Optical Properties and Gamma Radiation Shielding Capability of Transparent Barium Borosilicate Glass Composite
Abstract
1. Introduction
2. Materials and Methods
3. Theoretical Background
4. Results and Discussion
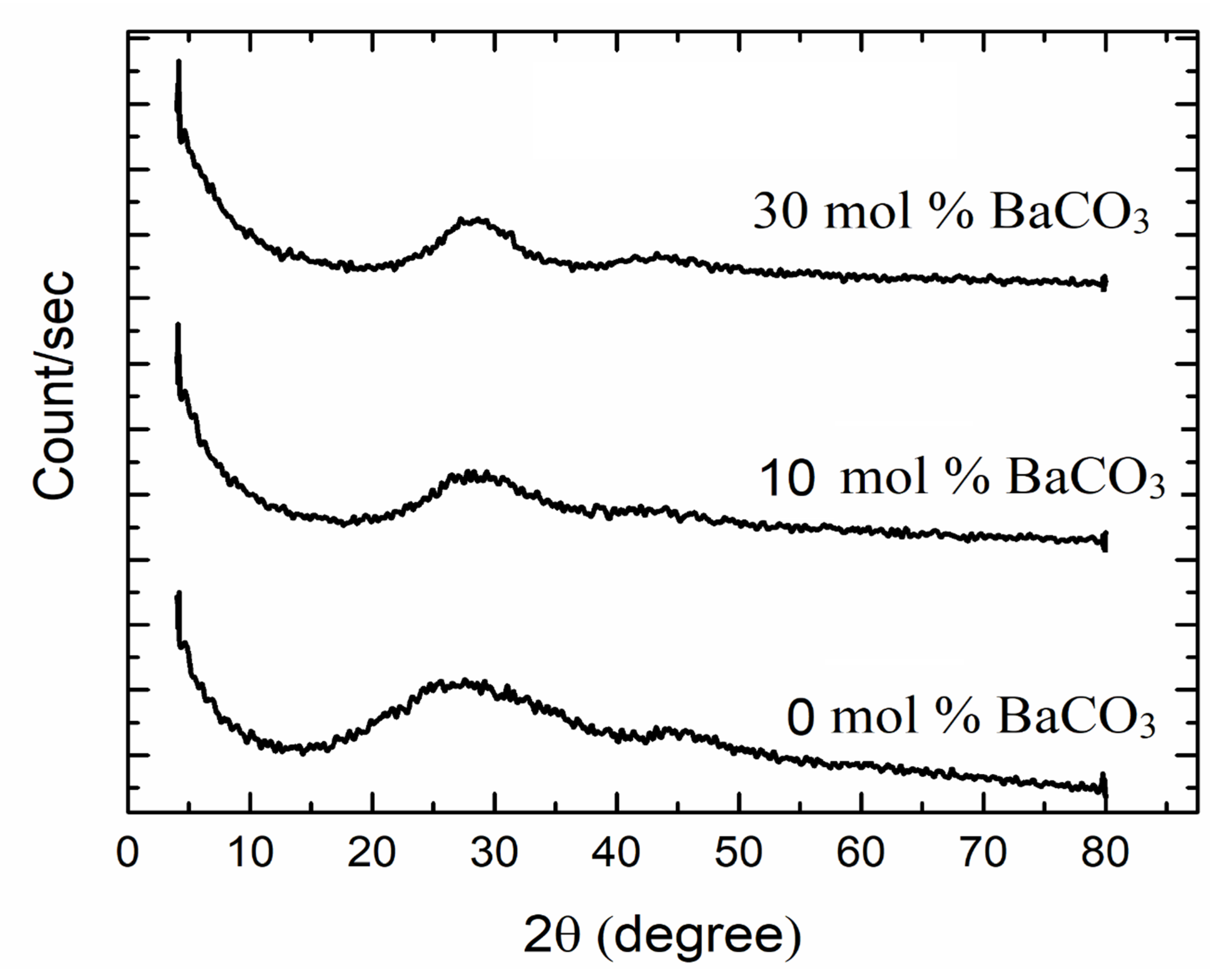
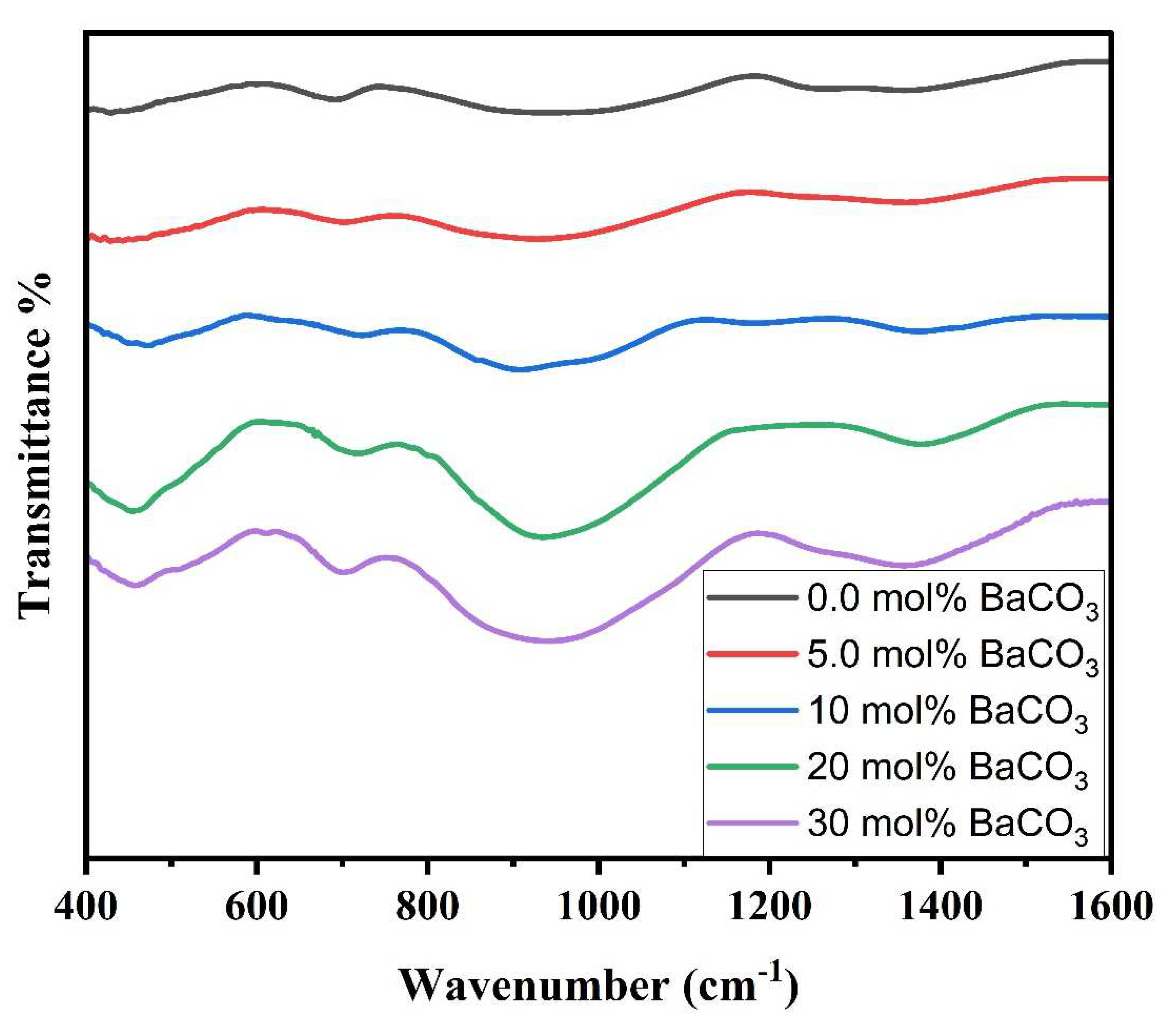
4.1. XRD Analysis and FTIR
