Solute Geochemistry and Water Quality Assessment of Groundwater in an Arid Endorheic Watershed on Tibetan Plateau
Abstract
1. Introduction
2. Materials and Methods
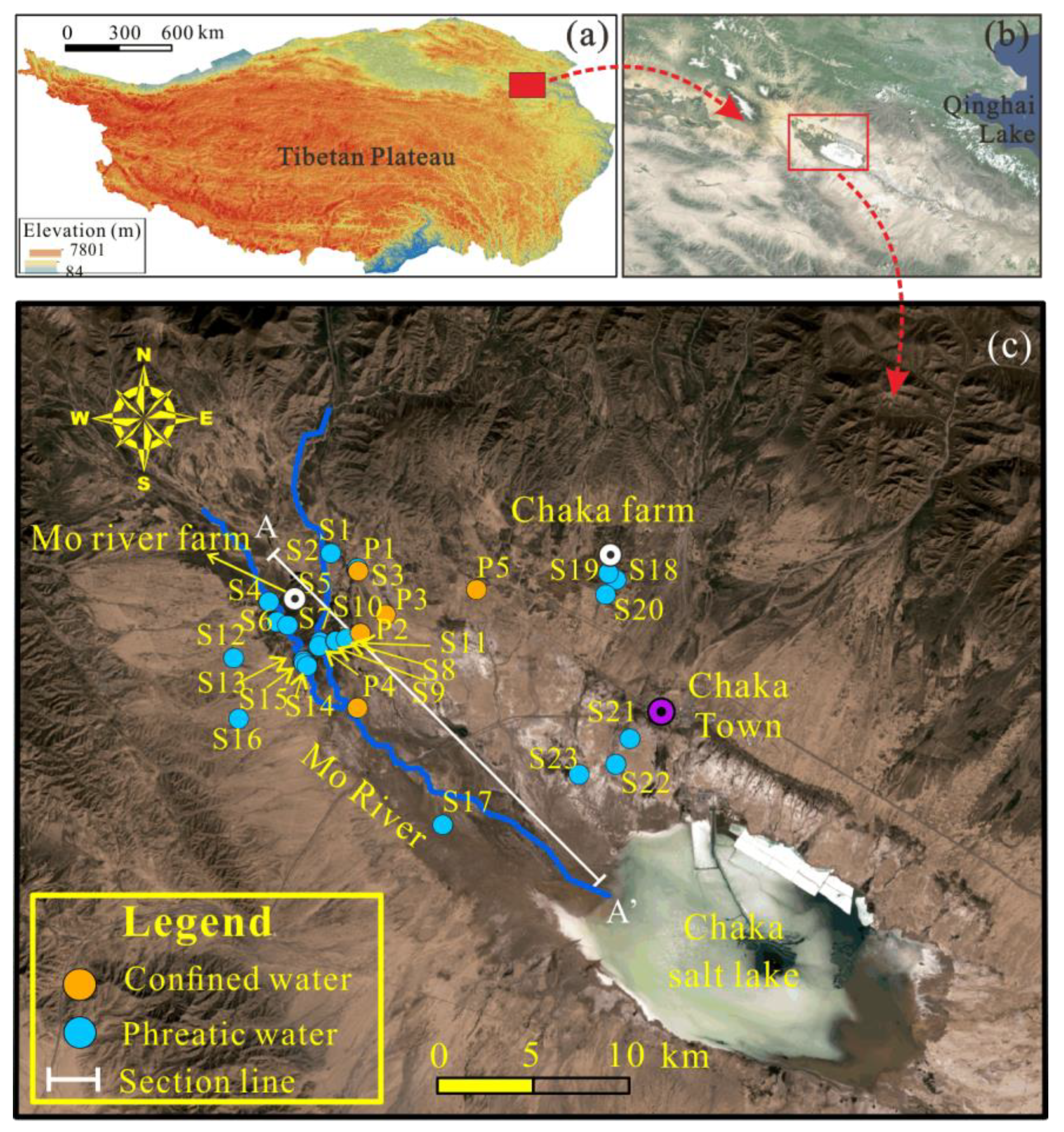
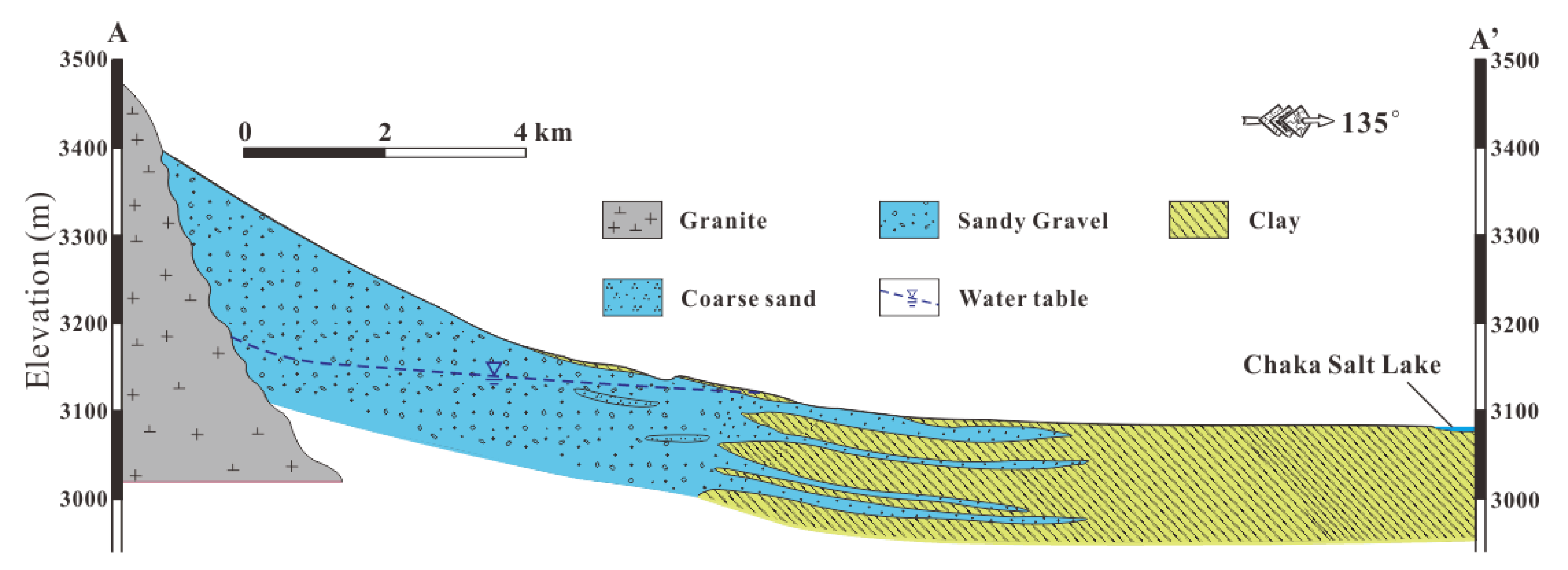
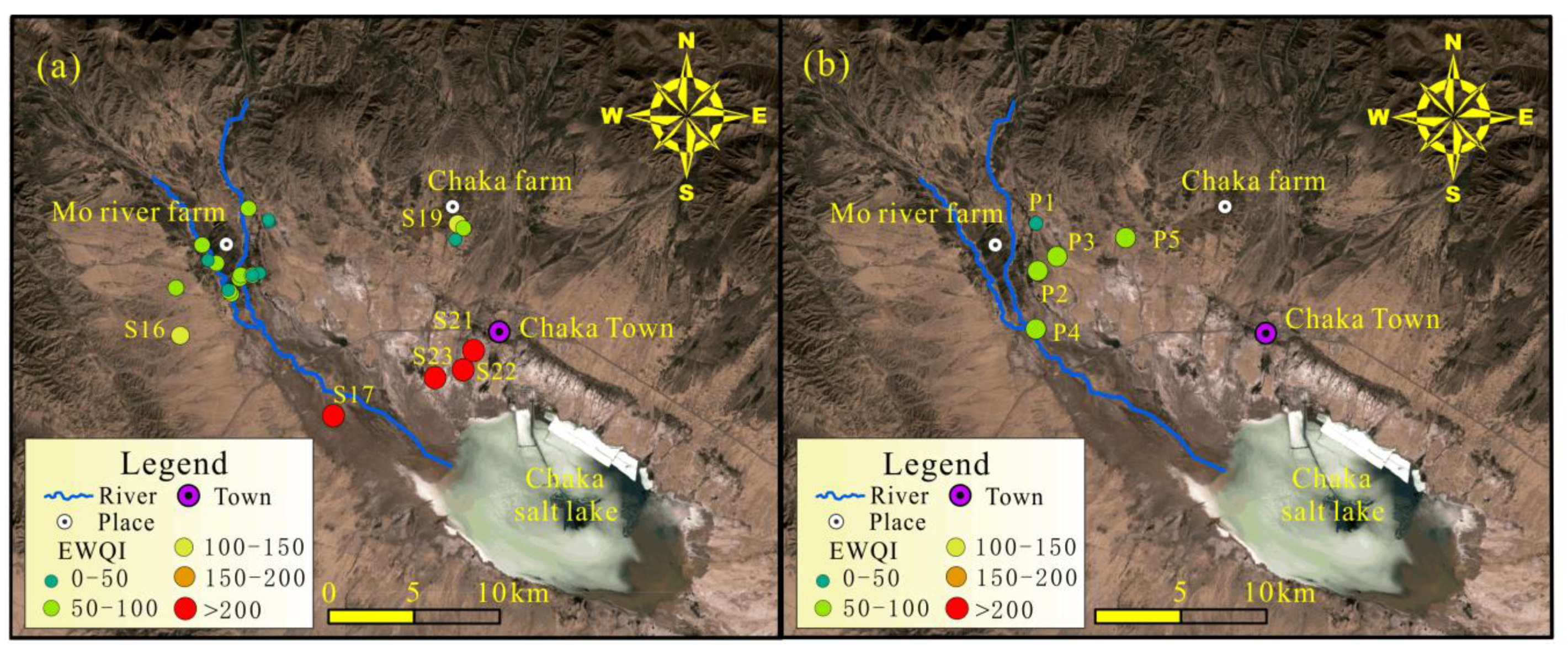
2.1. Description of the Study Area
2.2. Sampling and Analytical Techniques
3. Results and Discussion
3.1. General Chemistry of Groundwater
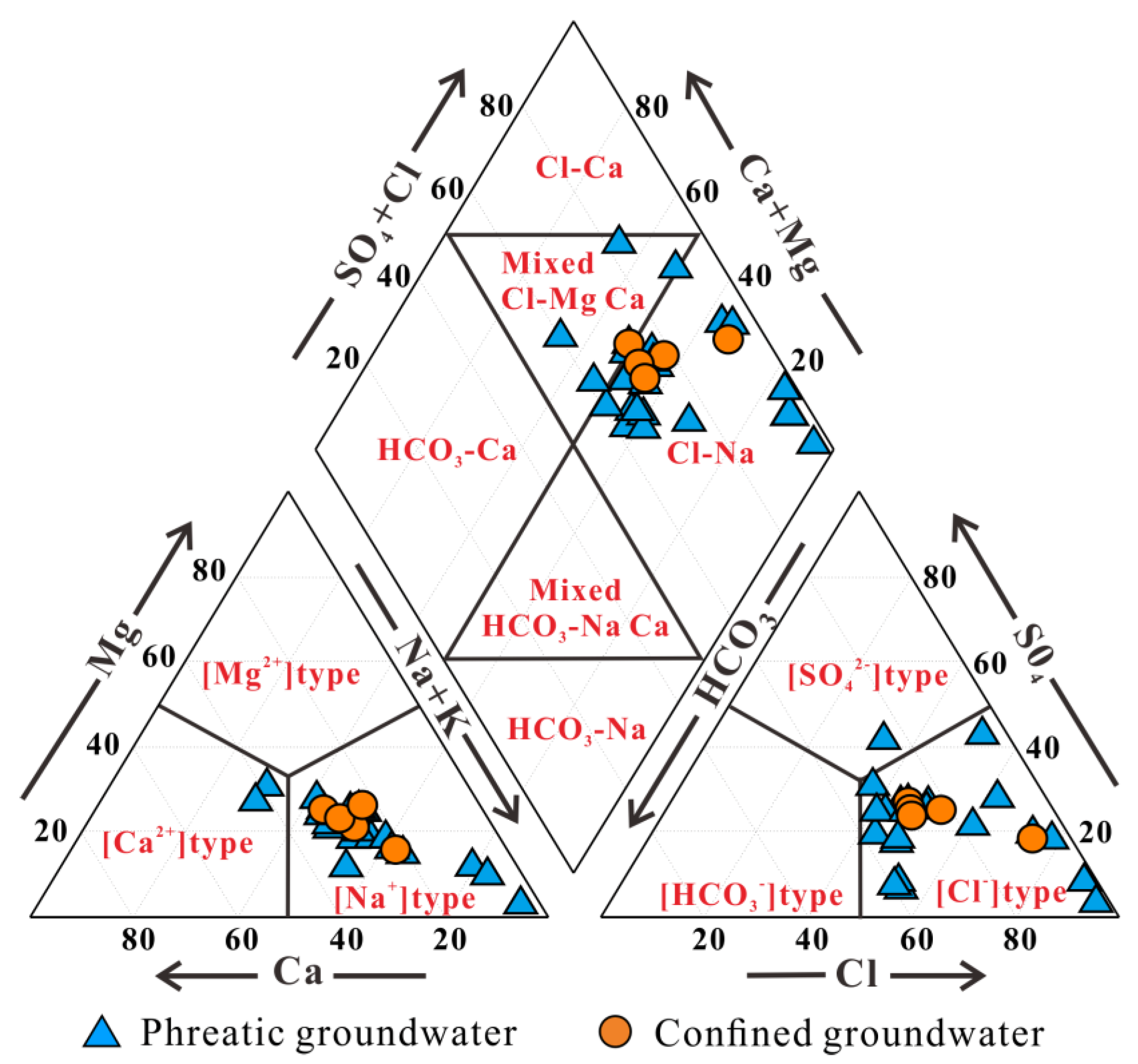
3.2. Hydrogeochemical Facies
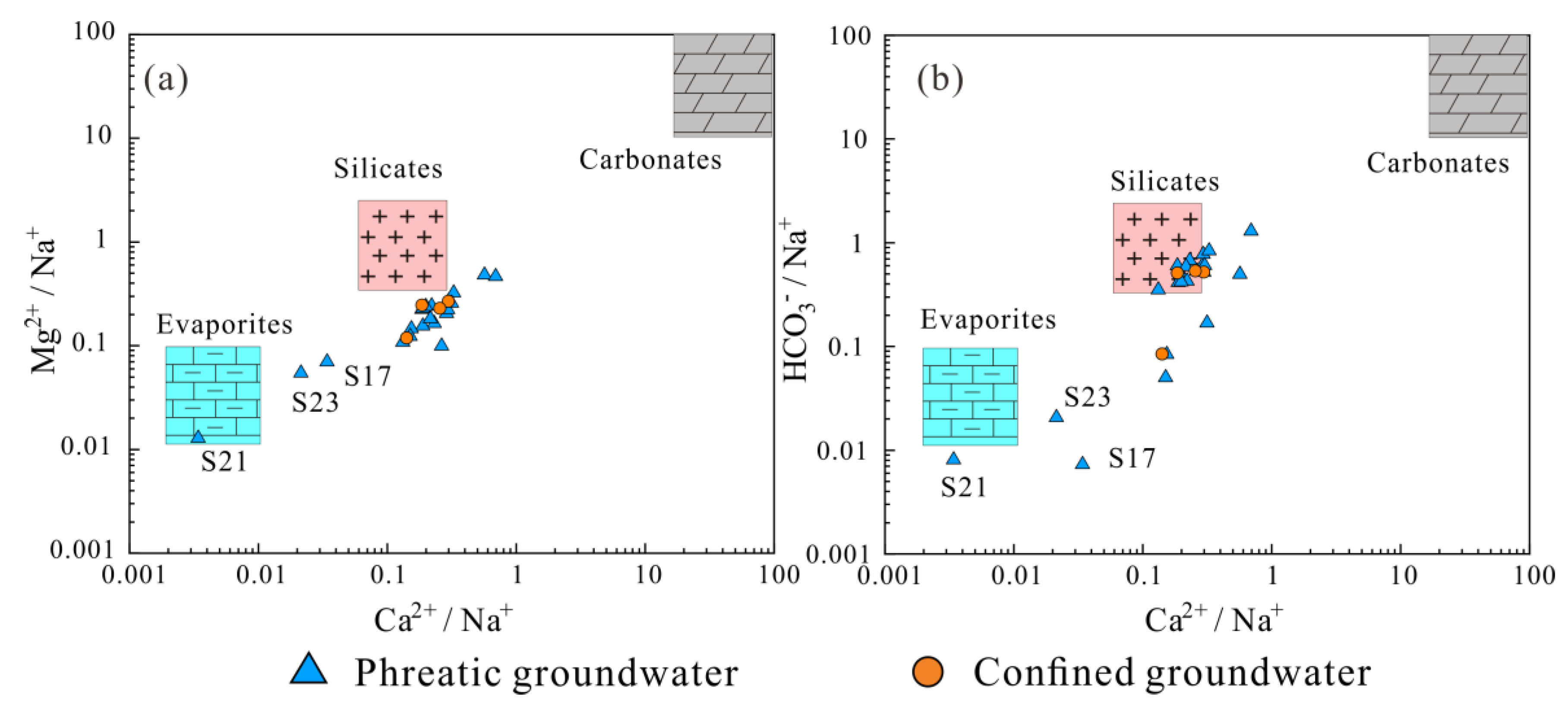
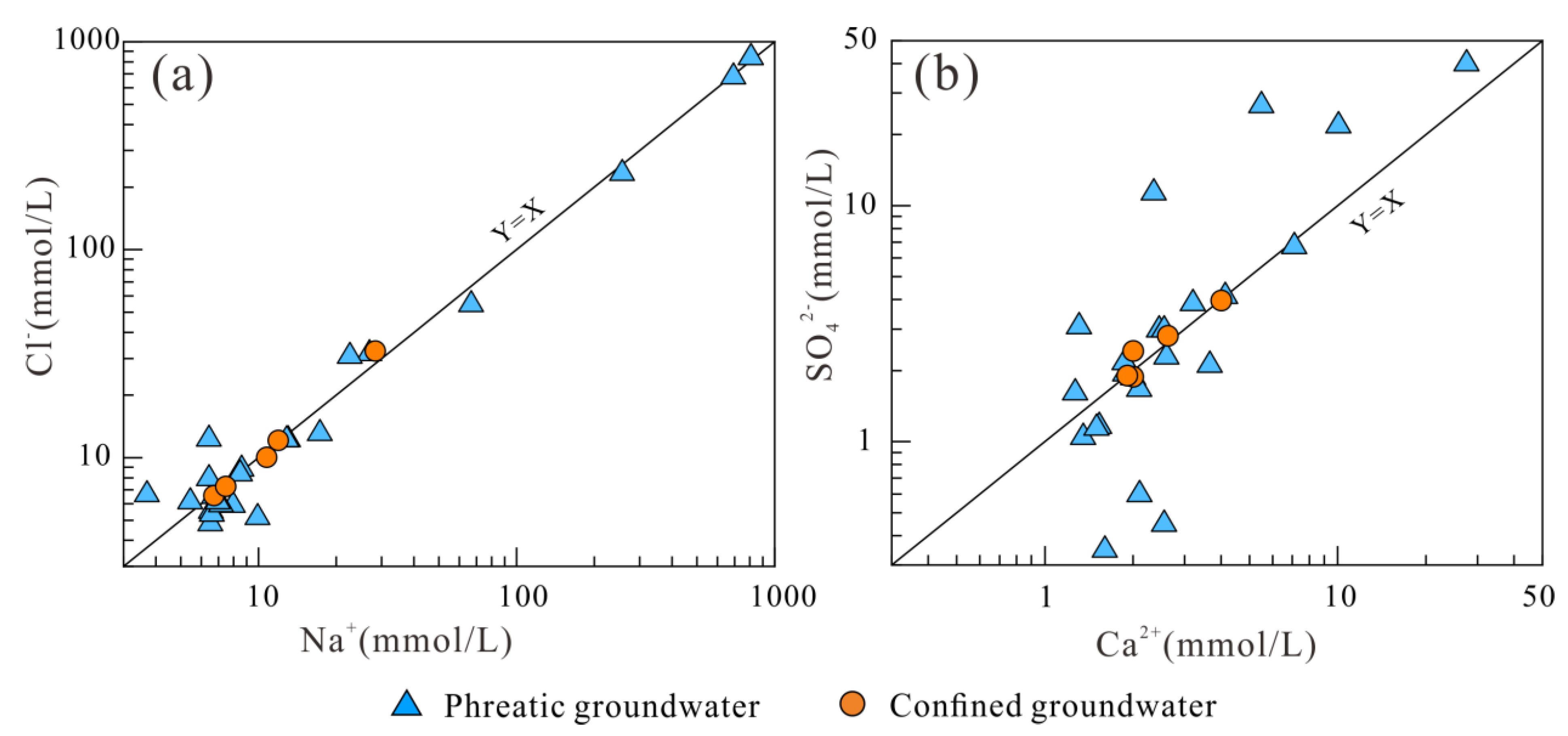
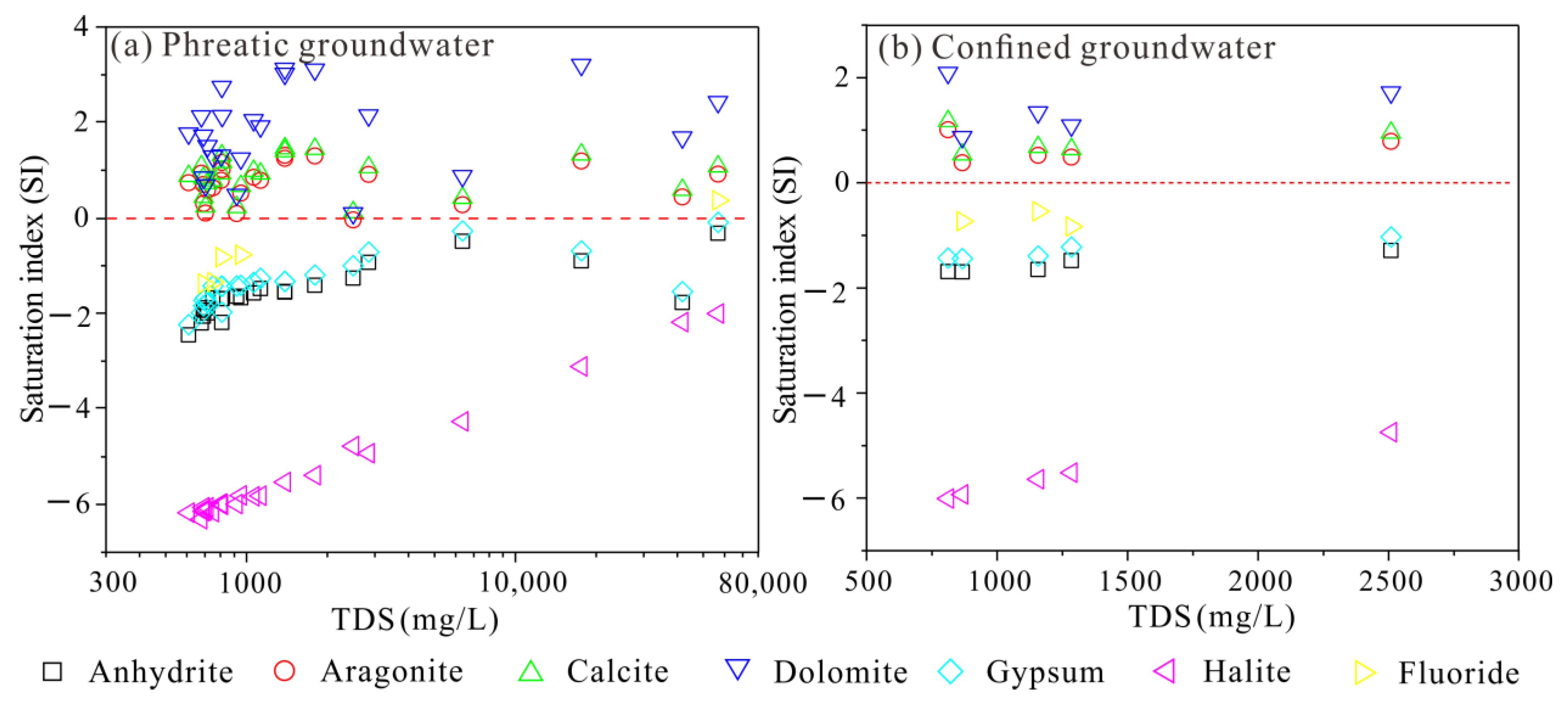
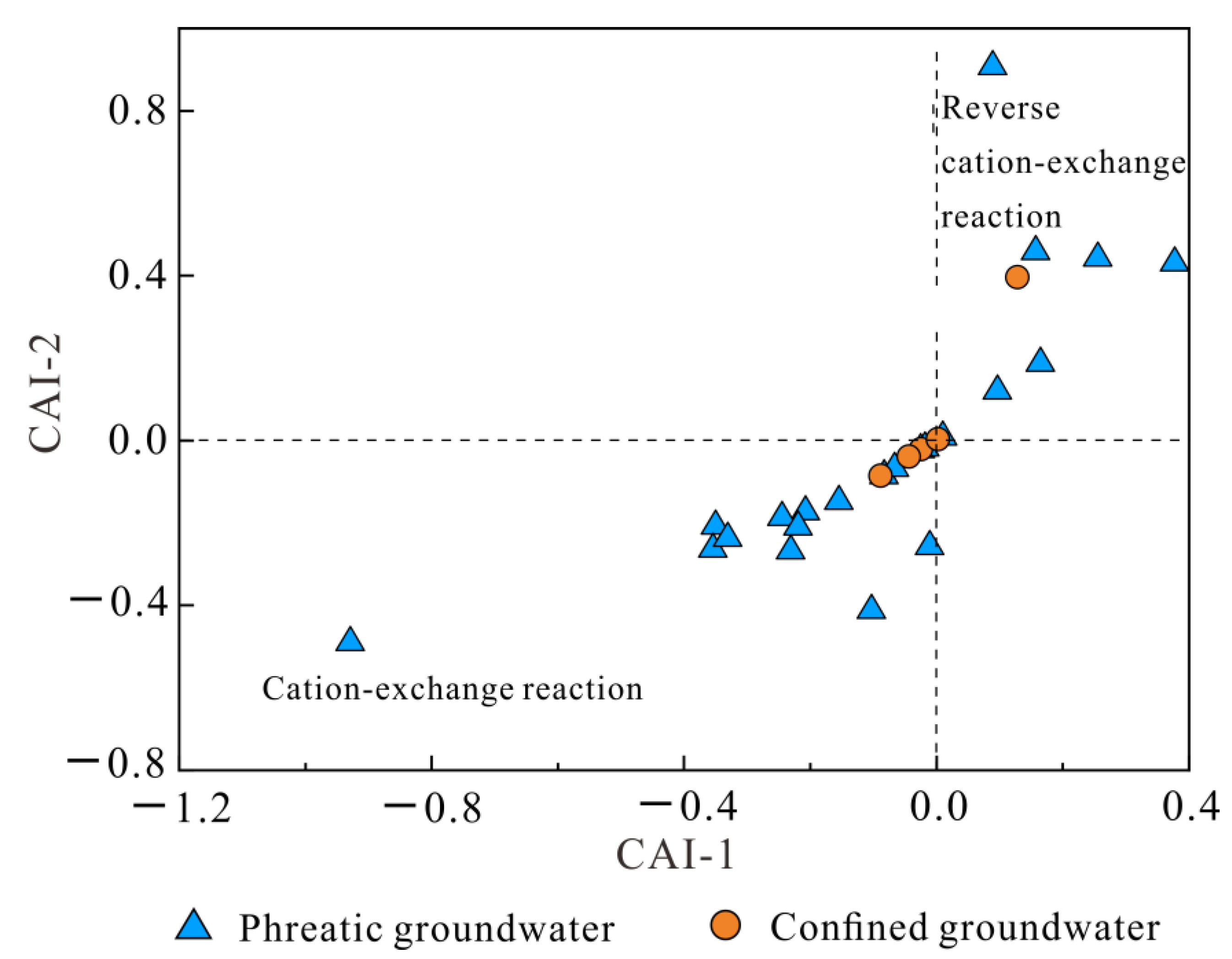
3.3. Hydrogeochemical Mechanisms Governing Groundwater Chemistry
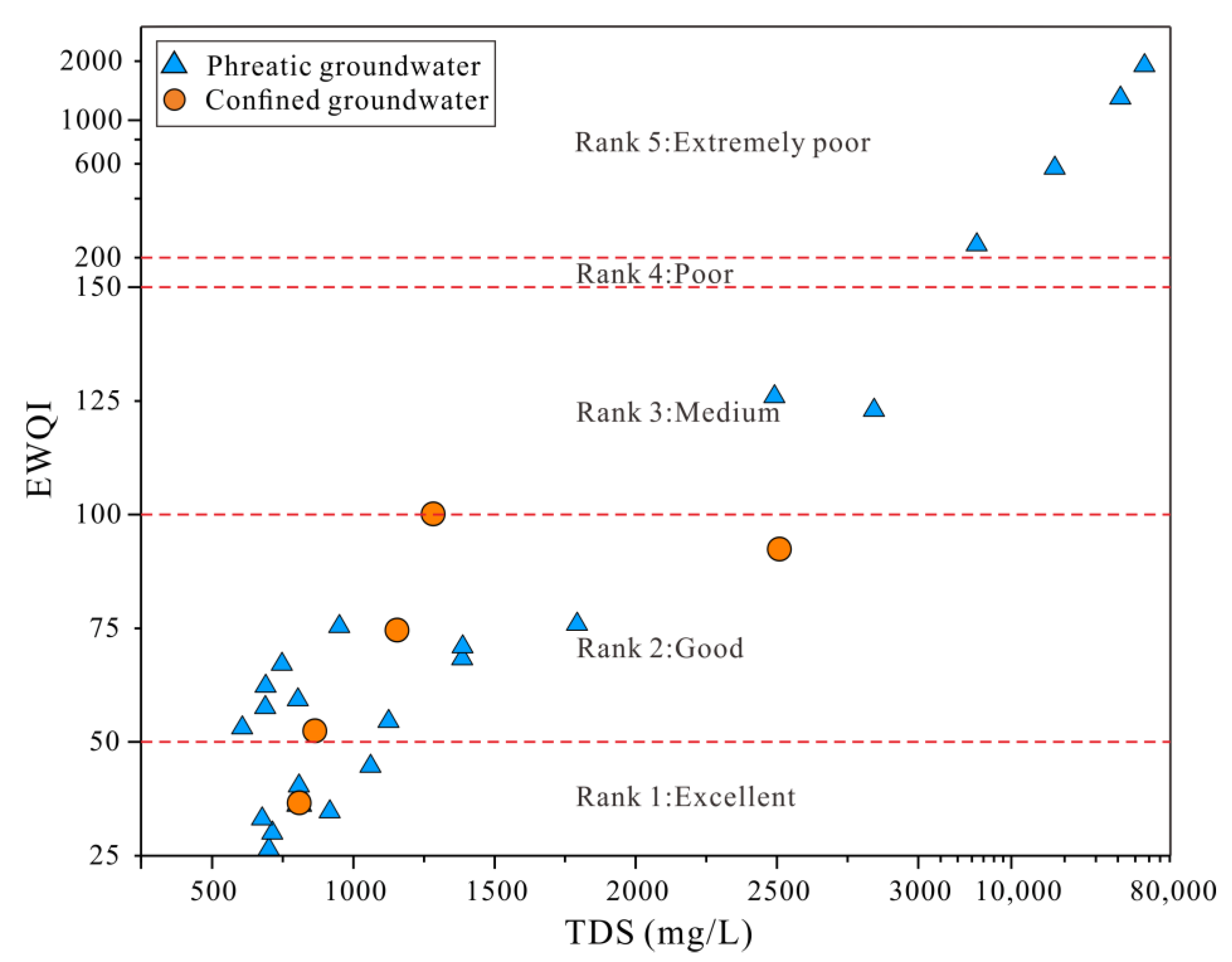
3.4. Groundwater Quality Assessment
3.4.1. Drinking Purposes
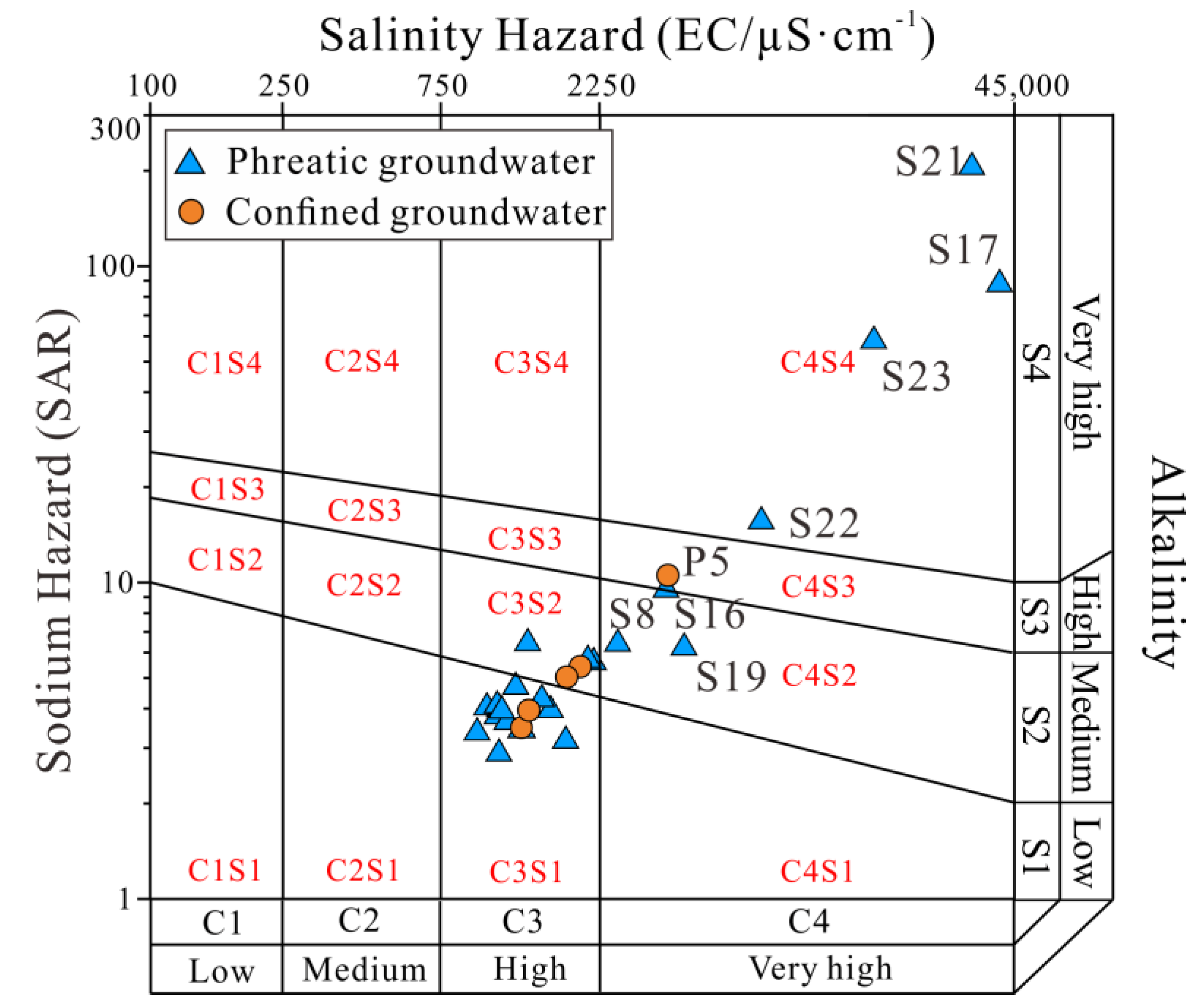
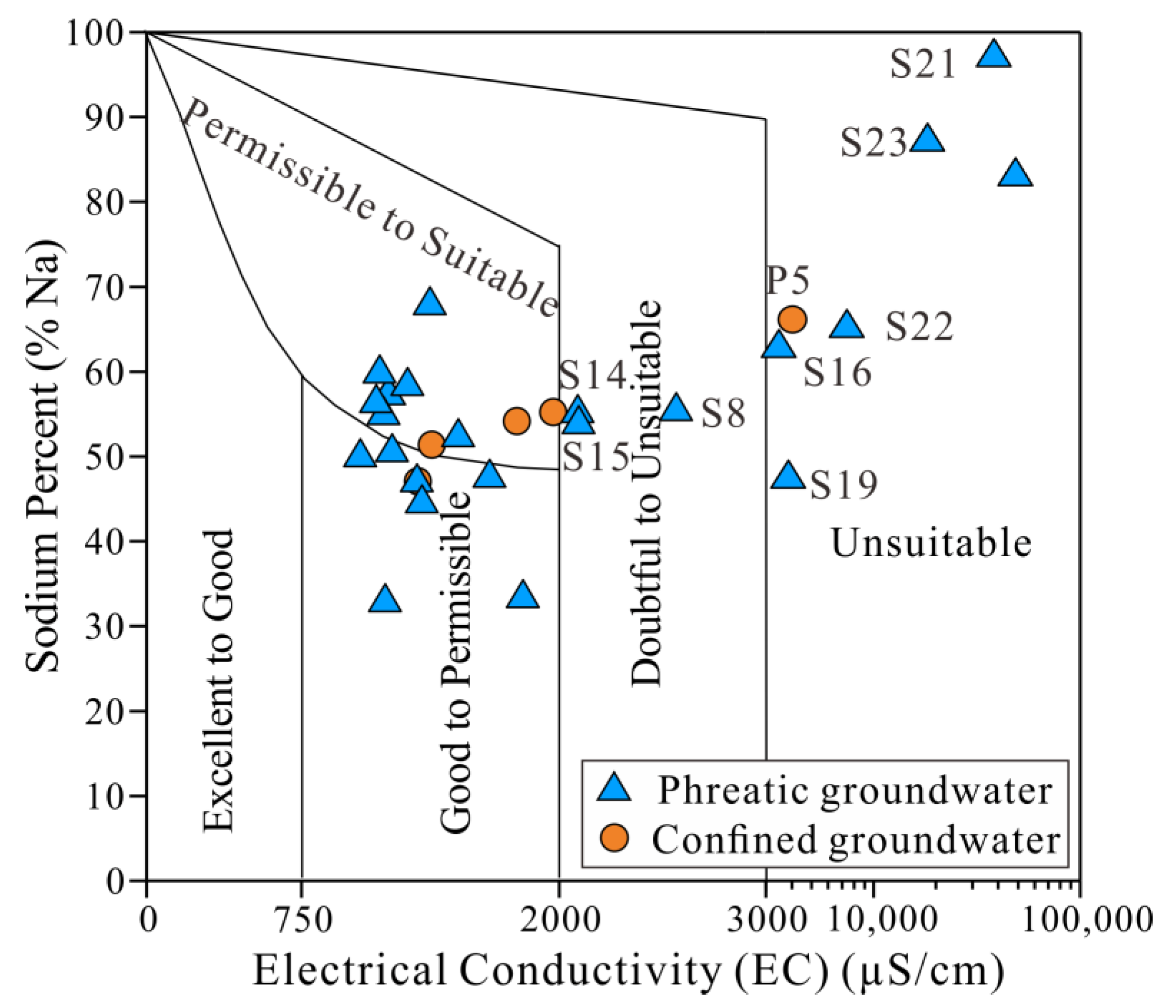
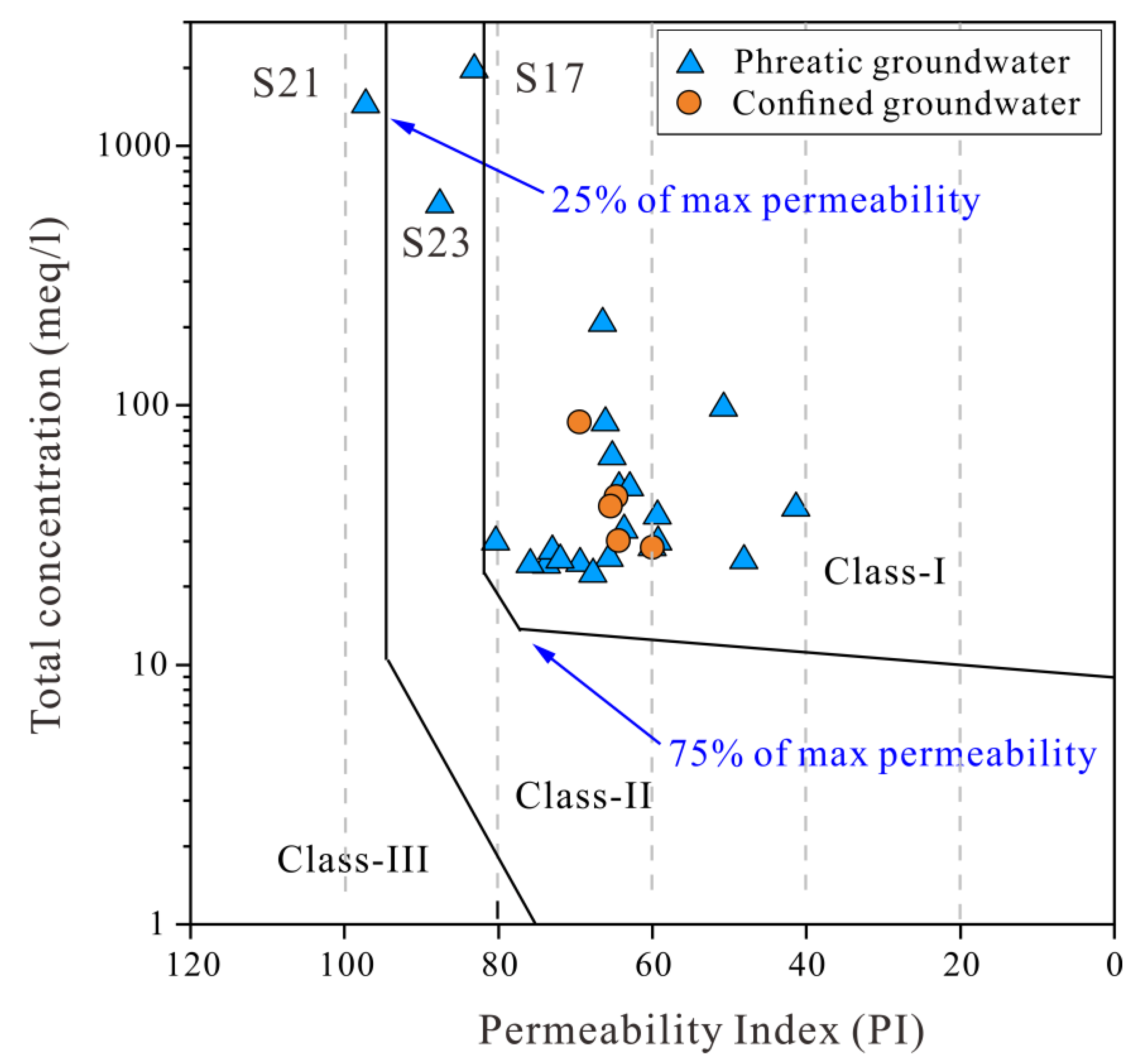
3.4.2. Irrigation Purposes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Avci, H.; Dokuz, U.E.; Avci, A.S. Hydrochemistry and groundwater quality in a semiarid calcareous area: An evaluation of major ion chemistry using a stoichiometric approach. Environ. Monit. Assess. 2018, 190, 641. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, P.; Qian, H. Groundwater Quality Assessment Using Improved Water Quality Index (WQI) and Human Health Risk (HHR) Evaluation in a Semi-arid Region of Northwest China. Expo. Health 2020, 12, 487–500. [Google Scholar] [CrossRef]
- Banadkooki, F.B.; Xiao, Y.; Malekinezhad, H.; Hosseini, M.M. Optimal allocation of regional water resources in an arid basin: Insights from Integrated Water Resources Management. J. Water Supply Res. Technol.-Aqua 2022, 71, 910–925. [Google Scholar] [CrossRef]
- Yin, S.; Gu, X.; Xiao, Y.; Wu, W.; Pan, X.; Shao, J.; Zhang, Q. Geostatistics-based spatial variation characteristics of groundwater levels in a wastewater irrigation area, northern China. Water Supply 2017, 17, 1479–1489. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, K.; Zhang, Y.; Yang, H.; Wang, S.; Qi, Z.; Hao, Q.; Wang, L.; Luo, Y.; Yin, S. Numerical investigation of groundwater flow system and its evolution under the climate change in the arid Golmud river watershed on Tibetan Plateau. Front. Earth Sci. 2022, 10, 943075. [Google Scholar] [CrossRef]
