Review of Life Cycle Assessments for Steel and Environmental Analysis of Future Steel Production Scenarios
Abstract
1. Introduction
2. Environmental Allocation Approaches for Primary and Secondary Steel Production
- The recycled content approach: The scrap does not have an environmental burden, which means neither an environmental footprint is taken into account when scrap is used nor the recycling credit at the end-of-life is considered.
- The end-of-life recycling approach: Scrap has an environmental footprint. Therefore, an environmental burden has to be considered when scrap is used, and credit is given when the material is recycled at the end-of-life.
- The demand of scrap is far above the supply. Scrap has a high economic value, which means that where scrap is recovered it will be used for recycling. Consequently, there is no need to additionally create a demand for recycled material since this market is already mature. For metals where there is a limited supply of recycled feedstock, market stimulation is ineffective and may result in inefficient processing and unnecessary transportation.
- Steel has inherent properties so it can be recycled almost an unlimited number of times. Although, in general, within the primary steel production routes, higher steel grades can be produced in comparison to the secondary steel production route, secondary steel production replaces primary steel production. As long as the scrap is recycled and the products are in demand, it does not matter in which area of application the steel is used.
3. Life Cycle Assessment (LCA) of State-of-the-Art Steel Production Routes
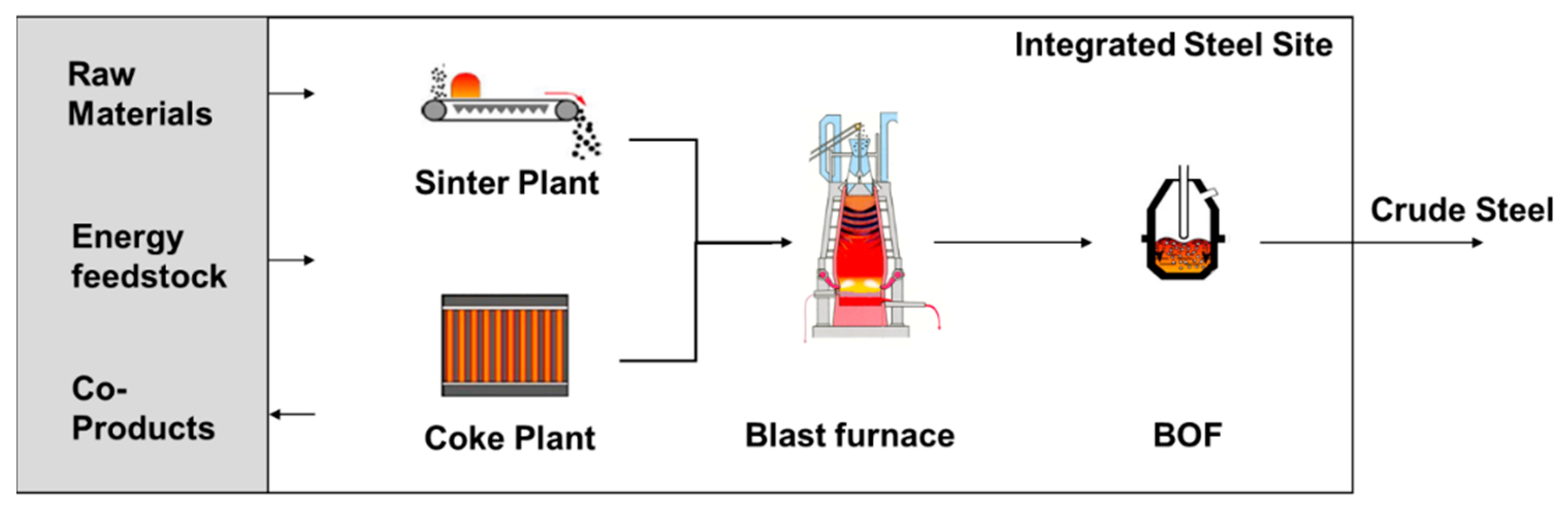
3.1. LCA Overview of the Primary Blast Furnace-Related Steel Production Route
| Study | Year | Product | Methodology | Impact Categories | |||||
|---|---|---|---|---|---|---|---|---|---|
| kg | Scrap | Co-Products | Impact Method | GWP kg CO2 eq | AP kg SO2 eq | ADPf MJ | CED MJ | ||
| [32] | 2007 | Steel | n. s. | n. s. | n. s. | 2.3 | 0.020 | n. a. | 23 |
| [21] | 2012 | HRC | MRA | SE | CML 16 | 1.0 | 3.0 × 10−3 | 12 | 15 |
| [21] | 2012 | HRC | RC | SE | CML 16 | 1.7 | 4.0 × 10−3 | 24 | 24 |
| [33] | 2013 | Steel | n. s. | SE | Recipe Midpoint | 1.7 | 5.0 × 10−3 | n. a. | 25 |
| [34] | 2016 | Steel | n. s. | Allocation | ILCD | 1.6 | n. a. | n. a. | 23 |
| [36] | 2019 | HRC | RC | n. s. | CML 16 | 2.1 | 1.6 × 10−4 | 5.3 | n. a. |
| [38] | 2021 | HRC | RC | SE | CML 16 | 2.1 | 4.8 × 10−3 | 21 | n. a. |
3.2. LCA Overview of the Secondary Scrap-Based Steel Production Route
4. Modifications of the Blast Furnace Steel Production Route
4.1. Use of Hydrogen in a Blast Furnace
4.2. Use of Pre-Reduced Iron Ores in the Blast Furnace
5. Direct Reduced Iron (DRI) Production with Electrical Melting
5.1. Natural Gas-Based Direct Reduction with Electrical Melting
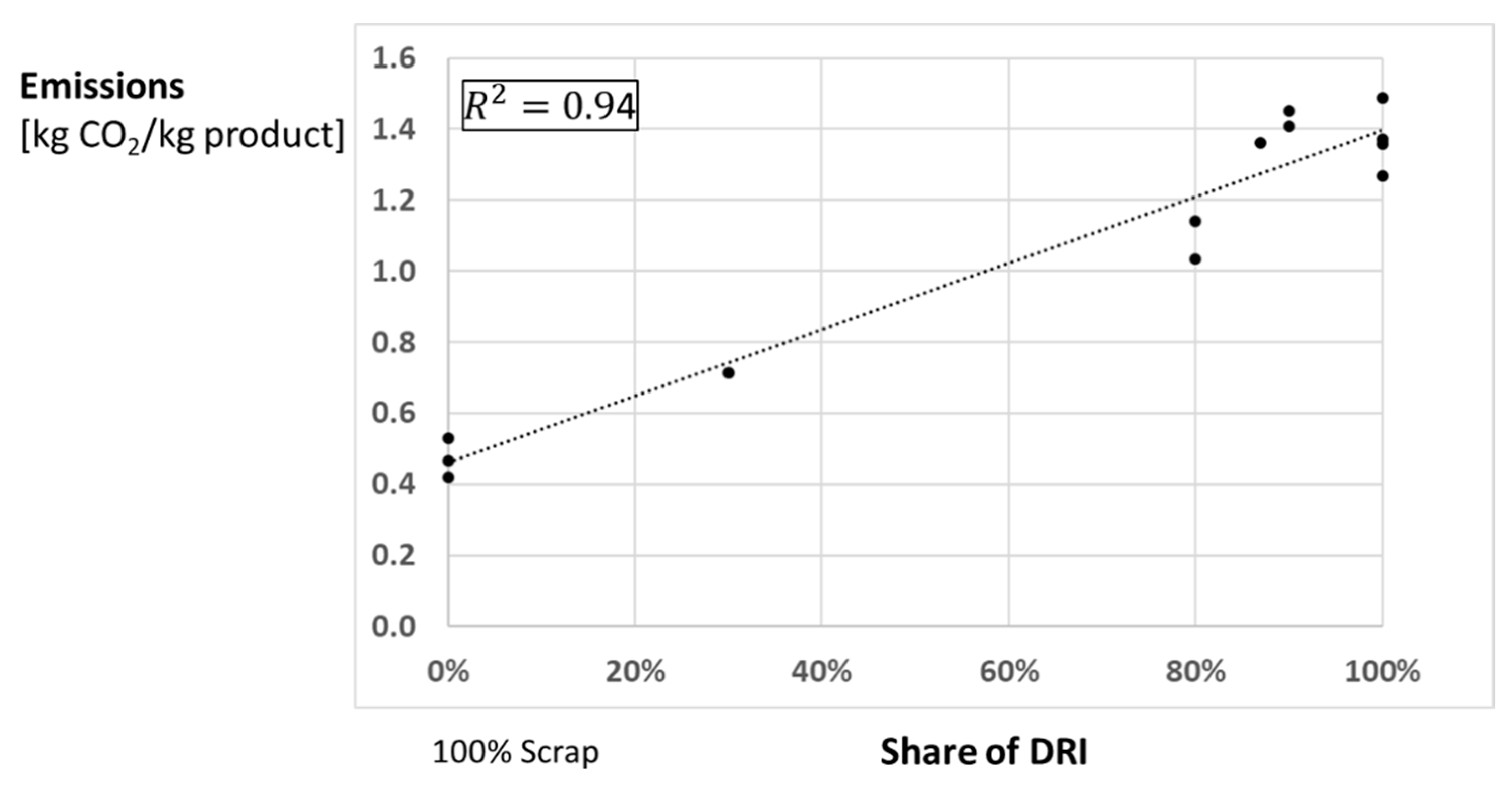
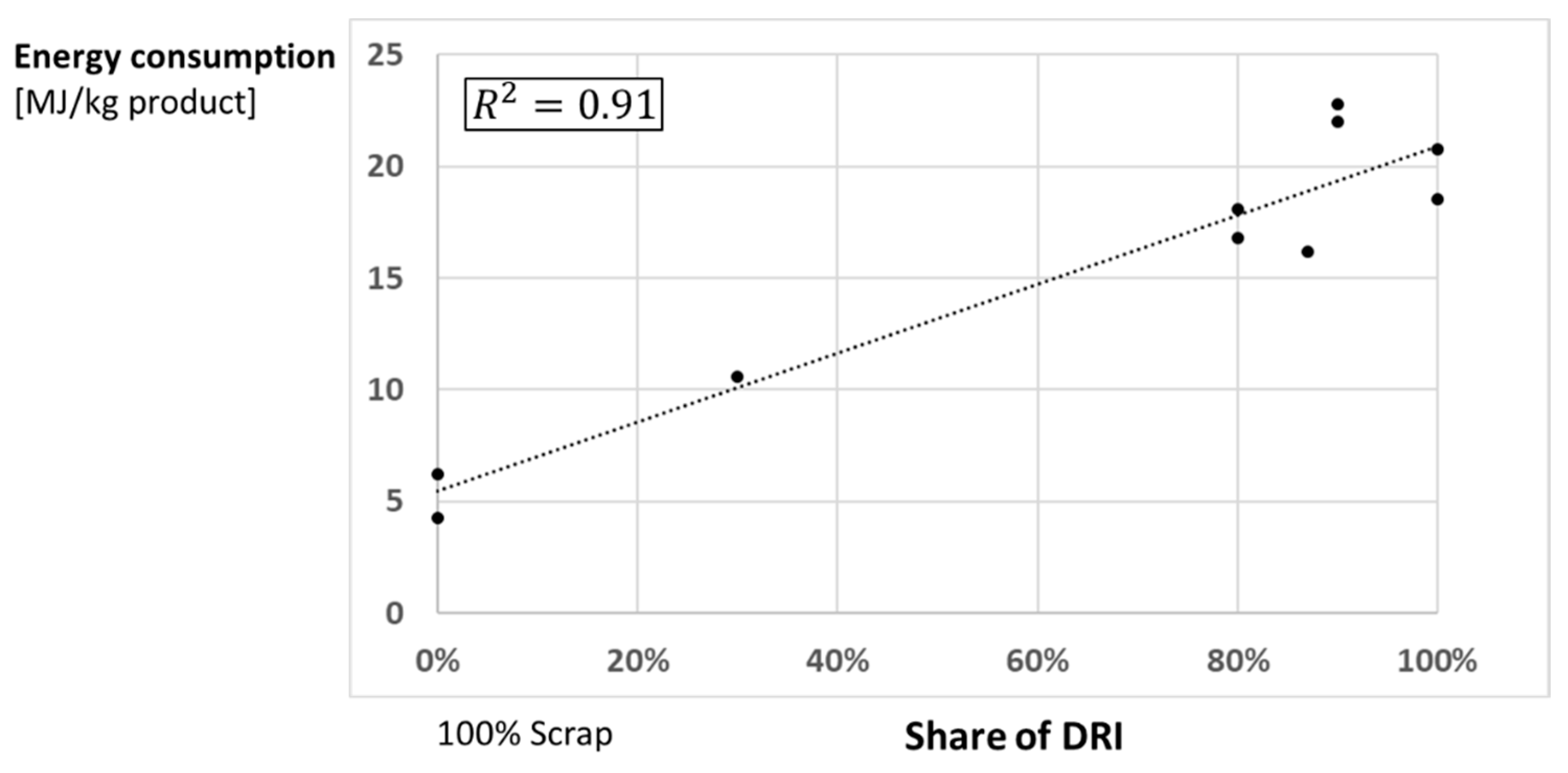
5.2. Hydrogen-Based Direct Reduction with Electrical Melting
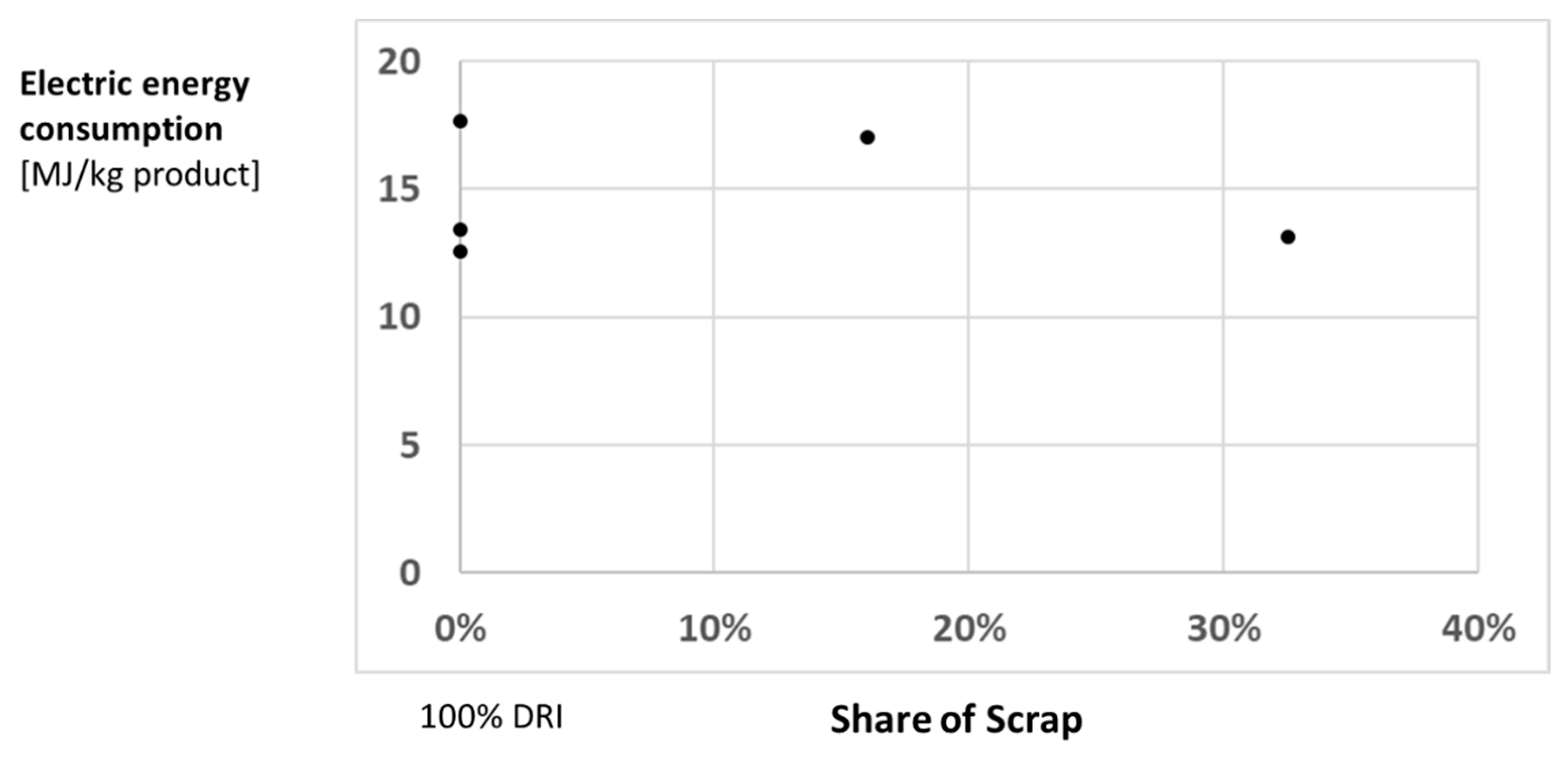
