Behavioural Responses of Common Dolphins Delphinus delphis to a Bio-Inspired Acoustic Device for Limiting Fishery By-Catch
Abstract
1. Introduction
2. Materials and Methods
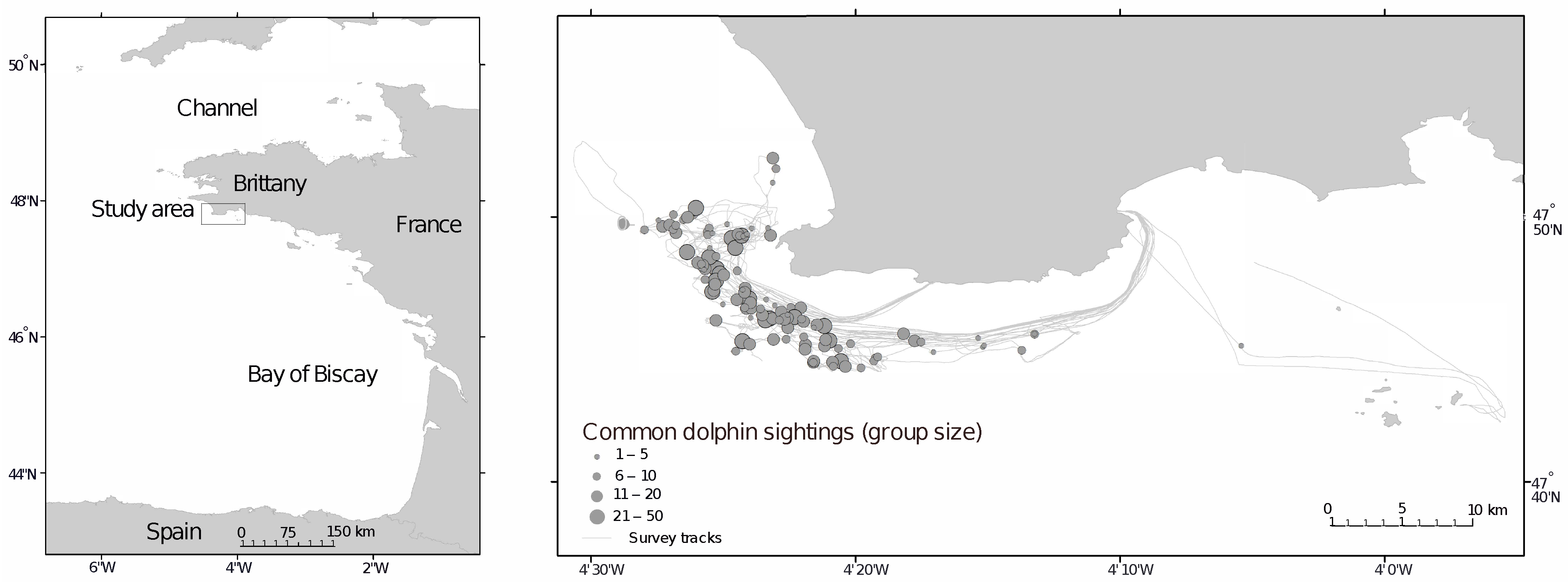
2.1. Study Area
2.2. Observations
2.3. Data Extraction
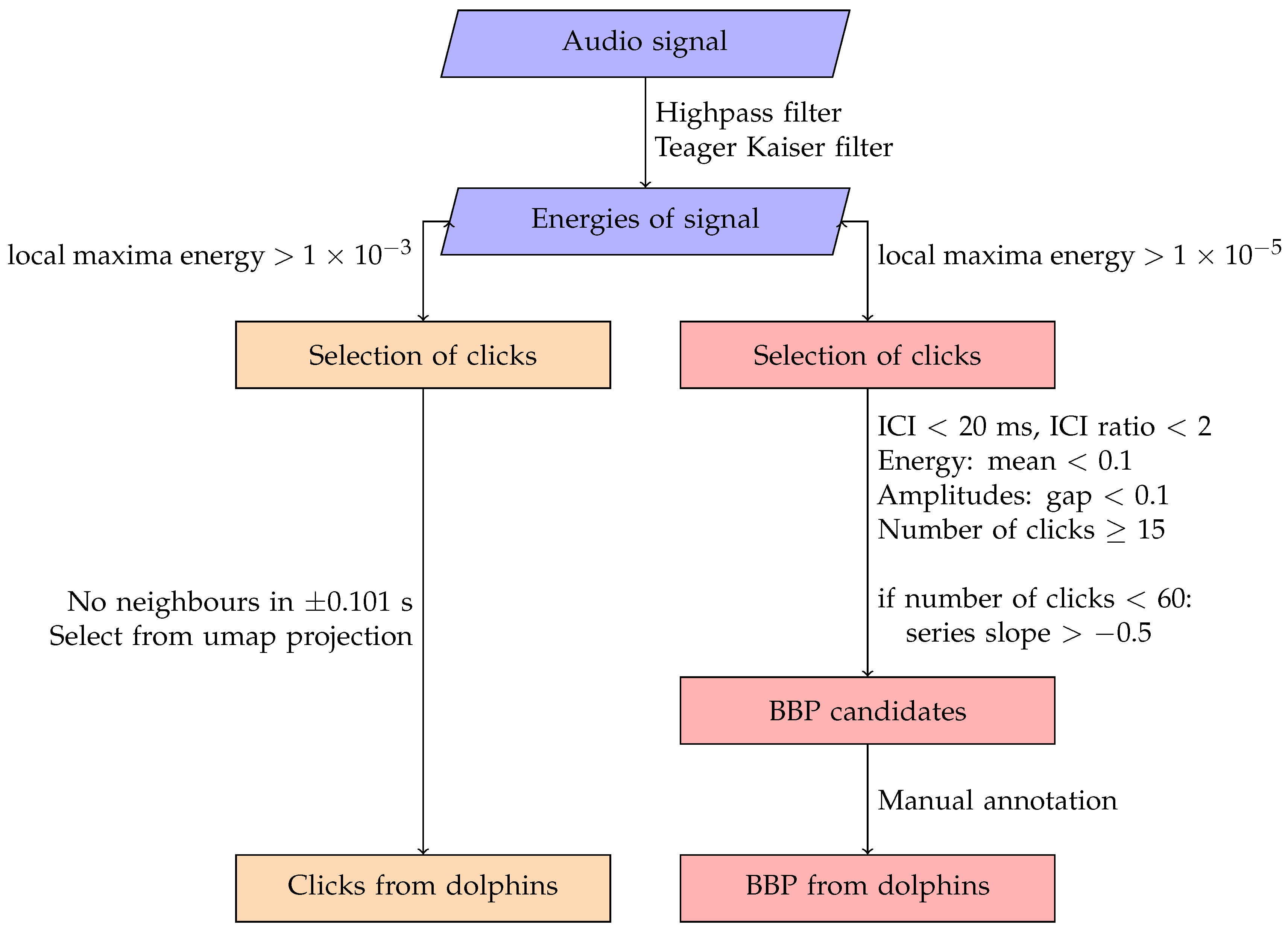
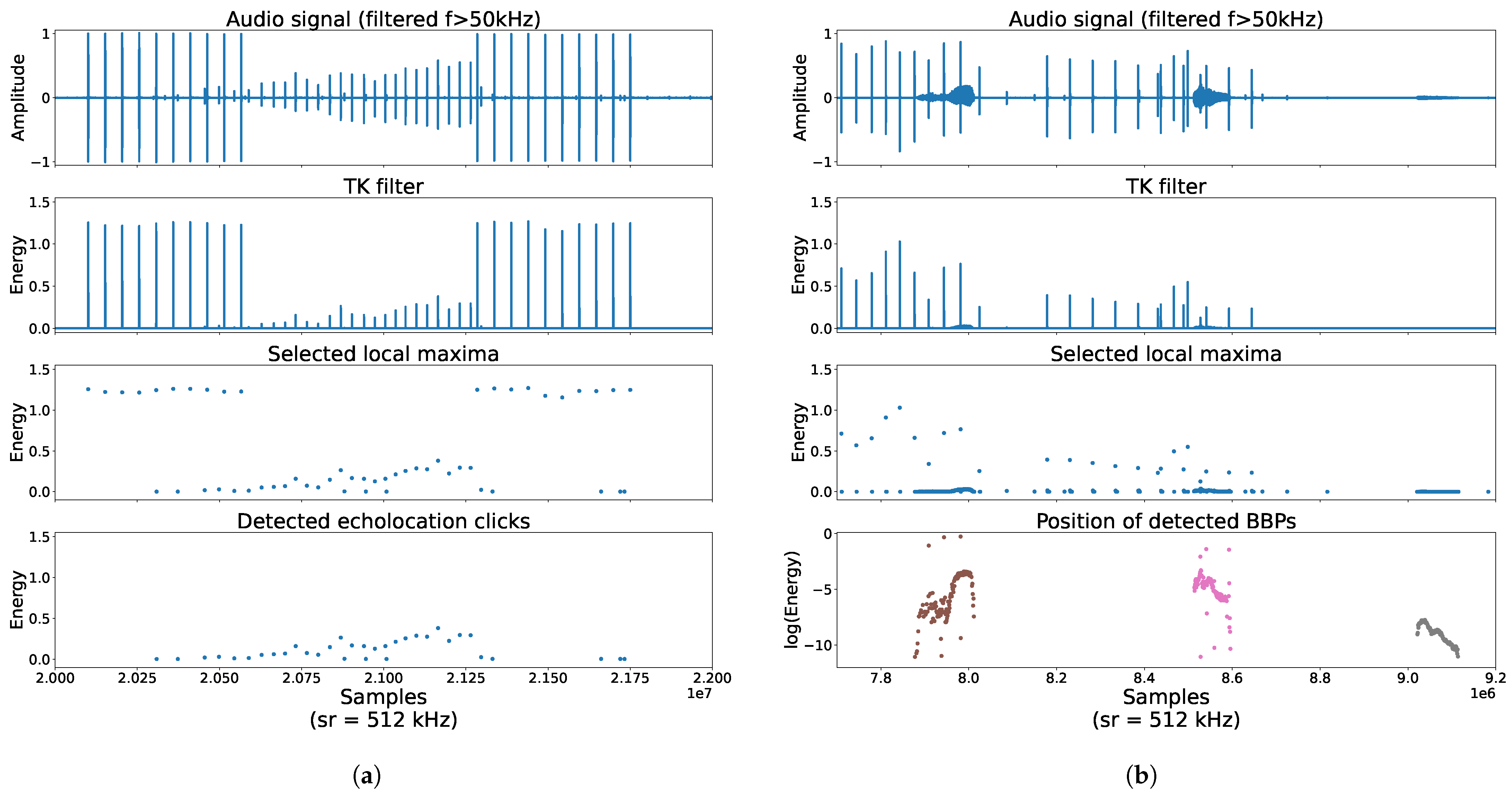
2.3.1. Detection and Identification of Echolocation Click, Buzz and Burst-Pulse
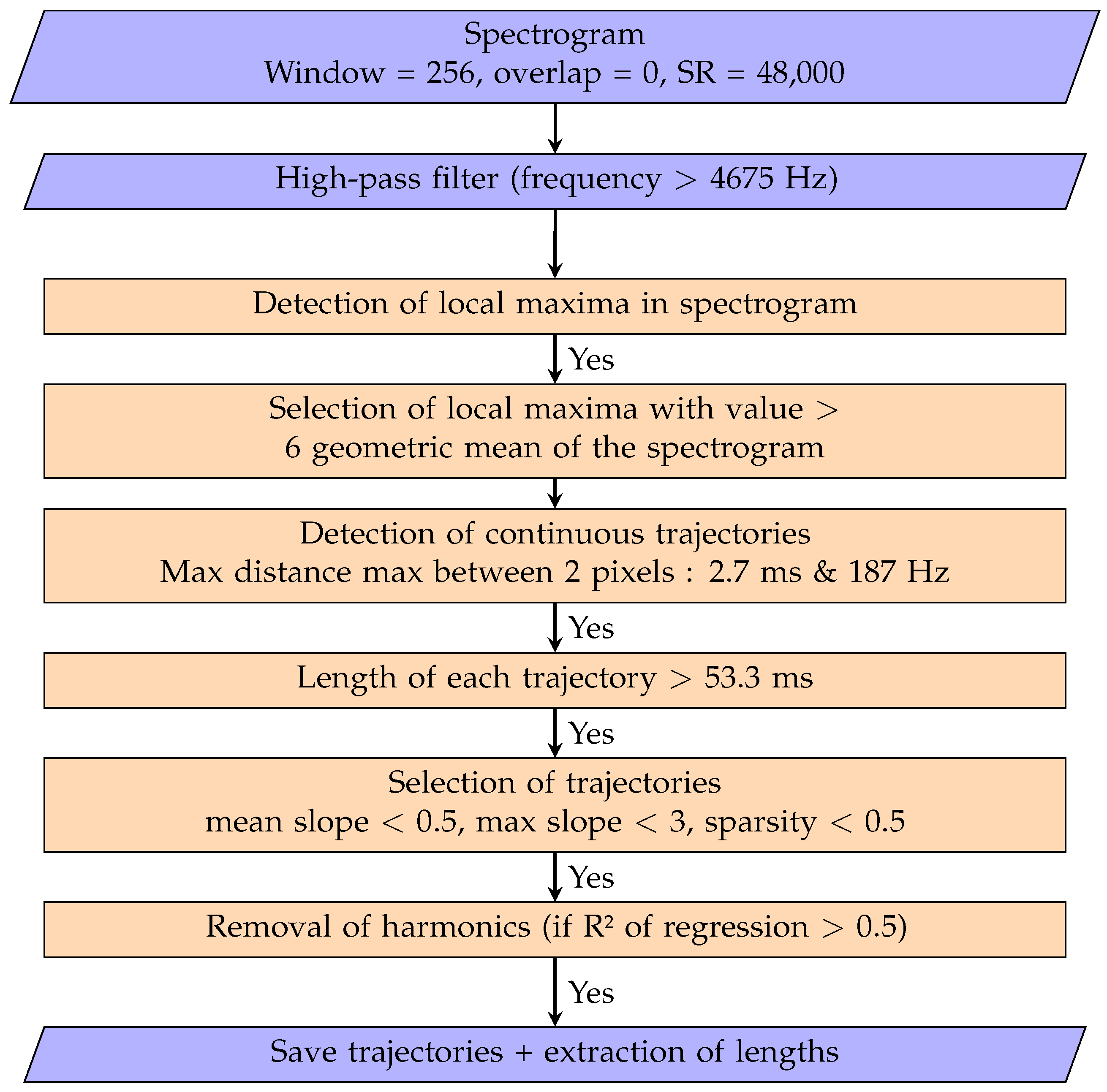
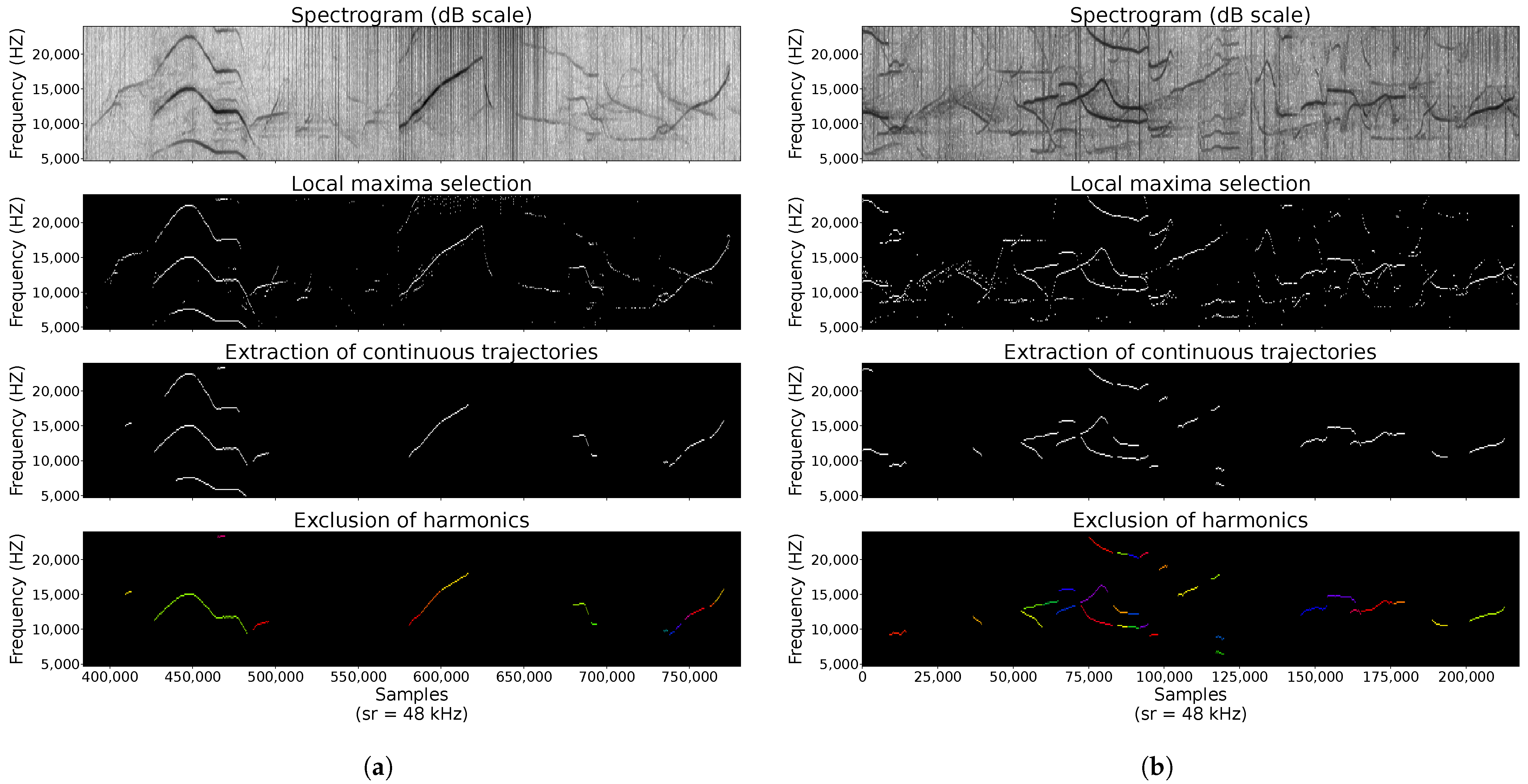
2.3.2. Vocalisation Detection
2.4. Data Analysis
3. Results
3.1. Surface Visual Observations
3.2. Acoustic Behaviours of Dolphins
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BBP | Buzz and Burst-Pulse |
| ICI | Inter-Click Interval |
| SR | Sampling Rate |
| STD | Standard Deviation |
| BEF | Before beacon activation |
| DUR | During beacon activation |
| AFT | After beacon activation |
| NB GLM | Negative Binomial Generalised Linear Model |
| ZINB | Zero-Inflated Negative Binomial model |
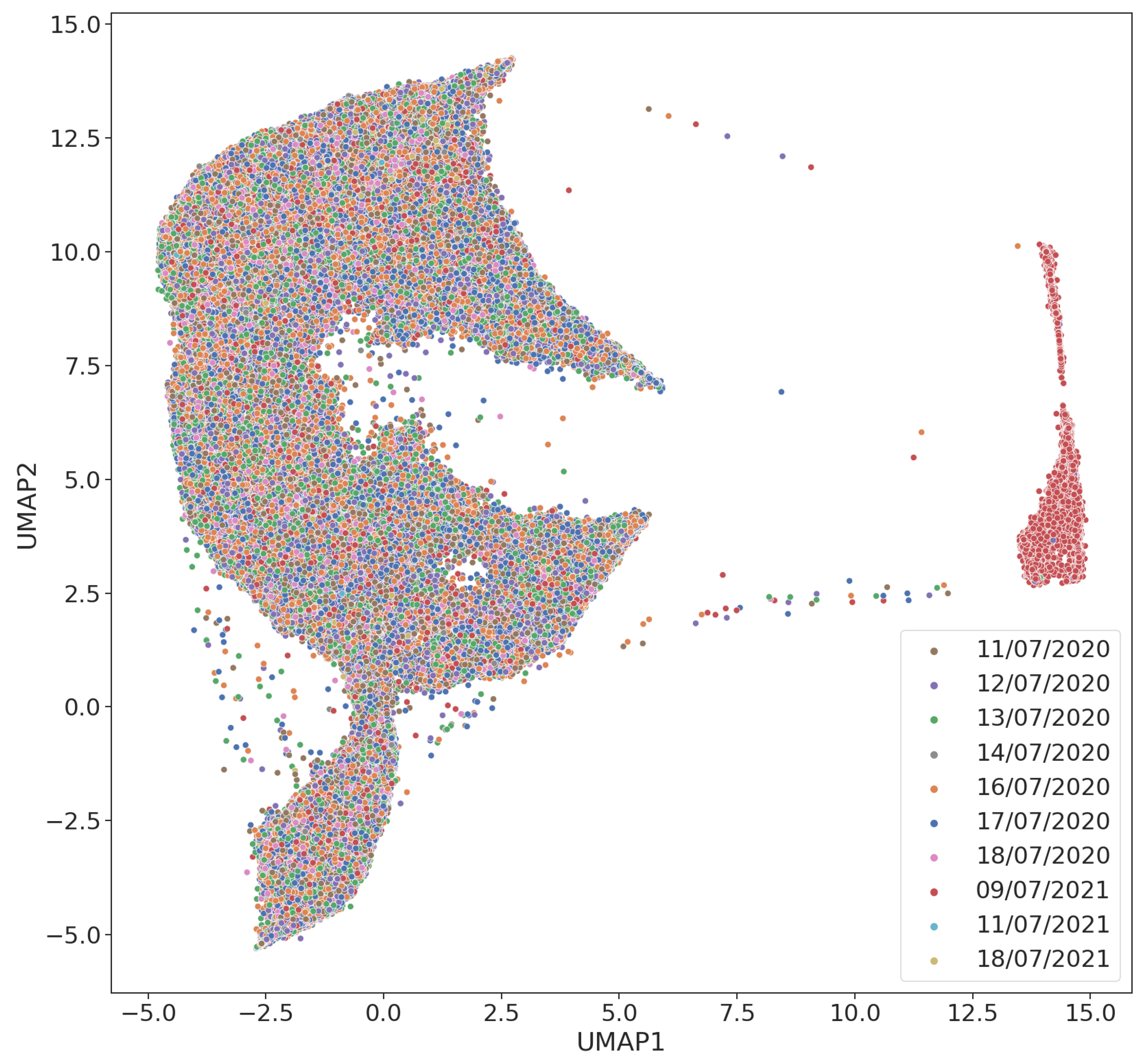
Appendix A. Projection of Clicks
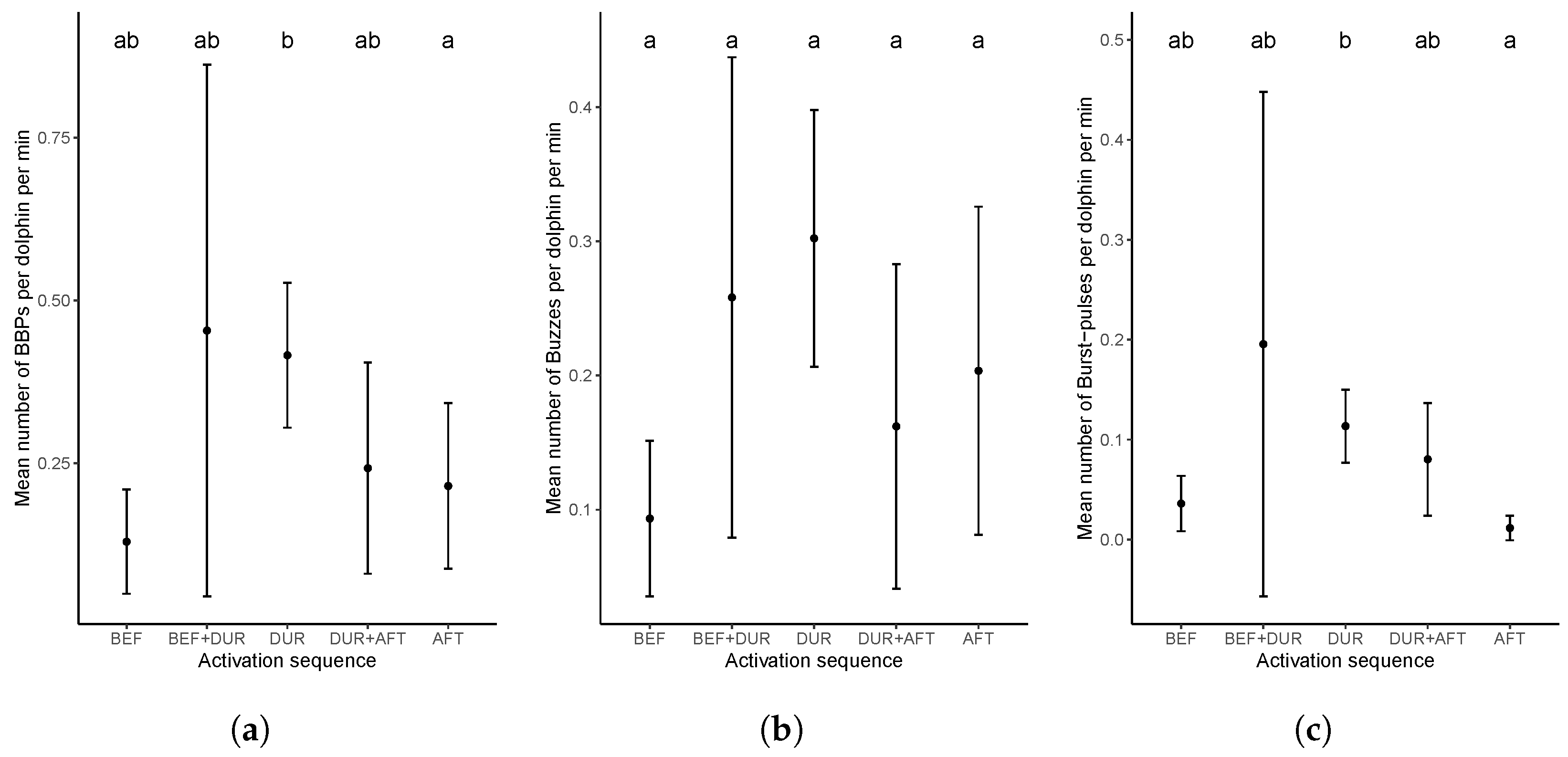
Appendix B. Buzz and Burst-Pulse Characteristics

References
- Read, A.J.; Drinker, P.; Northridge, S. Bycatch of Marine Mammals in U.S. and Global Fisheries: Bycatch of Marine Mammals. Conserv. Biol. 2006, 20, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.E.; Heinemann, D.; Francis, T.B.; Hammond, P.S.; Long, K.J.; Punt, A.E.; Reeves, R.R.; Sepúlveda, M.; Sigurðsson, G.M.; Siple, M.C.; et al. Estimating Bycatch Mortality for Marine Mammals: Concepts and Best Practices. Front. Mar. Sci. 2021, 8, 1793. [Google Scholar] [CrossRef]
- Hamilton, S.; Baker, G.B. Technical mitigation to reduce marine mammal bycatch and entanglement in commercial fishing gear: Lessons learnt and future directions. Rev. Fish Biol. Fish 2019, 29, 223–247. [Google Scholar] [CrossRef]
- Reeves, R.R.; Read, A.J.; Notarbartolo di Sicara, G. Report of the Workshop on Interactions between Dolphins and Fisheries in the Mediterranean: Evaluation of Mitigation Alternatives; Technical Report; Istituto Centrale per la Riiiirca Applicata al Mare: Roma, Italy, 2001. [Google Scholar]
- Mackay, A.I.; Knuckey, I.A. Mitigation of Marine Mammal Bycatch in Gillnet Fisheries Using Acoustic Devices—Literature Review; Technical Report; Final Report to the Australian Fisheries Management Authority; AFMA: Canberra, Australia, 2013. [Google Scholar]
- Barlow, J.; Cameron, G.A. Field Experiments Show that Acoustic Pingers Reduce Marine Mammal Bycatch in the California Drift Gill Net Fishery. Mar. Mamm. Sci. 2003, 19, 265–283. [Google Scholar] [CrossRef]
- Dawson, S.; Northridge, S.; Waples, D.; Read, A. To ping or not to ping: The use of active acoustic devices in mitigating interactions between small cetaceans and gillnet fisheries. Endanger. Species Res. 2013, 19, 201–221. [Google Scholar] [CrossRef]
- Morizur, Y.; Le Niliot, P.; Buanic, M.; Pianalto, S. Expérimentations de Répulsifs Acoustiques Commerciaux sur les Filets Fixes à Baudroies en mer d’Iroise; Technical Report; Ifremer, Centre de Brest: Plouzané, France, 2009. [Google Scholar]
- Berrow, S.; Cosgrove, R.; Leeney, R.H.; O’Brien, J.; Mcgrath, D.; Dalgard, J. Effect of acoustic deterrents on the behaviour of common dolphins (Delphinus delphis). J. Cetacean Res. Manag. 2008, 10, 227–233. [Google Scholar]
- Leeney, R.H.; Berrow, S.; McGrath, D.; O’Brien, J.; Cosgrove, R.; Godley, B.J. Effects of pingers on the behaviour of bottlenose dolphins. J. Mar. Biolog. Assoc. United Kingd. 2007, 87, 129–133. [Google Scholar] [CrossRef]
- Van Marlen, B. NEphrops and CEtacean Species Selection Information and TechnologY—Final Publishable Activity Report; Scientific Support to Policy (SSP), IMARES: Yerseke, The Netherlands, 2007. [Google Scholar]
