Innovative Phosphate Fertilizer Technologies to Improve Phosphorus Use Efficiency in Agriculture
Abstract
1. Introduction
2. Worldwide Market of Technologies for Phosphate Fertilizers
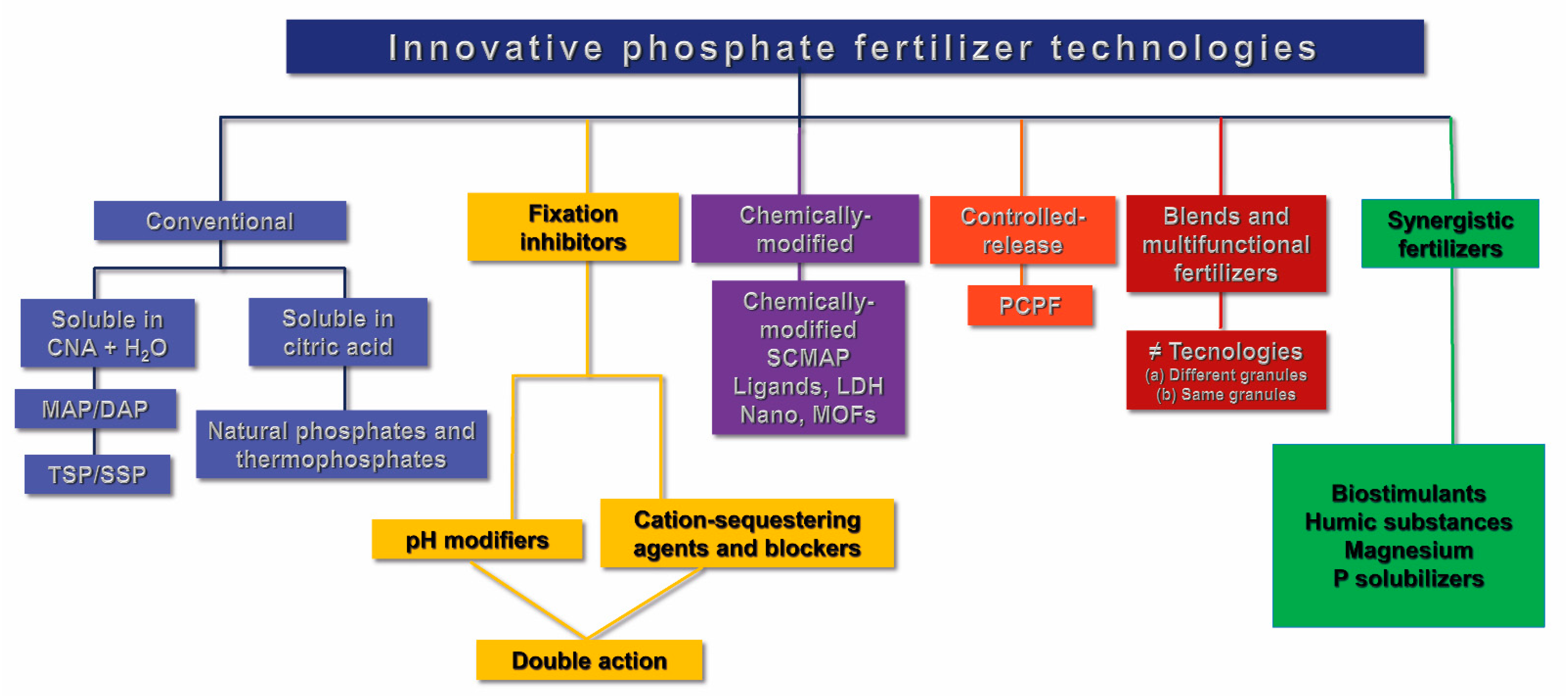
3. Classification of the Technologies for Phosphate Fertilizers
- (A)
- Conventional phosphate fertilizers: These are fertilizers obtained from phosphate rocks that are solubilized by chemical processes, using sulfuric, phosphoric, and nitric acids or thermal processes. The most used are SSP, TSP, MAP, DAP, thermophosphates, and natural phosphates that are merely ground up, whether followed by partial acidulation or not. We emphasize here that the aim of this publication is not to develop a specific discussion regarding conventional phosphate fertilizers.
