1. Introduction
Significant changes in global energy markets have recently been reflected as the growing need for energy, to develop and improve the efficiency of technology, to toughen environmental standards, etc. [
1]. This shows that energy security and well-being are increasingly complex and multifaceted. These issues are increasingly included in the priorities discussed by leaders of all countries, at the level of congresses, forums, symposia, and other meetings in business circles, as well as in scientific communities.
The concept of the Energy Trilemma developed by the World Energy Council focuses on three main vectors of development of the fuel and energy complex: energy security, energy availability, and environmental sustainability. Economics, politics, and environmental protection are the three competing objectives of the energy trilemma [
2,
3] and their balance is the subject of years of contention from different perspectives. With the United Nations Sustainable Development Goals and the Paris Climate Agreement already being implemented in the world [
4], the need for energy to contribute to the stable development of the world community should be clear and significant. Energy generation is now determined not only by economic indicators of feasibility but also by the growing social and environmental factors of society, corresponding to several side-by-side sustainable development paradigms presented by the UNO. The link between goal 7, “Achieving universal access to affordable, reliable, sustainable and modern energy for all”, and goal 13, “Taking urgent action to combat climate change and its consequences”, supported by the Paris Agreement international climate change treaty, is clear.
At the same time, there are justifiably more stringent requirements for ensuring energy security for facilities and farms that operate in territories in difficult conditions, in conditions of extreme climates, and in infrastructure isolation [
5,
6]. The creation of comfortable conditions for the functioning of human society and quality of life is coupled with a number of difficulties. The survival of mankind as a whole depends significantly on how much we can learn to live in a new way, that is, to protect nature and resources as much as possible, to introduce and use environmental and knowledge-intensive technologies, and to generate income with limited physical and energy resources.
The importance of addressing interrelated issues in this direction occurs due to the problem of a reliable energy supply in the developing northern and Arctic territories. Strategic guidelines for sustainable energy development are interesting and are identified and traced in the strategies of the countries of the Arctic region group: Russia, Canada, the USA, and Scandinavian countries [
7,
8,
9]. This largely explains the particular importance of energy security, as expressed in the declaration of international organizations and regional associations, as well as in national energy strategies and laws. Energy systems located in remote areas can face many specific challenges, such as transport logistics problems, including limited fuel and spare parts supply during the year and the dependence of these supplies on weather conditions, and the lack of qualified field maintenance personnel [
10,
11].
We conclude that it is necessary to jointly study environmental and economic opportunities for the development of the energy complex, including renewable energy source (RES) technologies, which is consistent with the position of the authors of the study [
12]. It is also necessary to do this individually for each case or region, as development decisions should be made on the basis of the reasonable use of natural capital, reduction in emissions, conservation of biological diversity, as well as the payback and efficiency of the project.
For example, due to the fact that the northern and Arctic zones of Eastern Siberia are climatically severe, underdeveloped, and poorly populated territories with large explored and exploited oil and gas reserves (33 hydrocarbon deposits of the Krasnoyarsk Territory) [
13], the problems of the fuel and energy complex here are extreme. In the cold season, the rivers and the Northern Sea Route freeze, and only aviation and winter roads (routes along frozen rivers) are the modern transport routes to the north of Eastern Siberia. Fuel imported to the fields and purchased by oil and gas companies is necessary for the operation of numerous technological units, machines, and equipment, as well as for their own needs (heating and electricity generation). Thus, a significant carbon footprint is generated in these fields. At the same time, an even greater carbon footprint is made by the burning flares of the oil production by-product, i.e., the associated gas and methane, carbon dioxide, and other gases formed as a result of combustion, which are due to the limited technological capabilities for the use and storage of gas [
14].
Table 1 shows the countries with the highest emissions from gas combustion in 2020 and 2021 at oil production facilities [
15]. It follows that Russia is the leader in these emissions and does not have a downward trend, unlike most other countries. Flare gases, and in particular CO
2, are referred to as greenhouse gases, and emissions into the atmosphere of this by-product are the root cause of global climate change [
16,
17]. At the same time, climate change contributes to the increase in the number of fires in Eastern Siberia, which, in turn, adds to the obvious harmful environmental impact and contributes to the increase in emissions [
17,
18,
19].
Given that most of the mentioned deposits have yet to be commissioned, this problem is significant for the sustainable development of Arctic territory society, nature, and fauna. Today, the need to dispose of man-made carbon dioxide, in the framework of the Paris Climate Agreement signed by the world community, is more important than ever before. At the same time, many countries, such as Russia and China, adhere to the strategy of a more moderate transition to the reduction in greenhouse gas emissions and the introduction of RES by 2060 [
20]. This is naturally dictated by the dependence of the economies of these countries on hydrocarbons and the sluggish dynamics of the introduction of electric generating capacities operating on renewable energy. At the same time, the Order of the Government of the Russian Federation dated 1 June 2021 No. 1447-r states, in particular, about the upcoming increase in oil production, “On approval of the action plan for the implementation of the Energy Strategy of the Russian Federation for the period up to 2035” [
21].
In this situation, following the logical reasoning of the authors of the study [
22], it can be assumed that an increase in the share of RES will lead to a decrease in CO
2 emissions. At the same time, considering the existing technologies in the field of renewable energy in relation to the northern and Arctic zones of Eastern Siberia, we do not see ready-made solutions. In particular, unique solutions are known in the field of solar energy production and storage in the Arctic zone of Alaska [
23,
24]. However, in winter (on Polar Night), the potential of solar energy in the Arctic drops significantly. This means that the energy systems of the Arctic cannot be completely dependent on solar energy, even in combination with batteries or other storage solutions, i.e., in winter, a backup power source is always required in the Arctic. In addition, snow deposition on batteries during temperature spikes and regular blizzards requires frequent regular maintenance. Ensuring the dynamic development of wind energy in the Arctic is also a big question. There is growing concern about the ability of wind farms to withstand and restore their performance in the event of failures [
25]. Issues of the reliability of wind turbines also have a significant role; in particular, problems with their icing [
26] and failure of bearings [
27] are known.