- Díaz-Alcaide, S.; Martínez-Santos, P. Review: Advances in groundwater potential mapping. Hydrogeol. J. 2019, 27, 2307–2324. [Google Scholar] [CrossRef]
- Hao, Q.; Xiao, Y.; Chen, K.; Zhu, Y.; Li, J. Comprehensive Understanding of Groundwater Geochemistry and Suitability for Sustainable Drinking Purposes in Confined Aquifers of the Wuyi Region, Central North China Plain. Water 2020, 12, 3052. [Google Scholar] [CrossRef]
- Barbieri, M.; Barberio, M.D.; Banzato, F.; Billi, A.; Boschetti, T.; Franchini, S.; Gori, F.; Petitta, M. Climate change and its effect on groundwater quality. Environ. Geochem. Health 2021. [Google Scholar] [CrossRef]
- Zhao, W.; Lin, Y.-z.; Zhou, P.-p.; Wang, G.-c.; Dang, X.-y.; Gu, X.-f. Characteristics of groundwater in Northeast Qinghai-Tibet Plateau and its response to climate change and human activities: A case study of Delingha, Qaidam Basin. China Geol. 2021, 4, 377–388. [Google Scholar] [CrossRef]
- Gu, X.; Xiao, Y.; Yin, S.; Hao, Q.; Liu, H.; Hao, Z.; Meng, G.; Pei, Q.; Yan, H. Hydrogeochemical Characterization and Quality Assessment of Groundwater in a Long-Term Reclaimed Water Irrigation Area, North China Plain. Water 2018, 10, 1209. [Google Scholar] [CrossRef]
- Li, P. Meeting the environmental challenges. Hum. Ecol. Risk Assess. 2020, 26, 2303–2315. [Google Scholar] [CrossRef]
- Adimalla, N. Groundwater Quality for Drinking and Irrigation Purposes and Potential Health Risks Assessment: A Case Study from Semi-Arid Region of South India. Expo. Health 2019, 11, 109–123. [Google Scholar] [CrossRef]
- Cui, W.; Hao, Q.; Xiao, Y.; Zhu, Y.; Li, J.; Zhang, Y. Combining river replenishment and restrictions on groundwater pumping to achieve groundwater balance in the Juma River Plain, North China Plain. Front. Earth Sci. 2022, 10, 902034. [Google Scholar] [CrossRef]
