Germination Performances of 14 Wildflowers Screened for Shaping Urban Landscapes in Mountain Areas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Collection
2.2. Thousand Seed Weight (TSW)
2.3. Germination Test
2.4. Tetrazolium Assay
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Environment Agency (EEA). The European Environment-State and Outlook 2010: Synthesis; EEA: Copenhagen, Denmark, 2010; ISBN 978-92-9213-114-2.
- Bretzel, F.; Vannucchi, F.; Romano, D.; Malorgio, F.; Benvenuti, S.; Pezzarossa, B. Wildflowers: From conserving biodiversity to urban greening—A review. Urban For. Urban Green. 2016, 20, 428–436. [Google Scholar] [CrossRef]
- Toscano, S.; Romano, D.; Tribulato, A.; Cavallaro, V. Assessing and modeling seed germination of Mediterranean wildflowers for low input landscape restoration. Restor. Ecol. 2018, 26, 525–536. [Google Scholar] [CrossRef]
- Scarici, E.; Ruggeri, R.; Provenzano, M.E.; Rossini, F. Germination and performance of seven native wildflowers in the Mediterranean landscape plantings. Ital. J. Agron. 2017, 11, 163–171. [Google Scholar] [CrossRef]
- Piotto, D.; Craven, D.; Montagnini, F.; Alice, F. Silvicultural and economic aspects of pure and mixed native tree species plantations on degraded pasturelands in humid Costa Rica. New For. 2010, 39, 369–385. [Google Scholar] [CrossRef]
- Benvenuti, S. Wildflower green roofs for urban landscaping, ecological sustainability and biodiversity. Landsc. Urban Plan. 2014, 124, 151–161. [Google Scholar] [CrossRef]
- Bretzel, F.; Malorgio, F.; Vannucchi, F.; Pezzarossa, B. Wildflowers: From biodiversity conservation to landscape planning. Ital. Hort. 2013, 20, 17–31. [Google Scholar]
- Haaland, C.; Bersier, L.-F. What can sown wildflower strips contribute to butterfly conservation? An example from a Swiss lowland agricultural landscape. J. Insect Conserv. 2010, 15, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Rhind, P.; Jones, R. A framework for the management of sand dune systems in Wales. J. Coast. Conserv. 2009, 13, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Benvenuti, S. Seed ecology of Mediterranean hind dune wildflowers. Ecol. Eng. 2016, 91, 282–293. [Google Scholar] [CrossRef]
- Giupponi, L.; Leoni, V. VegeT: An Easy Tool to Classify and Facilitate the Management of Seminatural Grasslands and Dynamically Connected Vegetation of the Alps. Land 2020, 9, 473. [Google Scholar] [CrossRef]
- Ruggeri, R.; Provenzano, M.; Rossini, F. Effect of mulch on initial coverage of four groundcover species for low input landscaping in a Mediterranean climate. Urban For. Urban Green. 2016, 19, 176–183. [Google Scholar] [CrossRef]
- Bretzel, F.; Pezzarossa, B.; Benvenuti, S.; Bravi, A.; Malorgio, F. Soil influence on the performance of 26 native herbaceous plants suitable for sustainable Mediterranean landscaping. Acta Oecol. 2009, 35, 657–663. [Google Scholar] [CrossRef]
- Bretzel, F.; Caudai, C.; Tassi, E.; Rosellini, I.; Scatena, M.; Pini, R. Culture and horticulture: Protecting soil quality in urban gardening. Sci. Total Environ. 2018, 644, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Schröder, R.; Glandorf, S.; Kiehl, K. Temporal revegetation of a demolition site—A contribution to urban restoration? J. Urban Ecol. 2018, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Ladouceur, E.; Jiménez-Alfaro, B.; Marin, M.; De Vitis, M.; Abbandonato, H.; Iannetta, P.P.; Bonomi, C.; Pritchard, H.W. Native Seed Supply and the Restoration Species Pool. Conserv. Lett. 2018, 11, e12381. [Google Scholar] [CrossRef]
- Hitchmough, J.; Woudstra, J. The ecology of exotic herbaceous perennials grown in managed, native grassy vegetation in urban landscapes. Landsc. Urban Plan. 1999, 45, 107–121. [Google Scholar] [CrossRef]