It is also known that energy can be obtained from various types of raw biomass materials, including agricultural waste, livestock waste, solid household waste, and other types of organic waste [
28,
29,
30]. Due to a variety of processes, this feedstock can be used directly for the production of electricity or heat, or can be used for the production of gaseous, liquid, or solid fuels; biodiesel, bioethanol, and biogas are considered the most promising [
31,
32,
33]. The most promising biogas-based fuels are hydrogen mixtures and hydrogen, which can reduce greenhouse gas emissions when replacing natural gas without changing the combustion technology [
34]. Thus, pure biofuels, or, more commonly, biofuels mixed with oil-based fuels, can be used in liquid fuel systems for use in process equipment, transport, or heating. The range of bioenergy technologies is wide, and the level of their technical development varies considerably [
35]. For example, according to existing research findings [
36,
37], the effect of hydrogen enrichment leads to a reduction in hydrocarbon emissions and soot from the gas engine and contributes to improved engine energy efficiency and fuel economy. This is particularly relevant to the Eastern Siberian Arctic area where a large number of diesel generator sets are used for heating and oil tankers run on diesel engines, i.e., the relevance of converting engines and generators to a gas-diesel format or using gas engines, in general, is increasing.
Due to its harsh climatic conditions, the Eastern Siberian Arctic is not rich in raw materials for the production of biofuels, however, this study shows non-trivial approaches to its production. In particular, among a number of suitable raw materials for the production of biofuels in the Eastern Siberian Arctic, the authors include wastes from the petroleum industry, which is further discussed below. It is worth noting that hydrogen and hydrogen-containing mixtures are quite difficult to transport and store [
38], but in this case, the harsh climate conditions of the Eastern Siberian Arctic best contribute to the development of technologies for storing and transporting hydrogen in a gas-hydrate state [
39].
In addition to flare gas, the petroleum industry produces large quantities of other industrial waste, i.e., oil sludge [
40], as well as physically and chemically similar and unprofitable to extract and transport heavy oil. Oil sludge is a hazardous toxic waste on the one hand and a valuable energy resource on the other [
40,
41]. One approach to the environmentally friendly treatment of sludge is deep thermal decomposition based on the pyrolysis process [
42]. Steam treatment or dissolving carbon dioxide CO
2 is known to enhance the physical properties of heavy oil [
43,
44]. For high-viscosity oil, the degree of viscosity reduction when carbon dioxide is dissolved in it is comparable to the reduction in viscosity when exposed to heat [
45]. Steam treatment in an area of permafrost can lead to serious equipment accidents [
46]. It is also known that CO
2 injection can accelerate biomass thermal cracking which increases the yield of gaseous pyrolysis products hydrogen and methane [
47,
48]. These prerequisites predetermine the need to develop a comprehensive technology for waste management, processing, and utilization in the Eastern Siberian Arctic fields, with an assessment of the energy resources obtained on the basis of known biofuel production technologies.
The authors of the study [
49] predicted a serious confrontation between the green lobby, the shale industry, and the traditional oil industry in the near future. We already see signs of this confrontation in connection with the Arctic development strategy published by the European Union where there is a call for an early ban on the exploration and production of oil, gas, and coal in the Arctic [
50]. Such political statements do not facilitate dialogue with supporters of a moderate transition strategy and, against the background of the European energy crisis of 2021/2022 [
51,
52], look unstable, since, for example, a significant share of gas imported by Europe is produced and supplied on the Yamal Peninsula, one of the key gas production centers of the Arctic Zone [
53]. One of the tasks set in our study was the desire to show that confronting various energy lobbies is not mandatory and that it is necessary to find compromises, in particular, by finding and introducing innovative technologies to develop broadly, not for the sake of individual positions, but for the benefit of society. As the authors of the study [
54] showed, this is especially relevant due to the increasing share of hard-to-recover hydrocarbon reserves (heavy and high-viscosity oil) widely represented in fields in the Arctic since the low temperature in such reservoirs prevents the ideal maturation of hydrocarbons. It also limits the ability to extract hard-to-recover hydrocarbons and increase oil recovery by thermal techniques which can cause permafrost melting and can lead to wellbore collapse, requiring the development of unique solutions and/or other techniques such as CO
2 injection [
55].
The set of non-trivial solutions presented in this study is based on the introduction of green environmental technologies into established mining processes, leading not only to a decrease in the carbon footprint but also to potential economic benefits for oil and gas companies. The main purpose of this study is to evaluate an oilfield-adapted integrated biofuel technology based on the supercritical extraction and thermal decomposition of the feedstock—waste products. The choice is justified by the analysis of the Eastern Siberian Arctic bio-resource potential stability for the region’s sustainable development.
To achieve this goal, it is necessary to present a concept for the production of biofuels on an industrial scale in the conditions of hydrocarbon deposits in the Eastern Siberian Arctic. The stage of this task was the development of technology for the production of biofuels in the conditions of the East Siberian Arctic field by adapting known methods for the production of biofuels and studying the sources of suitable raw materials. To predict the behavior of the biofuel production system in Arctic fields based on the proposed technology, the task was set to develop an experimental bench for the study of the well extraction process. The next task was to experimentally investigate the process of extracting hydrocarbon substances from heavy petroleum components by extracting carbon dioxide in a supercritical state, followed by heat treatment of the obtained substances to obtain useful products. A qualitative and quantitative composition assessment was then performed on the products obtained. In addition, one of the main tasks of this study was to demonstrate the possibilities for synthesizing existing mining technologies and green energy.
