Milk Fat Depression and Trans-11 to Trans-10 C18:1 Shift in Milk of Two Cattle Farming Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sampling and Chemical Composition of Diets
2.3. Milk Sampling and Chemical Analysis
2.4. Fatty Acids Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition of Milk
3.2. Fatty Acid Classes
3.3. Content of Principal Saturated Fatty Acids
3.4. Trans and Cis Isomers of Oleic Acid (C18:1), Linoleic Acid (C18:2) and Coniugated Linoleic Fatty Acids (CLA)
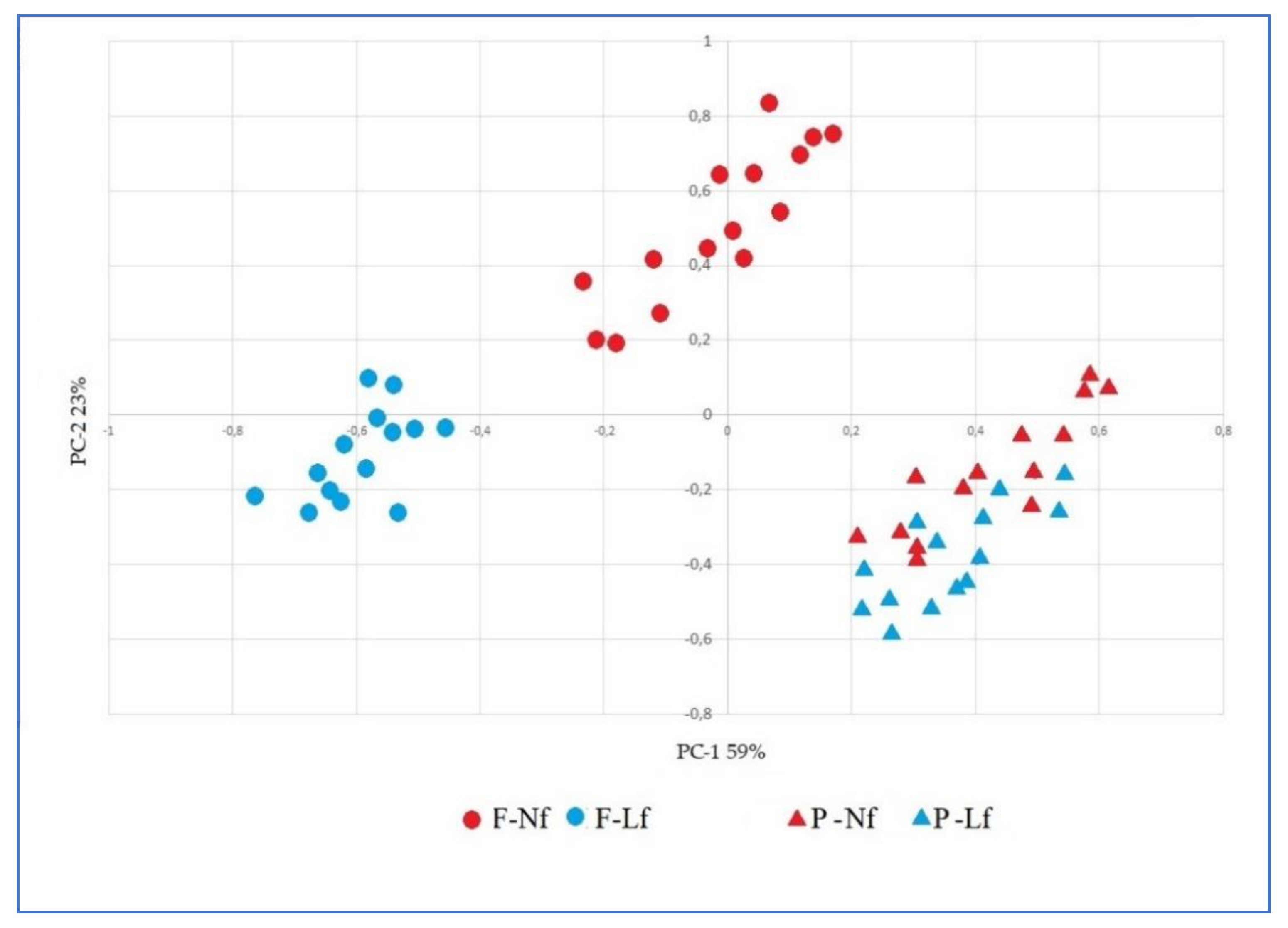
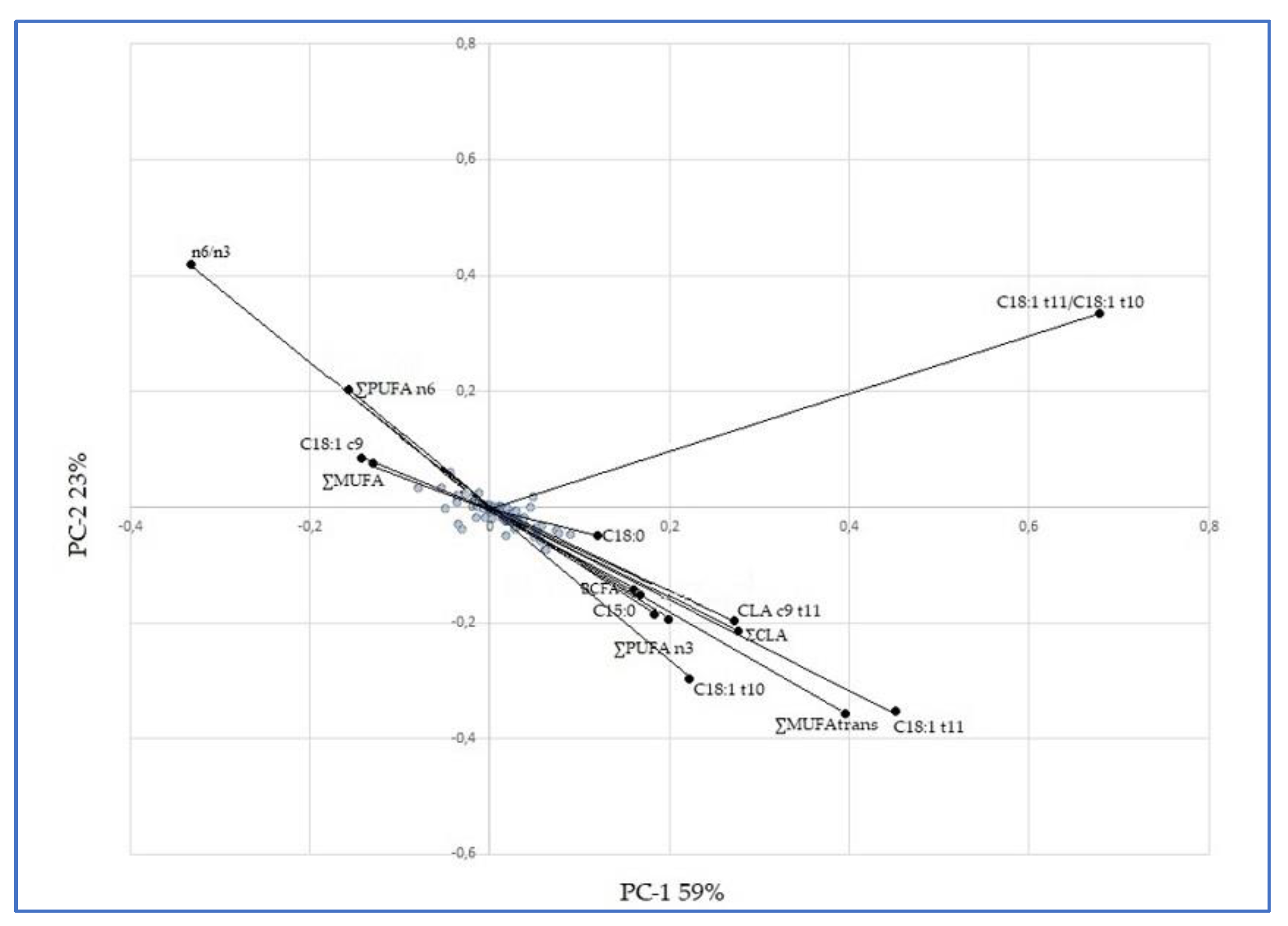
3.5. Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakry, I.A.; Yang, L.; Farag, M.A.; Korma, S.A.; Khalifa, I.; Cacciotti, I.; Ziedan, N.I.; Jin, J.; Jin, Q.; Wei, W.; et al. A comprehensive review of the composition, nutritional value, and functional properties of camel milk fat. Foods 2021, 10, 2158. [Google Scholar] [CrossRef] [PubMed]
- Månsson, L.H. Fatty acids in bovine milk fat. Food Nutr. Res. 2008, 52, 1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo-González, A.R.; Burrola-Barraza, M.E.; Domínguez-Viveros, J.; Chávez-Martínez, A. Rumen microorganisms and fermentation. Arch. Med. Vet. 2014, 46, 349–361. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Wang, D.; Wang, L.; Wei, X.; Li, X.; Cai, C.; Lei, X.; Yao, J. Physically effective neutral detergent fiber improves chewing activity, rumen fermentation, plasma metabolites, and milk production in lactating dairy cows fed a high-concentrate diet. J. Dairy Sci. 2021, 104, 5631–5642. [Google Scholar] [CrossRef]
- Peterson, D.G.; Matitashvili, E.A.; Bauman, D.E. Diet-induced milk fat depression in dairy cows results in increased trans-10,cis-12CLA in milk fat and coordinate suppression of mRNA abundance for mammary enzymes involved in milk fat synthesis. J. Nutr. 2003, 133, 3098–3102. [Google Scholar] [CrossRef] [Green Version]
- Weimer, P.J.; Stevenson, D.M.; Mertens, D.R. Shifts in bacterial community composition in the rumen of lactating dairy cows under milk fat-depressing conditions. J. Dairy Sci. 2010, 93, 265–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kairenius, P.; Ärölä, A.; Leskinen, H.; Toivonen, V.; Ahvenjärvi, S.; Vanhatalo, A.; Huhtanen, P.; Hurme, T.; Griinari, J.M.; Shingfield, K.J. Dietary fish oil supplements depress milk fat yield and alter milk fatty acid composition in lactating cows fed grass silage-based diets. J. Dairy Sci. 2015, 98, 5653–5671. [Google Scholar] [CrossRef] [PubMed]