- Benvenuti, S. Weed dynamics in the Mediterranean urban ecosystem: Ecology, biodiversity and management. Weed Res. 2004, 44, 341–354. [Google Scholar] [CrossRef]
- Bretzel, F.; Malorgio, F.; Paoletti, L.; Pezzarossa, B. Response of sowed, flowering herbaceous communities suitable for anthropic Mediterranean areas under different mowing regimes. Landsc. Urban Plan. 2012, 107, 80–88. [Google Scholar] [CrossRef]
- Tudela-Isanta, M.; Fernández-Pascual, E.; Wijayasinghe, M.; Orsenigo, S.; Rossi, G.; Pritchard, H.W.; Mondoni, A. Habitat-related seed germination traits in alpine habitats. Ecol. Evol. 2017, 8, 150–161. [Google Scholar] [CrossRef] [Green Version]
- Groffman, P.M.; Cavender-Bares, J.; Bettez, N.D.; Grove, J.M.; Hall, S.J.; Heffernan, J.B.; Hobbie, S.E.; Larson, K.L.; Morse, J.L.; Neill, C.; et al. Ecological homogenization of urban USA. Front. Ecol. Environ. 2014, 12, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Grimm, N.B.; Foster, D.; Groffman, P.; Grove, J.M.; Hopkinson, C.S.; Nadelhoffer, K.J.; Pataki, D.E.; Peters, D.P. The changing landscape: Ecosystem responses to urbanization and pollution across climatic and societal gradients. Front. Ecol. Environ. 2008, 6, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Benvenuti, S.; Bacci, D. Initial agronomic performances of Mediterranean xerophytes in simulated dry green roofs. Urban Ecosyst. 2010, 13, 349–363. [Google Scholar] [CrossRef]
- Petropoulos, S.; Karkanis, A.; Martins, N.; Ferreira, I. Halophytic herbs of the Mediterranean basin: An alternative approach to health. Food Chem. Toxicol. 2018, 114, 155–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scariot, V.; Gaino, W.; Demasi, S.; Caser, M.; Ruffoni, B. Flowers for edible gardens: Combinations of species and colours for northwestern Italy. Acta Hortic. 2018, 1215, 363–368. [Google Scholar] [CrossRef]
- Ceccanti, C.; Landi, M.; Benvenuti, S.; Pardossi, A.; Guidi, L. Mediterranean Wild Edible Plants: Weeds or “New Functional Crops”? Molecules 2018, 23, 2299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demasi, S.; Falla, N.M.; Caser, M.; Scariot, V. Postharvest aptitude of Begonia semperflorens and Viola cornuta edible flowers. Adv. Hortic. Sci. 2020, 34, 13–20. [Google Scholar]
- Demasi, S.; Caser, M.; Donno, D.; Enri, S.R.; Lonati, M.; Scariot, V. Exploring wild edible flowers as a source of bioactive compounds: New perspectives in horticulture. Folia Hortic. 2021, 33, 27–48. [Google Scholar] [CrossRef]
- Falla, N.M.; Demasi, S.; Caser, M.; Scariot, V. Preliminary Observations on Viola calcarata as a Source of Bioactive Compounds: Antioxidant Activity and Phytochemical Profile of Two Alpine Subspecies. Agronomy 2021, 11, 2241. [Google Scholar] [CrossRef]
- Jørgensen, D. Ecological restoration in the Convention on Biological Diversity targets. Biodivers. Conserv. 2013, 22, 2977–2982. [Google Scholar] [CrossRef]
- Wu, G.-L.; Du, G.-Z.; Shi, Z.-H. Germination strategies of 20 alpine species with varying seed mass and light availability. Aust. J. Bot. 2013, 61, 404–411. [Google Scholar] [CrossRef]
- Colbach, N.; Chauvel, B.; Dürr, C.; Richard, G. Effect of environmental conditions on Alopecurus myosuroides germination. I. Effect of temperature and light. Weed Res. 2002, 42, 210–221. [Google Scholar] [CrossRef]
- Thanos, C.A.; Georghiou, K.; Skarou, F. Glaucium flavum Seed Germination—An Ecophysiological Approach. Ann. Bot. 1989, 63, 121–130. [Google Scholar] [CrossRef]
- International Rules for Seed Testing 2014; ISTA: Antalya, Turkey, 2013; ISSN 2310-3655; Available online: http://www.seedtest.org/seedhealthmethods (accessed on 19 January 2021).