- Berg Soto, A.; Cagnazzi, D.; Everingham, Y.; Parra, G.; Noad, M.; Marsh, H. Acoustic alarms elicit only subtle responses in the behaviour of tropical coastal dolphins in Queensland, Australia. Endanger. Species Res. 2013, 20, 271–282. [Google Scholar] [CrossRef]
- Cox, T.M.; Read, A.J.; Solow, A.; Tregenza, N. Will harbour porpoises (Phocoena phocoena) habituate to pingers? J. Cetacean Res. Manag. 2001, 3, 81–86. [Google Scholar]
- Carlström, J.; Berggren, P.; Tregenza, N.J. Spatial and temporal impact of pingers on porpoises. Can. J. Fish. Aquat. Sci. 2009, 66, 72–82. [Google Scholar] [CrossRef]
- Read, A. REVIEW Development of conservation strategies to mitigate the bycatch of harbor porpoises in the Gulf of Maine. Endanger. Species Res. 2013, 20, 235–250. [Google Scholar] [CrossRef]
- Carretta, J.V.; Barlow, J. Long-Term Effectiveness, Failure Rates, and “Dinner Bell” Properties of Acoustic Pingers in a Gillnet Fishery. Mar. Technol. Soc. J 2011, 45, 7–19. [Google Scholar] [CrossRef]
- Cox, T.M.; Read, A.J.; Swanner, D.; Urian, K.; Waples, D. Behavioral responses of bottlenose dolphins, Tursiops truncatus, to gillnets and acoustic alarms. Biol. Conserv. 2004, 115, 203–212. [Google Scholar] [CrossRef]
- Kraus, S.O. The Once and Future Ping: Challenges for the Use of Acoustic Deterrents in Fisheries. Mar. Technol. Soc. J. 1999, 33, 90–93. [Google Scholar] [CrossRef]
- Buscaino, G.; Buffa, G.; Sarà, G.; Bellante, A.; Tonello, A.J.; Hardt, F.A.S.; Cremer, M.J.; Bonanno, A.; Cuttitta, A.; Mazzola, S. Pinger affects fish catch efficiency and damage to bottom gill nets related to bottlenose dolphins. Fish Sci. 2009, 75, 537–544. [Google Scholar] [CrossRef]
- Findlay, C.; Ripple, H.; Coomber, F.; Froud, K.; Harries, O.; van Geel, N.; Calderan, S.; Benjamins, S.; Risch, D.; Wilson, B. Mapping widespread and increasing underwater noise pollution from acoustic deterrent devices. Mar. Pollut. Bull. 2018, 135, 1042–1050. [Google Scholar] [CrossRef]
- Peltier, H.; Authier, M.; Dabin, W.; Dars, C.; Demaret, F.; Doremus, G.; Canneyt, O.V.; Laran, S.; Mendez-Fernandez, P.; Spitz, J.; et al. Can modelling the drift of bycaught dolphin stranded carcasses help identify involved fisheries? An exploratory study. Glob. Ecol. Conserv. 2020, 21, e00843. [Google Scholar] [CrossRef]
- ICES. Workshop on fisheries Emergency Measures to minimize BYCatch of short-beaked common dolphins in the Bay of Biscay and harbor porpoise in the Baltic Sea (WKEMBYC). In ICES Scientific Reports; ICES: Copenhagen, Denmark, 2020; Volume 2, p. 354. [Google Scholar] [CrossRef]
- ICES. OSPAR request to estimate bycatch mortality of marine mammals (harbour porpoise Phocoena phocoena, common dolphin Delphinus delphis, grey seal Halichoerus grypus) within the OSPAR maritime area. In ICES Advice: Special Requests; ICES: Copenhagen, Denmark, 2021; pp. 1–6. [Google Scholar] [CrossRef]
- Peltier, H.; Authier, M.; Caurant, F.; Dabin, W.; Dars, C.; Demaret, F.; Meheust, E.; Ridoux, V.; Van Canneyt, O.; Spitz, J. Etat des Connaissances sur les Captures Accidentelles de Dauphins Communs dans le Golfe de Gascogne—Synthèse 2019; Technical Report; Observatoire PELAGIS—UMS 3462, La Rochelle Université/CNRS: La Rochelle, France, 2019. [Google Scholar]
- Peltier, H.; Authier, M.; Caurant, F.; Dabin, W.; Daniel, P.; Dars, C.; Demaret, F.; Meheust, E.; Van Canneyt, O.; Spitz, J.; et al. In the Wrong Place at the Wrong Time: Identifying Spatiotemporal Co-occurrence of Bycaught Common Dolphins and Fisheries in the Bay of Biscay (NE Atlantic) From 2010 to 2019. Front. Mar. Sci. 2021, 8, 617342. [Google Scholar] [CrossRef]
