Microbial Fuel Cells for Electrical Energy: Outlook on Scaling-Up and Application Possibilities towards South African Energy Grid
Abstract
Highlights
- MFC breakdown, electrochemical principles for generating bioelectricity.
- MFC operation mechanism and applications.
- MFCs previous perspectives.
- RSA energy crisis, MFC technology viable solution.
- MFC suggests that, in theory, bioelectricity can be produced from organic content using just chemical energy.
- MFC can be used in plant operations on a daily basis to cut operational costs.
- Existing research shows that MFCs are viable, but scaling-up is very necessary.
- For the current energy crisis in South Africa, MFCs may be a workable sustainable energy source.
Abstract
1. Introduction
2. Microbial Electrochemical Technologies Blueprint
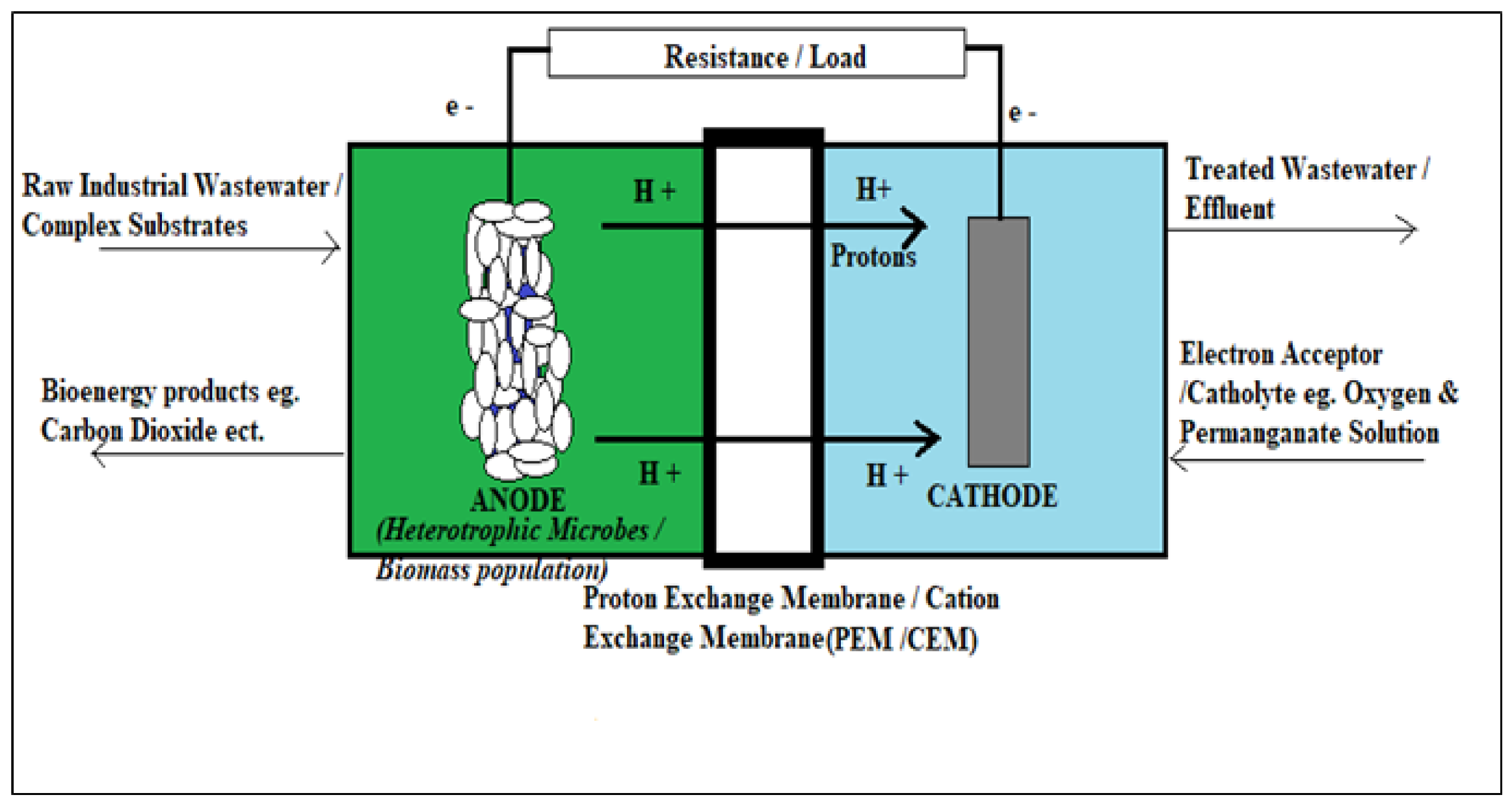
2.1. Microbial Fuel Cell (MFC) Principle and Anatomical Mechanisms
2.2. Analysis of the MFC Compartments
2.2.1. Anode Chamber
2.2.2. Cathode Chamber
2.2.3. Cation Exchange Membrane (CEM)/Proton Exchange Membrane (PET)
2.3. Summary of Challenges and Improvements of Electrode Materials of Construction towards Scaling Up
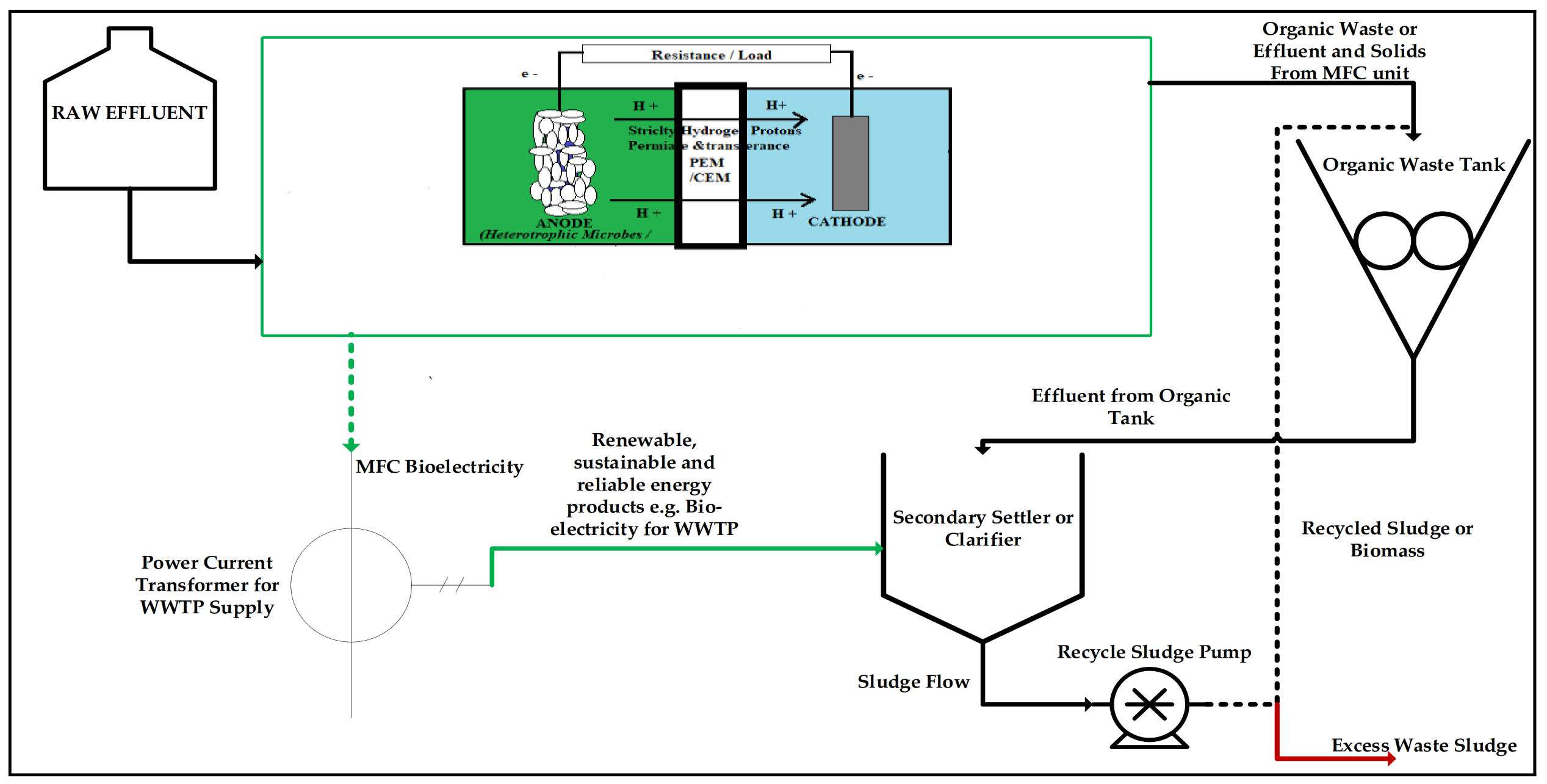
3. Current Applications of Microbial Fuel Cells Commercially
Waste Management Hierarchy That Incorporates MFC Technology as a Viable Solution to Both Waste and Energy Shortage
4. Previous Outlooks on MFC Electricity Generation and Scaling-Up Capacity
5. Current Outlooks on MFC Scaling-Up—Application Challenges
- ○
- Need for the influent to reach the entire anode matrix without disturbance.