- Wang, J.H.; Baskin, C.C.; Cui, X.L.; Du, G.Z. Effect of phylogeny, life history and habitat correlates on seed germination of 69 arid and semi-arid zone species from northwest China. Evol. Ecol. 2008, 23, 827–846. [Google Scholar] [CrossRef]
- Milberg, P.; Andersson, L.; Thompson, K. Large-seeded species are less dependent on light for germination than small-seeded ones. Seed Sci. Res. 2000, 10, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Bu, H.; Du, G.; Chen, X.; Xu, X.; Liu, K.; Wen, S. Community-wide germination strategies in an alpine meadow on the eastern Qinghai-Tibet plateau: Phylogenetic and life-history correlates. Plant Ecol. 2007, 195, 87–98. [Google Scholar] [CrossRef]
- Caser, M.; Dente, F.; Ghione, G.G.; Mansuino, A.; Giovannini, A.; Scariot, V. Shorteninng of selection time of Rosa hybrida by in vitro culture of isolated embryos and immature seeds. Prop. Ornam. Plants 2014, 14, 139–144. [Google Scholar]
- Kumar, B.; Verma, S.K.; Singh, H. Effect of temperature on seed germination parameters in Kalmegh (Andrographis paniculata Wall. ex Nees.). Ind. Crop. Prod. 2011, 34, 1241–1244. [Google Scholar] [CrossRef]
- Laboriau, L.G.; Valadares, M.E.B. On the germination of seeds of Calotropis procera (Ait.) Ait. f. AGRIS 1976, 48, 263–284. [Google Scholar]
- Wharton, M.J. The use of tetrazolium test for determining the viability of seeds of the genus Brassica. Proc. Int. Seed Test. Assoc. 1955, 20, 81–88. [Google Scholar]
- Wu, G.-L.; Du, G.-Z. Seed mass in Kobresia-dominated communities in alpine meadows at two different elevations. Isr. J. Ecol. Evol. 2009, 55, 31–40. [Google Scholar] [CrossRef]
- Luna, B.; Moreno, J.M. Light and nitrate effects on seed germination of Mediterranean plant species of several functional groups. Plant Ecol. 2008, 203, 123–135. [Google Scholar] [CrossRef]
- Honda, Y.; Katoh, K. Strict requirement of fluctuating temperatures as a reliable gap signal in Picris hieracioides var. japonica seed germination. Plant Ecol. 2007, 193, 147–156. [Google Scholar] [CrossRef]
- Silvertown, J.; Fenner, M. Seeds: The Ecology of Regeneration in Plant Communities. J. Ecol. 1993, 81, 384. [Google Scholar] [CrossRef]
- Bu, H.; Chen, X.; Xu, X.; Liu, K.; Jia, P.; Du, G. Seed mass and germination in an alpine meadow on the eastern Tsinghai–Tibet plateau. Plant Ecol. 2007, 191, 127–149. [Google Scholar] [CrossRef]
