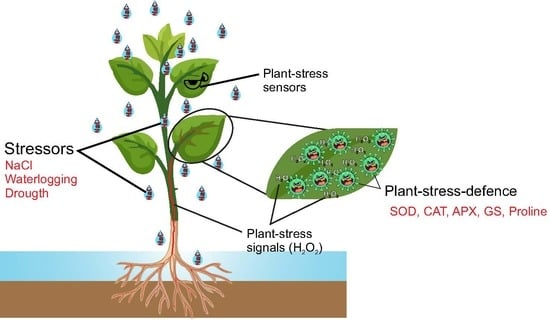
Can Chlorophyll a Fluorescence and Photobleaching Be a Stress Signal under Abiotic Stress in Vigna unguiculata L.?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Plant Material
2.2. Leaf Gas Exchange Parameters and Chlorophyll a Fluorescence
2.3. Biochemical Analysis
2.4. Assaying the Activity of Antioxidant Enzymes
2.5. RT-PCR
2.6. Total Biomass and Leaf Area Estimation
2.7. Experimental Design and Statistical Analyses
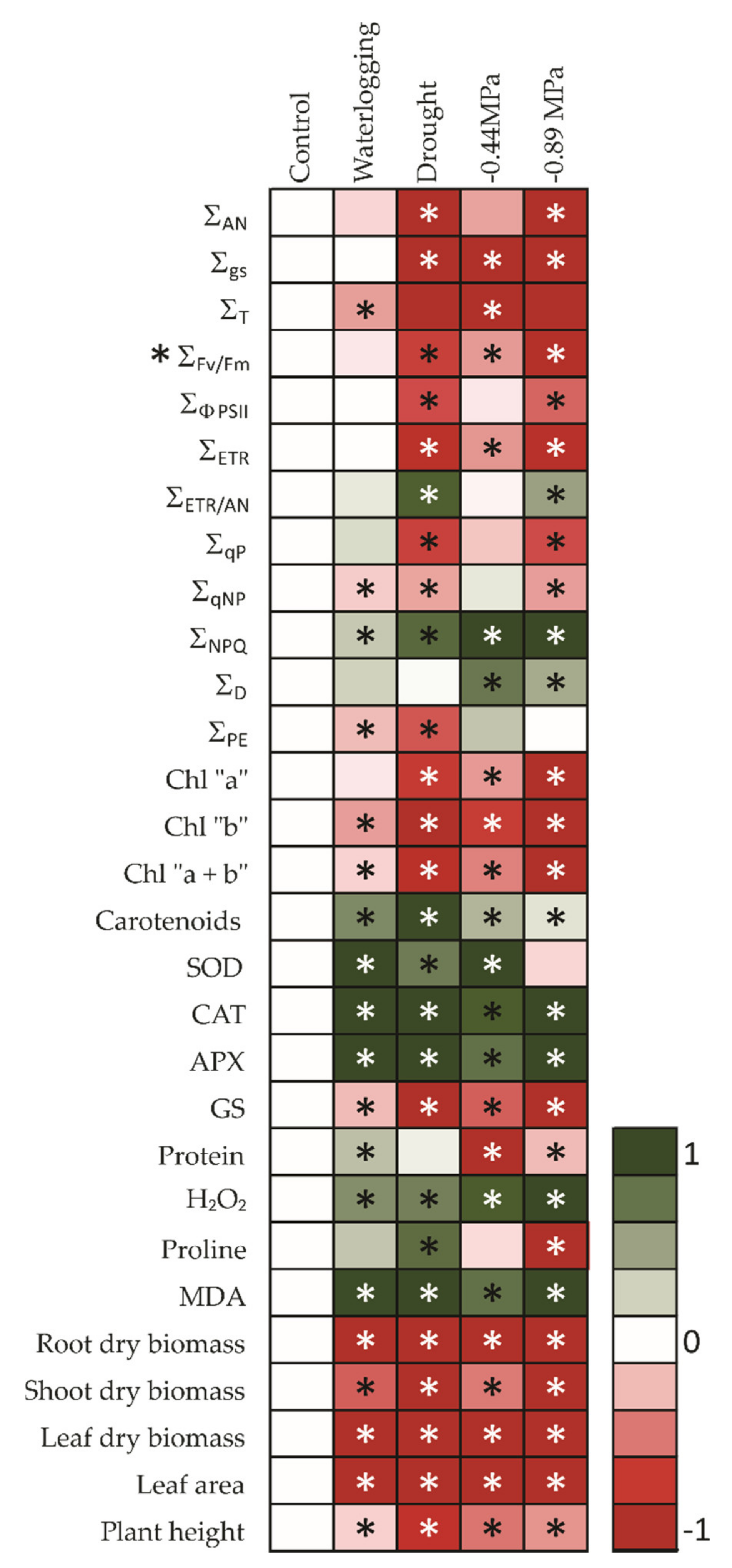
3. Results
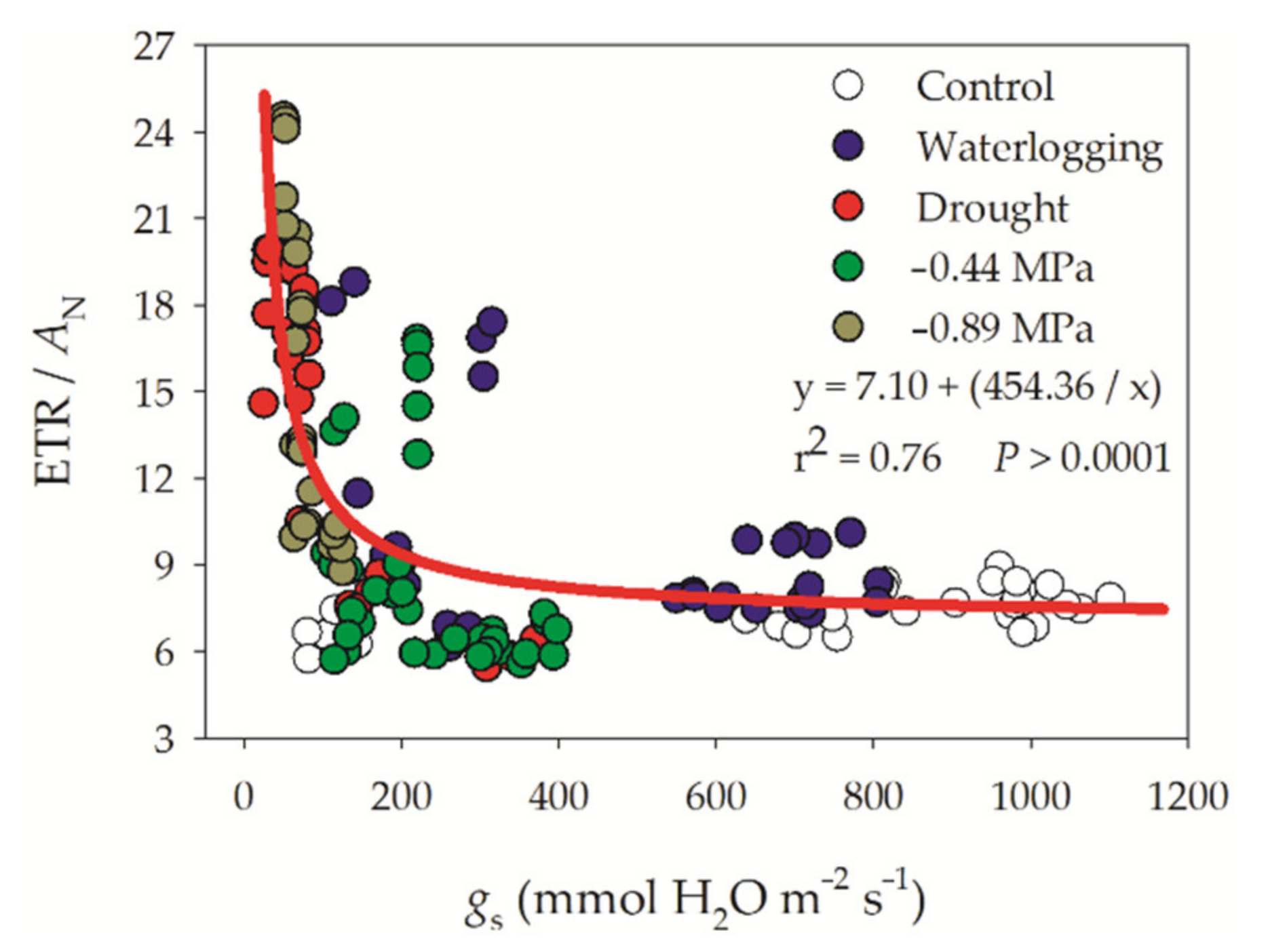
3.1. Gas Exchange Parameters
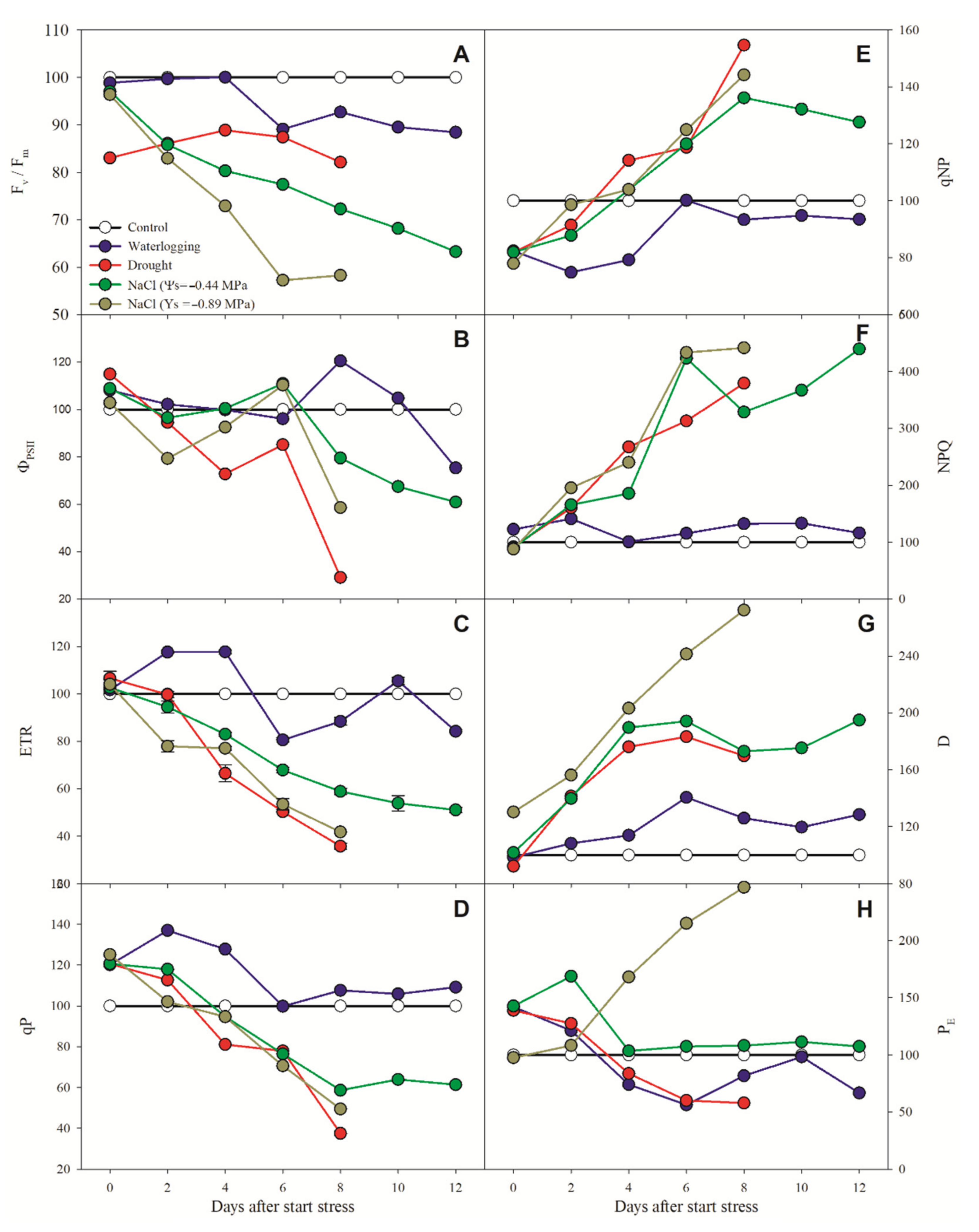
3.2. Chlorophyll a Fluorescence Parameters
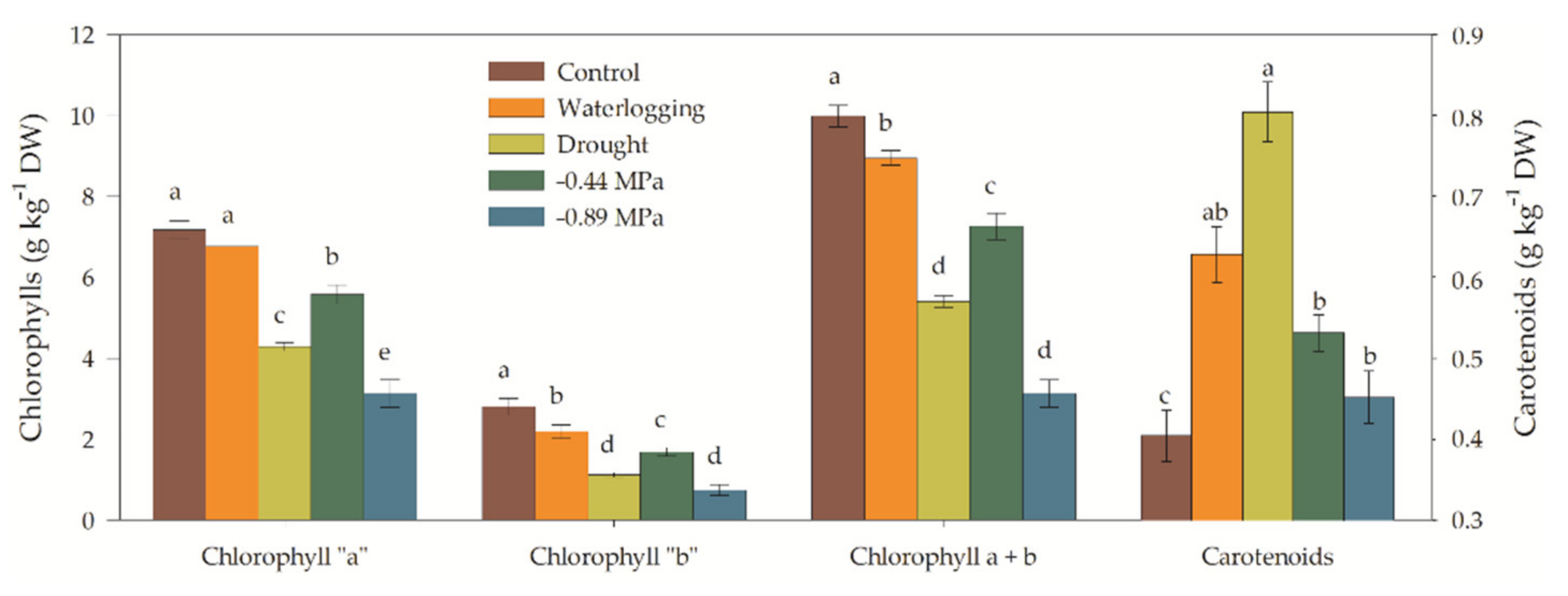
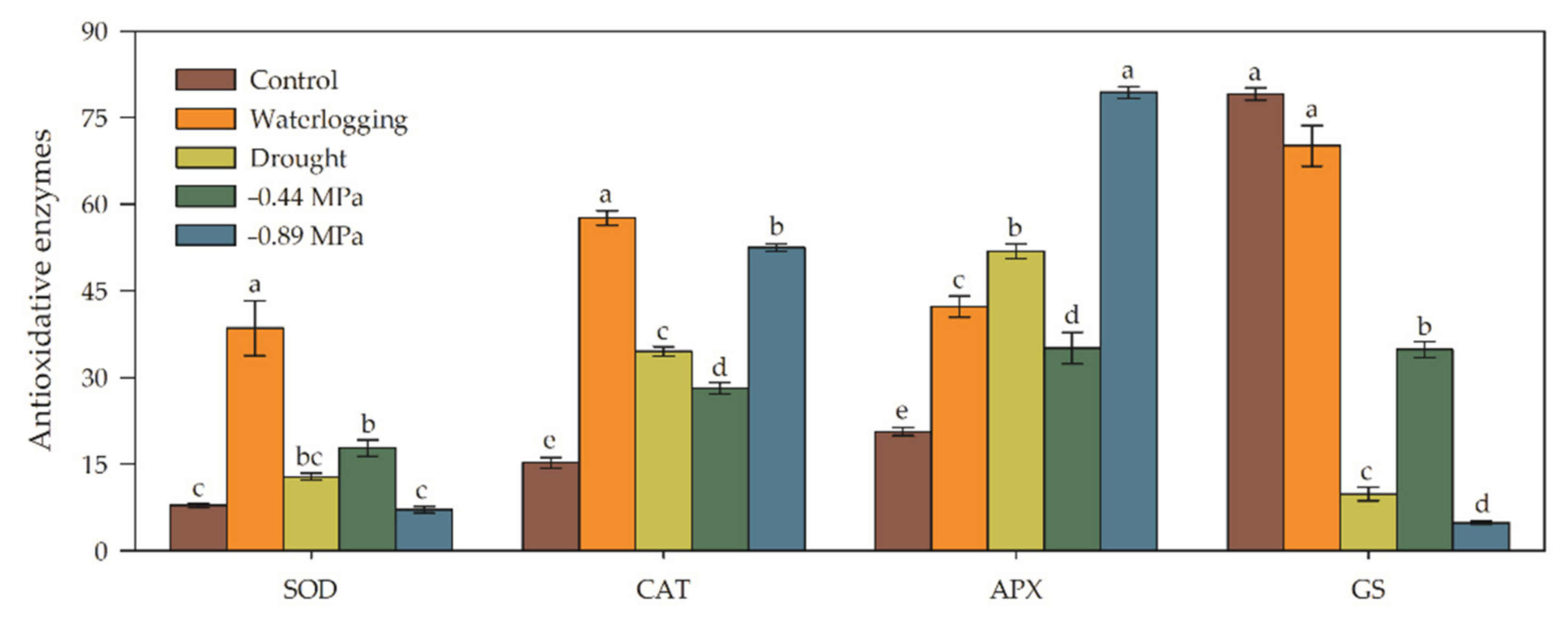
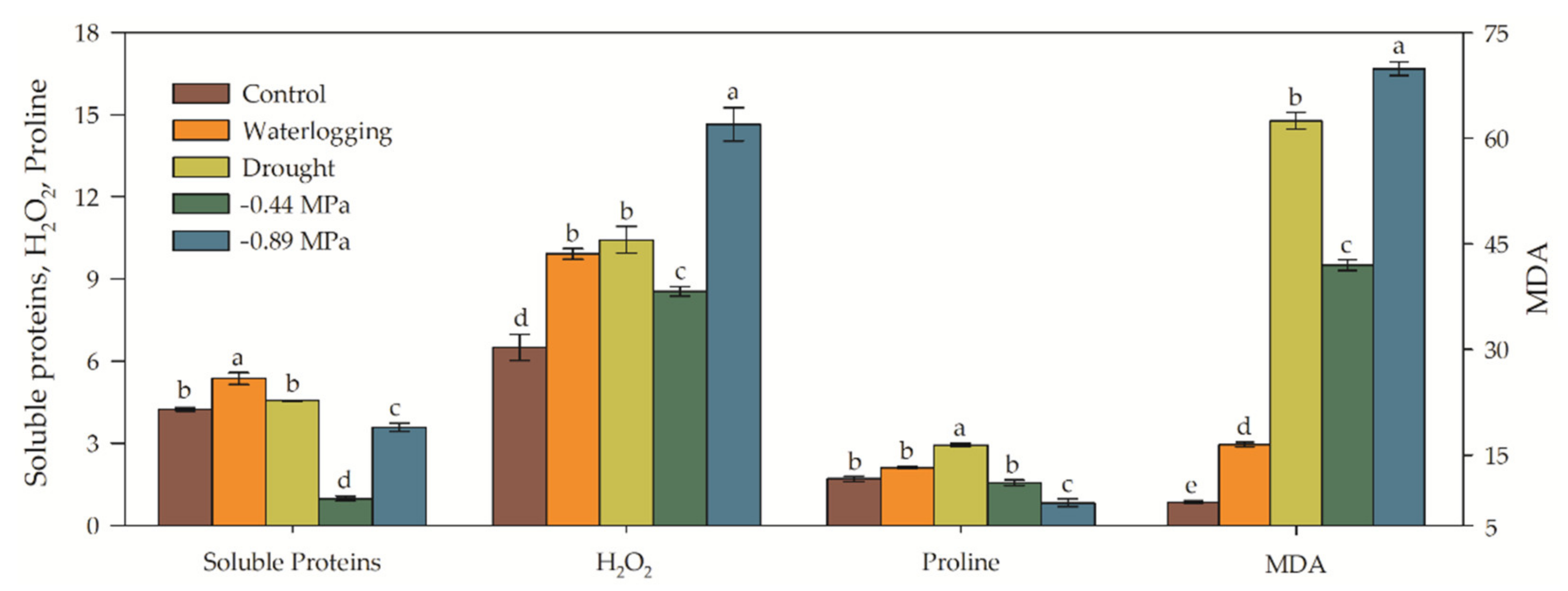
3.3. Biochemical and Enzymatic Analysis
3.4. Total Biomass and Leaf Area Estimation
3.5. RT-PCR

