Study on the Resistance of ‘Cabernet Sauvignon’ Grapevine with Different Rootstocks to Colomerus vitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experiments
2.2. Infestation Evaluation
2.3. Biochemistry Methods
2.4. Data Analysis
3. Results
3.1. GEM Field Identification Results of ‘Cabernet Sauvignon’ with Different Rootstocks
3.2. Effects of Different Rootstocks on Activities of SOD, PPO, CAT, and POD in Leaves of ‘Cabernet Sauvignon’ under Different Levels of GEM Injury
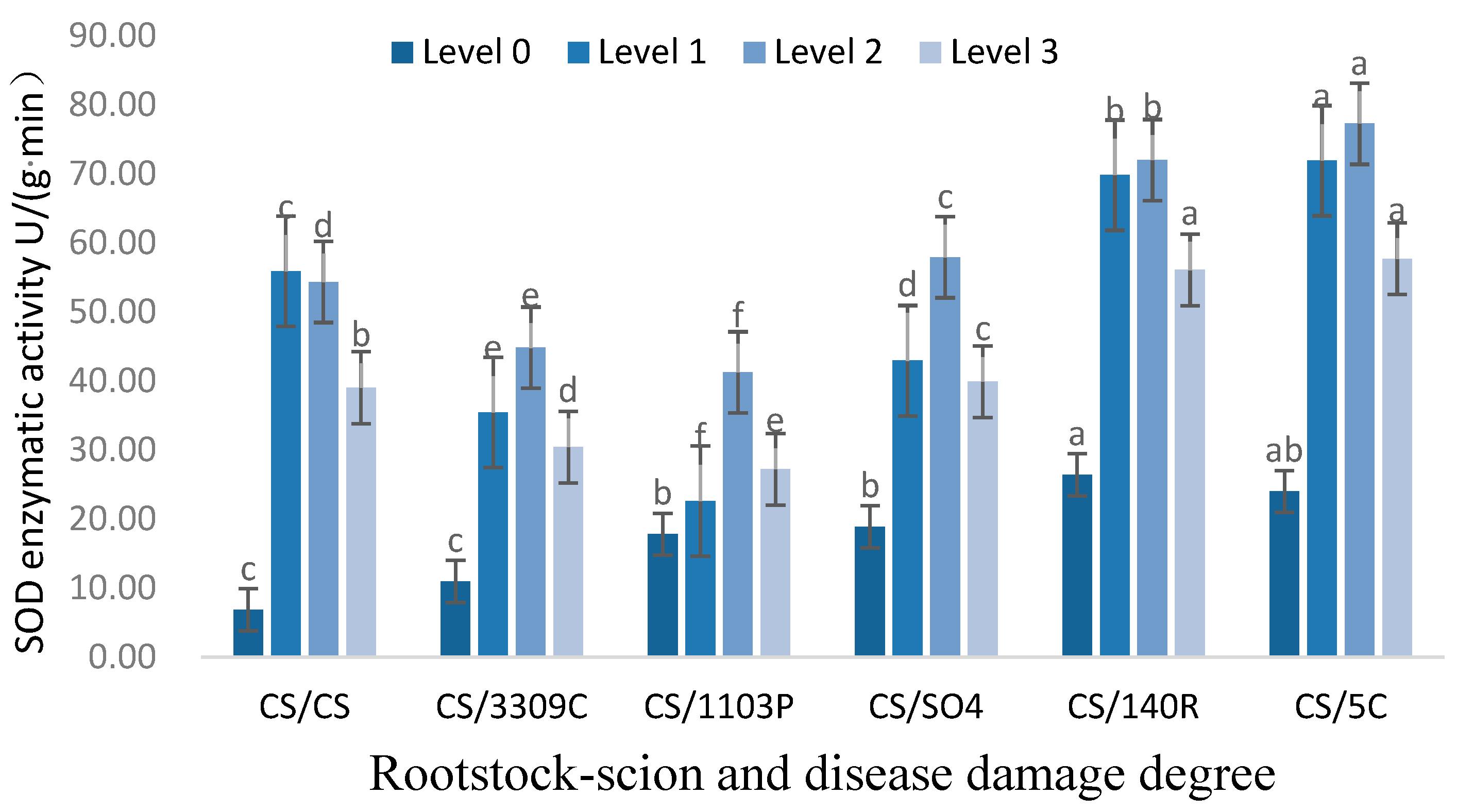
3.2.1. Analysis of SOD Activity in Leaves of ‘Cabernet Sauvignon’ with Different Rootstocks and GEM Injury Levels
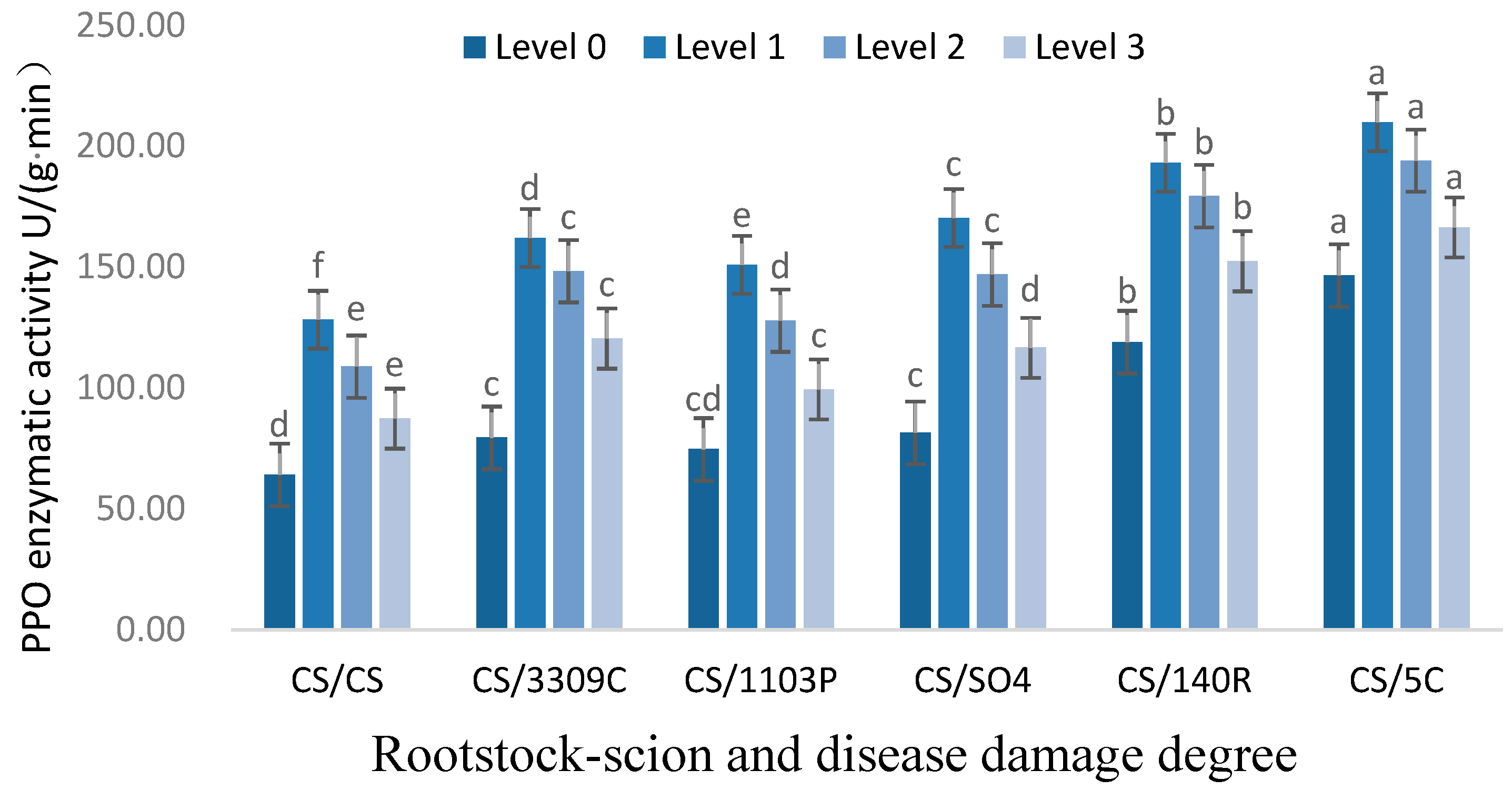
3.2.2. Analysis of PPO Enzyme Activity in Leaves of ‘Cabernet Sauvignon’ with Different Rootstocks and GEM Injury Grades
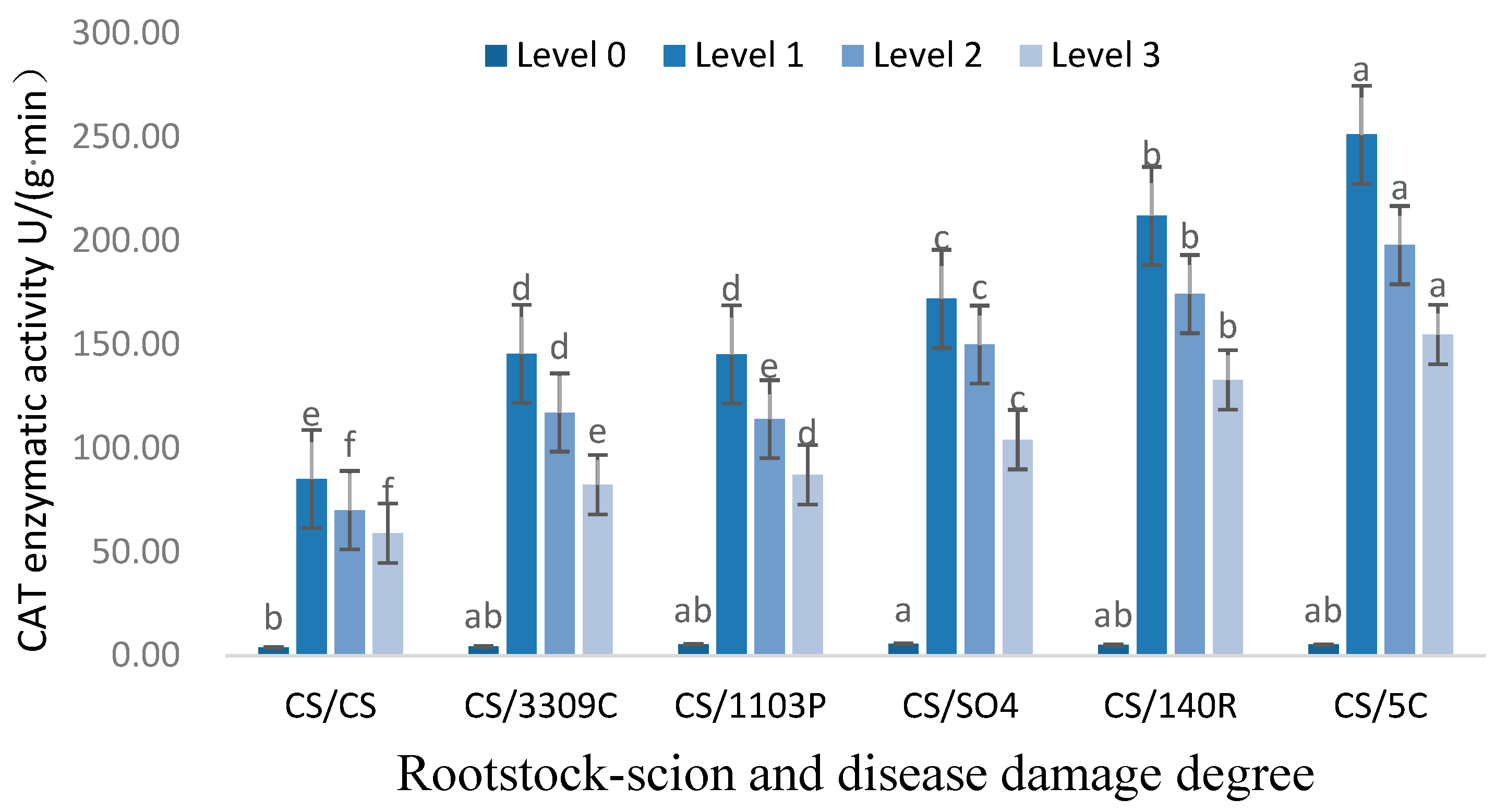
3.2.3. Analysis of CAT Enzyme Activity in Leaves of ‘Cabernet Sauvignon’ with Different Rootstocks and GEM Injury Grades
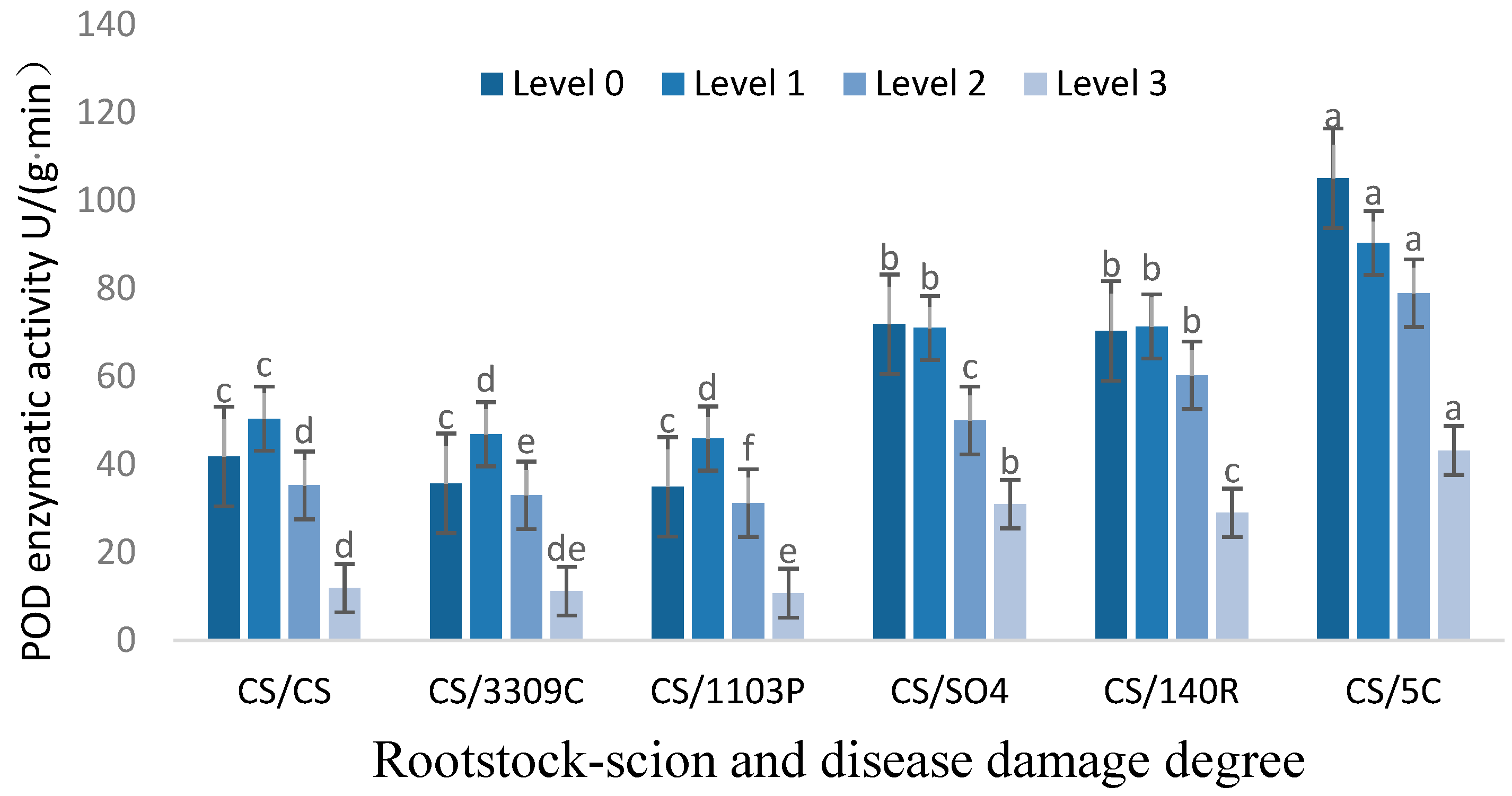
3.2.4. Analysis of POD Enzyme Activity in Leaves of ‘Cabernet Sauvignon’ with Different Rootstocks and GEM Injury Grades
3.3. Effects of Different Rootstocks on the Relative Expression of the Res and RS Genes in Leaves of ‘Cabernet Sauvignon’ Grapevine under Different GEM Injury Levels
3.3.1. Changes of Res Content in GEM Leaves of ‘Cabernet Sauvignon’ with Different Rootstocks and Injury Grades
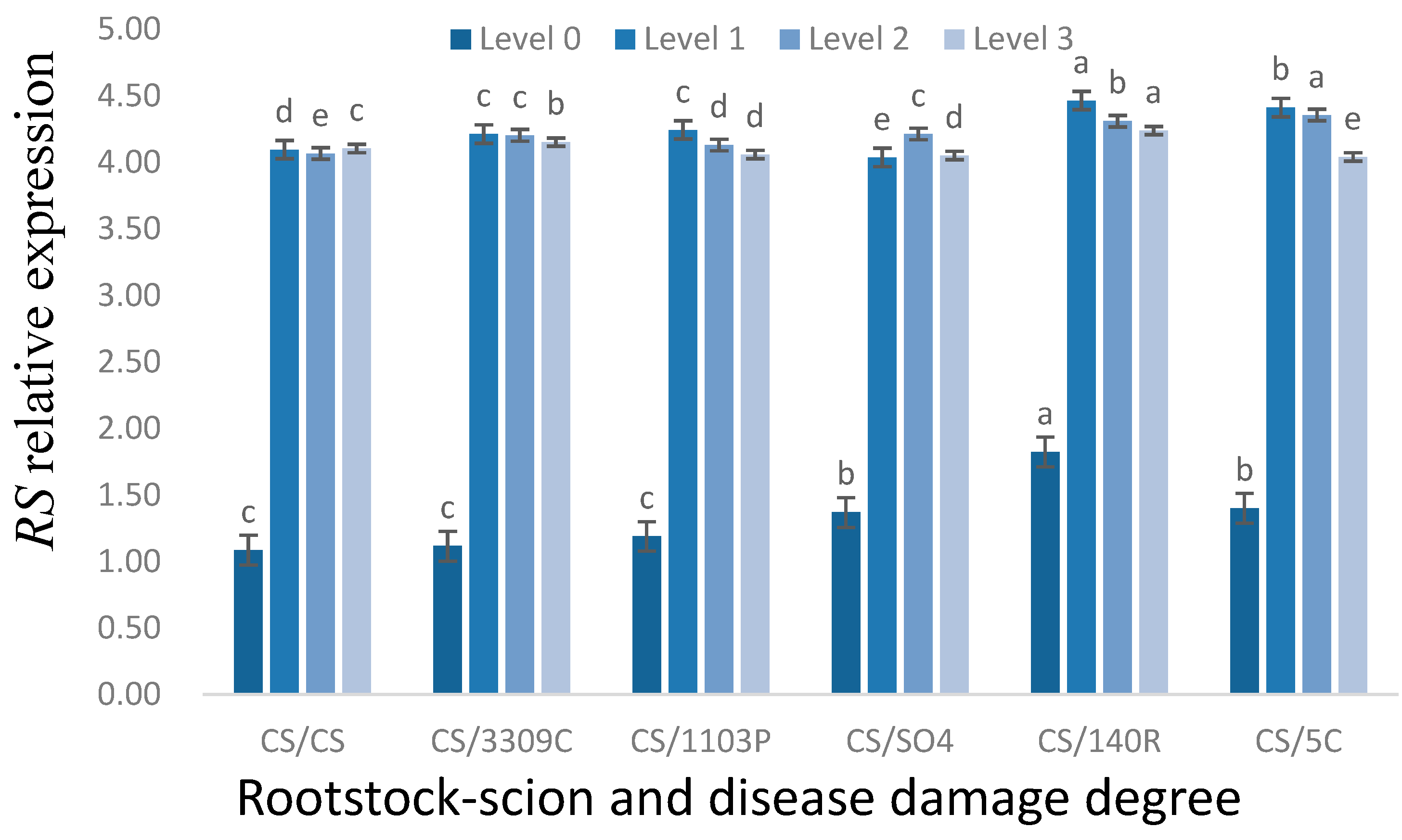
3.3.2. Expression of the RS Gene under Different GEM Injury Grades in the ‘Cabernet Sauvignon’ Leaves of Different Rootstocks
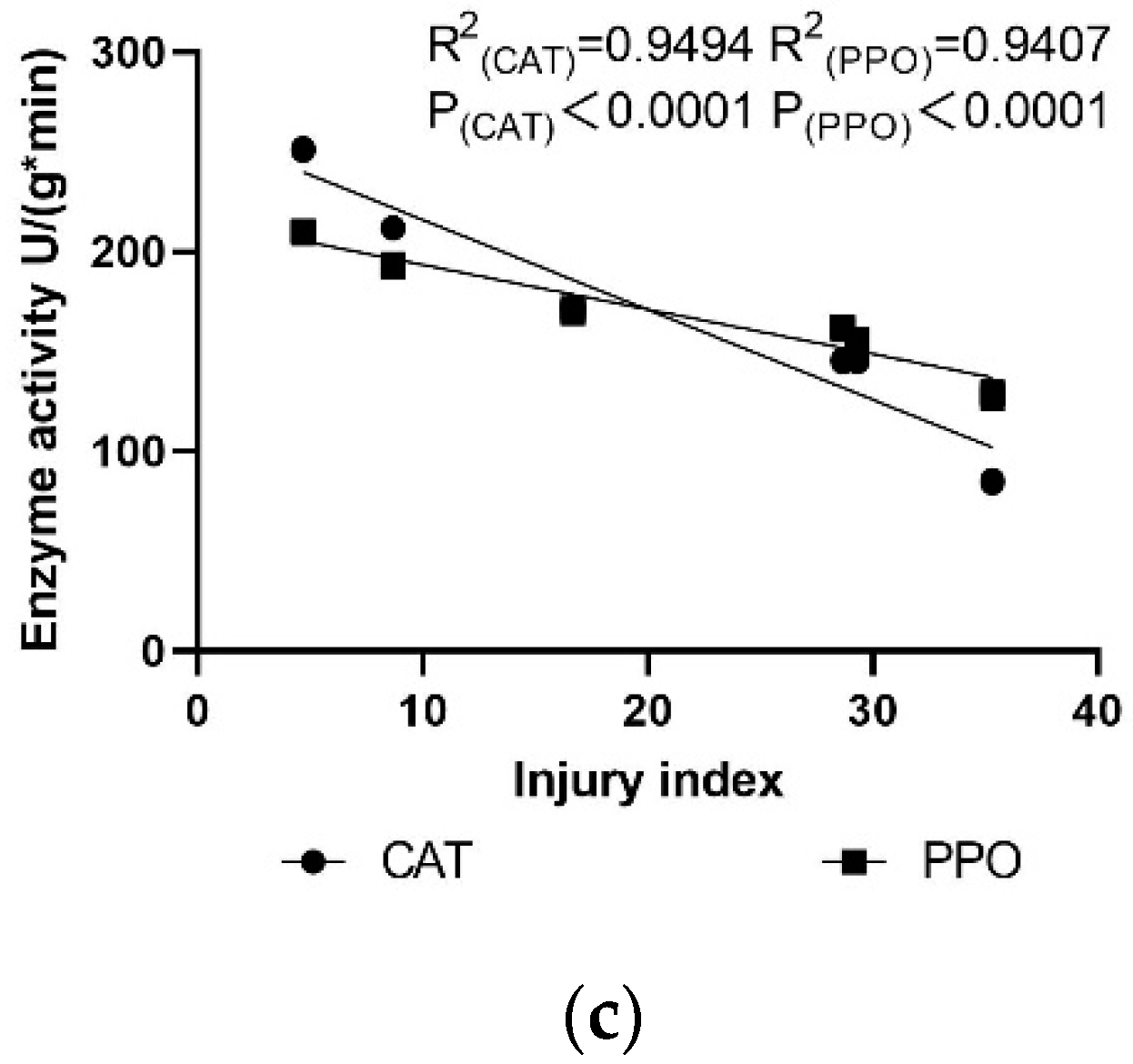
3.4. Correlation Analysis between Res Content, Resistance Enzyme Activity, and Injury Index of ‘Cabernet Sauvignon’ Grapevine Leaves with Different Rootstocks
4. Discussion