- Hoyle, G.L.; Steadman, K.; Good, R.B.; McIntosh, E.; Galea, L.M.E.; Nicotra, A.B. Seed germination strategies: An evolutionary trajectory independent of vegetative functional traits. Front. Plant Sci. 2015, 6, 731. [Google Scholar] [CrossRef] [Green Version]
- Lan, Q.; Yin, S.; He, H.; Tan, Y.; Liu, Q.; Xia, Y.; Wen, B.; Baskin, C.C.; Baskin, J.M. Seed dormancy-life form profile for 358 species from the Xishuangbanna seasonal tropical rainforest, Yunnan Province, China compared to world database. Sci. Rep. 2018, 8, 4674. [Google Scholar] [CrossRef] [Green Version]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: San Diego, CA, USA, 2014; 1600p. [Google Scholar]
- Bosco, R.; Caser, M.; Ghione, G.G.; Mansuino, A.; Giovannini, A.; Scariot, V. Dynamics of abscisic acid and indole-3-acetic acid during the early-middle stage of seed development in Rosa hybrida. Plant Growth Regul. 2014, 75, 265–270. [Google Scholar] [CrossRef]
- Gairola, S.; Shabana, H.A.; Mahmoud, T.; Santo, A. Seed Germination of Kickxia Acerbiana, a Rare Annual of The Arabian Desert. Seed Sci. Technol. 2019, 47, 53–58. [Google Scholar] [CrossRef]
- Jaganathan, G.K.; Liu, B. Role of seed sowing time and microclimate on germination and seedling establishment of Dodonaea viscosa (Sapindaceae) in a seasonal dry tropical environment—An insight into restoration efforts. Botany 2015, 93, 23–29. [Google Scholar] [CrossRef]
- Schwienbacher, E.; Navarro-Cano, J.A.; Neuner, G.; Erschbamer, B. Seed dormancy in alpine species. Flora Morphol. Distrib. Funct. Ecol. Plants 2011, 206, 845–856. [Google Scholar] [CrossRef] [Green Version]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Keller, M.; Kollmann, J. Effects of seed provenance on germination of herbs for agricultural compensation sites. Agric. Ecosyst. Environ. 1999, 72, 87–99. [Google Scholar] [CrossRef]
- Barekat, T.; Otroshy, M.; Samsam-Zadeh, B.; Sadrarhami, A.; Mokhtari, A. A novel approach for breaking seed dormancy and germination in Viola odorata (A medicinal plant). J. Novel Appl. Sci. 2013, 2, 513–516. [Google Scholar]
- Benech, R.L.; Giallorenzi, M.; Frank, J.; Rodrigvez, V. Termination of hull–imposed dormancy in developing barley grains is correlated with changes in embryonic ABA levels and sensitivity. Seed Sci. Res. 1998, 9, 39–47. [Google Scholar] [CrossRef]
- Mares, D.J. Quarterly reports on plant growth regulation and activities of the PGRSA 32nd Annual Conference. Plant Growth Regul. Soc. Am. 2005, 33, 78–89. [Google Scholar]
