Biomonitoring-Supported Land Restoration to Reduce Land Degradation in Intensively Mined Areas of India
Abstract
1. Introduction
- (i)
- The bioaccumulation potential of metals in non-edible plants;
- (ii)
- The bioaccumulation of metals in edible plants (agriculture and horticulture);
- (iii)
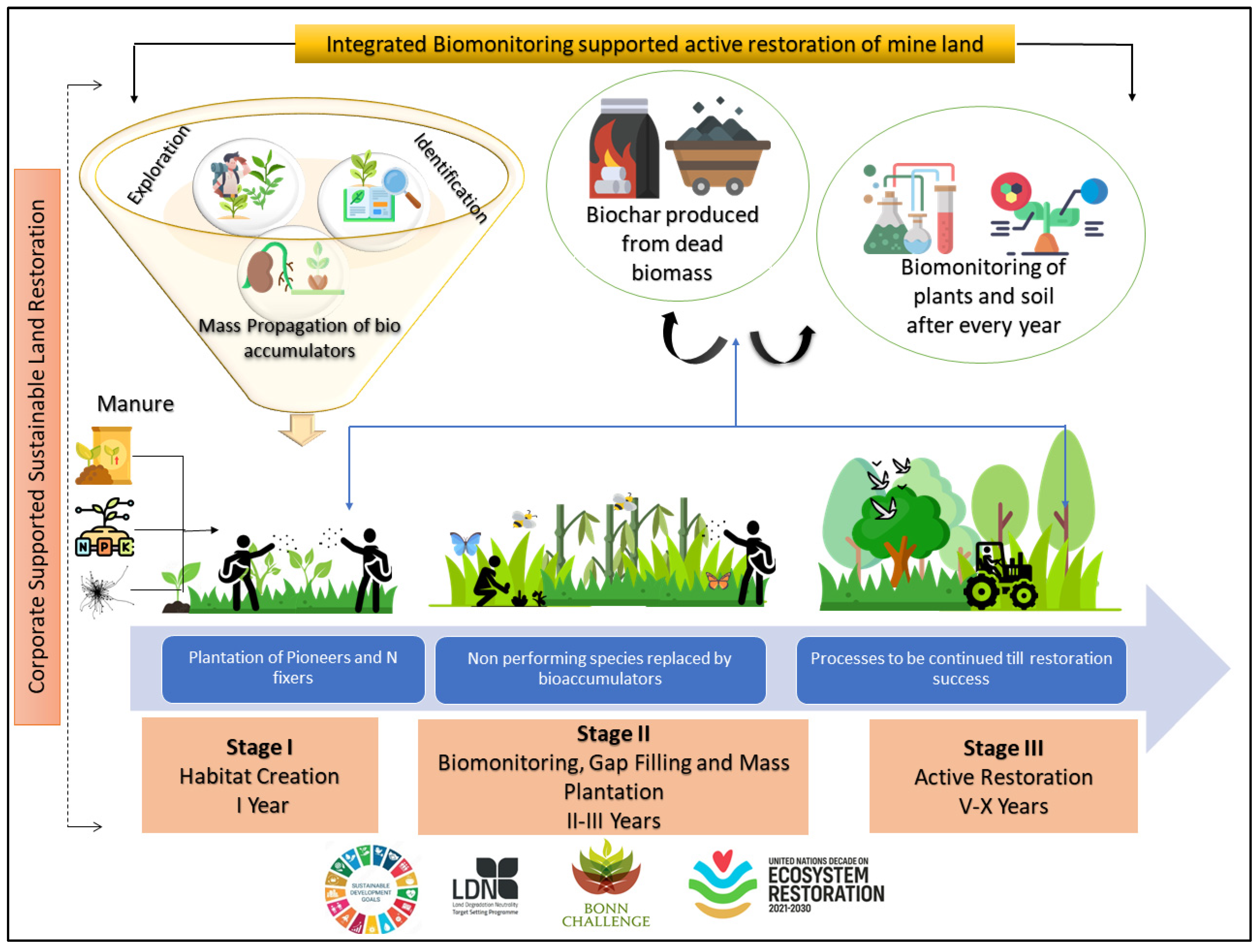
- A biomonitoring-supported restoration model for improve sustainable land restoration (SLR)
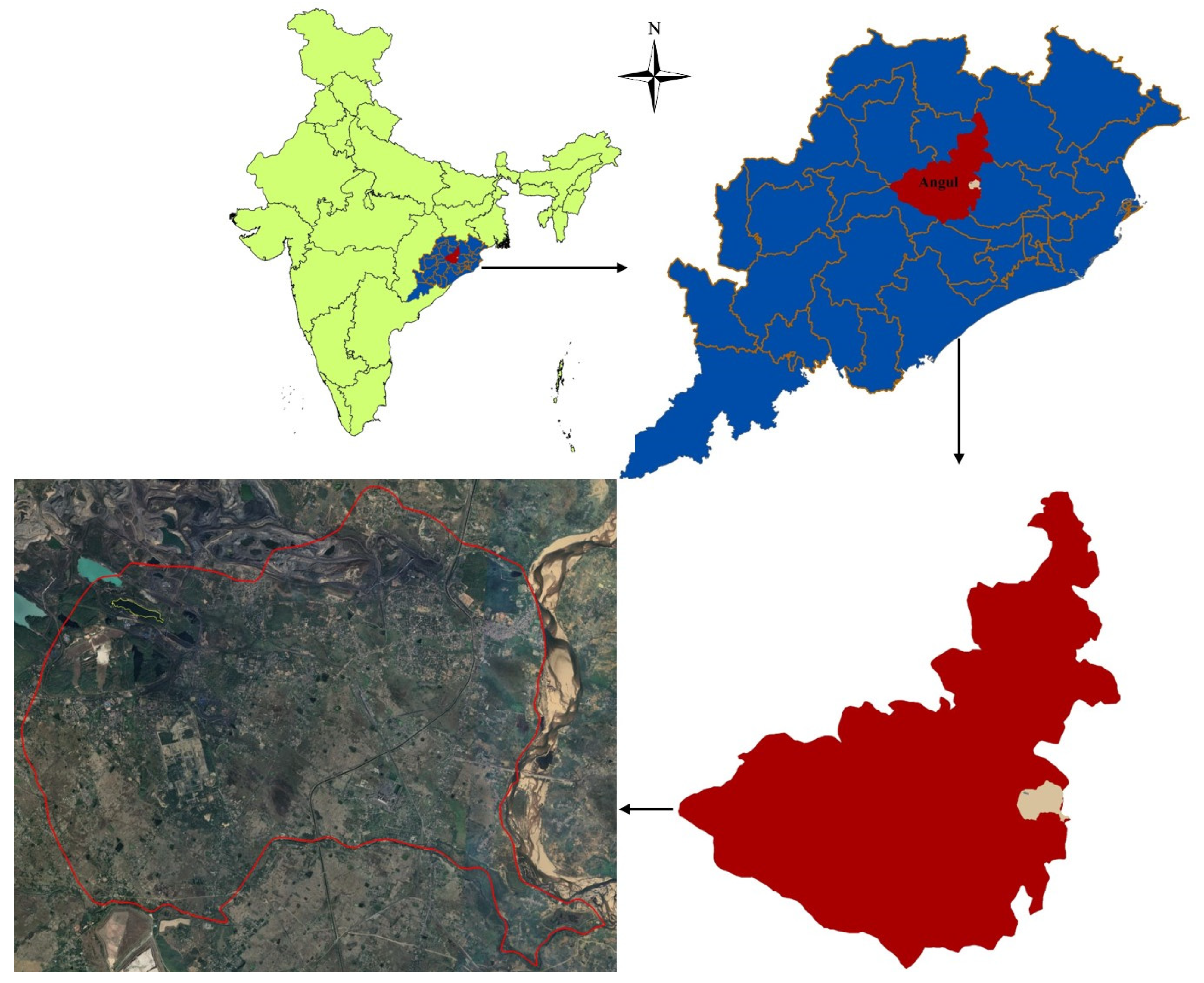
2. Study Area and Climate
3. Materials and Methods
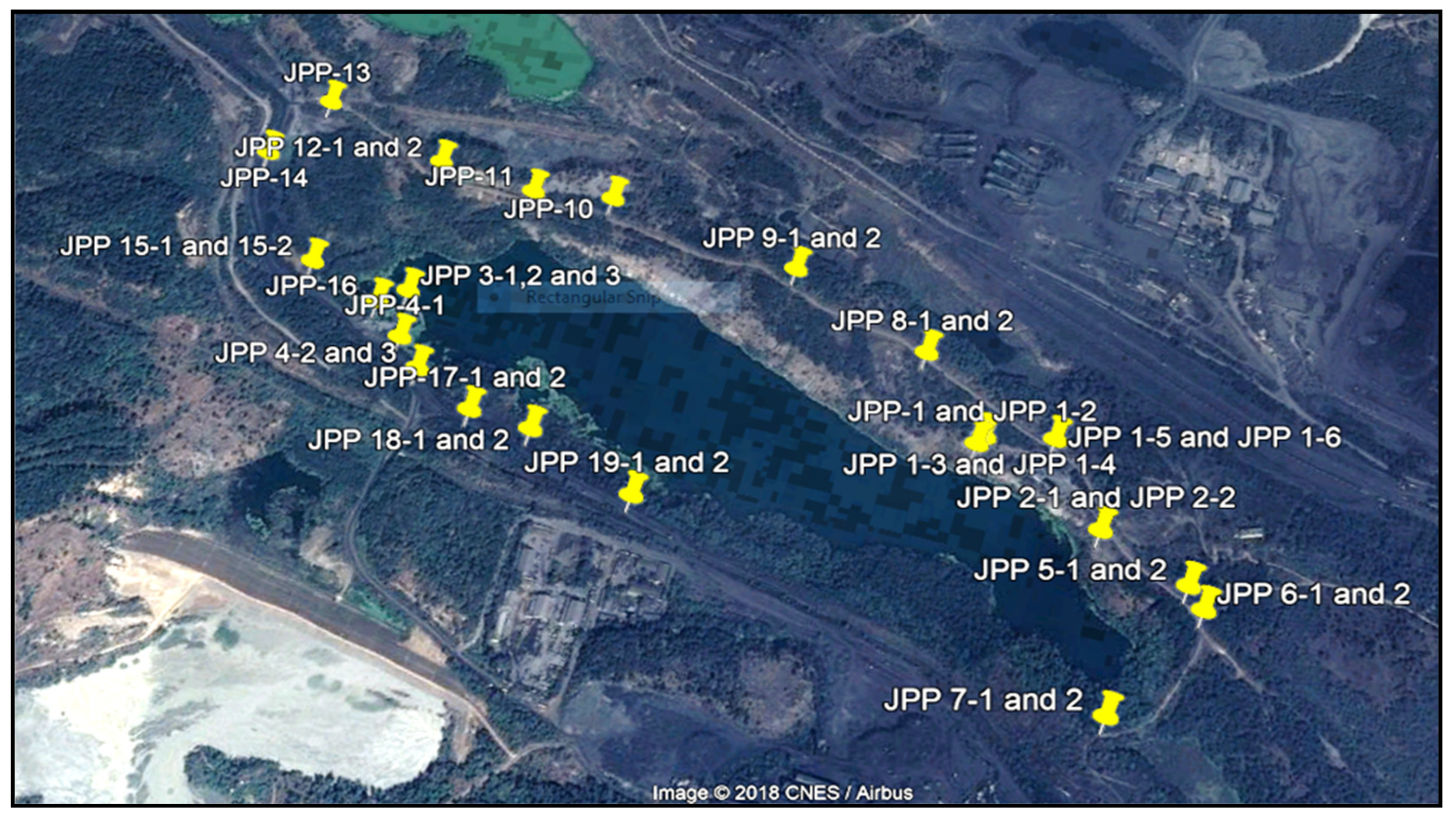
3.1. Sample Collection and Analysis
3.2. Sample Digestion
3.3. Bioaccumulation Factor for Metal in Plant Samples
4. Results and Discussion
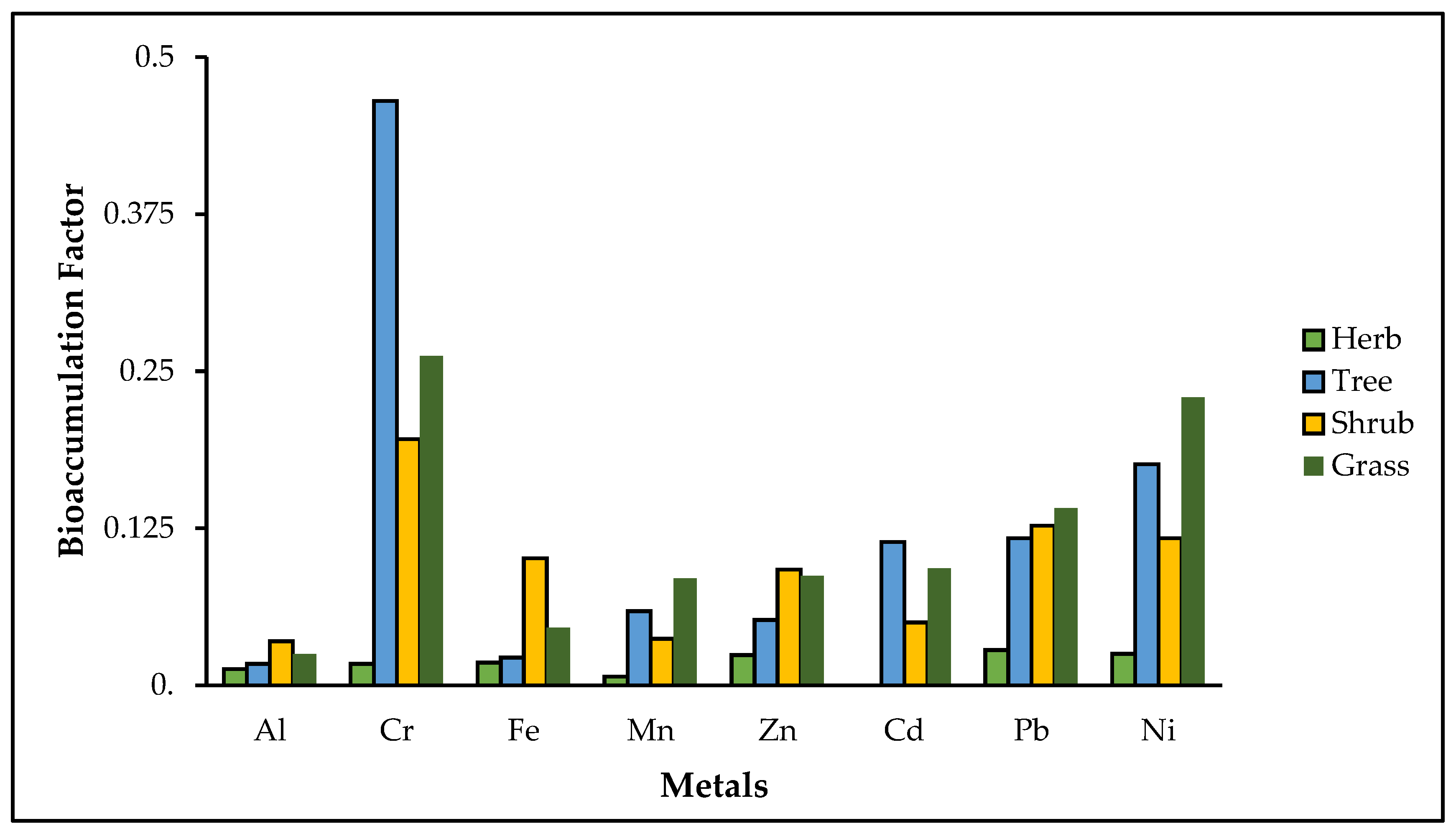
4.1. Bioaccumulation Factor for Metals in Non-Edible Plant Samples
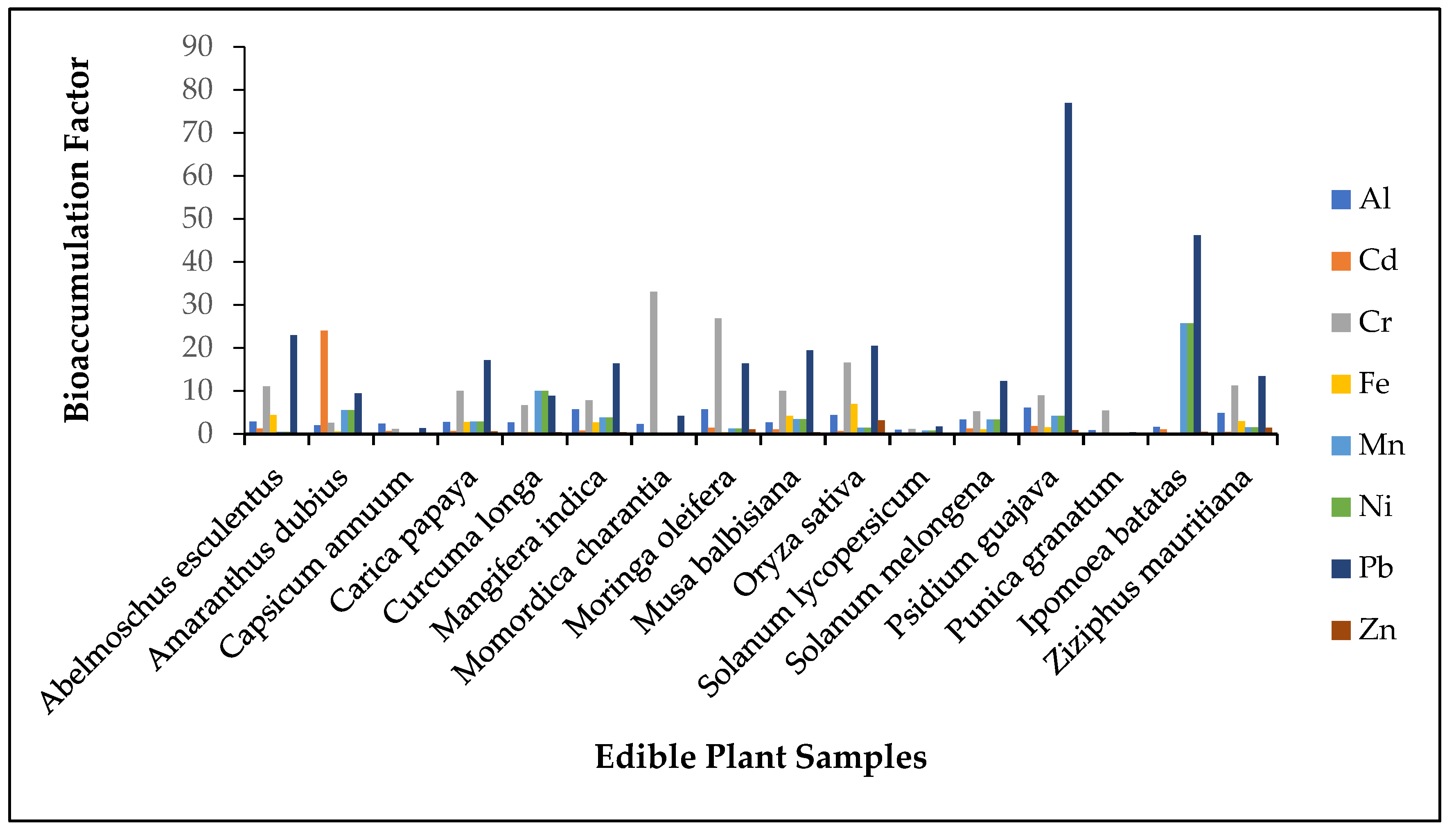
4.2. Bioaccumulation Factor for Metals in Edible Plant Samples
5. Biomonitoring-Supported Sustainable Land Restoration
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sl. No | Plant Species | Botanical Name | Al | Cd | Cr | Fe | Mn | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Billygoat-weed | Ageratum conyzoides | 1.054 | 0.250 | 0.011 | 0.023 | 0.250 | 0.262 | 0.115 | 0.133 |
| 2 | Indian Lilac | Azadirachta indica | 2.625 | 0.470 | 8.593 | 2.130 | 24.294 | 3.981 | 9.396 | 0.353 |
| 3 | Crown Flower | Calotropis gigantea | 4.222 | 1.000 | 13.133 | 3.599 | 6.745 | 1.619 | 5.577 | 0.526 |
| 4 | Common hackberry | Celtis occidentalis | 0.924 | BDL | 0.778 | 0.033 | 0.408 | 0.095 | 0.115 | 0.032 |
| 5 | Dhoob | Cynodon dactylon | 2.506 | 0.675 | 5.815 | 1.342 | 34.709 | 11.448 | 6.823 | 0.251 |
| 6 | Nut grass | Cyperus rotundus | 3.025 | 1.075 | 2.911 | 0.639 | 2.997 | 3.262 | 9.327 | 0.546 |
| 7 | North Indian Rosewood | Dalbergia sissoo | 1.400 | 0.500 | 32.833 | 0.399 | 0.883 | 4.000 | 10.385 | 0.241 |
| 8 | Non edible Crabgrass | Digitaria ciliaris | 4.407 | 1.667 | 18.926 | 4.337 | 8.464 | 0.841 | 2.821 | 0.602 |
| 9 | Royal Poinciana | Delonix regia | 1.006 | 1.000 | 44.667 | 0.439 | 6.820 | 2.905 | 10.769 | 0.566 |
| 10 | Peepal | Ficus religiosa | 1.366 | 3.500 | 5.556 | 0.342 | 9.276 | 1.429 | 0.462 | 0.224 |
| 11 | Weeping fig | Ficus benjamina | 2.226 | BDL | 7.778 | 2.780 | 0.714 | 0.952 | BDL | 0.138 |
| 12 | Spanish Flag | Lantana camara | 3.503 | 1.150 | 19.931 | 2.001 | 2.816 | 1.095 | 16.045 | 0.313 |
| 13 | Bitter vine | Mikania micrantha | 0.341 | BDL | 0.111 | 0.042 | 1.223 | 0.095 | 0.038 | 0.043 |
| 14 | Rangoon Creeper | Quisqualis indica | 0.341 | BDL | 0.111 | 0.042 | 1.325 | 0.095 | BDL | 0.043 |
| 15 | Black Locust | Robinia pseudoacacia | 1.916 | 0.150 | 2.222 | 0.112 | 0.418 | 0.143 | 2.692 | 0.064 |
| 16 | Non edible Sugarcane | Saccharum spontaneum | 1.890 | 1.000 | 10.278 | 1.776 | 12.370 | 1.333 | 13.077 | 0.378 |
| 17 | Indian Grass | Sorghastrum nutans | 0.682 | BDL | 0.556 | BDL | 0.408 | 0.048 | 1.154 | 0.053 |
| 18 | Narrowleaf Cattail | Typha angustifolia | 1.587 | 0.250 | 1.704 | 0.233 | 1.903 | 0.413 | 2.051 | 0.121 |
| 19 | Broadleaf cattail | Typha latifolia | 1.770 | BDL | 0.778 | 1.971 | 0.408 | 0.476 | 2.885 | 0.075 |
| 20 | Indian stinging nettle | Tragia involucrata | 5.747 | 0.500 | 33.611 | 9.398 | 11.871 | 4.452 | 2.462 | 0.594 |
| SL. No | Plant Species | Botanical Name | Al | Cd | Cr | Fe | Mn | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Okra | Abelmoschus esculentus | 2.874 | 1.271 | 11.022 | 4.404 | 0.497 | 0.497 | 22.912 | 0.284 |
| 2 | Spleen amaranth | Amaranthus dubius | 2.041 | 24.000 | 2.622 | 0.568 | 5.486 | 5.486 | 9.462 | 0.213 |
| 3 | Capsicum | Capsicum annuum | 2.406 | 0.700 | 1.111 | 0.255 | BDL | BDL | 1.346 | 0.186 |
| 4 | Papaya | Carica papaya | 2.745 | 0.643 | 10.000 | 2.737 | 2.905 | 2.905 | 17.154 | 0.600 |
| 5 | Turmeric | Curcuma longa | 2.701 | 0.350 | 6.667 | 0.512 | 10.000 | 10.000 | 8.846 | 0.394 |
| 6 | Mango | Mangifera indica | 5.745 | 0.775 | 7.778 | 2.637 | 3.810 | 3.810 | 16.346 | 0.410 |