- ○
- Protons must diffuse rapidly towards the membrane.
- ○
- Suitable electrical contact must be made between the suspended bacterial masses and the anode.
- ○
- To have functional power, sufficient voltage has to be achieved across the MFC.
- ○
- The installation of an aeration device should primarily be re-examined.
Application Challenges
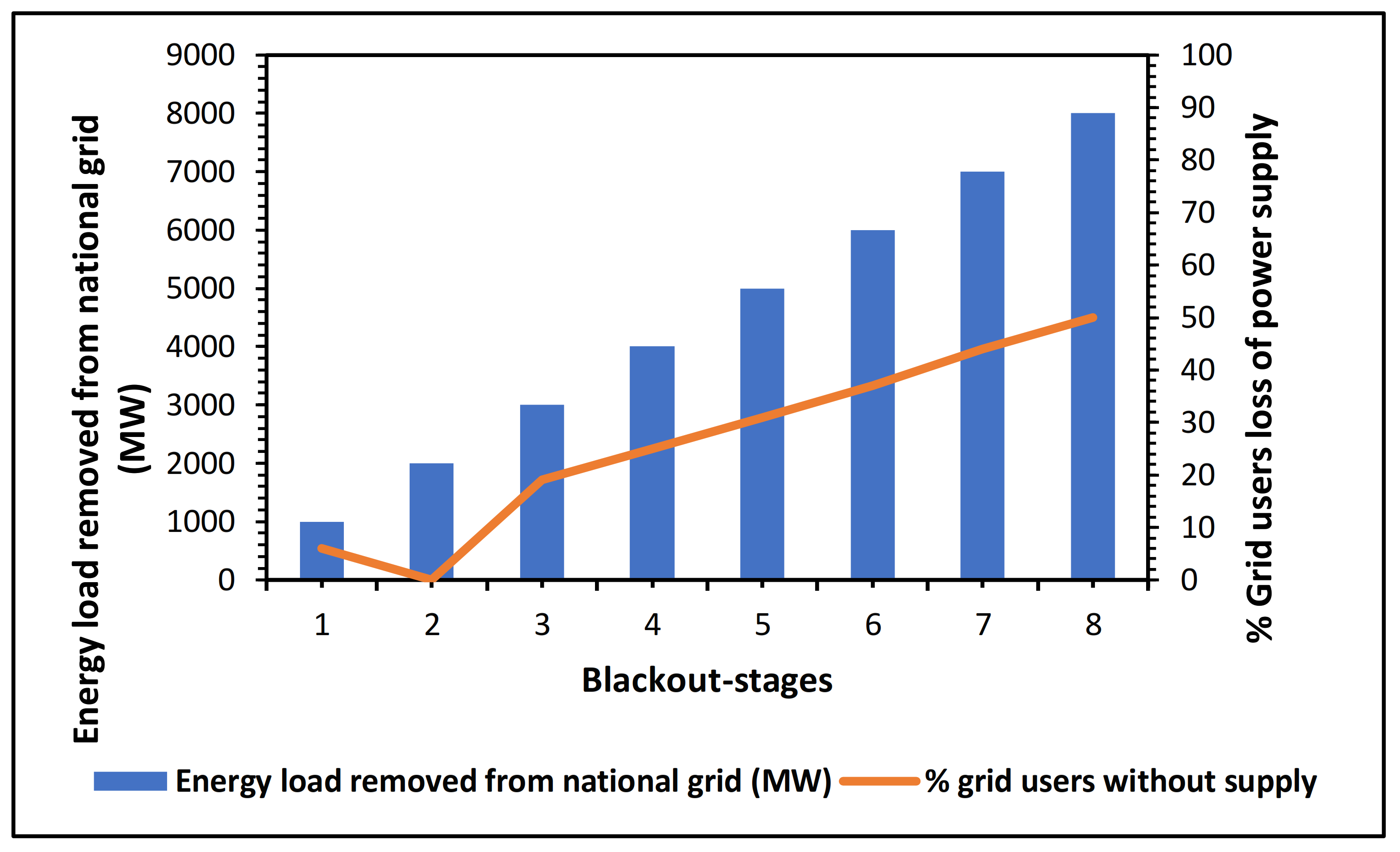
6. Perspectives on the Current South African Energy Crisis: MFC Technology as a Potential Solution
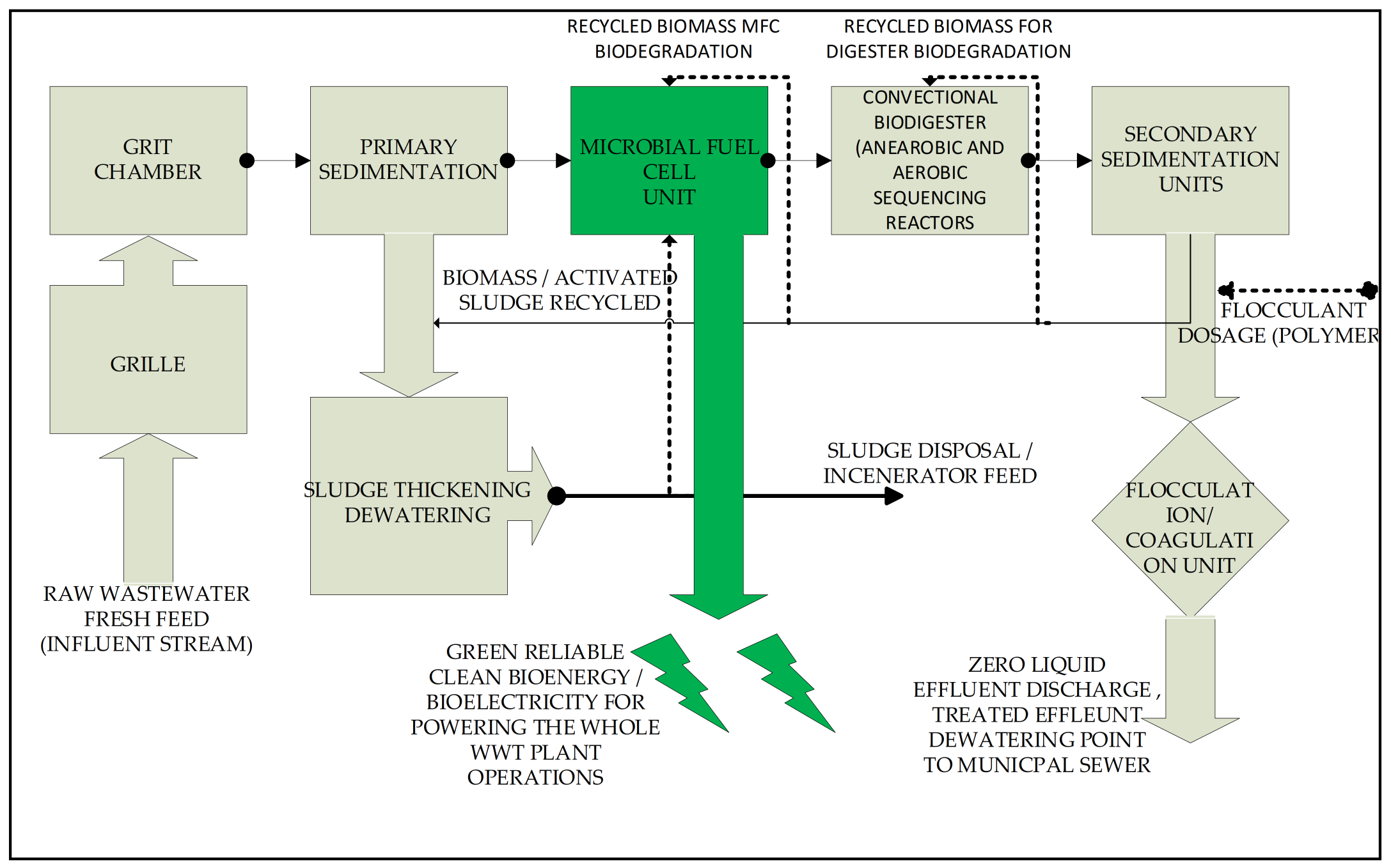
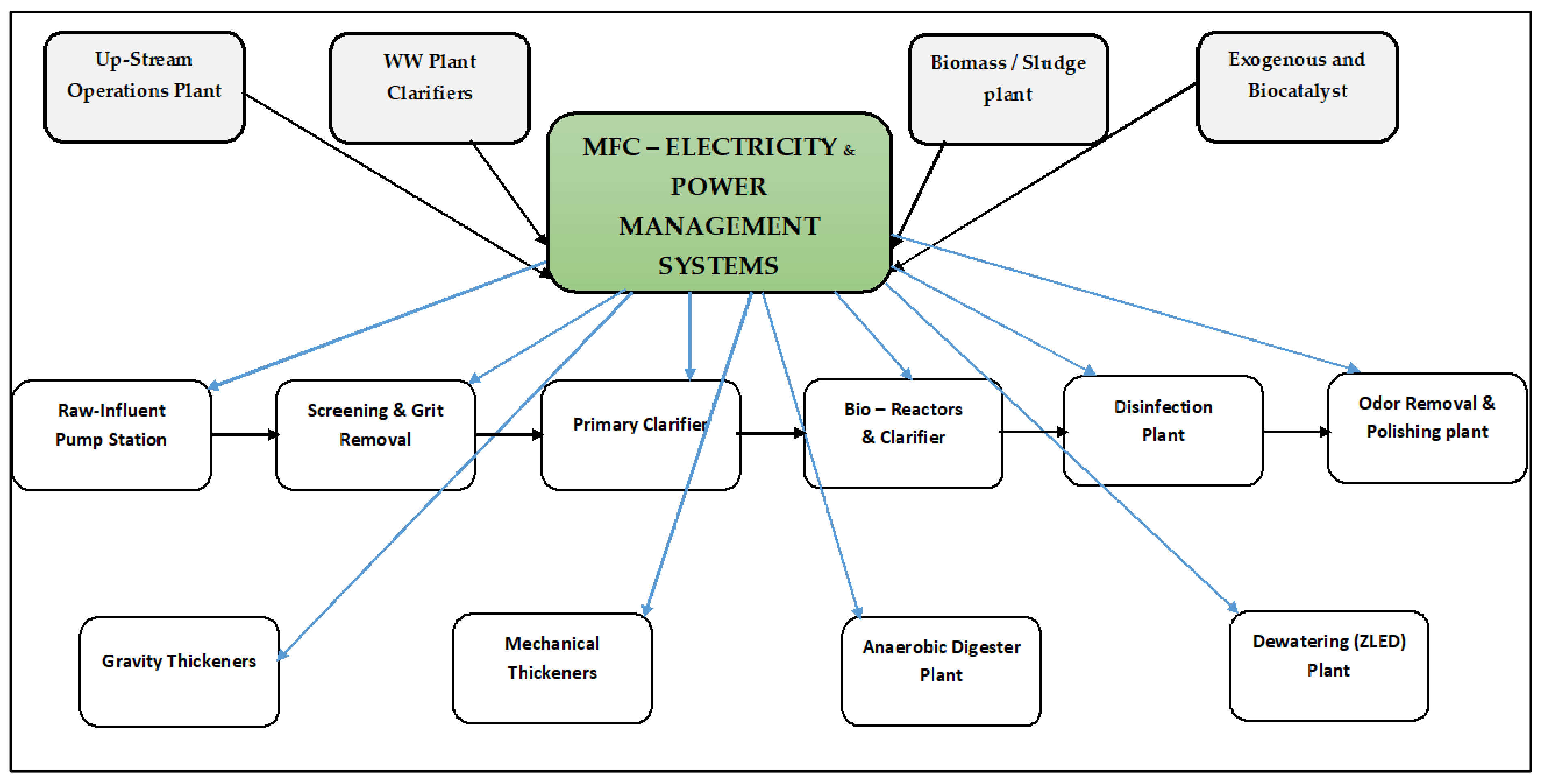
Proposed—Hybrid Technology: MFC Integrated Method for Electricity Scaling–Up Possibilities and Future Work
7. Future Work
8. Conclusions
- ○
- For both the anode and cathode compartments, a good electrode material of construction that is highly conducive, less corrosive, affordable, and easily accessible would be a good choice.
- ○
- Previous authors have vehemently argued that a solid grasp of microbial electrochemistry is essential for scaling up bioenergy and obtaining realistic power densities for MFC commercial applications.
- ○
- Since the MFC technology converts simple waste into bioenergy—specifically electricity—it is more dependable, sustainable, and multifarious, which can substantially benefit the current local South African national grid deficits.
- ○
- Achieving practicable power densities to make MFC technology an economically feasible strategy is one of its present limits, in addition to its expensive effectual process operating units.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AVSS | Active volatile suspended solids |
| ATP | Adenosine triphosphates |
| ABR | Active biomass ration |
| MFC | Microbial fuel cell |
| SMFC | Single-chamber microbial fuel cell |
| DCMFC | Double-chamber microbial fuel cell |
| LMFC | Large microbial fuel cell |
| SMFC | Small microbial fuel cell |
| FPMFC | Flat-plate microbial fuel cell |
| COD | Chemical oxygen demand (mgCOD/L) |
| BOD | Biological oxygen demand (mgBOD/L) |
| TOC | Total organic carbon (mgTOC/L) |
| CE | Coulombic efficiency (%) |
| OLR | Organic loading rates (kgCOD/L.day) |
| EMF | Electromotive force potential |
| MET | Microbial electrochemical technologies |
| MEC | Microbial electrolysis cell |
| WWT | Wastewater treatment plant |
| O2 | Oxygen |
| CO2 | Carbon dioxide |
| H2 | Hydrogen |
| HRT | Hydraulic retention time (hours or days) |
| PEM | Proton exchange membrane |
| CEM | Cation exchange membrane |
| ML-MFC | Membraneless microbial fuel cell |
| H2O | Water |
| NH4-N | Ammonium nitrate |
References
- Rahimnejad, M.; Najafpour, G.D.; Ghoreyshi, A.A.; Jafari, T.; Haghparast, F. Microbial Fuel Cell a Reliable Source for Recovery of Electrical Power from Synthetic Wastewater. Linnaeus Eco-Tech. 2010, 627–635. [Google Scholar] [CrossRef]
- Tamboli, E.; Eswari, J.S. Microbial fuel cell configurations: An overview. In Microbial Electrochemical Technology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 407–435. [Google Scholar]
- Wang, H.; Ren, Z.J. A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol. Adv. 2013, 31, 1796–1807. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Venkatramanan, V.; Prasad, R. Microbial fuel cell: Sustainable green technology for bioelectricity generation and wastewater treatment. In Sustainable Green Technologies for Environmental Management; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Rahimnejad, M.; Ghoreyshi, A.A.; Najafpour, G.; Jafary, T. Power generation from organic substrate in batch and continuous flow microbial fuel cell operations. Appl. Energy 2011, 88, 3999–4004. [Google Scholar] [CrossRef]
- Gudiukaite, R.; Nadda, A.K.; Gricajeva, A.; Shanmugam, S.; Nguyen, D.D.; Lam, S.S. Bioprocesses for the recovery of bioenergy and value-added products from wastewater: A review. J. Environ. Manag. 2021, 300, 113831. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Logan, B.E. Effectiveness of domestic wastewater treatment using microbial fuel cells at ambient and mesophilic temperatures. Bioresour. Technol. 2010, 101, 469–475. [Google Scholar] [CrossRef]
- Rabaey, K.; Rodríguez, J.; Blackall, L.L.; Keller, J.; Gross, P.; Batstone, D.; Verstraete, W.; Nealson, K.H. Microbial ecology meets electrochemistry: Electricity-driven and driving communities. ISME J. 2007, 1, 9–18. [Google Scholar] [CrossRef]
- Du, Z.; Li, H.; Gu, T. A state of the art review on microbial fuel cells: A promising technology for wastewater treatment and bioenergy. Biotechnol. Adv. 2007, 25, 464–482. [Google Scholar] [CrossRef]