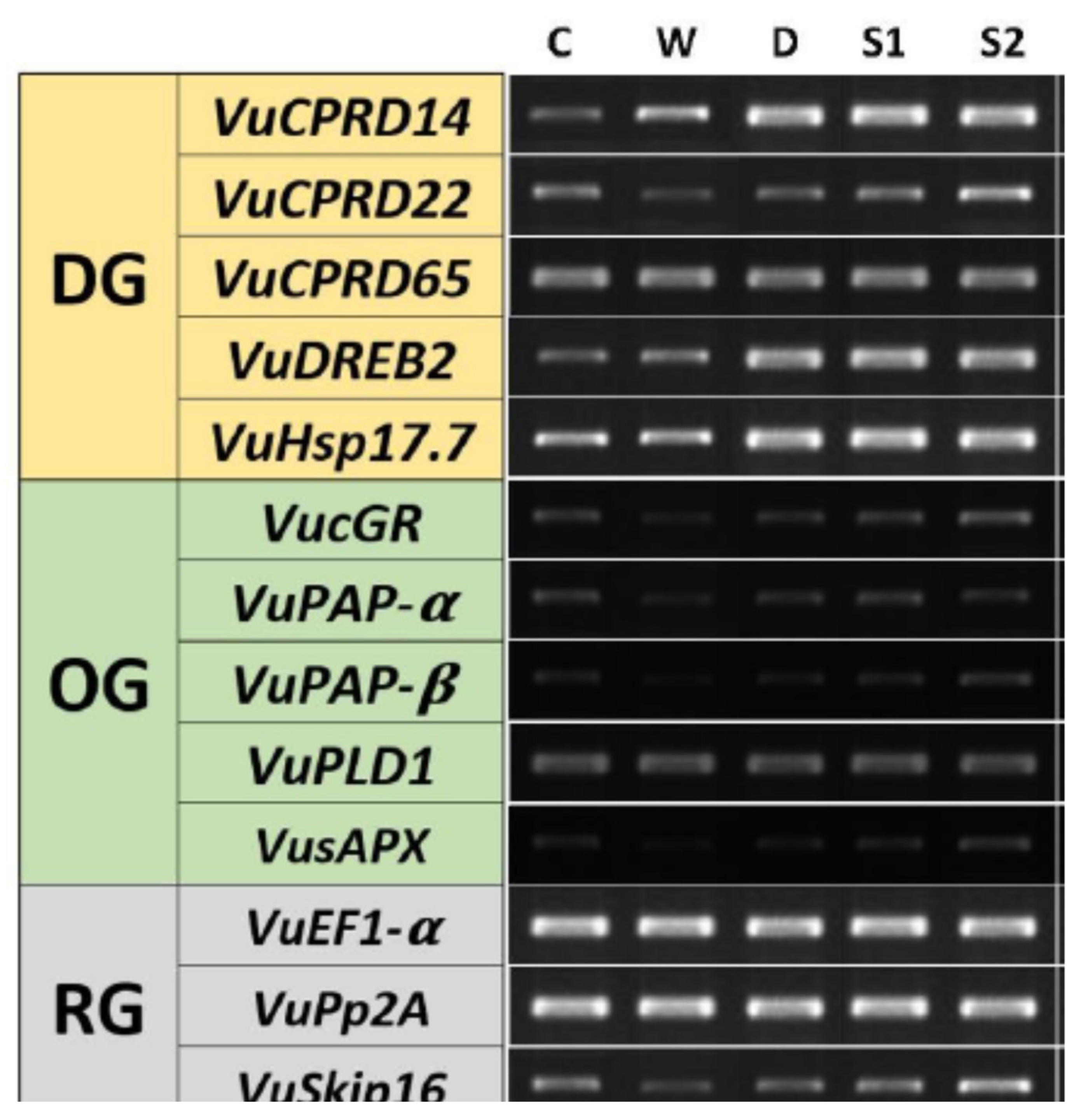
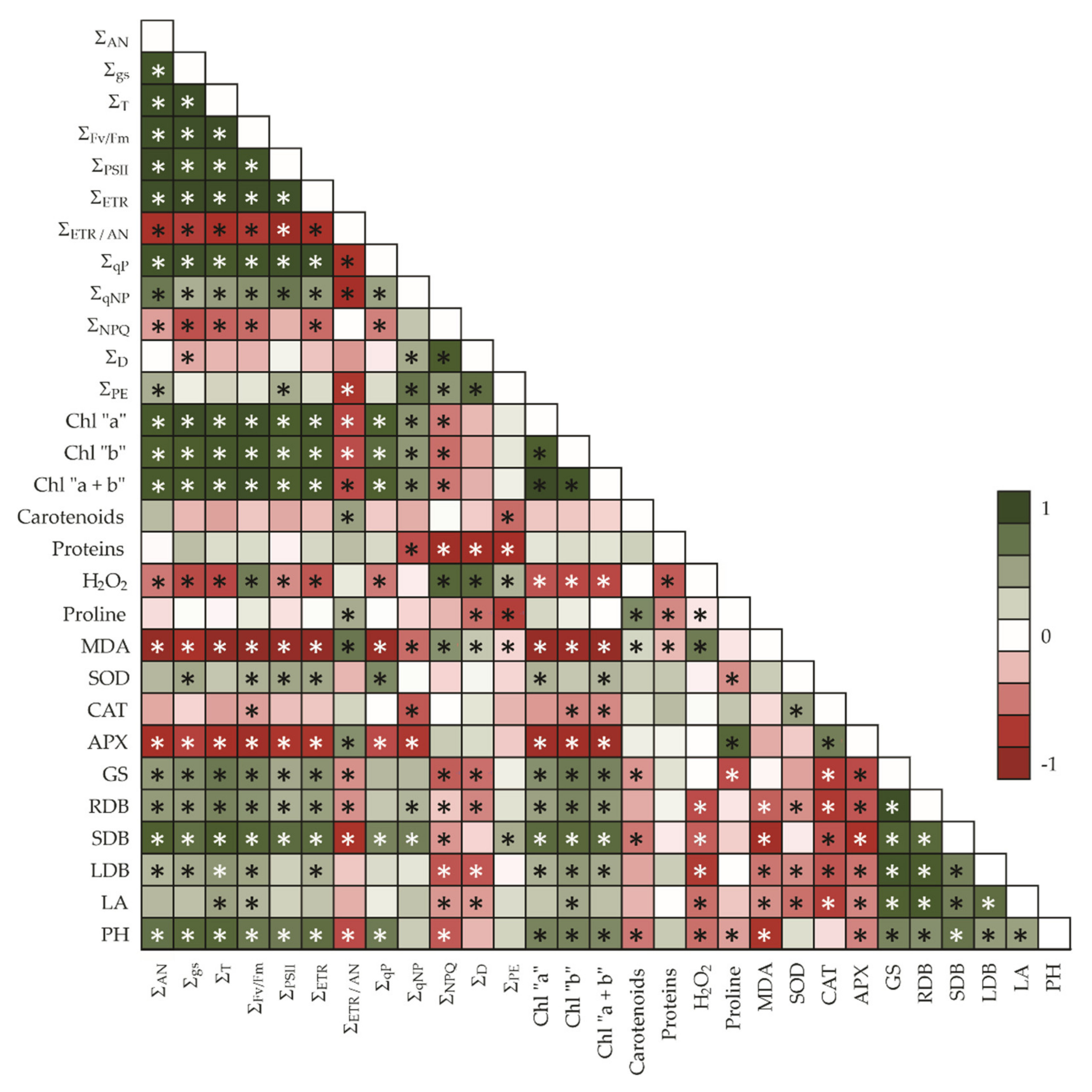
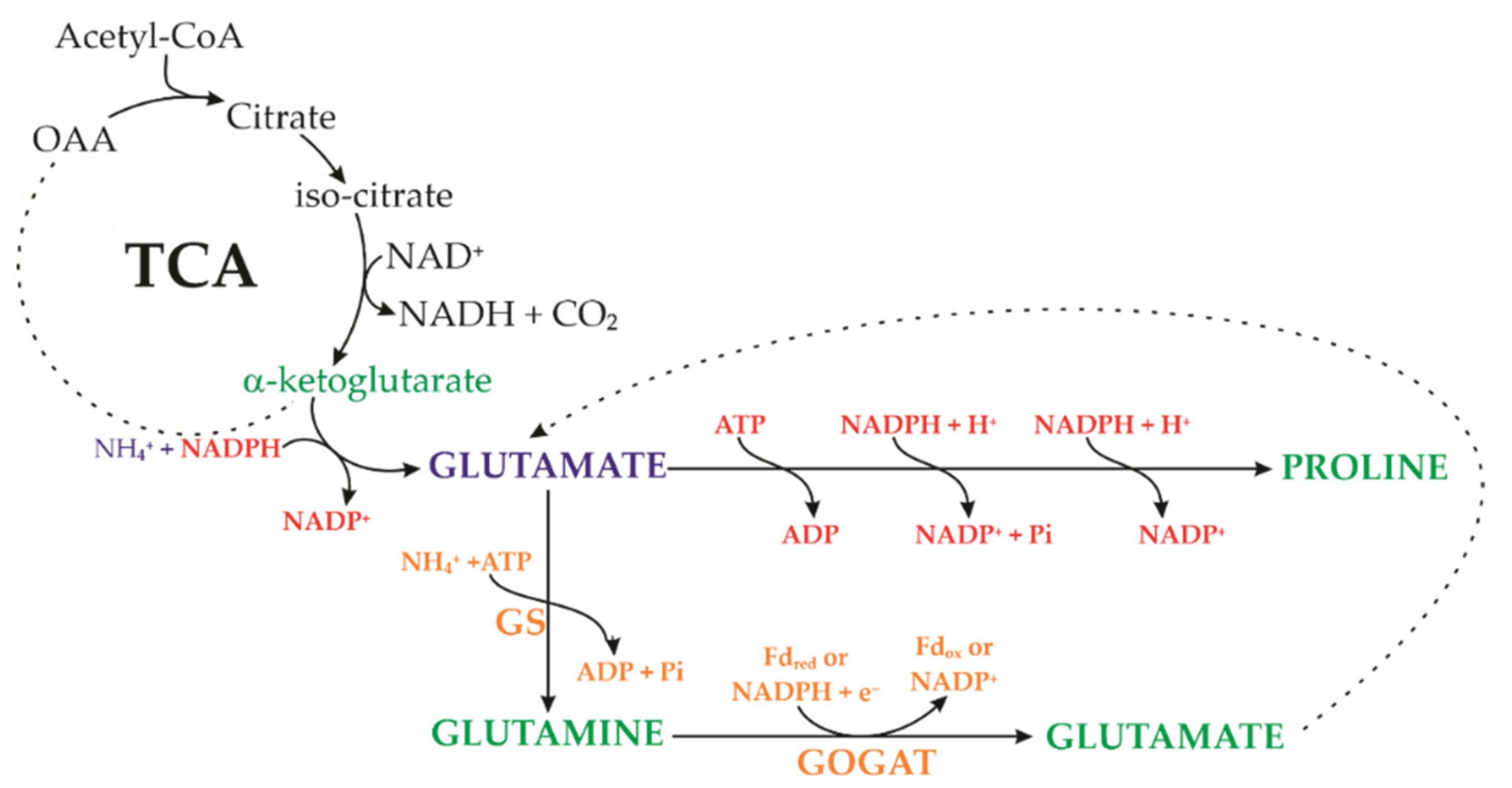
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. UN Climate Science Talks Open Amid Heatwaves, Floods and Drought. UN News 2021. Available online: https://news.un.org/en/story/2021/07/1096422 (accessed on 6 August 2022).
- Bustamante, M.M.C.; Nobre, C.A.; Smeraldi, R.; Aguiar, A.P.D.; Barioni, L.G.; Ferreira, L.G.; Longo, K.; May, P.; Pinto, A.S.; Ometto, J.P. Estimating greenhouse gas emissions from cattle raising in Brazil. Clim Chang. 2012, 115, 559–577. [Google Scholar] [CrossRef]
- UN Peace, Dignity and Equality on a Healthy Planet. 2020. Available online: https://www.un.org/en/global-issues/population (accessed on 6 August 2022).
- Majedul Islam, M.M. Threats to humanity from climate change. In Climate Change; Bandh, S.A., Ed.; Springer: New York, NY, USA, 2022. [Google Scholar]