| 7 | Bitter Gourd | Momordica charantia | 2.274 | 0.050 | 33.037 | 0.212 | 0.159 | 0.159 | 4.231 | 0.146 |
| 8 | Drumstick | Moringa oleifera | 5.672 | 1.400 | 26.889 | 0.354 | 1.238 | 1.238 | 16.410 | 1.032 |
| 9 | Banana | Musa balbisiana | 2.678 | 1.083 | 10.000 | 4.173 | 3.463 | 3.463 | 19.397 | 0.414 |
| 10 | Rice | Oryza sativa | 4.374 | 0.667 | 16.583 | 6.931 | 1.452 | 1.452 | 20.513 | 3.166 |
| 11 | Tomato | Solanum lycopersicum | 0.911 | BDL | 1.111 | 0.237 | 0.738 | 0.738 | 1.731 | 0.096 |
| 12 | Brinjal | Solanum melongena | 3.320 | 1.250 | 5.222 | 1.085 | 3.341 | 3.341 | 12.308 | 0.250 |
| 13 | Guava | Psidium guajava | 6.112 | 1.833 | 8.963 | 1.557 | 4.238 | 4.238 | 76.923 | 0.849 |
| 14 | Pomegranate | Punica granatum | 0.873 | BDL | 5.444 | 0.047 | 0.143 | 0.143 | 0.385 | 0.064 |
| 15 | Sweet Potato | Ipomoea batatas | 1.658 | 1.070 | BDL | 0.012 | 25.714 | 25.714 | 46.154 | 0.479 |
| 16 | Indian Jujube | Ziziphus mauritiana | 4.880 | 0.500 | 11.278 | 2.982 | 1.524 | 1.524 | 13.462 | 1.457 |
References
- Xie, H.; Zhang, Y.; Wu, Z.; Lv, T. A bibliometric analysis on land degradation: Current status, development, and future directions. Land 2020, 9, 28. [Google Scholar] [CrossRef]
- Dhyani, S.; Lahoti, S.; Khare, S.; Pujari, P.; Verma, P. Ecosystem based Disaster Risk Reduction approaches (EbDRR) as a prerequisite for inclusive urban transformation of Nagpur City, India. Int. J. Disaster Risk Reduct. 2018, 32, 95–105. [Google Scholar] [CrossRef]
- Dhyani, S.; Bartlett, D.; Kadaverugu, R.; Dasgupta, R.; Pujari, P.; Verma, P. Integrated climate sensitive restoration framework for transformative changes to sustainable land restoration. Restor Ecol. 2020, 28, 1026–1031. [Google Scholar] [CrossRef]
- Braganza, C. Desertification-An Ecological Cataclysm. Int. J. Novel Res. Devel. 2022, 7, 662–666. [Google Scholar]
- Luckeneder, S.; Giljum, S.; Schaffartzik, A.; Maus, V.; Tost, M. Surge in global metal mining threatens vulnerable ecosystems. Glob. Environ. Change 2021, 69, 0959–3780. [Google Scholar] [CrossRef]
- Ranjan, A.K.; Sahoo, D.; Gorai, A.K. Quantitative assessment of landscape transformation due to coal mining activity using earth observation satellite data in Jharsuguda coal mining region, Odisha, India. Environ. Dev. Sustain. 2020, 23, 4484–4499. [Google Scholar] [CrossRef]
- Ahamad, A.; Raju, N.J.; Madhav, S.; Khan, A.H. Heavy elements contamination in groundwater and associated human health risk in the industrial region of southern Sonbhadra, Uttar Pradesh, India. Environ. Geochem. Health 2020, 42, 3373–3391. [Google Scholar] [CrossRef]
- Bishwal, R.M.; Sen, P.; Jawed, M. Future challenges of overburden waste management in Indian coal mines. In Waste Management and Resource Efficiency; Springer: Singapore, 2019; pp. 1003–1011. [Google Scholar] [CrossRef]
- Gasparotto, J.; Martinello, K.D.B. Coal as an energy source and its impacts on human health. Energy Geosci. 2021, 2, 113–120. [Google Scholar] [CrossRef]
- Pandey, V.C.; Sahu, N.; Singh, D.P. Physiological profiling of invasive plant species for ecological restoration of fly ash deposits. Urban For. Urban Green 2020, 54, 126773. [Google Scholar] [CrossRef]
- Dhyani, S.; Singh, S.; Kadaverugu, R.; Pujari, P.; Verma, P. Habitat suitability modelling and nature-based solutions: An efficient combination to realise the targets of Bonn challenge and SDGs in South Asia. In Nature-based Solutions for Resilient Ecosystems and Societies; Springer: Singapore, 2020; pp. 347–364. [Google Scholar] [CrossRef]
- Wu, B.; Peng, H.; Sheng, M.; Luo, H.; Wang, X.; Zhang, R.; Xu, F.; Xu, H. Evaluation of phytoremediation potential of native dominant plants and spatial distribution of heavy metals in abandoned mining area in Southwest China. Ecotoxicol. Environ. Saf. 2020, 220, 112368. [Google Scholar] [CrossRef]