- Laran, S.; Authier, M.; Blanck, A.; Doremus, G.; Falchetto, H.; Monestiez, P.; Pettex, E.; Stephan, E.; Van Canneyt, O.; Ridoux, V. Seasonal distribution and abundance of cetaceans within French waters- Part II: The Bay of Biscay and the English Channel. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 141, 31–40. [Google Scholar] [CrossRef]
- Hammond, P.; Lacey, C.; Gilles, A.; Viquerat, S.; Börjesson, P.; Herr, H.; Macleod, K.; Ridoux, V.; Santos, M.; Scheidat, M.; et al. Estimates of Cetacean Abundance in European Atlantic Waters in Summer 2016 from the SCANS-III Aerial and Shipboard Surveys; Technical Report; Sea Mammal Research Unit, University of St Andrews: St Andrews, UK, 2017. [Google Scholar]
- ASCOBANS. Developing a Shared Understanding on the Use of Thresholds/Environmental Limits; Technical Report Part I; ASCOBANS: London, UK, 2015. [Google Scholar]
- Ministère de la Transition écologique et Solidaire. Arrêté du 9 Septembre 2019 Relatif à la Définition du bon état écologique des eaux Marines et aux Normes Méthodologiques D’évaluation; Journal Officiel: Paris, France, 2019. [Google Scholar]
- European Parliament, Council of the European Union. Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 Establishing a Framework for Community Action in the Field of Marine Environmental Policy (Marine Strategy Framework Directive) (Text with EEA Relevance); Official Journal of the European Union: Strasbourg, France, 2008. [Google Scholar]
- Altherr, S.; Hodgins, N. Small Cetaceans, Big Problems: A Global Review of the Impacts of Hunting on Small Whales, Dolphins and Porpoises; Technical Report; Whale and Dolphin Conservation (WDC): Chippenham, UK, 2018. [Google Scholar]
- Rimaud, T.; Authier, M.; Mehault, S.; Peltier, H.; Canneyt, O.V. RAPPORT Final du Projet PIC; Technical Report; Les Pêcheurs de Bretagne: Lorient, France, 2019. [Google Scholar]
- Rouby, E.; Dubroca, L.; Cloâtre, T.; Demanèche, S.; Genu, M.; Macleod, K.; Peltier, H.; Ridoux, V.; Authier, M. Estimating Bycatch From Non-representative Samples (II): A Case Study of Pair Trawlers and Common Dolphins in the Bay of Biscay. Front. Mar. Sci. 2022, 8, 795942. [Google Scholar] [CrossRef]
- Au, W.L.; Jones, L. Acoustic Reflectivity of Nets: Implications Concerning Incidental Take of Dolphins. Mar. Mamm. Sci. 1991, 7, 258–273. [Google Scholar] [CrossRef]
- Au, W.W.; Hastings, M.C. Emission of Social Sounds by Marine Animals. In Principles of Marine Bioacoustics; Springer: New York, NY, USA, 2008; pp. 401–499. [Google Scholar] [CrossRef]
- Schevill, W.E.; McBride, A.F. Evidence for echolocation by cetaceans. Deep Sea Res. 1956, 3, 153–154. [Google Scholar] [CrossRef]
- Mooney, T.A.; Nachtigall, P.E.; Au, W.W. Target Strength of a Nylon Monofilament and an Acoustically Enhanced Gillnet: Predictions of Biosonar Detection Ranges. Aquat. Mamm. 2004, 30, 220–226. [Google Scholar] [CrossRef]
- Mooney, T.A.; Au, W.W.L.; Nachtigall, P.E.; Trippel, E.A. Acoustic and stiffness properties of gillnets as they relate to small cetacean bycatch. ICES J. Mar. Sci. 2007, 64, 1324–1332. [Google Scholar] [CrossRef]
- Aubauer, R.; Au, W.W.L. Phantom echo generation: A new technique for investigating dolphin echolocation. J. Acoust. Soc. Am. 1998, 104, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Aubauer, R.; Au, W.W.L.; Nachtigall, P.E.; Pawloski, D.A.; DeLong, C.M. Classification of electronically generated phantom targets by an Atlantic bottlenose dolphin (Tursiops truncatus). J. Acoust. Soc. Am. 2000, 107, 2750–2754. [Google Scholar] [CrossRef]
- Muller, M.W.; Au, W.W.L.; Nachtigall, P.E.; Allen, J.S.; Breese, M. Phantom echo highlight amplitude and temporal difference resolutions of an echolocating dolphin, Tursiops truncatus. J. Acoust. Soc. Am. 2007, 122, 2255–2262. [Google Scholar] [CrossRef]
