Enhancing Bread Quality and Shelf Life via Glucose Oxidase Immobilized on Zinc Oxide Nanoparticles—A Sustainable Approach towards Food Safety
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
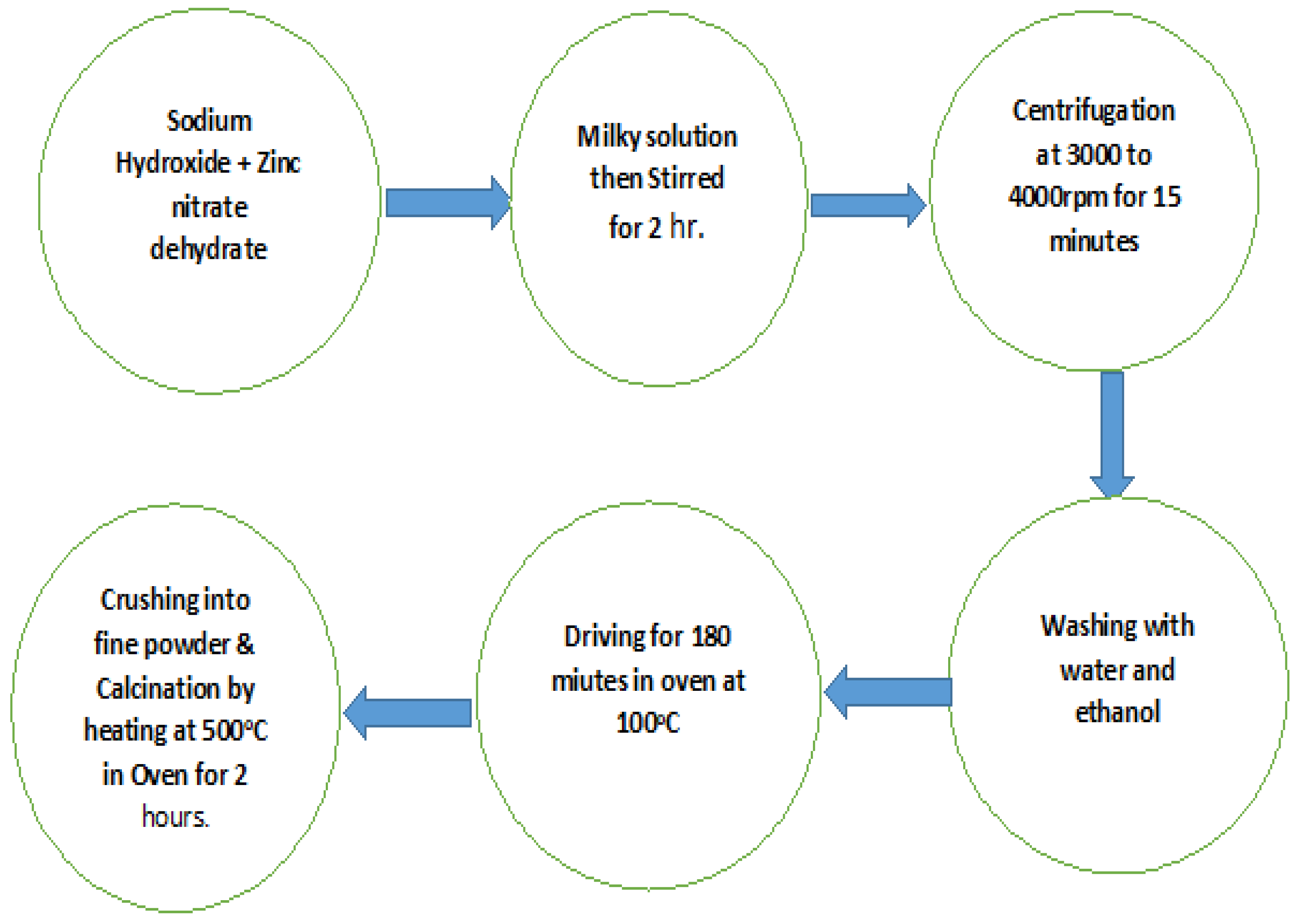
2.2. Preparationof ZnO Nanoparticles and Surface Modification
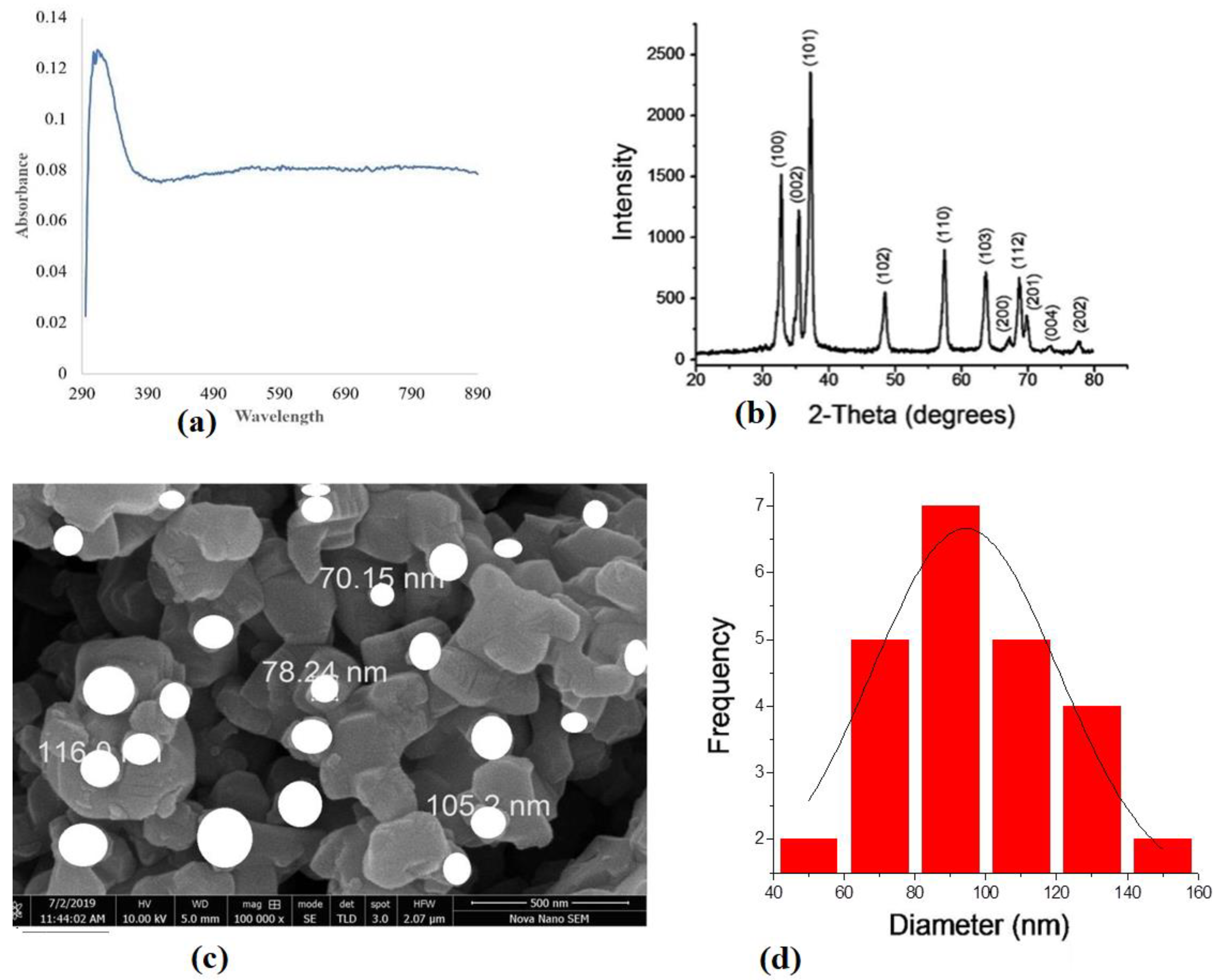
2.3. ZnO Nanoparticles Characterization
2.4. GOx Immoblization on Modified ZnONPs
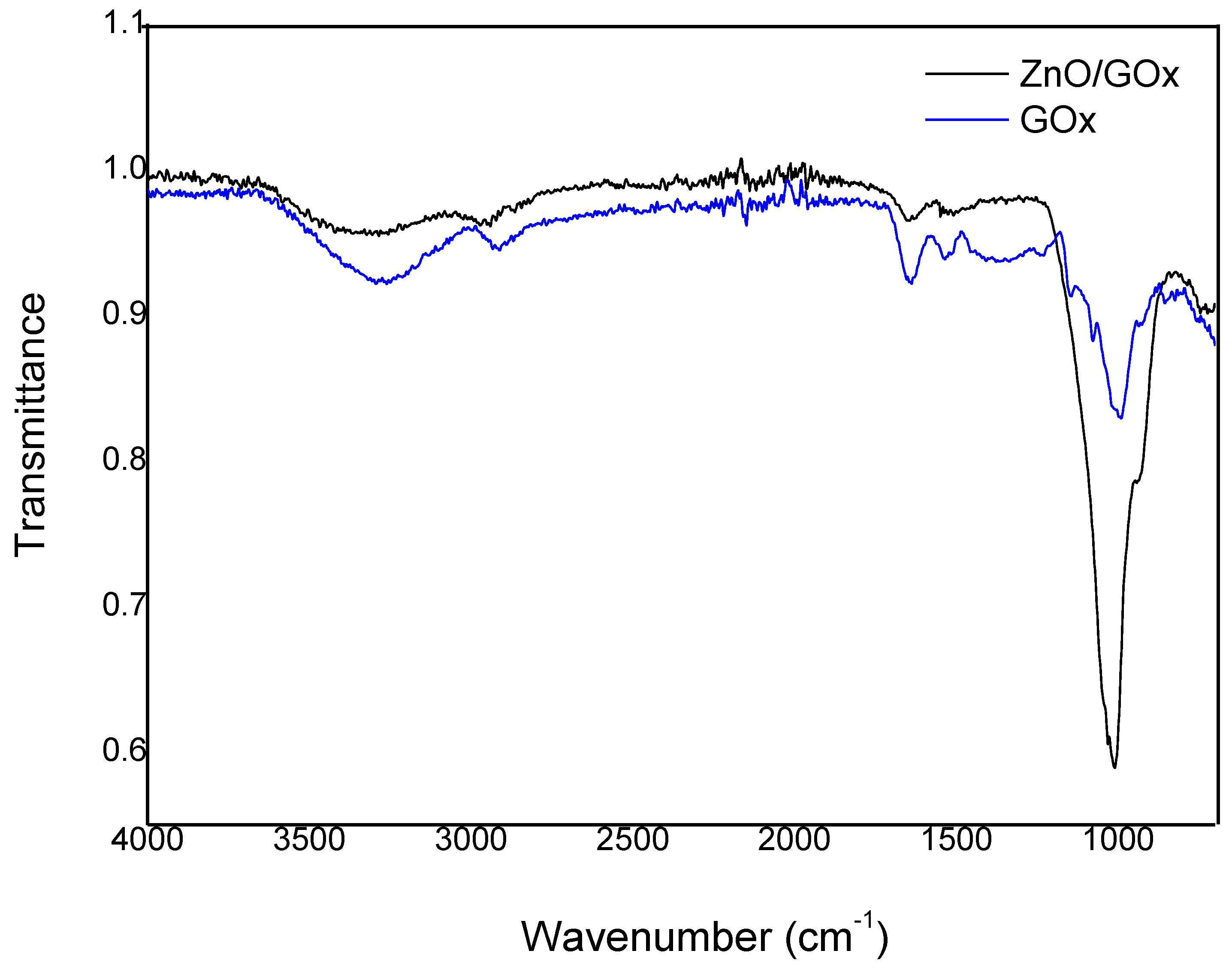
2.5. FTIR Analysis
2.6. Assessement of Enzymatic Activities
2.7. The Bioconjugate (GOx/ZnONPs) Preparation
2.8. GOx/ZnONPs Experimental Design and Application of solution in Bread Making
2.9. Volume of Bread Was Measured by Seed Displacement Apparatus