3. Methodology and Materials of Experimental Studies of Biofuel Production in Downhole Conditions
3.1. Raw Materials
To study the extraction process, samples of heavy oil components of the Vankor field of the Krasnoyarsk Territory were used as raw materials. They are a dark brown physicochemical mixture, consisting mainly of petroleum products, with a water content of about 9%, and mechanical inclusions of less than 1% (
Figure 5).
The main properties of the raw materials used are shown in
Table 3.
3.2. Purpose and Functions of Borehole Extraction Test Bench
The test bench (
Figure 6) was designed for the testing process operations of the extraction method in biofuel production and includes a process control system.
The bench was designed to investigate the parameters of the extraction process in the well casing using tubing, as well as methods of obtaining and processing information about these processes using the operator console (
Figure 7) and PC with the aim of further modeling and forecasting the parameters of the extraction process.
The bench is a collection of hardware, process control, monitoring tools, measuring sensors, steel structures, and software.
The bench provides the following process functions:
- -
Ability to test extraction process operations in biofuel production in close to actual well process conditions.
- -
Tests of the extraction process system at the specified physical parameters including the pressure of the injected process liquid and the flow rate (or volume flow rate) of the process liquid.
- -
Possibility of supercritical CO2 production by compression.
- -
Display of process progress information, i.e., process parameters and equipment states, on the operator console.
- -
Remote control of technical devices from the operator console.
- -
Transfer of stand parameters to the database.
- -
Emergency disconnection of all technical devices and devices to ensure safety in case of emergency situations.
3.3. Installation of Test Bench for Testing of Borehole Extraction Processes
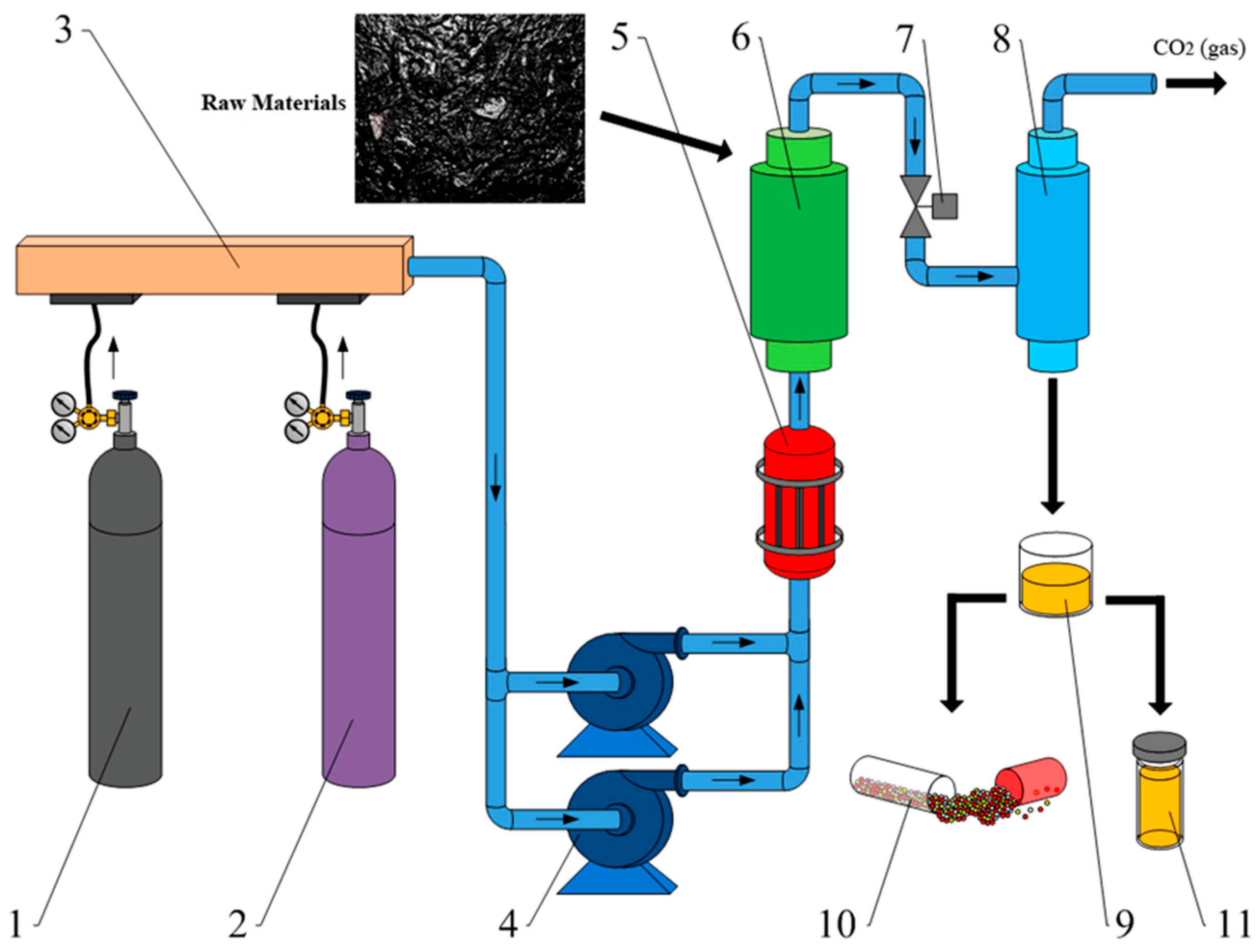
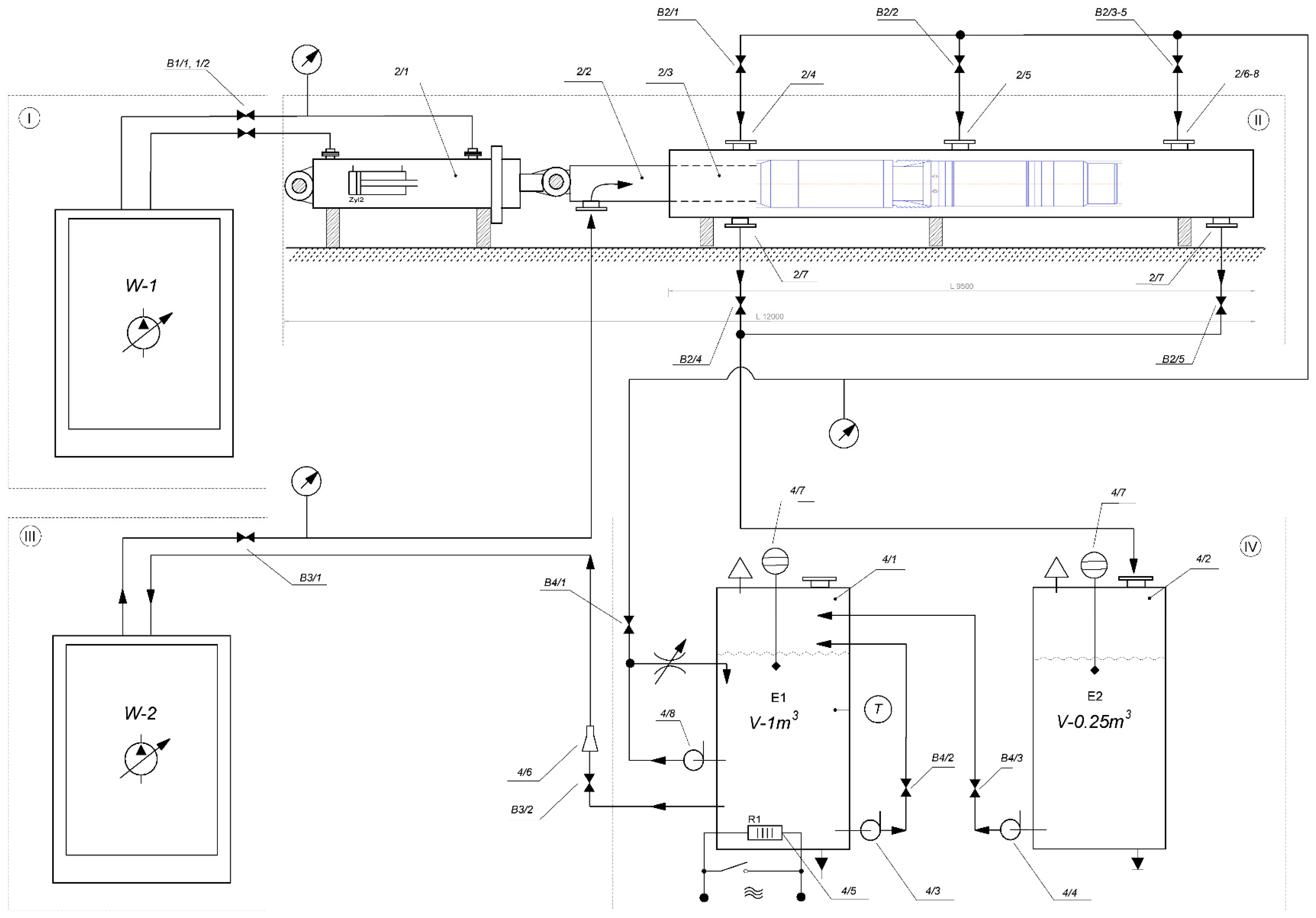
The stand (
Figure 8) consists of four units: I is hydraulic station of the hydraulic cylinder; II is well simulator; III is high-pressure hydraulic station; and IV is tank farm.
Pump station W-1 feeds a two-way hydraulic cylinder 2/1. Hydraulic cylinder 2/1 is mechanically connected to tubing 2/2 in casing 2/3.