- Li, X.; Lei, S.; Liu, F.; Wang, W. Analysis of plant and soil restoration process and degree of refuse dumps in open-pit coal mining areas. Int. J. Environ. Res. Public Health 2020, 17, 1975. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Das, G.; Shin, H.S.; Kumar, P.; Patra, J.K. Diversity of plant species in the steel city of Odisha, India: Ethnobotany and implications for conservation of urban bio-resources. Braz. Arch. Biol. Technol. 2018, 61. [Google Scholar] [CrossRef]
- Mishra, M.C.; Senapati, S.; Rao, B.H. Odisha. In Geotechnical Characteristics of Soils and Rocks of India; CRC Press: Leiden, The Netherlands, 2021; pp. 511–527. [Google Scholar]
- Mahalik, G.; Satapathy, K.B.; Sahoo, S. Floral diversity and quantitative analysis of tree diversity of northern tropical semi-evergreen forests in Dhenkanal district of Odisha, India. Int. J. Botany Stud. 2018, 3, 15–19. [Google Scholar]
- Anning, A.K.; Akoto, R. Assisted phytoremediation of heavy metals contaminated soil from a mined site with Typha latifolia and Chrysopogon zizanioides. Ecotoxicol. Environ. Saf. 2018, 148, 97–104. [Google Scholar] [CrossRef]
- Aladesanmi, O.T.; Oroboade, J.G.; Osisiogu, C.P.; Osewole, A.O. Bioaccumulation factor of selected heavy metals in Zea mays. J. Health Pollut. 2019, 9, 191207. [Google Scholar] [CrossRef]
- Gebeyehu, H.R.; Bayissa, L.D. Levels of heavy metals in soil and vegetables and associated health risks in Mojo area, Ethiopia. PLoS ONE 2020, 15, e0227883. [Google Scholar] [CrossRef]
- Khan, Z.I.; Ugulu, I.; Zafar, A.; Mehmood, N.; Bashir, H.; Ahmad, K.; Sana, M. Biomonitoring of heavy metals accumulation in wild plants growing at soon valley, Khushab, Pakistan. Pak. J. Bot. 2021, 53, 247–252. [Google Scholar] [CrossRef]
- Liu, J.; Dong, Y.; Xu, H.; Wang, D.; Xu, J. Accumulation of Cd, Pb and Zn by 19 wetland plant species in constructed wetland. J. Hazard. Mater. 2007, 147, 947–953. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60. [Google Scholar] [CrossRef]
- World Health Organization. Quality Control Methods for Medicinal Plant Materials; WHO: Geneva, Switzerland, 1998. [Google Scholar]
- Shah, A.; Niaz, A.; Ullah, N.; Rehman, A.; Akhlaq, M.; Zakir, M.; Suleman, M. Comparative study of heavy metals in soil and selected medicinal plants. J. Chem. 2013, 2013, 621265. [Google Scholar] [CrossRef]
- Zhan, F.; Li, B.; Jiang, M.; Li, T.; He, Y.; Li, Y.; Wang, Y. Effects of arbuscular mycorrhizal fungi on the growth and heavy metals accumulation of bermudagrass [Cynodon dactylon (L.) Pers.] grown in a lead–zinc mine wasteland. Int. J. Phytoremediation 2019, 21, 849–856. [Google Scholar] [CrossRef]
- Mahohi, A.; Raiesi, F. The performance of mycorrhizae, rhizobacteria, and earthworms to improve Bermuda grass (Cynodon dactylon) growth and Pb uptake in a Pb-contaminated soil. Environ. Sci. Pollut. Res. 2021, 28, 3019–3034. [Google Scholar] [CrossRef]
- Dehdezi, A.A.; Alaei, E.; Azadi, P.; Shavandi, M.; Mousavi, S.A. Role of Phytoremediation in Reducing Cadmium and Nickle Toxicity in Soil Using Species (Cynodon dactylon L.). J. Hum. Environ. Health Promot. 2021, 7, 213–220. [Google Scholar]
- Maiti, S.K.; Nandhini, S. Bioavailability of metals in fly ash and their bioaccumulation in naturally occurring vegetation: A pilot scale study. Environ. Monit. Assess. 2006, 116, 263–273. [Google Scholar] [CrossRef]
- Tariq, M.; Farooq, U.; Athar, M.; Salman, M.; Tariq, M.; Shahida, S.; Farooqi, Z.H. Lab-scale continuous flow studies for comparative biosorption of cadmium (II) on untreated and xanthated Ficus religiosa biomass. Water Environ. Res. 2021, 93, 2681–2695. [Google Scholar] [CrossRef]
- Salman, S.M.; Muhammad, S.; Iqbal, M.; Aijaz, M.; Siddique, M.; Ali, A.; Nawaz, S.; Kamran, A.W. Biosorption of Pb (II) and Cd (II) Ions from Aqueous Solution by Chemically Modified Syzygium cumini Leaves and its Equilibrium, Kinetic and Thermodynamic Studies. Pak. J. Sci. Ind. Res. A Phys. Sci. 2020, 63, 18–29. [Google Scholar] [CrossRef]