- Dewanckele, L.; Toral, P.G.; Vlaeminck, B.; Fievez, V. Invited review: Role of rumen biohydrogenation intermediates and rumen microbes in diet-induced milk fat depression: An update. J. Dairy Sci. 2020, 103, 7655–7681. [Google Scholar] [CrossRef]
- Bauman, D.E.; Griinari, J.M. Regulation and nutritional manipulation of milk fat low-fat milk syndrome. Livest. Prod. Sci. 2001, 70, 15–29. [Google Scholar] [CrossRef]
- Lock, A.L.; Overton, T.R.; Harvatine, K.J.; Giesy, J.; Bauman, D.E. Milk fat depression: Impact of dietary components and their interaction during rumen fermentation. In Proceedings of the Cornell Nutrition Conference, Ithaca, NY, USA, 18–20 October 2006; pp. 75–85. [Google Scholar]
- Rivero, M.J.; Anrique, R. Milk fat depression syndrome and the particular case of grazing cows: A review. Acta Agric. Scand. Sect. A—Anim. Sci. 2015, 65, 42–54. [Google Scholar] [CrossRef]
- Chilliard, Y.; Ferlay, A. Dietary lipids and forages interactions on cow and goat milk fatty acid composition and sensory properties. Reprod. Nutr. Dev. 2004, 44, 467–492. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Corl, B.A.; Dwyer, D.A.; Saebø, A.; Bauman, D.E. Identification of the conjugated linoleic acid isomer that inhibits milk fat synthesis. Am. J. Physiol. 2000, 278, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Bauman, D.E.; Griinari, J.M. Nutritional regulation of milk fat synthesis. Annu. Rev. Nutr. 2003, 23, 203–227. [Google Scholar] [CrossRef] [Green Version]
- Conte, G.; Dimauro, C.; Serra, A.; Macciotta, N.P.P.; Mele, M. A canonical discriminant analysis to study the association between milk fatty acids of ruminal origin and milk fat depression in dairy cows. J. Dairy Sci. 2018, 101, 6497–6510. [Google Scholar] [CrossRef] [Green Version]
- De Andrade, P.V.D.; Schmidely, P. Effect of duodenal infusion of trans10,cis12-CLA on milk performance and milk fatty acid profile in dairy goats fed high or low concentrate diet in combination with rolled canola seed. Reprod. Nutr. Dev. 2006, 46, 31–48. [Google Scholar] [CrossRef] [Green Version]