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- IPCC. Climate Change 2021: The Physical Science Basis. In Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- IEA (International Energy Agency). Iron and Steel Technology Roadmap—Towards More Sustainable Steelmaking. 2020, pp. 38–43. Available online: https://iea.blob.core.windows.net/assets/eb0c8ec1-3665-4959-97d0-187ceca189a8/Iron_and_Steel_Technology_Roadmap.pdf (accessed on 26 January 2022).
- Wang, P.; Ryberg, M.; Yang, Y.; Feng, K.; Kara, S.; Hauschild, M.; Chen, W.-Q. Efficiency stagnation in global steel production urges joint supply- and demand-side mitigation efforts. Nat. Commun. 2021, 12, 2066. [Google Scholar] [CrossRef] [PubMed]
- Nidheesh, P.V.; Kumar, M.S. An overview of environmental sustainability in cement and steel production. J. Clean. Prod. 2019, 231, 856–871. [Google Scholar] [CrossRef]
- Hasanbeigi, A.; Arens, M.; Price, L. Alternative emerging ironmaking technologies for energy-efficiency and carbon dioxide emissions reduction: A technical review. Renew. Sustain. Energy Rev. 2014, 33, 645–658. [Google Scholar] [CrossRef]
- Ariyama, T.; Takahashi, K.; Kawashiri, Y.; Nouchi, T. Diversification of the Ironmaking Process Toward the Long-Term Global Goal for Carbon Dioxide Mitigation. J. Sustain. Met. 2019, 5, 276–294. [Google Scholar] [CrossRef]
- Pardo, N.; Moya, J.A. Prospective scenarios on energy efficiency and CO2 emissions in the European Iron & Steel industry. Energy 2013, 54, 113–128. [Google Scholar] [CrossRef]
- ISO 14040; DIN EN ISO 14040:2006 + Amd 1:2020. Environmental Management—Life Cycle Assessment—Principles and Framework. ISO: Geneva, Switzerland, 2006.
- ISO 14044; DIN EN ISO 14044:2006 + Amd 1:2017 + Amd 2:2020. Environmental Management—Life Cycle Assessment—Requirements and Guidelines. ISO: Geneva, Switzerland, 2006.