- (B)
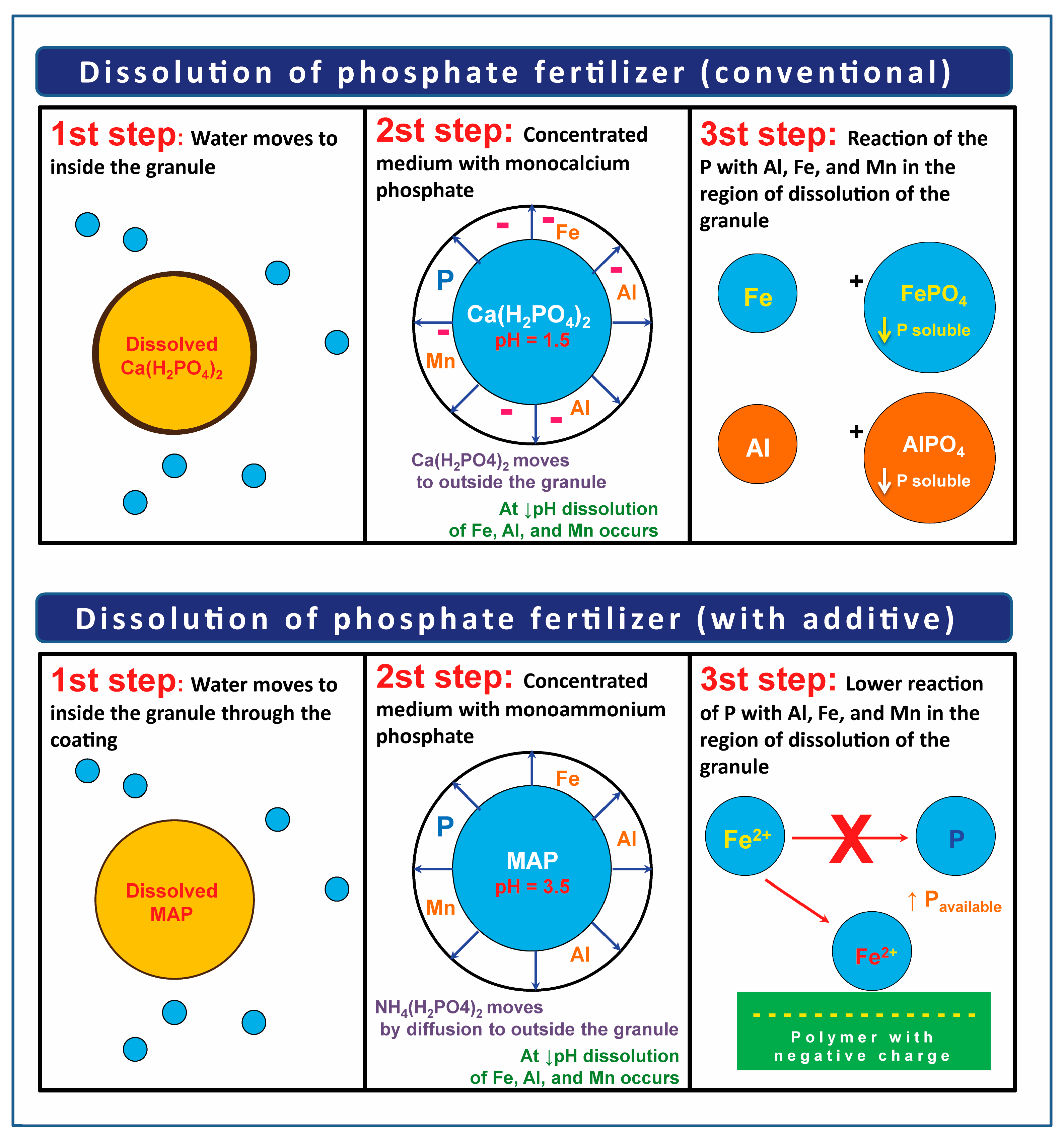
- Phosphate fertilizers with fixation inhibitors: These are phosphate fertilizers with additives to reduce reactions of precipitation and adsorption of P in the soil. Figure 2 illustrates the mode of action of this technology. It was created based on the theoretical concepts that are presented in this sub-topic. This group is subdivided into pH-modifying additives and the materials that generate negative charges around the fertilizer granules, which act as agents in sequestering metals such as iron, aluminum, and manganese or blockers of adsorption in the soil. As an example of pH modifiers, we have the materials with acidity neutralizing power, such as oxysulfates, carbonates, oxides, and calcium and magnesium oxides and hydroxides that are applied together with a liquid binder on the surface of the phosphate fertilizer granules.
- (C)
- Synergistic phosphate fertilizers: These are conventional phosphate fertilizers with the addition of nutrients, microorganisms, nanoparticles, or biostimulants that lead to improvement in the efficiency of P use by plants. As examples, we can cite magnesium, which is a P transporter through cell membranes of the roots, rooting agents associated with soluble calcium in the coating of the MAP to enhance root growth and interception, organic acids, and microorganisms efficient in the solubilization and mineralization of P in the soil.
- (D)
- Chemically modified phosphate fertilizers: These are P sources that in their production process pass through physical, chemical, or physical–chemical reactions that modify or promote the interaction of P with other chemical compounds that alter its solubility and/or chemical form. That is, the raw materials used in the production process pass through a reorganization in their chemical forms and bonds. Examples of these technologies are the layered double hydroxides (LDH’s) [27,28,29], nanoparticles [30,31], graphene oxide [32,33], and metal-organic frameworks (MOFs) [34,35].