- Xitco, M.J.; Roitblat, H.L. Object recognition through eavesdropping: Passive echolocation in bottlenose dolphins. Anim. Learn. Behav. 1996, 24, 355–365. [Google Scholar] [CrossRef]
- Götz, T.; Verfuß, U.K.; Schnitzler, H.U. ‘Eavesdropping’ in wild rough-toothed dolphins (Steno bredanensis)? Biol. Lett. 2006, 2, 5–7. [Google Scholar] [CrossRef]
- Bearzi, G.; Eddy, L.; Piwetz, S.; Reggente, M.A.L.; Cozzi, B. Cetacean Behavior Toward the Dead and Dying. In Encyclopedia of Animal Cognition and Behavior; Vonk, J., Shackelford, T., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–8. [Google Scholar] [CrossRef]
- Reggente, M.A.L.; Alves, F.; Nicolau, C.; Freitas, L.; Cagnazzi, D.; Baird, R.W.; Galli, P. Nurturant behavior toward dead conspecifics in free-ranging mammals: New records for odontocetes and a general review. J. Mammal. 2016, 97, 1428–1434. [Google Scholar] [CrossRef]
- Reggente, M.A.L.V.; Papale, E.; McGinty, N.; Eddy, L.; de Lucia, G.A.; Bertulli, C.G. Social relationships and death-related behaviour in aquatic mammals: A systematic review. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 1–6. [Google Scholar] [CrossRef]
- Culik, B.; von Dorrien, C.; Müller, V.; Conrad, M. Synthetic communication signals influence wild harbour porpoise (Phocoena phocoena) behaviour. Bioacoustics 2015, 24, 201–221. [Google Scholar] [CrossRef]
- Shane, S.H. Behavior and Ecology of the Bottlenose Dolphin at Sanibel Island, Florida. In The Bottlenose Dolphin; Elsevier: Amsterdam, The Netherlands, 1990; pp. 245–265. [Google Scholar] [CrossRef]
- Filby, N.E.; Bossley, M.; Stockin, K.A. Behaviour of free-ranging short-beaked common dolphins (Delphinus delphis) in Gulf St Vincent, South Australia. Aust. J. Zool. 2013, 61, 291. [Google Scholar] [CrossRef]
- Stockin, K.; Lusseau, D.; Binedell, V.; Wiseman, N.; Orams, M. Tourism affects the behavioural budget of the common dolphin Delphinus sp. in the Hauraki Gulf, New Zealand. Mar. Ecol. Prog. Ser. 2008, 355, 287–295. [Google Scholar] [CrossRef]
- Van Canneyt, O.; Larnaud, P.; Le Gall, Y.; Morizur, Y. Effets des Dispositifs de Dissuasion Acoustiques sur le Comportement du Dauphin Commun, Delphinus Delphis; Technical Report; CRMM, Contrat IFREMER 2005 2 22734206; Pelagis: La Rochelle, France, 2006. [Google Scholar]
- Kinze, C.C. Photographic Guide to the Marine Mammals of the North Atlantic; Oxford University Press: Totnes, UK, 2002. [Google Scholar]
- Nowak, R.M.; Walker, E.P. Walker’s Marine Mammals of the World; JHU Press: Baltimore, MD, USA, 2003. [Google Scholar]
- Jones, G. Echolocation. Curr. Biol. 2005, 15, R484–R488. [Google Scholar] [CrossRef]
- Martin, M.J.; Elwen, S.H.; Kassanjee, R.; Gridley, T. To buzz or burst-pulse? The functional role of Heaviside’s dolphin, Cephalorhynchus heavisidii, rapidly pulsed signals. Anim. Behav. 2019, 150, 273–284. [Google Scholar] [CrossRef]
- Arranz, P.; DeRuiter, S.L.; Stimpert, A.K.; Neves, S.; Friedlaender, A.S.; Goldbogen, J.A.; Visser, F.; Calambokidis, J.; Southall, B.L.; Tyack, P.L. Discrimination of fast click series produced by tagged Risso’s dolphins (Grampus griseus) for echolocation or communication. J. Exp. Biol. 2016, 18, 2898–2907. [Google Scholar] [CrossRef] [PubMed]
- Rankin, S.; Oswald, J.; Barlow, J.; Lammers, M. Patterned burst-pulse vocalizations of the northern right whale dolphin, Lissodelphis borealis. J. Acoust. Soc. Am. 2007, 121, 1213–1218. [Google Scholar] [CrossRef]
- Ridgway, S.; Samuelson, D.; Van Alstyne, K.; Price, D. On doing two things at once: Dolphin brain and nose coordinate sonar clicks, buzzes, and emotional squeals with social sounds during fish capture. J. Exp. Biol. 2015, 218, 3987–3995. [Google Scholar] [CrossRef]
