Cambridge, UK, 2002; pp. 327–409. [Google Scholar]
- Kim, J.K.; Kim, H.S. Algal Flora of Korea. Volume 6, Number 2 Chlorophyta: Chlorophyceae: Chlorococcales I: Micractiniaceae, Botryococcaceae, Characiaceae, Hydrodictyaceae; figs 1–92; National Institute of Biological Resources: Incheon, Republic of Korea, 2012. [Google Scholar]
- Komárek, J.; Fott, B. Chlorophyceae (Grünalgen) Ordnung: Chlorococcales. Das Phytoplankton des Süsswassers. In Das Phytoplankton des Süsswassers (Die Binnengewässer) XVI. 7. Teil 1. Hälfte; Huber-Pestalozzi, G., Ed.; E. Schweizerbart’sche Verlangbuchhandlung (Nägele u. Obermiller): Stuttgart, Germany, 1983. [Google Scholar]
- Korshikov, A.A. Viznachnik Prisnovodnihk Vodorostey Ukrainsykoi RSR [Vyp] V. Pidklas Protokokovi (Protococcineae). Bakuol’ni (Vacuolales) ta Protokokovi (Protococcales) [The Freshwater Algae of the Ukrainian SSR. V. Sub-Class Protococcineae. Vacuolales and Protococcales]; Akad. NAUK URSR: Kyjv [Kiev], Ukraine, 1953; pp. 1–439. [Google Scholar]
- Pankow, H. Algenflora der Ostsee. II. Plankton (Einschliesslich Benthischer Kieselalgen); Gustav Fischer: Jena, Germany, 1976; pp. 1–493. [Google Scholar]
- Tsarenko, P.M.; John, D.M. Order Sphaeropleales sensu lato [Phylum Chlorophyta]. In The Freshwater Algal Flora of the British Isles. An Identification Guide to Freshwater and Terrestrial Algae, 2nd ed.; John, D.M., Whitton, B.A., Brook, A.J., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 419–475. [Google Scholar]
- Tsarenko, P.M. Sphaeropleales. In Algae of Ukraine: Diversity, Nomenclature, Taxonomy, Ecology and Geography. Volume 3: Chlorophyta; Tsarenko, P.M., Wasser, S.P., Nevo, E., Eds.; A.R.A. Gantner Verlag K.-G.: Ruggell, Liechtenstein, 2011; pp. 280–355. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase; World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 2022; Available online: https://www.algaebase.org (accessed on 24 September 2022).
| Chl a Quota, µg L−1 | Chl b Quota, µg L−1 | µ, day−1 Chl a | µ, day−1 Chl b | µChl a:µChl b | DW (g L−1) | BP (g L−1 d−1) | P(Chl) (µg L−1 d−1) | C:N:P | N:P | x,y | Fitted Model Equation | r | R2 | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | 1803 ± 75.9 | 649.2 ± 28.2 | 1.687 ± 0.04 | 1.552 ± 0.02 | 1.09 | 7.66 ± 0.05 | 1.02 ± 0.12 | 0.25 ± 0.00 | 40.5:10.8:1 | 10.8:1 | (abc) | log10 (Chla) = 3.268 − 0.2787 × TP − 0.001 × COD + 0.0143 × TN | * | 92.087 | 0.0102 |
| S | 434.3 ± 22.1 | 179.1 ± 6.06 | 0.939 ± 0.02 | 0.91 ± 0.03 | 1.03 | 7.5 ± 0.09 | 0.83 ± 0.19 | 0.05 ± 0.00 | 36.7:6.5:1. | 9.0:1 | (abc) | log10 (Chla) = 2.94 + 0.00036 × COD + 0.00084 × TN − 0.164 × TP | * | 92.529 | 0.0005 |
| E | 444.6 ± 14.1 | 139.6 ± 10.9 | 0.917 ± 0.01 | 0.731 ± 0.03 | 1.25 | 6.50 ± 0.05 | 0.58 ± 0.09 | 0.06 ± 0.00 | 37:8.9:1 | 8.9:1 | (abc) | log10 (Chla) = 1.121 + 0.0685 x TN − 0.334 × TP − 0.008 × COD | * | 88.332 | 0.0245 |
| C | 218.2 ± 13.1 | 84.69 ± 3.33 | 0.547 ± 0.03 | 0.479 ± 0.01 | 1.14 | 2.33 ± 0.02 | 0.085 ± 0.06 | 0.02 ± 0.00 | 22.8:8.4:1 | 8.4:1. | (abc) | log10 (Chla) =1.8024 + 0.0254 × TN − 0.5562 × TP − 0.0002 × COD | * | 95.788 | 0.0033 |
| Parameter | Wastewater for Microalgae Cultivation | |||
|---|---|---|---|---|
| P | S | E | C | |
| pH | 9.4 ± 0.0 | 9.1 ± 0.0 | 9.0 ± 0.06 | 8.7 ± 0.06 |
| T °C | 24.1 ± 0.0 | 24.1 ± 0.0 | 24.1 ± 0.0 | 24.1 ± 0.5 |
| D.O (mg L−1) | 8.3 ± 0.0 | 8.8 ± 0.0 | 8.7 ± 0.0 | 8.8 ± 0.03 |
| NO2-N (µg L−1) | 40 ± 0.0 | 110 ± 0.0 | 90 ± 0.0 | 53.30 ± 5.77 |
| NO3-N (mg L−1) | 38.3 ± 4.5 | 26.2 ± 1.37 | 23.7 ± 0.57 | 138 ± 5.77 |
| NH4-N (mg L−1) | 53 ± 0.0 | 35 ± 0.0 | 21 ± 1.73 | 9 ± 2.64 |
| PO4-P (mg L−1) | 8.5 ± 0.0 | 7.9 ± 0.0 | 5 ± 0.0 | 16.7 ± 0.25 |
| COD (mg L−1) | 344 ± 0.0 | 290 ± 0.0 | 185 ± 0.0 | 402 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sisman-Aydin, G.; Simsek, K. Investigation of the Phycoremediation Potential of Freshwater Green Algae Golenkinia radiata for Municipal Wastewater. Sustainability 2022, 14, 15705. https://doi.org/10.3390/su142315705
Sisman-Aydin G, Simsek K. Investigation of the Phycoremediation Potential of Freshwater Green Algae Golenkinia radiata for Municipal Wastewater. Sustainability. 2022; 14(23):15705. https://doi.org/10.3390/su142315705
Chicago/Turabian StyleSisman-Aydin, Goknur, and Kemal Simsek. 2022. "Investigation of the Phycoremediation Potential of Freshwater Green Algae Golenkinia radiata for Municipal Wastewater" Sustainability 14, no. 23: 15705. https://doi.org/10.3390/su142315705
APA StyleSisman-Aydin, G., & Simsek, K. (2022). Investigation of the Phycoremediation Potential of Freshwater Green Algae Golenkinia radiata for Municipal Wastewater. Sustainability, 14(23), 15705. https://doi.org/10.3390/su142315705







