Stevia rebaudiana under a CO2 Enrichment Atmosphere: Can CO2 Enrichment Overcome Stomatic, Mesophilic and Biochemical Barriers That Limit Photosynthesis?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Environmental Conditions
2.2. CO2-Enriched Atmosphere Chambers and Treatments
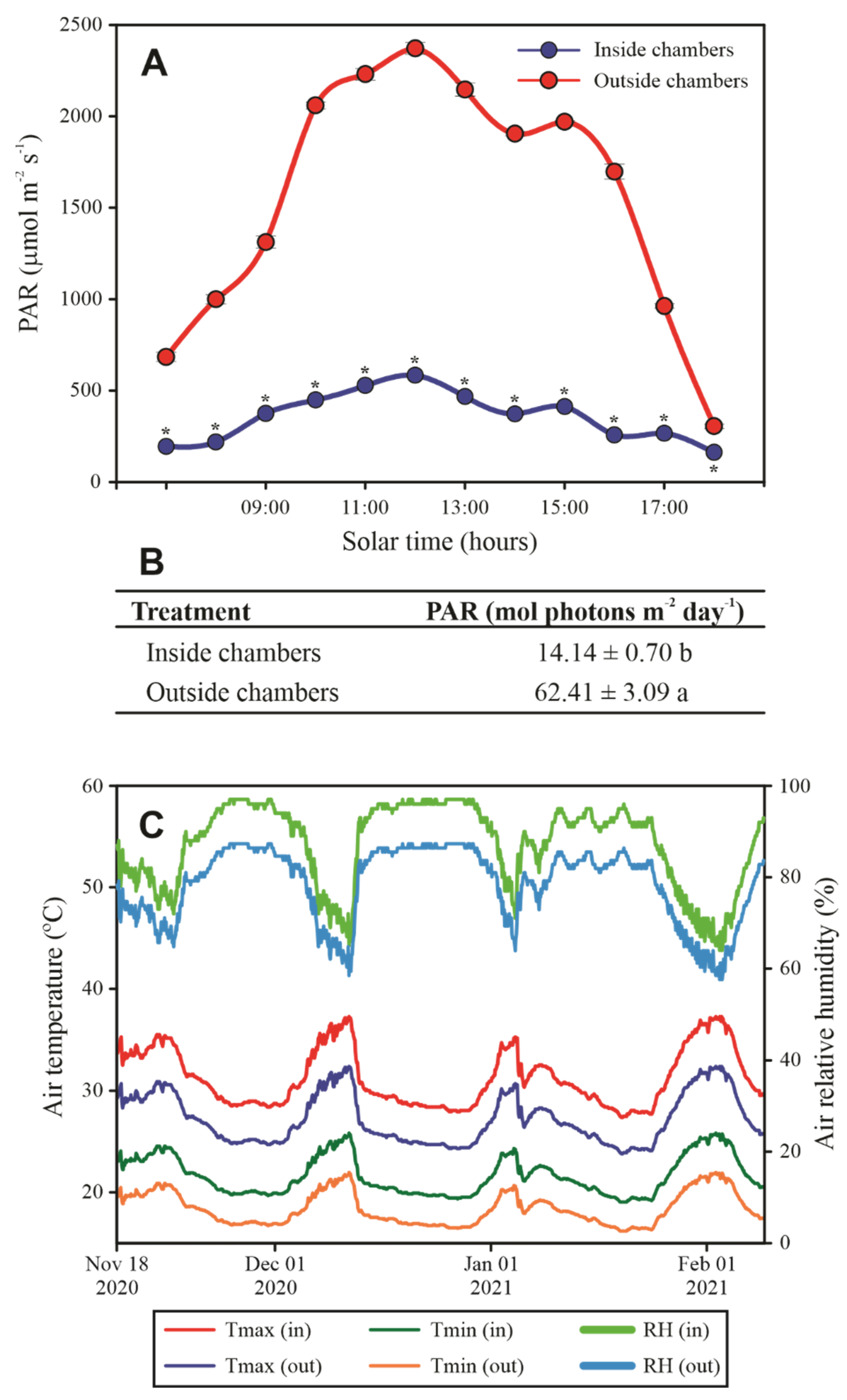
2.3. Estimation of CO2 Concentration and PAR Saturation
2.4. Leaf Gas Exchange Parameters and Chlorophyll a Fluorescence
2.5. Biochemical Measurements
2.5.1. Photosynthetic Pigments and Xanthophyll Pools
2.5.2. Calvin Cycle Enzyme Activity
2.5.3. Glucose, Fructose and Sucrose Measurements
2.5.4. Stevioside and Rebaudioside-A Measurements
2.6. Biomass Quantification
2.7. Experimental Design and Statistical Analyses
3. Results
3.1. Previous Test
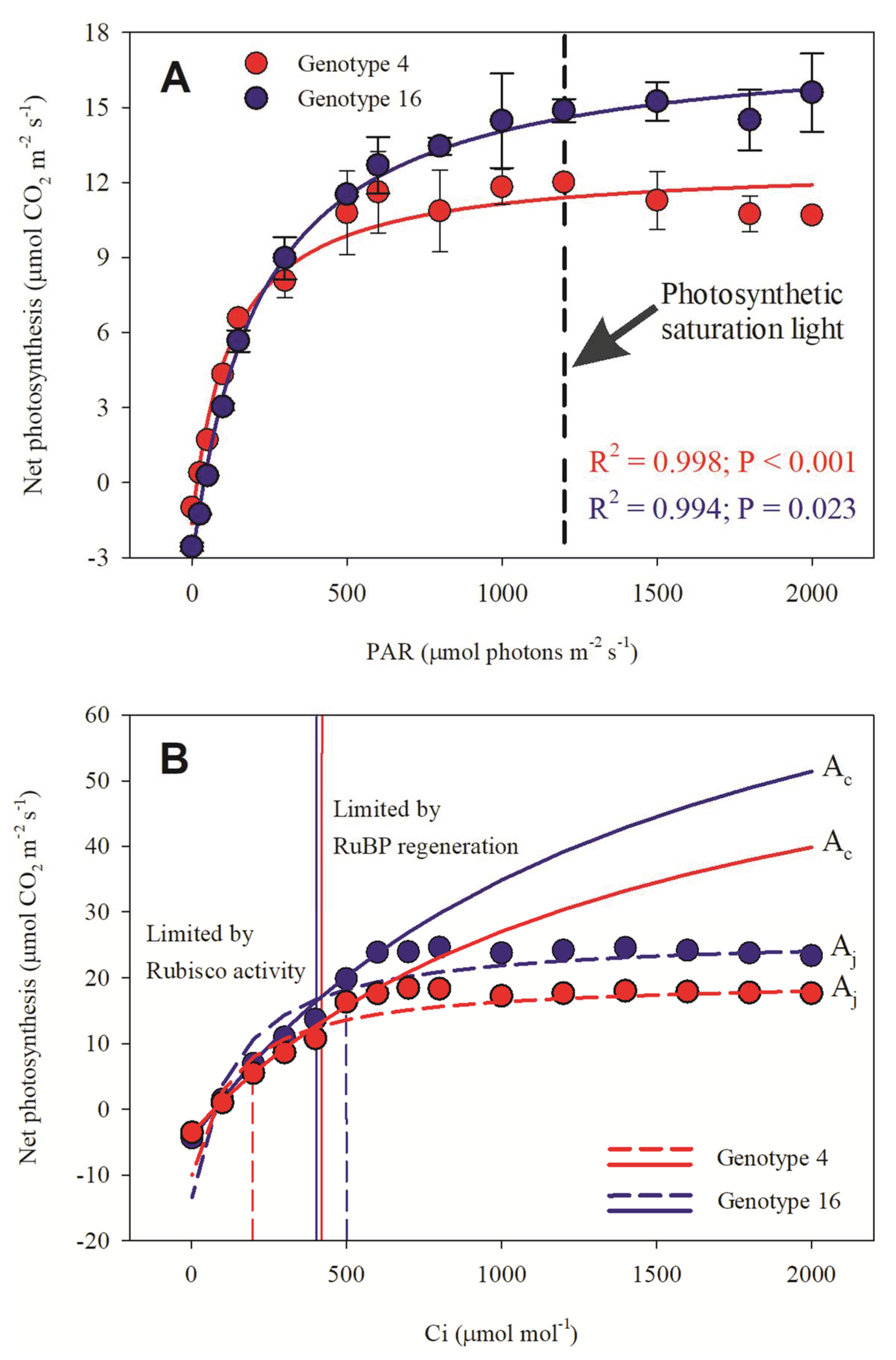
3.2. AN x PAR and AN x Ci Results
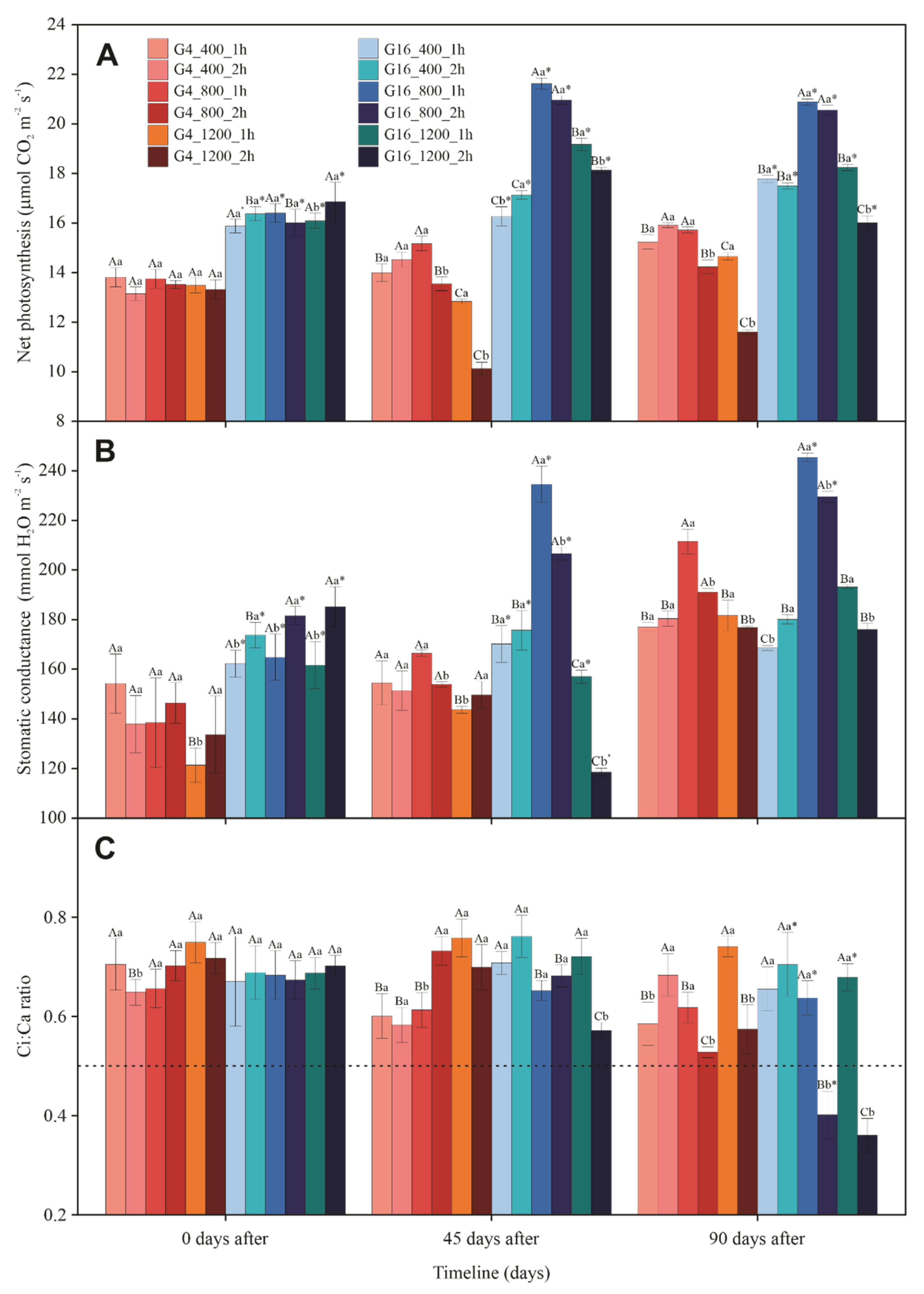
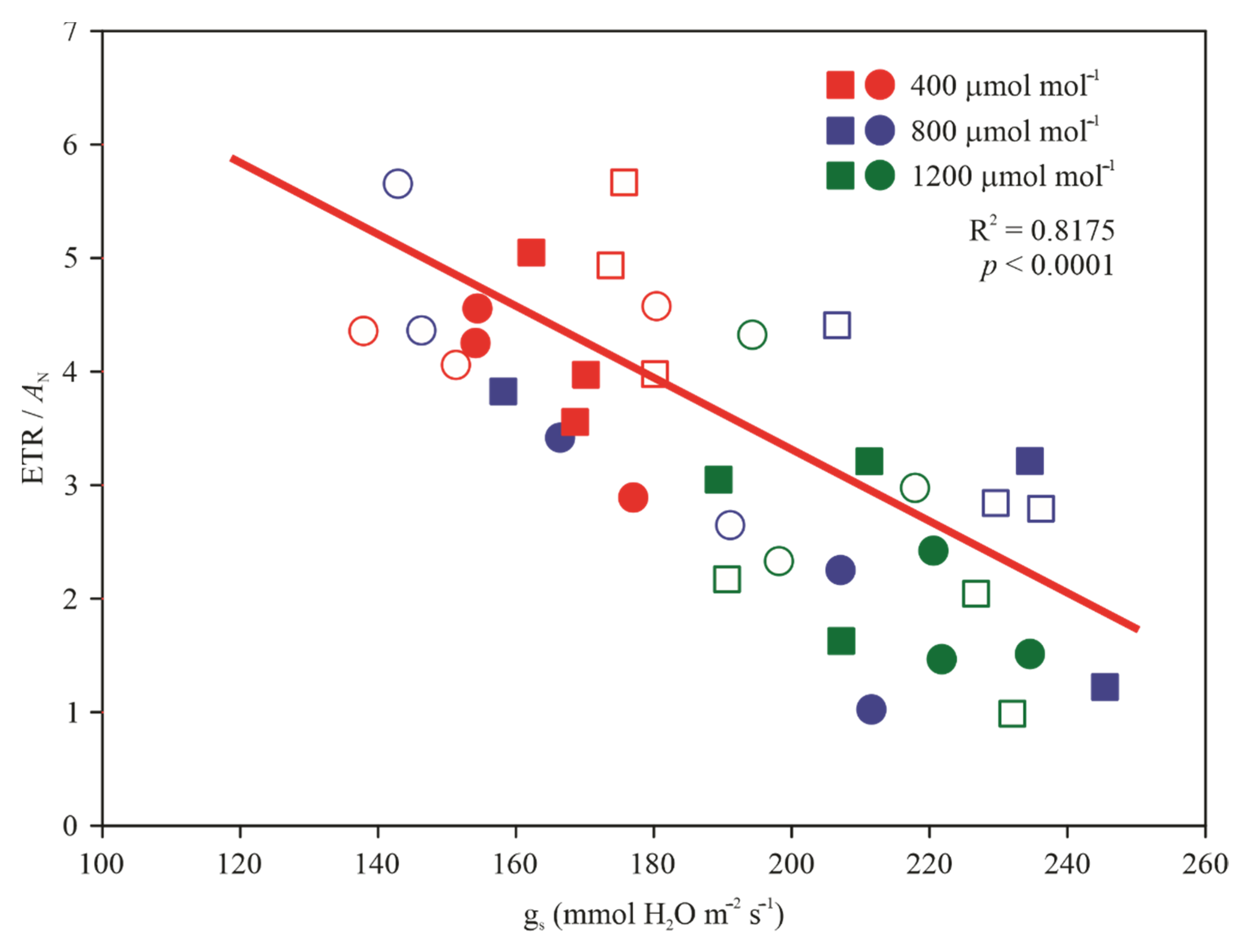
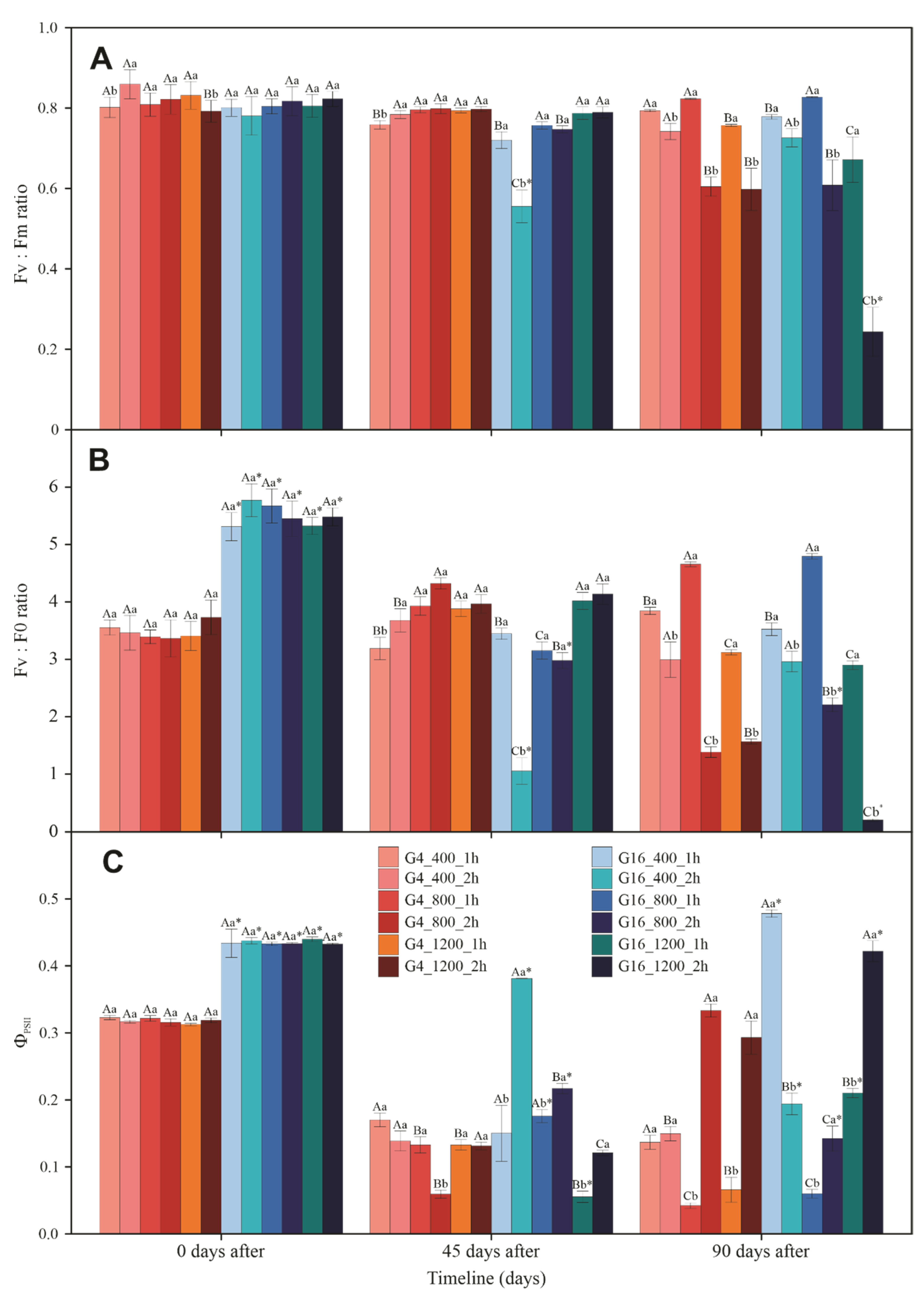
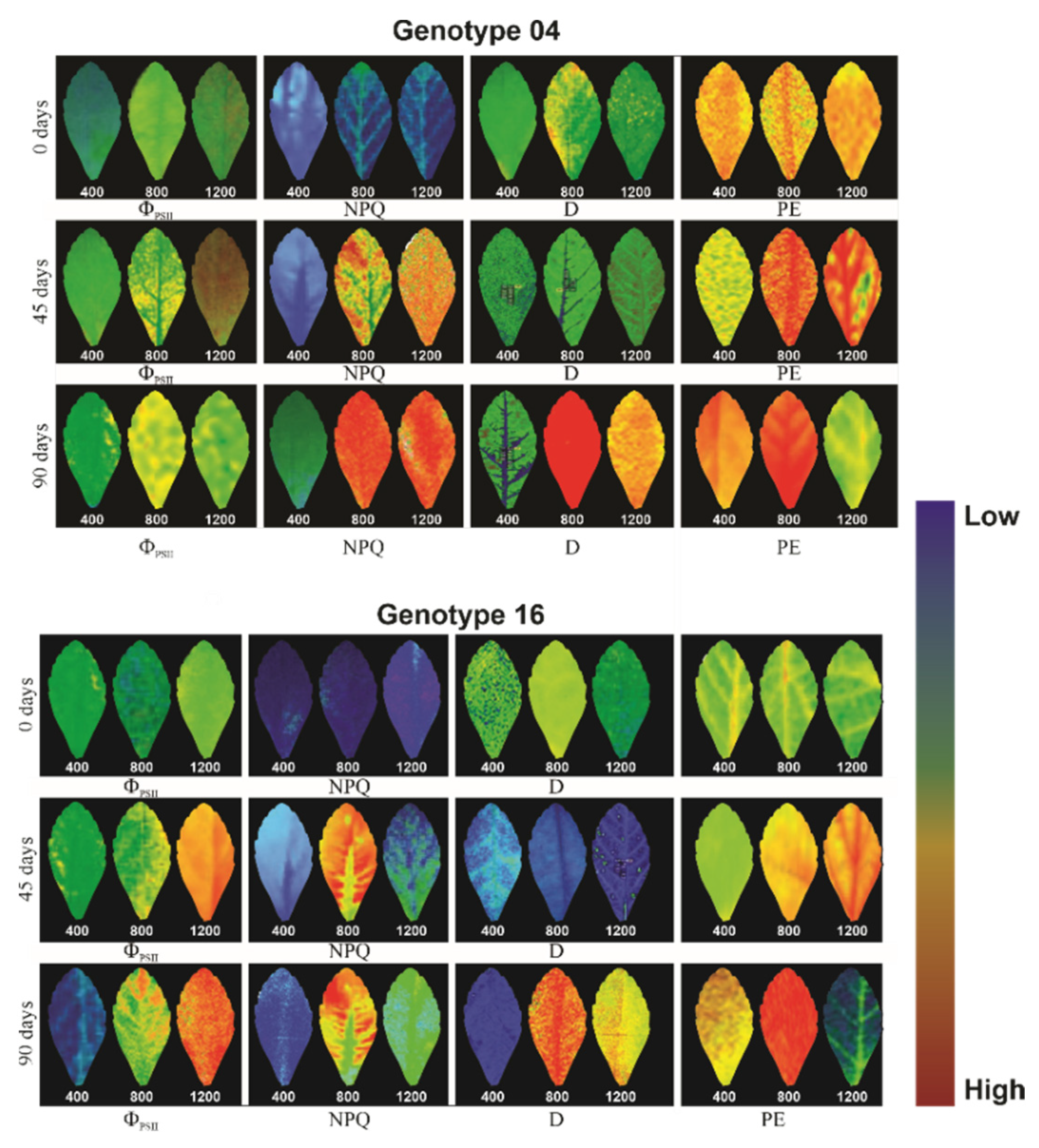
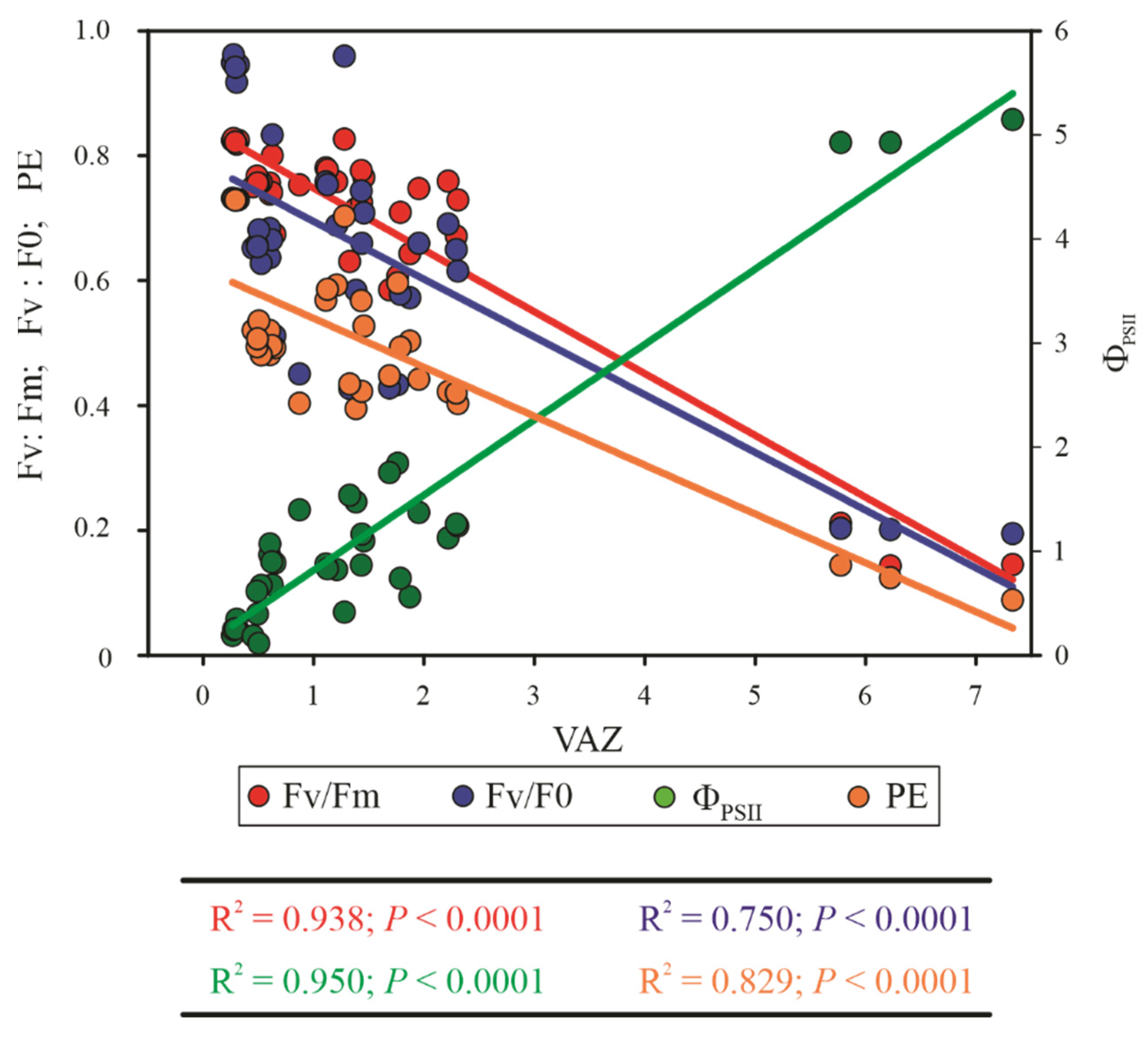
3.3. Gas Exchange and Chlorophyll a Fluorescence
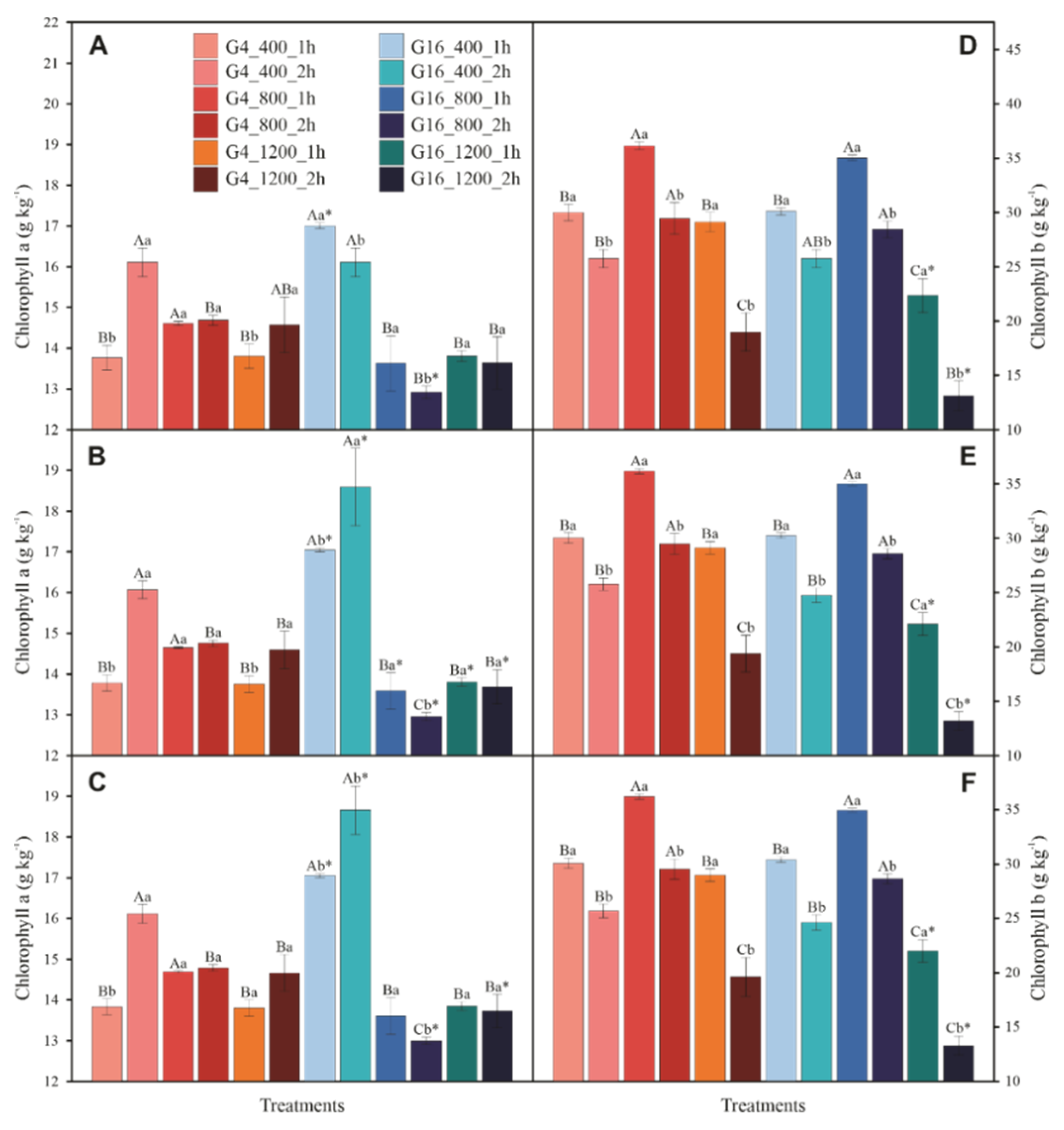
3.4. Photosynthetic Pigments and Violaxanthin Pool Cycle
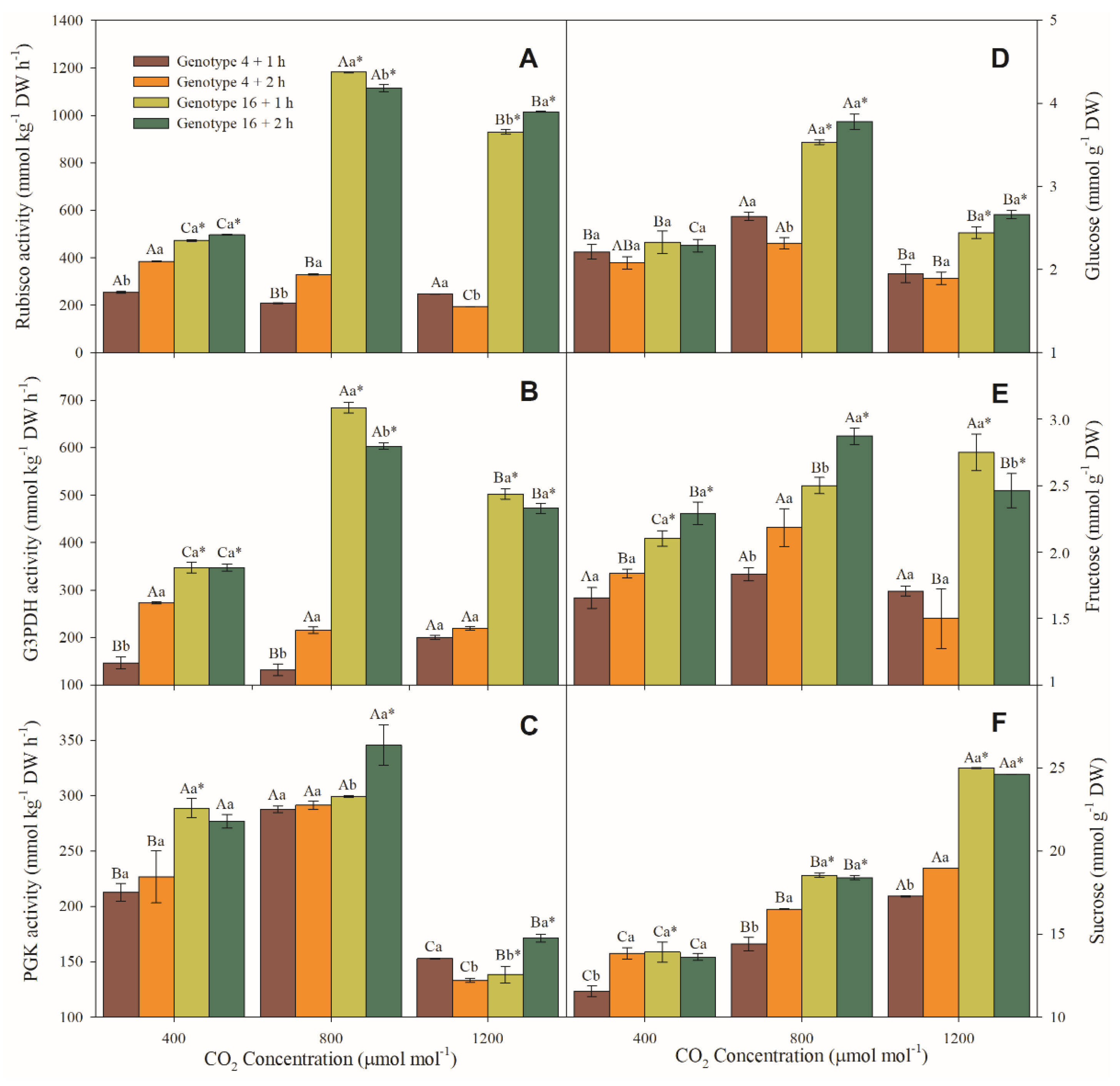
3.5. Enzymatic and Sugar Content Measurements
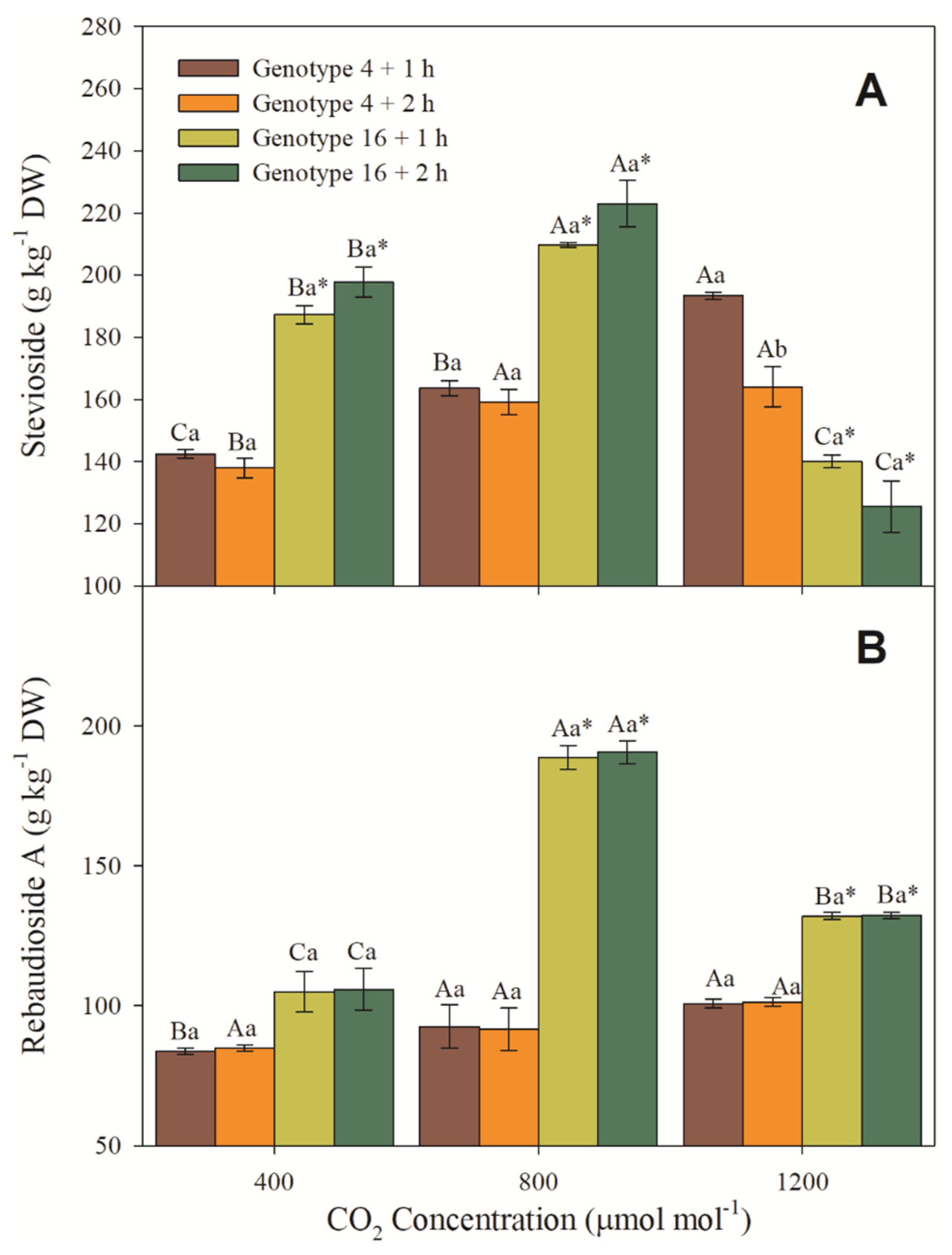
3.6. Steviosides
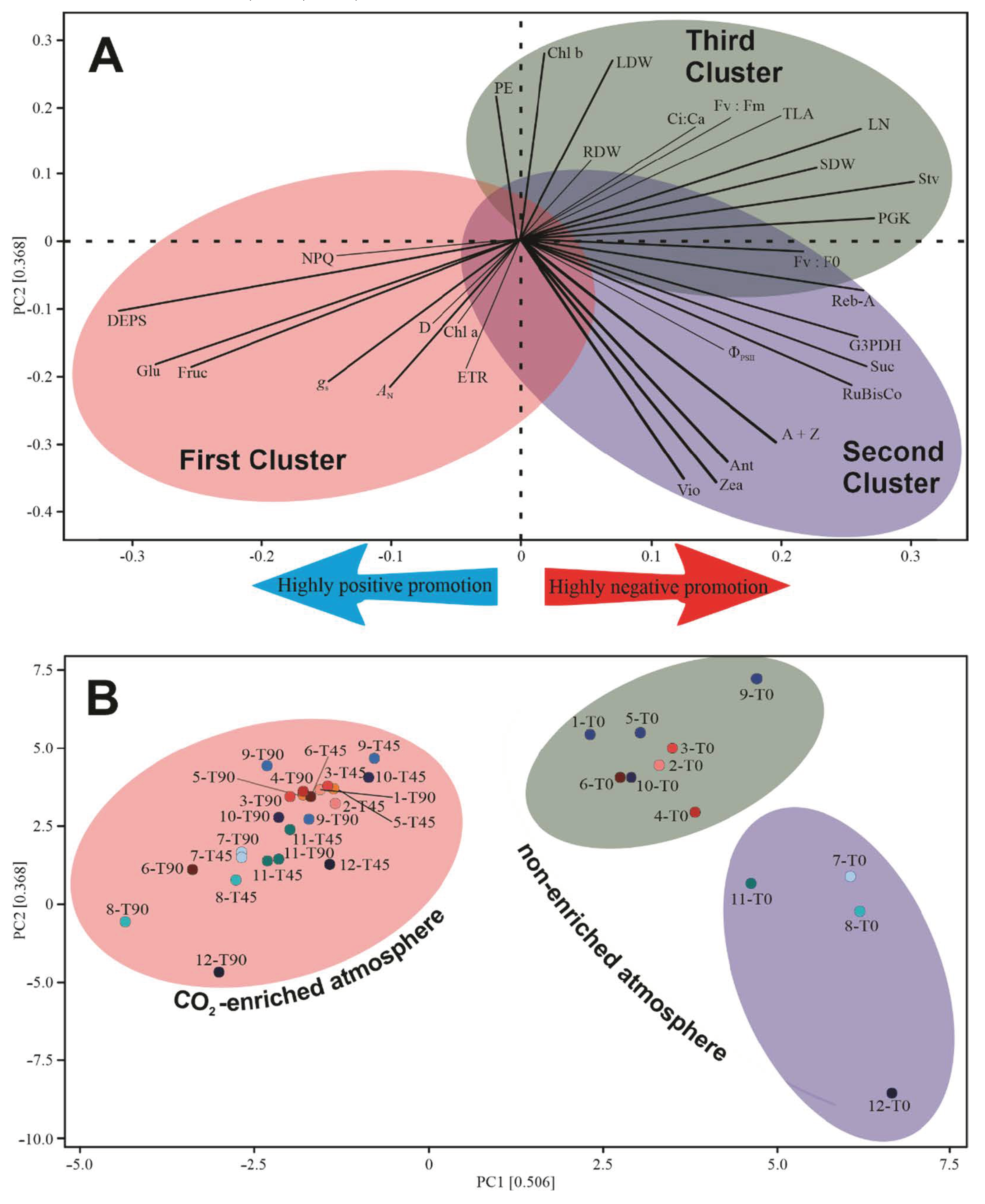
3.7. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernacchi, C.J.; Singsaas, E.L.; Pimentel, C.; Portis, A.R., Jr.; Long, S.P. Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant Cell Environ. 2001, 24, 253–259. [Google Scholar] [CrossRef]
- Jarma-Orozco, A.; Combatt-Baballero, E.; Jaraba-Navas, J. Growth and development of Stevia rebaudiana Bert., in high and low levels of radiation. Curr. Plant Biol. 2020, 22, 100144. [Google Scholar] [CrossRef]