- (E)
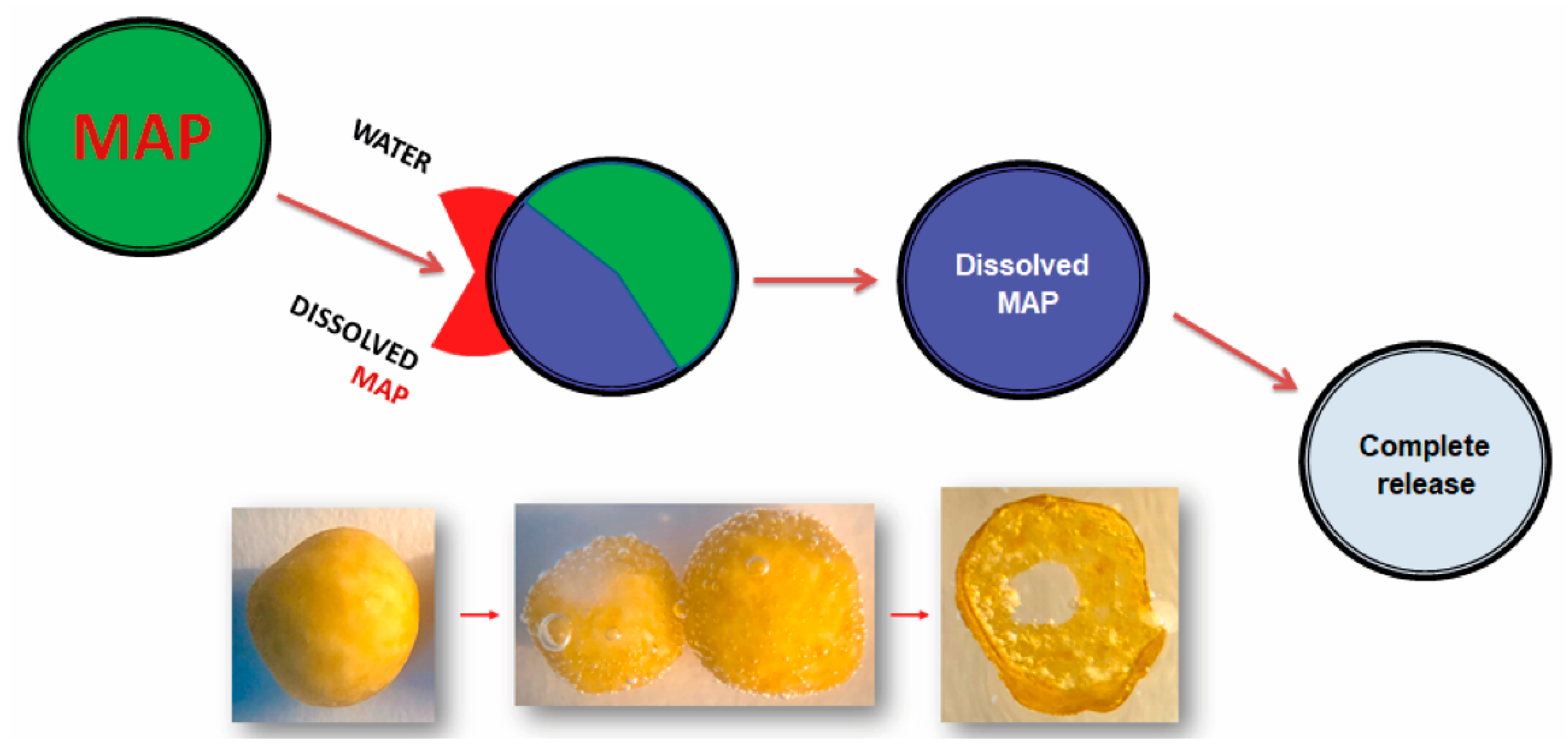
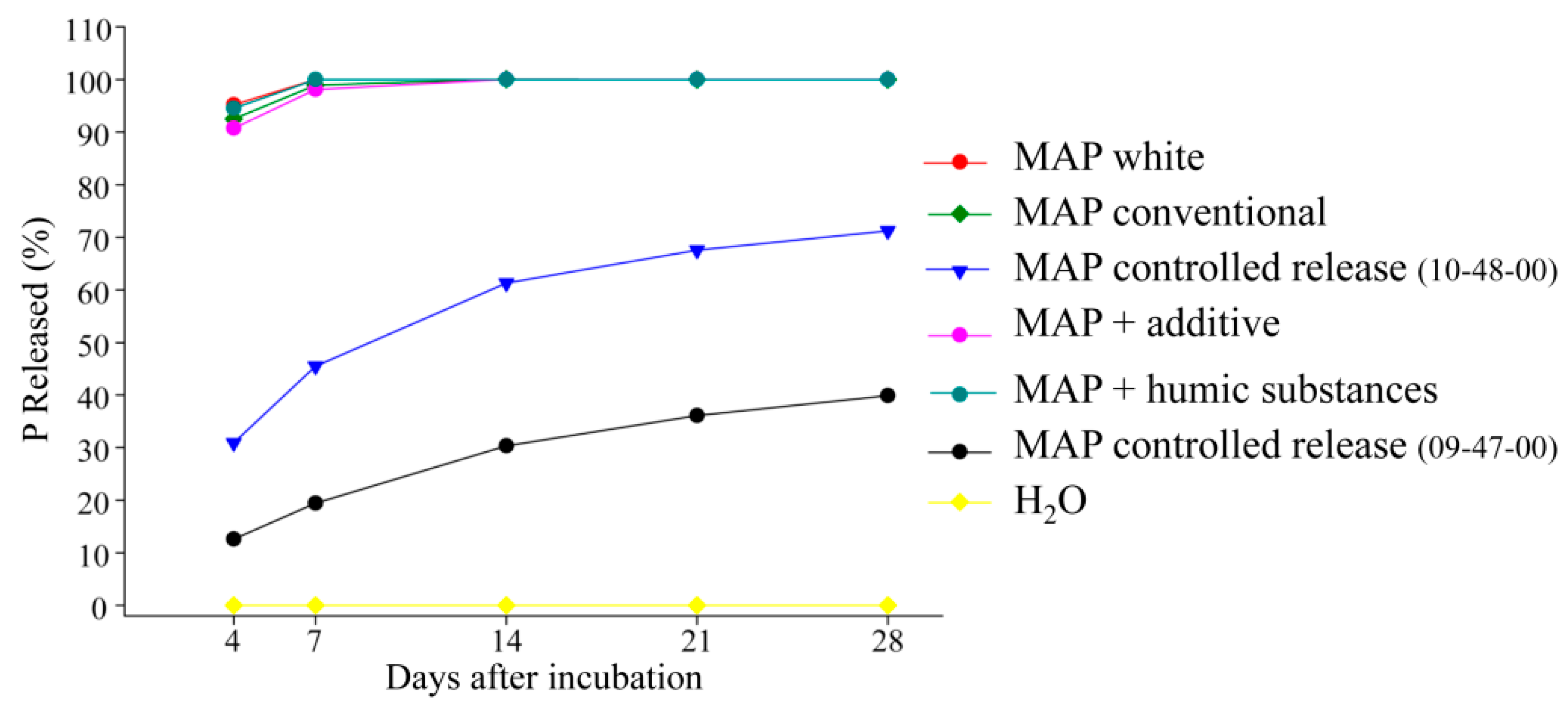
- Controlled-release phosphate fertilizers: These are conventional fertilizers, such as MAP, that have high solubility in CNA + water, to which compounds are added for coating the granule, which serve as a physical barrier and control the flow of P through the coating by diffusion.There are several compounds that can be used for the coating, such as elemental sulfur (S0), plastic resins, thermoplastics, polyurethane [36,37], polyethylene [38], polyesters, and others [39,40]. The choice of the coating suitable for the phosphate fertilizers is a long and diversified route for innovation in fertilizers.An ideal controlled-release fertilizer should be covered with biodegradable materials that allow suitable control of the release of nutrients under different environments and growing conditions. The nutrient-release curve should be similar to the phosphorus absorption curve of the crop in which the fertilizer is applied to maximize uptake by the plants and reduce P losses.The controlled-release fertilizers have a release rate of the nutrient from inside the coating to the soil basically affected by the type and thickness of the coating, the form of application on annual and perennial crops (incorporated or broadcast on the surface), soil temperature and moisture, and consequently, rainfall amounts.Therefore, the longevity or the time of release of the nutrient from the fertilizer to the soil is an important characteristic of this type of technology and can vary from days, months, or even to years, depending on the type of product and manufacturer.