- Wisniewska, D.M.; Johnson, M.; Nachtigall, P.E.; Madsen, P.T. Buzzing during biosonar-based interception of prey in the delphinids Tursiops truncatus and Pseudorca crassidens. J. Exp. Biol. 2014, 217, 4279–4282. [Google Scholar] [CrossRef]
- Lammers, M.O.; Au, W.W.L.; Herzing, D.L. The broadband social acoustic signaling behavior of spinner and spotted dolphins. J. Acoust. Soc. Am. 2003, 114, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Lammers, M.O.; Schotten, M.; Au, W.W.L. The spatial context of free-ranging Hawaiian spinner dolphins (Stenella longirostris) producing acoustic signals. J. Acoust. Soc. Am. 2006, 119, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Overstrom, N.A. Association between burst-pulse sounds and aggressive behavior in captive Atlantic bottlenosed dolphins (Tursiops truncatus). Zoo Biol. 1983, 2, 93–103. [Google Scholar] [CrossRef]
- Van Rossum, G.; Drake, F.L. Python 3 Reference Manual; CreateSpace: Scotts Valley, CA, USA, 2009. [Google Scholar]
- Poupard, M.; Ferrari, M.; Best, P.; Glotin, H. Passive acoustic monitoring of sperm whales and anthropogenic noise using stereophonic recordings in the Mediterranean Sea, North West Pelagos Sanctuary. Sci. Rep. 2022, 12, 2007. [Google Scholar] [CrossRef] [PubMed]
- Kandia, V.; Stylianou, Y. Detection of sperm whale clicks based on the Teager–Kaiser energy operator. Appl. Acoust. 2006, 67, 1144–1163. [Google Scholar] [CrossRef]
- Kaiser, J. On a simple algorithm to calculate the ’energy’ of a signal. In Proceedings of the International Conference on Acoustics, Speech, and Signal Processing, Albuquerque, NM, USA, 3–6 April 1990; IEEE: Albuquerque, NM, USA, 1990; pp. 381–384. [Google Scholar] [CrossRef]
- McInnes, L.; Healy, J.; Melville, J. UMAP: Uniform Manifold Approximation and Projection for Dimension Reduction. arXiv 2020, arXiv:1802.03426. [Google Scholar]
- Henderson, E.E.; Hildebrand, J.A.; Smith, M.H.; Falcone, E.A. The behavioral context of common dolphin (Delphinus sp.) vocalizations. Mar. Mamm. Sci. 2012, 28, 439–460. [Google Scholar] [CrossRef]
- Datta, S.; Sturtivant, C. Dolphin whistle classification for determining group identities. Signal Process. 2002, 82, 251–258. [Google Scholar] [CrossRef]
- Roch, M.A.; Scott Brandes, T.; Patel, B.; Barkley, Y.; Baumann-Pickering, S.; Soldevilla, M.S. Automated extraction of odontocete whistle contours. J. Acoust. Soc. Am. 2011, 130, 2212–2223. [Google Scholar] [CrossRef]
- Dadouchi, F.; Gervaise, C.; Ioana, C.; Huillery, J.; Mars, J.I. Automated segmentation of linear time-frequency representations of marine-mammal sounds. J. Acoust. Soc. Am. 2013, 134, 2546–2555. [Google Scholar] [CrossRef]
- Halkias, X.C.; Ellis, D.P. Call detection and extraction using Bayesian inference. Appl. Acoust. 2006, 67, 1164–1174. [Google Scholar] [CrossRef]
- Gruden, P.; White, P.R. Automated tracking of dolphin whistles using Gaussian mixture probability hypothesis density filters. J. Acoust. Soc. Am. 2016, 140, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Erbe, C.; King, A.R. Automatic detection of marine mammals using information entropy. J. Acoust. Soc. Am. 2008, 124, 2833–2840. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, D.; Caillat, M.; Gordon, J.; White, P. Automatic detection and classification of odontocete whistles. J. Acoust. Soc. Am. 2013, 134, 2427–2437. [Google Scholar] [CrossRef] [PubMed]
- Mellinger, D.K.; Martin, S.W.; Morrissey, R.P.; Thomas, L.; Yosco, J.J. A method for detecting whistles, moans, and other frequency contour sounds. J. Acoust. Soc. Am. 2011, 129, 4055–4061. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.T.; Chu, W.Y.; Li, W.L.; Huang, Y.H.; Hu, W.C.; Chen, C.F. A Case Study of Whistle Detection and Localization for Humpback Dolphins in Taiwan. J. Mar. Sci. Eng. 2021, 9, 725. [Google Scholar] [CrossRef]