- Liang, T.; Wang, S.; Lu, C.; Jiang, N.; Long, W.; Zhang, M.; Zhang, R. Environmental impact evaluation of an iron and steel plant in China: Normalized data and direct/indirect contribution. J. Clean. Prod. 2020, 264, 121697. [Google Scholar] [CrossRef]
- Olmez, G.M.; Dilek, F.B.; Karanfil, T.; Yetis, U. The environmental impacts of iron and steel industry: A life cycle assessment study. J. Clean. Prod. 2016, 130, 195–201. [Google Scholar] [CrossRef]
- Koltun, P.; Klymenko, V. Cradle-to-gate life cycle assessment of the production of separated mix of rare earth oxides based on Australian production route. Min. Miner. Deposits 2020, 14, 1–15. [Google Scholar] [CrossRef]
- Tönjes, A.; Lechtenböhmer, S.; Leipprand, A.; Zelt, O. Klimaneutraler Stahl Made in Germany: Transformationsherausforderungen im Kontext steigender Marktanforderungen. 2022. Available online: https://epub.wupperinst.org/frontdoor/deliver/index/docId/7924/file/7924_Toenjes.pdf (accessed on 10 October 2022).
- Liu, W.; Zuo, H.; Wang, J.; Xue, Q.; Ren, B.; Yang, F. The production and application of hydrogen in steel industry. Int. J. Hydrogen Energy 2021, 46, 10548–10569. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, Y.; Babich, A.; Senk, D.; Fan, X. Hydrogen direct reduction (H-DR) in steel industry—An overview of challenges and opportunities. J. Clean. Prod. 2021, 329, 129797. [Google Scholar] [CrossRef]
- WSA. World Steel Association: World Steel in Figures. 2021. Available online: https://worldsteel.org/wp-content/uploads/2021-World-Steel-in-Figures.pdf (accessed on 26 January 2022).
- WSA. World Steel Association: Life Cycle Assessment Methodology Report; World Steel Association: Brussels, Belgium, 2011. [Google Scholar]
- WSA. World Steel Association: World Steel Life Cycle Inventory Methodology Report; World Steel Association: Brussels, Belgium, 2000. [Google Scholar]
- Atherton, J. Declaration by the Metals Industry on Recycling Principles. Int. J. Life Cycle Assess. 2007, 12, 59–60. [Google Scholar] [CrossRef]
- Birat, J.-P.; Prum, N.; Yonezawa, K.; Aboussouan, L. The value of recycling to society and its internalization into LCA methodology. Rev. Met. 2006, 103, 50–61. [Google Scholar] [CrossRef]
- Neugebauer, S.; Finkbeiner, M. Ökobilanz nach ISO 14040/44 für das Multirecycling von Stahl; Wirtschaftsvereinigung Stahl: Düsseldorf, Germany, 2012. [Google Scholar]
- Finkbeiner, M.; Bach, V.; Lehmann, A. Environmental Footprint. Der Umweltfußabdruck von Produkten und Dienstleistungen—Abschlussbericht; On behalf of German Federal Environment Agency; Umweltbundesamt: Dessau-Roßlau, Germany, 2018; ISSN 1862-4804. [Google Scholar]
- European Union (EU). Product Environmental Footprint Category Rules (PEFCR) for Metal Sheets for Various Applications. 2019. Available online: https://ec.europa.eu/environment/eussd/smgp/PEFCR_OEFSR_en.htm (accessed on 26 January 2022).
- Frischknecht, R. LCI modelling approaches applied on recycling of materials in view of environmental sustainability, risk perception and eco-efficiency. Int. J. Life Cycle Assess. 2010, 15, 666–671. [Google Scholar] [CrossRef]
- Yellishetty, M.; Mudd, G.M.; Ranjith, P.; Tharumarajah, A. Environmental life-cycle comparisons of steel production and recycling: Sustainability issues, problems and prospects. Environ. Sci. Policy 2011, 14, 650–663. [Google Scholar] [CrossRef]
- Reale, F.; Buttol, P.; Cortesi, S.; Mengarelli, M.; Masoni, P.; Scalbi, S.; Zamagni, A. Dealing with LCA Modeling for the end of life of mechatronic products. Environ. Eng. Manag. J. 2015, 14, 1691–1704. [Google Scholar] [CrossRef]
- Mengarelli, M.; Neugebauer, S.; Finkbeiner, M.; Germani, M.; Buttol, P.; Reale, F. End-of-life modelling in life cycle assessment—Material or product-centred perspective? Int. J. Life Cycle Assess. 2016, 22, 1288–1301. [Google Scholar] [CrossRef]
- Volkhausen, W. Methodische Beschreibung und Bewertung der umweltgerechten Gestaltung von Stahlwerkstoffen und Stahlerzeugnissen. 2003. Available online: https://nbn-resolving.org/urn:nbn:de:swb:105-0625177 (accessed on 28 October 2022).
- Larsson, M.; Grip, C.-E.; Ohlsson, H.; Rutqvist, S.; Wikström, J.-O.; Ångström, S. Comprehensive Study Regarding Greenhouse Gas Emission from Iron Ore Based Production at the Integrated Steel Plant SSAB Tunnplåt AB. Int. J. Green Energy 2006, 3, 171–183. [Google Scholar] [CrossRef]
- Guidehouse. Gas Decarbonisation Pathways 2020–2050. Gas for Climate. 2020. Available online: https://gasforclimate2050.eu/wp-content/uploads/2020/04/Gas-for-Climate-Gas-Decarbonisation-Pathways-2020-2050.pdf (accessed on 28 October 2022).
- Agora. No-Regret Hydrogen. Charting Early Steps for H2 Infrastructure in Europe. 2021. Available online: https://static.agora-energiewende.de/fileadmin/Projekte/2021/2021_02_EU_H2Grid/A-EW_203_No-regret-hydrogen_WEB.pdf (accessed on 1 June 2022).
- Norgate, T.E.; Jahanshahi, S.; Rankin, W.J. Assessing the environmental impact of metal production processes. J. Clean. Prod. 2007, 15, 838–848. [Google Scholar] [CrossRef]
- Burchart-Korol, D. Life cycle assessment of steel production in Poland: A case study. J. Clean. Prod. 2013, 54, 235–243. [Google Scholar] [CrossRef]
- Renzulli, P.A.; Notarnicola, B.; Tassielli, G.; Arcese, G.; Di Capua, R. Life Cycle Assessment of Steel Produced in an Italian Integrated Steel Mill. Sustainability 2016, 8, 719. [Google Scholar] [CrossRef]
- DIN EN 19694-2; Stationary Source Emissions—Greenhouse Gas (GHG) Emissions in Energy-Intensive Industries—Part 2: Iron and Steel Industry. Deutsches Institut für Normung: Berlin, Germany, 2016.