- Murru, E.; Carta, G.; Manca, C.; Sogos, V.; Pistis, M.; Melis, M.; Banni, S. Conjugated Linoleic Acid and Brain Metabolism: A Possible Anti-Neuroinflammatory Role Mediated by PPARα Activation. Front. Pharmacol. 2021, 11, 587140. [Google Scholar] [CrossRef] [PubMed]
- Morales, R.; Ungerfeld, E.M. Use of tannins to improve fatty acids profile of meat and milk quality in ruminants: A review. Chil. J. Agric. Res. 2015, 75, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, G.F.; Delahoy, J.E.; Vidaurreta, I.; Bargo, F.; Gagliostro, G.A.; Muller, L.D. Milk fatty acid composition of cows fed a total mixed ration or pasture plus concentrates replacing corn with fat. J. Dairy Sci. 2003, 86, 3237–3248. [Google Scholar] [CrossRef]
- Infascelli, L.; Tudisco, R.; Iommelli, P.; Capitanio, F. Milk quality and animal welfare as a possible marketing lever for the economic development of rural areas in southern Italy. Animals 2021, 11, 1059. [Google Scholar] [CrossRef] [PubMed]
- Sturaro, E.; Marchiori, E.; Cocca, G.; Penasa, M.; Ramanzin, M.; Bittante, G. Dairy systems in mountainous areas: Farm animal biodiversity, milk production and destination, and land use. Livest. Sci. 2013, 158, 157–168. [Google Scholar] [CrossRef]
- Brito, L.F.; Bedere, N.; Douhard, F.; Oliveira, H.R.; Arnal, M.; Peñagaricano, F.; Schinckel, A.P.; Baes, C.F.; Miglior, F. Review: Genetic selection of high-yielding dairy cattle toward sustainable farming systems in a rapidly changing world. Animal 2021, 15, 100292. [Google Scholar] [CrossRef]
- Cifuni, G.F.; Napolitano, F.; Riviezzi, A.M.; Braghieri, A.; Girolami, A. Fatty acid profile, cholesterol content and tenderness of meat from Podolian young bulls. Meat Sci. 2004, 67, 289–297. [Google Scholar] [CrossRef]
- Cosentino, C.; D’Adamo, C.; Naturali, S.; Pecora, G.; Paolino, R.; Musto, M.; Adduci, F.; Freschi, P. Podolian cattle: Reproductive activity, milk and future prospects. Ital. J. Agron. 2018, 13, 200–207. [Google Scholar] [CrossRef]
- Quinto, M.; Sevi, A.; Di Caterina, R.; Albenzio, M.; Muscio, A.; Rotunno, T. Quality of milk and Caciocavallo cheese from farms rearing Podolica and Italian Friesian cows. Ital. J. Food Sci. 2003, 15, 485–498. [Google Scholar]
- Pistoia, A.; Casarosa, P.; Poli, P.; Mani, D.; Ferruzzi, G. Caratteristiche qualitative del latte e del formaggio Caciocavallo nella razza bovina Podolica. Quality aspects of milk and Caciocavallo cheese in Podolica cattle. Large Anim. Rev. 2015, 21, 63–66. [Google Scholar]