4.2. Density and Molar Volume
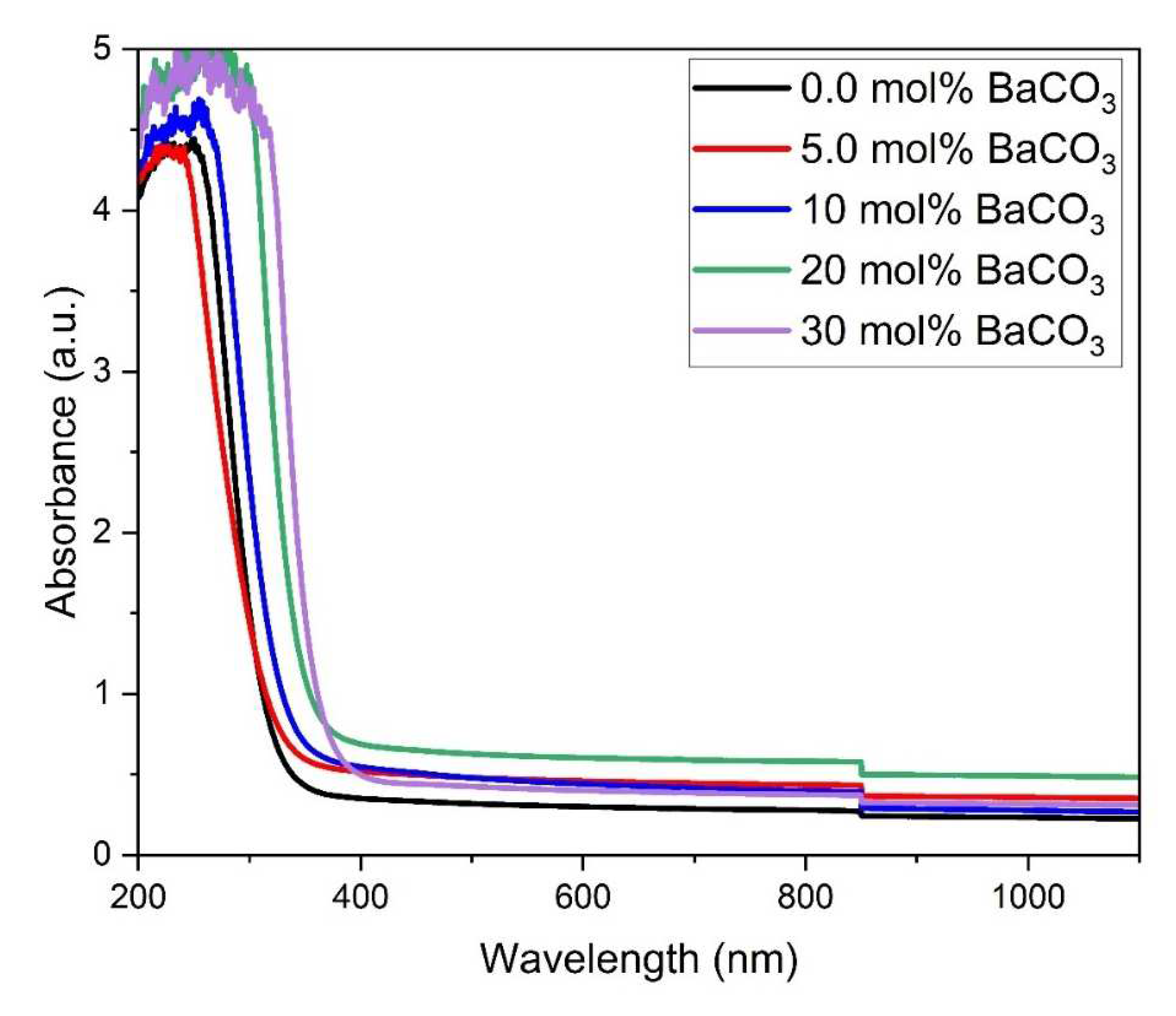
4.3. Optical Absorption Spectra
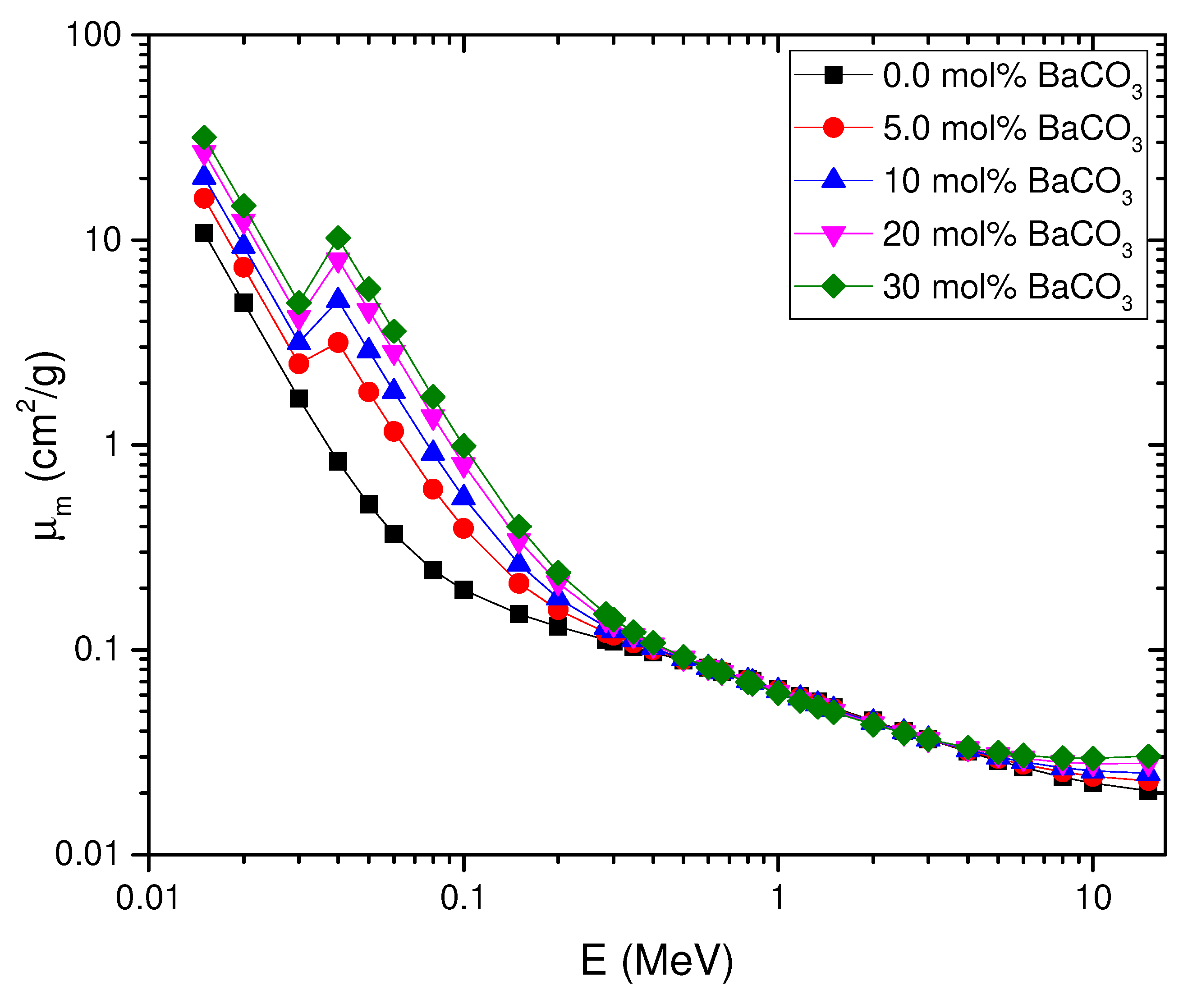
4.4. Mass Attenuation Coefficient
4.5. Half Value Layer (VL) and Effective Atomic Number (Zeff)
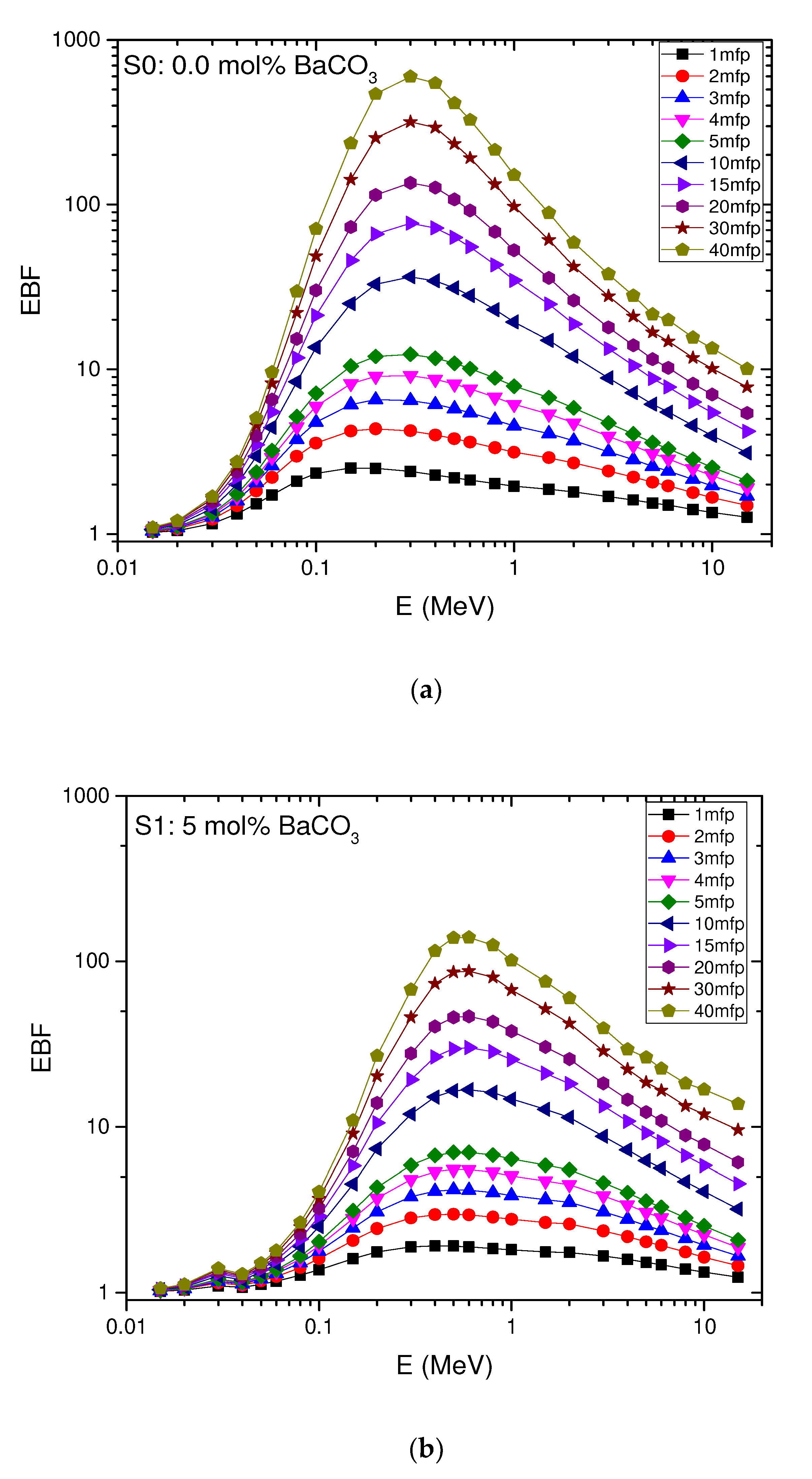
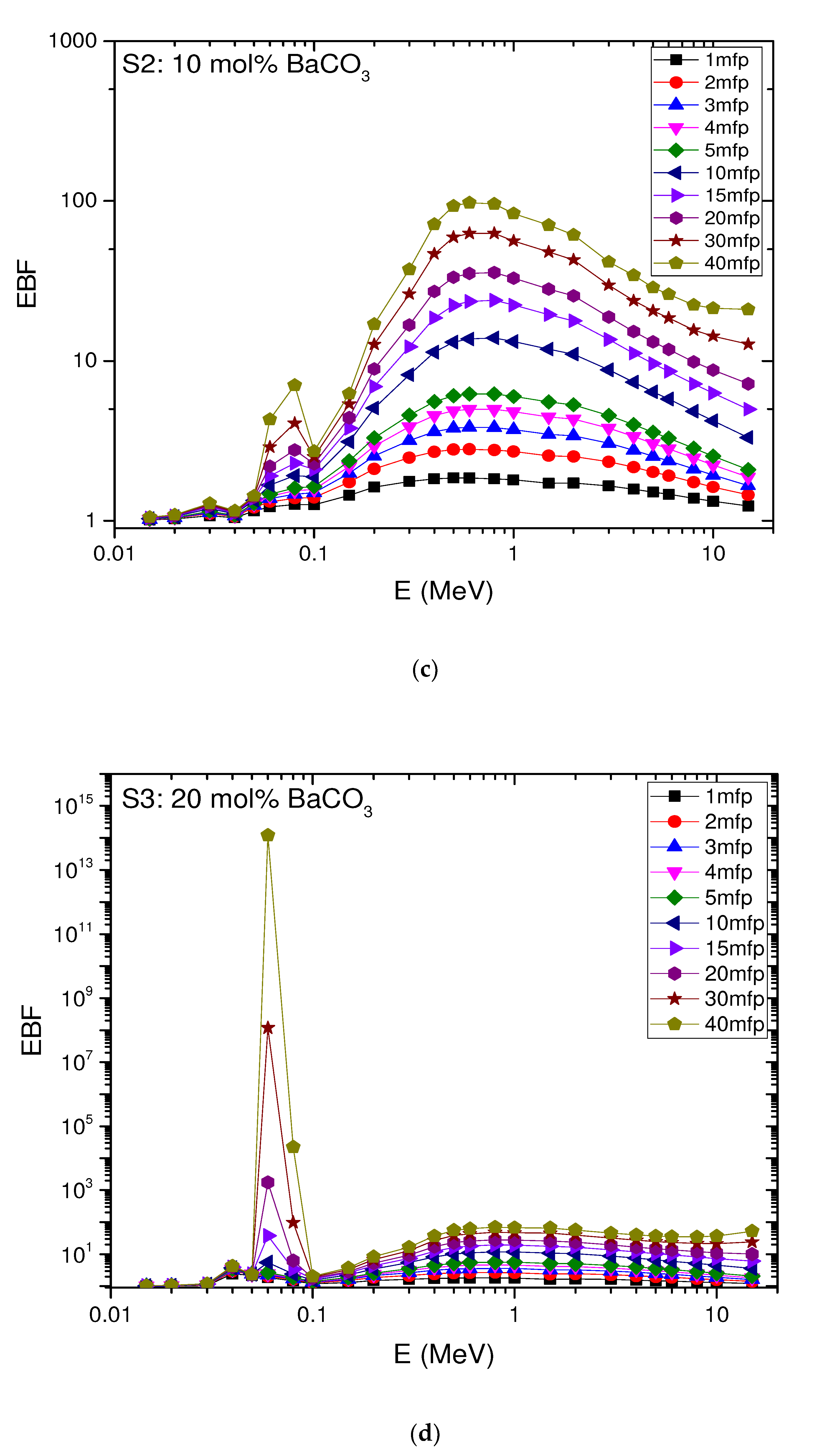