- Rao, K.S.; Anand, S.; Venkateswarlu, P. Adsorption of cadmium from aqueous solution by Ficus religiosa leaf powder and characterization of loaded biosorbent. CLEAN–Soil Air Water 2011, 39, 384–391. [Google Scholar] [CrossRef]
- Tariq, M.; Farooq, U.; Athar, M.; Salman, M.; Tariq, M. Biosorption of Cu (II) from aqueous solution onto immobilized Ficus religiosa branch powder in a fixed bed column: Breakthrough curves and mathematical modeling. Korean J. Chem. Eng. 2019, 36, 48–55. [Google Scholar] [CrossRef]
- Chidi, O.; Kelvin, R. Surface interaction of sweet potato peels (Ipomoea batata) with Cd (II) and Pb (II) ions in aqueous medium. Chem. Int. 2018, 4, 221–229. [Google Scholar]
- Chuma, F. Assessment of heavy metals concentration in ipomoea batatas and spinach consumed in Zanzibar by Energy Dispersive X-ray Fluorescence (EDXRF). Braz. J. Radiat. Sci. 2021, 9, 1–17. [Google Scholar]
- Zhou, J.; Du, B.; Liu, H.; Cui, H.; Zhang, W.; Fan, X.; Cui, J.; Zhou, J. The bioavailability and contribution of the newly deposited heavy metals (copper and lead) from atmosphere to rice (Oryza sativa L.). J. Hazard. Mater. 2020, 384, 121285. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, C.; Wang, Z.; Gao, T.; Liu, Y.; Xia, Y.; Yin, R.; Qi, M. Zinc regulation of iron uptake and translocation in rice (Oryza sativa L.): Implication from stable iron isotopes and transporter genes. Environ. Pollut. 2022, 297, 118818. [Google Scholar] [CrossRef]
- Xu, B.; Wang, F.; Zhang, Q.; Lan, Q.; Liu, C.; Guo, X.; Cai, Q.; Chen, Y.; Wang, G.; Ding, J. Influence of iron plaque on the uptake and accumulation of chromium by rice (Oryza sativa L.) seedlings: Insights from hydroponic and soil cultivation. Ecotoxicol. Environ. Saf. 2018, 162, 51–58. [Google Scholar] [CrossRef]
- Saralathambavani, D.; Prathipa, V. Strategies of heavy metals uptake by plants growing under urban environment. Asian J. Soil Sci. 2012, 7, 304–311. [Google Scholar]
- Krishnani, K.K.; Choudhary, K.; Boddu, V.M.; Moon, D.H.; Meng, X. heavy metals biosorption mechanism of partially delignified products derived from mango (Mangifera indica) and guava (Psidium guiag) barks. Environ. Sci. Pollut. Res. 2021, 28, 32891–32904. [Google Scholar] [CrossRef]
- Yap, C.K.; Razali, A.; Nulit, R.; Peng, S.H.T.; Yap, C.W.; Okamura, H.; Ismail, M.S. Biomonitoring of heavy metals in the Guava (Psidium guajava) for Their Health Risk Assessment in Kluang, Malaysia. Food Sci. Eng. 2021, 13–20. [Google Scholar] [CrossRef]
- Maiti, S.K.; Kumar, A.; Ahirwal, J. Bioaccumulation of metals in timber and edible fruit trees growing on reclaimed coal mine overburden dumps. Int. J. Min. Reclam. Environ. 2016, 30, 231–244. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, S. Bioremediation of heavy metals from industrial effluents by endophytes and their metabolic activity: Recent advances. Bioresour. Technol. 2021, 339, 125589. [Google Scholar] [CrossRef]
- Garba, S.T.; Gudusu, M.; Inuwa, L.B. Accumulation Ability of the Native Grass Species, Cyperus rotundus for the heavy metals; Zinc (Zn), Cadmium (Cd), Nickel (Ni) and Lead (Pb). Int. Res. J. Pure Appl. Chem 2018, 17, 1–15. [Google Scholar] [CrossRef]
- Sultana, T.; Majumdar, S.; Mitra, A.K. Phytoremediation potential of nickel by Cyperus rotundus along with its rhizospheric fungi. J. Mycopathol. Res. 2018, 55, 383–389. [Google Scholar]
- Banerjee, R.; Jana, A.; De, A.; Mukherjee, A. Phytoextraction of heavy metals from coal fly ash for restoration of fly ash dumpsites. Bioremediat. J. 2020, 24, 41–49. [Google Scholar] [CrossRef]
- Khodijah, N.S.; Suwignyo, R.A.; Harun, M.U.; Robiartini, L. Phytoremediation potential of some grasses on lead heavy metals in tailing planting media of former tin mining. Biodiversitas J. Biol. Diver. 2019, 20, 1973–1982. [Google Scholar] [CrossRef]