- Li, P.; He, X.; Li, Y.; Xiang, G. Occurrence and Health Implication of Fluoride in Groundwater of Loess Aquifer in the Chinese Loess Plateau: A Case Study of Tongchuan, Northwest China. Expo. Health 2019, 11, 95–107. [Google Scholar] [CrossRef]
- Yao, R.; Yan, Y.; Wei, C.; Luo, M.; Xiao, Y.; Zhang, Y. Hydrochemical Characteristics and Groundwater Quality Assessment Using an Integrated Approach of the PCA, SOM, and Fuzzy c-Means Clustering: A Case Study in the Northern Sichuan Basin. Front. Environ. Sci. 2022, 10, 907872. [Google Scholar] [CrossRef]
- Li, S.; Su, H.; Li, Z. Hydrochemical characteristics and groundwater quality in the thick loess deposits of China. Environ. Sci. Pollut. Res. 2022, 29, 8831–8850. [Google Scholar] [CrossRef]
- Li, C.; Gao, X.; Li, S.; Bundschuh, J. A review of the distribution, sources, genesis, and environmental concerns of salinity in groundwater. Environ. Sci. Pollut. Res. 2020, 27, 41157–41174. [Google Scholar] [CrossRef]
- Keesari, T.; Roy, A.; Pant, D.; Sinha, U.K.; Kumar, P.V.N.; Rao, L.V. Major ion, trace metal and environmental isotope characterization of groundwater in selected parts of Uddanam coastal region, Andhra Pradesh, India. J. Earth Syst. Sci. 2020, 129, 205. [Google Scholar] [CrossRef]
- Subba Rao, N.; Gugulothu, S.; Das, R. Deciphering artificial groundwater recharge suitability zones in the agricultural area of a river basin in Andhra Pradesh, India using geospatial techniques and analytical hierarchical process method. Catena 2022, 212, 106085. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, K.; Hao, Q.; Li, Y.; Xiao, D.; Zhang, Y. Occurrence, controlling factors and health hazards of fluoride-enriched groundwater in the lower flood plain of Yellow River, Northern China. Expo. Health 2022, 14, 345–358. [Google Scholar] [CrossRef]
- Hao, Q.; Lu, C.; Zhu, Y.; Xiao, Y.; Gu, X. Numerical investigation into the evolution of groundwater flow and solute transport in the Eastern Qaidam Basin since the Last Glacial Period. Geofluids 2018, 2018, 9260604. [Google Scholar] [CrossRef]