- Rivera-Avilez, J.A.; Jarma-Orozco, A.; Pompelli, M.F. Stevia rebaudiana Bertoni: The interaction of night interruption on gas exchange, flowering delay, and steviol glycosides synthesis. Horticulturae 2021, 7, 543. [Google Scholar] [CrossRef]
- Jagadish, K.S.V.; Kadam, N.N.; Xiao, G.; Melgar, R.J.; Bahuguna, R.N.; Quinones, C.; Tamilselvan, A.; Prasad, P.V.V. Agronomic and physiological responses to high temperature, drought, and elevated CO2 interactions in cereals. In Advances in Agronomy; Sparks, D., Ed.; Academic Press: London, UK, 2014; Volume 127, pp. 111–156. [Google Scholar]
- Carr, H.; Björk, M. Parallel changes in non-photochemical quenching properties, photosynthesis and D1 levels at sudden, prolonged irradiance exposures in Ulva fasciata Delile. J. Photoch. Photobio. B 2007, 87, 18–26. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Allakhverdiev, S.I.; Murata, N. A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim. Biophys. Acta 2006, 1757, 742–749. [Google Scholar] [CrossRef] [Green Version]
- Pokorska, B.; Romanowska, E. Photoinhibition and D1 protein degradation in mesophyll and agranal bundle sheath thylakoids of maize. Funct. Plant Biol. 2007, 34, 844–852. [Google Scholar] [CrossRef]
- Rintamäki, E.; Salo, R.; Aro, E.-M. Rapid turnover of the D1 reaction-center protein of photosystem II as a protection mechanisms against photoinhibition in a moss Ceratodon purpureus (Hedw.) Brid. Planta 1994, 193, 520–529. [Google Scholar] [CrossRef]
- Bernacchi, C.J.; Portis, A.R.; Nakano, H.; von Caemmerer, S.; Long, S.P. Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitation to photosynthesis in vivo. Plant Physiol. 2002, 130, 1992–1998. [Google Scholar] [CrossRef] [Green Version]
- DaMatta, F.M.; Maestri, M.; Mosquim, P.R.; Barros, R.S. Photosynthesis in coffee (Coffea arabica and C. canephora) as affected by winter and summer conditions. Plant Sci. 1997, 128, 43–50. [Google Scholar] [CrossRef]