2.10. Enumeration of Yeast and Mold in Bread by Colony Count—GLA Method
2.11. Sensory Evaluation of Bread Quality Attributes
2.12. Analytical Statistics
3. Findings and Analysis
3.1. Assay Strategy
3.2. ZnONPs Synthesis
3.3. GOx Immobilization on ZnONPs
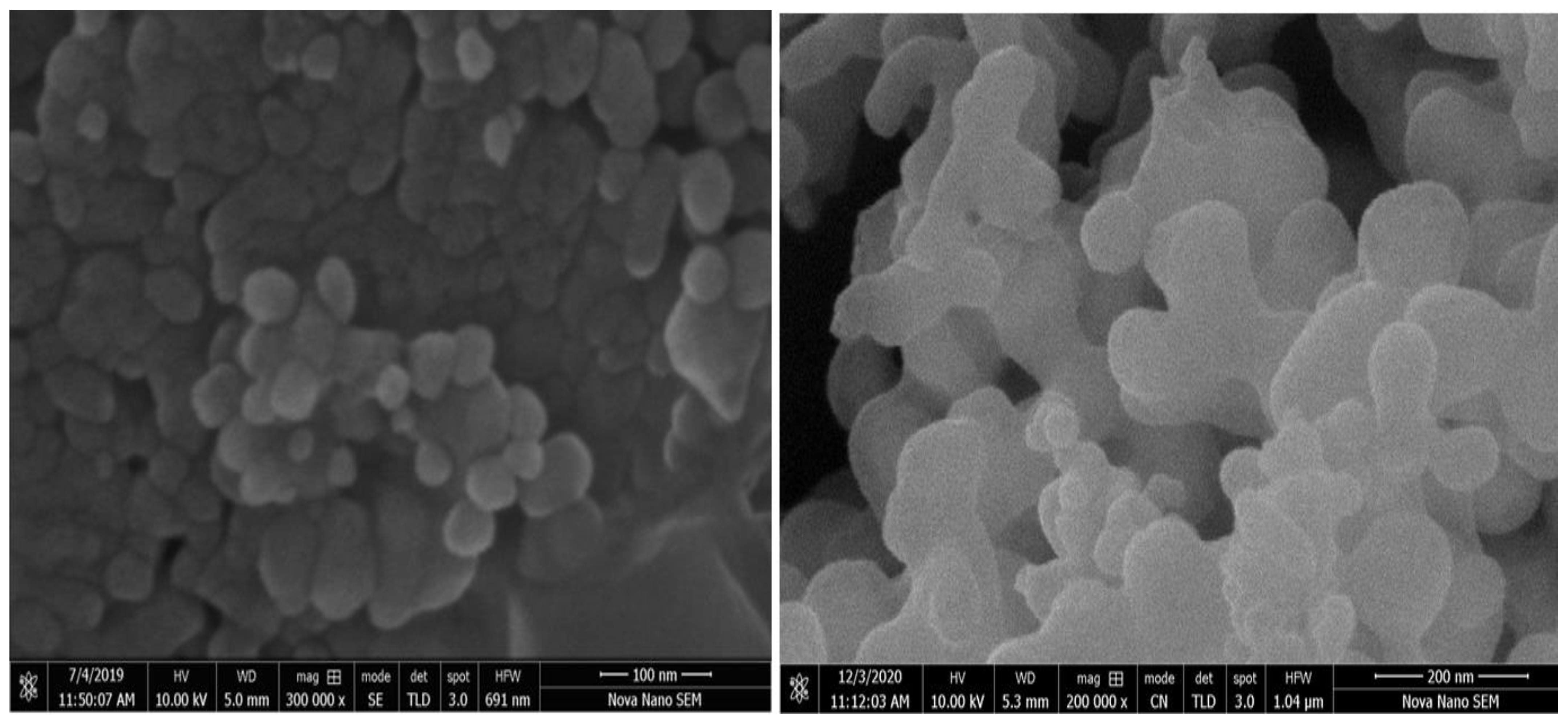
3.4. GOx/ZnONPs Bioconjugate SEM Imaging
3.5. Analysis of the Free and Adsorbed Enzyme’s Function
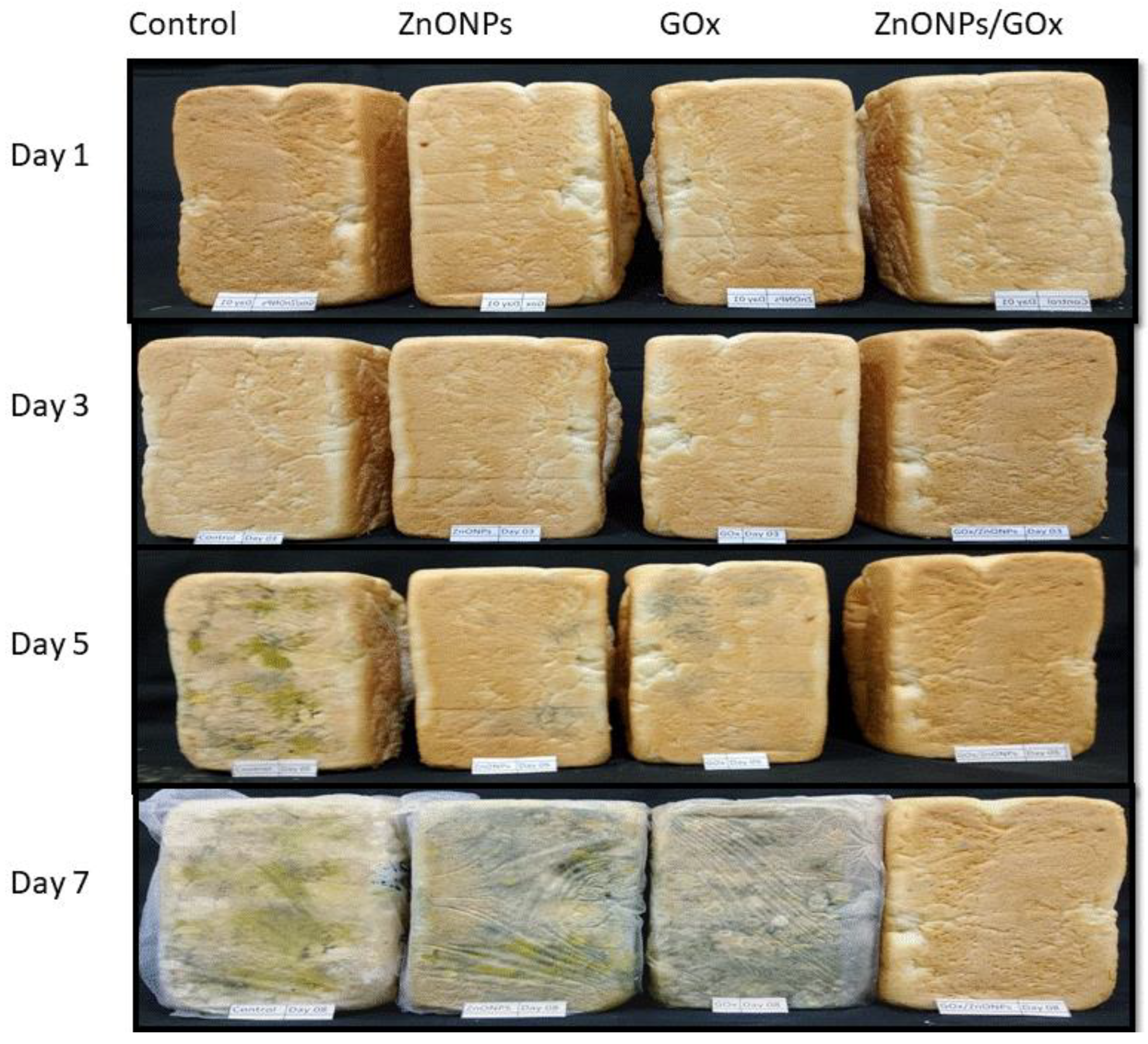
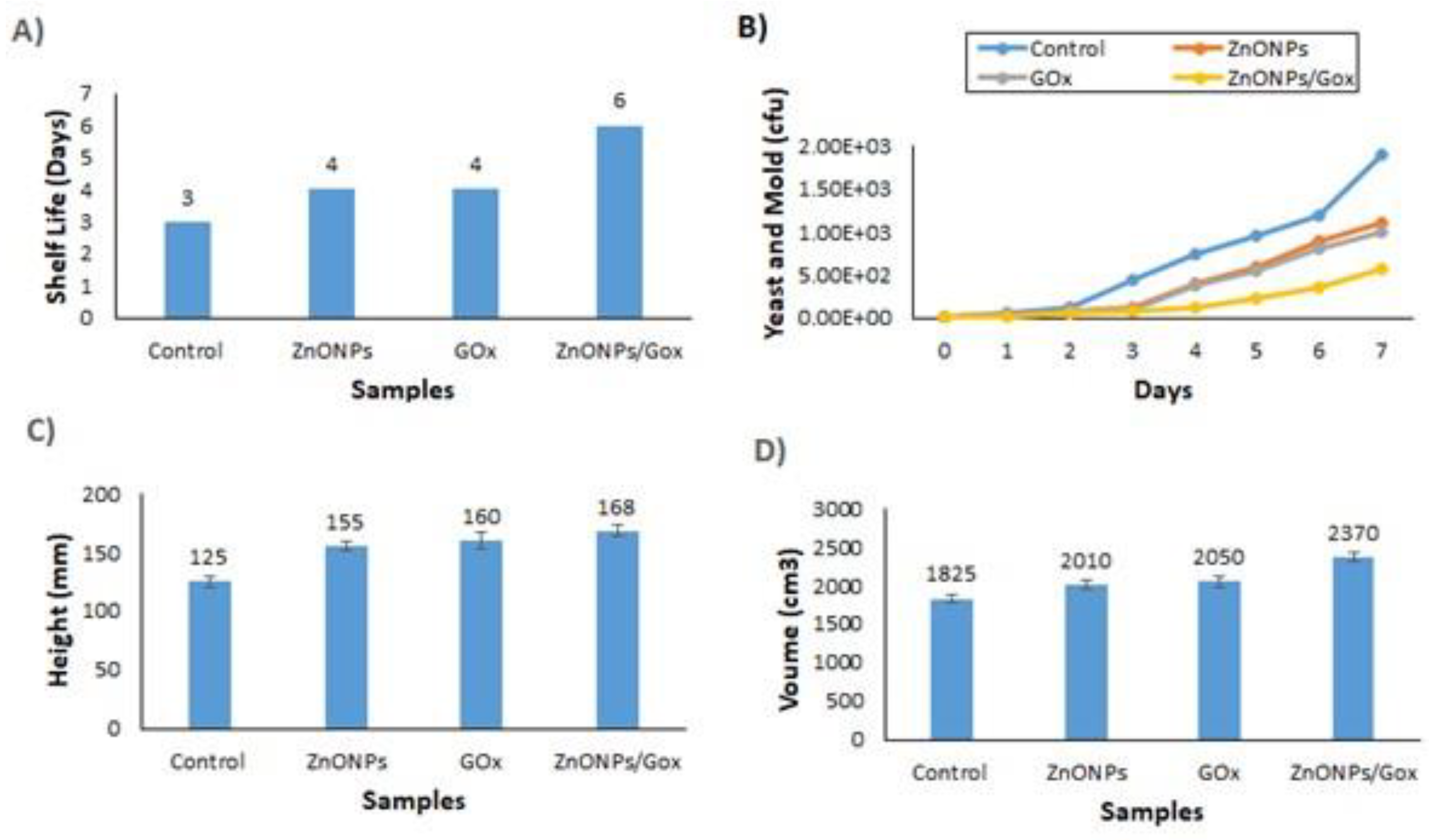
3.6. Impact on Bread’s Shelf Life of GOx/ZnONPs Bioconjugate Solution
3.7. Bread Quality Parameters
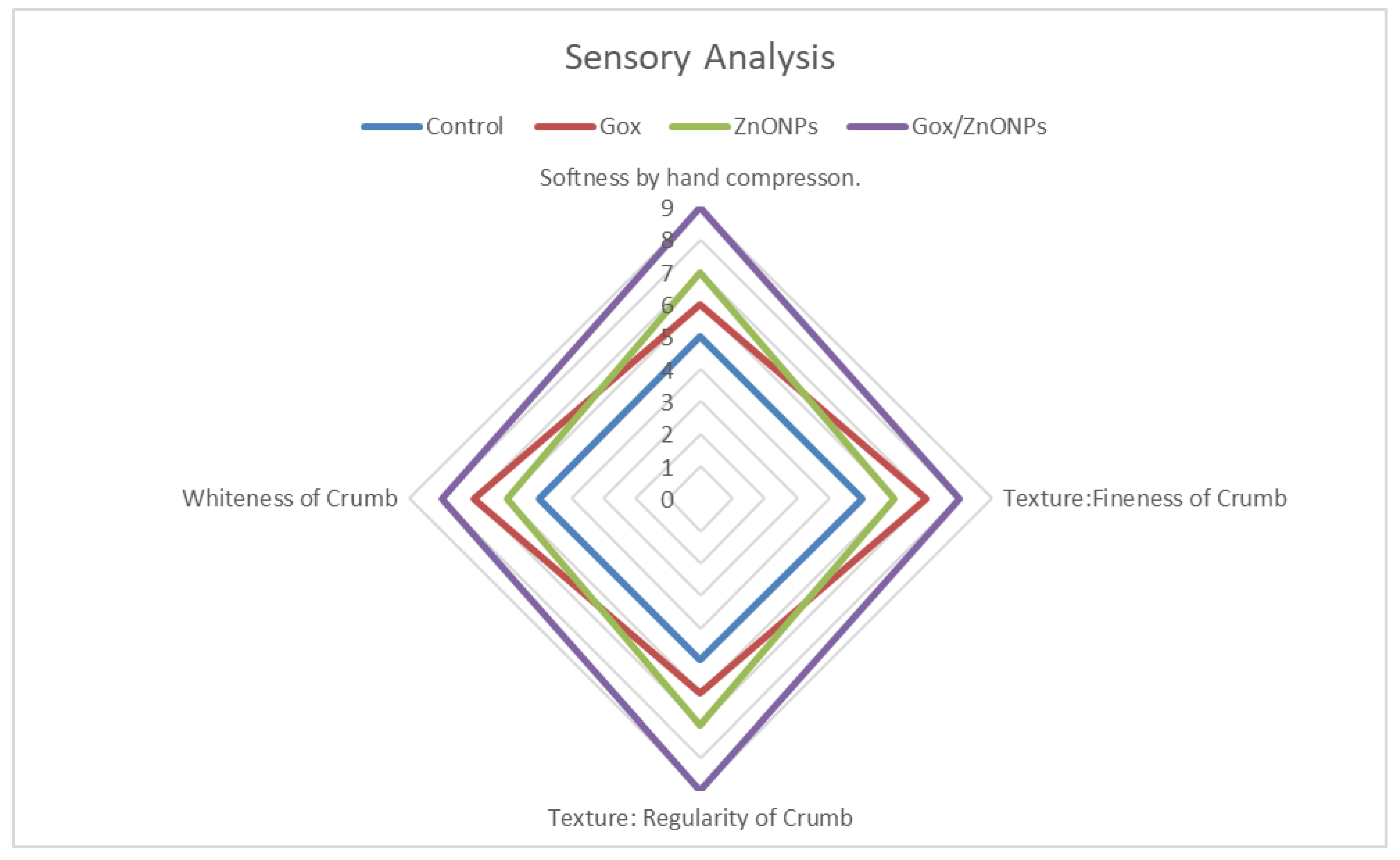
3.8. Sensory Evaluation of Bread Quality Attributes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, L.; Zhang, M.; Bhandari, B.; Gao, Z. Recent developments in novel shelf-life extension technologies of fresh-cut fruits and vegetables. Trends Food Sci. Technol. 