Historical Lead Smelting Slag Harmlessness and Valuable Metals Recovery: A Co-Treatment of Lead Slag and Zinc-Bearing Material in Rotary Kiln
Abstract
1. Introduction
2. Materials and Methods
2.1. Lead Smelting Slag Sampling and Zinc-Bearing Material
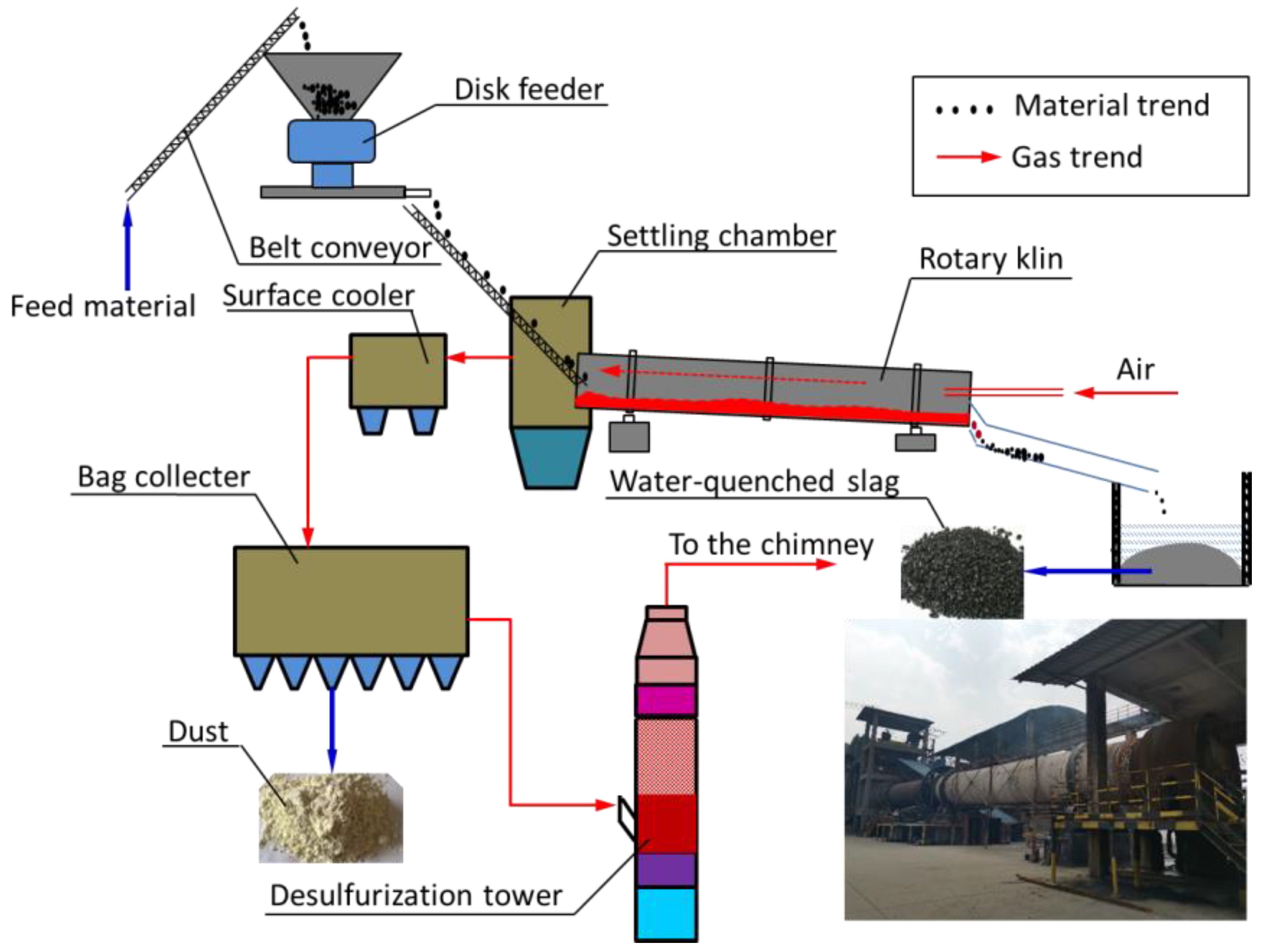
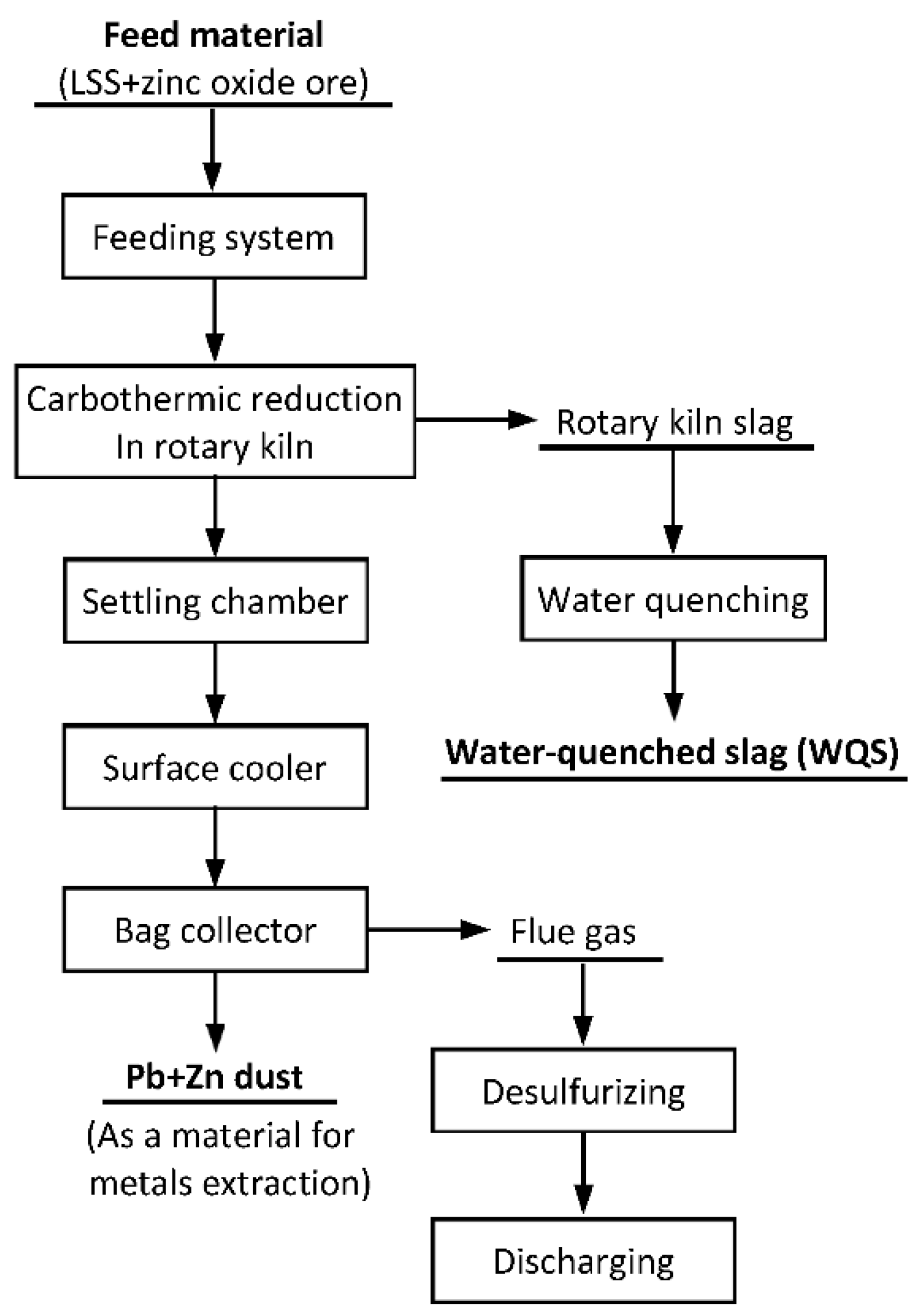
2.2. Rotary Kiln Tests and Sampling
2.3. Leaching Tests
2.4. Analysis Methods
3. Results and Discussion
3.1. Main Chemical Compositions of Products
3.1.1. Water-Quenched Slag
3.1.2. Dust
3.2. Metals Distribution during Rotary Kiln Treatment
3.2.1. Valuable Metals Recovery
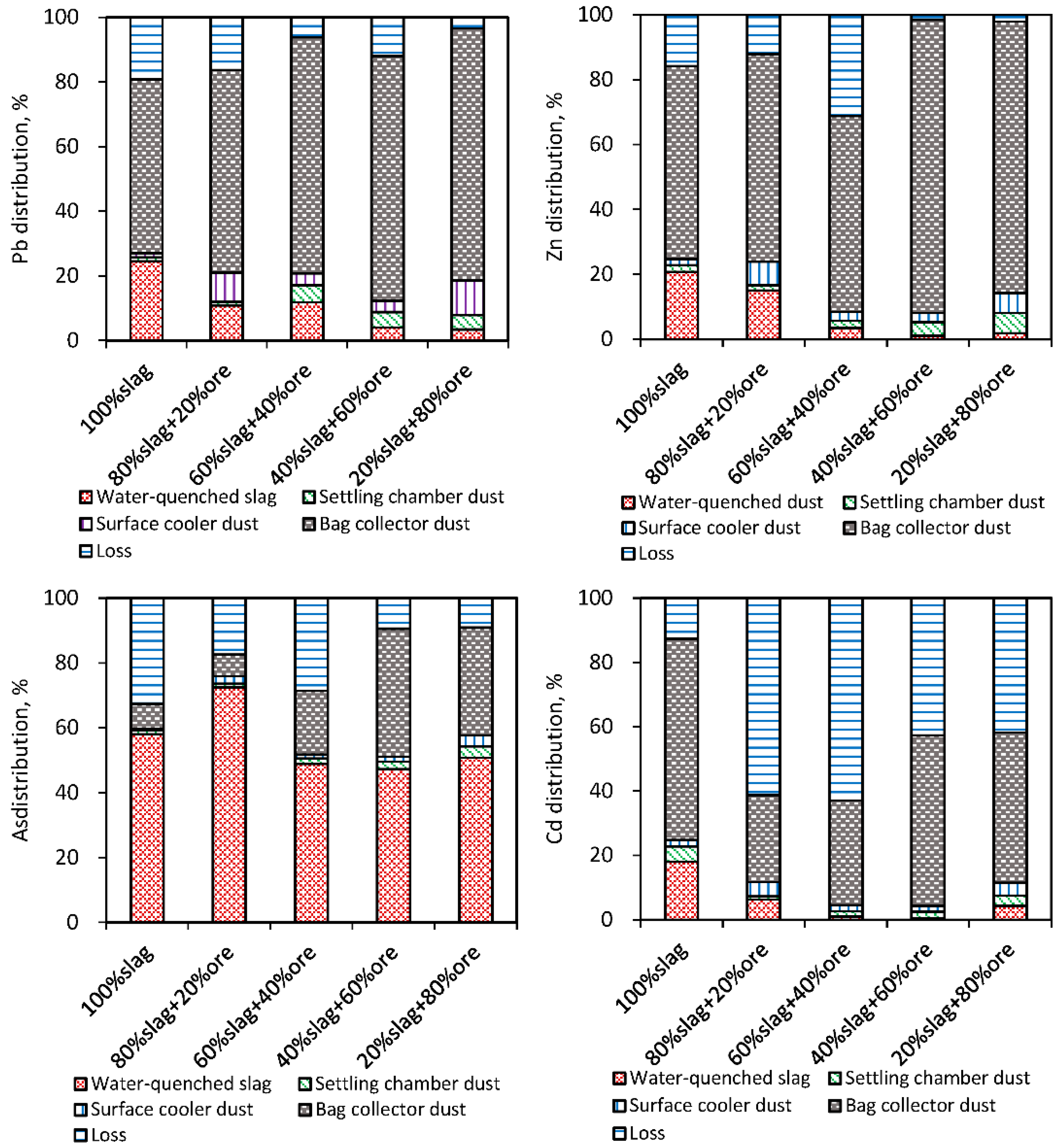
3.2.2. Metals Distribution
3.3. Migration and Solidification of Pb, Zn, As, and Cd during Carbothermic Reduction
3.4. Solidification Characterization of Water Quenched Slag from Rotary Kiln
3.5. Economic Evaluation
3.5.1. Energy Consumption
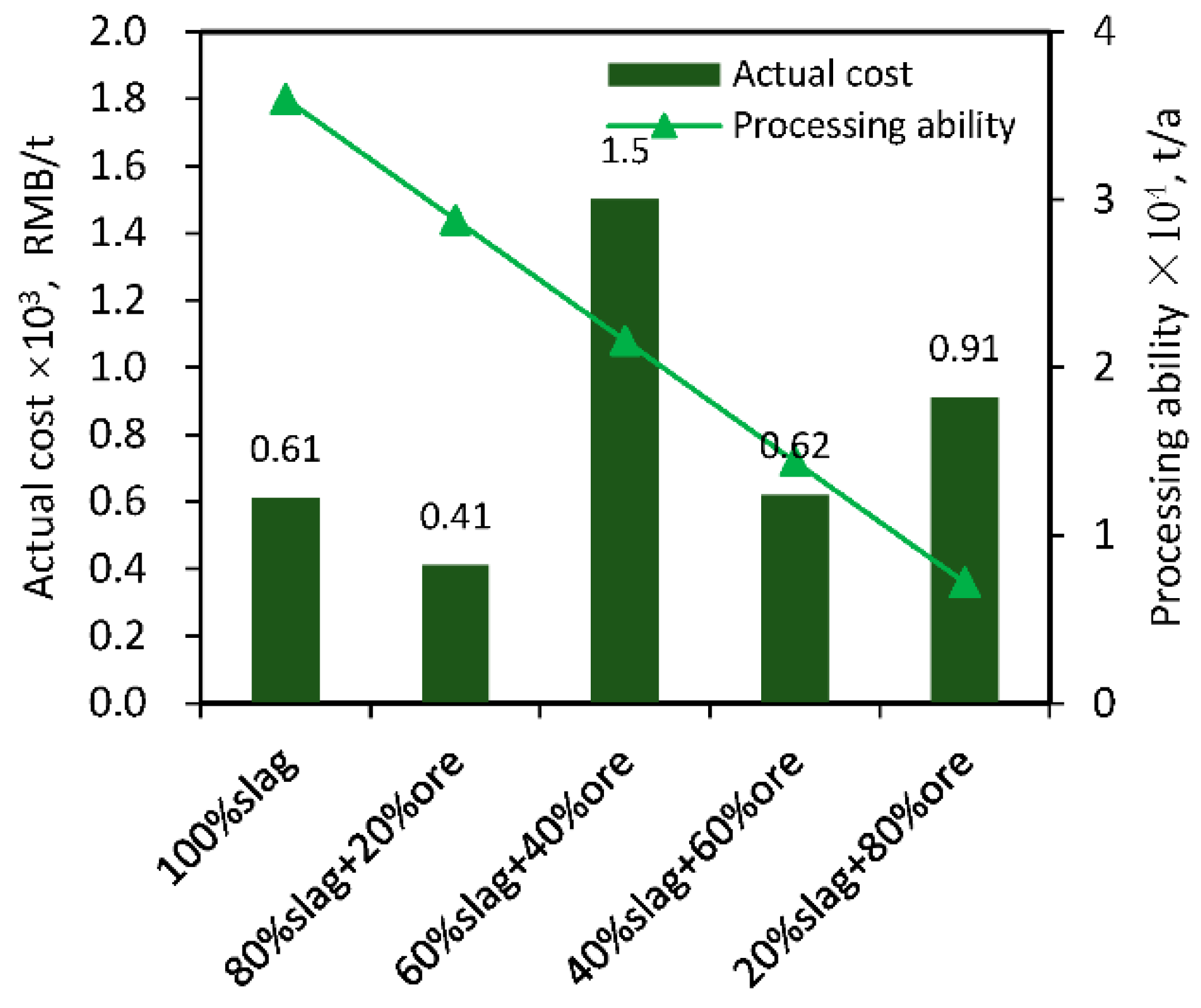
3.5.2. Actual Cost
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, J.; Zuo, R.; Chen, S.; Kreuzer, O.P. Application of the tectono-geochemistry method to mineral prospectivity mapping: A case study of the Gaosong tin-polymetallic deposit, Gejiu district, SW China. Ore Geol. Rev. 2015, 71, 719–734. [Google Scholar] [CrossRef]
- Mao, J.; Cheng, Y.; Guo, C.; Yang, Z.; Zhao, H. Gejiu tin polymetallic ore-field: Deposit model and discussion. Acta Geol. Sin. 2008, 81, 1456–1468, (In Chinese with English abstract). [Google Scholar]
- Cheng, Y.; Mao, J.; Chang, Z.; Pirajno, F. The origin of the world class tin- polymetallic deposits in the Gejiu district, SW China: Constraints from metal zoning characteristics and 40Ar−39Ar geochronology. Ore Geol. Rev. 2013, 53, 50–62. [Google Scholar] [CrossRef]
- Zhou, Y. Lead Smelting in Blast Furnace and Environmental Protection. Yunnan Metall. 2002, 31, 23–25, (In Chinese with English abstract). [Google Scholar]
- De Andrade Lima, L.R.P.; Bernardez, L.A. Characterization of the lead smelter slag in Santo Amaro, Bahia, Brazil. J. Hazard. Mater. 2011, 189, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Scheinert, M.; Kupsch, H.; Bletz, B. Geochemical investigations of slags from the historical smelting in Freiberg, Erzgebirge (Germany). Chem. Erde 2009, 69, 81–90. [Google Scholar] [CrossRef]
- Sobanska, S.; Ledésert, B.; Deneele, D.; Laboudigue, A. Alteration in soil of slag particles resulting from lead smelting. C. R. Acad. Sci. Paris 2000, 331, 271–278. [Google Scholar] [CrossRef]