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riaz, S.; Pap, D.; Uretsky, J.; Laucou, V.; Boursiquot, J.-M.; Kocsis, L.; Andrew Walker, M. Genetic diversity and parentage analysis of grape rootstocks. Theor. Appl. Genet. 2019, 132, 1847–1860. [Google Scholar] [CrossRef] [PubMed]
- Klimek, K.; Postawa, K.; Kapłan, M.; Kułażyński, M. Evaluation of the Influence of Rootstock Type on the Yield Parameters of Vines Using a Mathematical Model in Nontraditional Wine-Growing Conditions. Appl. Sci. 2022, 12, 7293. [Google Scholar] [CrossRef]
- Moussa, A.; Quaglino, F.; Faccincani, M.; Serina, F.; Torcoli, S.; Miotti, N.; Passera, A.; Casati, P.; Mori, N. Grafting of recovered shoots reduces bois noir disease incidence in vineyard. Crop Prot. 2022, 161, 106058. [Google Scholar] [CrossRef]
- Wallis Christopher, M.; Wallingford Anna, K.; Jianchi, C. Grapevine rootstock effects on scion sap phenolic levels, resistance to Xylella fastidiosa infection, and progression of Pierce’s disease. Front. Plant Sci. 2013, 4, 502. [Google Scholar] [CrossRef]
- Dandekar, A.M.; Jacobson, A.; Ibáñez, A.M.; Gouran, H.; Dolan, D.L.; Agüero, C.B.; Uratsu, S.L.; Just, R.; Zaini, P.A. Trans-graft protection against Pierce’s disease mediated by transgenic grapevine rootstocks. Front. Plant Sci. 2019, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Provost, C.; Campbell, A.; Dumont, F. Rootstocks impact yield, fruit composition, nutrient deficiencies, and winter survival of hybrid cultivars in eastern Canada. Horticulturae 2021, 7, 237. [Google Scholar] [CrossRef]
- Zongmin, F.; Junli, S.; Baolong, Z.; Huaifeng, L.; Kun, Y.; Zhijun, Z.; Jingjing, L. Evaluationofcoldresistanceofone-yearshoots from‘CabernetSauvignon’grape vine grafted on different rootstocks. J. Fruit Sci. 2020, 37, 215–225. [Google Scholar] [CrossRef]