- Eklund, J.; Jones, J.P.G.; Räsänen, M.; Geldmann, J.; Jokinen, A.-P.; Pellegrini, A.; Rakotobe, D.; Sarobidy Rakotonarivo, O.; Toivonen, T.; Balmford, A. Elevated fires during COVID-19 lockdown and the vulnerability of protected areas. Nat. Sustain. 2022, 5, 603–609. [Google Scholar] [CrossRef]
- Kromdijk, J.; Głowacka, K.; Leonelli, L.; Gabilly, S.T.; Iwai, M.; Niyogi, K.K.; Long, S.P. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 2016, 354, 857–861. [Google Scholar] [CrossRef]
- Brighenti, S.; Tolotti, M.; Bruno, M.C.; Wharton, G.; Pusch, M.; Bertoldi, W. Ecosystem shifts in Alpine streams under glacier retreat and rock glacier thaw: A review. Sci. Total Environ. 2019, 675, 542–559. [Google Scholar] [CrossRef]
- Jagadish, K.S.V.; Kadam, N.N.; Xiao, G.; Melgar, R.J.; Bahuguna, R.N.; Quinones, C.; Tamilselvan, A.; Prasad, P.V.V. Agronomic and physiological responses to high temperature, drought, and elevated CO2 interactions in cereals. In Advances in Agronomy; Sparks, D., Ed.; Academic Press: London, UK, 2014; Volume 127, pp. 111–156. [Google Scholar]
- Sánchez-Reinoso, A.D.; Ligarreto-Moreno, G.A.; Restrepo-Díaz, H. Evaluation of drought indices to identify tolerant genotypes in common bean bush (Phaseolus vulgaris L.). J. Integr. Agric. 2020, 19, 99–107. [Google Scholar] [CrossRef]
- Heuer, B. Role of proline in plant response to drought and salinity. In Handbook of Plant and Crop Stress, 4th ed.; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 213–238. [Google Scholar]
- Munns, R. Plant adaptation to salt and water stress: Differences and commonalities. Adv. Bot. Res. 2011, 57, 1–32. [Google Scholar]
- Silva, E.N.; Ribeiro, R.V.; Ferreira-Silva, S.L.; Viégas, R.A.; Silveira, J.A.G. Comparative effects of salinity and water stress on photosynthesis, water relations and growth of Jatropha curcas plants. J. Arid. Environ. 2010, 74, 1130–1137. [Google Scholar] [CrossRef]
- Huang, L.; Li, Z.; Liu, Q.; Pu, G.; Zhang, Y.; Li, J. Research on the adaptive mechanism of photosynthetic apparatus under salt stress: New directions to increase crop yield in saline soils. Ann. Appl. Biol. 2019, 175, 1–17. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Hui, W.; Zhao, F.; Wang, P.; Su, C.; Gong, W. Physiology of plant responses to water stress and related genes: A Review. Forests 2022, 3, 324. [Google Scholar] [CrossRef]
- USAID. Agriculture and Food Security; USAID: Washington, DC, USA, 2022. [Google Scholar]
- Cole, M.B.; Augustin, M.A.; Robertson, M.J.; Manners, K.M. The science of food security. NPJ Sci. Food 2018, 2, 14. [Google Scholar] [CrossRef]
- FAOSTAT Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 22 July 2022).