- Seignez, N.; Gauthier, A.; Bulteel, D.; Buatier, M.; Recourt, P.; Damidot, D.; Potdevin, J.L. Effect of Pb-rich and Fe-rich entities during alteration of a partially vitrified metallurgical waste. J. Hazard. Mater. 2007, 149, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Seignez, N.; Gauthier, A.; Bulteel, D.; Buatier, M.; Damidot, D.; Potdevin, J.L. Leaching of lead metallurgical slag and pollutant mobility far from equilibrium conditions. Appl. Geochem. 2008, 23, 3699–3711. [Google Scholar] [CrossRef]
- Ettler, V.; Legendre, O.; Bodénan, F.; Touray, J.C. Primary phases and natural weathering of old lead–zinc pyrometallurgical slag from Pribram, Czech Republic. Can. Mineral. 2001, 39, 873–888. [Google Scholar] [CrossRef]
- Ettler, V.; Mihaljevic, M.; Touray, J.C.; Piantone, P. Leaching of polished sections: An integrated approach for studying the liberation of heavy metals from lead–zinc metallurgical slags. Bull. Soc. Geol. Fr. 2002, 173, 161–169. [Google Scholar]
- Möller, A.C.; Friedrich, B. Long term reactivity of land filled slag from lead production. Erzmetall World Metall. 2009, 62, 316–319. [Google Scholar]
- Lewis, A.E.; Hugo, A. Characterization and batch testing of a secondary lead slag. J. S. Afr. Inst. Min. Metall. 2000, 100, 365–370. [Google Scholar]
- Kucha, H.; Martens, A.; Ottenburgs, R.; De Vo, W.; Viaene, W. Primary minerals of Zn–Pb mining and metallurgical dumps and their environmental behavior at Plombieres, Belgium. Environ. Geol. 1996, 27, 1–15. [Google Scholar] [CrossRef]
- Gee, C.; Ramsey, M.H.; Maskall, J.; Thornton, I. Mineralogy and weathering processes in historical smelting slag and their effect on the lead mobilization. J. Geochem. Explor. 1997, 58, 249–257. [Google Scholar]
- Ettler, V.; Johan, Z.; Kříbek, B.; Šebek, O. Mihaljevič, M. Mineralogy and environmental stability of slags from the Tsumeb smelter, Namibia. Appl. Geochem. 2009, 24, 1–15. [Google Scholar]
- Zou, Z.; Huang, W. Research on process mineralogy and mineral processing of blast furnace lead smelting slag. Mod. Min. 2011, 506, 126–127. [Google Scholar]
- Lin, W. Ecological degeneration and heavy metals pollution in zinc smelting areas. Ecol. Environ. Sci. 2009, 18, 149–153. [Google Scholar]
- Liao, G.; Liao, D.; Li, Q. Heavy metals contamination characteristics in soil of different mining activity zones. Trans. Nonferrous Met. Soc. China 2008, 18, 207–211. [Google Scholar]