- Association of Official Analytical Chemist (AOAC). Official Methods of Analysis, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- ISO 9622, IDF 141; Milk and Liquid Milk Products—Guidelines for the Application of Mid-Infrared Spectrometry. International Organization for Standardization: Geneva, Switzerland; International Dairy Federation: Brussels, Belgium, 2013. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, S.G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Statistical Analysis System Institute (SAS). User’s Guide: Statistics; Version 9.3; SAS Institute Inc.: Cary, NC, USA, 2011. [Google Scholar]
- Loften, J.R.; Linn, J.G.; Drackley, J.K.; Jenkins, T.C.; Soderholm, C.G.; Kertz, A.F. Invited review: Palmitic and stearic acid metabolism in lactating dairy cows. J. Dairy Sci. 2014, 97, 4661–4674. [Google Scholar] [CrossRef] [PubMed]
- Piantoni, P.; Lock, A.L.; Allen, M.S. Milk production responses to dietary stearic acid vary by production level in dairy cattle. J. Dairy Sci. 2015, 98, 1938–1949. [Google Scholar] [CrossRef]
- Toral, P.G.; Belenguer, A.; Shingfield, K.J.; Hervás, G.; Toivonen, V.; Frutos, P. Fatty acid composition and bacterial community changes in the rumen fluid of lactating sheep fed sunflower oil plus incremental levels of marine algae. J. Dairy Sci. 2012, 95, 794–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toral, P.G.; Hervás, G.; Carreño, D.; Frutos, P. Does supplemental 18:0 alleviate fish oil-induced milk fat depression in dairy ewes? J. Dairy Sci. 2016, 99, 1133–1144. [Google Scholar] [CrossRef] [Green Version]
- Palmquist, D.L.; Jenkins, T.C. A 100-Year Review: Fat feeding of dairy cows. J. Dairy Sci. 2017, 100, 10061–10077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanh, L.P.; Suksombat, W. Milk yield, composition, and fatty acid profile in dairy cows fed a high-concentrate diet blended with oil mixtures rich in polyunsaturated fatty acids. Asian-Australas. J. Anim. Sci. 2015, 28, 796–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlaeminck, B.; Fievez, V.; Cabrita, A.R.J.; Fonseca, A.J.M.; Dewhurst, R.J. Factors affecting odd- and branched-chain fatty acids in milk: A review. Anim. Feed Sci. Technol. 2006, 131, 389–417. [Google Scholar] [CrossRef]