- Boukar, O.; Belko, N.; Chamarthi, S.; Togola, A.; Batieno, J.; Owusu, E.; Haruna, M.; Diallo, S.; Umar, M.L.; Olufajo, O.; et al. Cowpea (Vigna unguiculata): Genetics, genomics and breeding. Plant Breed 2017, 138, 415–424. [Google Scholar] [CrossRef]
- Hashim, A.M.; Alharbi, B.M.; Abdulmajeed, A.M.; Elkelish, A.; Hozzein, W.N.; Hassan, H.M. Oxidative stress responses of some endemic plants to high altitudes by intensifying antioxidants and secondary metabolites content. Plants 2020, 9, 869. [Google Scholar] [CrossRef]
- El-Taher, A.M.; Abd El-Raouf, H.S.; Osman, N.A.; Azoz, S.N.; Omar, M.A.; Elkelish, A.; Abd El-Hady, M.A.M. Effect of salt stress and foliar application of salicylic acid on morphological, biochemical, anatomical, and productivity characteristics of cowpea (Vigna unguiculata L.) plants. Plants 2022, 11, 115. [Google Scholar] [CrossRef]
- Chen, C.; Tao, C.; Peng, T.; Ding, Y. Genetic analysis of salt stress responses in asparagus bean (Vigna unguiculata (L.) ssp. sesquipedalis Verdc.). J. Hered. 2007, 98, 655–665. [Google Scholar] [CrossRef]
- De Abreu, C.E.B.; Araújo, G.S.; Monteiro-Moreira, A.C.O.; Costa, J.H.; Leite, H.B.; Moreno, F.B.M.B.; Prisco, J.T.; Gomes-Filho, E. Proteomic analysis of salt stress and recovery in leaves of Vigna unguiculata cultivars differing in salt tolerance. Plant Cell Rep. 2014, 33, 1289–1306. [Google Scholar] [CrossRef]
- Murillo-Amador, B.; Jones, H.G.; Kaya, C.; Aguilar, R.L.; García-Hernández, J.L.; Troyo-Diéguez, E.; Ávila-Serrano, N.Y.; Rueda-Puente, E. Effects of foliar application of calcium nitrate on growth and physiological attributes of cowpea (Vigna unguiculata L. Walp.) grown under salt stress. Enrironm. Exp. Bot. 2006, 58, 188–196. [Google Scholar] [CrossRef]
- Holden, M. The breakdown of chlorophyll by chlorophyllase. Biochem. J. 1961, 78, 359–364. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Osmond, B.; Badger, M.; Maxwell, K.; Björkman, O.; Leegood, R. Too many photons: Photorespiration, photoinhibition and photooxidation. Trends Plant Sci. 1997, 2, 119–121. [Google Scholar] [CrossRef]
- Carvalho, M.; Castro, I.; Moutinho-Pereira, J.; Correia, C.; Egea-Cortines, M.; Matos, M.; Rosa, E.; Carnide, V.; Lino-Neto, T. Evaluating stress responses in cowpea under drought stress. J. Plant Physiol. 2019, 241, 153001. [Google Scholar] [CrossRef]
- Meteoblue Tiempo Montería. Available online: https://n9.cl/meteoblue (accessed on 25 June 2022).
- De-Martonne, E. Aérisme et indice d’aridité. C R Acad. Sci. 1926, 182, 1395–1398. [Google Scholar]
- Pompelli, M.F.; Mendes, K.R.; Ramos, M.V.; Santos, J.N.B.; Youssef, D.T.A.; Pereira, J.D.; Endres, L.; Jarma-Orozco, A.; Solano-Gomes, R.; Jarma-Arroyo, B.; et al. Mesophyll thickness and sclerophylly among Calotropis procera morphotypes reveal water-saved adaptation to environments. J. Arid. Land 2019, 11, 795–810. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Ferreira, P.P.B.; Chaves, A.R.M.; Figueiredo, R.C.Q.Q.; Martins, A.O.; Jarma-Orozco, A.; Batista-Silva, W.; Endres, L.; Araújo, W.L. Physiological, metabolic, and stomatal adjustments in response to salt stress in Jatropha curcas. Plant Physiol. Bioch. 2021, 168, 116–127. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Martins, S.C.V.; Antunes, W.C.; Chaves, A.R.M.; DaMatta, F.M. Photosynthesis and photoprotection in coffee leaves is affected by nitrogen and light availabilities in winter conditions. J. Plant Physiol. 2010, 167, 1052–1060. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and quantitative method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anual Biochem. 1976, 72, 248–252. [Google Scholar]
- Peksen, E. Non-destructive leaf area estimation model for faba bean (Vicia faba L.). Sci. Hortic. 2007, 113, 322–328. [Google Scholar] [CrossRef]
- Desoky, E.-S.M.; EL-Maghraby, L.M.M.; Awad, A.E.; Abdo, A.I.; Rady, M.M.; Semida, W.M. Fennel and ammi seed extracts modulate antioxidant defence system and alleviate salinity stress in cowpea (Vigna unguiculata). Sci. Hortic. 2020, 272, 109576. [Google Scholar] [CrossRef]
- Olorunwa, O.J.; Adhikari, B.; Shi, A.; Barickman, T.C. Screening of cowpea (Vigna unguiculata (L.) Walp.) genotypes for waterlogging tolerance using morpho-physiological traits at early growth stage. Plant Sci. 2022, 315, 11136. [Google Scholar] [CrossRef]
- Hamidou, F.; Zombre, G.; Braconnier, S. Physiological and biochemical responses of cowpea genotypes to water stress under glasshouse and field conditions. J. Agron. Crop Sci. 2007, 193, 229–237. [Google Scholar] [CrossRef]
- Awala, S.K.; Yamane, K.; Izumi, Y.; Fujioka, Y.; Watanabe, Y.; Wada, K.C.; Kawato, Y.; Mwandemele, O.D.; Iijima, M. Field evaluation of mixed-seedlings with rice to alleviate flood stress for semi-arid cereals. Eur. J. Agron. 2016, 80, 105–112. [Google Scholar] [CrossRef]
- Sivakumar, P.; Sharmila, P.; Pardha-Saradhi, P. Proline alleviates salt-stress-induced enhancement in Ribulose-1,5-bisphosphate oxygenase activity. Biochem. Biophys. Res. Commun. 2000, 279, 512–515. [Google Scholar] [CrossRef]
- Lin, J.; Li, J.P.; Yuan, F.; Yang, Z.; Wang, B.S.; Chen, M. Transcriptome profiling of genes involved in photosynthesis in Elaeagnus angustifolia L. under salt stress. Photosynthetica 2018, 56, 998–1009. [Google Scholar] [CrossRef]
- Sairam, R.K.; Kumutha, D.; Ezhilmathi, K.; Chinnusamy, V.; Meena, R.C. Waterlogging induced oxidative stress and antioxidant enzyme activities in pigeon pea. Biol. Plant 2009, 53, 493–504. [Google Scholar] [CrossRef]
- Murillo-Amador, B.; Troyo-Dieguez, E.; Lopez-Cortes, A.; Jones, H.A.-C.F.; Tinoco-Ojanguren, C. Salt tolerance of cowpea genotypes in the emergence stage. Anim. Product Sci. 2001, 41, 81–88. [Google Scholar] [CrossRef]
- Iqbal, W.; Afridi, M.Z.; Jamal, A.; Mihoub, A.; Saeed, M.F.; Székely, Á.; Zia, A.; Khan, M.A.; Jarma-Orozco, A.; Pompelli, M.F. Canola seed priming and its effect on gas exchange, chlorophyll photobleaching, and enzymatic activities in response to salt stress. Sustainabiity 2022, 14, 9377. [Google Scholar] [CrossRef]