- Niyogi, K.K.; Björkman, O.; Grossman, A.E. The roles of specific xanthophylls in photoprotection. Proc. Natl. Acad. Sci. USA 1997, 94, 14162–14167. [Google Scholar] [CrossRef]
- Pinto, A.M.F.; von Sperling, E.; Moreira, R.M. Chlorophyll-a determination via continuous measurement of plankton fluorescence: Methodology development. Water Res. 2001, 35, 3977–3981. [Google Scholar] [CrossRef]
- Wullschleger, S.D. Biochemical limitations to carbon assimilation in C3 plants—A retrospective analysis of the A/Ci curves from 109 species. J. Exp. Bot. 1993, 44, 907–920. [Google Scholar] [CrossRef]
- Levine, L.H.; Kasahara, H.; Kopka, J.; Erban, A.; Fehrl, I.; Kaplan, F.; Zhao, W.; Littelle, R.C.; Guy, C.; Wheeler, R.; et al. Physiologic and metabolic responses of wheat seedlings to elevated and super-elevated carbon dioxide. Adv. Space Res. 2008, 42, 1917–1928. [Google Scholar] [CrossRef]
- Possell, M.; Hewitt, C.N. Gas exchange and photosynthetic performance of the tropical tree Acacia nigrescens when grown in different CO2 concentrations. Planta 2009, 229, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Díaz-Espejo, A.; Conesa, M.A.; Coopman, R.E.; Douthe, C.; Gago, J.; Gallé, A.; Galmés, J.; Medrano, H.; Ribas-Carbo, M.; et al. Mesophyll conductance to CO2 and Rubisco as targets for improving intrinsic water use efficiency in C3 plants. Plant Cell Environ. 2016, 39, 965–982. [Google Scholar] [CrossRef]
- Grassi, G.; Magnani, F. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ. 2005, 28, 834–849. [Google Scholar] [CrossRef]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vu, J.C.V.; Allen, L.H., Jr.; Boote, K.J.; Bowes, G. Effects of elevated CO2 and temperature on photosynthesis and Rubisco in rice and soybean. Plant Cell Environ. 1997, 20, 68–76. [Google Scholar] [CrossRef]
- Fan, Y.; Zhong, Z.; Zhang, X. Determination of photosynthetic parameters Vcmax and Jmax for a C3 plant (spring hulless barley) at two altitudes on the Tibetan Plateau. Agric. For. Meteorol. 2011, 151, 1481–1487. [Google Scholar] [CrossRef]