- Ghasemi, F.F.; Dobaradaran, S.; Saeedi, R.; Nabipour, I.; Nazmara, S.; Abadi, D.R.V.; Arfaeinia, H.; Ramavandi, B.; Spitz, J.; Mohammadi, M.J.; et al. Levels and ecological and health risk assessment of PM 2.5-bound heavy metals in the northern part of the Persian Gulf. Environ. Sci. Pollut. Res. 2020, 27, 5305–5313. [Google Scholar] [CrossRef]
- Dobaradaran, S.; Naddafi, K.; Nazmara, S.; Ghaedi, H. Heavy metals (Cd, Cu, Ni and Pb) content in two fish species of Persian gulf in Bushehr Port, Iran. Afr. J. Biotechnol. 2010, 37, 6191–6193. [Google Scholar]
- Dobaradaran, S.; Schmidt, T.C.; Nabipour, I.; Khajeahmadi, N.; Tajbakhsh, S.; Saeedi, R.; Mohammadi, M.J.; Keshtkar, M.; Khorsand, M.; Ghasemi, F.F. Characterization of plastic debris and association of metals with microplastics in coastline sediment along the Persian Gulf. Waste Manag. 2018, 78, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Dobaradaran, S.; Soleimani, F.; Nabipour, I.; Saeedi, R.; Mohammadi, M.J. Heavy metal levels of ballast waters in commercial ships entering Bushehr port along the Persian Gulf. Mar. Pollut. Bull. 2018, 126, 74–76. [Google Scholar] [CrossRef]
- Arfaeinia, H.; Nabipour, I.; Ostovar, A.; Asadgol, Z.; Abuee, E.; Keshtkar, M.; Dobaradaran, S. Assessment of sediment quality based on acid-volatile sulfide and simultaneously extracted metals in heavily industrialized area of Asaluyeh, Persian Gulf: Concentrations, spatial distributions, and sediment bioavailability/toxicity. Environ. Sci. Pollut. Res. 2016, 23, 9871–9890. [Google Scholar] [CrossRef] [PubMed]
- Arfaeinia, H.; Dobaradaran, S.; Moradi, M.; Pasalari, H.; Mehrizi, E.A.; Taghizadeh, F.; Esmaili, A.; Ansarizadeh, M. The effect of land use configurations on concentration, spatial distribution, and ecological risk of heavy metals in coastal sediments of northern part along the Persian Gulf. Sci. Total Environ. 2019, 653, 783–791. [Google Scholar] [CrossRef]
- Karbasdehi, V.N.; Dobaradaran, S.; Nabipour, I.; Arfaeinia, H.; Mirahmadi, R.; Keshtkar, M. Data on metal contents (As, Ag, Sr, Sn, Sb, and Mo) in sediments and shells of Trachycardium lacunosum in the northern part of the Persian Gulf. Data Brief. 2016, 8, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Akhbarizadeh, R.; Dobaradaran, S.; Parhizgar, G.; Schmidt, T.C.; Mallaki, R. Potentially toxic elements leachates from cigarette butts into different types of water: A threat for aquatic environments and ecosystems? Environ. Res. 2021, 202, 11170. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; WU, X.; Hou, J.; Ji, C.; Wang, S.; Chen, C. Study on heavy metal content and chemical forms of soil around typical lead smelting blast furnace. Sichuan Environ. 2018, 37, 25–28. [Google Scholar]