- Chisalita, D.-A.; Petrescu, L.; Cobden, P.; van Dijk, H.; Cormos, A.-M.; Cormos, C.-C. Assessing the environmental impact of an integrated steel mill with post-combustion CO2 capture and storage using the LCA methodology. J. Clean. Prod. 2019, 211, 1015–1025. [Google Scholar] [CrossRef]
- IEA (International Energy Agency). Iron and Steel CCS Study. (Techno-Economis Integrated Steel Mill). 2013. Available online: https://ieaghg.org/docs/General_Docs/Reports/2013-19.pdf (accessed on 26 January 2022).
- Backes, J.; Suer, J.; Pauliks, N.; Neugebauer, S.; Traverso, M. Life Cycle Assessment of an Integrated Steel Mill Using Primary Manufacturing Data: Actual Environmental Profile. Sustainability 2021, 13, 3443. [Google Scholar] [CrossRef]
- Bach, V.; Finkbeiner, M. Approach to qualify decision support maturity of new versus established impact assessment methods—Demonstrated for the categories acidification and eutrophication. Int. J. Life Cycle Assess. 2017, 22, 387–397. [Google Scholar] [CrossRef]
- Khalid, Y.; Wu, M.; Silaen, A.; Martinez, F.; Okosun, T.; Worl, B.; Low, J.; Zhou, C.; Johnson, K.; White, D. Oxygen enrichment combustion to reduce fossil energy consumption and emissions in hot rolling steel production. J. Clean. Prod. 2021, 320, 128714. [Google Scholar] [CrossRef]
- Schmitz, N.; Sankowski, L.; Kaiser, F.; Schwotzer, C.; Echterhof, T.; Pfeifer, H. Towards CO2-neutral process heat generation for continuous reheating furnaces in steel hot rolling mills—A case study. Energy 2021, 224, 120155. [Google Scholar] [CrossRef]
- Dutta, S.K.; Chokshi, Y.B. Basic Concepts of Iron and Steel Making; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- MacRosty, R.D.M.; Swartz, C.L.E. Dynamic optimization of electric arc furnace operation. AIChE J. 2007, 53, 640–653. [Google Scholar] [CrossRef]
- Babich, A. Blast furnace injection for minimizing the coke rate and CO2 emissions. Ironmak. Steelmak. 2021, 48, 728–741. [Google Scholar] [CrossRef]
- Remus, R.; Aguado-Monsonet, M.; Roudier, S.; Sancho, L.D. Best Available Techniques (BAT) Reference Document for Iron and Steel Production: Industrial Emissions Directive 2010/75/EU (Integrated Pollution Prevention and Control). (No. JRC69967); Joint Research Centre (Seville Site): Seville, Spain, 2013. [Google Scholar] [CrossRef]
- Lüngen, H.B.; Schmöle, P. Comparison of Blast Furnace Operation Modes in the World. Steel Res. Int. 2020, 91, 2000182. [Google Scholar] [CrossRef]
- Gudenau, H.W.; Gebel, U.; Gerlach, W.; Grandin, F.H.; Guntermann, K.; Hoberg, H.; Kim, S.; Koerfer, M.; Mulanza, J.P.; Pintsch, S.; et al. Eisenhüttenmännische Verfahrenstechnik. Vom Erz zum Stahl; Druck-& Verlagshaus MAINZ GmbH: Aachen, Germany, 1989. [Google Scholar]
- Thyssenkrupp Steel Europe AG. Wasserstoff Statt Kohle. 2019. Available online: https://www.thyssenkrupp-steel.com/de/newsroom/pressemitteilungen/wasserstoff-statt-kohle.html (accessed on 26 January 2022).
- Spreitzer, D.; Schenk, J. Reduction of Iron Oxides with Hydrogen—A Review. Steel Res. Int. 2019, 90, 1900108. [Google Scholar] [CrossRef]
- Nogami, H.; Kashiwaya, Y.; Yamada, D. Simulation of Blast Furnace Operation with Intensive Hydrogen Injection. ISIJ Int. 2012, 52, 1523–1527. [Google Scholar] [CrossRef]
- Bernasowski, M. Theoretical Study of the Hydrogen Influence on Iron Oxides Reduction at the Blast Furnace Process. Steel Res. Int. 2014, 85, 670–678. [Google Scholar] [CrossRef]
- Yilmaz, C.; Wendelstorf, J.; Turek, T. Modeling and simulation of hydrogen injection into a blast furnace to reduce carbon dioxide emissions. J. Clean. Prod. 2017, 154, 488–501. [Google Scholar] [CrossRef]
- Schmöle, P. The blast furnace—Fit for the future? Stahl und Eisen 2016, 136, 31–40. [Google Scholar]
- de Castro, J.A.; Takano, C.; Yagi, J.-I. A theoretical study using the multiphase numerical simulation technique for effective use of H 2 as blast furnaces fuel. J. Mater. Res. Technol. 2017, 6, 258–270. [Google Scholar] [CrossRef]
- Mehmeti, A.; Angelis-Dimakis, A.; Arampatzis, G.; McPhail, S.J.; Ulgiati, S. Life Cycle Assessment and Water Footprint of Hydrogen Production Methods: From Conventional to Emerging Technologies. Environments 2018, 5, 24. [Google Scholar] [CrossRef]
- Jampani, M.; Gibson, J.; Pistorius, P.C. Increased Use of Natural Gas in Blast Furnace Ironmaking: Mass and Energy Balance Calculations. Met. Mater. Trans. A 2019, 50, 1290–1299. [Google Scholar] [CrossRef]
- De Castro, J.A.; Nogami, H.; Yagi, J.-I. Numerical Investigation of Simultaneous Injection of Pulverized Coal and Natural Gas with Oxygen Enrichment to the Blast Furnace. ISIJ Int. 2002, 42, 1203–1211. [Google Scholar] [CrossRef]
- Nogami, H.; Yagi, J.-I.; Kitamura, S.-Y.; Austin, P.R. Analysis on Material and Energy Balances of Ironmaking Systems on Blast Furnace Operations with Metallic Charging, Top Gas Recycling and Natural Gas Injection. ISIJ Int. 2006, 46, 1759–1766. [Google Scholar] [CrossRef]
- Efetürk, M.; Janz, A.; Sprecher, M.; Peter, R.; Hölsken, T. Verwendung von Koksofengas als Reduktionsmittel im Hochofenprozess. Stahl + Technik 2. 2020. Available online: https://www.stahl-und-technik.de/artikel/verwendung-von-koksofengas-als-reduktionsmittel-im-hochofenprozess (accessed on 28 September 2022).