The high-pressure station W-2 provides an injection of process fluid into tubing 2/2. Tubing string 2/2 has windows (through holes) that open in casing string 2/3 when hydraulic cylinder 2/1 moves together with pipe 2/2 to the right. The piston is installed on tubing 2/2 (not shown conventionally).
The bench is equipped with a 1 m3 4/1 flow tank and a 0.25 m3 4/2 drain tank. Pumping of liquid from drain tank 4/2 to flow tank 4/1 is performed by drain pump 4/4. Tank 4/1 is equipped with a system of automatic heating of the process fluid 4/5 and a circulation pump 4/3 for medium temperature averaging. The centrifugal pump-compressor used 4/8 to pump liquid or gas into casing 2/3.
All hydraulic systems are equipped with valves (positions “B” in the diagram of
Figure 8). Pressure in hydraulic systems is controlled by pressure gauges. The volume of liquid flow is controlled by an ultrasonic flowmeter 4/6. Tanks 4/1 and 4/2 are equipped with level indication devices 4/7. Test bench monitoring devices and controls are reduced to the operator panel.
Grades and models of the main equipment used and equipment of the bench are summarized in
Table 4.
Controlled bench parameters:
- -
Pressure levels of hydraulic supply systems of the hydraulic cylinder and high-pressure station of process fluid supply;
- -
Operating medium temperature in the service tank;
- -
Liquid levels in the flow and drain tanks;
- -
Critical level of liquid in the service tank;
- -
Ambient temperature of the bench location at three measurement points;
- -
Process fluid flow volume by ultrasonic flowmeter;
- -
Availability of power supply on electric motors of pumps;
- -
Control of the linear stroke of the hydraulic cylinder rod.
The dispatching and monitoring of system operation parameters are organized on the operator console.
3.4. Operation of the Stand
Process sequence on the bench:
- (1)
Feed to tubing 2/2;
- (2)
Supply of solvent (supercritical CO2) prepared in advance in consumption tank 4/1 to casing 2/3;
- (3)
Mixing of raw material and solvent by the movement of hydraulic cylinder 2/1 with tubing 2/2 to the right. At the same time, pressure increases due to the piston installed on the tubing;
- (4)
Maintenance and production of extract;
- (5)
Opening of valve B2/4 and overflow of extract into drain tank 4/2.