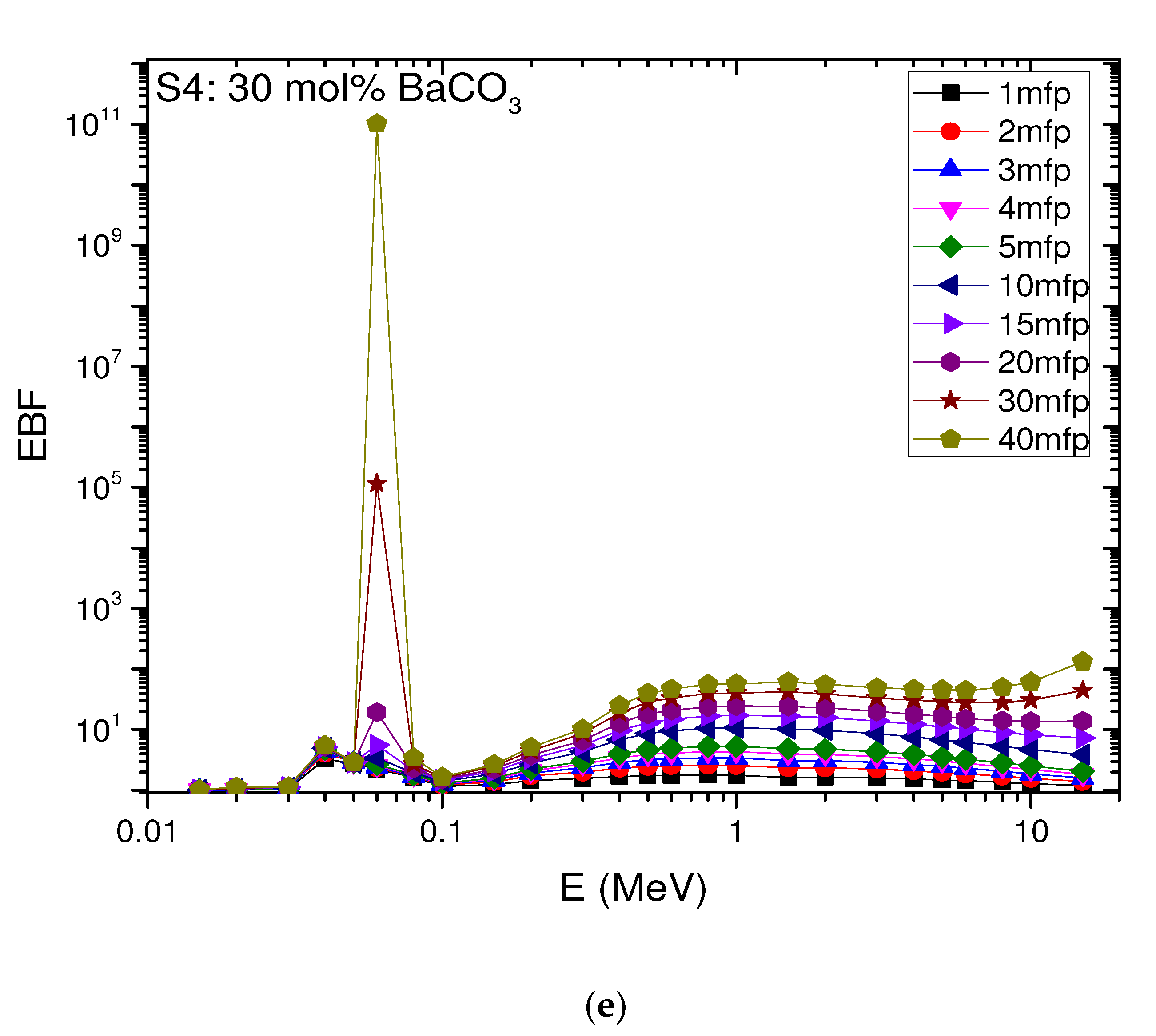
4.6. The Exposure Build-Up Factor (EBF)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Majeed, K.F.; Salama, E.; Elfiki, S.A.; Al-Bakhat, Y.M.Z. Natural radioactivity assessment around the petroleum-producing areas of The-Qar province, Iraq. Environ. Earth Sci. 2021, 80, 64. [Google Scholar] [CrossRef]
- Farah, K.; Mejri, A.; Hosni, F.; Ben Ouada, H.; Fuochi, P.G.; Lavalle, M.; Kovács, A. Characterization of a silicate glass as a high dose dosimeter. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2010, 614, 137–144. [Google Scholar] [CrossRef]
- DWK Life Sciences Glassware Technical Information. Glas. Types Prop. 2022, 12–19.