- Srednicka-Tober, D.; Barański, M.; Seal, C.J.; Sanderson, R.; Benbrook, C.; Steinshamn, H.; Gromadzka-Ostrowska, J.; Rembiałkowska, E.; Skwarło-Sońta, K.; Eyre, M.; et al. Higher PUFA and n-3 PUFA, conjugated linoleic acid, α-tocopherol and iron, but lower iodine and selenium concentrations in organic milk: A systematic literature review and meta- and redundancy analyses. Br. J. Nutr. 2016, 115, 1043–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markey, O.; Souroullas, K.; Fagan, C.C.; Kliem, K.E.; Vasilopoulou, D.; Jackson, K.G.; Humphries, D.J.; Grandison, A.S.; Givens, D.I.; Lovegrove, J.A.; et al. Consumer acceptance of dairy products with a saturated fatty acid–reduced, monounsaturated fatty acid–enriched content. J. Dairy Sci. 2017, 100, 7953–7966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlay, A.; Bernard, L.; Meynadier, A.; Malpuech-Brug Ere, C. Production of trans and conjugated fatty acids in dairy ruminants and their putative effects on human health: A review. Biochimie 2017, 141, 107–120. [Google Scholar] [CrossRef]
- Bernard, A.; Leroux, C.; Chilliard, Y. Expression and nutritional regulation of Stearoyl-CoA desaturase genes in the ruminant mammary gland: Relationship with milk fatty acid composition. In Stearoyl-CoA Desaturase Genes in Lipid Metabolism; Ntambi, J.M., Ed.; Springer Science Business Media: New York, NY, USA, 2013; pp. 161–193. ISBN 9781461479697. [Google Scholar]
- Frutos, P.; Toral, P.G.; Hervás, G. Individual variation of the extent of milk fat depression in dairy ewes fed fish oil: Milk fatty acid profile and mRNA abundance of candidate genes involved in mammary lipogenesis. J. Dairy Sci. 2017, 100, 9611–9622. [Google Scholar] [CrossRef] [Green Version]
- Shingfield, K.J.; Chilliard, Y.; Toivonen, V.; Kairenius, P.; Givens, D.I. Trans fatty acids and bioactive lipids in ruminant milk. Adv. Exp. Med. Biol. 2008, 606, 3–65. [Google Scholar] [CrossRef]
| Unifeed | Pasture | |
|---|---|---|
| Ingredient (% of total diet) | ||
| Triticale silage | 49.2 | - |
| Alfalfa hay, second cut | 12.8 | - |
| Poliphyte hay | 2.1 | - |
| Barley grain | 10.7 | - |
| Corn grain | 8.6 | - |
| Sorghum grain | 6.4 | - |
| Soybean meal | 8.6 | - |
| Metho-Fat 1 | 0.75 | - |
| Mineral vitamin supplement 2 | 0.85 | - |