- (F)
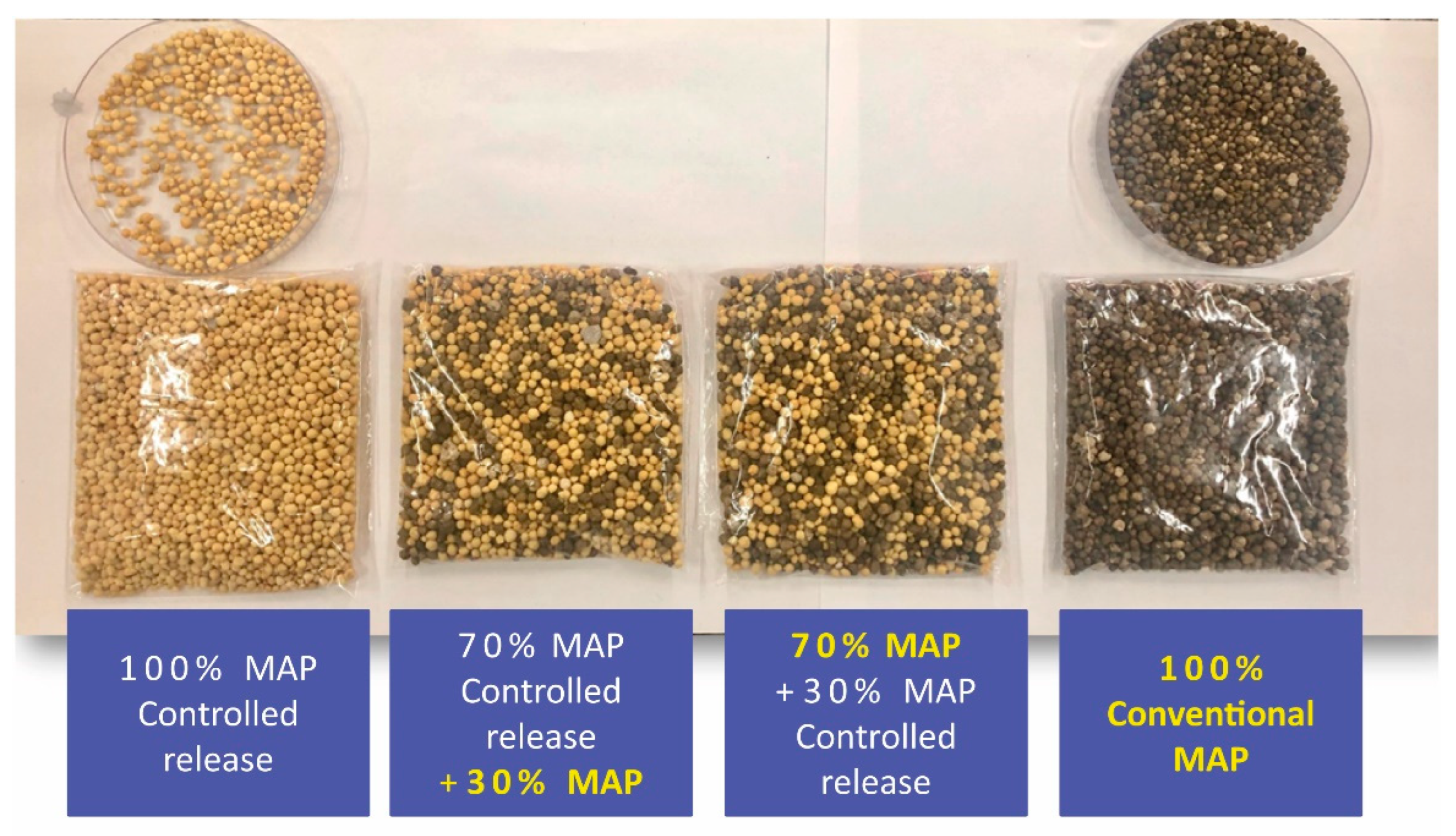
- Blends and multifunctional fertilizers: Blends are produced from physical mixture of conventional phosphate fertilizer granules and those that have some type of technology, as for example, controlled-release fertilizers. Physical mixture is made at different proportions among the fertilizers, which are pre-defined and in accordance with the absorption curve of the crop and their technologies. The proportion of conventional fertilizers and controlled-release fertilizers are the main factors that affect the time of release of P from the fertilizer to the soil and determine the final price of the blends. Multifunctional fertilizers have the most diverse types of technologies, though used on the same fertilizer granule through coating and/or granulation.