- Nag, S.; Roy, M.B.; Roy, P.K. Study on the functionality of land use land cover over the evaluation of groundwater potential zone: A fuzzy AHP based approach. J. Earth Syst. Sci. 2022, 131, 146. [Google Scholar] [CrossRef]
- Ntona, M.M.; Busico, G.; Mastrocicco, M.; Kazakis, N. Modeling groundwater and surface water interaction: An overview of current status and future challenges. Sci. Total Environ. 2022, 846, 157355. [Google Scholar] [CrossRef]
- Khorchani, H.; Kamel, S. Modeling of groundwater yield by the electrical method case of the Triassic sandstone aquifer (Tataouine South-Eastern Tunisia). J. Water Supply Res. Technol.-Aqua 2021, 70, 600–618. [Google Scholar] [CrossRef]
- Marinović, V.; Stevanović, Z. Karst groundwater quantity assessment and sustainability: The approach appropriate for river basin management plans. Environ. Earth Sci. 2019, 78, 362. [Google Scholar] [CrossRef]
- Yin, S.; Xiao, Y.; Gu, X.; Hao, Q.; Liu, H.; Hao, Z.; Meng, G.; Pan, X.; Pei, Q. Geostatistical analysis of hydrochemical variations and nitrate pollution causes of groundwater in an alluvial fan plain. Acta Geophys. 2019, 67, 1191–1203. [Google Scholar] [CrossRef]
- Adimalla, N.; Manne, R.; Zhang, Y.; Xu, P.; Qian, H. Evaluation of groundwater quality and its suitability for drinking purposes in semi-arid region of Southern India: An application of GIS. Geocarto Int. 2022, 1–12. [Google Scholar] [CrossRef]
- Li, Y.; Li, P.; Cui, X.; He, S. Groundwater quality, health risk, and major influencing factors in the lower Beiluo River watershed of northwest China. Hum. Ecol. Risk Assess. 2021, 27, 1987–2013. [Google Scholar] [CrossRef]
- Luo, Y.; Xiao, Y.; Hao, Q.; Zhang, Y.; Zhao, Z.; Wang, S.; Dong, G. Groundwater geochemical signatures and implication for sustainable development in a typical endorheic watershed on Tibetan plateau. Environ. Sci. Pollut. Res. 2021, 28, 48312–48329. [Google Scholar] [CrossRef]
- Ray, R.K.; Syed, T.H.; Saha, D.; Sarkar, B.C.; Reddy, D.V. Recharge mechanism and processes controlling groundwater chemistry in a Precambrian sedimentary terrain: A case study from Central India. Environ. Earth Sci. 2017, 76, 136. [Google Scholar] [CrossRef]