- Lay, C.H.; Kokko, M.E.; Puhakka, J.A. Power generation in fed-batch and continuous up-flow microbial fuel cell from synthetic wastewater. Energy 2015, 91, 235–241. [Google Scholar] [CrossRef]
- Azwar, M.Y.; Hussain, M.A.; Abdul-Wahab, A.K. Development of biohydrogen production by photobiological, fermentation and electrochemical processes: A review. Renew. Sustain. Energy Rev. 2014, 31, 158–173. [Google Scholar] [CrossRef]
- Kumar, G.; Saratale, R.G.; Kadier, A.; Sivagurunathan, P.; Zhen, G.; Kim, S.H.; Saratale, G.D. A review on bio-electrochemical systems (BESs) for the syngas and value added biochemicals production. Chemosphere 2017, 177, 84–92. [Google Scholar] [CrossRef]
- Min, B.; Kim, J.R.; Oh, S.E.; Regan, J.M.; Logan, B.E. Electricity generation from swine wastewater using microbial fuel cells. Water Res. 2005, 39, 4961–4968. [Google Scholar] [CrossRef] [PubMed]
- Noori, M.T.; Ganta, A.; Tiwari, B.R. Recent Advances in the Design and Architecture of Bioelectrochemical Systems to Treat Wastewater and to Produce Choice-Based Byproducts. J. Hazard. Toxic Radioact. Waste 2020, 24, 04020023. [Google Scholar] [CrossRef]
- Pant, D.; Singh, A.; Van Bogaert, G.; Gallego, Y.A.; Diels, L.; Vanbroekhoven, K. An introduction to the life cycle assessment (LCA) of bioelectrochemical systems (BES) for sustainable energy and product generation: Relevance and key aspects. Renew. Sustain. Energy Rev. 2011, 15, 1305–1313. [Google Scholar] [CrossRef]
- McCarty, P.L.; Bae, J.; Kim, J. Domestic wastewater treatment as a net energy producer-can this be achieved? Environ. Sci. Technol. 2011, 45, 7100–7106. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, N.; Islam, N.; Ahmed, S. Progress in microbial fuel cells for sustainable management of industrial effluents. Process Biochem. 2021, 106, 20–41. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Islam, N.; Parisa, T.A.; Rafa, N.; Bokhari, A.; Klemeš, J.J.; Mahlia, T.M.I. Insights into the development of microbial fuel cells for generating biohydrogen, bioelectricity, and treating wastewater. Energy 2022, 254, 124163. [Google Scholar] [CrossRef]
- Wang, H.; Ren, Z.J. Bioelectrochemical metal recovery from wastewater: A review. Water Res. 2014, 66, 219–232. [Google Scholar] [CrossRef]
- Ogugbue, C.J.; Ebode, E.E.; Leera, S. Electricity generation from swine wastewater using microbial fuel cell. J. Ecol Eng. 2015, 16, 26–33. [Google Scholar] [CrossRef]
- Wang, H.; Park, J.; Do, R.Z.J. Practical energy harvesting for microbial fuel cells: A review. Environ. Sci. Technol. 2015, 49, 3267–3277. [Google Scholar] [CrossRef]
- Wang, H.; Luo, H.; Fallgren, P.H.; Jin, S.; Ren, Z.J. Bioelectrochemical system platform for sustainable environmental remediation and energy generation. Biotechnol. Adv. 2015, 33, 317–334. [Google Scholar] [CrossRef]
- Ren, L.; Ahn, Y.; Logan, B.E. A two-stage microbial fuel cell and anaerobic fluidized bed membrane bioreactor (MFC-AFMBR) system for effective domestic wastewater treatment. Environ. Sci. Technol. 2014, 48, 4199–4206. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Hu, H.; Liu, H. Enhanced Coulombic efficiency and power density of air-cathode microbial fuel cells with an improved cell configuration. J. Power Sources 2007, 171, 348–354. [Google Scholar] [CrossRef]
- Sharif, H.M.A.; Farooq, M.; Hussain, I.; Ali, M.; Mujtaba, M.A.; Sultan, M.; Yang, B. Recent innovations for scaling up microbial fuel cell systems: Significance of physicochemical factors for electrodes and membranes materials. J. Taiwan Inst. Chem. Eng. 2021, 129, 207–226. [Google Scholar] [CrossRef]
- Zhuang, L.; Zheng, Y.; Zhou, S.; Yuan, Y.; Yuan, H.; Chen, Y. Scalable microbial fuel cell (MFC) stack for continuous real wastewater treatment. Bioresour. Technol. 2012, 106, 82–88. [Google Scholar] [CrossRef]
- Cheng, S.; Logan, B.E. Ammonia treatment of carbon cloth anodes to enhance power generation of microbial fuel cells. Electrochem. Commun. 2007, 9, 492–496. [Google Scholar] [CrossRef]
- Wang, C.-T.; Huang, R.-Y.; Lee, Y.-C.; Zhang, C.-D. Electrode Material of Carbon Nanotube/Polyaniline Carbon Paper Applied in Microbial Fuel Cells. J. Clean Energy Technol. 2013, 1, 206–210. [Google Scholar] [CrossRef]
- Zhou, M.; Chi, M.; Luo, J.; He, H.; Jin, T. An overview of electrode materials in microbial fuel cells. J. Power Sources 2011, 196, 4427–4435. [Google Scholar] [CrossRef]
- Pattanayak, P.; Pramanik, N.; Kumar, P.; Kundu, P.P. Fabrication of cost-effective non-noble metal supported on conducting polymer composite such as copper/polypyrrole graphene oxide (Cu2O/PPy–GO) as an anode catalyst for methanol oxidation in DMFC. Int. J. Hydrog. Energy 2018, 43, 11505–11519. [Google Scholar] [CrossRef]