3.1. Phosphate Fertilizers with Fixation Inhibitors
3.1.1. pH Modifiers
3.1.2. Cation-Sequestering Agents or Blockers
3.2. Chemically Modified Phosphate Fertilizers
3.3. Synergistic Phosphate Fertilizers
3.4. Controlled-Release Phosphate Fertilizers
3.5. Blends and Multifunctional Fertilizers
4. Final Considerations and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Dedication
Abbreviations
References
- Krafft, F. Phosphorus. From elemental light to chemical element. In Angewandte Chemie—International Edition in English; Wiley: New York, NY, USA, 1969; pp. 660–671. [Google Scholar]
- Sharpley, A.; Jarvie, H.; Flaten, D.; Kleinman, P.; Sharpley, A. Celebrating the 350th anniversary of phosphorus discovery: A conundrum of deficiency and excess. J. Environ. Qual. 2018, 47, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Powers, J. Searching for the Philosopher’s Stone. The Story behind Joseph Wright’s Alchymist; Quandary Books: Derbyshire, UK, 2017. [Google Scholar]
- Emsley, J. The 13th Element: The Sordid Tale of Murder, Fire, and Phosphorus; John Wiley & Sons: New York, NY, USA, 2000; ISBN 0471394556. [Google Scholar]
- Wisniak, J. Phosphorus-From discovery to commodity. Indian J. Chem. Technol. 2005, 12, 108–122. [Google Scholar]
- John, H.; Payne, J. Fertilizer Manufacture; Interscien; van Mayer, J., Ed.; Springer: Dordrecht, The Netherlands; New York, NY, USA, 1961; Available online: https://link.springer.com/chapter/10.1007/978-94-011-0121-9_18 (accessed on 8 September 2022).
- Leibig, J. Organic Chemistry in Its Application to Agriculture and Physiology; Playfair, L., Ed.; Taylor and Walton: London, UK, 1840. [Google Scholar]
- Lawes, J.B. Lawes’s Patent Manures; Gardeners’ Chronicle: London, UK, 1843; 442p. [Google Scholar]
- Lawes, J.B.; Gilbert, J.H. The Rothamsted Experiments: Being an Account of Some of the Results of the Agricultural Investigations, Conducted at Rothamsted in the Field, the Feeding Shed, and the Laboratory over a Period of Fifty Years; Fifth Series; Transactions Highland and Agricultural Society of Scotland: London, UK, 1895. [Google Scholar]
- Rothamsted Research. e-RA: The Electronic Rothamsted Archive. 2019. Available online: http://www.era.rothamsted.ac.uk/ (accessed on 8 September 2022).
- Johnston, A.E.; Poulton, P.R. Phosphorus in Agriculture: A Review of Results from 175 Years of Research at Rothamsted, UK. J. Environ. Qual. 2019, 48, 1133–1144. [Google Scholar] [CrossRef]
- Ivell, D.M. Phosphate fertilizer production—From the 1830′s to 2011 and beyond. Procedia Eng. 2012, 46, 166–171. [Google Scholar] [CrossRef][Green Version]
- Jacob, K.D. Predecessors of superphosphate. In Superphosphate: Its History, Chemistry, and Manufacture; U.S. Department of Agriculture: Washington, DC, USA; Tennessee Valley Authority: Knoxville, TN, USA; U.S. Government Printing Office: Washington, DC, USA, 1964. [Google Scholar]
- Jacob, K.D. History and status of the superphosphate industry. In Superphosphate: Its History, Chemistry, and Manufacture; U.S. Government Printing Office: Washington, DC, USA, 1964. [Google Scholar]
- Leikam, D.F.; Achorn, F.P. Phosphate fertilizers: Production, characteristics, and technologies. Phosphorus Agric. Environ. 2015, 46, 23–50. [Google Scholar]
- Slack, A.V.; Hardesty, J.O. Status of superphosphate in relation to other fertilizer phosphates. In Superphosphate: Its History, Chemistry, and Manufacture; U.S. Department of Agriculture: Washington, DC, USA; Tennessee Valley Authority: Knoxville, TN, USA; U.S. Government Printing Office: Washington, DC, USA, 1964. [Google Scholar]
- Fageria, N.K.; Baligar, V.C. Improving nutrient use efficiency of annual crops in brazilian acid soils for sustainable crop production. Commun. Soil Sci. Plant Anal. 2007, 32, 1303–1319. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Elser, J.J.; Hilton, J.; Ohtake, H.; Schipper, W.J.; Van Dijk, K.C. Greening the global phosphorus cycle: How green chemistry can help achieve planetary P sustainability. Green Chem. 2015, 17, 2087–2099. [Google Scholar] [CrossRef]
- Tiecher, T.; dos Santos, D.R.; Calegari, A. Soil organic phosphorus forms under different soil management systems and winter crops, in a long term experiment. Soil Tillage Res. 2012, 124, 57–67. [Google Scholar] [CrossRef]