- Suopajärvi, H.; Umeki, K.; Mousa, E.; Hedayati, A.; Romar, H.; Kemppainen, A.; Wang, C.; Phounglamcheik, A.; Tuomikoski, S.; Norberg, N.; et al. Use of biomass in integrated steelmaking—Status quo, future needs and comparison to other low-CO2 steel production technologies. Appl. Energy 2018, 213, 384–407. [Google Scholar] [CrossRef]
- Asanuma, M.; Ariyama, T.; Sato, M.; Murai, R.; Nonaka, T.; Okochi, I.; Tsukiji, H.; Nemoto, K. Development of Waste Plastics Injection Process in Blast Furnace. ISIJ Int. 2000, 40, 244–251. [Google Scholar] [CrossRef]
- Babich, A.; Senk, D.; Knepper, M.; Benkert, S. Conversion of injected waste plastics in blast furnace. Ironmak. Steelmak. 2016, 43, 11–21. [Google Scholar] [CrossRef]
- Duarte, P.; Becerra, J.; Scarnati, T. ENERGIRON direct reduction technology—Economical, flexible, environmentally friendly. Acero LatinoAmericano 2008, 6, 52–58. [Google Scholar]
- Martinez, J.; Duarte, P. Tenova HYL NEWS; Tenova HYL (HYL Technologies, S.A. de C.V.): Monterrey, Mexico, 2017. [Google Scholar]
- Schmöle, P.; Lüngen, H.B. Use of pre-reduced material in the blast furnace: Metallurgical, ecological and economic aspects. In Stahl und Eisen 2007, 127, 47–54. [Google Scholar]
- Yilmaz, C.; Turek, T. Modeling and simulation of the use of direct reduced iron in a blast furnace to reduce carbon dioxide emissions. J. Clean. Prod. 2017, 164, 1519–1530. [Google Scholar] [CrossRef]
- Dutta, S.K.; Sah, R. Direct Reduced Iron: Production. In Encyclopedia of Iron, Steel, and Their Alloys; Taylor and Francis: New York, NY, USA, 2016; pp. 1082–1108. [Google Scholar] [CrossRef]
- Müller, N.; Herz, G.; Reichelt, E.; Jahn, M. CO2 Emission Reduction Potential in the Steel Industry by Integration of a Direct Reduction Process into Existing Steel Mills (No. DGMK--2018-2). 2018. Available online: https://inis.iaea.org/collection/NCLCollectionStore/_Public/49/107/49107596.pdf?r=1 (accessed on 29 January 2022).
- Griesser, A.; Buergler, T. Use of HBI in Blast Furnace. BHM Berg- und Hüttenmännische Monatshefte 2019, 164, 267–273. [Google Scholar] [CrossRef]
- Kobe Steel. KOBELCO Group’s CO2 Reduction Solution for Blast Furnace Ironmaking. 16 February 2021. Available online: https://www.kobelco.co.jp/english/releases/files/20210216_e.pdf (accessed on 29 January 2022).