| Leguminosae | - | 44.0 |
| Gramineae | - | 34.3 |
| Asteraceae | - | 10.0 |
| Other plants | - | 11.7 |
| Chemical composition | ||
| Dry matter % | 59.43 | 44.53 |
| Ash (g/kg) | 61.6 | 58.5 |
| Ether extract (g/kg) | 35.0 | 26.0 |
| Crude protein (g/kg) | 180.2 | 150.4 |
| Non-structural carbohydrate (NSC) (g/kg) | 460.0 | 187.1 |
| ADF 3 (g/kg) | 171.0 | 307.0 |
| NDF 3 (g/kg) | 264.0 | 578.0 |
| Fatty acids (% of total FAME 4) | ||
| C14:0 | 0.78 | 1.88 |
| C16:0 | 16.90 | 18.50 |
| C18:0 | 2.89 | 3.30 |
| Total SFA 4 | 22.08 | 27.09 |
| C18:1 n-9 | 18.13 | 8.97 |
| Total MUFA 4 | 19.53 | 10.45 |
| C18:2 n-6 | 47.56 | 17.23 |
| C18:3 n-6 | 1.90 | 11.06 |
| C18:3 n-3 | 7.86 | 31.69 |
| Total PUFA 4 | 57.32 | 59.98 |
| Holstein Friesian | Podolica | Root MSE | p-Values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lf | Nf | Sign. | Lf | Nf | Sign. | Breed | Fat Level | Breed × Fat Level | ||
| Fat % | 2.86 | 4.22 | *** | 2.97 | 4.63 | *** | 0.41 | 0.042 | 0.001 | 0.075 |
| Protein % | 2.89 | 3.37 | *** | 3.33 | 3.44 | ns | 0.23 | 0.001 | 0.001 | 0.008 |
| Lactose % | 4.59 | 4.77 | ns | 5.01 | 4.89 | ns | 0.30 | 0.031 | 0.950 | 0.085 |
| F/P 1 | 1.03 | 1.27 | *** | 0.90 | 1.35 | *** | 0.13 | 0.393 | 0.001 | 0.004 |
| Fatty Acids | Holstein Friesian | Podolica | Root MSE | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lf | Nf | Sign | Lf | Nf | Sign | Breed | Fat Level | Breed × Fat Level | ||
| SCFA | 8.94 | 10.02 | ns | 11.66 | 10.43 | ns | 1.14 | 0.001 | 0.980 | 0.032 |
| BCFA | 1.73 | 1.81 | ns | 3.11 | 3.12 | ns | 0.30 | 0.001 | 0.537 | 0.279 |
| OCFA | 2.14 | 2.06 | ns | 2.59 | 2.76 | ns | 0.27 | 0.002 | 0.547 | 0.260 |
| ∑SFA | 63.96 | 66.67 | * | 65.66 | 65.32 | ns | 3.16 | 0.474 | 0.378 | 0.021 |
| cis MUFA | 27.21 | 24.27 | *** | 18.82 | 18.92 | ns | 2.24 | 0.001 | 0.112 | 0.004 |
| trans MUFA | 1.55 | 1.55 | ns | 5.81 | 5.96 | ns | 0.73 | 0.001 | 0.712 | 0.743 |
| ∑MUFA | 29.60 | 26.49 | ** | 25.35 | 26.60 | ns | 2.49 | 0.004 | 0.150 | 0.001 |
| n-6 PUFA | 3.14 | 3.55 | ns | 1.80 | 1.75 | ns | 0.66 | 0.001 | 0.299 | 0.191 |
| n-3 PUFA | 0.62 | 0.58 | ns | 1.70 | 1.68 | ns | 0.17 | 0.001 | 0.474 | 0.783 |
| n6/n3 | 6.31 | 7.46 | ns | 2.02 | 2.08 | ns | 2.38 | 0.001 | 0.157 | 0.272 |
| ∑PUFA | 4.53 | 4.91 | ns | 5.15 | 5.19 | ns | 0.74 | 0.027 | 0.273 | 0.257 |