Heavy oil extraction efficiency was analyzed by assessing the viscosity of the residual oil. The residual oil is the oil immobilized after the extraction process. CO2 dissolution was carried out at a pressure of 10, 20, and 30 MPa. Residual oil sample viscosity was measured at 30 °C using a Brookfield rotary viscometer CAP-1000+.
To produce biofuels, the obtained extract is treated by thermal methods. In our case, the process of low-temperature destruction was used.
3.5. Processing of the Hydrocarbon Component of the Obtained Extract
The process of low-temperature destruction (distillation) was carried out in reaction equipment at a temperature of 550 ± 20 °C and atmospheric pressure. The process of destruction itself was divided into two stages:
The organic part of the extract is distilled to produce hydrocarbon gases and the raw material is held in the reaction zone for 10, 20, and 30 min.
The production of hydrogen by the reaction of light hydrocarbons with a catalyst. The duration of the process was determined by reducing the amount of hydrogen produced. The process was carried out in the temperature range of 550 to 650 °C.
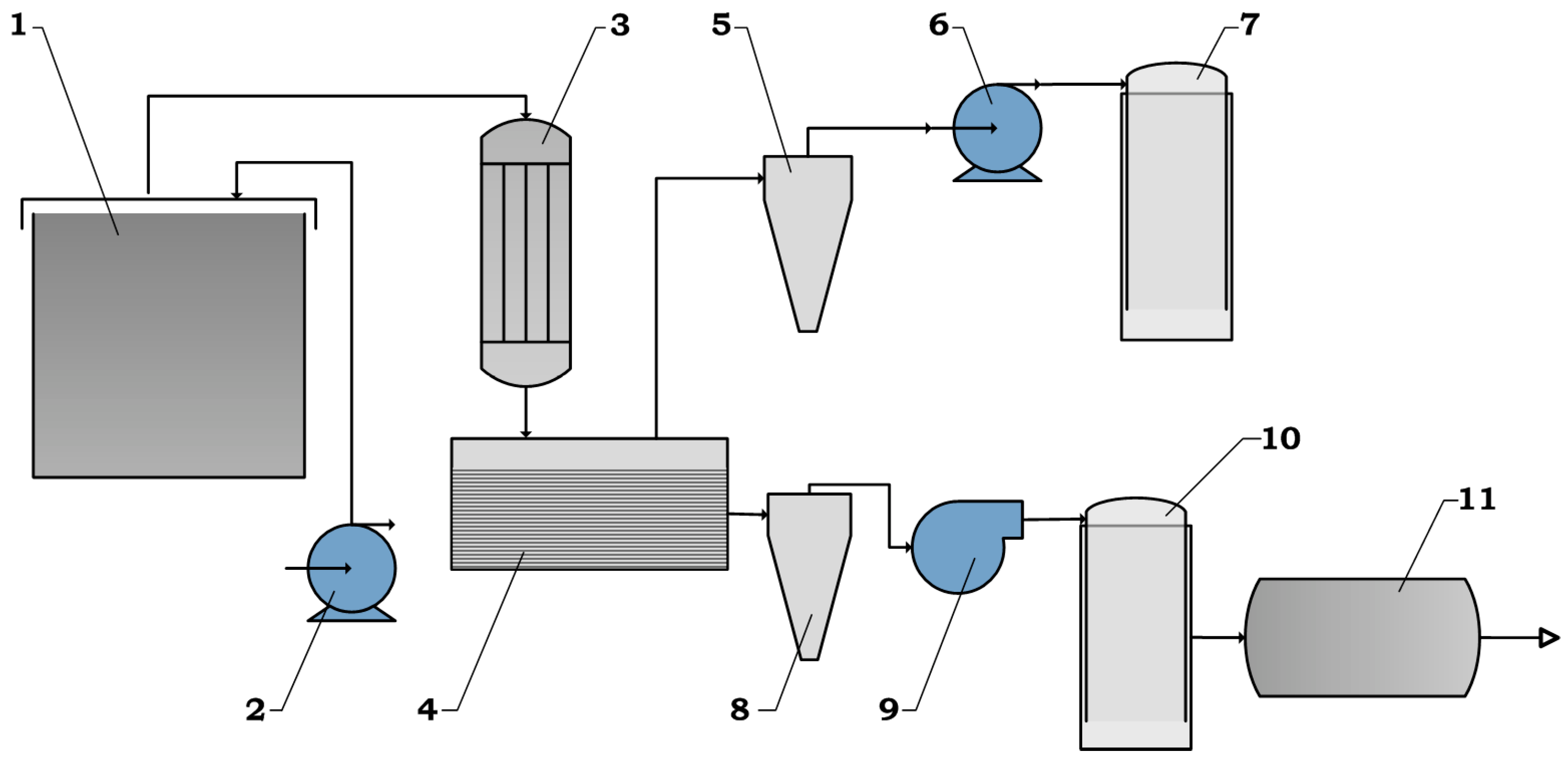
The experimental bench for processing the hydrocarbon component of the obtained extract is shown in
Figure 9.
The stand works as follows:
The obtained extract is fed to pyrolysis reactor 1 by means of pump 2. The heaters provide the necessary amount of heat to start the destruction process. Thermocouples monitor the temperature parameters. The heat-insulating material retains heat in the reactor, thereby reducing energy costs to maintain the pyrolysis process.
The released reaction products are fed to heat exchanger 3 to separate the liquid and gaseous pyrolysis products. Then, they enter settling tank 4, where, by means of pump 6, liquid products pass through the hydrocyclone 5 and enter receiver 7 for temporary storage.
In turn, the gaseous products, by means of the high-pressure fan 9, pass through cyclone 8 and enter the sampler 10.
In order to obtain hydrogen, the remainder is sent to reactor 11 for processing.