- Jubier, N.J. Estimation of Radiation Shielding Properties for Composites Material Based Unsaturated Polyester Filled with Granite and Iron Particles. J. Multidiscip. Eng. Sci. Stud. 2017, 3, 1309–1316. [Google Scholar]
- Khanna, A.; Bhatti, S.S.; Singh, K.J.; Thind, K.S. Gamma-ray attenuation coefficients in some heavy metal oxide borate glasses at 662 keV. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 1996, 114, 217–220. [Google Scholar] [CrossRef]
- Oto, B.; Gür, A.; Kavaz, E.; Çakır, T.; Yaltay, N. Determination of gamma and fast neutron shielding parameters of magnetite concretes. Prog. Nucl. Energy 2016, 92, 71–80. [Google Scholar] [CrossRef]
- Mesbahi, A.; Ghiasi, H. Shielding properties of the ordinary concrete loaded with micro-and nano-particles against neutron and gamma radiations. Appl. Radiat. Isot. 2018, 136, 27–31. [Google Scholar] [CrossRef]
- Shimizu, A.; Onda, T.; Sakamoto, Y. Calculation of gamma-ray buildup factors up to depths of 100 mfp by the method of invariant embedding, (III). J. Nucl. Sci. Technol. 2004, 41, 413–424. [Google Scholar] [CrossRef]
- Singh, K.; Singh, H.; Sharma, V.; Nathuram, R.; Khanna, A.; Kumar, R.; Singh Bhatti, S.; Singh Sahota, H. Gamma-ray attenuation coefficients in bismuth borate glasses. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2002, 194, 1–6. [Google Scholar] [CrossRef]
- Durani, L. Update to ANSI/ANS-6.4.3-1991 for Low-Z and Compound Materials and Review of Particle Transport Theory. Master’s Thesis, University of Nevada, Las Vegas, NV, USA, 2009. [Google Scholar]
- Dawoud, M.M.A.; Hegazi, M.M.; Saleh, H.M.; El Helew, W.K. Removal of stable and radio isotopes from wastewater by using modified microcrystalline cellulose based on Taguchi L16. Int. J. Environ. Sci. Technol. 2022, 1–12. [Google Scholar] [CrossRef]
- Dawoud, M.M.A.; Hegazy, M.M.; Helew, W.K.; Saleh, H.M. Overview of Environmental Pollution and Clean Management of Heavy Metals and Radionuclides by using Microcrystalline Cellulose. J. Nucl. Energy Sci. Power Gener. Technol. 2021, 3, 2. [Google Scholar]
- Saleh, H.M.; Bondouk, I.I.; Salama, E.; Esawii, H.A. Consistency and shielding efficiency of cement-bitumen composite for use as gamma-radiation shielding material. Prog. Nucl. Energy 2021, 137, 103764. [Google Scholar] [CrossRef]
- Reda, S.M.; Saleh, H.M. Calculation of the gamma radiation shielding efficiency of cement-bitumen portable container using MCNPX code. Prog. Nucl. Energy 2021, 142, 104012. [Google Scholar] [CrossRef]
- Eid, M.S.; Bondouk, I.I.; Saleh, H.M.; Omar, K.M.; Sayyed, M.I.; El-Khatib, A.M.; Elsafi, M. Implementation of waste silicate glass into composition of ordinary cement for radiation shielding applications. Nucl. Eng. Technol. 2021, 54, 1456–1463. [Google Scholar] [CrossRef]
- Eskander, S.B.; Saleh, H.M.; Tawfik, M.E.; Bayoumi, T.A. Towards potential applications of cement-polymer composites based on recycled polystyrene foam wastes on construction fields: Impact of exposure to water ecologies. Case Stud. Constr. Mater. 2021, 15, e00664. [Google Scholar] [CrossRef]
- Saleh, H.; Salman, A.; Faheim, A.; El-Sayed, A. Polymer and polymer waste composites in nuclear and industrial applications. J. Nucl. Energy Sci. Power Gener. Technol. 2020, 9, 1000199. [Google Scholar]
- Saleh, H.M.; Eskander, S.B. Impact of water flooding on hard cement-recycled polystyrene composite immobilizing radioactive sulfate waste simulate. Constr. Build. Mater. 2019, 222, 522–530. [Google Scholar] [CrossRef]
- Saleh, H.M.; El-Saied, F.A.; Salaheldin, T.A.; Hezo, A.A. Influence of severe climatic variability on the structural, mechanical and chemical stability of cement kiln dust-slag-nanosilica composite used for radwaste solidification. Constr. Build. Mater. 2019, 218, 556–567. [Google Scholar] [CrossRef]
- Saleh, H.M.; El-Sheikh, S.M.; Elshereafy, E.E.; Essa, A.K. Performance of cement-slag-titanate nanofibers composite immobilized radioactive waste solution through frost and flooding events. Constr. Build. Mater. 2019, 223, 221–232. [Google Scholar] [CrossRef]
- Saleh, H.M.; Salman, A.A.; Faheim, A.A.; El-Sayed, A.M. Sustainable composite of improved lightweight concrete from cement kiln dust with grated poly (styrene). J. Clean. Prod. 2020, 277, 123491. [Google Scholar] [CrossRef]
- Saleh, H.M.; Salman, A.A.; Faheim, A.A.; El-Sayed, A.M. Influence of aggressive environmental impacts on clean, lightweight bricks made from cement kiln dust and grated polystyrene. Case Stud. Constr. Mater. 2021, 15, e00759. [Google Scholar] [CrossRef]
- Singh, V.P.; Badiger, N.M.; Kaewkhao, J. Radiation shielding competence of silicate and borate heavy metal oxide glasses: Comparative study. J. Non-Cryst. Solids 2014, 404, 167–173. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Tekin, H.O.; Kılıcoglu, O.; Agar, O.; Zaid, M.H.M. Shielding features of concrete types containing sepiolite mineral: Comprehensive study on experimental, XCOM and MCNPX results. Results Phys. 2018, 11, 40–45. [Google Scholar] [CrossRef]