- Rajmohan, N.; Masoud, M.H.Z.; Niyazi, B.A.M. Impact of evaporation on groundwater salinity in the arid coastal aquifer, Western Saudi Arabia. Catena 2021, 196, 104864. [Google Scholar] [CrossRef]
- Venkatramanan, S.; Chung, S.Y.; Selvam, S.; Lee, S.Y.; Elzain, H.E. Factors controlling groundwater quality in the Yeonjegu District of Busan City, Korea, using the hydrogeochemical processes and fuzzy GIS. Environ. Sci. Pollut. Res. 2017, 24, 23679–23693. [Google Scholar] [CrossRef]
- Liu, R.-p.; Zhu, H.; Liu, F.; Dong, Y.; El-Wardany, R.M. Current situation and human health risk assessment of fluoride enrichment in groundwater in the Loess Plateau: A case study of Dali County, Shaanxi Province, China. China Geol. 2021, 4, 487–497. [Google Scholar] [CrossRef]
- Xiao, Y.; Shao, J.; Frape, S.; Cui, Y.; Dang, X.; Wang, S.; Ji, Y. Groundwater origin, flow regime and geochemical evolution in arid endorheic watersheds: A case study from the Qaidam Basin, northwestern China. Hydrol. Earth Syst. Sci. 2018, 22, 4381–4400. [Google Scholar] [CrossRef]
- Wang, S.; Xie, Z.; Wang, F.; Zhang, Y.; Wang, W.; Liu, K.; Qi, Z.; Zhao, F.; Zhang, G.; Xiao, Y. Geochemical Characteristics and Quality Appraisal of Groundwater From Huatugou of the Qaidam Basin on the Tibetan Plateau. Front. Earth Sci. 2022, 10, 874881. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, B.; Zhou, X. The effects of climate change and groundwater exploitation on the spatial and temporal variations of heavy metal content in maize in the Luan River catchment of China. Environ. Sci. Pollut. Res. 2019, 27, 1035–1052. [Google Scholar] [CrossRef]
- Manikandan, E.; Rajmohan, N.; Anbazhagan, S. Monsoon impact on groundwater chemistry and geochemical processes in the shallow hard rock aquifer. Catena 2020, 195, 104766. [Google Scholar] [CrossRef]
- Mthembu, P.P.; Elumalai, V.; Brindha, K.; Li, P. Hydrogeochemical Processes and Trace Metal Contamination in Groundwater: Impact on Human Health in the Maputaland Coastal Aquifer, South Africa. Expo. Health 2020, 12, 403–426. [Google Scholar] [CrossRef]