- Valverde, T.; Silvertown, J. Spatial Variation in the Seed Ecology of a Woodland Herb (Primula vulgaris) in Relation to Light Environment. Funct. Ecol. 1995, 9, 942. [Google Scholar] [CrossRef]
- Godefroid, S.; Van de Vyver, A.; Vanderborght, T. Germination capacity and viability of threatened species collections in seed banks. Biodivers. Conserv. 2009, 19, 1365–1383. [Google Scholar] [CrossRef]
- Moles, A.T.; Westoby, M. Seedling survival and seed size: A synthesis of the literature. J. Ecol. 2004, 92, 372–383. [Google Scholar] [CrossRef]
- Benvenuti, S.; Pardossi, A. Germination ecology of nutraceutical herbs for agronomic perspectives. Eur. J. Agron. 2016, 76, 118–129. [Google Scholar] [CrossRef]
- Letchamo, W.; Gosselin, A. Light, temperature and duration of storage govern the germination and emergence of Taraxacum officinale seed. J. Hortic. Sci. 1996, 71, 373–377. [Google Scholar] [CrossRef]
- Keller, M. The Importance of Seed Source in Programmes to Increase Species Diversity in Arable Systems. Doctoral Thesis, ETH Zürich, Zürich, Switzerland, 1999. [Google Scholar] [CrossRef]
- Bratcher, C.B.; Dole, J.M.; Cole, J.C. Stratification Improves Seed Germination of Five Native Wildflower Species. HortScience 1993, 28, 899–901. [Google Scholar] [CrossRef] [Green Version]
- Nagase, A.; Dunnett, N. Performance of geophytes on extensive green roofs in the United Kingdom. Urban For. Urban Green. 2013, 12, 509–521. [Google Scholar] [CrossRef]
- Fetouh, M.I. Edible Landscaping in Urban Horticulture. In Urban Horticulture; Nandwani, D., Ed.; Sustainable Development and Biodiversity; Springer: Cham, Switzerland, 2018; Volume 18. [Google Scholar] [CrossRef]

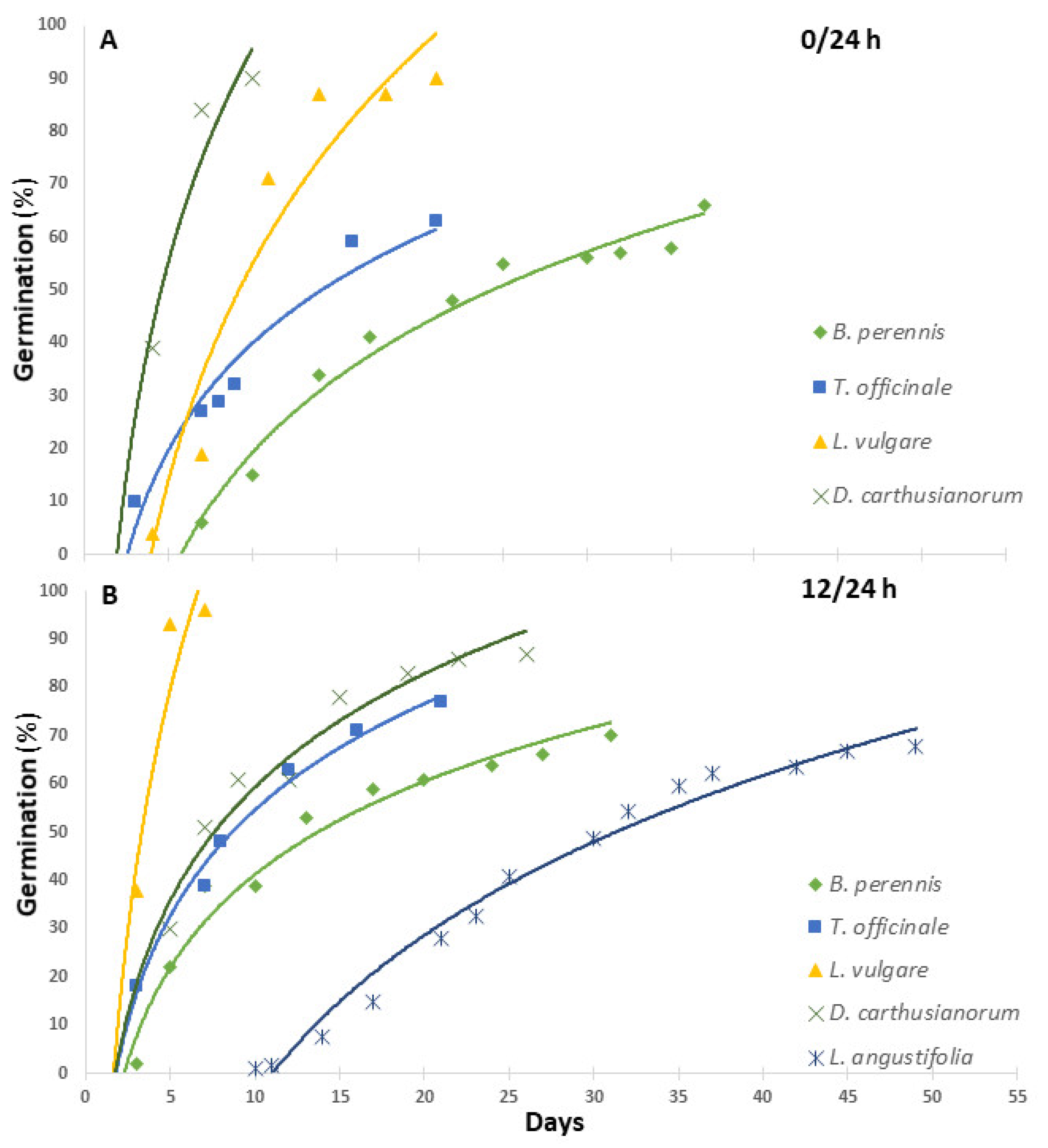
| Plant Species | Family | Site of Collection | Geographic References | Altitude (m a.s.l.) |
|---|---|---|---|---|
| Allium schoenoprasum L. | Amaryllidaceae | Castelmagno (CN) | Long 7.122 Lat 44.390 | 2293 |
| Bellis perennis L. | Asteraceae | Grugliasco (TO) | Long 7.592 Lat 45.065 | 285 |
| Centaurea cyanus L. | Asteraceae | Ivrea (TO) | Long 7.896 Lat 45.475 | 240 |
| Cichorium intybus L. | Asteraceae | Chivasso (TO) | Long 7.843 Lat 45.192 | 189 |
| Dianthus carthusianorum L. | Caryophyllaceae | Balme (TO) | Long 7.219 Lat 45.301 | 1435 |