- Niu, X.; Wu, X.; Wang, W.; Ai, Z.; Wang, S.; Hou, J.; Zhou, T. Study on enrichment characteristics of heavy metals from dominant plants around the waste slag yard of lead smelting in a typical blast furnace. Ecol. Environ. Sci. 2021, 30, 1293–1298. [Google Scholar]
- Omar, W.A.; Zaghloul, K.H.; Abdel-Khalek, A.A.; Abo-Hegab, S. Risk Assessment and toxic effects of metal pollution in two cultured and wild fish species from highly degraded aquatic habitats. Arch. Environ. Contam. Toxicol. 2013, 65, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Farombi, E.O.; Adelowo, O.A.; Ajimoko, Y.R. Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in African Cat Fish (Clariasgariepinus) from Nigeria Ogun River. Int. J. Env. Res. Pub. Health 2007, 4, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Sivry, Y.; Guyot, F.; Lens, P.N.L.; van Hullebusch, E.D. Evaluation on chemical stability of lead blast furnace (LBF) and imperial smelting furnace (ISF) slags. J. Environ. Manag. 2016, 180, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Liu, D.; Ke, Y.; Li, Y.; Wang, Z.; Fei, J.; Xu, H.; Min, X. Synthesis and hydration characteristic of geopolymer based on lead smelting slag. Int. J. Environ. Res. Public Health 2020, 17, 2762. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.; Wu, J.; Wu, Y.; Tang, C.; Yang, W. Environmental risk assessment on slag and iron-rich matte produced from reducing-matting smelting of lead-bearing wastes and iron-rich wastes. Trans. Nonferrous Met. Soc. China 2015, 25, 3429–3435. [Google Scholar] [CrossRef]
- Li, Y.; Min, X.; Chai, L.; Shi, M.; Tang, C.; Wang, Q.; Liang, Y.; Lei, J.; Liyang, W. Co-treatment of gypsum sludge and Pb/Zn smelting slag for the solidification of sludge containing arsenic and heavy metals. J. Environ. Manag. 2016, 181, 756–761. [Google Scholar] [CrossRef]
- Saikia, N.; Cornelis, G.; Cizer, Ö.; Vandecasteele, C.; Van Gemert, D.; Van Balen, K.; Van Gerven, T. Use of Pb blast furnace slag as a partial substitute for fine aggregate in cement mortar. J. Mater. Cycles Waste Manag. 2012, 14, 102–112. [Google Scholar] [CrossRef]
- Pan, D.; Li, L.; Wu, Y.; Liu, T.; Yu, H. Characteristics and properties of glass-ceramics using lead fuming slag. J. Clean. Prod. 2018, 175, 251–256. [Google Scholar] [CrossRef]