- Suer, J.; Traverso, M.; Ahrenhold, F. Carbon footprint of scenarios towards climate-neutral steel according to ISO 14067. J. Clean. Prod. 2021, 318, 128588. [Google Scholar] [CrossRef]
- Agora Energiewende. Klimaneutrale Industrie. Schlüsseltechnologien und Politikoptionen für Stahl, Chemie und Zement. Berlin, November 2019. Available online: https://www.agora-energiewende.de/veroeffentlichungen/klimaneutrale-industrie-hauptstudie/ (accessed on 8 March 2022).
- Berger, R. The Future of Steelmaking. How the European Steel Industry Can Achieve Carbon Neutrality; Roland Berger GMBH: Munich, Germany, 2020. [Google Scholar]
- Suer, J.; Ahrenhold, F.; Traverso, M. Carbon Footprint and Energy Transformation Analysis of Steel Produced via a Direct Reduction Plant with an Integrated Electric Melting Unit. J. Sustain. Met. 2022, 1–14. [Google Scholar] [CrossRef]
- Barati, M. Energy intensity and greenhouse gases footprint of metallurgical processes: A continuous steelmaking case study. Energy 2010, 35, 3731–3737. [Google Scholar] [CrossRef]
- Harada, T.; Tanaka, H. Future Steelmaking Model by Direct Reduction Technologies. ISIJ Int. 2011, 51, 1301–1307. [Google Scholar] [CrossRef]
- Arens, M.; Worrell, E.; Eichhammer, W.; Hasanbeigi, A.; Zhang, Q. Pathways to a low-carbon iron and steel industry in the medium-term—The case of Germany. J. Clean. Prod. 2017, 163, 84–98. [Google Scholar] [CrossRef]
- Kirschen, M.; Badr, K.; Pfeifer, H. Influence of direct reduced iron on the energy balance of the electric arc furnace in steel industry. Energy 2011, 36, 6146–6155. [Google Scholar] [CrossRef]
- Cardenas, J.G.G.; Conejo, A.N.; Gnechi, G.G. Optimization of energy consumption in electric arc furnaces operated with 100% DRI. Metal 2007, 2017, 1–7. [Google Scholar]
- Sarkar, S.; Bhattacharya, R.; Roy, G.G.; Sen, P.K. Modeling MIDREX Based Process Configurations for Energy and Emission Analysis. Steel Res. Int. 2017, 89, 1700248. [Google Scholar] [CrossRef]
- WSA. World Steel Association: CO2 Data Collection. User Guide, Version 10; World Steel Association: Brussels, Belgium, 2021. [Google Scholar]
- Suer, J.; Jäger, N.; Traverso, M. Carbon footprint assessment of hydrogen and related hydrogen-based steel production. 2022; in press. [Google Scholar]
- Fischedick, M.; Marzinkowski, J.; Winzer, P.; Weigel, M. Techno-economic evaluation of innovative steel production technologies. J. Clean. Prod. 2014, 84, 563–580. [Google Scholar] [CrossRef]