| Fatty Acids | Holstein Friesian | Podolica | Root MSE | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lf | Nf | Sign. | Lf | Nf | Sign. | Breed | Fat Level | Breed × Fat Level | ||
| C4:0 | 1.54 | 1.62 | ns | 2.20 | 2.14 | ns | 0.50 | 0.001 | 0.970 | 0.649 |
| C6:0 | 1.48 | 1.54 | ns | 1.70 | 1.64 | ns | 0.25 | 0.007 | 0.961 | 0.386 |
| C8:0 | 0.98 | 1.08 | ns | 1.22 | 1.11 | ns | 0.19 | 0.003 | 0.905 | 0.060 |
| C10:0 | 2.23 | 2.67 | * | 3.02 | 2.54 | ** | 0.52 | 0.015 | 0.925 | 0.003 |
| C12:0 | 2.67 | 3.11 | * | 3.52 | 3.00 | * | 0.67 | 0.058 | 0.973 | 0.012 |
| C14:0 iso | 0.13 | 0.15 | ns | 0.19 | 0.21 | ns | 0.03 | 0.001 | 0.019 | 0.825 |
| C14:0 | 9.88 | 10.20 | ns | 11.49 | 10.04 | ns | 1.53 | 0.181 | 0.312 | 0.054 |
| C15:0 iso | 0.23 | 0.28 | ns | 0.44 | 0.52 | ** | 0.08 | 0.001 | 0.005 | 0.352 |
| C15:0 anteiso | 0.43 | 0.50 | ns | 1.24 | 1.19 | ns | 0.22 | 0.001 | 0.899 | 0.338 |
| C15:0 | 1.03 | 1.00 | ns | 1.27 | 1.43 | * | 0.19 | 0.001 | 0.286 | 0.082 |
| C16:0 | 32.04 | 33.18 | ns | 26.01 | 24.45 | ns | 2.80 | 0.001 | 0.986 | 0.035 |
| C17:0 iso | 0.37 | 0.35 | ns | 0.60 | 0.59 | ns | 0.11 | 0.001 | 0.759 | 0.907 |
| C17:0 anteiso | 0.37 | 0.32 | ns | 0.34 | 0.32 | ns | 0.17 | 0.810 | 0.457 | 0.675 |
| C17:0 | 0.64 | 0.62 | ns | 0.76 | 0.80 | ns | 0.11 | 0.001 | 0.849 | 0.139 |
| C18:0 | 9.26 | 8.71 | ns | 11.20 | 13.87 | *** | 1.95 | 0.001 | 0.051 | 0.004 |
| C20:0 | 0.20 | 0.19 | ns | 0.26 | 0.32 | * | 0.06 | 0.001 | 0.195 | 0.003 |
| Holstein Friesian | Podolica | Root MSE | p-Values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lf | Nf | Sign. | Lf | Nf | Sign. | Breed | Fat Level | Breed × Fat Level | ||
| C18:1 cis-9 | 23.15 | 20.18 | *** | 15.39 | 16.33 | ns | 2.11 | 0.001 | 0.067 | 0.001 |
| C18:1 cis-11 | 0.69 | 0.57 | * | 0.53 | 0.58 | ns | 0.11 | 0.021 | 0.243 | 0.001 |
| C18:1 cis-12 | 0.24 | 0.21 | ns | 0.12 | 0.14 | ns | 0.05 | 0.001 | 0.418 | 0.265 |
| C18:1 trans-9 | 0.26 | 0.22 | ns | 0.41 | 0.42 | ns | 0.13 | 0.001 | 0.747 | 0.368 |
| C18:1 trans-10 | 0.50 | 0.32 | ** | 1.65 | 1.62 | ns | 0.16 | 0.001 | 0.512 | 0.182 |
| C18:1 trans-11 | 0.33 | 0.68 | ** | 3.31 | 3.47 | ns | 0.31 | 0.001 | 0.289 | 0.453 |
| C18:1 trans-12 | 0.17 | 0.22 | ns | 0.30 | 0.24 | ns | 0.09 | 0.008 | 0.734 | 0.024 |
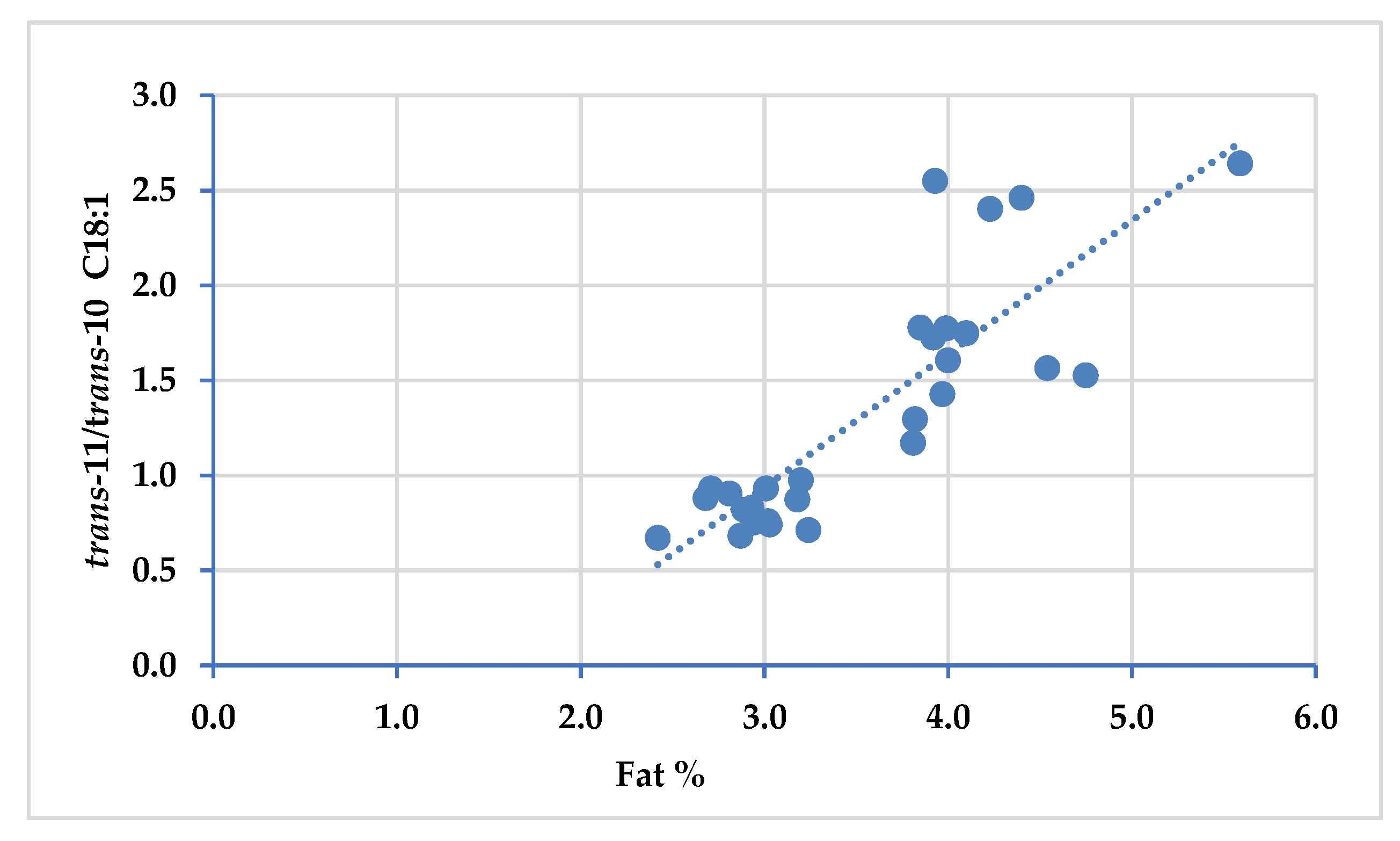