The “Clarus 600” chromatograph was used to measure the concentration of gaseous hydrocarbons in the gases generated by the distillation of hydrocarbons produced by the extraction of heavy petroleum components formed in the first stage. The chromatographic condition is shown in
Table 5.
The catalytic pyrolysis method was used to decompose the gasified oil pyrolysis product, methane. Proven effective in previous hydrogen production, high-percentage nickel catalysts were used [
111]; they are characterized by a high product yield efficiency and low cost compared to catalysts containing noble metals.
The amount of hydrogen released was determined using the ”EMIS VIKHR 200” (EMIS, Russia) vortex flowmeter.
The lowest combustion heat of the resulting hydrocarbon gas mixture was determined using a flow gas calorimeter Rhadox 7300 (“AMS Analysen-, Mess- und Systemtechnik GmbH”, Dielheim, Germany).
4. Results and Discussion
The main result of the laboratory studies was confirmation that the developed biofuel production technology works under borehole conditions. In particular, a number of technological processes were successfully tested in the laboratory facilities developed, namely:
Supercritical heavy oil extraction technology adapted to downhole conditions;
Thermal destruction technology of carbonated oil including mode parameter development (reaction times) to determine optimum conditions for the process;
Catalytic pyrolysis technology for the thermal degradation product of carbonated oil and hydrocarbon gases, determining different catalysts’ effect on hydrogen yield.
Research results on these processes are explored consistently in this section.
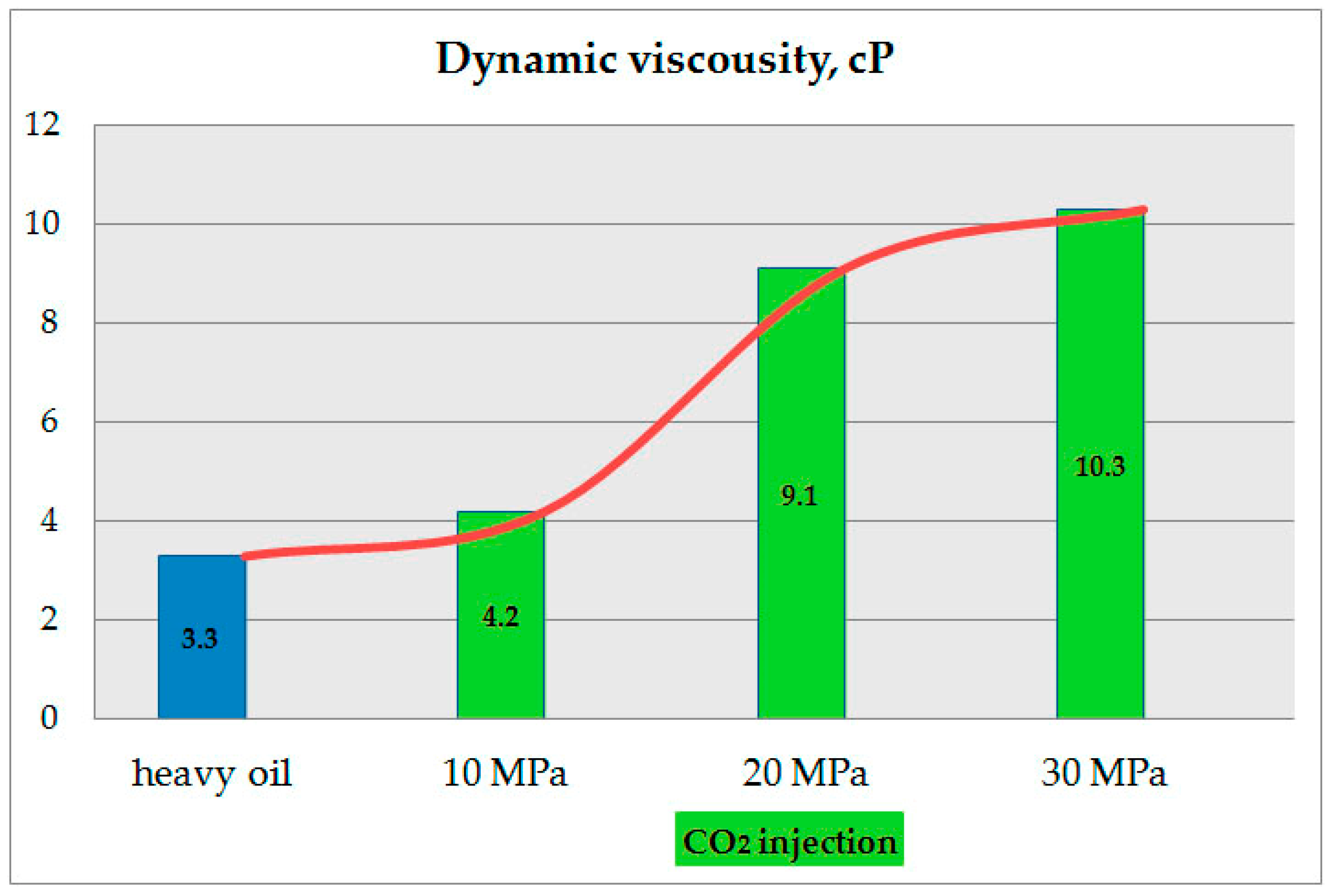
The results of the residual oil viscosity change after exposure to CO
2 at 10, 20, and 30 MPa compared to the viscosity of unexposed oil are shown in
Figure 10.
According to the results (
Figure 10), higher pressure increases the solubility of CO
2 in heavy oil. Significant results are obtained at a pressure of 20 MPa. However, data obtained at 30 MPa indicate that the trend in residual oil viscosity with increasing pressure is likely to have a rapid limit, i.e., further pressure increases are ineffective, requiring additional residual oil operations such as repeated extraction or additional heating. Dissolving CO
2 increases the proportion of light hydrocarbons, mobilizing them and overcoming heavy oil’s low mobility. CO
2 extraction thus contributes to raw materials’ manageability, in particular, their transportability. It should be noted that the maximum working pressure of most tubing types exceeds 25 MPa.