- Zhang, F.; Brastad, K.S.; He, Z. Integrating forward osmosis into microbial fuel cells for wastewater treatment, water extraction and bioelectricity generation. Environ. Sci. Technol. 2011, 45, 6690–6696. [Google Scholar] [CrossRef]
- Cabrera, J.; Irfan, M.; Dai, Y.; Zhang, P.; Zong, Y.; Liu, X. Bioelectrochemical system as an innovative technology for treatment of produced water from oil and gas industry: A review. Chemosphere 2021, 285, 131428. [Google Scholar] [CrossRef]
- Kumar, G.; Bakonyi, P.; Zhen, G.; Sivagurunathan, P.; Koók, L.; Kim, S.H.; Tóth, G.; Nemestóthy, N.; Bélafi-Bakó, K. Microbial electrochemical systems for sustainable biohydrogen production: Surveying the experiences from a start-up viewpoint. Renew. Sustain. Energy Rev. 2017, 70, 589–597. [Google Scholar] [CrossRef]
- Mathuriya, A.S. Eco-affectionate face of microbial fuel cells. Crit. Rev. Environ. Sci. Technol. 2014, 44, 97–153. [Google Scholar] [CrossRef]
- Lai, M.F.; Lou, C.W.; Lin, J.H. Improve 3D electrode materials performance on electricity generation from livestock wastewater in microbial fuel cell. Int. J. Hydrog. Energy 2018, 43, 11520–11529. [Google Scholar] [CrossRef]
- Kalathil, S.; Lee, J.; Cho, M.H. Granular activated carbon based microbial fuel cell for simultaneous decolorization of real dye wastewater and electricity generation. N. Biotechnol. 2011, 29, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Chang, I.S.; Gadd, G.M. Challenges in microbial fuel cell development and operation. Appl. Microbiol. Biotechnol. 2007, 76, 485–494. [Google Scholar] [CrossRef]
- Chung, K.; Okabe, S. Continuous power generation and microbial community structure of the anode biofilms in a three-stage microbial fuel cell system. Appl. Microbiol. Biotechnol. 2009, 83, 965–977. [Google Scholar] [CrossRef]
- Scott, K.; Rimbu, G.A.; Katuri, K.P.; Prasad, K.K.; Head, I.M. Application of modified carbon anodes in microbial fuel cells. Process Saf. Environ. Prot. 2007, 85, 481–488. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, H.; Logan, B.E. Increased power generation in a continuous flow MFC with advective flow through the porous anode and reduced electrode spacing. Environ. Sci. Technol. 2006, 40, 2426–2432. [Google Scholar] [CrossRef]
- Logan, B.; Cheng, S.; Watson, V.; Estadt, G. Graphite fiber brush anodes for increased power production in air-cathode microbial fuel cells. Environ. Sci. Technol. 2007, 41, 3341–3346. [Google Scholar] [CrossRef]
- Huggins, T.M.; Pietron, J.J.; Wang, H.; Ren, Z.J.; Biffinger, J.C. Graphitic biochar as a cathode electrocatalyst support for microbial fuel cells. Bioresour. Technol. 2015, 195, 147–153. [Google Scholar] [CrossRef]
- Ter Heijne, A.; Liu, F.; Van Rijnsoever, L.S.; Saakes, M.; Hamelers, H.V.M.; Buisman, C.J.N. Performance of a scaled-up Microbial Fuel Cell with iron reduction as the cathode reaction. J. Power Sources 2011, 196, 7572–7577. [Google Scholar] [CrossRef]
- HaoYu, E.; Cheng, S.; Scott, K.; Logan, B. Microbial fuel cell performance with non-Pt cathode catalysts. J. Power Sources 2007, 171, 275–281. [Google Scholar] [CrossRef]
- Salahuddin, M.; Uddin, M.N.; Hwang, G.; Asmatulu, R. Superhydrophobic PAN nanofibers for gas diffusion layers of proton exchange membrane fuel cells for cathodic water management. Int. J. Hydrog. Energy 2018, 43, 11530–11538. [Google Scholar] [CrossRef]
- Gu, H.Y.; Zhang, X.W.; Li, Z.J.; Lei, L.C. Studies on treatment of chlorophenol-containing wastewater by microbial fuel cell. Chin. Sci. Bull. 2007, 52, 3448–3451. [Google Scholar] [CrossRef]
- Xiao, B.; Yang, F.; Liu, J. Enhancing simultaneous electricity production and reduction of sewage sludge in two-chamber MFC by aerobic sludge digestion and sludge pretreatments. J. Hazard. Mater. 2011, 189, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.K.; Dec, J.; Bruns, M.A.; Logan, B.E. Removal of odors from swine wastewater by using microbial fuel cells. Appl. Environ. Microbiol. 2008, 74, 2540–2543. [Google Scholar]
- Anwar, A.; Siddique, M.; Eyup Dogan Sharif, A. The moderating role of renewable and non-renewable energy in environment-income nexus for ASEAN countries: Evidence from Method of Moments Quantile Regression. Renew. Energy 2021, 164, 956–967. [Google Scholar] [CrossRef]
- Muchtar, A.; Tambunan, A.H.; Machfud, M. Preliminary Analysis of Single-Flash Geothermal Power Plant by Using Exergy Method: A Case Study from Ulubelu Geothermal Power Plant in Indonesia Bioethanol from Brown Seaweed View project Production System Design of Banana based Energy Bar Industry View Project. 2018. Available online: https://www.researchgate.net/publication/335175433 (accessed on 21 January 2022).