2017, 64, 23–28. [Google Scholar] [CrossRef]
- Dewettinck, K.; Van Bockstaele, F.; Kühne, B.; Van de Walle, D.; Courtens, T.M.; Gellynck, X. Nutritional value of bread: Influence of processing, food interaction and consumer perception. J. Cereal Sci. 2008, 48, 243–257. [Google Scholar] [CrossRef]
- Bakare, A.H.; Osundahunsi, O.F.; Joseph, O. Olusanya Rheological, baking, and sensory properties of composite bread dough with breadfruit (Artocarpus communis Forst) and wheat flours. Food Sci. Nutr. 2016, 4, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Kouassi-Koffi, J.D.; Sturza, A.; Păucean, A.; Man, S.; Mureșan, A.E.; Petruț, G.; Mureșan, V.; Muste, S. Effect of glucose oxidase addition on the textural characteristics of wheat-maize dough and bread. Food Sci. Technol. 2018, 39, 127–133. [Google Scholar] [CrossRef]
- Pejcz, E.; Burešová, I. Rheological Characteristics of Model Gluten-Free Dough with Plantago Seeds and Husk Incorporation. Foods 2022, 11, 536. [Google Scholar] [CrossRef]
- Balarabe, M.M.; Mohammed, S.S.D.; Orukotan, A.A. Physico-Chemical Analysis and Sensory Evaluation of Bread Produced Using Different Indigenous Yeast Isolates. Sci. World J. 2017, 12, 33–37. [Google Scholar]
- Steffolani, M.E.; Ribotta, P.D.; Pérez, G.T.; León, A.E. Combinations of glucose oxidase, alpha-amylase and xylanase affect dough properties and bread quality. Int. J. Food Sci. 2012, 47, 525–534. [Google Scholar] [CrossRef]