| Dianthus pavonius Tauesch | Caryophyllaceae | Castelmagno (CN) | Long 7.122 Lat 44.391 | 2393 |
| Lavandula angustifolia Mill. | Lamiaceae | Grugliasco (TO) | Long 7.592 Lat 45.065 | 287 |
| Leucanthemum vulgare Lam. | Asteraceae | Grugliasco (TO) | Long 7.593 Lat 45.065 | 285 |
| Mentha aquatica L. | Lamiaceae | Caselette (TO) | Long 7.485 Lat 45.120 | 364 |
| Primula veris L. | Primulaceae | Cesana Torinese (TO) | Long 6.802 Lat 44.968 | 1395 |
| Primula vulgaris Hudson | Primulaceae | Cesana Torinese (TO) | Long 7.379 Lat 45.145 | 774 |
| Taraxacum officinale Weber | Asteraceae | Grugliasco (TO) | Long 7.593 Lat 45.064 | 285 |
| Trifolium alpinum L. | Fabaceae | Castelmagno (CN) | Long 7.122 Lat 44.390 | 2385 |
| Viola odorata L. | Violaceae | Grugliasco (TO) | Long 7.591 Lat 45.065 | 287 |
| Germination Index | Formula | Explanation | Reference |
|---|---|---|---|
| Final germination percentage (FGP) | FGP = 100 ∗ GN/SN | GN = total number of germinated seeds; SN = total number of seeds tested. | [35] |
| Relative light germination percentage (RLGP) | RLGP = Pl/(Pd + Pl) | RLGP is an expression of the light requirement for germination. Pl = percentage germination in light; Pd = percentage germination in shade. | [36] |
| First germination time (FGT) | Number of days from the beginning of the experiment to first germination. | [37] | |
| Half time of germination (T50) | Number of days from the beginning of the experiment to the count reached 50% of the final germination. | [38] | |
| Germination period (GPD) | Number of days from the beginning of the experiment to the maximum number of seeds germinated. | [39] | |
| Mean germination time (MGT) | MGT = Σ(NS * DAS)/GN | NS = number of germinated seeds; DAS = days after sowing; GN = total number of germinated seeds. Calculation is based on the daily count of normal seedling until the final date of the germination test. | [40] |
| Plant Species | TSW (g) | Seeds g−1 (n.) |
|---|---|---|
| Allium schoenoprasum | 0.968 ± 0.011 | 1033 |
| Bellis perennis | 0.615 ± 0.030 | 1626 |
| Centaurea cyanus | 3.440 ± 0.030 | 290 |
| Cichorium intybus | 0.117 ± 0.001 | 8547 |
| Dianthus carthusianorum | 0.086 ± 0.001 | 11,628 |
| Dianthus pavonius | 0.534 ± 0.042 | 1873 |
| Lavandula angustifolia | 0.096 ± 0.001 | 10,417 |
| Leucanthemum vulgare | 0.023 ± 0.001 | 43,478 |
| Mentha aquatica | 0.122 ± 0.001 | 8197 |
| Primula veris | 0.734 ± 0.023 | 1362 |