- Huggins, T.; Wang, H.; Kearns, J.; Jenkins, P.; Ren, Z.J. Biochar as a sustainable electrode material for electricity production in microbial fuel cells. Bioresour. Technol. 2014, 157, 114–119. [Google Scholar] [CrossRef]
- Wang, B.; Han, J.I. A single chamber stackable microbial fuel cell with air cathode. Biotechnol. Lett. 2009, 31, 387–393. [Google Scholar] [CrossRef]
- Kaku, N.; Yonezawa, N.; Kodama, Y.; Watanabe, K. Plant/microbe cooperation for electricity generation in a rice paddy field. Appl. Microbiol. Biotechnol. 2008, 79, 43–49. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X.; Logan, B.E.; Lee, H. Brewery wastewater treatment using air-cathode microbial fuel cells. Appl. Microbiol. Biotechnol. 2008, 78, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Cheng, S.; Call, D.; Logan, B.E. Tubular membrane cathodes for scalable power generation in microbial fuel cells. Environ. Sci. Technol. 2007, 41, 3347–3353. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Regan, J.M. Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol. 2006, 14, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Clauwaert, P.; Aelterman, P.; Verstraete, W. Tubular microbial fuel cells for efficient electricity generation. Environ. Sci. Technol. 2005, 39, 8077–8082. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, H.; Zhou, S.; Shi, C.; Wang, C.; Ni, J. A novel UASB-MFC-BAF integrated system for high strength molasses wastewater treatment and bioelectricity generation. Bioresour. Technol. 2009, 100, 5687–5693. [Google Scholar] [CrossRef]
- Sivasankaran, A.; Sangeetha, D.; Ahn, Y.H. Nanocomposite membranes based on sulfonated polystyrene ethylene butylene polystyrene (SSEBS) and sulfonated SiO2 for microbial fuel cell application. Chem. Eng. J. 2016, 289, 442–451. [Google Scholar] [CrossRef]
- Sil, A.; Ijeri, V.S.; Srivastava, A.K. Coated-wire iron(III) ion-selective electrode based on iron complex of 1,4,8,11-tetraazacyclotetradecane. Sens. Actuators B Chem. 2005, 106, 648–653. [Google Scholar]
- Rossi, R.; Wang, X.; Logan, B.E. High performance flow through microbial fuel cells with anion exchange membrane. J. Power Sources 2020, 475, 228633. [Google Scholar] [CrossRef]
- Nandy, A.; Kumar, V.; Kundu, P.P. Effect of electric impulse for improved energy generation in mediatorless dual chamber microbial fuel cell through electroevolution of Escherichia coli. Biosens. Bioelectron. 2016, 79, 796–801. [Google Scholar] [CrossRef]
- Pham, T.H.; Rabaey, K.; Aelterman, P.; Clauwaert, P.; De Schamphelaire, L.; Boon, N.; Verstraete, W. Microbial fuel cells in relation to conventional anaerobic digestion technology. Eng. Life Sci. 2006, 6, 285–292. [Google Scholar] [CrossRef]
- Suzuki, K.; Tanaka, Y.; Osada, T.; Waki, M. Removal of phosphate, magnesium and calcium from swine wastewater through crystallization enhanced by aeration. Water Res. 2002, 36, 2991–2998. [Google Scholar] [CrossRef]
- Bakare, B.F.; Shabangu, K.; Chetty, M. Brewery wastewater treatment using laboratory scale aerobic sequencing batch reactor. S. Afr. J Chem Eng. 2017, 24, 128–134. [Google Scholar] [CrossRef]
- Dosta, J.; Rovira, J.; Galí, A.; Macé, S.; Mata-Álvarez, J. Integration of a Coagulation/Flocculation step in a biological sequencing batch reactor for COD and nitrogen removal of supernatant of anaerobically digested piggery wastewater. Bioresour. Technol. 2008, 99, 5722–5730. [Google Scholar] [CrossRef] [PubMed]
- Ringsmuth, A.K.; Landsberg, M.J.; Hankamer, B. Can photosynthesis enable a global transition from fossil fuels to solar fuels, to mitigate climate change and fuel-supply limitations? Renew. Sustain. Energy Rev. 2016, 62, 134–163. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, H.; Logan, B.E. Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem. Commun. 2006, 8, 489–494. [Google Scholar] [CrossRef]
- Logan, B.E.; Rabaey, K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef]
- Allami, S.; Hasan, B.; Redah, M.; Hamody, H.; Abd Ali, Z.D. Using Low Cost Membrane in Dual-Chamber Microbial Fuel Cells (MFCs) for Petroleum Refinery Wastewater Treatment. J. Phys. Conf. Ser. 2018, 1032, 012061. [Google Scholar] [CrossRef]
- Jafary, T.; Ghoreyshi, A.A.; Najafpour, G.D.; Fatemi, S.; Rahimnejad, M. Investigation on performance of microbial fuel cells based on carbon sources and kinetic models. Int. J. Energy Res. 2013, 37, 1539–1549. [Google Scholar] [CrossRef]
- Tessema, T.D.; Yemata, T.A. Experimental dataset on the effect of electron acceptors in energy generation from brewery wastewater via a microbial fuel cell. Data Brief 2021, 37, 107272. [Google Scholar] [CrossRef]
- Rozendal, R.A.; Hamelers, H.V.M.; Rabaey, K.; Keller, J.; Buisman, C.J.N. Towards practical implementation of bioelectrochemical wastewater treatment. Trends Biotechnol. 2008, 26, 450–459. [Google Scholar] [CrossRef]
- Lu, N.; Zhou, S.G.; Zhuang, L.; Zhang, J.T.; Ni, J.R. Electricity generation from starch processing wastewater using microbial fuel cell technology. Biochem. Eng. J. 2009, 43, 246–251. [Google Scholar] [CrossRef]
- Puig, S.; Serra, M.; Coma, M.; Balaguer, M.D.; Colprim, J. Simultaneous domestic wastewater treatment and renewable energy production using microbial fuel cells (MFCs). Water Sci. Technol. 2011, 64, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Logan, B.E. Continuous Electricity Generation from Domestic Wastewater and Organic Substrates in a Flat Plate Microbial Fuel Cell. Environ. Sci. Technol. 2004, 38, 5809–5814. [Google Scholar] [CrossRef]
- Dong, J.; Wu, Y.; Wang, C.; Lu, H.; Li, Y. Three-dimensional electrodes enhance electricity generation and nitrogen removal of microbial fuel cells. Bioprocess Biosyst. Eng. 2020, 43, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- Butti, S.K.; Velvizhi, G.; Sulonen, M.L.K.; Haavisto, J.M.; Oguz Koroglu, E.; Yusuf Cetinkaya, A.; Singh, S.; Arya, D.; Modestra, J.A.; Krishna, K.V.; et al. Microbial electrochemical technologies with the perspective of harnessing bioenergy: Maneuvering towards upscaling. Renew. Sustain. Energy Rev. 2016, 53, 462–476. [Google Scholar] [CrossRef]
- Venkatramanan, V.; Shah, S.; Prasad, R. A Critical Review on Microbial Fuel Cells Technology: Perspectives on Wastewater Treatment. Open Biotechnol. J. 2021, 15, 131–141. [Google Scholar] [CrossRef]
- Gul, H.; Raza, W.; Lee, J.; Azam, M.; Ashraf, M.; Kim, K.H. Progress in microbial fuel cell technology for wastewater treatment and energy harvesting. Chemosphere 2021, 281, 130828. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Qi, R.; Tandoi, V.; Yang, M. Sludge Bulking Impact on Relevant Bacterial Populations in a Full-Scale Municipal Wastewater Treatment Plant. Process Biochem. 2014, 49, 2258–2265. [Google Scholar] [CrossRef]
- Filamentous and Non-Filamentous Bulking of Activated Sludge Encountered Under Nutrients Limitation or Deficiency Conditions|Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/S1385894714008067?token=4D0DE471E017CBA9AFFE44200063C1294125B65E92E7653D829FB6F2D253FF651E32660F281D947ED9A6B45D6ED437F0&originRegion=eu-west-1&originCreation=20220121175337 (accessed on 21 January 2022).