- Kershenbaum, A.; Roch, M.A. An image processing based paradigm for the extraction of tonal sounds in cetacean communications. J. Acoust. Soc. Am. 2013, 134, 4435–4445. [Google Scholar] [CrossRef]
- Serra, O.; Martins, F.; Padovese, L. Active contour-based detection of estuarine dolphin whistles in spectrogram images. Ecol. Inform. 2020, 55, 101036. [Google Scholar] [CrossRef]
- Mallawaarachchi, A.; Ong, S.H.; Chitre, M.; Taylor, E. Spectrogram denoising and automated extraction of the fundamental frequency variation of dolphin whistles. J. Acoust. Soc. Am. 2008, 124, 1159–1170. [Google Scholar] [CrossRef]
- Lin, T.H.; Chou, L.S.; Akamatsu, T.; Chan, H.C.; Chen, C.F. An automatic detection algorithm for extracting the representative frequency of cetacean tonal sounds. J. Acoust. Soc. Am. 2013, 134, 2477–2485. [Google Scholar] [CrossRef]
- Baumgartner, M.F.; Mussoline, S.E. A generalized baleen whale call detection and classification system. J. Acoust. Soc. Am. 2011, 129, 2889–2902. [Google Scholar] [CrossRef] [PubMed]
- Halkias, X.C.; Paris, S.; Glotin, H. Classification of mysticete sounds using machine learning techniques. J. Acoust. Soc. Am. 2013, 134, 3496–3505. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, X.; Palmer, K.J.; Fleishman, E.; Gillespie, D.; Nosal, E.M.; Shiu, Y.; Klinck, H.; Cholewiak, D.; Helble, T.; et al. Learning Deep Models from Synthetic Data for Extracting Dolphin Whistle Contours. In Proceedings of the 2020 International Joint Conference on Neural Networks (IJCNN), Glasgow, UK, 19–24 July 2020; IEEE: Glasgow, UK, 2020; pp. 1–10. [Google Scholar] [CrossRef]
- Han, K.; Wang, D. Neural Network Based Pitch Tracking in Very Noisy Speech. IEEE/ACM Trans. Audio Speech Lang. Process. 2014, 22, 2158–2168. [Google Scholar] [CrossRef]
- Abeille, R.; Chamroukhi, F.; Doh, Y.; Dufour, O.; Giraudet, P.; Halkias, X.; Lotin, H.G.; Prévot, J.; Rabouy, C.; Razik, J. Detection et Classification sur Transect Audio-Visuel de Populations de Cétacés du Nord Pelagos-Iles d’Or; DECAV Pelagos Research Report N°11-031 83400 PC; Université du Sud Toulon Var: La Garde, France, 2012. [Google Scholar]
- Anderson, M.J. Permutation tests for univariate or multivariate analysis of variance and regression. Can. J. Fish. Aquat. Sci. 2001, 58, 626–639. [Google Scholar] [CrossRef]
- Hotelling, H. Analysis of a complex of statistical variables into principal components. J. Educ. Psychol. 1933, 24, 417–441. [Google Scholar] [CrossRef]
- Thurstone, L.L. Multiple factor analysis. Psychol. Rev. 1931, 38, 406–427. [Google Scholar] [CrossRef]
- Guan, H.; Zhang, Y.; Xian, M.; Cheng, H.D.; Tang, X. SMOTE-WENN: Solving class imbalance and small sample problems by oversampling and distance scaling. Appl. Intell. 2021, 51, 1394–1409. [Google Scholar] [CrossRef]
- Quick, N.J.; Janik, V.M. Whistle rates of wild bottlenose dolphins (Tursiops truncatus): Influences of group size and behavior. J. Comp. Psychol. 2008, 122, 305–311. [Google Scholar] [CrossRef]
- Greene, W.H. Accounting for Excess Zeros and Sample Selection. NYU Work. Pap. 1994, EC-94-10, 1–37. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio Team: Boston, MA, USA, 2019. [Google Scholar]
- Poupard, M.; de Montgolfier, B.; Glotin, H. Ethoacoustic by bayesian non parametric and stochastic neighbor embedding to forecast anthropic pressure on dolphins. In Proceedings of the OCEANS, Marseille, France, 17–20 June 2019; pp. 1–5. [Google Scholar] [CrossRef]