- Maiti, D.; Pandey, V.C. Metal remediation potential of naturally occurring plants growing on barren fly ash dumps. Environ. Geochem. Health 2021, 43, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shen, Q. Phytoremediation of cadmium-contaminated wetland soil with Typha latifolia L. and the underlying mechanisms involved in the heavy-metal uptake and removal. Environ. Sci. Pollut. Res. 2020, 27, 4905–4916. [Google Scholar] [CrossRef]
- Mani, D.; Kumar, C. Biotechnological advances in bioremediation of heavy metals contaminated ecosystems: An overview with special reference to phytoremediation. Int. J. Environ. Sci. Tech. 2014, 11, 843–872. [Google Scholar] [CrossRef]
- Teimoory, H.; Kodikara, K.A.S.; De Silva, P.M.C.S.; Madarasinghe, S.K.; Ranasinghe, P.; Somasiri, H.P.P.S.; Danee, M.; Dahdouh-Guebas, F.; Jayatissa, L.P. Heavy metals Contents in Surface Sediments of Kalametiya Lagoon, Sri Lanka and heavy metals Uptake by Typha angustifolia L., A Wetland Sedge. In 18th Academisc Sessions; University of Ruhuna: Matara, Sri Lanka, 2021. [Google Scholar]
- Sharma, V.; Pant, D. Biocompatible metal decontamination from soil using Ageratum conyzoides. Environ. Sci. Pollut. Res. 2018, 25, 22294–22307. [Google Scholar] [CrossRef]
- Parihar, J.K.; Parihar, P.K.; Pakade, Y.B.; Katnoria, J.K. Bioaccumulation potential of indigenous plants for heavy metals phytoremediation in rural areas of Shaheed Bhagat Singh Nagar, Punjab (India). Environ. Sci. Pollut. Res. 2021, 28, 2426–2442. [Google Scholar] [CrossRef]
- Mahato, A.; Ghosh, D.; Maiti, S.K. Phytoremediation and environmental bioremediation. In Phytoremediation Technology for the Removal of Heavy Metals and Other Contaminants from Soil and Water; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–18. [Google Scholar]
- Vandana, U.K.; Gulzar, A.B.M.; Singha, L.P.; Bhattacharjee, A.; Mazumder, P.B.; Pandey, P. Hyperaccumulation of arsenic by Pteris vittata, a potential strategy for phytoremediation of arsenic-contaminated soil. Environ. Sust. 2020, 3, 169–178. [Google Scholar] [CrossRef]
- Zeng, P.; Guo, Z.; Xiao, X.; Peng, C.; Feng, W.; Xin, L.; Xu, Z. Phytoextraction potential of Pteris vittata L. co-planted with woody species for As, Cd, Pb and Zn in contaminated soil. Sci. Total Environ. 2019, 650, 594–603. [Google Scholar] [CrossRef]
- Zhao, X.; Joo, J.C.; Lee, J.K.; Kim, J.Y. Mathematical estimation of heavy metals accumulations in Helianthus annuus L. with a sigmoid heavy metals uptake model. Chemosphere 2019, 220, 965–973. [Google Scholar] [CrossRef]
- Mani, D.; Sharma, B.; Kumar, C. Phytoaccumulation, interaction, toxicity and remediation of cadmium from Helianthus annuus L. (sunflower). Bull. Environ. Contam. Toxicol. 2007, 79, 71–79. [Google Scholar] [CrossRef]
- Selvam, A.; Wong, J.W.C. Phytochelatin systhesis and cadmium uptake of Brassica napus. Environ. Tech. 2008, 29, 765–773. [Google Scholar] [CrossRef]
- Kamran, M.; Malik, Z.; Parveen, A.; Huang, L.; Riaz, M.; Bashir, S.; Mustafa, A.; Abbasi, G.H.; Ali, U. Ameliorative effects of biochar on rapeseed (Brassica napus L.) growth and heavy metals immobilization in soil irrigated with untreated wastewater. J. Plant Growth Regul. 2020, 39, 266–281. [Google Scholar] [CrossRef]
- Liu, D.; Zou, J.; Wang, M.; Jiang, W. Hexavalent chromium uptake and its effects on mineral uptake, antioxidant defence system and photosynthesis in Amaranthus viridis L. Bioresour. Technol. 2008, 99, 2628–2636. [Google Scholar] [CrossRef]
- Sangeetha, P.; Venkatachalam, P.; Geetha, N. Exploring the phytoremediation potential of Calotropis gigantea L. Using a combined FTIR and principal component analysis. In vitro Plant Breeding Towards Novel Agronomic Traits; Springer: Singapore, 2019; pp. 75–82. [Google Scholar]
- Pandey, S.K.; Bhattacharya, T. Effect of two biodegradable chelates on metals uptake, translocation and biochemical changes of Lantana Camara growing in fly ash amended soil. Int. J. Phytoremediation 2018, 20, 214–224. [Google Scholar] [CrossRef]