- Trapp, S.; Feificova, D.; Rasmussen, N.F.; Bauer-Gottwein, P. Plant uptake of NaCl in relation to enzyme kinetics and toxic effects. Environ. Exp. Bot. 2008, 64, 1–7. [Google Scholar] [CrossRef]
- Campos, H.; Trejo, C.; Peña-Valdivia, C.B.; García-Nava, R.; Conde-Martínez, F.V.; Cruz-Ortega, M.R. Stomatal and non-stomatal limitations of bell pepper (Capsicum annuum L.) plants under water stress and re-watering: Delayed restoration of photosynthesis during recovery. Enrironm. Exp. Bot. 2014, 98, 56–64. [Google Scholar] [CrossRef]
- Batra, N.G.; Sharma, V.; Kumari, N. Drought-induced changes in chlorophyll fluorescence, photosynthetic pigments, and thylakoid membrane proteins of Vigna radiata. J. Plant Interac. 2014, 9, 712–721. [Google Scholar] [CrossRef]
- Johnson, M.P.; Davison, P.A.; Ruban, A.V.; Horton, P. The xanthophyll cycle pool size controls the kinetics of non-photochemical quenching in Arabidopsis thaliana. Febs. Lett. 2008, 582, 262–266. [Google Scholar] [CrossRef]
- Saga, G.; Giorgetti, A.; Fufezan, C.; Giacometti, G.M.; Bassi, R.; Morosinotto, T. Mutation analysis of violaxanthin de-epoxidase identifies substrate-binding sites and residues involved in catalysis. J. Biol. Chem. 2010, 285, 23763–23770. [Google Scholar] [CrossRef]
- Rady, M.O.A.; Semida, W.M.; El-mageed, T.A.A.; Hemida, K.A.; Rady, M.M. Upregulation of antioxidative defense systems by glycine betaine foliar application in onion plants confer tolerance to salinity stress. Sci. Hortic. 2018, 240, 614–622. [Google Scholar] [CrossRef]
- Nair, A.S.; Abraham, T.K.; Jaya, D.S. Studies on the changes in lipid peroxidation and antioxidants in drought stress induced cowpea (Vigna unguiculata L.) varieties. J. Environ. Biol. 2008, 29, 689–691. [Google Scholar]
- Smirnoff, N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef]
- Pandey, S.; Fartyal, D.; Agarwal, A.; Shukla, T.; James, D.; Kaul, T.; Negi, Y.K.; Arora, S.; Reddy, M.K. Abiotic stress tolerance in plants: Myriad roles of ascorbate peroxidase. Front. Plant Sci. 2017, 8, 581. [Google Scholar] [CrossRef]
- Szarka, A.; Tomasskovics, B.; Bánhegyi, G. Settings the ascorbate-glutathione-α-tocopherol triad in abiotic stress response. Int. J. Mol. Sci. 2012, 13, 4458–4483. [Google Scholar] [CrossRef]
- Li, X.; Huang, J.; May, J.M. Ascorbic acid spares α-tocopherol and decreases lipid peroxidation in neuronal cells. Biochem. Biophys. Res. Commun. 2003, 305, 656–661. [Google Scholar] [CrossRef]
- Foyer, C.; Rowell, J.; Walker, D. Measurement of the ascorbate content of spinach leaf protoplasts and chloroplasts during illumination. Planta 1983, 157, 239–244. [Google Scholar] [CrossRef]
- Berteli, F.; Corrales, E.; Guerrero, C.; Ariza, M.J.; Pliego, F.; Valpuesta, V. Salt stress increases ferredoxin-dependent glutamate synthase activity and protein level in the leaves of tomato. Physiol. Plant 1995, 93, 259–264. [Google Scholar] [CrossRef]
- Mwenye, O.J.; van Rrensburg, L.; van Biljon, A.; Van der Merwe, R. The role of proline and root traits on selection for drought-stress tolerance in soybeans: A review. S. Afr. J. Plant Soil. 2016, 33, 245–256. [Google Scholar] [CrossRef]
- Somal, T.L.C.; Yapa, P.A.J. Accumulation of proline in cowpea under nutrient, drought, and saline stresses. J. Plant Nutr. 1998, 21, 2465–2473. [Google Scholar] [CrossRef]
- Souza, R.P.; Machado, E.C.; Silva, J.A.B.; Lagôa, A.M.M.A.; Silveira, J.A.G. Photosynthetic gas exchange, chlorophyll fluorescence and some associated metabolic changes in cowpea (Vigna unguiculata) during water stress and recovery. Environ. Exp. Bot. 2004, 51, 45–56. [Google Scholar] [CrossRef]
- Su, X.; Wei, F.; Huo, Y.; Xia, Z. Comparative physiological and molecular analyses of two contrasting flue-cured Tobacco genotypes under progressive drought stress. Front. Plant Sci. 2017, 8, 827. [Google Scholar] [CrossRef]
- Furlan, A.L.; Bianucci, E.; Giordano, W.; Castro, S.; Becker, D.F. Proline metabolic dynamics and implications in drought tolerance of peanut plants. Plant Physiol. Biochem. 2020, 151, 566–578. [Google Scholar] [CrossRef]
- Figueiredo, M.V.B.; Burity, H.A.; Martínez, C.R.; Chanway, C.P. Drought stress response on some key enzymes of cowpea (Vigna unguiculata L. Walp.) nodule metabolism. World J. Microbiol. Biotechnol. 2007, 23, 187–193. [Google Scholar] [CrossRef]
- Kumar, R.G.; Shah, K.; Dubey, R.S. Salinity induced behavioural changes in malate dehydrogenase and glutamate dehydrogenase activities in rice seedlings of differing salt tolerance. Plant Sci. 2000, 156, 23–34. [Google Scholar] [CrossRef]
- Gulati, A.; Jaiwal, P.K. Effect of NaCI on nitrate reductase, glutamate dehydrogenase and glutamate synthase in Vigna radiata calli. Biol. Plant 1996, 38, 177–183. [Google Scholar] [CrossRef]
- Chiulele, R.M.; Agenbag, G.A. Plant water relations and proline accumulation on two cowpea (Vigna unguiculata (L.) Walp.) cultivars as a response to water stress. S. Afr. J. Plant Soil. 2004, 21, 109–112. [Google Scholar] [CrossRef]
- Lobato, A.K.S.; Oliveira Neto, C.F.; Costa, R.C.L.; Santos Filho, B.G.; Cruz, F.J.R.; Laughinghous, H.D. Biochemical and physiological behavior of Vigna unguiculata (L.) Walp under water stress during the devetative phase. Asian J. Plant Sci. 2008, 7, 44–49. [Google Scholar] [CrossRef]
- Zhu, X.; Tang, G.; Granier, F.; Bouchez, D.; Galili, G. A T-DNA insertion knockout of the bifunctional lysine-ketoglutarate reductase/saccharopine dehydrogenase gene elevates lysine levels in Arabidopsis seeds. Plant Phvsiol. 2001, 126, 1539–1545. [Google Scholar] [CrossRef][Green Version]
- Li, P.; Wu, G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Anino Acids 2018, 50, 29–38. [Google Scholar] [CrossRef]
- Lancien, M.; Gadal, P.; Hodges, M. Enzyme redundancy and the importance of 2-oxoglutarate in higher plant ammonium assimilation. Plant Phvsiol. 2000, 123, 817–824. [Google Scholar] [CrossRef]
- Ayala, C.E.C.; Villadiego, C.E.C.; Tatis, H.A.; Camacho, M.M.E. Growth, biomass distribution, gas exchange and chlorophyll fluorescence in cowpea (Vigna unguiculata (L.) Walp.) under drought conditions. Aust. J. Crop Sci. 2020, 14, 371–381. [Google Scholar] [CrossRef]