- Sample, E.C.; Soper, R.J.; Racz, G.J. Reactions of phosphate fertilizers in soils. In The Role of Phosphorus in Agriculture; Khasawneh Chairman, F.E., Sample, E.C., Kamprath, E.J., Eds.; American Society of Agronomy, Inc./Crop Science Society of America, Inc./Soil Science Society of America Inc.: Madison, WI, USA, 1980; pp. 263–310. [Google Scholar] [CrossRef]
- Novais, R.F.; Smyth, T.J.; Nunes, F.N. Fósforo. In Fertilidade Do Solo; Novais, R.F., Alvarez, V.V.H., Barros, N.F., Fontes, R.L.F., Cantarutti, R.B., Neves, J.C.L., Eds.; SBCS: Viçosa, MG, Brazil, 2007; pp. 471–550. (In Portuguese) [Google Scholar]
- Wu, P.; Ata-Ul-Karim, S.T.; Singh, B.P.; Wang, H.; Wu, T.; Liu, C.; Fang, G.; Zhou, D.; Wang, Y.; Chen, W. A scientometric review of biochar research in the past 20 years (1998–2018). Biochar 2019, 1, 23–43. [Google Scholar] [CrossRef]
- Lyons, G.; Genc, Y. Commercial humates in agriculture: Real substance or smoke and mirrors? Agronomy 2016, 6, 50. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, M.; Meng, S.; Yang, Y.; Li, Y.C.; Tong, Z. Biowaste-derived, nanohybrid-reinforced double-function slow-release fertilizer with metal-adsorptive function. Chem. Eng. J. 2022, 450, 138084. [Google Scholar] [CrossRef]
- Doydora, S.; Hesterberg, D.; Klysubun, W. Phosphate solubilization from poorly crystalline iron and aluminum hydroxides by AVAIL copolymer. Soil Sci. Soc. Am. J. 2017, 81, 20–28. [Google Scholar] [CrossRef]
- Koilraj, P.; Antonyraj, C.A.; Gupta, V.; Reddy, C.R.K.; Kannan, S. Novel approach for selective phosphate removal using colloidal layered double hydroxide nanosheets and use of residue as fertilizer. Appl. Clay Sci. 2013, 86, 111–118. [Google Scholar] [CrossRef]
- Nalawade, P.; Aware, B.; Kadam, V.J.; Hirlekar, R.S. Layered double hydroxides: A review. J. Sci. Ind. Res. 2009, 68, 267–272. [Google Scholar]
- Kong, L.; Tian, Y.; Wang, Y.; Li, N.; Liu, Y.; Pang, Z.; Huang, X.; Li, M.; Zhang, J.; Zuo, W. Periclase-induced generation of flowerlike clay-based layered double hydroxides: A highly efficient phosphate scavenger and solid-phase fertilizer. Chem. Eng. J. 2019, 359, 902–913. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Synthetic apatite nanoparticles as a phosphorus fertilizer for soybean (Glycine max). Sci. Rep. 2014, 4, 5686. [Google Scholar] [CrossRef] [PubMed]
- Montalvo, D.; McLaughlin, M.J.; Degryse, F. Efficacy of Hydroxyapatite Nanoparticles as Phosphorus Fertilizer in Andisols and Oxisols. Soil Sci. Soc. Am. J. 2015, 79, 551–558. [Google Scholar] [CrossRef]
- Andelkovic, I.B.; Kabiri, S.; Tavakkoli, E.; Kirby, J.K.; McLaughlin, M.J.; Losic, D. Graphene oxide-Fe(III) composite containing phosphate—A novel slow release fertilizer for improved agriculture management. J. Clean. Prod. 2018, 185, 97–104. [Google Scholar] [CrossRef]
- Kabiri, S.; Degryse, F.; Tran, D.N.H.; Da Silva, R.C.; McLaughlin, M.J.; Losic, D. Graphene Oxide: A New Carrier for Slow Release of Plant Micronutrients. ACS Appl. Mater. Interfaces 2017, 9, 43325–43335. [Google Scholar] [CrossRef]
- Anstoetz, M.; Rose, T.J.; Clark, M.W.; Yee, L.H.; Raymond, C.A.; Vancov, T. Novel applications for oxalate-phosphate-amine metal-organic-frameworks (OPA-MOFs): Can an iron-based OPA-MOF be used as slow-release fertilizer? PLoS ONE 2015, 10, e0144169. [Google Scholar] [CrossRef]
- Wu, K.; Du, C.; Ma, F.; Shen, Y.; Liang, D.; Zhou, J. Degradation of Metal-Organic Framework Materials as Controlled-Release Fertilizers in Crop Fields. Polymers 2019, 11, 947. [Google Scholar] [CrossRef] [PubMed]
- Du, C.W.; Zhou, J.M.; Shaviv, A. Release characteristics of nutrients from polymer-coated compound controlled release fertilizers. J. Polym. Environ. 2006, 14, 223–230. [Google Scholar] [CrossRef]
- Xie, J.; Yang, Y.; Gao, B.; Wan, Y.; Li, Y.C.; Xu, J.; Zhao, Q. Biomimetic Superhydrophobic Biobased Polyurethane-Coated Fertilizer with Atmosphere “Outerwear”. ACS Appl. Mater. Interfaces 2017, 9, 15868–15879. [Google Scholar] [CrossRef] [PubMed]
- Dahnke, W.C.; Attoe, O.J.; Engelbert, L.E.; Groskopp, M.D. Controlling release of fertilizer constituents by means of coatings and capsules. Agron. J. 1963, 55, 242–244. [Google Scholar] [CrossRef]