- Bowes, G. Facing the inevitable: Plants and increasing atmospheric CO2. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1993, 44, 309–332. [Google Scholar] [CrossRef]
- Gorthi, A.; Volenec, J.J.; Welp, L.R. Stomatal response in soybean during drought improves leaf-scale and field-scale water use efficiencies. Agric. For. Meteorol. 2019, 27, 107629. [Google Scholar] [CrossRef]
- Aranjuelo, I.; Erice, G.; Nogués, S.; Morales, F.; Irigoyen, J.J.; Sánchez-Díaz, M. The mechanism(s) involved in the photoprotection of PSII at elevated CO2 in nodulated alfalfa plants. Environ. Exp. Bot. 2008, 64, 295–306. [Google Scholar] [CrossRef] [Green Version]
- Long, S.P. Modification of the response of photosynthetic productivity to rising temperature by atmospheric CO2 concentrations: Has its importance been underestimated? Plant Cell Environ. 1991, 14, 729–739. [Google Scholar] [CrossRef]
- Alonso, A.; Perez, P.; Morcuende, R.; Martinez-Carrasco, R. Future CO2 concentrations, though not warmer temperatures, enhance wheat photosynthesis temperature responses. Physiol. Plant 2008, 132, 102–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Caemmerer, S.; Lawson, T.; Oxborough, K.; Baker, N.R.; Andrews, T.J.; Raines, C.A. Stomatal conductance does not correlate with photosynthetic capacity in transgenic tobacco with reduced amounts of rubisco. J. Exp. Bot. 2004, 55, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Medlyn, B.E.; Badeck, F.-H.; De Pury, D.G.G.; Barton, C.V.M.; Broadmeadow, M.; Ceulemans, R.; De Angelis, P.; Forstreuter, M.; Jach, M.E.; Kellomäki, S.; et al. Effects of elevated [CO2] on photosynthesis in European forest species: A meta-analysis of model parameters. Plant Cell Environ. 1999, 22, 1475–1495. [Google Scholar] [CrossRef]