- Singh, S.K.; Reddy, K.R. Regulation of photosynthesis, fluorescence, stomatal conductance and water-use efficiency of cowpea (Vigna unguiculata [L.] Walp.) under drought. J. Photoch. Photobio. B 2011, 105, 40–50. [Google Scholar] [CrossRef]
- Saxena, I.; Srikanth, S.; Chen, Z. Cross talk between H2O2 and interacting signal molecules under plant stress response. Front. Plant Sci. 2016, 7, 570. [Google Scholar] [CrossRef]
- Davey, M.W.; Stals, E.; Panis, B.; Keulemans, J.; Swennen, R.L. High-throughput determination of malondialdehyde in plant tissues. Anal. Biochem. 2005, 347, 201–207. [Google Scholar] [CrossRef]
- Da Silva, H.A.P.; Galisa, P.S.; Oliveira, R.S.S.; Vidal, M.S.; Simões-Araújo, J.L. Gene expression induced by abiotic stress in cowpea nodules. Pesq. Agropec. Bras. 2012, 47, 797–807. [Google Scholar]
- Iuchi, S.; Kobayashi, M.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A stress-inducible gene for 9-cis-epoxycarotenoid dioxygenase involved in abscisic acid biosynthesis under water stress in drought-tolerant cowpea. Plant Physiol. 2000, 123, 553–562. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. III The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996, 1, 21–26. [Google Scholar] [CrossRef]
- Simões-Araújo, J.L.; Alves-Ferreira, M.; Rumjanek, N.G.; Margis-Pinheiro, M. VuNIP1 (NOD26-like) and VuHSP17.7 gene expression are regulated in response to heat stress in cowpea nodule. Environ. Exp. Bot. 2008, 63, 256–265. [Google Scholar] [CrossRef]
- Chaudhari, R.S.; Jangale, B.L.; Krishna, B.; Sane, P.V. Improved abiotic stress tolerance in Arabidopsis by constitutive active form of a banana DREB2 type transcription factor, MaDREB20.CA, than its native form, MaDREB20. Protoplasma 2022, 20, 10–42. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Gomaa, M.A. Combining high tolerance to drought with high tolerance to salinity in Egyptian wheat (Triticum aestivum L.) cultivars. Cereal. Res. Commun. 2022, 20, 24–41. [Google Scholar] [CrossRef]
- Mafakheri, K.; Valizadeh, M.; Mohammadi, S.A. Evaluation of catalase and DREB-2 gene expression in maize (Zea mays L.) genotypes under water deficit stress condition. Crop Biotech. 2022, 11, 75–93. [Google Scholar]
- Yang, Z.; Du, H.; Sun, J.; Xiang, X.; Kong, Y.; Li, W.; Zhang, C. A nodule-localized small heat shock protein GmHSP17.1 confers nodule development and nitrogen fixation in soybean. Front Plant Sci. 2022, 13, 838718. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, Y.; Pan, H.; Hu, W.; Zhang, Q. Cloning and characterisation of a Primula heat shock protein gene, PfHSP17.1, which confers heat, salt and drought tolerance in transgenic Arabidopsis thaliana. Acta Physiol. Plant 2013, 35, 3191–3200. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Bioch. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Chaerle, L.; Leinonen, I.; Jones, H.G.; Van Der Straeten, D. Monitoring and screening plant populations with combined thermal and chlorophyll fluorescence imaging. J. Exp. Bot. 2007, 58, 773–784. [Google Scholar] [CrossRef]
- Shi, X.L.; Zhou, D.Y.; Guo, P.; Zhang, H.; Dong, J.L.; Ren, J.Y.; Jiang, C.J.; Zhong, C.; Zhao, X.H.; Yu, H.Q. External potassium mediates the response and tolerance to salt stress in peanut at the flowering and needling stages. Photosynthetica 2020, 58, 1141–1149. [Google Scholar] [CrossRef]
- Antunes, W.C.; Mendes, K.R.; Chaves, A.R.M.; Ometto, J.P.; Jarma-Orozco, A.; Pompelli, M.F. Spondias tuberosa trees grown in tropical, wet environments are more susceptible to drought than those grown in arid environments. Rev. Col. Ciên. Hortic. 2016, 10, 9–27. [Google Scholar]
- Short, A.H.; Fay, T.P.; Crisanto, T.; Hall, J.; Steen, C.J.; Niyogi, K.K.; Limmer, D.T.; Fleming, G.R. Xanthophyll-cycle based model of the rapid photoprotection of Nannochloropsis in response to regular and irregular light/dark sequences. J. Chem. Phys. 2022, 156, 205102. [Google Scholar] [CrossRef]
- Kumar, P.; Pal, M. Comparative analysis of photoprotection mediated by photosynthetic pigments during summer midday heat stress in rice and wheat. Vegetos 2022. [Google Scholar] [CrossRef]
- Wang, X.; Ren, P.; Ji, L.; Zhu, B.; Xie, G. OsVDE, a xanthophyll cycle key enzyme, mediates abscisic acid biosynthesis and negatively regulates salinity tolerance in rice. Planta 2022, 255, 6. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pompelli, M.F.; Arrieta, D.V.; Rodríguez, Y.Y.P.; Ramírez, A.M.J.; Bettin, A.M.V.; Avilez, M.A.Q.; Cárcamo, J.A.A.; Garcia-Castaño, S.G.; González, L.M.M.; Cordero, E.D.F.; et al. Can Chlorophyll a Fluorescence and Photobleaching Be a Stress Signal under Abiotic Stress in Vigna unguiculata L.? Sustainability 2022, 14, 15503. https://doi.org/10.3390/su142315503
Pompelli MF, Arrieta DV, Rodríguez YYP, Ramírez AMJ, Bettin AMV, Avilez MAQ, Cárcamo JAA, Garcia-Castaño SG, González LMM, Cordero EDF, et al. Can Chlorophyll a Fluorescence and Photobleaching Be a Stress Signal under Abiotic Stress in Vigna unguiculata L.? Sustainability. 2022; 14(23):15503. https://doi.org/10.3390/su142315503
Chicago/Turabian StylePompelli, Marcelo F., Daniela Vegliante Arrieta, Yirlis Yadeth Pineda Rodríguez, Ana Melisa Jiménez Ramírez, Ana Milena Vasquez Bettin, María Angélica Quiñones Avilez, Jesús Adolfo Ayala Cárcamo, Samuel Giovanny Garcia-Castaño, Lina María Mestra González, Elias David Florez Cordero, and et al. 2022. "Can Chlorophyll a Fluorescence and Photobleaching Be a Stress Signal under Abiotic Stress in Vigna unguiculata L.?" Sustainability 14, no. 23: 15503. https://doi.org/10.3390/su142315503
APA StylePompelli, M. F., Arrieta, D. V., Rodríguez, Y. Y. P., Ramírez, A. M. J., Bettin, A. M. V., Avilez, M. A. Q., Cárcamo, J. A. A., Garcia-Castaño, S. G., González, L. M. M., Cordero, E. D. F., Montaño, M. J. P., Mendoza, C. C. P., González, A. R. A., Coley, A. J. T., Jarma-Orozco, A., & Rodriguez Paez, L. A. (2022). Can Chlorophyll a Fluorescence and Photobleaching Be a Stress Signal under Abiotic Stress in Vigna unguiculata L.? Sustainability, 14(23), 15503. https://doi.org/10.3390/su142315503