- Yin, S.; Xiao, Y.; Han, P.; Hao, Q.; Gu, X.; Men, B.; Huang, L. Investigation of Groundwater Contamination and Health Implications in a Typical Semiarid Basin of North China. Water 2020, 12, 1137. [Google Scholar] [CrossRef]
- Li, H.-x.; Han, S.-b.; Wu, X.; Wang, S.; Liu, W.-p.; Ma, T.; Zhang, M.-n.; Wei, Y.-t.; Yuan, F.-q.; Yuan, L.; et al. Distribution, characteristics and influencing factors of fresh groundwater resources in the Loess Plateau, China. China Geol. 2021, 4, 509–526. [Google Scholar] [CrossRef]
- Gu, X.; Xiao, Y.; Yin, S.; Liu, H.; Men, B.; Hao, Z.; Qian, P.; Yan, H.; Hao, Q.; Niu, Y.; et al. Impact of Long-Term Reclaimed Water Irrigation on the Distribution of Potentially Toxic Elements in Soil: An In-Situ Experiment Study in the North China Plain. Int. J. Environ. Res. Public Health 2019, 16, 649. [Google Scholar] [CrossRef]
- Xiao, Y.; Hao, Q.; Zhang, Y.; Zhu, Y.; Yin, S.; Qin, L.; Li, X. Investigating sources, driving forces and potential health risks of nitrate and fluoride in groundwater of a typical alluvial fan plain. Sci. Total Environ. 2022, 802, 149909. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, H.; Sheng, Y.; Ma, Z.; Zhang, J.; Liu, F.; Chen, S.; Meng, Q.; Bai, Y. Distribution, Source Apportionment, and Health Risk Assessment of Heavy Metals in Groundwater in a Multi-mineral Resource Area, North China. Expo. Health 2022, 14, 807–827. [Google Scholar] [CrossRef]
- Jiang, W.; Sheng, Y.; Liu, H.; Ma, Z.; Song, Y.; Liu, F.; Chen, S. Groundwater quality assessment and hydrogeochemical processes in typical watersheds in Zhangjiakou region, northern China. Environ. Sci. Pollut. Res. 2022, 29, 3521–3539. [Google Scholar] [CrossRef]
- Jin, X.; He, C.; Zhang, L.; Zhang, B. A Modified Groundwater Module in SWAT for Improved Streamflow Simulation in a Large, Arid Endorheic River Watershed in Northwest China. Chin. Geogr. Sci. 2018, 28, 47–60. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, K.; Yan, H.; Zhou, B.; Huang, X.; Hao, Q.; Zhang, Y.; Zhang, Y.; Liao, X.; Yin, S. Hydrogeochemical constraints on groundwater resource sustainable development in the arid Golmud alluvial fan plain on Tibetan plateau. Environ. Earth Sci. 2021, 80, 750. [Google Scholar] [CrossRef]