- Sharkey, T.D. What gas exchange data can tell us about photosynthesis. Plant Cell Environ. 2015, 39, 1161–1163. [Google Scholar] [CrossRef]
- Bellasio, C.; Beerling, D.J.; Griffiths, H. An Excel tool for deriving key photosynthetic parameters from combined gas exchange and chlorophyll fluorescence: Theory and practice. Plant Cell Environ. 2016, 39, 1180–1197. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Taylor, J.M.; Wilkins, D.; Besford, R.T. Effects of elevated CO2 on chloroplast components, gas exchange and growth of oak and cherry. Tree Physiol. 1997, 17, 319–325. [Google Scholar] [CrossRef] [Green Version]
- Sharkey, T.D. Photosynthesis in intact leaves of C3 plants: Physics, physiology and rate limitations. Bot. Rev. 1985, 51, 53–105. [Google Scholar] [CrossRef]
- Shaheen, H.; Mustafa, A.; Ulfat, A. Crop production in response to elevated CO2: Grain yield and quality. In Sustainable Crop Productivity and Quality Under Climate Change Responses of Crop Plants to Climate Change; Liu, F., li, X., Hogy, P., Jiang, D., Brestic, M., liu, B., Eds.; Academic Press: London, UK, 2022; pp. 21–42. [Google Scholar]
- Pimentel, C. Carbon metabolism of crops and the increase of atmospheric CO2 and O3: Situation and previsions. Bragantia 2011, 70, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Babani, F.; Langsdorf, G.; Knapp, M.; Buschmann, C.; Lichtenthaler, H.K. UV-A induced fluorescence images in sun and shade leaves. BMC Plant Biol. 2005, 5, S3. [Google Scholar] [CrossRef] [Green Version]
- Hajihashemi, S.; Noedoost, F.; Geuns, J.M.C.; Djalovic, I.; Siddique, K.H.M. Effect of cold stress on photosynthetic traits, carbohydrates, morphology, and anatomy in nine cultivars of Stevia rebaudiana. Front. Plant Sci. 2018, 9, 1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Agronet. La Estevia en Colombia; Oferta y Cosumo. Available online: https://www.agronet.gov.co/Paginas/inicio.aspx (accessed on 23 October 2022).