- Otto, A.; Robinius, M.; Grube, T.; Schiebahn, S.; Praktiknjo, A.; Stolten, D. Power-to-Steel: Reducing CO2 through the Integration of Renewable Energy and Hydrogen into the German Steel Industry. Energies 2017, 10, 451. [Google Scholar] [CrossRef]
- Hölling, M.; Weng, M.; Gellert, S. Bewertung der Herstellung von Eisenschwamm unter Verwendung von Wasserstoff. Stahl Und Eisen 2017, 137, 47–53. [Google Scholar]
- Vogl, V.; Åhman, M.; Nilsson, L.J. Assessment of hydrogen direct reduction for fossil-free steelmaking. J. Clean. Prod. 2018, 203, 736–745. [Google Scholar] [CrossRef]
- Bhaskar, A.; Assadi, M.; Somehsaraei, H.N. Decarbonization of the Iron and Steel Industry with Direct Reduction of Iron Ore with Green Hydrogen. Energies 2020, 13, 758. [Google Scholar] [CrossRef]

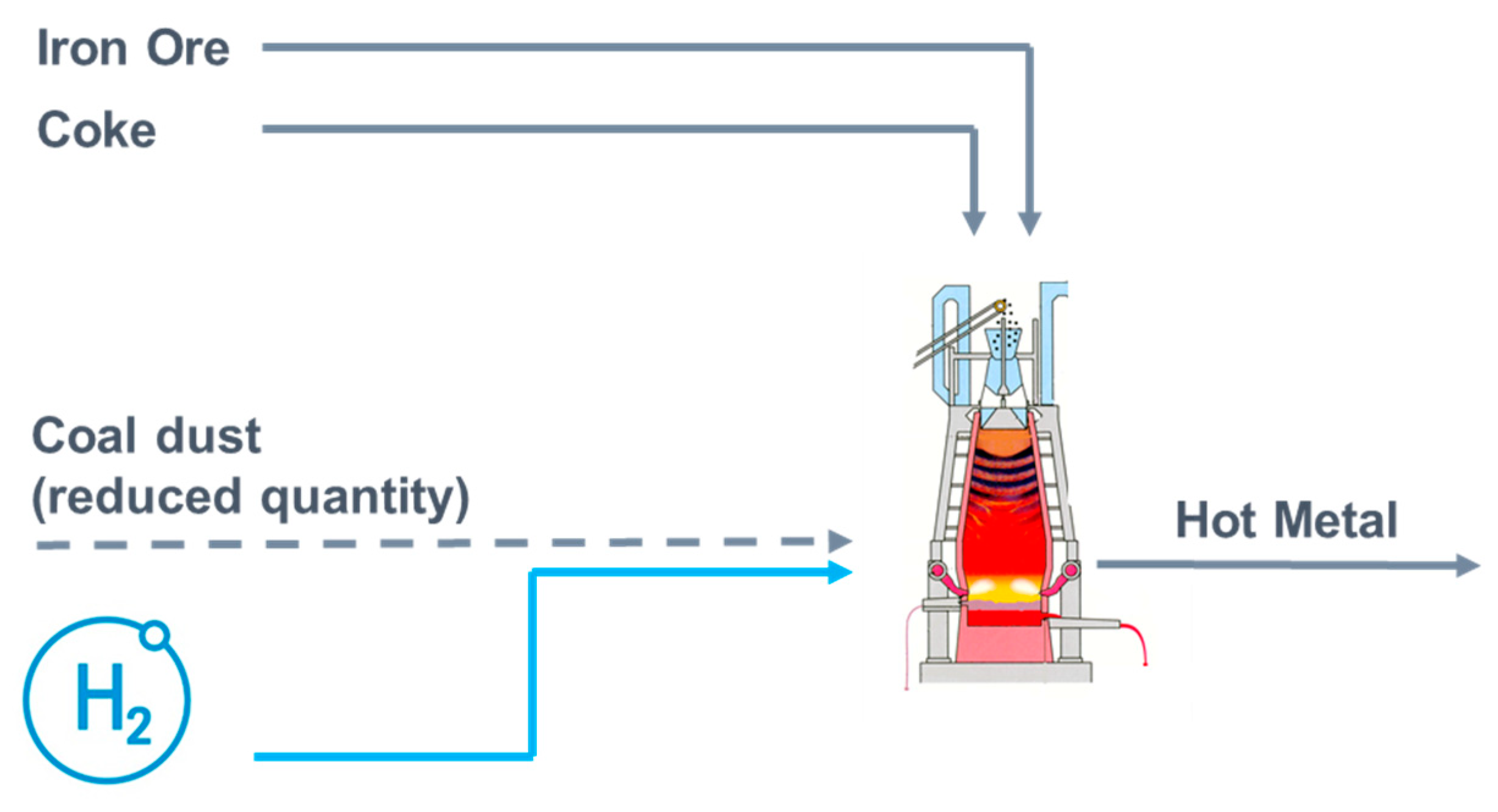
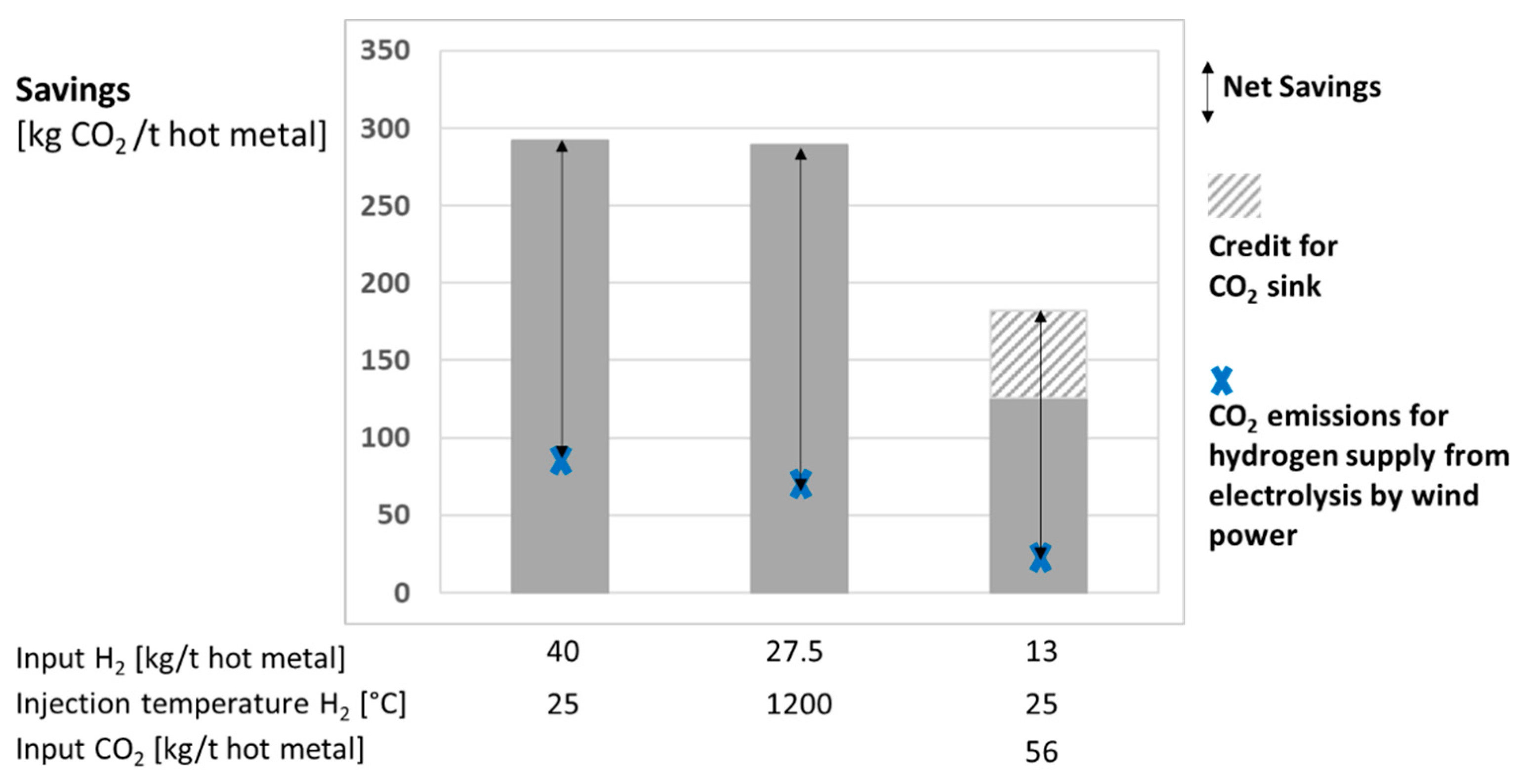

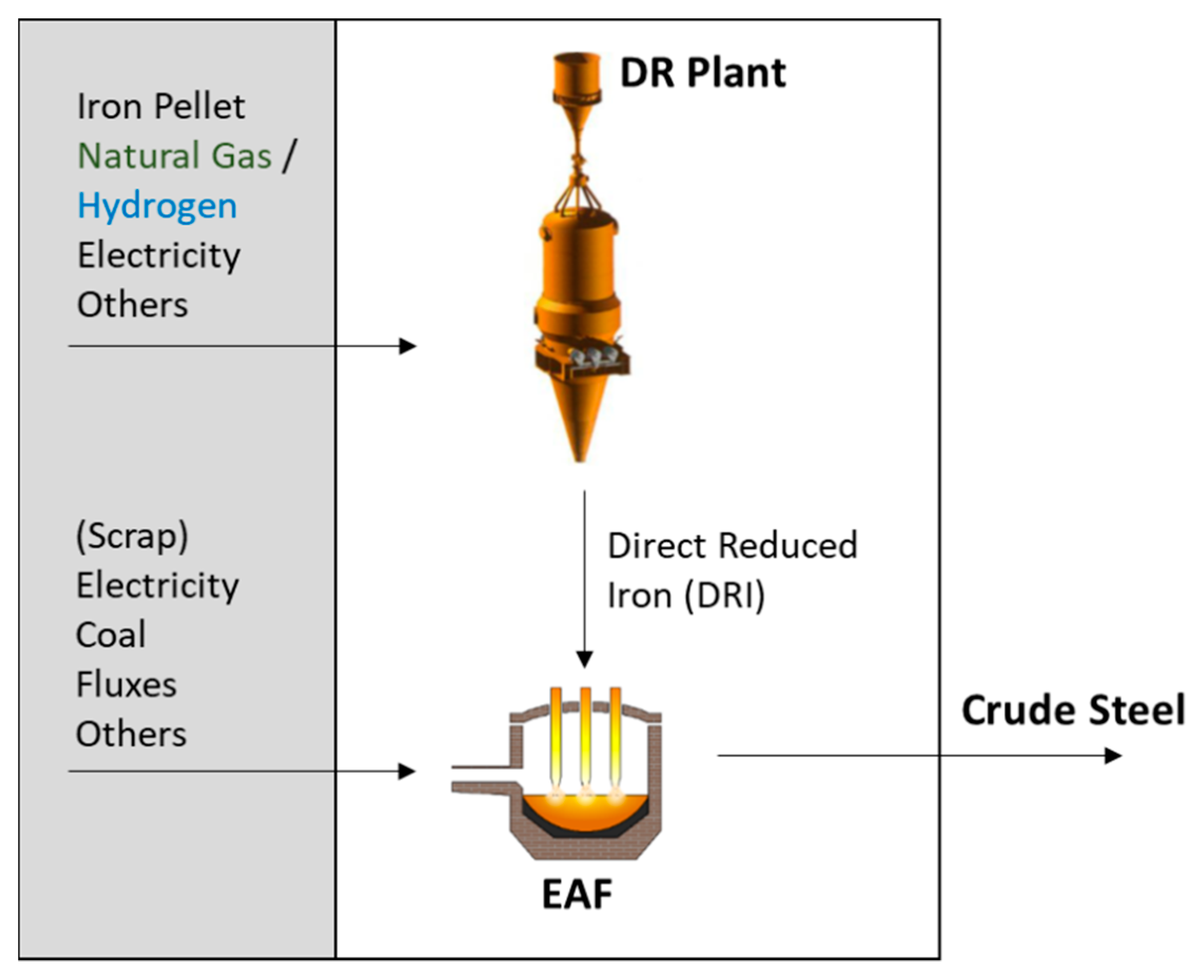
| Study | Year | Product | Methodology | Impact Categories | |||||
|---|---|---|---|---|---|---|---|---|---|
| kg | Scrap | Co-Products | Impact Method | GWP kg CO2 eq | AP kg SO2 eq | ADPf MJ | CED MJ | ||
| [21] | 2012 | HRC | RC | n. a. | CML 16 | 0.74 | 0.0020 | 7.5 | 11 |
| [33] | 2013 | Steel | n. s. | Credit for EAF Slag | Recipe Midpoint | 0.77 | 0.0025 | n. a. | 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suer, J.; Traverso, M.; Jäger, N. Review of Life Cycle Assessments for Steel and Environmental Analysis of Future Steel Production Scenarios. Sustainability 2022, 14, 14131. https://doi.org/10.3390/su142114131
Suer J, Traverso M, Jäger N. Review of Life Cycle Assessments for Steel and Environmental Analysis of Future Steel Production Scenarios. Sustainability. 2022; 14(21):14131. https://doi.org/10.3390/su142114131
Chicago/Turabian StyleSuer, Julian, Marzia Traverso, and Nils Jäger. 2022. "Review of Life Cycle Assessments for Steel and Environmental Analysis of Future Steel Production Scenarios" Sustainability 14, no. 21: 14131. https://doi.org/10.3390/su142114131
APA StyleSuer, J., Traverso, M., & Jäger, N. (2022). Review of Life Cycle Assessments for Steel and Environmental Analysis of Future Steel Production Scenarios. Sustainability, 14(21), 14131. https://doi.org/10.3390/su142114131