- Hanumantharayappa, C. Study of gamma, X-ray and neutron shielding parameters of some alloys. IJPAP 2018, 56, 631–634. [Google Scholar]
- Abouhaswa, A.S.; Tekin, H.O.; Ahmed, E.M.; Kilicoglu, O.; Rammah, Y.S. Synthesis, physical, linear optical and nuclear radiation shielding characteristics of B2O3–BaO–PbO–SrO2 glasses. J. Mater. Sci. Mater. Electron. 2021, 32, 18163–18177. [Google Scholar] [CrossRef]
- Al-Hadeethi, Y.; Sayyed, M.I. Analysis of borosilicate glasses doped with heavy metal oxides for gamma radiation shielding application using Geant4 simulation code. Ceram. Int. 2019, 45, 24858–24864. [Google Scholar] [CrossRef]
- Zaid, M.H.M.; Matori, K.A.; Abdul Aziz, S.H.; Zakaria, A.; Ghazali, M.S.M. Effect of ZnO on the physical properties and optical band gap of soda lime silicate glass. Int. J. Mol. Sci. 2012, 13, 7550–7558. [Google Scholar] [CrossRef]
- Dong, M.G.; Sayyed, M.I.; Lakshminarayana, G.; Çelikbilek Ersundu, M.; Ersundu, A.E.; Nayar, P.; Mahdi, M.A. Investigation of gamma radiation shielding properties of lithium zinc bismuth borate glasses using XCOM program and MCNP5 code. J. Non-Cryst. Solids 2017, 468, 12–16. [Google Scholar] [CrossRef]
- Tekin, H.O.; Altunsoy, E.E.; Kavaz, E.; Sayyed, M.I.; Agar, O.; Kamislioglu, M. Photon and neutron shielding performance of boron phosphate glasses for diagnostic radiology facilities. Results Phys. 2019, 12, 1457–1464. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Rammah, Y.S.; Abouhaswa, A.S.; Tekin, H.O.; Elbashir, B.O. ZnO-B2O3-PbO glasses: Synthesis and radiation shielding characterization. Phys. B Condens. Matter 2018, 548, 20–26. [Google Scholar] [CrossRef]
- Hussein, K.I.; Alqahtani, M.S.; Alzahrani, K.J.; Alqahtani, F.F.; Zahran, H.Y.; Alshehri, A.M.; Yahia, I.S.; Reben, M.; Yousef, E.S. The Effect of ZnO, MgO, TiO2, and Na2O Modifiers on the Physical, Optical, and Radiation Shielding Properties of a TeTaNb Glass System. Materials 2022, 15, 1844. [Google Scholar] [CrossRef]
- Tijani, S.A.; Kamal, S.M.; Al-Hadeethi, Y.; Arib, M.; Hussein, M.A.; Wageh, S.; Dim, L.A. Radiation shielding properties of transparent erbium zinc tellurite glass system determined at medical diagnostic energies. J. Alloys Compd. 2018, 741, 293–299. [Google Scholar] [CrossRef]
- Shams, T.; Eftekhar, M.; Shirani, A. Investigation of gamma radiation attenuation in heavy concrete shields containing hematite and barite aggregates in multi-layered and mixed forms. Constr. Build. Mater. 2018, 182, 35–42. [Google Scholar] [CrossRef]
- Kindrat, I.I.; Padlyak, B.V.; Drzewiecki, A. Intrinsic luminescence of un-doped borate glasses. J. Lumin. 2017, 187, 546–554. [Google Scholar] [CrossRef]
- Singh, N.; Singh, K.J.; Singh, K.; Singh, H. Comparative study of lead borate and bismuth lead borate glass systems as gamma-radiation shielding materials. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2004, 225, 305–309. [Google Scholar] [CrossRef]
- AlBuriahi, M.S.; Hegazy, H.H.; Alresheedi, F.; Olarinoye, I.O.; Algarni, H.; Tekin, H.O.; Saudi, H.A. Effect of CdO addition on photon, electron, and neutron attenuation properties of boro-tellurite glasses. Ceram. Int. 2021, 47, 5951–5958. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, S.; Pandey, O.P. Structural variation in gamma ray irradiated PbO-Na2O-B2O3-SiO2 glasses. Solid State Commun. 2014, 188, 40–44. [Google Scholar] [CrossRef]
- Marzouk, S.Y.; Seoudi, R.; Said, D.A.; Mabrouk, M.S. Linear and non-linear optics and FTIR characteristics of borosilicate glasses doped with gadolinium ions. Opt. Mater. 2013, 35, 2077–2084. [Google Scholar] [CrossRef]
- Bootjomchai, C.; Laopaiboon, J.; Yenchai, C.; Laopaiboon, R. Gamma-ray shielding and structural properties of barium-bismuth-borosilicate glasses. Radiat. Phys. Chem. 2012, 81, 785–790. [Google Scholar] [CrossRef]
- Salama, E.; Soliman, H.A.; Youssef, G.M.; Hamad, S. Thermoluminescence Properties of Borosilicate Glass Doped with ZnO Thermoluminescence properties of borosilicate glass doped with ZnO. J. Lumin. 2018, 186, 164–169. [Google Scholar] [CrossRef]
- Alajerami, Y.S.M.; Hashim, S.; Ghoshal, S.K.; Bradley, D.A.; Mhareb, M.; Saleh, M.A. Copper doped borate dosimeters revisited. J. Lumin. 2014, 155, 141–148. [Google Scholar] [CrossRef]
- Mhareb, M.H.A.; Alqahtani, M.; Alshahri, F.; Alajerami, Y.S.M.; Saleh, N.; Alonizan, N.; Sayyed, M.I.; Ashiq, M.G.B.; Ghrib, T.; Al-Dhafar, S.I.; et al. The impact of barium oxide on physical, structural, optical, and shielding features of sodium zinc borate glass. J. Non-Cryst. Solids 2020, 541, 120090. [Google Scholar] [CrossRef]
- Rammah, Y.S.; Tekin, H.O.; Sriwunkum, C.; Olarinoye, I.; Alalawi, A.; Al-Buriahi, M.S.; Nutaro, T.; Tonguc, B.T. Investigations on borate glasses within SBC-Bx system for gamma-ray shielding applications. Nucl. Eng. Technol. 2021, 53, 282–293. [Google Scholar] [CrossRef]