The results of the chromatographic analysis, in the first step, show that with an increase in the residence time of the feedstock, from 10 to 20 min in the reaction chamber, the total concentration of light hydrocarbons increases (
Table 6 and
Table 7).
A longer residence time of up to 30 min resulted in a decrease in the total concentration of the gaseous fraction (
Table 8).
This can be explained by the conversion of minor organic products to significant ones by chemical interaction at a given temperature (recorded by chromatographic analysis) as well as losses.
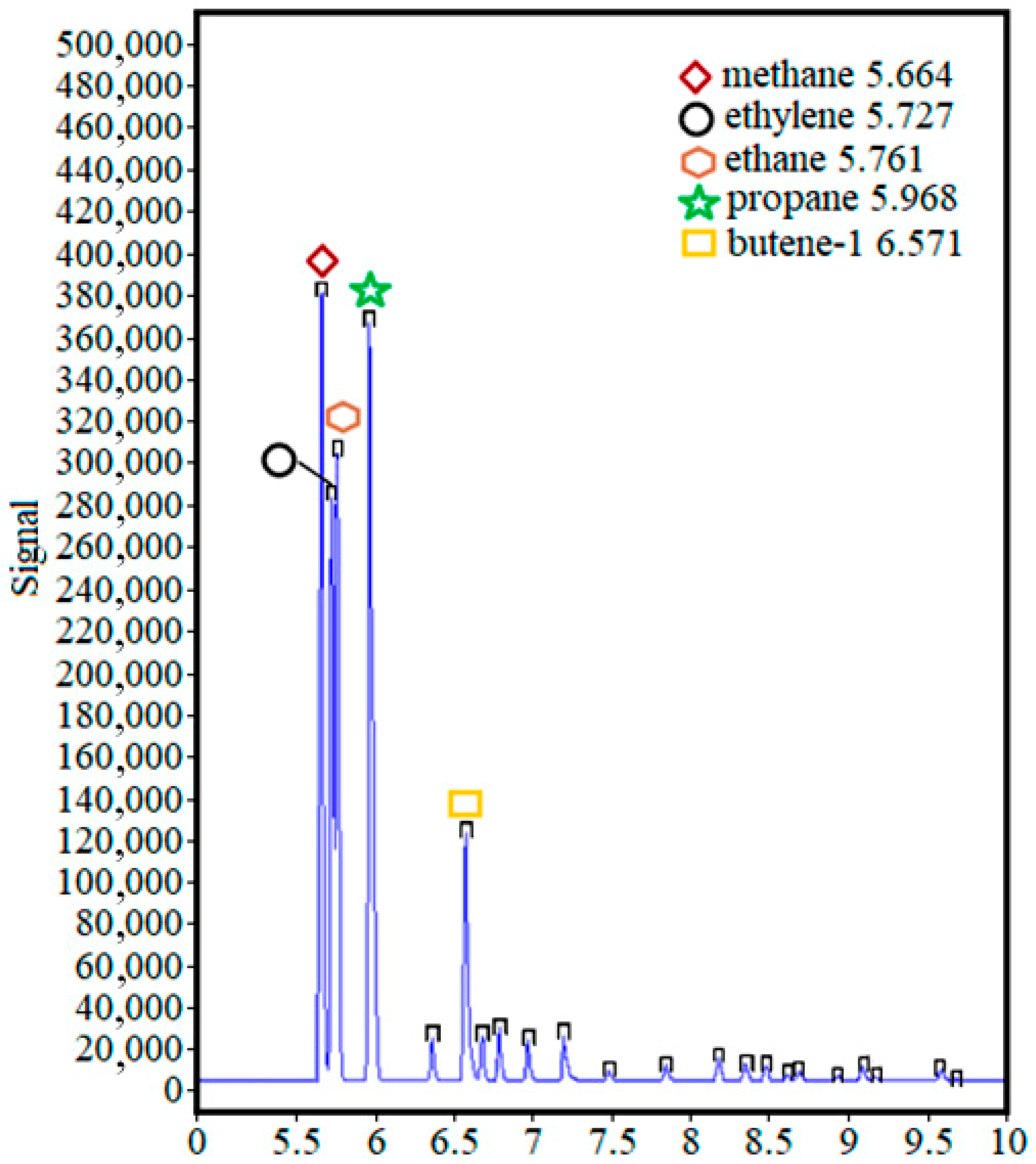
The main components of the gas fraction were methane (CH
4), ethylene (C
2H
4), ethane (C
2H
6), propane (C
3H
8), and butylene (C
4H
8). In the entire range of holding time, only their percentage changed (
Figure 11).
Thus, a suitable holding time was about 20 min (ranging from 20 to 30 min). At the same time, it should be understood that increased residence time leads to increased energy consumption, which can affect the efficiency of the process, thereby spending more energy than is obtained. At the same time, the concentration of the remaining hydrocarbons was below 0.5%, or traces were observed.
In addition to the formation of gaseous products, liquid hydrocarbons and a solid residue are also formed. The liquid fraction consisted of saturated, unsaturated, cyclic, and aromatic hydrocarbons. In turn, the solid fraction consisted of coke; the combustion of which produced ash. The present paper did not examine the liquid and solid fractions, but only determined their quantitative distribution. Therefore, the distribution of fractions during distillation was as follows: 60%—liquid, 20%—gaseous, and 20%—solid, with an error of ±2%.