- Jin, S.; Wang, Y.; He, J.; Yang, Y.; Yu, X.; Yue, G. Preparation and properties of a degradable interpenetrating polymer networks based on starch with water retention, amelioration of soil, and slow release of nitrogen and phosphorus fertilizer. J. Appl. Polym. Sci. 2013, 128, 407–415. [Google Scholar] [CrossRef]
- Ma, Z.; Jia, X.; Hu, J.; Liu, Z.; Wang, H.; Zhou, F. Mussel-inspired thermosensitive polydopamine- graft -poly(N -isopropylacrylamide) coating for controlled-release fertilizer. J. Agric. Food Chem. 2013, 61, 12232–12237. [Google Scholar] [CrossRef]
- Owino-Gerroh, C.; Gascho, G.J. Effect of silicon on low pH soil phosphorus sorption and on uptake and growth of maize. Commun. Soil Sci. Plant Anal. 2004, 35, 2369–2378. [Google Scholar] [CrossRef]
- Novais, R.F.; Alvarez, V.V.H.; de Barros, N.F.; Fontes, R.L.F.; Cantarutti, R.B.; Neves, J.C.L. (Eds.) Fertilidade Do Solo; Sociedade Brasileira de Ciência do Solo: Viçosa, MG, Brazil, 2007; Volume 1, 1017p, ISBN 978-85-8650-408-2. [Google Scholar]
- Horowitz, N.; Meurer, E.J. Uso do enxofre elementar como fertilizante. Informações Agronômicas. Inf. Agronômicas 2005, 112, 4–7. [Google Scholar]
- Vitti, G.C.; Otto, R.; Savieto, J. Manejo do enxofre na agricultura. Inf. Agronômicas 2015, 152, 2–12. [Google Scholar]
- Mitchell, J.; Dehm, J.E.; Dion, H. The effect of small additions of elemental sulphur on the availability of phosphate fertilizers. Sci. Agric. 1952, 32, 311–316. [Google Scholar]
- Chien, S.H.; Edmeades, D.; McBride, R.; Sahrawat, K.L. Review of maleic-itaconic acid copolymer purported as urease inhibitor and phosphorus enhancer in soils. Agron. J. 2014, 106, 423–430. [Google Scholar] [CrossRef]
- Mclaughlin, M.J.; Mcbeath, T.M.; Smernik, R.; Stacey, S.P.; Ajiboye, B.; Guppy, C.; Mclaughlin, M.J.; Mcbeath, T.M.; Smernik, R.; Stacey, S.P.; et al. The chemical nature of P accumulation in agricultural soils-implications for fertiliser management and design: An Australian perspective. Plant Soil 2011, 349, 69–87. [Google Scholar] [CrossRef]
- Weeks, J.J.; Hettiarachchi, G.M. A review of the latest in phosphorus fertilizer technology: Possibilities and pragmatism. J. Environ. Qual. 2019, 48, 1300–1313. [Google Scholar] [CrossRef]
- Peacock, L. Method for Producing a Fertilizer with Micronutrients. US7497891B2, 3 March 2009. [Google Scholar]
- Peacock, L.A.; Stacey, S.; Mclaughlin, M. Fertilizer Composition Containing Micronutrients and Methods of Making Same. US2016/0083308A1, 24 March 2016. [Google Scholar]
- Mao, X.; Jiang, R.; Xiao, W.; Yu, J. Use of surfactants for the remediation of contaminated soils: A review. J. Hazard. Mater. 2015, 285, 419–435. [Google Scholar] [CrossRef]
- Bordes, R.; Holmberg, K. Amino acid-based surfactants—Do they deserve more attention? Adv. Colloid Interface Sci. 2015, 222, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Degryse, F.; Ajiboye, B.; Armstrong, R.D.; McLaughlin, M.J. Sequestration of phosphorus-binding cations by complexing compounds is not a viable mechanism to increase phosphorus efficiency. Soil Sci. Soc. Am. J. 2013, 77, 2050–2059. [Google Scholar] [CrossRef]
- Chien, S.H.; Rehm, G.W.; Chien, S.H.; Rehm, G.W. Theoretical equilibrium considerations explain the failure of the maleic-itaconic copolymer to increase efficiency of fertiliser phosphorus applied to soils. Soil Res. 2016, 54, 120–124. [Google Scholar] [CrossRef]
- Hopkins, B.G.; Fernelius, K.J.; Hansen, N.C.; Eggett, D.L. AVAIL phosphorus fertilizer enhancer: Meta-analysis of 503 field evaluations. Agron. J. 2018, 110, 389–398. [Google Scholar] [CrossRef]
- Pierzynski, J.; Hettiarachchi, G.M. Reactions of phosphorus fertilizers with and without a fertilizer enhancer in three acidic soils with high phosphorus-fixing capacity. Soil Sci. Soc. Am. J. 2018, 82, 1124–1139. [Google Scholar] [CrossRef]
- Urrutia, O.; Guardado, I.; Erro, J.; Mandado, M.; García-Mina, J.M. Theoretical chemical characterization of phosphate-metal-humic complexes and relationships with their effects on both phosphorus soil fixation and phosphorus availability for plants. J. Sci. Food Agric. 2013, 93, 293–303. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- International Humic Substances Society. What Are Humic Substances. 2007. Available online: https://humic-substances.org/ (accessed on 12 October 2022).