- Carew, M.E.; Goodisman, M.A.; Hoffmann, A.A. Species status and population genetic structure of grapevine eriophyoid mites. Entomol. Exp. Appl. 2004, 111, 87–96. [Google Scholar] [CrossRef]
- Cooper, M.; Hobbs, M.; Strode, B.; Varela, L. Grape erineum mite: Postharvest sulfur use reduces subsequent leaf blistering. Calif. Agric. 2020, 74, 94–100. [Google Scholar] [CrossRef]
- Kunugi, Y.; Asari, S.; Terai, Y.; Shinkai, A. Studies on the grapevine berry inner necrosis virus disease, 2: Transmission of grapevine berry inner necrosis virus by the grape erineum mite, Colomerus vitis in Yamanashi [Japan]. Bull. Yamanashi Fruit Tree Exp. Stn. 2000, 10, 57–63. [Google Scholar]
- Malagnini, V.; de Lillo, E.; Saldarelli, P.; Beber, R.; Duso, C.; Raiola, A.; Zanotelli, L.; Valenzano, D.; Giampetruzzi, A.; Morelli, M. Transmission of grapevine Pinot gris virus by Colomerus vitis (Acari: Eriophyidae) to grapevine. Arch. Virol. 2016, 161, 2595–2599. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Sun, J.; Jiang, Y.; Duan, X. Role of Reactive Oxygen Species against Pathogens in Relation to Postharvest Disease of Papaya Fruit. Horticulturae 2022, 8, 205. [Google Scholar] [CrossRef]
- El-Gendi, H.; Al-Askar, A.A.; Király, L.; Samy, M.A.; Moawad, H.; Abdelkhalek, A. Foliar Applications of Bacillus subtilis HA1 Culture Filtrate Enhance Tomato Growth and Induce Systemic Resistance against Tobacco mosaic virus Infection. Horticulturae 2022, 8, 301. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; Zhao, R.; Li, R.; Zhang, S.; Yu, W.; Sheng, J.; Shen, L. Melatonin induces disease resistance to Botrytis cinerea in tomato fruit by activating jasmonic acid signaling pathway. J. Agric. Food Chem. 2019, 67, 6116–6124. [Google Scholar] [CrossRef]
- Zhang, Q.; Yong, D.; Zhang, Y.; Shi, X.; Li, B.; Li, G.; Liang, W.; Wang, C. Streptomyces rochei A-1 induces resistance and defense-related responses against Botryosphaeria dothidea in apple fruit during storage. Postharvest Biol. Technol. 2016, 115, 30–37. [Google Scholar] [CrossRef]