- Basharat, S.; Huang, Z.; Gong, M.; Lv, X.; Ahmed, A.; Hussain, I.; Li, J.; Du, G.; Liu, L. A review on current conventional and biotechnical approaches to enhance biosynthesis of steviol glycosides in Stevia rebaudiana. Chin. J. Chem. Eng. 2021, 30, 92–104. [Google Scholar] [CrossRef]
- Benhmimou, A.; Ibriz, M.; Al Faïz, C.; Gaboun, F.; Douaik, A.; Amchra, F.Z.; Khiraoui, A.; Lage, M. Effects of planting density and harvesting time on productivity of natural sweetener plant (Stevia rebaudiana Bertoni.) in Larache Region, Morocco. Int. J. Plant Res. 2017, 7, 83–89. [Google Scholar] [CrossRef]
- Rodriguez-Paez, L.A.; Hernandez-Burgos, J.L.; Caballero, E.M.C.; Jarma-Orozco, A.; Santos, J.M.P. Rendimiento y calidad de hojas de Stevia rebaudiana Bert. bajo la oferta edafológica y dos niveles de radiación en cinco regiones de Colombia. Rev. UDCA Act. Div. Cient. 2016, 9, 77–85. [Google Scholar]
- Cantabella, D.; Piqueras, A.; Acosta-Motos, J.R.; Bernal-Vicente, A.; Hernández, J.A.; Díaz-Vivancos, P. Salt-tolerance mechanisms induced in Stevia rebaudiana Bertoni: Effects on mineral nutrition, antioxidative metabolism and steviol glycoside content. Plant Physiol. Biochem. 2017, 115, 484–496. [Google Scholar] [CrossRef]
- Castillejo-Morales, A.; Jarma-Orozco, A.; Pompelli, M.F. Physiological and morphological features denote that salt stress in Stevia rebaudiana is based on nonstomatic instead of stomatic limitation. Rev. Colombiana Cien Hortíc 2021, 15, e12928. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Geuns, J.M.C. Gene transcription and steviol glycoside accumulation in Stevia rebaudiana under polyethylene glycol-induced drought stress in greenhouse cultivation. FEBS Open Bio 2016, 6, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, Y.; Shimizu, H.; Nakashima, H.; Miyasaka, J.; Ohdoi, K. Effects of light intensity and photoperiod on improving steviol glycosides content in Stevia rebaudiana (Bertoni) Bertoni while conserving light energy consumption. J. Appl. Res. Med. Aromat. Plants 2017, 7, 64–73. [Google Scholar] [CrossRef]
- Ceunen, S.; Werbrouck, S.; Geuns, J.M.C. Stimulation of steviol glycoside accumulation in Stevia rebaudiana by red LED light. J Plant Physiol. 2012, 169, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Pico-González, A.I.; Aramendiz-Tatis, H.; Pérez-Polo, D.J. Caracterización morfoagronómica de 25 clones de estevia (Stevia rebaudiana Bertoni.), en condiciones del valle del Sinú medio. Temas Agrarios 2020, 25, 106–116. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Mendes, K.R.; Ramos, M.V.; Santos, J.N.B.; Youssef, D.T.A.; Pereira, J.D.; Endres, L.; Jarma-Orozco, A.; Solano-Gomes, R.; Jarma-Arroyo, B.; et al. Mesophyll thickness and sclerophylly among Calotropis procera morphotypes reveal water-saved adaptation to environments. J. Arid Land 2019, 11, 795–810. [Google Scholar] [CrossRef] [Green Version]
- Caballero, E.M.C. Distribución espacial de la salinidad en suelos del área de influencia de la desembocadura del río Sinú (Córdoba, Colombia). Rev. UDCA Act. Div. Cient. 2014, 17, 433–443. [Google Scholar]
- Garten, M. Take urgent action to combat climate change and its impacts. In United Nations Statistics Division Development Data and Outreach Branch; United Nations: New York, NY, USA, 2020; Available online: https://unstats.un.org/sdgs/report/2021/goal-13/ (accessed on 10 September 2022).
- Moualeu-Ngangue, D.P.; Chen, T.-W.; Stützel, H. A new method to estimate photosynthetic parameters through net assimilation rate—Intercellular space CO2 concentration (A-Ci) curve and chlorophyll fluorescence measurements. New Phytol. 2017, 213, 1543–1554. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Ferreira, P.P.B.; Chaves, A.R.M.; Figueiredo, R.C.Q.Q.; Martins, A.O.; Jarma-Orozco, A.; Batista-Silva, W.; Endres, L.; Araújo, W.L. Physiological, metabolic, and stomatal adjustments in response to salt stress in Jatropha curcas. Plant Physiol. Biochem. 2021, 168, 116–127. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.; Pan, D.; Zhang, Y.; Luo, B.; Ji, J. Effects of drought stress on photosynthesis and chlorophyll fluorescence images of soybean (Glycine max) seedlings. Int. J. Agric. Biol. Eng. 2018, 11, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Borek, M.; Baczek-Kwinta, R.; Rapacz, M. Photosynthetic activity of variegated leaves of Coleus × hybridus hort. cultivars characterised by chlorophyll fluorescence techniques. Photosynthetica 2016, 54, 331–339. [Google Scholar] [CrossRef] [Green Version]
- Pompelli, M.F.; Martins, S.C.V.; Antunes, W.C.; Chaves, A.R.M.; DaMatta, F.M. Photosynthesis and photoprotection in coffee leaves is affected by nitrogen and light availabilities in winter conditions. J. Plant Physiol. 2010, 167, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Pompelli, M.F.; França, S.C.S.; Tigre, R.C.; Oliveira, M.T.; Sacilot, M.; Pereira, E.C.G. Spectrophotometric determinations of chloroplastidic pigments in acetone, ethanol and dimethylsulphoxide. Rev. Bras. Biol. 2013, 11, 52–58. [Google Scholar]
- Geigenberger, P.; Stitt, M. Sucrose synthase catalyses a readily reversible reaction in vivo in developing potato tubers and other plant tissues. Planta 1993, 189, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Sulpice, R.; Tschoep, H.; Von Korff, M.; Bussis, D.; Usadel, B.; Hohne, M.; Witucka-Wall, H.; Altmann, T.; Stitt, M.; Gibon, Y. Description and applications of a rapid and sensitive non-radioactive microplate-based assay for maximum and initial activity of D-Ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Cell Environ. 2007, 30, 1163–1175. [Google Scholar] [CrossRef] [PubMed]
- Vescovi, M.; Zaffagnini, M.; Festa, M.; Trost, P.; Lo Schiavo, F.; Costa, A. Nuclear accumulation of cytosolic glyceraldehyde-3-phosphate dehydrogenase in cadmium-stressed Arabidopsis roots. Plant Phvsiol. 2013, 162, 333–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulbe, K.D.; Bojanovski, M. 3-Phosphoglycerate kinase from bovine liver and yeast. Methods Enzymol. 1982, 90, 115–120. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Cunha, R.L.; Antunes, W.C.; Martins, S.C.V.; Araujo, W.L.; Fernie, A.R.; Moraes, G.A.B.K. In field-grown coffee trees source-sink manipulation alters photosynthetic rates, independently of carbon metabolism, via alterations in stomatal function. New Phytol. 2008, 178, 348–357. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Fernandéz, I.A.; Jarma-Orozco, A.; Pompelli, M.F. Allometric models for non-destructive leaf area measurement of stevia: An in depth and complete analysis. Hortic Bras. 2021, 39, 207–217. [Google Scholar] [CrossRef]
- CoreTeam. The R Project for Statistical Computing Version 2022.07. Available online: https://www.r-project.org/about.html (accessed on 15 April 2020).