| C18:1 trans-11/trans-10 | 0.82 | 1.83 | *** | 2.01 | 2.14 | ns | 0.37 | 0.001 | 0.001 | 0.001 |
| ΣC18:2 trans trans | 0.08 | 0.10 | ns | 0.18 | 0.21 | ns | 0.05 | 0.001 | 0.192 | 0.798 |
| ΣC18:2 cis trans | 0.21 | 0.19 | ns | 0.35 | 0.36 | ns | 0.08 | 0.001 | 0.712 | 0.649 |
| ΣC18:2 trans cis | 0.22 | 0.20 | ns | 0.55 | 0.60 | ns | 0.15 | 0.001 | 0.341 | 0.218 |
| C18:2 cis-9 cis-12 | 2.67 | 2.97 | ns | 1.40 | 1.34 | ns | 0.59 | 0.001 | 0.437 | 0.259 |
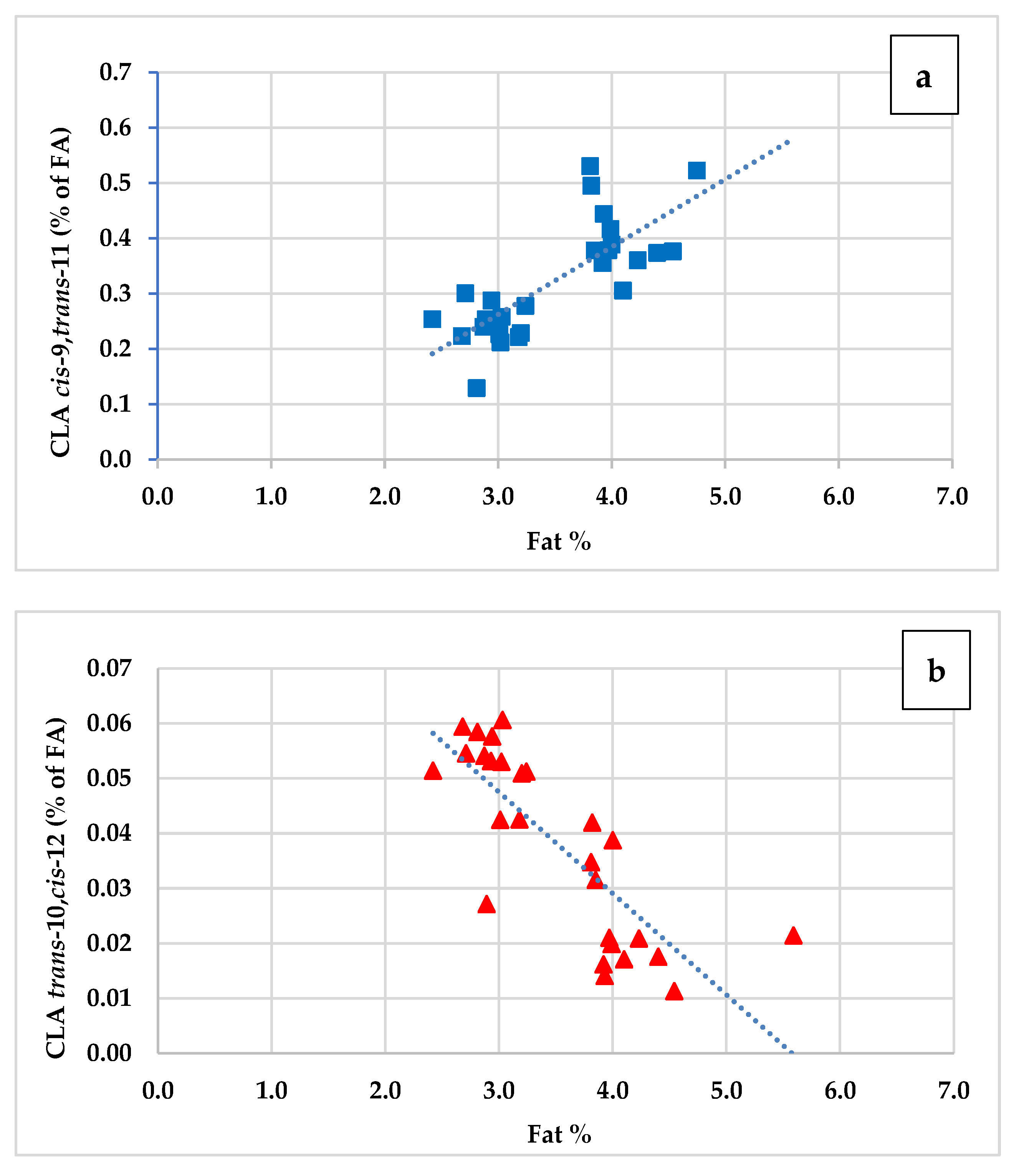
| CLA cis-9 trans-11 | 0.24 | 0.40 | ** | 1.40 | 1.48 | ns | 0.10 | 0.001 | 0.164 | 0.171 |
| CLA trans-7 cis-9 | 0.02 | 0.02 | ns | 0.01 | 0.01 | ns | 0.01 | 0.001 | 0.330 | 0.992 |
| CLA trans-8 cis-10 | 0.02 | 0.02 | ns | 0.02 | 0.02 | ns | 0.01 | 0.093 | 0.449 | 0.708 |
| CLA trans-10 cis-12 | 0.05 | 0.03 | ** | 0.07 | 0.06 | ns | 0.02 | 0.001 | 0.179 | 0.091 |
| CLA trans-11 cis-13 | 0.02 | 0.02 | ns | 0.05 | 0.05 | ns | 0.01 | 0.001 | 0.163 | 0.545 |
| ΣCLA cis cis | 0.03 | 0.03 | ns | 0.12 | 0.12 | ns | 0.03 | 0.001 | 0.871 | 0.520 |
| ΣCLA trans trans | 0.05 | 0.05 | ns | 0.11 | 0.09 | ns | 0.05 | 0.001 | 0.876 | 0.051 |
| ΣCLA | 0.46 | 0.60 | * | 1.89 | 1.94 | ns | 0.14 | 0.001 | 0.270 | 0.163 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinaldi, S.; Contò, M.; Claps, S.; Marchitelli, C.; Renzi, G.; Crisà, A.; Failla, S. Milk Fat Depression and Trans-11 to Trans-10 C18:1 Shift in Milk of Two Cattle Farming Systems. Sustainability 2022, 14, 977. https://doi.org/10.3390/su14020977
Rinaldi S, Contò M, Claps S, Marchitelli C, Renzi G, Crisà A, Failla S. Milk Fat Depression and Trans-11 to Trans-10 C18:1 Shift in Milk of Two Cattle Farming Systems. Sustainability. 2022; 14(2):977. https://doi.org/10.3390/su14020977
Chicago/Turabian StyleRinaldi, Simona, Michela Contò, Salvatore Claps, Cinzia Marchitelli, Gianluca Renzi, Alessandra Crisà, and Sebastiana Failla. 2022. "Milk Fat Depression and Trans-11 to Trans-10 C18:1 Shift in Milk of Two Cattle Farming Systems" Sustainability 14, no. 2: 977. https://doi.org/10.3390/su14020977
APA StyleRinaldi, S., Contò, M., Claps, S., Marchitelli, C., Renzi, G., Crisà, A., & Failla, S. (2022). Milk Fat Depression and Trans-11 to Trans-10 C18:1 Shift in Milk of Two Cattle Farming Systems. Sustainability, 14(2), 977. https://doi.org/10.3390/su14020977