- Sato, C.; Paucar, N.E.; Chiu, S.; Mahmud, M.Z.I.M.; Dudgeon, J. Single-Chamber Microbial Fuel Cell with Multiple Plates of Bamboo Charcoal Anode: Performance Evaluation. Processes 2021, 9, 2194. [Google Scholar] [CrossRef]
- Yang, Z.; Tsapekos, P.; Zhang, Y.; Zhang, Y.; Angelidaki, I.; Wang, W. Bio-electrochemically extracted nitrogen from residual resources for microbial protein production. Bioresour. Technol. 2021, 337, 125353. [Google Scholar] [CrossRef]
- Complete Nitrogen Removal by Simultaneous Nitrification and Denitrification in Flat-Panel Air-Cathode Microbial Fuel Cells Treating Domestic Wastewater|Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/S1385894717301626?token=5F85DD284D224B87D998AB8F229CC975D821C421C01C566B48F471370C637BA9844E70E2926BD9580FD01518DD7E4350&originRegion=eu-west-1&originCreation=20220121162726 (accessed on 21 January 2022).
- Negassa, L.W.; Mohiuddin, M.; Tiruye, G.A. Treatment of brewery industrial wastewater and generation of sustainable bioelectricity by microbial fuel cell inoculated with locally isolated microorganisms. J. Water Process. Eng. 2021, 41, 102018. [Google Scholar] [CrossRef]
- Zhao, F.; Slade, R.C.T.; Varcoe, J.R. Techniques for The Study and Development of Microbial Fuel Cells: An Electrochemical Perspective. Chem. Soc. Rev. 2009, 38, 1926–1939. [Google Scholar] [CrossRef] [PubMed]
- Vinayak, V.; Khan, M.J.; Varjani, S.; Saratale, G.D.; Saratale, R.G.; Bhatia, S.K. Microbial fuel cells for remediation of environmental pollutants and value addition: Special focus on coupling diatom microbial fuel cells with photocatalytic and photoelectric fuel cells. J. Biotechnol. 2021, 338, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Apollon, W.; Luna-Maldonado, A.I.; Kamaraj, S.K.; Vidales-Contreras, J.A.; Rodríguez-Fuentes, H.; Gómez-Leyva, J.F.; Aranda-Ruíz, J. Progress and recent trends in photosynthetic assisted microbial fuel cells: A review. Biomass Bioenergy 2021, 148, 106028. [Google Scholar] [CrossRef]
- Munoz-Cupa, C.; Hu, Y.; Xu, C.; Bassi, A. An overview of microbial fuel cell usage in wastewater treatment, resource recovery and energy production. Sci. Total Environ. 2021, 754, 142429. [Google Scholar] [CrossRef]
- Bensaida, K.; Maamoun, I.; Eljamal, R.; Falyouna, O.; Sugihara, Y.; Eljamal, O. New insight for electricity amplification in microbial fuel cells (MFCs) applying magnesium hydroxide coated iron nanoparticles. Energy Convers. Manag. 2021, 249, 114877. [Google Scholar] [CrossRef]
- Sumisha, A.; Haribabu, K. Energy Generation and Iron Removal in Batch and Continuous Single-Chamber Microbial Fuel Cells. Chem. Eng. Technol. 2021, 44, 258–264. [Google Scholar] [CrossRef]
- Jung, R.K.; Zuo, Y.; Regan, J.M.; Logan, B.E. Analysis of ammonia loss mechanisms in microbial fuel cells treating animal wastewater. Biotechnol. Bioeng. 2008, 99, 1120–1127. [Google Scholar]
- Ye, Y.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Varjani, S.; Liu, Q.; Bui, X.T.; Hoang, N.B. Bio-membrane integrated systems for nitrogen recovery from wastewater in circular bioeconomy. Chemosphere 2022, 289, 133175. [Google Scholar] [CrossRef]
- Kong, W.; Guo, Q.; Wang, X.; Yue, X. Electricity generation from wastewater using an anaerobic fluidized bed microbial fuel cell. Ind. Eng. Chem. Res. 2011, 50, 12225–12232. [Google Scholar] [CrossRef]
- Khandaker, S.; Das, S.; Hossain, M.T.; Islam, A.; Miah, M.R.; Awual, M.R. Sustainable approach for wastewater treatment using microbial fuel cells and green energy generation–A comprehensive review. J. Mol. Liq. 2021, 344, 117795. [Google Scholar] [CrossRef]
- An, J.; Kim, D.; Chun, Y.; Lee, S.J.; Ng, H.Y.; Chang, I.S. Floating-type microbial fuel cell (FT-MFC) for treating organic-contaminated water. Environ. Sci. Technol. 2009, 43, 1642–1647. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.; Ieropoulos, I.; Greenman, J.; Cristiani, P.; Vadas, T.; Mackay, A.; Li, B. Power generation and contaminant removal in single chamber microbial fuel cells (SCMFCs) treating human urine. Int. J. Hydrog. Energy 2013, 38, 11543–11551. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.J.; Lee, H. Electricity production from beer brewery wastewater using single chamber microbial fuel cell. Water Sci. Technol. 2008, 57, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Ting, M.B.; Byrne, R. Eskom and the rise of renewables: Regime-resistance, crisis and the strategy of incumbency in South Africa’s electricity system. Energy Res. Soc. Sci. 2020, 60, 101333. [Google Scholar] [CrossRef]
- Mbuli, N.; Mendu, B.; Seitshiro, M.; Pretorius, J.H.C. Statistical analysis of forced outage durations of Eskom subtransmission transformers. Energy Rep. 2020, 6, 321–329. [Google Scholar] [CrossRef]
- Valet, R. Factors Influencing the Societal Acceptance of Renewable Energies Related Papers Background Paper on Technology Roadmaps for UNFCCC. Available online: www.createacceptance.net (accessed on 25 August 2022).
- Khimulu, R.M. Assesment of The Nexus Of Energy, Climate Change, Urbanization And Industrialization For Africa’s Transformation. 2017. Available online: http://repository.pauwes-cop.net/handle/1/133 (accessed on 25 August 2022).
- Eskom: Electricity and Technopolitics in South Africa-Sylvy Jaglin, Alain Dubresson-Google Books. Available online: https://books.google.co.za/books?hl=en&lr=&id=bfZyDwAAQBAJ&oi=fnd&pg=PP5&dq=Eskom+captured&ots=VicQqqSAQu&sig=EJHDUCUWSNUY-oohlOo307yDOK8&redir_esc=y#v=onepage&q=Eskomcaptured&f=false (accessed on 25 August 2022).
- South Africa Comes to Standstill with Eskom’s Load Shedding-ProQuest. Available online: https://www.proquest.com/openview/88be753c6894745beeaa1cea8e53d700/1?pq-origsite=gscholar&cbl=39 (accessed on 25 August 2022).
- Du Venage, G. South Africa Comes to Standstill with Eskom’s Load Shedding. 2020. Available online: www.e-mj.com (accessed on 25 August 2022).