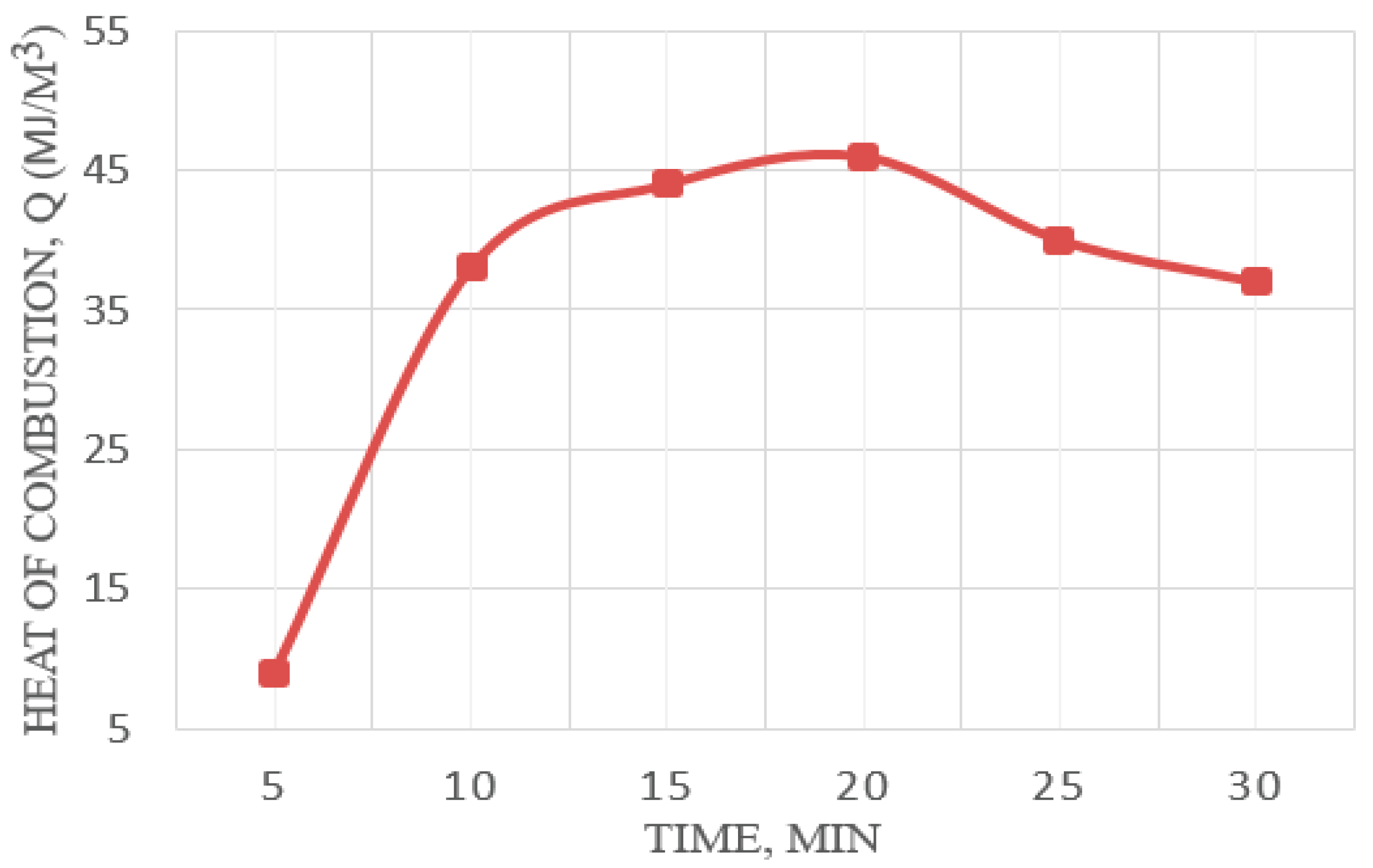
In addition to the quantitative characterization of the formed light hydrocarbons, a qualitative assessment of the gaseous pyrolysis products was carried out. For this purpose, the heat of combustion of the gas and the mixture was determined. The kinetics of combustion heat change for pyrolysis gaseous products at 550 °C is shown in
Figure 12.
As a result of the calorimetric analysis, it was found that the lowest combustion heat of the resulting gas mixture reaches its maximum value at around 20 min (
Figure 12). Further holding time leads to a gradual reduction in the combustion heat value, i.e., the calorific gas value is reduced. This phenomenon can be explained by a deeper breakdown of hydrocarbons and an increased yield of low-molecular-weight gaseous products. The combustion heat measurement error was ±1.52%.
As mentioned above, in the second step, light hydrocarbons are converted to hydrogen by reacting with the catalyst. To do this, the distillation products were discharged from the reaction apparatus, the catalyst was charged, and by running the gas fraction (methane) in the presence of a catalyst, a process of hydrogen formation took place and the carbon nanomaterials were formed.
The results and catalysts used are shown in
Table 9.
The obtained hydrogen yield of the test extract ranged from 44 to 118 L/h depending on the catalyst (
Table 9). As can be seen from the results, with an increase in the process temperature, there was a roughly twofold increase in the hydrogen yield. This is explained, as in the previous case, by deeper conversion of raw materials. Additionally, with an increase in the reaction temperature, an increase in the residual methane content of the reaction products was observed. Furthermore, the increase in temperature affects the effective operation time of the catalyst, leading to its faster deactivation. Further adsorption of methane can be carried out on carbon plates in order to separate the gas fraction.
According to
Table 9, the nickel-based catalyst on aluminum oxide had the lowest hydrogen yield. The low efficiency of the monometallic catalyst in gaseous hydrocarbon decomposition can be explained by its rapid deactivation. It can be due to the catalyst’s low active metal content, and the use of aluminum oxide as a carrier which, in its turn, has weak acidic properties and can itself take part in the chemical reaction leading to by-product formation. The nickel catalyst with silicon dioxide has the highest hydrogen production efficiency. It contains a larger amount of active metal and uses silicon dioxide as a carrier. Silicon dioxide, compared to aluminum oxide, has a higher specific surface area and is characterized by greater inertness towards the active metal deposited thereon.