- Albitar, M.; Mohamed Ali, M.S.; Visintin, P.; Drechsler, M. Effect of granulated lead smelter slag on strength of fly ash-based geopolymer concrete. Constr. Build. Mater. 2015, 83, 128–135. [Google Scholar] [CrossRef]
- Alwaeli, M. Application of granulated lead–zinc slag in concrete as an oppor-tunity to save natural resources. Radiat. Phys. Chem. 2013, 83, 54–60. [Google Scholar] [CrossRef]
- Penpolcharoen, M. Utilization of secondary lead slag as construction material. Cem. Concr. Res. 2005, 35, 1050–1055. [Google Scholar] [CrossRef]
- GB 5085.3−2007; Identification Standards for Hazardous Wastes-Identification for Extraction Toxicity. State Environmental Protection Administration State Administration of Quality Supervision. Inspection and Quarantine: Beijing, China, 2007. (In Chinese)
- Hu, H.; Deng, Q.; Li, C.; Xie, Y.; Dong, Z.; Zhang, W. The recovery of Zn and Pb and the manufacture of lightweight bricks from zinc smelting slag and clay. J. Hazard. Mater. 2014, 271, 220–227. [Google Scholar] [CrossRef]
- She, X.; Wang, J.; Wang, G.; Xue, Q.; Zhang, X. Removal mechanism of Zn, Pb and Alkalis from metallurgical dusts in direct reduction process. J. Iron Steel Res. Int. 2014, 21, 488–495. [Google Scholar] [CrossRef]
- Li, S.; Pan, J.; Zhu, D.; Guo, Z.; Xu, J.; Chou, J. A novel process to upgrade the copper slag by direct reduction-magnetic separation with the addition of Na2CO3 and CaO. Powder Technol. 2019, 347, 159–169. [Google Scholar] [CrossRef]
- Gu, M.; Zhong, Y.; Wang, L.; Guo, Z. Kinetics study of heavy metal removal from the lead smelting slag by carbothermic reduction: Effect of phase transformation. J. Environ. Chem. Eng. 2021, 9, 106516. [Google Scholar] [CrossRef]
- Gu, M.; Zhong, Y.; Wang, L.; Guo, Z. Separation of heavy metals from hazardous lead slag by carbothermic reduction and thermal volatilization: Effect of phase transformation on Sn, Pb, Zn removal. Proc. Saf. Environ. Prot. 2021, 156, 330–339. [Google Scholar] [CrossRef]
- U.S. EPA. Toxicity Characteristic Leaching Procedure, Appendix 1. Fed. Register 1986, 51, 216. [Google Scholar]
- U.S. EPA. Characteristics of EP Toxicity. Paragraph 261.24. Federal Register 1990, 45, 98. [Google Scholar]
- HJ/T 299−2007; Solid Waste-Extraction Procedure for Leaching Toxicity-Sulphuric Acid & Nitric Acid Method. State Environmental Protection Administration: Beijing, China, 2007. (In Chinese)
- Editorial Board of Lead Zinc Metallurgy. Metallurgy of Lead and Zinc; China Science Press: Beijing, China, 2003. [Google Scholar]