- Alaribe, F.O.; Agamuthu, P. Lantana camara—An ecological bioindicator plant for decontamination of Pb-impaired soil under organic waste-supplemented scenarios. Pedosphere 2019, 29, 248–258. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Liu, R.; Zhou, J.; Jiang, Z. Biomonitoring and phytoremediation potential of the leaves, bark, and branch bark of street trees for heavy metals pollution in urban areas. Environ. Monit. Assess. 2022, 194, 1–14. [Google Scholar] [CrossRef]
- Leung, H.M.; Yue, P.Y.K.; Sze, S.C.W.; Au, C.K.; Cheung, K.C.; Chan, K.L.; Yung, K.L.K.; Li, W.C. The potential of Mikania micrantha (Chinese creeper) to hyperaccumulate heavy metals in soil contaminated by electronic waste. Environ. Sci. Pollut. Res. 2019, 26, 35275–35280. [Google Scholar] [CrossRef]
- Usman, K.; Al-Ghouti, M.A.; Abu-Dieyeh, M.H. The assessment of cadmium, chromium, copper, and nickel tolerance and bioaccumulation by shrub plant Tetraena qataranse. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Naseem, K.; Imran, Q.; Ur Rehman, M.Z.; Tahir, M.H.; Najeeb, J. Adsorptive removal of heavy metals and dyes from wastewater using Azadirachta indica biomass. Int. J. Environ. Sci. Techn. 2022, 1–24. [Google Scholar] [CrossRef]
- Sun, W.; Ji, B.; Khoso, S.A.; Tang, H.; Liu, R.; Wang, L.; Hu, Y. An extensive review on restoration technologies for mining tailings. Environ. Sci. Pollut. Res. 2018, 5, 33911–33925. [Google Scholar] [CrossRef]
- Morales-Estupiñan, M.J.; Recalde, S.; Orozco, K.; Ponce, W. Analysis of heavy metals in Azadirachta indica A. Juss Leaves, as Bioindicator for Monitoring Enviromental Pollution in Guayaquil, Ecuador 2020. In Proceedings of the 6th World Congress on New Technologies, Online, 19–21 August 2020. [Google Scholar]
- Luo, J.; Cai, L.; Qi, S.; Wu, J.; Gu, X.S. heavy Metal remediation with Ficus microcarpa through transplantation and its environmental risks through field scale experiment. Chemosphere 2018, 193, 244–250. [Google Scholar] [CrossRef]
- Ultra Jr, V. Fly ash and compost amendments and mycorrhizal inoculation enhanced the survival and growth of Delonix regia in Cu-Ni mine tailings. Philipine J. Sci. 2020, 149, 479–489. [Google Scholar]
- Kalam, S.U.; Naushin, F.; Khan, F.A.; Rajakaruna, N. Long-term phytoremediating abilities of Dalbergia sissoo Roxb. (Fabaceae). SN Appl. Sci. 2019, 1, 1–8. [Google Scholar] [CrossRef]
- Fan, M.; Liu, Z.; Nan, L.; Wang, E.; Chen, W.; Lin, Y.; Wei, G. Isolation, characterization, and selection of heavy metals -resistant and plant growth-promoting endophytic bacteria from root nodules of Robinia pseudoacacia in a Pb/Zn mining area. Microbiol. Res. 2018, 217, 51–59. [Google Scholar] [CrossRef]
- Netty, N. Bioaccumulation of nickel by five wild plant species on nickel-contaminated soil. IOSR J. Eng. 2018, 260, 1–7. [Google Scholar] [CrossRef]
- Jambhulkar, H.P.; Juwarkar, A.A. Assessment of bioaccumulation of heavy metals by different plant species grown on fly ash dump. Ecotoxicol. Environ. Saf. 2009, 72, 1122–1128. [Google Scholar] [CrossRef]
| Sl. No. | Metals | Fly Ash | Soil | Non Edible Plants | Edible Plants (Crops/Vegetables/Fruits) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Concentration (mg/kg) Min–Max | X ± SD | Concentration (mg/kg) Min–Max | X ± SD | Concentration (mg/kg) Min–Max | X ± SD | Concentration (mg/kg) Min–Max | X ± SD | ||
| 1 | Al | 0.187–4.140 | 1.909 ± 1.494 | 123.120–238.420 | 172.430 ± 59.429 | 0.660–11.129 | 3.930 ± 2.702 | 1.690–11.836 | 6.205 ± 3.240 |
| 2 | Cd | 0–0.001 | 0 ± 0 | 0.010–0.020 | 0.016 ± 0.005 | 0–0.007 | 0.001 ± 0.002 | 0.000–0.048 | 0.005 ± 0.012 |
| 3 | Cr | 0.001–0.040 | 0.016 ± 0.017 | 0.150–0.410 | 0.323 ± 0.150 | 0–0.402 | 0.090 ± 0.113 | 0.000–0.297 | 0.089 ± 0.082 |
| 4 | Fe | 0.021–9.200 | 2.118 ± 3.566 | 99.870–224.460 | 171.486 ± 64.353 | 0–53.784 | 8.003 ±12.280 | 0.070–39.668 | 10.266 ± 11.558 |
| 5 | Mn | 0.084–1.050 | 0.533 ± 0.406 | 1.800–25.440 | 11.596 ± 12.328 | 0.025–3.405 | 0.561 ± 0.841 | 0.085–3.710 | 0.766 ± 0.844 |
| 6 | Ni | 0.003–0.052 | 0.029 ± 0.017 | 0.140–0.360 | 0.266 ± 0.113 | 0.001–0.240 | 0.036 ± 0.054 | 0.000–0.540 | 0.085 ± 0.133 |