- Xiao, Y.; Shao, J.; Cui, Y.; Zhang, G.; Zhang, Q. Groundwater circulation and hydrogeochemical evolution in Nomhon of Qaidam Basin, northwest China. J. Earth Syst. Sci. 2017, 126, 26. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, Y.; Ma, H.; Wan, Q.; Zhang, Z.; Sang, L.; Liu, Y.; Xu, Y. Effects of a chain of reservoirs on temporal and spatial variation in water chemistry within an endorheic basin. Ecol. Indic. 2021, 125, 107523. [Google Scholar] [CrossRef]
- Wu, C.; Wu, X.; Qian, C.; Zhu, G. Hydrogeochemistry and groundwater quality assessment of high fluoride levels in the Yanchi endorheic region, northwest China. Appl. Geochem. 2018, 98, 404–417. [Google Scholar] [CrossRef]
- Grizard, P.; Schmitt, J.-M.; Goblet, P. Hydrogeology of an arid endorheic basin (Tsagaan Els, Dornogobi, Mongolia): Field data and conceptualization, three-dimensional groundwater modeling, and water budget. Hydrogeol. J. 2019, 27, 145–160. [Google Scholar] [CrossRef]
- Huang, F.; Ochoa, C.G.; Chen, X.; Zhang, D. Modeling oasis dynamics driven by ecological water diversion and implications for oasis restoration in arid endorheic basins. J. Hydrol. 2021, 593, 125774. [Google Scholar] [CrossRef]
- Gao, G.; Shen, Q.; Zhang, Y.; Pan, N.; Ma, Y.; Jiang, X.; Fu, B. Determining spatio-temporal variations of ecological water consumption by natural oases for sustainable water resources allocation in a hyper-arid endorheic basin. J. Clean. Prod. 2018, 185, 1–13. [Google Scholar] [CrossRef]
- Ndehedehe, C.E.; Ferreira, V.G.; Onojeghuo, A.O.; Agutu, N.O.; Emengini, E.; Getirana, A. Influence of global climate on freshwater changes in Africa’s largest endorheic basin using multi-scaled indicators. Sci. Total Environ. 2020, 737, 139643. [Google Scholar] [CrossRef]
- GB/T 14848-2017; Standards for Groundwater Quality. General Administration of Quality Supervision (GAQS): Beijing, China, 2017.