- Wang, C.; Li, K.; Yang, H.; Li, C. Probing study on separating Pb, Zn, and Fe from lead slag by coal-based direct reduction. ISIJ Int. 2017, 57, 996–1003. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Liu, H.; Peng, B. Clean strengthening reduction of lead and zinc from smelting waste slag by iron oxide. J. Clean. Prod. 2017, 143, 311–318. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, Y.; Liu, H.; Peng, B.; Liu, Z. Iron extraction from lead slag by bath smelting. Trans. Nonferrous Met. Soc. China 2017, 27, 1862–1869. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Cui, K.; Fu, T.; Gao, J.; Hussain, S.; AlGarni, T.S. Pyrometallurgical recovery of zinc and valuable metals from electric arc furnace dust—A review. J. Clean. Prod. 2021, 298, 126788. [Google Scholar] [CrossRef]
- Zhang, S.; Lv, Q.; Hu, X. Thermodynamics of arsenic removal from arsenic-bearing iron ores. Trans. Nonferrous Met. Soc. China 2011, 21, 1705–1712. (In Chinese) [Google Scholar]
- Li, G.; You, Z.; Zhang, Y.; Rao, M.; Wen, P.; Guo, Y.; Jiang, T. Synchronous volatilization of Sn, Zn, and As, and preparation of direct reduction iron (DRI) from a complex iron concentrate via CO reduction. JOM 2014, 66, 1701–1710. [Google Scholar] [CrossRef]
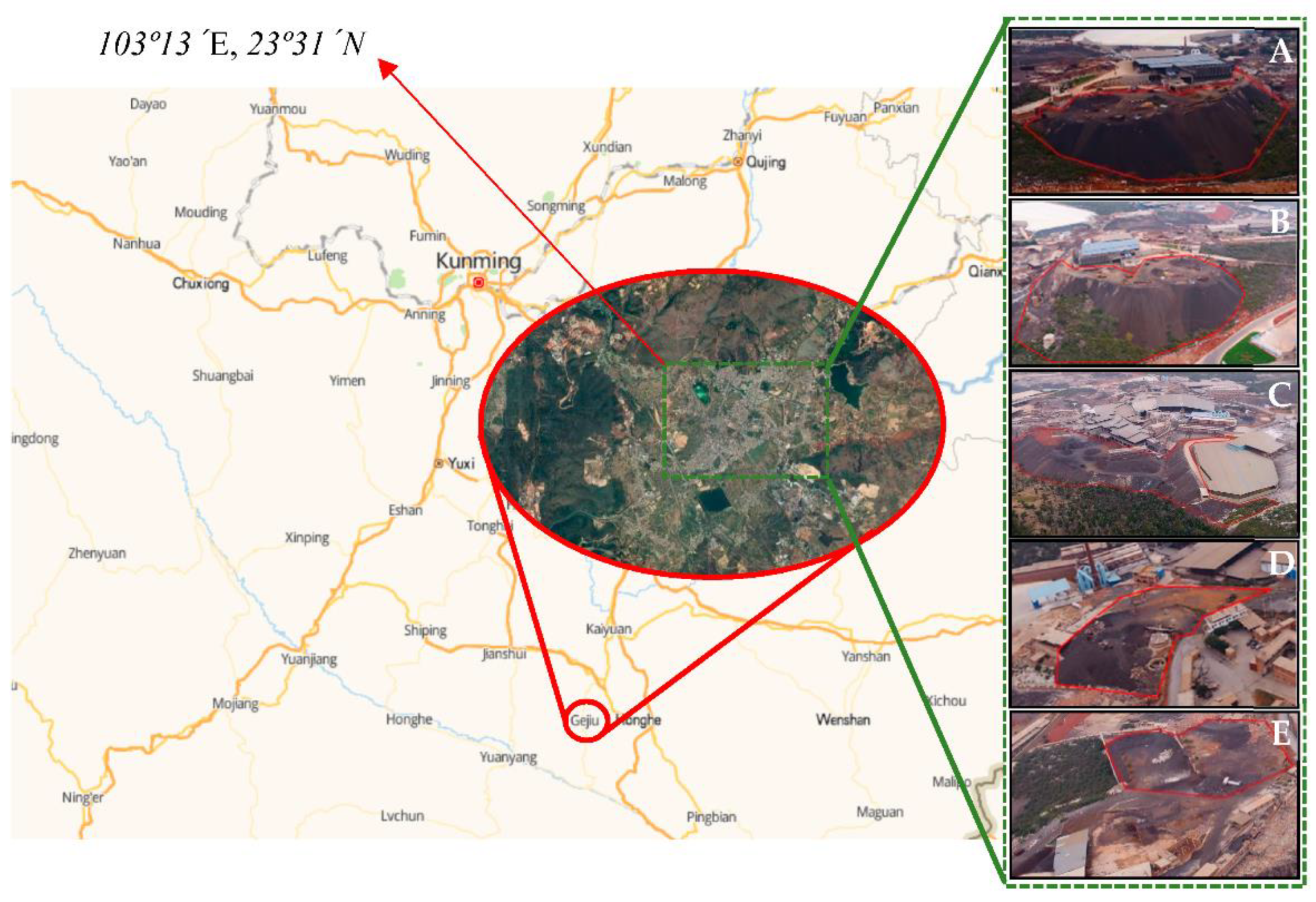

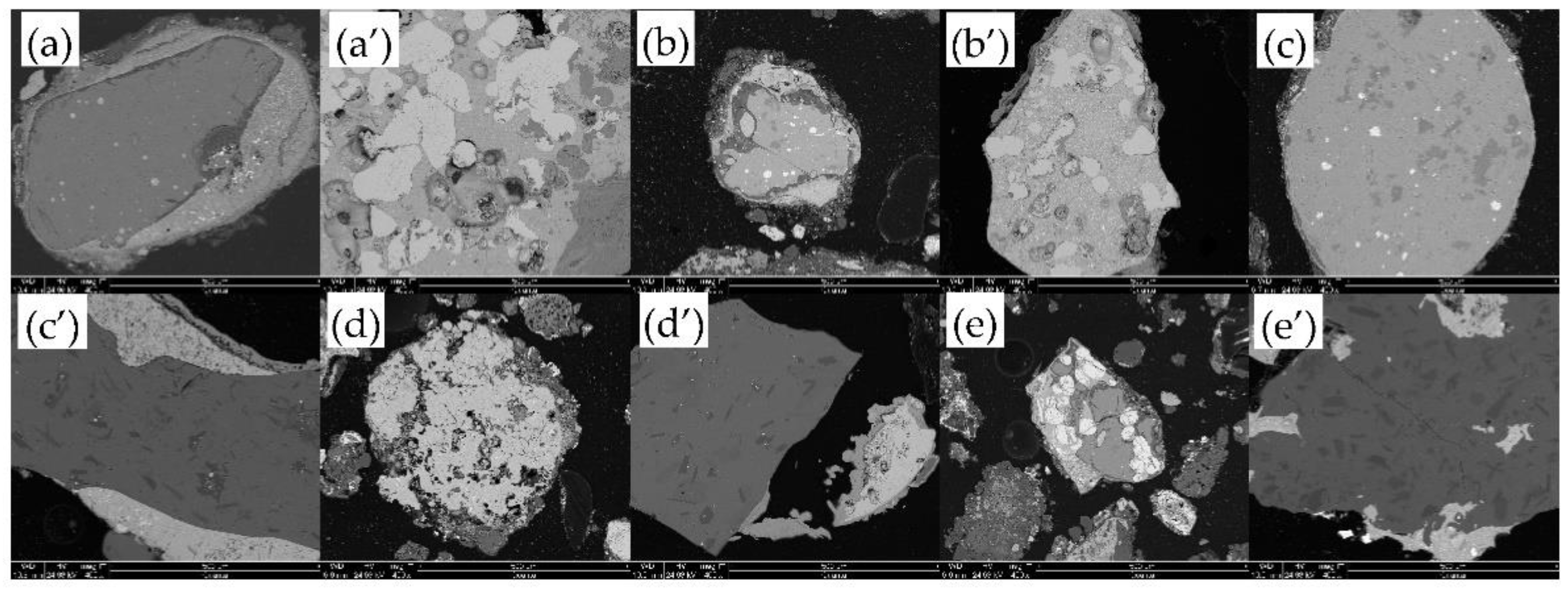
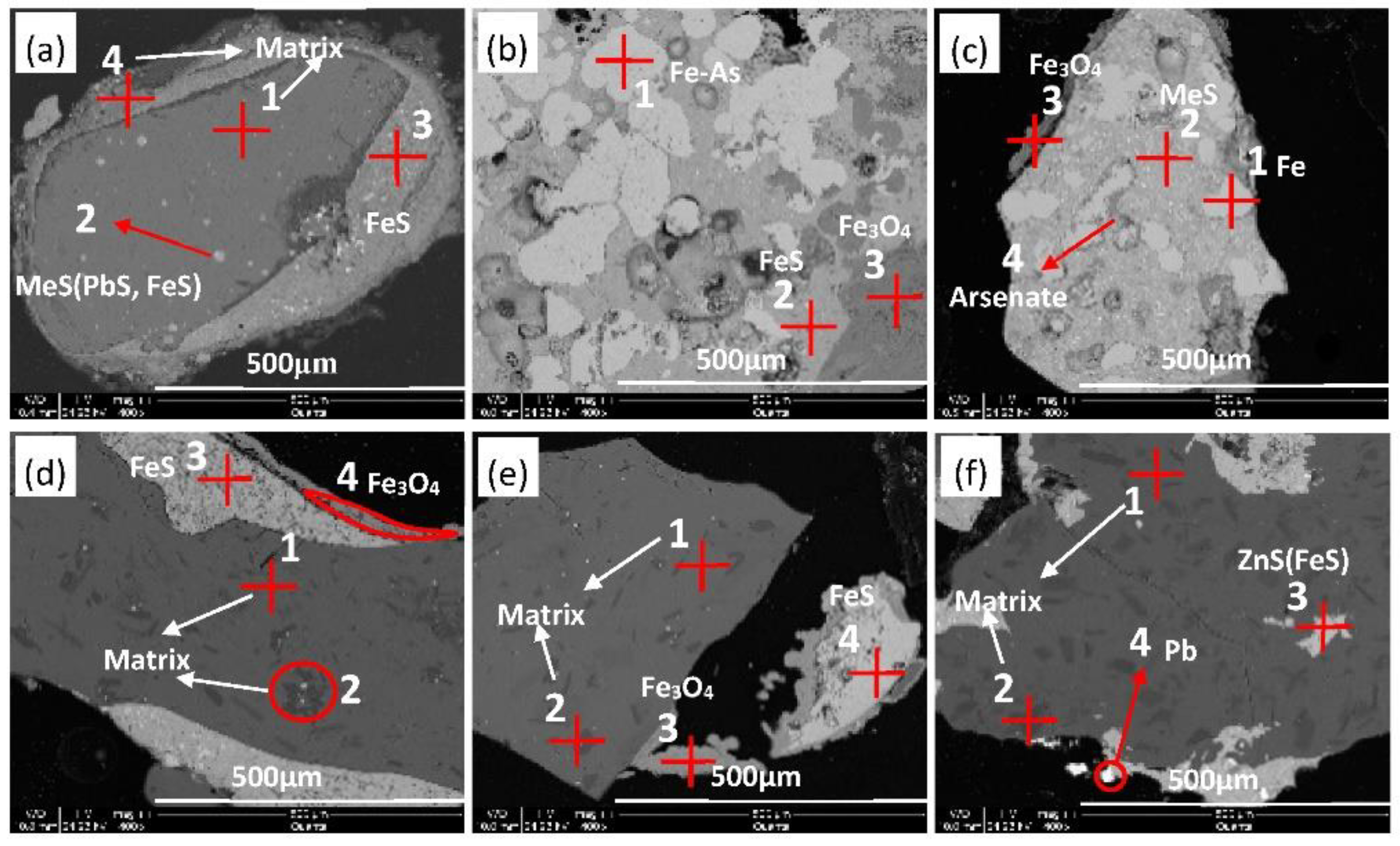
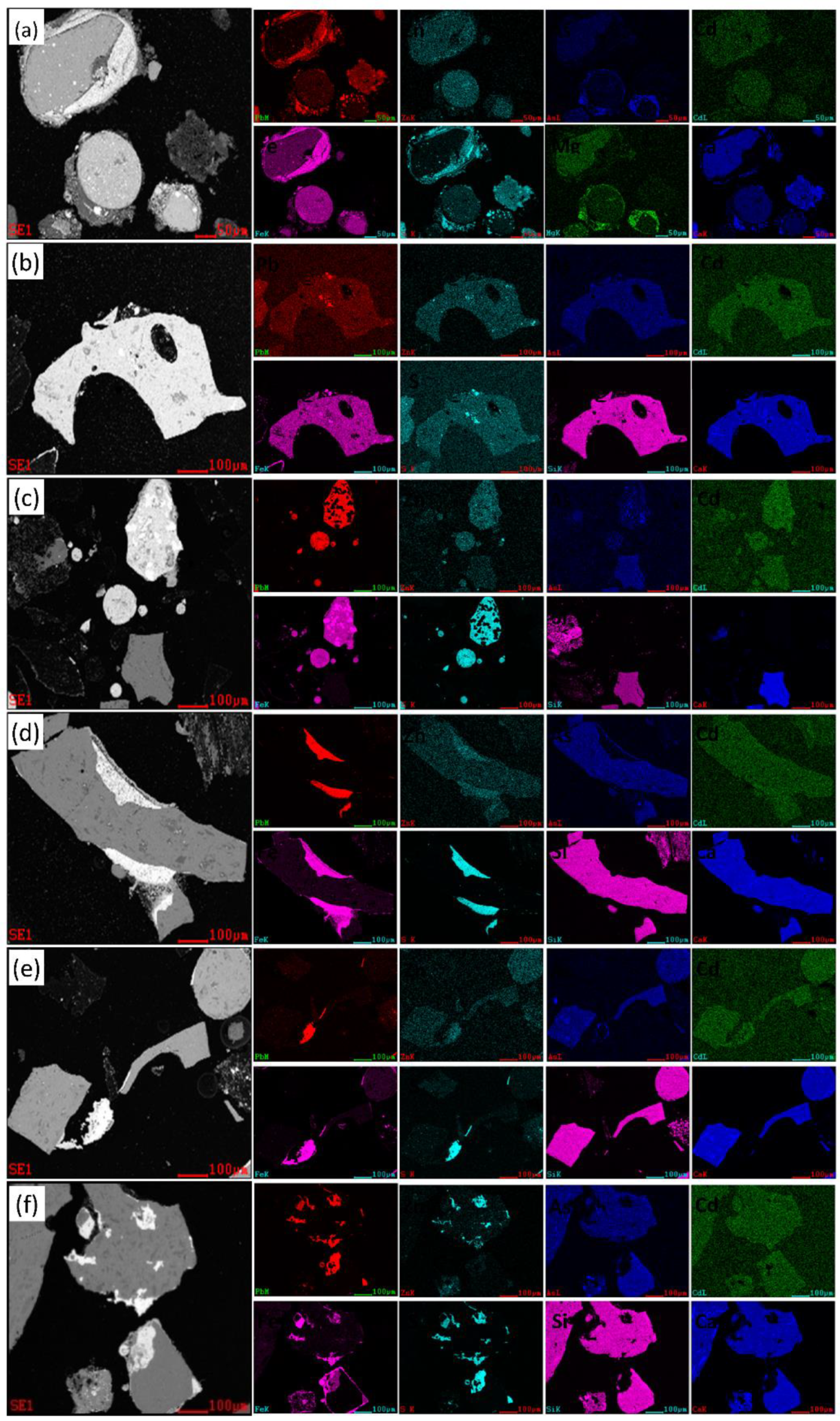

| Site | Storage Weight (Ten Thousand Tons) | Weight Proportion of Each Slag Heap (%) | Sampling Weight (t) |
|---|---|---|---|
| A | 15.29 | 8.83 | 106 |
| B | 68.40 | 39.51 | 474 |
| C | 77.00 | 44.48 | 533 |
| D | 6.84 | 3.95 | 47 |
| E | 5.58 | 3.22 | 38 |
| Total | 173.11 | 100.00 | 1198 |
| Element | Experiments with Different Ratios | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Feed Material (FM) | Water-Quenched Slag (WQS) | |||||||||
| 1# | 2# | 3# | 4# | 5# | 1# | 2# | 3# | 4# | 5# | |
| Pb | 1.19 | 1.26 | 0.54 | 1.02 | 1.33 | 0.44 | 0.19 | 0.10 | 0.075 | 0.068 |
| Zn | 1.74 | 2.56 | 4.32 | 5.51 | 6.45 | 0.55 | 0.53 | 0.24 | 0.10 | 0.18 |
| FeO | 38.76 | 35.28 | 31.69 | 28.43 | 25.71 | 33.30 | 30.86 | 34.97 | 26.61 | 18.90 |
| SiO2 | 15.42 | 15.96 | 12.10 | 12.38 | 10.35 | 19.82 | 19.49 | 18.87 | 19.36 | 18.19 |
| Al2O3 | 5.86 | 5.34 | 4.99 | 5.45 | 5.05 | 7.74 | 7.73 | 8.34 | 7.69 | 8.72 |
| CaO | 7.88 | 7.03 | 6.61 | 8.28 | 6.63 | 9.97 | 9.39 | 9.56 | 8.99 | 10.09 |
| MgO | 1.04 | 1.19 | 1.64 | 2.27 | 2.14 | 1.15 | 1.32 | 1.72 | 1.53 | 2.35 |
| F | 0.024 | 0.029 | 0.034 | 0.038 | 0.035 | 0.03 | 0.03 | 0.028 | 0.03 | 0.028 |