| 7 | Pb | 0–0.003 | 0.001 ± 0 | 0.030–0.170 | 0.113 ± 0.073 | 0–0.042 | 0.012 ± 0.013 | 0.001–0.200 | 0.047 ± 0.050 |
| Sl.No | Plant Species | Botanical Name | Al * | Cd ** | Cr ** | Fe ** | Mn* * | Ni ** | Pb ** | Zn ** |
|---|---|---|---|---|---|---|---|---|---|---|
| Permissible Limit | 15 | 0.02 | 1.30 | 20 | 200 | 10 | 2 | 0.60 | ||
| 1 | Billygoat weed | Ageratum conyzoides | 2.040 | BDL | BDL | 0.130 | 0.025 | 0.006 | BDL | 0.125 |
| 2 | Indian Lilac | Azadirachta indica | 5.083 | 0.001 | 0.077 | 12.188 | 2.383 | 0.084 | 0.024 | 0.331 |
| 3 | Crown Flower | Calotropis gigantea | 8.176 | 0.002 | 0.118 | 20.6 | 0.662 | 0.034 | 0.015 | 0.494 |
| 4 | Common hackberry | Celtis occidentalis | 1.790 | BDL | 0.007 | 0.190 | 0.040 | 0.002 | BDL | 0.030 |
| 5 | Dhoob | Cynodon dactylon | 4.853 | 0.001 | 0.052 | 7.678 | 3.405 | 0.240 | 0.018 | 0.236 |
| 6 | Nut grass | Cyperus rotundus | 5.858 | 0.002 | 0.026 | 3.657 | 0.294 | 0.069 | 0.024 | 0.513 |
| 7 | North Indian Rosewood | Dalbergia sissoo | 2.711 | 0.001 | 0.296 | 2.284 | 0.087 | 0.084 | 0.027 | 0.227 |
| 8 | Non edible Crabgrass | Digitaria ciliaris | 8.535 | 0.003 | 0.170 | 24.818 | 0.830 | 0.018 | 0.007 | 0.565 |
| 9 | Royal Poinciana | Delonix regia | 1.949 | 0.002 | 0.402 | 2.513 | 0.669 | 0.061 | 0.028 | 0.531 |
| 10 | Peepal | Ficus religiosa | 2.645 | 0.007 | 0.050 | 1.960 | 0.910 | 0.030 | 0.001 | 0.210 |
| 11 | Weeping fig | Ficus benjamina | 4.310 | BDL | 0.070 | 15.910 | 0.070 | 0.020 | BDL | 0.130 |
| 12 | Spanish Flag | Lantana camara | 6.783 | 0.002 | 0.179 | 11.450 | 0.276 | 0.023 | 0.042 | 0.294 |
| 13 | Bitter vine | Mikania micrantha | 0.660 | BDL | 0.001 | 0.240 | 0.120 | 0.002 | BDL | 0.040 |
| 14 | Rangoon Creeper | Combretum indicum | 0.660 | BDL | 0.001 | 0.240 | 0.130 | 0.002 | BDL | 0.040 |
| 15 | Black Locust | Robinia pseudoacacia | 3.710 | BDL | 0.020 | 0.640 | 0.041 | 0.003 | 0.007 | 0.060 |
| 16 | Non edible Sugarcane | Saccharum spontaneum | 3.660 | 0.002 | 0.093 | 10.163 | 1.214 | 0.028 | 0.034 | 0.355 |
| 17 | Indian Grass | Sorghastrum nutans | 1.320 | BDL | 0.005 | BDL | 0.040 | 0.001 | 0.003 | 0.050 |
| 18 | Narrowleaf Cattail | Typha angustifolia L. | 3.073 | BDL | 0.015 | 1.333 | 0.187 | 0.009 | 0.005 | 0.113 |
| 19 | Broadleaf cattail | Typha latifolia | 3.427 | BDL | 0.007 | 11.280 | 0.040 | 0.010 | 0.008 | 0.070 |
| 20 | Indian stinging nettle | Tragia involucrata | 11.129 | 0.001 | 0.303 | 53.784 | 1.165 | 0.094 | 0.006 | 0.558 |
| Sl. No. | Plant Species | Botanical Name | Al | Cd | Cr | Fe | Mn | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| Permissible Limit | 15 | 0.02 | 1.30 | 20 | 200 | 10 | 2 | 0.60 | ||
| 1 | Okra | Abelmoschus esculentus | 5.566 | 0.003 | 0.099 | 25.205 | 0.800 | 0.010 | 0.060 | 0.267 |
| 2 | Spleen amaranth | Amaranthus dubius | 3.953 | 0.048 | 0.024 | 3.248 | 0.884 | 0.115 | 0.025 | 0.200 |
| 3 | Capsicum | Capsicum annuum | 4.660 | 0.001 | 0.010 | 1.460 | 0.085 | 0.000 | 0.004 | 0.175 |
| 4 | Papaya | Carica papaya | 5.316 | 0.001 | 0.090 | 15.663 | 0.655 | 0.061 | 0.045 | 0.564 |
| 5 | Turmeric | Curcuma longa | 5.230 | 0.001 | 0.060 | 2.930 | 0.180 | 0.210 | 0.023 | 0.370 |
| 6 | Mango | Mangifera indica | 11.125 | 0.002 | 0.070 | 15.090 | 0.400 | 0.080 | 0.043 | 0.385 |
| 7 | Bitter Gourd | Momordica charantia | 4.403 | BDL | 0.297 | 1.215 | 0.104 | 0.003 | 0.011 | 0.137 |
| 8 | Drumstick | Moringa oleifera | 10.983 | 0.003 | 0.242 | 2.024 | 0.730 | 0.026 | 0.043 | 0.969 |
| 9 | Banana | Musa balbisiana | 5.186 | 0.002 | 0.090 | 23.881 | 0.540 | 0.073 | 0.050 | 0.389 |
| 10 | Rice | Oryza sativa | 8.470 | 0.001 | 0.149 | 39.668 | 0.696 | 0.031 | 0.053 | 2.973 |
| 11 | Tomato | Solanum lycopersicum | 1.765 | BDL | 0.010 | 1.355 | 1.000 | 0.016 | 0.005 | 0.090 |
| 12 | Brinjal | Solanum melongena | 6.430 | 0.003 | 0.047 | 6.208 | 0.978 | 0.070 | 0.032 | 0.235 |