- Kohlsdorf, D.; Herzing, D.; Starner, T. An Auto Encoder For Audio Dolphin Communication. In Proceedings of the 2020 International Joint Conference on Neural Networks (IJCNN), Glasgow, UK, 19–24 July 2020; pp. 1–7. [Google Scholar] [CrossRef]
- Herzing, D.L. Vocalizations and associated underwater behavior of free-ranging Atlantic spotted dolphins, Stenella frontalis and bottlenose dolphins, Tursiops truncatus. Aquat. Mamm. 1996, 22, 61–80. [Google Scholar]
- Ridgway, S.H.; Moore, P.W.; Carder, D.A.; Romano, T.A. Forward shift of feeding buzz components of dolphins and belugas during associative learning reveals a likely connection to reward expectation, pleasure and brain dopamine activation. J. Exp. Biol. 2014, 217, 2910–2919. [Google Scholar] [CrossRef] [PubMed]
- Townsend, S.W.; Manser, M.B. Functionally Referential Communication in Mammals: The Past, Present and the Future. Ethology 2013, 119, 1–11. [Google Scholar] [CrossRef]
- Martin, M.J.; Gridley, T.; Elwen, S.H.; Jensen, F.H. Heaviside’s dolphins (Cephalorhynchus heavisidii) relax acoustic crypsis to increase communication range. Proc. R. Soc. B Biol. Sci. 2018, 285, 20181178. [Google Scholar] [CrossRef] [PubMed]
- Bowles, A. Behavioral Responses and Habituation of Pinnipeds and Small Cetaceans to Novel Objects and Simulated Fishing Gear With and Without a Pinger. Aquat. Mamm. 2012, 38, 161–188. [Google Scholar] [CrossRef]
- ICES. Workshop on estimation of MOrtality of Marine MAmmals due to Bycatch. In ICES Scientific Reports; ICES: Copenhagen, Denmark, 2021; Volume 3, p. 107. [Google Scholar] [CrossRef]
- Cloatre, T.; Quinio-Scavinner, M.; Sagan, J.; Dubroca, L.; Billet, N.; Boiron-Leroy, A.; Martin-Baillet, V.; Bourdonnec, P.L.; Derridj, O.; Vigneau, J.; et al. Captures et Rejets des Métiers de Pêche Français Résultats des Observations à Bord des Navires de Pêche Professionnelle en 2020; Technical Report; OBSMER: Plouzané, France, 2020. [Google Scholar]
- Demanèche, S.; Berthou, P.; Le Blond, S.; Begot, E.; Weiss, J. Amélioration de la Connaissance de L’activité des Fileyeurs dans le Golfe de Gascogne; Technical Report Ref. DG/2019.350—Saisine DPMA 19-14259; DPMA—Direction des Pêches Maritimes et de l’Aquaculture: La Défense, France, 2019. [Google Scholar]
- PELAGIS. Bilan de L’hiver 2018–2019 Captures Accidentelles de Petits Cétacés en Atlantique; Technical Report; Pelagis: La Rochelle, France, 2019. [Google Scholar]
- Glotin, H.; Thellier, N.; Best, P.; Poupard, M.; Ferrari, M.; Viera, S.; Giés, V.; Oger, M.; Giraudet, P.; Mercier, M.; et al. Sphyrna-Odyssey 2019–2020, Rapport I: Découvertes Ethoacoustiques de Chasses Collaboratives de Cachalots en Abysse & Impacts en Mer du Confinement COVID-19; Rapport Scientifique 1, CNRS LIS; Université de Toulon: La Garde, France, 2020. [Google Scholar]
- Macaulay, J.; Kingston, A.; Coram, A.; Oswald, M.; Swift, R.; Gillespie, D.; Northridge, S. Passive acoustic tracking of the three-dimensional movements and acoustic behaviour of toothed whales in close proximity to static nets. Methods Ecol. Evol. 2022, 13, 1250–1264. [Google Scholar] [CrossRef]
- Maaten, L.v.d.; Hinton, G. Visualizing Data using t-SNE. J. Mach. Learn. Res. 2008, 9, 2579–2605. [Google Scholar]
- Becht, E.; McInnes, L.; Healy, J.; Dutertre, C.A.; Kwok, I.W.H.; Ng, L.G.; Ginhoux, F.; Newell, E.W. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 2019, 37, 38–44. [Google Scholar] [CrossRef]

| State | Definition |
|---|---|
| Foraging | Dolphins involved in any effort to pursue, capture and/or consume prey, as defined by observations of two or more of the following: fish chasing; erratic movements at the surface; multidirectional diving; coordinated deep diving; and rapid circle swimming. Prey often observed at the surface, as well as the presence of birds hunting. |