| Primula vulgaris | 0.966 ± 0.018 | 1035 |
| Taraxacum officinale | 0.061 ± 0.001 | 16,393 |
| Trifolium alpinum | 5.710 ± 0.202 | 175 |
| Viola odorata | 3.308 ± 0.078 | 302 |
| Species | FGP (%) | GP | RLGP (Classification) | |||
|---|---|---|---|---|---|---|
| 0/24 | 12/12 | p | 0/24 | 12/12 | ||
| Allium schoenoprasum | 0 f | 23.0 e | *** | Low | Moderate | 1.00 (LD) |
| Bellis perennis | 66.0 b | 70.0 bc | ns | Moderate | Moderate | 0.51 (LInt) |
| Centaurea cyanus | 0 f | 0 g | ns | Low | Low | - |
| Cichorium intybus | 41.0 c | 47.0 d | ns | Moderate | Moderate | 0.53 (LInt) |
| Dianthus carthusianorum | 90.0 a | 87.0 ab | ns | High | High | 0.49 (LInt) |
| Dianthus pavonius | 9.0 e | 8.0 f | ns | Low | Low | 0.47 (LInt) |
| Lavandula angustifolia | 26.3 d | 67.6 c | *** | Moderate | Moderate | 0.72 (LD) |
| Leucanthemum vulgare | 90.0 a | 96.0 a | ns | High | High | 0.52 (LInt) |
| Mentha aquatica | 0 f | 1.0 g | ns | Low | Low | - |
| Primula veris | 0 f | 0 g | ns | Low | Low | - |
| Primula vulgaris | 0 f | 0 g | ns | Low | Low | - |
| Taraxacum officinale | 63.0 b | 77.0 bc | * | Moderate | Moderate | 0.55 (LInt) |
| Trifolium alpinum | 15.0 de | 8.0 f | ns | Low | Low | 0.35 (LI) |
| Viola odorata | 0 f | 0 g | ns | Low | Low | - |
| p | *** | *** | ||||
| Plant Species | Seed Viability (%) |
|---|---|
| Centaurea cyanus | 96 |
| Dianthus pavonius | 95 |
| Mentha aquatica | 98 |
| Primula veris | 95 |
| Primula vulgaris | 97 |
| Trifolium alpinum | 94 |
| Viola odorata | 98 |
| Species | FGT (Days) | GPa | MGT (Days) | GRe | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0/24 | 12/12 | p | 0/24 | 12/12 | 0/24 | 12/12 | p | 0/24 | 12/12 | |
| Allium schoenoprasum | - | 17.8 a | - | S | S | - | 21.9 a | - | - | S |
| Bellis perennis | 10.0 b | 4.8 bc | *** | A | A | 18.5 b | 11.3 b | *** | M | M |
| Centaurea cyanus | - | - | - | - | - | - | - | - | - | - |
| Cichorium intybus | 4.0 d | 3.8 cd | ns | S | S | 5.8 d | 5.7 c | ns | F | F |
| Dianthus carthusianorum | 4.0 d | 6.4 b | * | S | S | 5.9 d | 9.2 b | * | F | F |
| Dianthus pavonius | 9.6 b | 22.5 a | * | S | A | 11.7 c | 25.7 a | ** | M | S |
| Lavandula angustifolia | 28.6 a | 16.4 a | *** | A | A | 35.2 a | 25.8 a | *** | S | S |
| Leucanthemum vulgare | 6.2 c | 3.0 d | *** | S | S | 10.8 c | 4.3 d | *** | M | F |
| Mentha aquatica | - | - | - | - | - | - | - | - | - | - |