- Zhang, Y.; Huber, D.J.; Hu, M.; Jiang, G.; Gao, Z.; Xu, X.; Jiang, Y.; Zhang, Z. Delay of postharvest browning in litchi fruit by melatonin via the enhancing of antioxidative processes and oxidation repair. J. Agric. Food Chem. 2018, 66, 7475–7484. [Google Scholar] [CrossRef]
- Wingerter, C.; Eisenmann, B.; Weber, P.; Dry, I.; Bogs, J. Grapevine Rpv3-, Rpv10-and Rpv12-mediated defense responses against Plasmopara viticola and the impact of their deployment on fungicide use in viticulture. BMC Plant Biol. 2021, 21, 470. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kurosaki, F.; Suh, D.-Y.; Sankawa, U.; Nishioka, M.; Akiyama, T.; Shibuya, M.; Ebizuka, Y. Cross-reaction of chalcone synthase and stilbene synthase overexpressed in Escherichia coli. FEBS Lett. 1999, 460, 457–461. [Google Scholar] [CrossRef]
- Gatto, P.; Vrhovsek, U.; Muth, J.; Segala, C.; Romualdi, C.; Fontana, P.; Pruefer, D.; Stefanini, M.; Moser, C.; Mattivi, F. Ripening and genotype control stilbene accumulation in healthy grapes. J. Agric. Food Chem. 2008, 56, 11773–11785. [Google Scholar] [CrossRef]
- Li, R.; Xie, X.; Ma, F.; Wang, D.; Wang, L.; Zhang, J.; Xu, Y.; Wang, X.; Zhang, C.; Wang, Y. Resveratrol accumulation and its involvement in stilbene synthetic pathway of Chinese wild grapes during berry development using quantitative proteome analysis. Sci. Rep. 2017, 7, 9295. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Cao, X.; Wu, D.; Hui, M.; Han, X.; Yao, F.; Li, Y.; Li, H.; Wang, H. Screening and Validation of SSR Molecular Markers for Identification of Downy Mildew Resistance in Intraspecific Hybrid F1 Progeny (V. vinifera). Horticulturae 2022, 8, 706. [Google Scholar] [CrossRef]