- Aly, P.; El-Kheshen, A.A.; Abou-Gabal, H.; Agamy, S. Structural investigation and measurement of the shielding effect of borosilicate glass containing PbO, SrO, and BaO against gamma irradiation. J. Phys. Chem. Solids 2020, 145, 109521. [Google Scholar] [CrossRef]
- Sayed El-Ahll, L.; Salama, E.; Saudi, H.A.; Alazab, H.A.; Ghany, H.A.A. The Effect of Barium on the Nuclear Radiation Shielding Capabilities of Nickel-Reinforced Borosilicate Glasses. Silicon 2022, 14, 8909–8917. [Google Scholar] [CrossRef]
- Kavas, T.; Alsufyani, S.J.; Alrowaili, Z.A.; Tamam, N.; Kurtulus, R.; Olarinoye, I.O.; Al-Buriahi, M.S. Influence of iron (III) oxide on the optical, mechanical, physical, and radiation shielding properties of sodium-barium-vanadate glass system. Optik 2022, 257. [Google Scholar] [CrossRef]
- Mhareb, M.H.A.; Alqahtani, M.; Alajerami, Y.S.M.; Alshahri, F.; Sayyed, M.I.; Mahmoud, K.A.; Saleh, N.; Alonizan, N.; Al-Buriahi, M.S.; Kaky, K.M. Ionizing radiation shielding features for titanium borosilicate glass modified with different concentrations of barium oxide. Mater. Chem. Phys. 2021, 272, 125047. [Google Scholar] [CrossRef]
- Aboalatta, A.; Asad, J.; Humaid, M.; Musleh, H.; Shaat, S.K.K.; Ramadan, K.; Sayyed, M.I.; Alajerami, Y.; Aldahoudi, N. Experimental investigation of zinc sodium borate glass systems containing barium oxide for gamma radiation shielding applications. Nucl. Eng. Technol. 2021, 53, 3058–3067. [Google Scholar] [CrossRef]
- Abdel-Baki, M.; Salem, A.M.; Abdel-Wahab, F.A.; El-Diasty, F. Bond character, optical properties and ionic conductivity of Li2O/B2O3/SiO2/Al2O3 glass: Effect of structural substitution of Li2O for LiCl. J. Non-Cryst. Solids 2008, 354, 4527–4533. [Google Scholar] [CrossRef]
- Singh, D.; Singh, K.; Singh, G.; Manupriya; Mohan, S.; Arora, M.; Sharma, G. Optical and structural properties of ZnO-PbO-B2O3 and ZnO-PbO-B2O3-SiO2 glasses. J. Phys. Condens. Matter 2008, 20, 075228. [Google Scholar] [CrossRef]
- Rammah, Y.S.; Sayyed, M.I.; Ali, A.A.; Tekin, H.O.; El-Mallawany, R. Optical properties and gamma-shielding features of bismuth borate glasses. Appl. Phys. A Mater. Sci. Process. 2018, 124, 832. [Google Scholar] [CrossRef]
- Rammah, Y.S.; Sayyed, M.I.; Abohaswa, A.S.; Tekin, H.O. FTIR, electronic polarizability and shielding parameters of B2O3 glasses doped with SnO2. Appl. Phys. A Mater. Sci. Process. 2018, 124, 650. [Google Scholar] [CrossRef]
- Bashter, I.I. Calculation of radiation attenuation coefficients for shielding concretes. Ann. Nucl. Energy 1997, 24, 1389–1401. [Google Scholar] [CrossRef]
- Thakur, S.; Thakur, V.; Kaur, A.; Singh, L. Structural, optical and thermal properties of nickel doped bismuth borate glasses. J. Non-Cryst. Solids 2019, 512, 60–71. [Google Scholar] [CrossRef]
- Gaafar, I.; El-Shershaby, A.; Zeidan, I.; El-Ahll, L.S. Natural radioactivity and radiation hazard assessment of phosphate mining, Quseir-Safaga area, Central Eastern Desert, Egypt. NRIAG J. Astron. Geophys. 2016, 5, 160–172. [Google Scholar] [CrossRef]
- Umar, S.A.; Halimah, M.K.; Chan, K.T.; Amirah, A.A.; Azlan, M.N.; Grema, L.U.; Hamza, A.M.; Ibrahim, G.G. Optical and structural properties of rice husk silicate incorporated borotellurite glasses doped with erbium oxide nanoparticles. J. Mater. Sci. Mater. Electron. 2019, 30, 18606–18616. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Qashou, S.I.; Khattari, Z.Y. Radiation shielding competence of newly developed TeO2-WO3 glasses. J. Alloys Compd. 2017, 696, 632–638. [Google Scholar] [CrossRef]
- Sathiyaraj, P.; Samuel, E.J.J.; Valeriano, C.C.S.; Kurudirek, M. Effective atomic number and buildup factor calculations for metal nano particle doped polymer gel. Vacuum 2017, 143, 138–149. [Google Scholar] [CrossRef]
- Kavaz, E.; Yorgun, N.Y. Gamma ray buildup factors of lithium borate glasses doped with minerals. J. Alloys Compd. 2018, 752, 61–67. [Google Scholar] [CrossRef]
- El-Kameesy, S.U.; Youssef, G.M.; El-Zaiat, S.Y.; Saudi, H.A.; Abd El-Kawy, F.S. Gamma Rays Attenuation Properties and the Associated Optical and Mechanical Behavior of Development (70-x) B2O3-10Al2O3-10Na2O-10ZnO-x PbO Glasses. Silicon 2018, 10, 1881–1886. [Google Scholar] [CrossRef]
- Harima, Y. An historical review and current status of buildup factor calculations and applications. Radiat. Phys. Chem. 1993, 41, 631–672. [Google Scholar] [CrossRef]
- Kaplan, M.F. Concrete Radiation Shielding: Nuclear Physics, Concrete Properties, Design and Construction; Longman Scientific & Technical: New York, NY, USA, 1989; ISBN 0470213388. [Google Scholar]
- Singh, V.P.; Badiger, N.M. Gamma ray and neutron shielding properties of some alloy materials. Ann. Nucl. Energy 2014, 64, 301–310. [Google Scholar] [CrossRef]
- ANSI/ANS-6.4.3; Gamma-Ray Attenuation Coefficients and Buildup Factors for Engineering Materials. American Nuclear Society: La Grange Park, IL, USA, 1991.