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Xiao, Y.; Yin, S.; Hao, Q.; Gu, X.; Pei, Q.; Zhang, Y. Hydrogeochemical appraisal of groundwater quality and health risk in a near-suburb area of North China. J. Water Supply Res. Technol.-Aqua 2020, 69, 55–69. [Google Scholar] [CrossRef]
- Qu, S.; Wang, G.; Shi, Z.; Xu, Q.; Guo, Y.; Ma, L.; Sheng, Y. Using stable isotopes (δD, δ18O, δ34S and 87Sr/86Sr) to identify sources of water in abandoned mines in the Fengfeng coal mining district, northern China. Hydrogeol. J. 2018, 26, 1443–1453. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Luo, M.; Wei, C.; Huang, X.; Xiao, Y.; Qin, L.; Pei, Q. Hydrochemistry and Entropy-Based Groundwater Quality Assessment in the Suining Area, Southwestern China. J. Chem. 2021, 2021, 5591892. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, Y.; Wang, Y.; Huang, X.; Xiao, Y.; Pei, Q. Hydrochemistry, quality and potential health risk appraisal of nitrate enriched groundwater in the Nanchong area, southwestern China. Sci. Total Environ. 2021, 784, 147186. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, K.; Hao, Q.; Xiao, D.; Zhu, Y.; Yin, S.; Zhang, Y. Hydrogeochemical insights into the signatures, genesis and sustainable perspective of nitrate enriched groundwater in the piedmont of Hutuo watershed, China. Catena 2022, 212, 106020. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Z.; Tian, H.; Huang, X.; Zhang, Z.; Liu, Y.; Xiao, Y.; Li, R. Hydrochemistry appraisal, quality assessment and health risk evaluation of shallow groundwater in the Mianyang area of Sichuan Basin, southwestern China. Environ. Earth Sci. 2021, 80, 576. [Google Scholar] [CrossRef]
- Qu, S.; Duan, L.; Shi, Z.; Liang, X.; Lv, S.; Wang, G.; Liu, T.; Yu, R. Hydrochemical assessments and driving forces of groundwater quality and potential health risks of sulfate in a coalfield, northern Ordos Basin, China. Sci. Total Environ. 2022, 835, 155519. [Google Scholar] [CrossRef]
- Mahammad, S.; Islam, A.; Shit, P.K. Geospatial assessment of groundwater quality using entropy-based irrigation water quality index and heavy metal pollution indices. Environ. Sci. Pollut. Res. 2022. [Google Scholar] [CrossRef]
- USSL. Diagnosis and Improvement of Saline and Alkali Soils; Agriculture Handbook; United States Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Qu, S.; Shi, Z.; Liang, X.; Wang, G.; Han, J. Multiple factors control groundwater chemistry and quality of multi-layer groundwater system in Northwest China coalfield—Using self-organizing maps (SOM). J. Geochem. Explor. 2021, 227, 106795. [Google Scholar] [CrossRef]


| Index | Unit | Min | Max | Mean | SD * | Guideline | % of the Sample Exceeding the Guideline | |
|---|---|---|---|---|---|---|---|---|
| Phreatic groundwater | pH | / | 7.42 | 8.66 | 7.98 | 0.31 | 6.5–8.5 ** | 8.70% |
| EC | μs/cm | 1033.50 | 44,205.00 | 5932.70 | 11,271.57 | / | ||
| TDS | mg/L | 609.19 | 56,715.34 | 6246.78 | 14,213.90 | 1000 ** | 47.83% | |
| K+ | mg/L | 1.50 | 68.81 | 13.51 | 14.44 | / | ||
| Na+ | mg/L | 85.00 | 18,610.00 | 2012.05 | 4972.36 | 200 ** | 43.48% | |
| Ca2+ | mg/L | 50.84 | 1102.20 | 160.48 | 221.62 | 75 *** | 69.57% | |
| Mg2+ | mg/L | 18.95 | 1358.37 | 135.53 | 277.33 | 50 *** | 47.83% | |
| Cl− | mg/L | 172.29 | 31,550.50 | 3177.79 | 8061.68 | 250 ** | 56.52% | |
| SO42− | mg/L | 33.62 | 3866.42 | 595.96 | 960.04 | 250 ** | 43.48% | |
| HCO3− | mg/L | 137.91 | 634.61 | 278.44 | 96.42 | / | ||
| NO3− | mg/L | 0.92 | 37.30 | 17.12 | 11.62 | 50.0 *** | 0% | |
| NO2− | mg/L | 0.00 | 0.08 | 0.01 | 0.02 | 0.02 ** | 8.70% | |
| NH4+ | mg/L | 0.00 | 0.20 | 0.07 | 0.09 | 0.2 ** | 17.93% | |
| F− | mg/L | 0.00 | 4.00 | 0.48 | 1.03 | 1.0 ** | 13.04% | |
| Confined groundwater | pH | / | 7.78 | 8.52 | 8.05 | 0.32 | 6.5–8.5 ** | 20% |
| EC | μs/cm | 1309.00 | 3608.10 | 2011.00 | 934.77 | / | ||
| TDS | mg/L | 811.86 | 2509.51 | 1325.94 | 690.32 | 1000 ** | 60% | |
| K+ | mg/L | 4.50 | 17.02 | 8.26 | 5.25 | / | ||
| Na+ | mg/L | 145.25 | 635.29 | 295.11 | 197.46 | 200 ** | 60% | |
| Ca2+ | mg/L | 76.55 | 160.32 | 100.52 | 35.37 | 75 *** | 100% | |
| Mg2+ | mg/L | 41.07 | 80.68 | 56.77 | 16.21 | 50 *** | 60% | |
| Cl− | mg/L | 232.55 | 1153.54 | 485.95 | 381.41 | 250 ** | 80% | |
| SO42− | mg/L | 180.59 | 379.44 | 248.80 | 81.83 | 250 ** | 40% | |
| HCO3− | mg/L | 146.45 | 335.61 | 244.08 | 71.03 | / | ||
| NO3− | mg/L | 4.00 | 16.00 | 9.20 | 4.60 | 50.0 *** | 0% | |
| NO2− | mg/L | 0.00 | 0.06 | 0.02 | 0.03 | 0.02 ** | 40% | |
| NH4+ | mg/L | 0.00 | 0.20 | 0.08 | 0.08 | 0.2 ** | 20% | |
| F− | mg/L | 1.00 | 1.60 | 1.27 | 0.31 | 1.0 ** | 60% |
| pH | TDS | K+ | Na+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | NO2− | NO3− | NH4+ | F− | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phreatic groundwater | pH | 1.00 | ||||||||||||
| TDS | −0.07 | 1.00 | ||||||||||||
| K+ | 0.19 | 0.67 | 1.00 | |||||||||||
| Na+ | −0.17 | 0.87 | 0.48 | 1.00 | ||||||||||
| Ca2+ | −0.17 | 0.77 | 0.78 | 0.56 | 1.00 | |||||||||
| Mg2+ | −0.08 | 0.90 | 0.85 | 0.69 | 0.85 | 1.00 | ||||||||
| Cl− | −0.08 | 0.87 | 0.86 | 0.71 | 0.86 | 0.96 | 1.00 | |||||||
| SO42− | −0.17 | 0.95 | 0.55 | 0.91 | 0.70 | 0.78 | 0.74 | 1.00 | ||||||
| HCO3- | 0.42 | 0.21 | 0.17 | 0.27 | 0.20 | 0.24 | 0.28 | 0.12 | 1.00 | |||||
| NO2− | 0.11 | 0.30 | −0.19 | 0.20 | 0.25 | 0.20 | 0.18 | 0.20 | 0.34 | 1.00 | ||||
| NO3− | 0.00 | 0.24 | 0.03 | 0.29 | 0.28 | 0.16 | 0.26 | 0.24 | 0.26 | 0.13 | 1.00 | |||
| NH4+ | −0.23 | −0.27 | −0.42 | 0.03 | −0.41 | −0.34 | −0.33 | −0.12 | −0.26 | −0.11 | −0.29 | 1.00 | ||
| F− | −0.12 | 0.08 | −0.37 | 0.19 | −0.08 | −0.18 | −0.20 | 0.20 | −0.08 | 0.26 | 0.31 | 0.58 | 1.00 | |
| Confined groundwater | pH | 1.00 | ||||||||||||
| TDS | 0.15 | 1.00 | ||||||||||||
| K+ | 0.66 | 0.84 | 1.00 | |||||||||||
| Na+ | 0.15 | 0.99 | 0.84 | 1.00 | ||||||||||
| Ca2+ | 0.22 | 0.98 | 0.85 | 0.97 | 1.00 | |||||||||
| Mg2+ | 0.04 | 0.92 | 0.71 | 0.92 | 0.84 | 1.00 | ||||||||
| Cl− | 0.21 | 0.99 | 0.87 | 0.99 | 0.98 | 0.90 | 1.00 | |||||||
| SO42− | 0.05 | 0.98 | 0.76 | 0.98 | 0.97 | 0.93 | 0.97 | 1.00 | ||||||
| HCO3− | −0.68 | −0.60 | −0.84 | −0.60 | −0.69 | −0.29 | −0.66 | −0.49 | 1.00 | |||||
| NO2− | 0.86 | −0.15 | 0.37 | −0.15 | −0.03 | −0.39 | −0.07 | −0.26 | −0.67 | 1.00 | ||||
| NO3− | −0.59 | −0.08 | −0.46 | −0.10 | −0.03 | 0.02 | −0.15 | 0.10 | 0.54 | −0.62 | 1.00 | |||
| NH4+ | −0.86 | −0.28 | −0.72 | −0.29 | −0.28 | −0.14 | −0.35 | −0.12 | 0.76 | −0.77 | 0.90 | 1.00 | ||
| F− | 0.84 | −0.11 | 0.65 | −0.07 | −0.68 | 0.54 | −0.25 | −0.24 | 0.74 | −0.50 | −0.50 | −0.50 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Yang, H.; Zhang, Y.; Wang, S.; Liu, K.; Qi, Z.; Chai, X.; Wang, L.; Wang, W.; Banadkooki, F.B.; et al. Solute Geochemistry and Water Quality Assessment of Groundwater in an Arid Endorheic Watershed on Tibetan Plateau. Sustainability 2022, 14, 15593. https://doi.org/10.3390/su142315593
Wang F, Yang H, Zhang Y, Wang S, Liu K, Qi Z, Chai X, Wang L, Wang W, Banadkooki FB, et al. Solute Geochemistry and Water Quality Assessment of Groundwater in an Arid Endorheic Watershed on Tibetan Plateau. Sustainability. 2022; 14(23):15593. https://doi.org/10.3390/su142315593
Chicago/Turabian StyleWang, Fenglin, Hongjie Yang, Yuqing Zhang, Shengbin Wang, Kui Liu, Zexue Qi, Xiaoran Chai, Liwei Wang, Wanping Wang, Fatemeh Barzegari Banadkooki, and et al. 2022. "Solute Geochemistry and Water Quality Assessment of Groundwater in an Arid Endorheic Watershed on Tibetan Plateau" Sustainability 14, no. 23: 15593. https://doi.org/10.3390/su142315593
APA StyleWang, F., Yang, H., Zhang, Y., Wang, S., Liu, K., Qi, Z., Chai, X., Wang, L., Wang, W., Banadkooki, F. B., Senapthi, V., & Xiao, Y. (2022). Solute Geochemistry and Water Quality Assessment of Groundwater in an Arid Endorheic Watershed on Tibetan Plateau. Sustainability, 14(23), 15593. https://doi.org/10.3390/su142315593