- Mengqi, L.; Fuli, M.; Fengying, W.; Changyue, J.; Yuejin, W. Expression of stilbene synthase VqSTS6 from wild Chinese Vitis quinquangularis in grapevine enhances resveratrol production and powdery mildew resistance. Planta 2019, 250, 1997–2007. [Google Scholar] [CrossRef]
- Zhijun, Z.; Huaifeng, L.; Junli, S.; Baolong, Z.; Lizhong, P.; Wang, H.; Jingjing, L. Analysis of Differential Metabolites in Cabernet Sauvignon Skins from Different Rootstock-Scion Combinations by Non-Targeted Metabolomics. Food Sci. 2020, 41, 22–30. [Google Scholar] [CrossRef]
- Adrian, M.; Jeandet, P.; Veneau, J.; Weston, L.A.; Bessis, R. Biological activity of resveratrol, a stilbenic compound from grapevines, against Botrytis cinerea, the causal agent for gray mold. J. Chem. Ecol. 1997, 23, 1689–1702. [Google Scholar] [CrossRef]
- Calderon, A.; Pedreno, M.; Barcelo, A.R.; Munoz, R. A spectrophotometric assay for quantitative analysis of the oxidation of 4-hydroxystilbene by peroxidase-H2O2 systems. J. Biochem. Biophys. Methods 1990, 20, 171–180. [Google Scholar] [CrossRef]
- Pezet, R.; Gindro, K.; Viret, O.; Richter, H. Effects of resveratrol, viniferins and pterostilbene on Plasmopara viticola zoospore mobility and disease development. VITIS-GEILWEILERHOF 2004, 43, 145–148. [Google Scholar]
- Pezet, R.; Gindro, K.; Viret, O.; Spring, J.-L. Glycosylation and oxidative dimerization of resveratrol are respectively associated to sensitivity and resistance of grapevine cultivars to downy mildew. Physiol. Mol. Plant Pathol. 2004, 65, 297–303. [Google Scholar] [CrossRef]
- Javadi Khederi, S.; Khanjani, M.; Gholami, M.; Panzarino, O.; de Lillo, E. Influence of the erineum strain of Colomerus vitis (Acari: Eriophyidae) on grape (Vitis vinifera) defense mechanisms. Exp. Appl. Acarol. 2018, 75, 1–24. [Google Scholar] [CrossRef]