- Li, S.; Wang, X.; Liu, X.; Thompson, A.J.; Liu, F. Elevated CO2 and high endogenous ABA level alleviate PEG-induced short-term osmotic stress in tomato plants. Environ. Exp. Bot. 2022, 194, 104763. [Google Scholar] [CrossRef]
- Abdelhakim, L.O.A.; Zhou, R.; Ottosen, C.-O. Physiological responses of plants to combined drought and heat under elevated CO2. Agronomy 2022, 12, 2526. [Google Scholar] [CrossRef]
- Rogers, A.; Humphries, S.W. A mechanistic evaluation of photosynthetic acclimation at elevated CO2. Glob. Chang. Biol. 2000, 6, 1005–1011. [Google Scholar] [CrossRef] [Green Version]
- Cen, Y.-P.; Sage, R.F. The regulation of rubisco activity in response to variation in temperature and atmospheric CO2 partial pressure in sweet potato. Plant Phvsiol. 2005, 139, 979–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, T.; Sakai, H.; Tokida, T.; Nakamura, H.; Zhu, C.; Usui, Y.; Yoshimoto, M.; Fukuoka, M.; Wakatsuki, H.; Katayanagi, N.; et al. Rice cultivar responses to elevated CO2 at two free-air CO2 enrichment (FACE) sites in Japan. Funct Plant Biol. 2013, 40, 148–159. [Google Scholar] [CrossRef]
- Kang, H.; Zhu, T.; Zhang, Y.; Ke, X.; Sun, W.; Hu, Z.; Zhu, X.; Shen, H.; Huang, Y.; Tang, Y. Elevated CO2 enhances dynamic photosynthesis in rice and wheat. Front. Plant Sci. 2021, 12, 727374. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.V.V.; Boote, K.J.; Allen, L.H. Longevity and temperature response of pollen as affected by elevated growth temperature and carbon dioxide in peanut and grain sorghum. Environ. Exp. Bot. 2011, 70, 51–57. [Google Scholar] [CrossRef]
- Tan, S.-L.; Huang, X.; Li, W.-Q.; Zhang, S.-B.; Huang, W. Elevated CO2 concentration alters photosynthetic performances under fluctuating light in Arabidopsis thaliana. Cells 2021, 10, 2329. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Li, C.; Portis, A.R. Arabidopsis thaliana expressing a thermostable chimeric Rubisco activase exhibits enhanced growth and higher rates of photosynthesis at moderately high temperatures. Photosynth Res. 2009, 100, 143–153. [Google Scholar] [CrossRef]
- Mendes, K.R.; Marenco, R.A.; Nascimento, H.C.S. Maximum carboxylation velocity of rubisco and maximum rate of electron transport in saplings in response to variations in environmental factors in central Amazonia. Cienc Florest 2017, 27, 947–959. [Google Scholar] [CrossRef] [Green Version]
- Ellsworth, D.S.; Reich, P.B.; Naumburg, E.S.; Koch, G.W.; Kubiske, M.E.; Smith, S.D. Photosynthesis, carboxylation and leaf nitrogen responses of 16 species to elevated pCO2 across four free-air CO2 enrichment experiments in forest, grassland and desert. Glob. Chang. Biol. 2004, 10, 2121–2138. [Google Scholar] [CrossRef] [Green Version]
- Onoda, Y.; Hikosaka, K.; Hirose, T. Seasonal change in the balance between capacities of RuBP carboxylation and RuBP regeneration affects CO2 response of photosynthesis in Polygonum cuspidatum. J. Exp. Bot. 2005, 56, 755–763. [Google Scholar] [CrossRef]
- Ruiz-Vera, U.M.; De Souza, A.P.; Ament, M.R.; Gleadow, R.M.; Ort, D.R. High sink strength prevents photosynthetic down-regulation in cassava grown at elevated CO2 concentration. J. Exp. Bot. 2021, 72, 542–560. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Kang, S.; Zhang, J. Interactive effects of elevated CO2, nitrogen and drought on leaf area, stomatal conductance, and evapotranspiration of wheat. Agric. Water Manag. 2004, 67, 221–233. [Google Scholar] [CrossRef]
- Ma, Y.; Xie, Y.; Ha, R.; Bing, C.; Lihua, S. Effects of elevated CO2 on photosynthetic accumulation, sucrose metabolism-related enzymes, and genes identification in goji berry (Lycium barbarum). Front. Plant Sci. 2021, 12, 643555. [Google Scholar] [CrossRef] [PubMed]
- Castaño, M.A.M.; Chica, R.H.A.; Obando, B.D.; Riaño, H.N.M. Gas exchange in Cordia alliodora, Alnus acuminata and Pachira quinata in Colombia. Cenicafe 2012, 63, 93–110. [Google Scholar]
- Madan, P.; Jagadish, S.V.K.; Craufurd, P.Q.; Fitzgerald, M.; Lafarge, T.; Wheeler, T.R. Effect of elevated CO2 and high temperature on seed-set and grain quality of rice. J. Exp. Bot. 2012, 63, 3843–3852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kizildeniz, T.; Pascual, I.; Irigoyen, J.J.; Morales, F. Future CO2, warming and water deficit impact white and red Tempranillo grapevine: Photosynthetic acclimation to elevated CO2 and biomass allocation. Physiol Plant 2021, 172, 1779–1794. [Google Scholar] [CrossRef]
- Van Oosten, J.-J.; Besford, R.T. Sugar feeding mimics effect of acclimation to high CO2-rapid down regulation of RuBisCo small subunit transcripts but not of the large subunit transcripts. J. Plant Physiol. 1994, 143, 306–312. [Google Scholar] [CrossRef]
- Rivero, R.M.; Mittler, R.; Blumwald, E.; Zandalinas, S.I. Developing climate-resilient crops: Improving plant tolerance to stress combination. Plant J. 2022, 109, 373–389. [Google Scholar] [CrossRef]
- Johnson, A.A.T. Enhancing the chelation capacity of rice to maximise iron and zinc concentrations under elevated atmospheric carbon dioxide. Funct. Plant Biol. 2013, 40, 101–108. [Google Scholar] [CrossRef]
- de Guzman, R.; Midmore, D.J.; Walsh, K.B. Do steviol glycosides act either as a carbon storage pool or in osmoregulation within leaves of Stevia rebaudiana? J. Nat. Prod. 2018, 81, 2357–2363. [Google Scholar] [CrossRef]
- Hussin, S.; Geissler, N.; El-Far, M.M.M.; Koyro, H.W. Effects of salinity and short-term elevated atmospheric CO2 on the chemical equilibrium between CO2 fixation and photosynthetic electron transport of Stevia rebaudiana Bertoni. Plant Physiol. Biochem. 2017, 118, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Marenco, R.A.; Gonçalves, J.F.C.; Vieira, G. Leaf gas exchange and carbohydrates in tropical trees differing in successional status in two light environments in central Amazonia. Tree Physiol. 2001, 21, 1311–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, J.; Grace, J. The effects of shade on the gas exchange of seedlings of four tropical trees from Mexico. Funct Ecol. 1990, 4, 667–677. [Google Scholar] [CrossRef]
- Terashima, I.; Hikosaka, K. Comparative ecophysiology of leaf and canopy photosynthesis. Plant Cell Environ. 1995, 18, 1111–1128. [Google Scholar] [CrossRef]
- Mendes, K.R.; Granja, J.A.A.; Ometto, J.P.; Antonino, A.C.D.; Menezes, R.S.C.; Pereira, E.C.; Pompelli, M.F. Croton blanchetianus modulates its morphophysiological responses to tolerate drought in a tropical dry forest. Funct. Plant Biol. 2017, 44, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Mendes, K.R.; Batista-Silva, W.; Dias-Pereira, J.; Pereira, M.P.S.; Souza, E.V.; Serrão, J.E.; Granja, J.A.A.; Pereira, E.C.; Gallacher, D.J.; Mutti, P.R.; et al. Leaf plasticity across wet and dry seasons in Croton blanchetianus (Euphorbiaceae) at a tropical dry forest. Sci. Rep. 2022, 12, 954. [Google Scholar] [CrossRef]
- Albert, K.R.; Ro-Poulsen, H.; Mikkelsen, T.N.; Michelsen, A.; Van Der Linden, L.; Beier, C. Effects of elevated CO2, warming and drought episodes on plant carbon uptake in a temperate heath ecosystem are controlled by soil water status. Plant Cell Environ. 2011, 34, 1207–1222. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef] [Green Version]
- Demmig-Adams, B.; Adams III, W.W. Photoprotection in an ecological context: The remarkable complexity of thermal energy dissipation. New Phytol. 2006, 172, 11–21. [Google Scholar] [CrossRef]
- Werner, C.; Ryel, R.J.; Correia, O.; Beyschlag, W. Effects of photoinhibition on whole-plant carbon gain assessed with a photosynthesis model. Plant Cell Environ. 2001, 24, 27–40. [Google Scholar] [CrossRef]
- Zu, X.G.; Ort, D.R.; Whitmarsh, J.; Long, S.P. The slow reversibility of photosystem II thermal energy dissipation on transfer from high to low light may cause large losses in carbon gain by crop canopies: A theoretical analysis. J. Exp. Bot. 2004, 55, 1167–1175. [Google Scholar] [CrossRef]