| Primula veris | - | - | - | - | - | - | - | - | - | - |
| Primula vulgaris | - | - | - | - | - | - | - | - | - | - |
| Taraxacum officinale | 4.2 d | 3.0 d | ns | S | S | 11.3 c | 9.5 b | ns | M | F |
| Trifolium alpinum | 4.5 cd | 5.2 cd | ns | S | S | 6.3 d | 5.7 cd | ns | F | F |
| Viola odorata | - | - | - | - | - | - | - | - | - | - |
| p | *** | *** | *** | *** | ||||||
| Species | T50 (Days) | GPD (Days) | ||||
|---|---|---|---|---|---|---|
| 0/24 | 12/12 | p | 0/24 | 12/12 | p | |
| Allium schoenoprasum | - | 20.8 a | - | - | 26.6 b | - |
| Bellis perennis | 16.8 b | 7.4 bc | *** | 31.8 b | 23.1 bc | * |
| Centaurea cyanus | - | - | - | - | - | - |
| Cichorium intybus | 4.6 d | 4.7 d | ns | 9.2 def | 9.3 d | ns |
| Dianthus carthusianorum | 5.8 d | 7.8 bc | ns | 8.5 ef | 16.2 c | ** |
| Dianthus pavonius | 9.7 c | 24.0 a | ** | 13.8 de | 30.5 ab | ns |
| Lavandula angustifolia | 35.0 a | 25 a | *** | 39.8 a | 36.6 a | * |
| Leucanthemum vulgare | 11.0 c | 5.6 cd | *** | 15.1 d | 5.6 d | *** |
| Mentha aquatica | - | - | - | - | - | - |
| Primula veris | - | - | - | - | - | - |
| Primula vulgaris | - | - | - | - | - | - |
| Taraxacum officinale | 11.0 c | 9.1 b | ns | 17.5 c | 15.5 c | ns |
| Trifolium alpinum | 5.0 d | 5.2 cd | ns | 6.0 f | 6.4 d | * |
| Viola odorata | - | - | - | - | - | - |
| p | *** | *** | *** | *** | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caser, M.; Demasi, S.; Mozzanini, E.; Chiavazza, P.M.; Scariot, V. Germination Performances of 14 Wildflowers Screened for Shaping Urban Landscapes in Mountain Areas. Sustainability 2022, 14, 2641. https://doi.org/10.3390/su14052641
Caser M, Demasi S, Mozzanini E, Chiavazza PM, Scariot V. Germination Performances of 14 Wildflowers Screened for Shaping Urban Landscapes in Mountain Areas. Sustainability. 2022; 14(5):2641. https://doi.org/10.3390/su14052641
Chicago/Turabian StyleCaser, Matteo, Sonia Demasi, Eric Mozzanini, Paola Maria Chiavazza, and Valentina Scariot. 2022. "Germination Performances of 14 Wildflowers Screened for Shaping Urban Landscapes in Mountain Areas" Sustainability 14, no. 5: 2641. https://doi.org/10.3390/su14052641
APA StyleCaser, M., Demasi, S., Mozzanini, E., Chiavazza, P. M., & Scariot, V. (2022). Germination Performances of 14 Wildflowers Screened for Shaping Urban Landscapes in Mountain Areas. Sustainability, 14(5), 2641. https://doi.org/10.3390/su14052641