- Reid, K.E.; Olsson, N.; Schlosser, J.; Peng, F.; Lund, S.T. An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biol. 2006, 6, 27. [Google Scholar] [CrossRef]
- Afzal, F.; Khurshid, R.; Ashraf, M.; Kazi, A.G. Reactive oxygen species and antioxidants in response to pathogens and wounding. In Oxidative Damage to Plants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 397–424. [Google Scholar]
- Zhang, L.; Yu, Y.; Chang, L.; Wang, X.; Zhang, S. Melatonin enhanced the disease resistance by regulating reactive oxygen species metabolism in postharvest jujube fruit. J. Food Process. Preserv. 2022, 46, e16363. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, M.-J.; Song, J.-B.; Zhang, S.-Y.; Guo, R.; Hou, D.-R.; Hao, C.-Y.; An, H.-L.; Huang, X. Roles of endogenous melatonin in resistance to Botrytis cinerea infection in an Arabidopsis model. Front. Plant Sci. 2021, 12, 683228. [Google Scholar] [CrossRef] [PubMed]
- Walling, L.L. The myriad plant responses to herbivores. J. Plant Growth Regul. 2000, 19, 195–216. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Shi, X.; Yong, D.; Zhang, Y.; Li, B.; Liang, W.; Wang, C. Induction of resistance against Glomerella cingulata in apple by endophytic actinomycetes strain A-1. Plant Physiol. Comm 2015, 51, 949–954. [Google Scholar]
- Mohammadi, M.; Kazemi, H. Changes in peroxidase and polyphenol oxidase activities in susceptible and resistant wheat heads inoculated with Fusarium graminearum and induced resistance. Plant Sci. 2002, 162, 491–498. [Google Scholar] [CrossRef]
- Gao, Y.; Yin, X.; Jiang, H.; Hansen, J.; Jørgensen, B.; Moore, J.P.; Fu, P.; Wu, W.; Yang, B.; Ye, W. Comprehensive Leaf Cell Wall Analysis Using Carbohydrate Microarrays Reveals Polysaccharide-Level Variation between Vitis Species with Differing Resistance to Downy Mildew. Polymers 2021, 13, 1379. [Google Scholar] [CrossRef]