- Miyata, K.; Noguchi, K.; Terashima, I. Cost and benefit of the repair of photodamaged photosystem II in spinach leaves: Roles of acclimation to growth light. Photosynth Res. 2012, 113, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, S.; Rodrigo, M.J.; Vives-Peris, V.; Gómez-Cadenas, A.; Zacarías, L.; Machado, N.; Moutinho-Pereira, J.; Dinis, L.-T. Fine-tuning of grapevine xanthophyll-cycle and energy dissipation under Mediterranean conditions by kaolin particle-film. Sci. Hortic. Amst. 2022, 291, 110584. [Google Scholar] [CrossRef]
- Filella, I.; Porcar-Castell, A.; Munné-Bosch, S.; Bäck, J.; Garbulsky, M.F.; Peñuelas, J. PRI assessment of long-term changes in carotenoids/chlorophyll ratio and short-term changes in de-epoxidation state of the xanthophyll cycle. Int. J. Remote Sens. 2009, 30, 4443–4455. [Google Scholar] [CrossRef]
- García-Plazaola, J.I.; López-Pozo, M.; Fernández-Marín, B. Xanthophyll cycles in the juniper haircap moss (Polytrichum juniperinum) and Antarctic hair grass (Deschampsia antarctica) on Livingston Island (South Shetland Islands, Maritime Antarctica). Polar Biol. 2022, 45, 1247–1256. [Google Scholar] [CrossRef]
- Rockholm, D.C.; Yamamoto, H.Y. Violaxanthin de-epoxidase (purification of a 43-kilodalton lumenal protein from lettuce by lipid-afinity precipitation with monogalactosyldiacylglyceride. Plant Physiol. 1996, 110, 697–703. [Google Scholar] [CrossRef] [Green Version]
- Kromdijk, J.; Głowacka, K.; Leonelli, L.; Gabilly, S.T.; Iwai, M.; Niyogi, K.K.; Long, S.P. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 2016, 354, 857–861. [Google Scholar] [CrossRef] [Green Version]
- Tsai, I.-H.; Murthy, S.N.P.; Steck, T.L. Effect of red cell membrane binding on the catalytic activit of glyceraldehyde-3-phosphate dehydrogenase. J. Biol. Chem. 1982, 257, 1438–1442. [Google Scholar] [CrossRef]
- Putnik, P.; Bezuk, I.; Barba, F.J.; Lorenzo, J.M.; Polunić, I.; Bursać, D.K. Sugar reduction: Stevia rebaudiana Bertoni as a natural sweetener. In Agri-Food Industry Strategies for Healthy Diets and Sustainability; Barba, F.J., Putnik, P., Kovačević, D.B., Eds.; Academic Press: San Diego, CA, USA, 2020; pp. 123–152. [Google Scholar]
- Francisco, F.; Pereira, G.P.; Machado, M.P.; Kanis, L.A.; Deschamps, C. Characterization of Stevia rebaudiana Bertoni A accessions cultived in southern Brazil. J. Agric. Sci. 2018, 10, 353–363. [Google Scholar] [CrossRef]
- Giraldo, C.; Marín, L.; Habeych, D. Obtención de edulcorantes de Stevia rebaudiana Bertoni. Rev. CENIC Cienc Biol. 2005, 36, 3–10. [Google Scholar]


| Parameters | Genotype 4 (G4) | Genotype 16 (G16) |
|---|---|---|
| Reference CO2 (mmol mol−1) | 400 | 400 |
| ANpot (μmol CO2 m−2 s−1) | 14.41 ± 0.30 b | 20.80 ± 0.90 a |
| gs(1200) (mol H2O m−2 s−1) | 0.54 ± 0.01 a | 0.39 ± 0.01 b |
| Light compensation point (LCP) (μmol photons m−2 s−1) | 8.81 ± 0.63 a | 8.82 ± 0.28 a |
| Light saturation point (LSP) (μmol photons m−2 s−1) | 1290.41 ± 106.67 b | 1977.05 ± 115.40 a |
| Maximum rate of electron transport (JMAX) (μmol m−2 s−1) | 92.62 ± 7.57 b | 167.99 ± 42.43 a |
| Saturation CO2 (Cisat) (μmol mol−1) | 799.60 ± 9.38 a | 701.51 ± 8.25 b |
| Maximum carboxylation rate of rubisco (VcMAX) (μmol m−2 s−1) | 57.67 ± 17.64 a | 45.18 ± 4.14 b |
| Electron transport rate (J) (μmol m−2 s−1) | 126.29 ± 2.79 a | 95.16 ± 5.20 b |
| Maximum rate of triose phosphate use (TPU) (μmol m−2 s−1) | 8.23 ± 0.79 a | 6.34 ± 0.40 b |
| Day respiration (Rd*) (μmol m−2 s−1) | 2.00 ± 0.01 a | 1.94 ± 0.10 a |
| Rubisco CO2 compensation point (Γ*) (Pa) | 3.88 ± 0.00 a | 3.64 ± 0.42 a |
| Parameters | Genotype 4 (G4) | Genotype 16 (G16) | ||
|---|---|---|---|---|
| 45 Days after | 90 Days after | 45 Days after | 90 Days after | |
| ANpot | 25.10 ± 2.40 Aa | 25.44 ± 2.33 Aa | 25.44 ± 0.88 Aa | 24.49 ± 1.46 Bb |
| LCP | 7.83 ± 2.22 Aa | 4.40 ± 0.54 Ab | 4.67 ± 0.16 Bb | 5.92 ± 1.02 Aa |
| LSP | 1602.97 ± 106.67 Aa | 1068.45 ± 39.24 Bb | 1161.75 ± 60.84 Ab | 1234.20 ± 52.64 Aa |
| Cisat | 809.37 ± 43.20 Ba | 1105.45.28 Aa | 101.20 ± 74.41 Aa | 891.67 ± 16.67 Ab |
| VcMAX | 50.51 ± 3.90 Ba | 66.60 ± 12.02 Aa | 52.84 ± 7.29 Aa | 66.67 ± 10.76 Aa |
| J | 48.20 ± 2.60 Aa | 43.97 ± 1.81 Ba | 43.14 ± 1.52 Ba | 47.50 ± 1.91 Aa |
| TPU | 3.51 ± 0.47 Aa | 3.12 ± 0.26 Aa | 3.27 ± 0.26 Aa | 3.81 ± 0.70 Aa |
| Rd* | 1.55 ± 0.30 Aa | 1.45 ± 0.29 Aa | 1.67 ± 0.33 Aa | 1.33 ± 0.33 Aa |
| Γ* | 3.34 ± 0.54 Aa | 2.11 ± 0.34 Aa | 2.80 ± 1.08 Aa | 2.08 ± 0.19 Aa |
| CO2 time | Violaxanthin (mg kg−1 DW) | |||||
| Genotype 4 | Genotype 16 | |||||
| 400 μm CO2 mol−1 | 800 μm CO2 mol−1 | 1200 μm CO2 mol−1 | 400 μm CO2 mol−1 | 800 μm CO2 mol−1 | 1200 μm CO2 mol−1 | |
| 1 h | 4.1 ± 0.1 Ca | 4.9 ± 0.1 Bb | 6.6 ± 0.3 Ab | 8.3 ± 0.1 Cb* | 9.8 ± 0.1 Bb* | 11.7 ± 0.1 Ab* |
| 2 h | 4.6 ± 0.3 Ca | 5.4 ± 0.4 Ba | 8.9 ± 0.6 Aa | 10.0 ± 0.4 Ca* | 11.7 ± 0.5 Ba* | 20.8 ± 0.6 Aa* |
| CO2 time | Antheraxanthin (mg kg−1 DW) | |||||
| Genotype 4 | Genotype 16 | |||||
| 400 μm CO2 mol−1 | 800 μm CO2 mol−1 | 1200 μm CO2 mol−1 | 400 μm CO2 mol−1 | 800 μm CO2 mol−1 | 1200 μm CO2 mol−1 | |
| 1 h | 2.9 ± 0.2 Cb | 3.4 ± 0.2 Bb | 5.0 ± 0.0 Ab | 20.2 ± 0.5 Ba* | 23.8 ± 0.6 Aa* | 20.3 ± 0.6 Bb* |
| 2 h | 5.2 ± 0.1 Ca | 6.1 ± 0.1 Ba | 8.1 ± 0.1 Aa | 16.8 ± 0.6 Cb* | 19.8 ± 0.7 Bb* | 51.9 ± 1.4 Aa* |
| CO2 time | Zeaxanthin (mg kg−1 DW) | |||||
| Genotype 4 | Genotype 16 | |||||
| 400 μm CO2 mol−1 | 800 μm CO2 mol−1 | 1200 μm CO2 mol−1 | 400 μm CO2 mol−1 | 800 μm CO2 mol−1 | 1200 μm CO2 mol−1 | |
| 1 h | 1.2 ± 0.0 Cb | 1.5 ± 0.0 Bb | 1.9 ± 0.1 Ab | 9.1 ± 0.5 Cb* | 10.6 ± 0.5 Bb* | 16.8 ± 0.2 Ab* |
| 2 h | 7.1 ± 0.2 Ca | 8.3 ± 0.2 Ba | 9.7 ± 0.3 Aa | 14.2 ± 0.5 Ca* | 16.7 ± 0.6 Ba* | 30.8 ± 0.5 Aa* |
| CO2 time | Violaxanthin + Zeaxanthin (mg kg−1 DW) | |||||
| Genotype 4 | Genotype 16 | |||||
| 400 μm CO2 mol−1 | 800 μm CO2 mol−1 | 1200 μm CO2 mol−1 | 400 μm CO2 mol−1 | 800 μm CO2 mol−1 | 1200 μm CO2 mol−1 | |
| 1 h | 5.4 ± 0.1 Cb | 6.3 ± 0.1 Bb | 8.6 ± 0.3 Ab | 17.4 ± 0.5 Cb* | 20.4 ± 0.5 Bb* | 28.5 ± 0.2 Ab* |
| 2 h | 11.7 ± 0.3 Ca | 13.8 ± 0.4 Ba | 18.7 ± 0.7 Aa | 24.2 ± 0.9 Ca* | 28.5 ± 1.0 Ba* | 51.6 ± 0.9 Aa* |
| CO2 time | DEPS | |||||
| Genotype 4 | Genotype 16 | |||||
| 400 μm CO2 mol−1 | 800 μm CO2 mol−1 | 1200 μm CO2 mol−1 | 400 μm CO2 mol−1 | 800 μm CO2 mol−1 | 1200 μm CO2 mol−1 | |
| 1 h | 0.3 ± 0.0 Ab | 0.3 ± 0.0 Ab | 0.3 ± 0.0 Ab | 0.4 ± 0.0 Aa | 0.5 ± 0.0 Aa* | 0.5 ± 0.1 Aa* |
| 2 h | 0.5 ± 0.0 Aa | 0.6 ± 0.0 Aa | 0.5 ± 0.0 Aa | 0.5 ± 0.0 Aa | 0.5 ± 0.0 Aa | 0.5 ± 0.1 Aa |
| CO2 time | (V + A + Z)/(Chl a + Chl b) (mmol mmol−1) | |||||
| Genotype 4 | Genotype 16 | |||||
| 400 μm CO2 mol−1 | 800 μm CO2 mol−1 | 1200 μm CO2 mol−1 | 400 μm CO2 mol−1 | 800 μm CO2 mol−1 | 1200 μm CO2 mol−1 | |
| 1 h | 0.29 ± 0.01 Bb | 0.29 ± 0.01 Bb | 0.49 ± 0.01 Ab | 1.22 ± 0.01 Cb* | 1.41 ± 0.03 Ba* | 2.09 ± 0.07 Ab* |
| 2 h | 0.62 ± 0.01 Ba | 0.69 ± 0.02 Ba | 1.25 ± 0.10 Aa | 1.44 ± 0.02 Ca* | 1.76 ± 0.03 Ba* | 6.05 ± 0.31 Aa* |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pompelli, M.F.; Espitia-Romero, C.A.; de Diós Jaraba-Navas, J.; Rodriguez-Paez, L.A.; Jarma-Orozco, A. Stevia rebaudiana under a CO2 Enrichment Atmosphere: Can CO2 Enrichment Overcome Stomatic, Mesophilic and Biochemical Barriers That Limit Photosynthesis? Sustainability 2022, 14, 14269. https://doi.org/10.3390/su142114269
Pompelli MF, Espitia-Romero CA, de Diós Jaraba-Navas J, Rodriguez-Paez LA, Jarma-Orozco A. Stevia rebaudiana under a CO2 Enrichment Atmosphere: Can CO2 Enrichment Overcome Stomatic, Mesophilic and Biochemical Barriers That Limit Photosynthesis? Sustainability. 2022; 14(21):14269. https://doi.org/10.3390/su142114269
Chicago/Turabian StylePompelli, Marcelo F., Carlos A. Espitia-Romero, Juán de Diós Jaraba-Navas, Luis Alfonso Rodriguez-Paez, and Alfredo Jarma-Orozco. 2022. "Stevia rebaudiana under a CO2 Enrichment Atmosphere: Can CO2 Enrichment Overcome Stomatic, Mesophilic and Biochemical Barriers That Limit Photosynthesis?" Sustainability 14, no. 21: 14269. https://doi.org/10.3390/su142114269
APA StylePompelli, M. F., Espitia-Romero, C. A., de Diós Jaraba-Navas, J., Rodriguez-Paez, L. A., & Jarma-Orozco, A. (2022). Stevia rebaudiana under a CO2 Enrichment Atmosphere: Can CO2 Enrichment Overcome Stomatic, Mesophilic and Biochemical Barriers That Limit Photosynthesis? Sustainability, 14(21), 14269. https://doi.org/10.3390/su142114269