| Cl | 0.062 | 0.088 | 0.050 | 0.026 | 0.015 | 0.01 | 0.028 | <0.01 | <0.01 | <0.01 |
| S | 4.02 | 3.29 | 4.59 | 2.65 | 2.36 | 3.75 | 4.34 | 3.89 | 4.08 | 2.63 |
| In(g/t) | 38.10 | 21.70 | 28.40 | 45.10 | 32.50 | 47.90 | 37.30 | 42.30 | 56.00 | 46.80 |
| Ge(g/t) | 13.70 | 17.70 | 11.90 | 17.40 | 19.00 | 10.10 | 12.60 | 10.60 | 12.70 | 11.00 |
| * Hg | 3.72 | 14.10 | 14.00 | 16.50 | 15.70 | 0.116 | 0.06 | 0.027 | 0.028 | 0.029 |
| * Ag | 1.50 | 6.10 | 8.70 | 8.30 | 9.80 | 20.90 | 20.20 | 19.10 | 22.70 | 17.80 |
| * Ni | 7.70 | 15.90 | 20.60 | 15.10 | 21.20 | 49.70 | 40.60 | 56.00 | 34.10 | 29.10 |
| * Cr | 14.90 | 15.80 | 22.50 | 17.20 | 20.20 | 40.60 | 37.20 | 56.80 | 40.10 | 26.50 |
| * As | 4880 | 3800 | 2850 | 1940 | 1760 | 4320 | 3800 | 2310 | 1710 | 1420 |
| * Ba | 174 | 147 | 177 | 132 | 260 | 504 | 451 | 503 | 546 | 442 |
| * Be | 1.00 | ND | 0.90 | 1.50 | 0.50 | 1.50 | ND | 3.00 | 1.00 | 0.70 |
| * Cd | 101 | 302 | 392 | 472 | 545 | 27.70 | 25.80 | 5.80 | 3.30 | 34.90 |
| * Cu | 361 | 369 | 334 | 275 | 261 | 2170 | 2230 | 2510 | 1410 | 969 |
| * Se | 6.40 | 7.80 | 5.80 | 4.20 | 6.60 | 5.80 | 47.30 | 18.60 | 23.20 | 15.70 |
| Dust Type | Element | Experiments with Different Ratios | ||||
|---|---|---|---|---|---|---|
| 1# (100% Slag) | 2# (80% Slag + 20% Ore) | 3# (60% Slag + 40% Ore) | 4# (40% Slag + 60% Ore) | 3# (20% Slag + 80% Ore) | ||
| Settling chamber dust (SCD1) | Pb | 2.21 | 3.08 | 3.19 | 3.93 | 2.58 |
| Zn | 6.12 | 9.35 | 10.90 | 18.30 | 17.70 | |
| * As | 9350 | 9400 | 5080 | 3560 | 2700 | |
| * Cd | 792 | 740 | 736 | 790 | 780 | |
| Surface cooler dust (SCD2) | Pb | 0.62 | 4.07 | 4.59 | 5.62 | 4.64 |
| Zn | 18.70 | 21.2 | 27.2 | 25.10 | 27.10 | |
| * As | 13900 | 9990 | 7740 | 4770 | 4080 | |
| * Cd | 1160 | 1520 | 1640 | 1250 | 1440 | |
| Bag collector dust (BCD) | Pb | 6.61 | 5.68 | 5.60 | 5.77 | 6.25 |
| Zn | 38.9 | 36.6 | 37.10 | 37.10 | 35.10 | |
| * As | 11000 | 5720 | 8000 | 5710 | 3800 | |
| * Cd | 1870 | 1820 | 1820 | 1870 | 1660 | |
| Sample | TCLP (mg/L) | SNAL (mg/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pb | Zn | As | Cd | Pb | Zn | As | Cd | ||
| Lead smelting slag (LSS) | 302.33 | 899.15 | 12.11 | 1.36 | 50.77 | 182.17 | 8.15 | 1.78 | |
| water-quenched slag (WQS) | 100% slag | 0.11 | 0.19 | 0.92 | 0.017 | 0.084 | 0.063 | 0.071 | 0.0014 |
| 20% slag + 80% ore | 0.15 | 0.16 | 0.83 | 0.014 | 0.063 | 0.050 | 0.14 | 0.0012 | |
| 40% slag + 60% ore | 0.14 | 0.21 | 0.86 | 0.018 | 0.096 | 0.059 | 0.15 | 0.0015 | |