- Khederi, S.J.; Khanjani, M.; Fayaz, B.A. Resistance of three grapevine cultivars to Grape Erineum Mite, Colomerus vitis (Acari: Eriophyidae), in field conditions. Persian J. Acarol. 2014, 3. [Google Scholar] [CrossRef]
- Petanović, R.; Kielkiewicz, M. Plant–eriophyoid mite interactions: Cellular biochemistry and metabolic responses induced in mite-injured plants. Part I. Exp. Appl. Acarol. 2010, 51, 61–80. [Google Scholar] [CrossRef]
- Akhtar, Y.; Isman, M.B.; Lee, C.-H.; Lee, S.-G.; Lee, H.-S. Toxicity of quinones against two-spotted spider mite and three species of aphids in laboratory and greenhouse conditions. Ind. Crops Prod. 2012, 37, 536–541. [Google Scholar] [CrossRef]
- Wang, H.; Junli, S.; Baolong, Z.; Zhijun, Z.; Lianling, L.; Lizhong, P.; Xinyi, C. Effects of rootstocks on the content of resveratrol and relative enzyme activities in ‘Cabernet Sauvignon’ grape. J. Fruit Sci. 2019, 6, 738–747. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, J.; Zhao, S.; Lu, Q.; Pan, L.; Zhao, B.; Yu, S. Effects of different rootstocks on phenolics in the skin of ‘Cabernet Sauvignon’ and widely targeted metabolome and transcriptome analysis. Hortic. Res. 2022, 9, uhac053. [Google Scholar] [CrossRef] [PubMed]
- Samira, H.; Carolyn, A.B.; Ulrike, M. Effectors of plant parasitic nematodes that re-program root cell development. Funct. Plant Biol. 2010, 37, 933–942. [Google Scholar]
- Jaouannet, M.; Magliano, M.; Arguel, M.J.; Gourgues, M.; Evangelisti, E.; Abad, P.; Rosso, M.-N. The root-knot nematode calreticulin Mi-CRT is a key effector in plant defense suppression. Mol. Plant-Microbe Interact. 2013, 26, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Brader, G.; Tas, E.; Palva, E.T. Jasmonate-dependent induction of indole glucosinolates in Arabidopsis by culture filtrates of the nonspecific pathogen Erwinia carotovora. Plant Physiol. 2001, 126, 849–860. [Google Scholar] [CrossRef]