- Inglesi, R. Aggregate electricity demand in South Africa: Conditional forecasts to 2030. Appl. Energy 2010, 87, 197–204. [Google Scholar] [CrossRef]


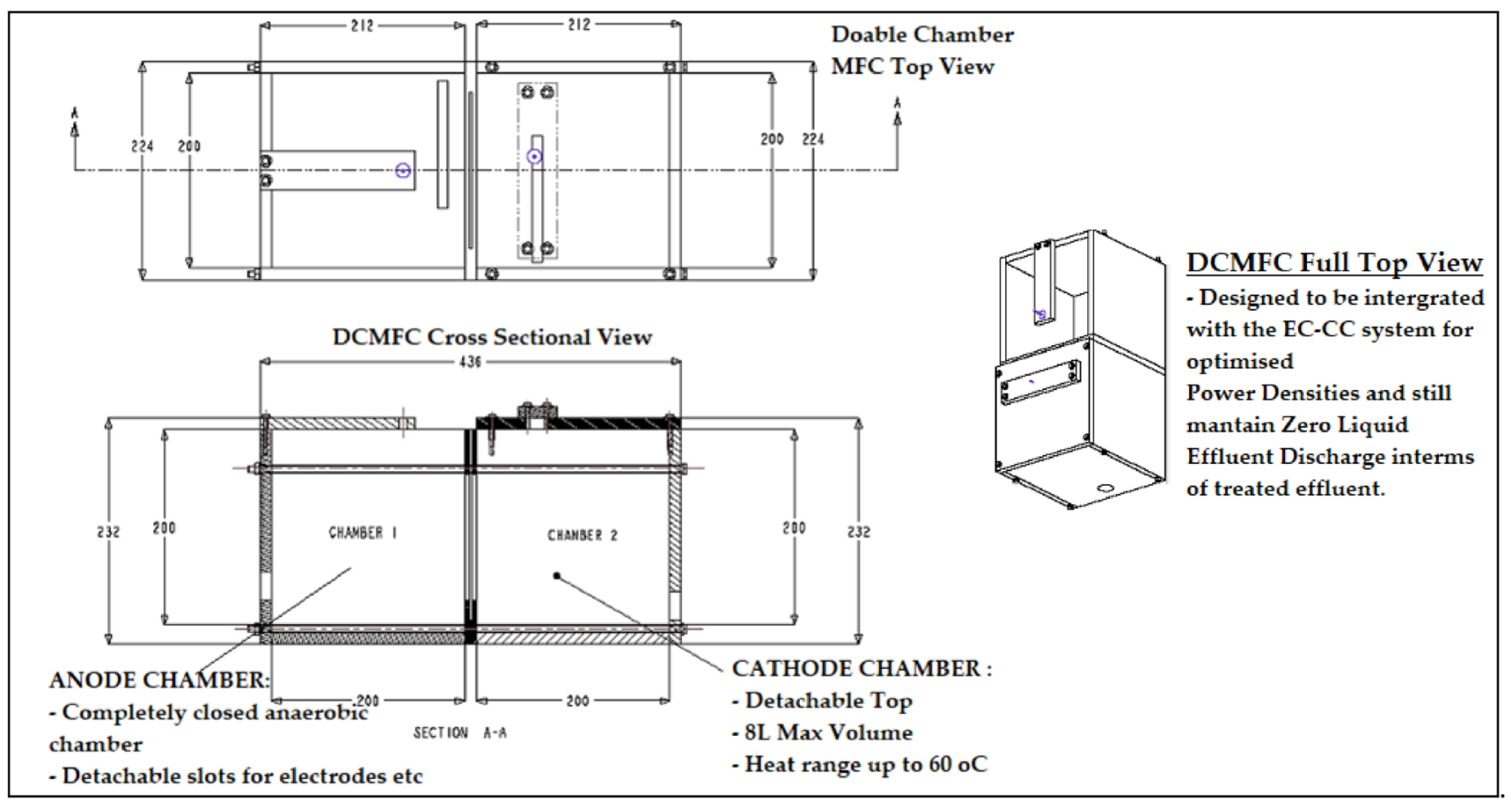
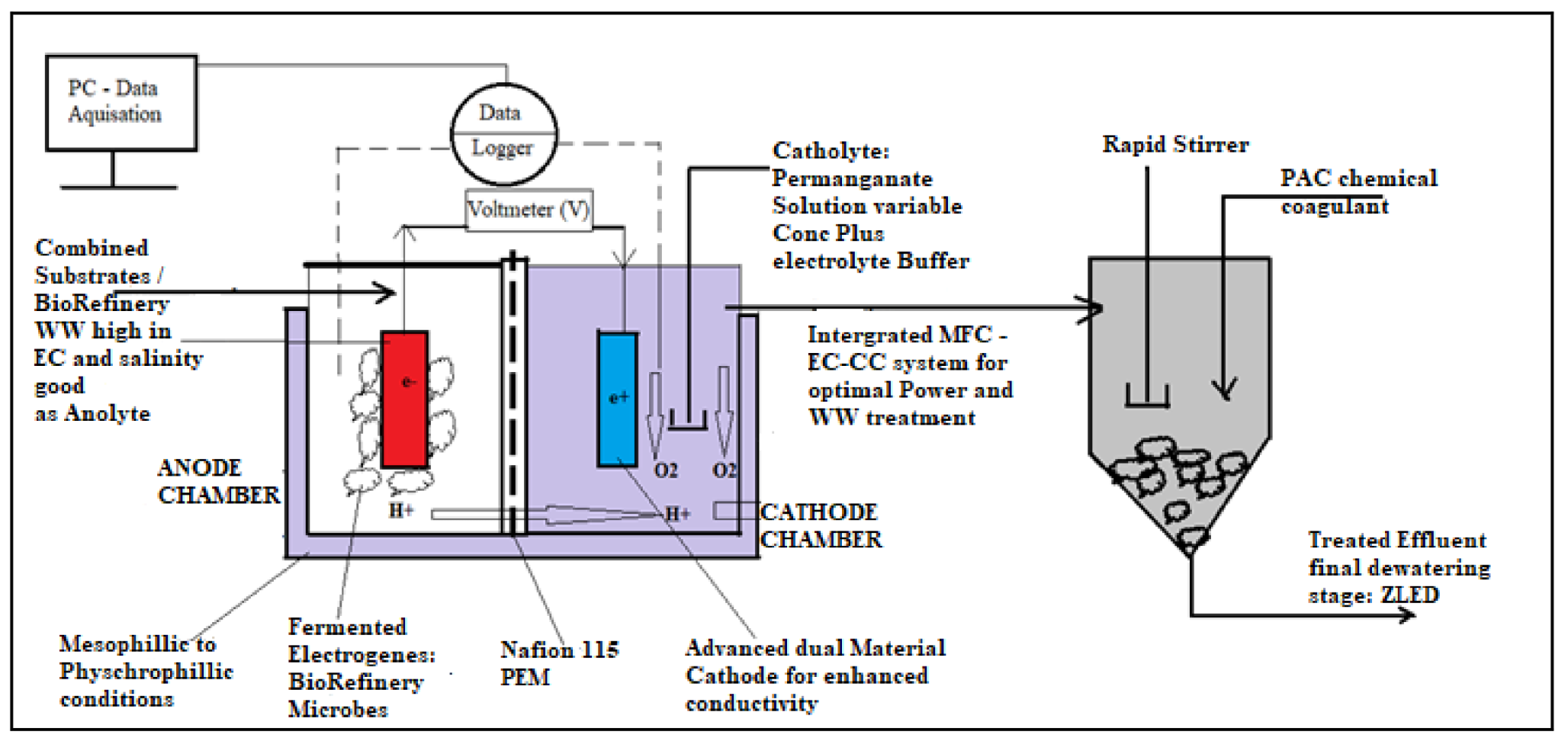
| Component | Design Parameters and Material | Most Common Electrode/Catalyst | Suppliers and Costs | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|---|
| ANODE- ELECTRODE | Conductive Material. Bio-compatible. Chemically stable metal, non-corrosive; gold, silver, nickel, s-steel (304), graphite, and polycrystalline. | Copper, aluminum, zinc, carbon, carbon paper, carbon brushes, graphene electrode, graphite: rods, plates, granules, fiber, etc. | E-TEK and Electro-Synthesis United Sates of America (USA) GEE Graphite Ltd., (USA). Dewsbury; United Kingdom, (UK). Morgan Grinbergen; (Belgium). Alfer–Aeser (Germany). Generally in-expensive simple materials, e.g., graphite. | Porous. Easy to handle. Large surface area. Allows efficient flow of electrons through the anode. Permits minimal oxygen into the chamber. | Poor biofilm growth. Poor electron flow. Recurrent Biofouling on the film. | [32,33,34,35,36,37,38] |
| CATHODE- ELECTRODE | Bio-compatible. Chemically stable metal/alloy, non-corrosive. | Copper, aluminum, zinc, carbon, carbon paper, carbon brushes, graphene electrode, graphite: rods, plates, granules, fiber, etc. | Low-cost for O2 but expensive for catalyst catholyte, e.g., Pt, etc. | Lower potential. Greatly impacts power generation in MFC. | Insufficient re-oxidation. Expensive chemical acceptors. Catalyst catholyte activity drops over time. Problems with binder for catalyst. | [5,39,40,41,42,43,44] |
| PEM/CEM | Ultrex CMI-7000 | Nafion 112/115/117 | DuPont USA Aldrich and Ion Power Highly Expensive | Permeability to Protons | External Biofouling Internal Biofouling Costly Upscaling and practical application Problems | [26,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47] |
| MFC Catalyst | Ferricyanide, Pt catalyst | Pt catalyst. Ferricyanide. Permanganate Solution. | Synthesis in local chemical suppliers. | Boost the conductivity of either anolyte or catholyte. Acts as a viable electrolyte. | Expensive for commercial-scale applications. Complex disposal procedures due to being environmentally unfriendly. | [44,45,46,47,48,49,50,51] |
| Parameters | Power Densities, COD Removals | Advantages | Disadvantages | References |
|---|---|---|---|---|
| One-month acclimation. HRT: 2.0 h. 0.22 mL/min flow rate. Influent COD: 2463 mg/L. FPMFC. | 58% mg COD/L 560 mW/m2 | Continuous electricity generation. Continuous organic WWT. 10% higher power density than other MFCs. | Need for up-scaling of power for application. | [38,39,40] |
| Double electrode chambers. Permeable PEM to O2. FPMFC. | 500 mW/m2 | Increased power potentials due to packed electrodes. | Poor MFC performance on WWT and power generation due to permeable PEM. | [31,32,33,34,35,36,58] |
| Air cathode. Fed-batch/continuous. Temp: Mesophilic: (23 + 1 and 30 + 1). OLR: 54 gCOD/L.d SCMFC. | 422 mW/m2 25% mg COD/L | Series configuration is operational and convenient for temperature phases. | Energy dependent on operating conditions (temp, OLR, HRT, etc.). | [7,19,59] |
| Lactate/glucose substrates. Eight graphite electrode setup. Air cathode. Continuous flow. HRT: 3–33 h. 50–220 mg COD/L influent. SCMFC. | 26 mW/m2 80% mg COD/L | Bioreactor seems a good technique for both electricity generation and WWT. | . Cathode-controlled power generation. Loss of organic matter without power generation. | [19,30,31,32,33,34,35,36,37,38,39,40,41] |
| Starch processed water (SPW). Resistance: 120 ohms. SPWMFC. | 293 mW/m2 98% mg COD/L 90% mg NH4/L | Higher nitrate removal. Good electrode activity of biofilm | O2 diffusion into anode impairs electricity generation. | [28,54,60] |
| Domestic WW. Non-PEM/CEM MFC SCMFC. | 26 mW/m2 80% mg COD/L 14 mW/m2 (non PEM) | Simple design and lower costs of PEM. | Scaling up bioenergy in the MFC. Non-PEM has zero WWT. Constant transfer/diffusion of O2 into anode from cathode. | [7,28,29,30,31,32,33,34,35,36,37,38,39,40,61] |
| Non-mixing system One-month acclimation. Short HRT. FPMFC. | Consistent COD removal with long HRTs. | Observed that prolonged hydraulic retention times (HRT) devastate the Coulombic efficiency (CE) generation and impair overall Emf production in the air-cathode MFC. Need to optimize MFC hydraulic retention times (HRT). | [25,26,27] |
| Research Output and Contribution | Operating Conditions | Limitations | References |
|---|---|---|---|
| Conversion of waste into bioelectricity and chemicals by using microbial electrochemical technologies. | Review manuscript on key advances in implementing exoelectrogenic bacterial species to produce bioenergy products. | Scaling-up and commercialization technique. | [12,23,37,63,69,70,71,72,73,74] |
| Investigated microbial fuel cell (MFC) in mesophilic conditions. | Two single-chamber MFCs with air cathodes were utilized. Gas diffusion electrodes (GDE) with a Pt load of 0.5 mg/cm2 served as the cathodes (GDE-LT-120EW, E-TEK Division, PEMEAS Fuel Cell Technologies, Somerset, NJ, USA). The anodes were made of a piece of carbon felt measuring 5 cm by 10 cm and with a thickness of 5 mm from Speer Canada in Kitchener, Ontario, Canada. Polycarbonate plates were used to create the anodic chambers. An amount of 110 mL of MFC (MFC-1) was assembled utilizing two plates. This MFC had 1.5 cm between electrodes, and the anode only took up 25% of the chamber space. | Effect of temperature on scaling-up the Eoemf. | [22,25,57,58,59,60,61,62,63,64,65,66,67,68,69,70] |