- Nunes, A.P.P.; Santos, C.F.; Guelfi, D. Interfaces between biodegradable organic matrices coating and MAP fertilizer for improve use efficiency. Sci. Total Environ. 2022, 804, 149896. [Google Scholar] [CrossRef] [PubMed]
- Benbi, D.K.; Gilkes, R.J. The movement into soil of P from superphosphate grains and its availability to plants. Fertil. Res. 1987, 12, 21–36. [Google Scholar] [CrossRef]
- Erro, J.; Baigorri, R.; Maria, J.; Freire, G.-M.; Yvin, J.-C. Phosphate Compounds and Use Thereof as Fertilizer. US20130104612A1, 2 May 2013. [Google Scholar]
- Urrutia-Sagardia, O.; Garces, J.E.; Ramirez, M.F.; Ekisoain, R.B.; Arregu, A.M.Z.; Yvin, J.C.; Freire, J.M.G.M. Humic Substance-Encapsulated Particles, Compositions and Method of Making the Same. WO2018149825A1, 23 August 2018. [Google Scholar]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Wang, P.; Hunter, M.N.; Kopittke, P.M. Bioavailability and movement of hydroxyapatite nanoparticles (HA-NPs) applied as a phosphorus fertiliser in soils. Environ. Sci. Nano 2018, 5, 2888–2898. [Google Scholar] [CrossRef]
- McKnight, M.M.; Qu, Z.; Copeland, J.K.; Guttman, D.S.; Walker, V.K. A practical assessment of nano-phosphate on soybean (Glycine max) growth and microbiome establishment. Sci. Rep. 2020, 10, 9151. [Google Scholar] [CrossRef] [PubMed]
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of nanoparticles with edible plants and their possible implications in the food. J. Agric. Food Chem. 2011, 59, 3485–3498. [Google Scholar] [CrossRef]
- Gogos, A.; Knauer, K.; Bucheli, T.D. Nanomaterials in plant protection and fertilization: Current state, foreseen applications, and research priorities. J. Agric. Food Chem. 2012, 60, 9781–9792. [Google Scholar] [CrossRef]
- Guelfi, D.R.; Chagas, W.F.T.; Lacerda, J.R.; Chagas, R.M.R.; de Souza, T.L.; Andrade, A.B. Monoammonium phosphate coated with polymers and magnesium for coffee plants. Ciência Agrotecnologia 2018, 42, 261–270. [Google Scholar] [CrossRef]
- Fageria, V.D. Nutrient interactions in crop plants. J. Plant Nutr. 2006, 24, 1269–1290. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar] [CrossRef]
- Richardson, A.E. Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Funct. Plant Biol. 2001, 28, 897–906. [Google Scholar] [CrossRef]
- Jacobson, K.L.; Hobb, T.W.; Balaban, L. Incorporation of Biological Agents in Fertilizers. EP3166911A1, 17 May 2017. [Google Scholar]
- Liu, S.; Wang, X.; Yin, X.; Savoy, H.J.; McClure, A.; Essington, M.E. Ammonia volatilization loss and corn nitrogen nutrition and productivity with efficiency enhanced uan and urea under no-tillage. Sci. Rep. 2019, 9, 6610. [Google Scholar] [CrossRef]
- Andrade, A.B.; Guelfi, D.R.; Chagas, W.F.T.; Cancellier, E.L.; de Souza, T.L.; Oliveira, L.S.S.; Faquin, V.; Du, C. Fertilizing maize croppings with blends of slow/controlled-release and conventional nitrogen fertilizers. J. Plant Nutr. Soil Sci. 2021, 184, 227–237. [Google Scholar] [CrossRef]
- Briassoulis, D.; Dejean, C. Critical review of norms and standards for biodegradable agricultural plastics. Part I. Biodegradation in soil. J. Polym. Environ. 2010, 18, 384–400. [Google Scholar] [CrossRef]
- Santos, C.F.; da Silva Aragao, O.O.; Silva, D.R.G.; da Conceição Jesus, E.; Chagas, W.F.T.; Correia, P.S.; de Souza Moreira, F.M. Environmentally friendly urea produced from the association of N-(n-butyl) thiophosphoric triamide with biodegradable polymer coating obtained from a soybean processing byproduct. J. Clean. Prod. 2020, 276, 123014. [Google Scholar] [CrossRef]
- Trenkel, M.E. Slow-and Controlled-Release and Stabilized Fertilizers: An Option for Enhancing Nutrient Use Efficiency in Agriculture; International Fertilizer Industry Association: Paris, France, 2010; 167p, ISBN 9782952313971. [Google Scholar]
- Fertahi, S.; Bertrand, I.; Ilsouk, M.; Oukarroum, A.; Amjoud, M.B.; Zeroual, Y.; Barakat, A. New generation of controlled release phosphorus fertilizers based on biological macromolecules: Effect of formulation properties on phosphorus release. Int. J. Biol. Macromol. 2020, 143, 153–162. [Google Scholar] [CrossRef]
- ISO 18644:2016; Fertilizers and Soil Conditioners—Controlled-Release Fertilizer—General Requirements. International Organization for Standardization: Geneva, Switzerland, 2016.
- ISO 21263:2017; Slow-Release Fertilizers—Determination of the Release of the Nutrients—Method for Coated Fertilizers. International Organization for Standardization: Geneva, Switzerland, 2017.
- Lopes Cancellier, E.; Degryse, F.; Silva, D.R.G.; Coqui Da Silva, R.; Mclaughlin, M.J. Rapid and low-cost method for evaluation of nutrient release from controlled-release fertilizers using electrical conductivity. J. Polym. Environ. 2018, 26, 4388–4395. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guelfi, D.; Nunes, A.P.P.; Sarkis, L.F.; Oliveira, D.P. Innovative Phosphate Fertilizer Technologies to Improve Phosphorus Use Efficiency in Agriculture. Sustainability 2022, 14, 14266. https://doi.org/10.3390/su142114266
Guelfi D, Nunes APP, Sarkis LF, Oliveira DP. Innovative Phosphate Fertilizer Technologies to Improve Phosphorus Use Efficiency in Agriculture. Sustainability. 2022; 14(21):14266. https://doi.org/10.3390/su142114266
Chicago/Turabian StyleGuelfi, Douglas, Ana Paula Pereira Nunes, Leonardo Fernandes Sarkis, and Damiany Pádua Oliveira. 2022. "Innovative Phosphate Fertilizer Technologies to Improve Phosphorus Use Efficiency in Agriculture" Sustainability 14, no. 21: 14266. https://doi.org/10.3390/su142114266
APA StyleGuelfi, D., Nunes, A. P. P., Sarkis, L. F., & Oliveira, D. P. (2022). Innovative Phosphate Fertilizer Technologies to Improve Phosphorus Use Efficiency in Agriculture. Sustainability, 14(21), 14266. https://doi.org/10.3390/su142114266