- Steffolani, M.E.; Ribotta, P.D.; Pérez, G.T.; León, A.E. Effect of glucose oxidase, transglutaminase, and pentosanase on wheat proteins: Relationship with dough properties and bread-making quality. J. Cereal Sci. 2010, 51, 366–373. [Google Scholar] [CrossRef]
- Singh, J.; Verma, N. Glucose oxidase from Aspergillus niger: Production, characterization, and immobilization for glucose oxidation. Adv. Appl. Sci. Res. 2013, 4, 250–257. [Google Scholar]
- Kerman, F.K.P.; Salehifar, M.; Mirzaei, M. Investigation of the possibility of glucose oxidase enzyme in combination of ascorbic acid for improving quality of frozen dough and its resulted bread. Eur. J. Exp. Biol. 2014, 4, 716–721. [Google Scholar]
- Wong, C.M.; Wong, K.H.; Chen, X.D. Glucose oxidase: Natural occurrence, function, properties and industrial applications. Appl. Microbiol. Biotechnol. 2008, 78, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, Y.; Wang, Y.; Huang, H.; Bai, Y.; Su, X.; Zhang, J.; Yao, B.; Tu, T.; Luo, H. Exploiting the activity-stability trade-off of glucose oxidase from Aspergillus niger using a simple approach to calculate thermostability of mutants. Food Chem. 2021, 342, 128270. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration—FDA; Center for Food Safety and Applied Nutrition—CFSAN. Agency Response Letter: GRAS Notice No. GRN 000106, Agency Response Letter: GRAS Notice No. GRN 000089; FDA: Silver Spring, MD, USA, 2002.