- Gomaa, H.M.; Yahia, I.S.; Zahren, H.Y.; Saudi, H.A.; El-Dosokey, A.H. Effect of replacement of SiO2 with BaTiO3 on the cadmium calcium-borate glass: Aiming to obtain an active glass for optical and shielding applications. Radiat. Phys. Chem. 2022, 193, 109955. [Google Scholar] [CrossRef]
- Huang, W.J.; Wen, Z.X.; Li, L.J.; Ashraf, G.A.; Chen, L.P.; Lei, L.; Guo, H.; Li, X.M. Photoluminescence and X-ray excited scintillating properties of Tb3+-doped borosilicate aluminate glass scintillators. Ceram. Int. 2022, 48, 17178–17184. [Google Scholar] [CrossRef]
- Shajan, D.; Murugasen, P.; Sagadevan, S. Analysis on the structural, spectroscopic, and dielectric properties of borate glass. Dig. J. Nanomater. Biostruct. 2016, 11, 177–183. [Google Scholar]
- Mustafa, I.S.; Kamari, H.M.; Wan Yusoff, W.M.D.; Aziz, S.A.; Rahman, A.A. Structural and optical properties of lead-boro-tellurrite glasses induced by Gamma-ray. Int. J. Mol. Sci. 2013, 14, 3201–3214. [Google Scholar] [CrossRef]
- Yadav, A.K.; Gautam, C.R. Structural and optical studies of Fe2O3 doped barium strontium titanate borosilicate glasses. Indian J. Pure Appl. Phys. 2015, 53, 42–48. [Google Scholar]
- Rani, S.; Sanghi, S.; Agarwal, A.; Seth, V.P. Study of optical band gap and FTIR spectroscopy of Li2O·Bi2O3·P2O5 glasses. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2009, 74, 673–677. [Google Scholar] [CrossRef]
- Yadav, A.K.; Gautam, C.R. Synthesis, structural and optical studies of barium strontium titanate borosilicate glasses doped with ferric oxide. Spectrosc. Lett. 2015, 48, 514–520. [Google Scholar] [CrossRef]
- Gautam, C.; Yadav, A.K.; Mishra, V.K.; Vikram, K. Synthesis, IR and Raman Spectroscopic Studies of (Ba,Sr)TiO3 Borosilicate Glasses with Addition of La2O3. Open J. Inorg. Non-Metallic Mater. 2012, 2, 47–54. [Google Scholar] [CrossRef]
- Gautam, C.R.; Kumar, D.; Parkash, O. IR study of Pb-Sr titanate borosilicate glasses. Bull. Mater. Sci. 2010, 33, 145–148. [Google Scholar] [CrossRef]
- Mandal, A.K.; Agrawal, D.; Sen, R. Preparation of homogeneous barium borosilicate glass using microwave energy. J. Non-Cryst. Solids 2013, 371–372, 41–46. [Google Scholar] [CrossRef]
- Study, S.; Consumption, L.E.; Part, D.U.; Utilization, D.; Yoshizawa, N.; Harimoto, K.; Ichihara, M.; Miki, Y.; Takase, K.; Inoue, T. Synthesis, Characterization and Bioactive Study of Borosilicate Sol-Gel Glass Khairy. Nat. Sci. 2015, 13, 475–476. [Google Scholar]
- Rada, S.; Dehelean, A.; Culea, E. FTIR and UV-VIS spectroscopy investigations on the structure of the europium-lead-tellurate glasses. J. Non-Cryst. Solids 2011, 357, 3070–3073. [Google Scholar] [CrossRef]
- Saudi, H.A.; Abd-Allah, W.M.; Shaaban, K.S. Investigation of gamma and neutron shielding parameters for borosilicate glasses doped europium oxide for the immobilization of radioactive waste. J. Mater. Sci. Mater. Electron. 2020, 31, 6963–6976. [Google Scholar] [CrossRef]
- Alzahrani, J.S.; Alrowaili, Z.A.; Olarinoye, I.O.; Alothman, M.A.; Al-Baradi, A.M.; Kebaili, I.; Al-Buriahi, M.S. Nuclear shielding properties and buildup factors of Cr-based ferroalloys. Prog. Nucl. Energy 2021, 141, 103956. [Google Scholar] [CrossRef]





| Sample | Na2CO3 | SiO2 | ZnO | H3BO3 | BaCO3 |
|---|---|---|---|---|---|
| S0 | 10 | 20 | 10 | 60 | 0 |
| S1 | 10 | 20 | 10 | 55 | 5 |
| S2 | 10 | 20 | 10 | 50 | 10 |
| S3 | 10 | 20 | 10 | 40 | 20 |
| S4 | 10 | 20 | 10 | 30 | 30 |
| Peak Position (cm−1) | Assignment | Reference Range |
|---|---|---|
| 1364 | Stretching relaxation of B–O bonds of trigonal BO3 units | 1170−1600 [68,69] |
| 950 | Stretching vibrations of B–O–Si linkages | 950−1050 [70,71] |
| 926 | Stretching vibrations of B–O bonds of tetrahedral BO4 units. | 800–1200 [43,71,72] |
| 705 | B–O–B vibrations of linkages in a borate network | ~700 [73,74] |
| 451 | Vibrations of the metal cations Ba+2 and Zn+2 | 400−600 [75−77] |
| Physical Parameter | BaCO3 mol% | ||||
|---|---|---|---|---|---|
| 0 | 5 | 10 | 20 | 30 | |
| Density (g/cm3) | 3.11 | 3.21 | 3.37 | 3.53 | 3.68 |
| Molar volume (cm3 mol−1) | 33.69 | 33.77 | 33.91 | 33.96 | 34.52 |
| Refractive index | 1.57 | 1.58 | 1.59 | 1.61 | 1.63 |
| Dielectric constant | 2.46 | 2.5 | 2.54 | 2.62 | 2.68 |
| Refraction loss (%) | 0.049 | 0.050 | 0.052 | 0.055 | 0.058 |
| Molar refraction (cm3) | 11.07 | 11.29 | 11.51 | 11.91 | 12.39 |
| The optical bandgap (eV) | 3.55 | 3.42 | 3.29 | 3.21 | 3.13 |
| BaCO3 (mol%) | 0.662 MeV | 1.173 MeV | 1.332 MeV | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Exp. | Theo. | % Diff, | Exp. | Theo. | % Diff | Exp. | Theo. | % Diff | |
| 0 | 0.078 ± 0.006 | 0.078 | 0.0 | 0.056 ± 0.006 | 0.059 | 5.4 | 0.055 ± 0.003 | 0.056 | 1.8 |
| 5 | 0.078 ± 0.006 | 0.078 | 0.0 | 0.059 ± 0.004 | 0.059 | 0.0 | 0.054 ± 0.002 | 0.055 | 1.9 |
| 10 | 0.078 ± 0.006 | 0.078 | 0.0 | 0.049 ± 0.004 | 0.058 | 17 | 0.056 ± 0.003 | 0.054 | 3.6 |
| 20 | 0.079 ± 0.006 | 0.078 | 1.3 | 0.054 ± 0.004 | 0.057 | 5.6 | 0.054 ± 0.002 | 0.053 | 1.9 |
| 30 | 0.083 ± 0.005 | 0.078 | 6.0 | 0.051 ± 0.004 | 0.056 | 7.8 | 0.054 ± 0.002 | 0.053 | 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehab, M.; Salama, E.; Ashour, A.; Attallah, M.; Saleh, H.M. Optical Properties and Gamma Radiation Shielding Capability of Transparent Barium Borosilicate Glass Composite. Sustainability 2022, 14, 13298. https://doi.org/10.3390/su142013298
Ehab M, Salama E, Ashour A, Attallah M, Saleh HM. Optical Properties and Gamma Radiation Shielding Capability of Transparent Barium Borosilicate Glass Composite. Sustainability. 2022; 14(20):13298. https://doi.org/10.3390/su142013298
Chicago/Turabian StyleEhab, Mohamed, Elsayed Salama, Ahmed Ashour, Mohamed Attallah, and Hosam M. Saleh. 2022. "Optical Properties and Gamma Radiation Shielding Capability of Transparent Barium Borosilicate Glass Composite" Sustainability 14, no. 20: 13298. https://doi.org/10.3390/su142013298
APA StyleEhab, M., Salama, E., Ashour, A., Attallah, M., & Saleh, H. M. (2022). Optical Properties and Gamma Radiation Shielding Capability of Transparent Barium Borosilicate Glass Composite. Sustainability, 14(20), 13298. https://doi.org/10.3390/su142013298