| 60% slag + 40% ore | 0.17 | 0.22 | 0.91 | 0.015 | 0.073 | 0.035 | 0.16 | 0.0012 | |
| 80% slag + 20% ore | 0.15 | 0.18 | 0.95 | 0.014 | 0.043 | 0.023 | 0.17 | ND | |
| Threshold value [U.S. EPA, 1986; U.S. EPA, 1990; GB 5085.3−2007] | 5.00 | 5.00 | 5.00 | 1.00 | 5.00 | 100.00 | 5.00 | 1.00 | |
| Ratios of Lead Smelting Slag to Zinc Oxide Ore | Lead Smelting Slag Handling Capacity (t) | Zinc Oxide Ore Addition (t) | Gypsum Addition (t) | Coal Powder Addition (t) | Coal-Feed Material Ratio (%) |
|---|---|---|---|---|---|
| 100% slag | 338.36 | 0.00 | 88.42 | 226.72 | 53.12 |
| 80% slag + 20% ore | 263.74 | 52.86 | 53.36 | 195.30 | 52.79 |
| 60% slag + 40% ore | 226.70 | 148.14 | 45.28 | 208.58 | 49.65 |
| 40% slag + 60% ore | 146.94 | 223.82 | 28.94 | 186.60 | 46.69 |
| 20% slag + 80% ore | 55.88 | 222.36 | 17.66 | 141.74 | 47.90 |
| Total capacity (t) | 1031.62 | 647.18 | 233.66 | 958.94 | 50.14 (Average) |
| Energy and Energy Consuming Medium Consumption | 100% Slag | 80% Slag+ 20% Ore | 60% Slag+ 40% Ore | 40% Slag+ 60% Ore | 20% Slag+ 80% Ore |
|---|---|---|---|---|---|
| * Electricity (kW·h) | 21367.5 | 22470.0 | 25147.5 | 27772.5 | 2814 |
| * Water (t) | 1296.0 | 588.0 | 1249.0 | 460.0 | 696.0 |
| * Coal powder (t) | 226.7 | 195.3 | 208.6 | 186.6 | 141.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, X.; Li, M.; Wang, H.; Ma, L.; Wang, S.; Zhou, T.; Wang, W. Historical Lead Smelting Slag Harmlessness and Valuable Metals Recovery: A Co-Treatment of Lead Slag and Zinc-Bearing Material in Rotary Kiln. Sustainability 2022, 14, 13647. https://doi.org/10.3390/su142013647
Niu X, Li M, Wang H, Ma L, Wang S, Zhou T, Wang W. Historical Lead Smelting Slag Harmlessness and Valuable Metals Recovery: A Co-Treatment of Lead Slag and Zinc-Bearing Material in Rotary Kiln. Sustainability. 2022; 14(20):13647. https://doi.org/10.3390/su142013647
Chicago/Turabian StyleNiu, Xuekui, Minting Li, Hongbin Wang, Liping Ma, Shuting Wang, Tao Zhou, and Wei Wang. 2022. "Historical Lead Smelting Slag Harmlessness and Valuable Metals Recovery: A Co-Treatment of Lead Slag and Zinc-Bearing Material in Rotary Kiln" Sustainability 14, no. 20: 13647. https://doi.org/10.3390/su142013647
APA StyleNiu, X., Li, M., Wang, H., Ma, L., Wang, S., Zhou, T., & Wang, W. (2022). Historical Lead Smelting Slag Harmlessness and Valuable Metals Recovery: A Co-Treatment of Lead Slag and Zinc-Bearing Material in Rotary Kiln. Sustainability, 14(20), 13647. https://doi.org/10.3390/su142013647