- Decamps, K.; Joye, I.J.; Courtin, C.M.; Delcour, J.A. Glucose and pyranose oxidase improve bread dough stability. J. Cereal Sci. 2012, 55, 380–384. [Google Scholar] [CrossRef]
- Figoni, P.I. Bleaching, and Maturing Agents: How Baking Works: Exploring the Fundamentals of Baking Science, 1st ed.; Wiley: Hoboken, NJ, USA, 2003; p. 71. [Google Scholar]
- Moore, M.M.; Chen, T. Mutagenicity of bromate: Implications for cancer risk assessment. Toxicology 2006, 221, 190–196. [Google Scholar] [CrossRef]
- Hanft, F.; Koehler, P. Studies on the effect of glucose oxidase in bread making. J. Sci. Food Agric. 2006, 86, 1699–1704. [Google Scholar] [CrossRef]
- Bonet, A.; Rosell, C.M.; Caballero, P.A.; Gómez, M.; Pérez-Munuera, I.; Lluch, M.A. Glucose oxidase effect on dough rheology and bread quality: A study from macroscopic to molecular level. Food Chem. 2006, 99, 408–415. [Google Scholar] [CrossRef]
- Vemulapalli, V.; Hoseney, R.C. Glucose oxidase effects on gluten and water solubles. Cereal Chem. 1998, 75, 859–862. [Google Scholar] [CrossRef]
- Rasiah, I.A.; Sutton, K.H.; Low, F.L.; Lin, H.M.; Gerrard, J.A. Crosslinking of wheat dough proteins by glucose oxidase and the resulting effects on bread and crissants. Food Chem. 2005, 89, 325–332. [Google Scholar] [CrossRef]
- Ge, J.; Jiang, X.; Liu, W.; Wang, Y.; Huang, H.; Bai, Y.; Su, X.; Yao, B.; Luo, H. Characterization, stability improvement, and bread baking applications of a novel cold-adapted glucose oxidase from Cladosporium neopsychrotolerans SL16. Food Chem. 2020, 310, 125970. [Google Scholar] [CrossRef]
- Szweda, R.T.; Schmidt, K.; Zorn, H. Bleaching of colored whey and milk by a multiple-enzyme system. Eur. Food Res. Technol. 2013, 237, 377–384. [Google Scholar] [CrossRef]
- Valencia, P.; Espinoza, K.; Ramirez, C.; Franco, W.; Urtubia, A. Technical feasibility of glucose oxidase as a prefermentation treatment for lowering the alcoholic degree of red wine. Am. J. Enol. Vitic. 2017, 68, 386–389. [Google Scholar] [CrossRef]
- Xu, D.; Sun, L.; Li, C.; Wang, Y.; Ye, R. Inhibitory effect of glucose oxidase from Bacillus sp. CAMT22370 on the quality deterioration of Pacific white shrimp during cold storage. LWT—Food Sci. Tech. 2018, 92, 339–346. [Google Scholar] [CrossRef]
- Visvanathan, R.; Jayathilake, C.; Liyanage, R.; Sivakanesan, R. Applicability and reliability of the glucose oxidase method in assessing α-amylase activity. Food Chem. 2019, 275, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Phiri, M.M.; Mulder, D.W.; Mason, S.; Vorster, B.C. Facile immobilization of glucose oxidase onto gold nanostars with enhanced binding affinity and optimal function. R. Soc. Open Sci. 2019, 6, 190205. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Amiri, R.; Bordbar, A.K.; Ranjbakhsh, E.; Khosropour, A.R. Improvement of the stability and activity of immobilized glucose oxidase on modified iron oxide magnetic nanoparticles. Appl. Surf. Sci. 2016, 364, 752–757. [Google Scholar] [CrossRef]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef]
- Golikova, E.P.; Lakina, N.V.; Grebennikova, O.V.; Matveeva, V.G.; Sulman, E.M. A study of biocatalysts based on glucose oxidase. Faraday Discuss. 2017, 202, 303–314. [Google Scholar] [CrossRef]
- Morshed, M.N.; Behary, N.; Bouazizi, N.; Guan, J.; Chen, G.; Nierstrasz, V. Surface modification of polyester fabric using plasma-dendrimer for robust immobilization of glucose oxidase enzyme. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, K.X.; Zhou, H.M. Immobilization of glucose oxidase in alginate-chitosan microcapsules. Int. J. Mol. Sci. 2011, 12, 3042–3054. [Google Scholar] [CrossRef]
- Kazmi, S.A.R.; Qureshi, M.Z.; Masson, J.F. Drug-Based Gold Nanoparticles Overgrowth for Enhanced SPR Biosensing of Doxycycline. Biosensors 2020, 10, 184. [Google Scholar] [CrossRef]
- Lee, C.K.; Au Duong, A.N. Enzyme Immobilization on Nanoparticles: Recent Applications. In Emerging Areas in Bioengineering; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 67–80. [Google Scholar]
- Akbar, A.; Anal, A.K. Zinc oxide nanoparticles loaded active packaging, a challenge study against Salmonella typhimurium and Staphylococcus aureus in ready-to-eat poultry meat. Food Control 2014, 38, 88–95. [Google Scholar] [CrossRef]