| 13 | Guava | Psidium guajava | 11.836 | 0.004 | 0.081 | 8.911 | 0.861 | 0.089 | 0.200 | 0.797 |
| 14 | Pomegranate | Punica granatum | 1.690 | BDL | 0.049 | 0.270 | 0.170 | 0.003 | 0.001 | 0.060 |
| 15 | Sweet Potato | Ipomoea batatas | 3.210 | 0.002 | BDL | 0.070 | 3.710 | 0.540 | 0.120 | 0.450 |
| 16 | Indian Jujube | Ziziphus mauritiana | 9.451 | 0.001 | 0.102 | 17.064 | 0.465 | 0.032 | 0.035 | 1.368 |
| Sl. No. | Species | Metals | References |
|---|---|---|---|
| Herbs | |||
| 1 | Tragia involucrata | Al, Cd, Cr, Fe, Mn, Ni, Pb Zn | Present Study, [43] |
| 2 | Digitaria sanguinalis | Al, Cd, Cr, Fe, Mn, Ni, Pb Zn | Present Study, [19,20] |
| 3 | Cynodon dactylon | Al, Cd, Cr, Fe, Mn, Ni, Pb Zn | Present Study, [24,25,26,27] |
| 4 | Cyperus rotundus | Al, Cd, Cr, Fe, Mn, Ni, Pb Zn | Present Study, [44,45] |
| 5 | Saccharum spontaneum | Al, Cd, Cr, Fe, Mn, Ni, Pb Zn | Present Study, [46,47,48] |
| 6 | Typha latifolia | Al, Cd, Cr, Fe, Mn, Ni, Pb Zn | Present Study, [49,50] |
| 7 | Typha angustifolia | Al, Cd, Cr, Fe, Mn, Ni, Pb Zn | Present Study, [51] |
| 8 | Ageratum conyzoides | Al, Cd, Cr, Fe, Mn, Ni, Pb Zn | Present Study, [52,53] |
| 9 | Sorghastrum nutans | Al, Cd, Cr, Fe, Mn, Ni, Pb Zn | Present Study, [54] |
| 10 | Pteris vittata | Cu, Ni, Zn, As | Present Study, [55,56] |
| 11 | Helianthus annuus | Cd | [57,58] |
| 12 | Brassica napus | Cd | [59,60] |
| 13 | Amaranthus viridis | Cd, Cr, Co, Cu, Fe, Zn, Mn | [61,52] |
| 14 | Medicago polymorpha | Cd, Cr, Co, Cu, Fe, Zn, Mn | [52] |
| 15 | Parthenium hysterophorus | Cd, Cr, Co, Cu, Fe, Zn, Mn | [52] |
| Shrubs | |||
| 1 | Calotropis gigantean | Al, Cd, Cr, Fe, Mn, Ni, Pb Zn | Present Study, [62] |
| 2 | Lantana camara | Al, Cd, Cr, Fe, Mn, Ni, Pb Zn | Present Study, [63,64] |
| 3 | Quisqualis indica | Al, Cd, Cr, Fe, Mn, Ni, Pb Zn | Present Study, [65] |
| 4 | Mikania micrantha | Al, Cd, Cr, Fe, Mn, Ni, Pb Zn | Present Study, [66] |
| 5 | Tetraena qatarensis | Cd, Cr, Cu, Ni | [67] |
| Trees | |||
| 1 | Azadirachta indica | Al, Cd, Cr, Fe, Mn, Ni, Pb Zn | Present Study, [68,69] |
| 2 | Ficus benjamina | Al, Cd, Cr, Fe, Mn, Ni, Pb Zn | Present Study, [70] |
| 3 | Delonix regia | Al, Cd, Cr, Fe, Mn, Ni, Pb Zn | Present Study, [71] |
| 4 | Ficus religiosa | Al, Cd, Cr, Fe, Mn, Ni, Pb Zn | Present Study, [28,29] |
| 5 | Dalbergia sissoo | Al, Cd, Cr, Fe, Mn, Ni, Pb Zn | Present Study, [72] |
| 6 | Robinia pseudoacacia | Al, Cd, Cr, Fe, Mn, Ni, Pb Zn | Present Study. [73] |
| 7 | Celtis occidentalis | Al, Cd, Cr, Fe, Mn, Ni, Pb Zn | Present Study, [74] |
| 8 | Millettia pinnata | Cr, Cu, Pb, Zn, Mn, Fe. Ni, Cd | [75] |
| 9 | Tectona grandis | Cr, Cu, Pb, Zn, Mn, Fe. Ni, Cd | [75] |
| 10 | Senna siamea | Cr, Cu, Pb, Zn, Mn, Fe. Ni, Cd | [75] |
| 11 | Dendrocalamus strictus | Cr, Cu, Pb, Zn, Mn, Fe. Ni, Cd | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.; Dhyani, S.; Janipella, R.; Chakraborty, S.; Pujari, P.R.; Shinde, V.M.; Singh, K. Biomonitoring-Supported Land Restoration to Reduce Land Degradation in Intensively Mined Areas of India. Sustainability 2022, 14, 13639. https://doi.org/10.3390/su142013639
Singh S, Dhyani S, Janipella R, Chakraborty S, Pujari PR, Shinde VM, Singh K. Biomonitoring-Supported Land Restoration to Reduce Land Degradation in Intensively Mined Areas of India. Sustainability. 2022; 14(20):13639. https://doi.org/10.3390/su142013639
Chicago/Turabian StyleSingh, Sunidhi, Shalini Dhyani, Ramesh Janipella, Soumya Chakraborty, Paras Ranjan Pujari, V. M. Shinde, and Kripal Singh. 2022. "Biomonitoring-Supported Land Restoration to Reduce Land Degradation in Intensively Mined Areas of India" Sustainability 14, no. 20: 13639. https://doi.org/10.3390/su142013639
APA StyleSingh, S., Dhyani, S., Janipella, R., Chakraborty, S., Pujari, P. R., Shinde, V. M., & Singh, K. (2022). Biomonitoring-Supported Land Restoration to Reduce Land Degradation in Intensively Mined Areas of India. Sustainability, 14(20), 13639. https://doi.org/10.3390/su142013639