- Schnee, S.; Viret, O.; Gindro, K. Role of stilbenes in the resistance of grapevine to powdery mildew. Physiol. Mol. Plant Pathol. 2008, 72, 128–133. [Google Scholar] [CrossRef]



| Genes | Forward Primer | Reverse Primer |
|---|---|---|
| actin1 | CTTGCATCCCTCAGCACCTT | TCCTGTGGACAATGGATGGA |
| RS | GCTATGCAGGTGGAACTGTCCTTC | CTCAGAGCACACCACAAGAACTCG |
| Processing Combination | Infestation Rate | Injury Index | Injury Resistance Degree |
|---|---|---|---|
| CS/CS | 68% c | 39.78 d | I |
| CS/3309C | 65% c | 33.11 cd | I |
| CS/1103P | 66% c | 34.22 d | I |
| CS/SO4 | 44% b | 23.01 bc | R |
| CS/140R | 29% b | 14.00 ab | R |
| CS/5C | 9% a | 4.89 a | HR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, W.; He, W.; Zhang, Z.; Sun, J.; Zhu, C.; Liu, Z.; Xu, Y.; Zhao, B. Study on the Resistance of ‘Cabernet Sauvignon’ Grapevine with Different Rootstocks to Colomerus vitis. Sustainability 2022, 14, 15193. https://doi.org/10.3390/su142215193
Shi W, He W, Zhang Z, Sun J, Zhu C, Liu Z, Xu Y, Zhao B. Study on the Resistance of ‘Cabernet Sauvignon’ Grapevine with Different Rootstocks to Colomerus vitis. Sustainability. 2022; 14(22):15193. https://doi.org/10.3390/su142215193
Chicago/Turabian StyleShi, Wenchao, Wang He, Zhijun Zhang, Junli Sun, Chunmei Zhu, Zhiyu Liu, Yeqing Xu, and Baolong Zhao. 2022. "Study on the Resistance of ‘Cabernet Sauvignon’ Grapevine with Different Rootstocks to Colomerus vitis" Sustainability 14, no. 22: 15193. https://doi.org/10.3390/su142215193
APA StyleShi, W., He, W., Zhang, Z., Sun, J., Zhu, C., Liu, Z., Xu, Y., & Zhao, B. (2022). Study on the Resistance of ‘Cabernet Sauvignon’ Grapevine with Different Rootstocks to Colomerus vitis. Sustainability, 14(22), 15193. https://doi.org/10.3390/su142215193