- Bezbradica, D.I.; Mateo, C.; Guisan, J.M. Novel support for enzyme immobilization prepared by chemical activation with cysteine and glutaraldehyde. J. Mol. Catal. 2014, 102, 218–224. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, H.; Huang, X.; Zhang, J.; Guo, S. Effect of substrate (ZnO) morphology on enzyme immobilization and its catalytic activity. Nanoscale Res. Lett. 2011, 6, 450. [Google Scholar] [CrossRef] [PubMed]
- Patil, H.V.; Nithin, K.S.; Sachhidananda, S.; Chandrashekara, K.T.; Kumar, S. Mycofabrication of bioactive silver nanoparticle: Photo catalysed synthesis and characterization to attest its augmented bio-efficacy. Arab. J. Chem. 2019, 12, 4596–4611. [Google Scholar] [CrossRef]
- Morris, E.O.; Eddy, A.A. Method for the measurement of wild yeast infection in pitching yeast. J. Inst. Brew. 1957, 63, 34–35. [Google Scholar] [CrossRef]
- Singh, R.P.; Shukla, V.K.; Yadav, R.S.; Sharma, P.K.; Singh, P.K.; Pandey, A.C. Biological approach of zinc oxide nanoparticles formation and its characterization. Adv. Mater. Lett. 2011, 2, 313–317. [Google Scholar] [CrossRef]
- Wang, H.; Li, C.; Zhao, H.; Li, R.; Liu, J. Synthesis, characterization, and electrical conductivity of ZnO with different morphologies. Powder Technol. 2013, 239, 266–271. [Google Scholar] [CrossRef]
- Baghayeri, M. Glucose sensing by a glassy carbon electrode modified with glucose oxidase and a magnetic polymeric nanocomposite. RSC Adv. 2015, 5, 18267–18274. [Google Scholar] [CrossRef]
- Paul, A.; Srivastava, D.N. Amperometric Glucose Sensing at Nanomolar Level Using MOF-Encapsulated TiO2 Platform. ACS Omega 2018, 3, 14634–14640. [Google Scholar] [CrossRef]
- Liu, H.; Tao, C.A.; Hu, Z.; Zhang, S.; Wang, J.; Zhan, Y. An electrochemical glucose biosensor based on graphene composites: Use of dopamine as reducing monomer and as site for covalent immobilization of enzyme. RSC Adv. 2014, 4, 43624–43629. [Google Scholar] [CrossRef]
- Kazmi, S.A.R.; Qureshi, M.Z.; Ali, S.; Masson, J.F. In Vitro Drug Release and Biocatalysis from pH-Responsive Gold Nanoparticles Synthesized Using Doxycycline. Langmuir 2019, 35, 16266–16274. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, J.; Wang, C.; Li, D.; Ding, L.; Han, Y. Immobilization of glucose oxidase using CoFe2O4/SiO2 nanoparticles as carrier. Appl. Surf. Sci. 2011, 257, 5739–5745. [Google Scholar] [CrossRef]
- Lopes, F.M.; Batista, K.d.A.; Batista, G.L.A.; Fernandes, K.F. Biosensor for determination of glucose in real samples of beverages. Food Sci. Tech. 2012, 32, 65–69. [Google Scholar] [CrossRef]
- Tiwari, V.; Mishra, N.; Gadani, K.; Solanki, P.S.; Shah, N.A.; Tiwari, M. Mechanism of anti-bacterial activity of zinc oxide nanoparticle against Carbapenem-Resistant Acinetobacter baumannii. Front. Microbiol. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Desikan, R.; Cheung, M.K.; Bright, J.; Henson, D.; Hancock, J.T.; Neill, S.J. ABA, hydrogen peroxide and nitric oxide signalling in stomatal guard cells. J. Exp. Bot. 2004, 55, 205–212. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, J.; Khurshid, S.; Sarwar, A.; Aziz, T.; Naveed, M.; Ali, U.; Makhdoom, S.I.; Nadeem, A.A.; Khan, A.A.; Sameeh, M.Y.; et al. Enhancing Bread Quality and Shelf Life via Glucose Oxidase Immobilized on Zinc Oxide Nanoparticles—A Sustainable Approach towards Food Safety. Sustainability 2022, 14, 14255. https://doi.org/10.3390/su142114255
Khan J, Khurshid S, Sarwar A, Aziz T, Naveed M, Ali U, Makhdoom SI, Nadeem AA, Khan AA, Sameeh MY, et al. Enhancing Bread Quality and Shelf Life via Glucose Oxidase Immobilized on Zinc Oxide Nanoparticles—A Sustainable Approach towards Food Safety. Sustainability. 2022; 14(21):14255. https://doi.org/10.3390/su142114255
Chicago/Turabian StyleKhan, Jahangir, Shazia Khurshid, Abid Sarwar, Tariq Aziz, Muhammad Naveed, Urooj Ali, Syeda Izma Makhdoom, Abad Ali Nadeem, Ayaz Ali Khan, Manal Y. Sameeh, and et al. 2022. "Enhancing Bread Quality and Shelf Life via Glucose Oxidase Immobilized on Zinc Oxide Nanoparticles—A Sustainable Approach towards Food Safety" Sustainability 14, no. 21: 14255. https://doi.org/10.3390/su142114255
APA StyleKhan, J., Khurshid, S., Sarwar, A., Aziz, T., Naveed, M., Ali, U., Makhdoom, S. I., Nadeem, A. A., Khan, A. A., Sameeh, M. Y., Alharbi, A. A., Filimban, F. Z., Rusu, A. V., Göksen, G., & Trif, M. (2022). Enhancing Bread Quality and Shelf Life via Glucose Oxidase Immobilized on Zinc Oxide Nanoparticles—A Sustainable Approach towards Food Safety. Sustainability, 14(21), 14255. https://doi.org/10.3390/su142114255