| Travelling | Dolphins engaged in persistent, directional movement making noticeable headway along a specific compass bearing. Group spacing varied and individuals swam with short (<20 s), relatively constant dive intervals. |
| Socialising | Animals were involved in active surface behaviour (frequent surfacing and breaching) that included physical interactions among group members and sometimes aerial behaviour. |
| Milling | Dolphins showed little movement, tended to remain in the same place and either spent floating at the surface or surfaced asynchronously. |
| Attraction | Dolphins came towards the boat and swam at a few metres along it, following its direction. |
| Variable Name | Nature | Definition | References |
|---|---|---|---|
| Structure of the group | Qualitative | ‘Compact’ if <5 body lengths between all individuals of a group or ‘Dispersed’ otherwise. | Adapted from [49] |
| Direction followed | Qualitative | ‘Variable’ or ‘Constant’. | - |
| Diving time | Qualitative | ‘Short’ if <1 min, ‘Long’ if >1 min, or ‘Variable’. | Threshold adapted from [52] |
| Speed of movement | Qualitative | ‘Slow’ if <10 km/h or ‘Fast’ if >10 km/h. 10 km/h being the travelling speed of common dolphins. | Travelling speed from [53] |
| Behavioural events | Quantitative | Dolphins of the group surfacing simultaneously, doing active swimming on the surface, diving, jumping. Estimated using percentage. | [12,51] |
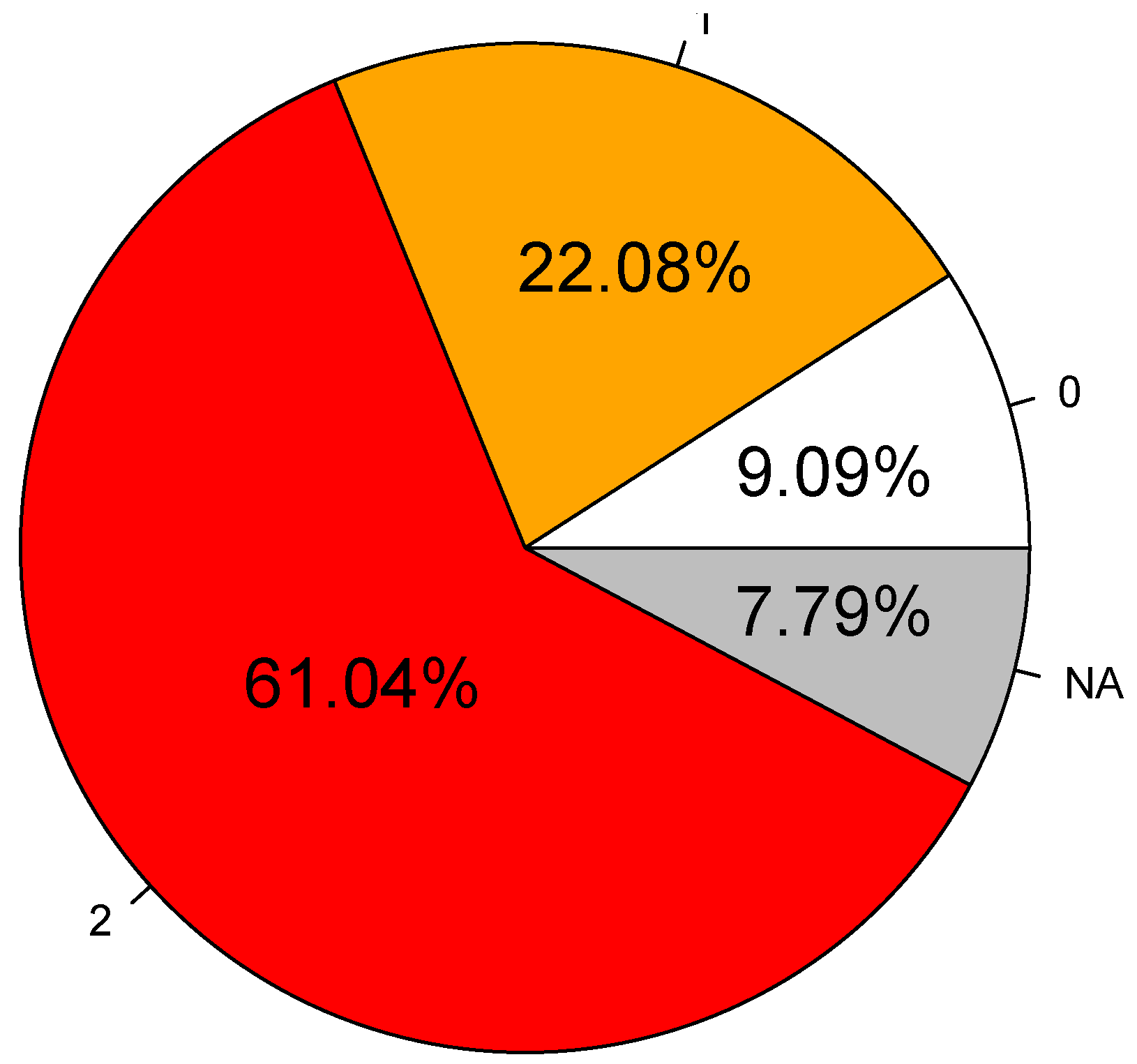
| Intensity of response | Semi- Quantitative | When the beacon emitted: ‘0’: no change in behaviour, ‘1’: attentive behaviour to the signal without moving 5 m away from the emission source, ‘2’: attentive behaviour to the signal and moves at least 5–30 m away from the emission source. Attentive behaviour means that the dolphins oriented themselves towards the emission source and were prospecting, doing back and forth movements in parallel with the emission source/boat, before calmly leaving. | Threshold adapted from click detection range [38] |
| Fishing Net | Treatment Sequence | Total Sequences | ||||
|---|---|---|---|---|---|---|
| BEF | BEF + DUR | DUR | DUR + AFT | AFT | ||
| Present | 12 | 26 | 103 | 27 | 39 | 207 |
| Absent | 26 | 26 | 73 | 18 | 11 | 154 |
| Total sequences | 38 | 52 | 176 | 45 | 50 | 361 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehnhoff, L.; Glotin, H.; Bernard, S.; Dabin, W.; Le Gall, Y.; Menut, E.; Meheust, E.; Peltier, H.; Pochat, A.; Pochat, K.; et al. Behavioural Responses of Common Dolphins Delphinus delphis to a Bio-Inspired Acoustic Device for Limiting Fishery By-Catch. Sustainability 2022, 14, 13186. https://doi.org/10.3390/su142013186
Lehnhoff L, Glotin H, Bernard S, Dabin W, Le Gall Y, Menut E, Meheust E, Peltier H, Pochat A, Pochat K, et al. Behavioural Responses of Common Dolphins Delphinus delphis to a Bio-Inspired Acoustic Device for Limiting Fishery By-Catch. Sustainability. 2022; 14(20):13186. https://doi.org/10.3390/su142013186
Chicago/Turabian StyleLehnhoff, Loïc, Hervé Glotin, Serge Bernard, Willy Dabin, Yves Le Gall, Eric Menut, Eleonore Meheust, Hélène Peltier, Alain Pochat, Krystel Pochat, and et al. 2022. "Behavioural Responses of Common Dolphins Delphinus delphis to a Bio-Inspired Acoustic Device for Limiting Fishery By-Catch" Sustainability 14, no. 20: 13186. https://doi.org/10.3390/su142013186
APA StyleLehnhoff, L., Glotin, H., Bernard, S., Dabin, W., Le Gall, Y., Menut, E., Meheust, E., Peltier, H., Pochat, A., Pochat, K., Rimaud, T., Sourget, Q., Spitz, J., Van Canneyt, O., & Mérigot, B. (2022). Behavioural Responses of Common Dolphins Delphinus delphis to a Bio-Inspired Acoustic Device for Limiting Fishery By-Catch. Sustainability, 14(20), 13186. https://doi.org/10.3390/su142013186