| Evaluation of nitrification and denitrification on MFCs. | Comprehensive review: Researchers have looked at various aspects of MFC-mediated denitrification, including different operating conditions, reactor configurations, presence/absence of oxygen, electron donor, and nitrate concentration. | Removal of non-biodegradable substances in MFCs. | [69,70,71,72,73,74,75,76,77] |
| Evaluation of the buffering capacity of brewery wastewater through phosphate addition. | The wastewater had a COD of 2250 ± 418 mg/L. In some tests, the wastewater was diluted with ultra-pure water (Milli-Q system; Millipore Corp., New Bedford, MA, USA) or a phosphate buffer (PBS; 50 or 200 mM) and was added to MFCs. | Ionic exchange strength of brewery wastewater. | [54,78,79] |
| Investigated the use of electrochemical measurement methods Tafel plots. | The identical electrode/electrolyte interface was used in the current experiment using the microelectrode approach, which was previously established to measure these parameters at the platinum/Nafion® contact at 25 °C. This study was conducted in the 30–80 °C temperature range with an oxygen pressure of 5 atm. | EIS for proper Eoemf measurement. | [43,44,45] |
| Bio-cathodic use for COD removal with better electro-generation capacity in the MFC. | The CEM that divided the two chambers had a sectional area of 5.6 × 5 = 28 cm2. Jiangsu Province, China-made GGs (55 m2/m3, diameter 1 to 5 mm) were used as the anode, and GFB (400 m2/m3) was used as the air-sparged cathode. The anode’s wet volume was 22 mL. Both GG and GFB were pre-soaked for 24 h in a solution of 1 mol L−1 HCl and NaOH, and then for another 48 h in nonionized water. To capture the electrons generated by microbial oxidation, a graphite rod with a 1 cm diameter and 5 cm length was introduced into the granule matrix. | Cathodic electrodes boost the electro-generation surface, hence up-scaling the production of electricity. | [69,70,71,72,73,74,75,76,77,78,79,80,81,82] |
| Study of the effect of HRT in an SCMFC. | During the test in continuous mode, wastewater was injected through the injection port using a peristaltic pump (Watson-Marlow, 520S) outfitted with Marprene II tubing at a flow rate ranging from 0.03 to 1 cm3/min1 (0.14 cm internal diameter). The MFCs were run at about 21 2 C, which is the temperature of ambient air. | HRT for increased electrical potential capacity Eoemf. | [6,17,41,42,43,44,45,46,47,48,49,50,51,52,53,54,83,84,85,86] |
| Wastewater treatment using air-cathode microbial fuel cells. | The cathode was composed of carbon cloth (30% wet proofed; ETEK, USA) with a Pt catalyst (0.35 mg/cm2; E-TEK), whereas the anode electrode (projected surface area = 7 cm2) was built of carbon cloth (without wet proofing). In the cylindrical chamber, the electrodes were positioned on opposing sides (4 cm long, 3 cm diameter; 28 mL liquid volume). The external circuit was connected to the carbon electrodes using titanium wire. | Knowledge of solution chemistry and operational parameters affecting MFCs towards scaling up. | [5,6,7,8,9,10,11,12,13,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,60,81,87,88,89,90,91,92] |
| Production of bioenergy and biochemicals from industrial and agricultural Wastewater | Critical review based on various industrial substrates and their inoculum with a clear power generation capacity display. | A need for commercializing and scaling-up bioenergy production in the MFC. | [13,61,86] |
| Waste and wastewater clean-up using microbial fuel cells | Batch feeding was used in the early investigations with the R-MFCs. Once or twice a week, MFCs were fed, and each time, 5 mL of old anolyte was removed before 5 mL of fresh feedstock was added to the reactors. Anolyte in the anodic chambers was thoroughly stirred during the feeding procedure to guarantee uniformity. | A need to improve anode performance and waste utilization. Need for MFC scale-up through multiple small-sized MFC units. | [47,81,83] |
| Electricity generation from starch processing wastewater using microbial fuel cell technology | In a temperature-controlled incubator, all MFCs were run in fed-batch mode at 30 °C (HPG-280H, China). The electrode was created by sandwiching a proton exchange membrane (PEM, Nafion 117, Dupont) between an anode (carbon paper) and a cathode (carbon paper containing 1.12 mg/cm2 of Pt catalyst) and then joining them together by hot pressing. The projected surface areas of the anode and cathode were 25 and 17 cm2, respectively. Except where stated, a set 1000 external resistance was used for all experiments. | Observed that prolonged hydraulic retention times (HRT) devastate the Coulombic efficiency (CE) generation and impair overall Emf production in the air-cathode MFC. Need to optimize MFC-HRT. | [10,11,12,19,55,56,57,58,59,60,73,74,75,76,77,78,79] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabangu, K.P.; Bakare, B.F.; Bwapwa, J.K. Microbial Fuel Cells for Electrical Energy: Outlook on Scaling-Up and Application Possibilities towards South African Energy Grid. Sustainability 2022, 14, 14268. https://doi.org/10.3390/su142114268
Shabangu KP, Bakare BF, Bwapwa JK. Microbial Fuel Cells for Electrical Energy: Outlook on Scaling-Up and Application Possibilities towards South African Energy Grid. Sustainability. 2022; 14(21):14268. https://doi.org/10.3390/su142114268
Chicago/Turabian StyleShabangu, Khaya Pearlman, Babatunde Femi Bakare, and Joseph Kapuku Bwapwa. 2022. "Microbial Fuel Cells for Electrical Energy: Outlook on Scaling-Up and Application Possibilities towards South African Energy Grid" Sustainability 14, no. 21: 14268. https://doi.org/10.3390/su142114268
APA StyleShabangu, K. P., Bakare, B. F., & Bwapwa, J. K. (2022). Microbial Fuel Cells for Electrical Energy: Outlook on Scaling-Up and Application Possibilities towards South African Energy Grid. Sustainability, 14(21), 14268. https://doi.org/10.3390/su142114268









