Abstract
During the production process of commercial carbon fiber reinforced polymers (CFRPs), a silane coupling agent is added to the carbon fiber at the sizing step as a binder to enhance the product’s physical properties. While improving strength, the silane coupling agent results in a silane residue on recovered carbon fibers (rCF) after recycling, which is a disadvantage when using recovered carbon fibers in the manufacture of new materials. In this study, the rCF is recovered from waste carbon fiber reinforced polymers (CFRPs) from the bicycle industry by a microwave pyrolysis method, applying a short reaction time and in an air atmosphere. Moreover, the rCF are investigated for their surface morphologies and the elements present on the surface. The silicon element content changes with pyrolysis temperature were 0.4, 0.9, and 0.2%, respectively, at 450, 550, and 650 °C. Additionally, at 950 °C, silicon content can be reduced to 0.1 ± 0.05%. The uniformity of microwave pyrolysis recycle treatment was compared with traditional furnace techniques used for bulk waste treatment by applying the same temperature regime. This work provides evidence that microwave pyrolysis can be used as an alternative method for the production of rCFs for reuse applications.
1. Introduction
In recent decades, carbon fiber-reinforced polymers (CFRPs) have been extensively utilized in various applications of the aerospace industry, transportation, infrastructure [1,2,3], energy, sport industries, defense, medical sector, and electronics [4,5]. The physical properties of CFRPs have led to increased use of these materials as replacements for more conventional options, including steel, aluminum, alloys, etc. These physical properties include low density and lightweight, superior strength to weight ratios and elastic modulus [6,7,8], stiffness, low expansion or shrinkage, thermal stability [9,10,11,12], electrical conductivity, superior resistance to corrosion and chemical attack, and exceptional endurance of physico–chemical properties [13,14,15,16]. Given these properties, they have been heavily utilized in the manufacture of automotive parts to reduce weight and improve fuel efficiency [17]. CFRPs’ composites are engineered materials that compose of carbon fibers (CFs) acting as reinforcing matrix materials within versed thermosetting polymer to form structures. [18]. The fibers act as load-carrying elements for their orientation and to resist environmental damage [11,19].
CFRPs’ composite materials are differentiated by silane with different properties, suitable for a variety of strength requirements. In response to increased demand to reduce CO2 emissions through weight saving measures and production related energy costs, the use of CFRP products and applications has a high potential to grow significantly over the coming years. Carbon fibers were first commercially produced in the late 1960s. They are now manufactured around the world. It is predicted that by 2025, revenues associated with use of CFRPs will exceed 25 billion dollars per year [20,21]. Recent annual growth rates of more than 10% underline these forecasts [22]. Given that the service life of CFRPs is approximately 50 years, as end of life is reached, this will bring about a considerable increase in CFRPs waste generation and present a significant challenge for the disposal and recycling of CFRPs based components [6,23].
With increasing global focus on a circular economy model at domestic and foreign scale, the problems of recycling and waste reduction are becoming more and more prominent, with industry increasingly considering the impact of growth on finite resources. The presence of carbon fibers in composite materials is a challenge when it comes to recycling and separating the fibrous material from epoxy resins, plastics, and other materials. In recent years, industries have speedily adopted these materials. However, it has displayed no proper awareness and consideration of their disposal and possible recycling. The main treatment methods for waste CFRPs have been landfill disposal and incineration [9,24]. Economic viability, new legislations, limited landfill capacity, and fuel incineration, and ecological aspects are driving towards recycling and processing for CFRPs waste [25,26]. Thus, the treatment methodology of CFRP waste is becoming a more critical issue, with industry currently lacking in the skills and methodologies to respond effectively. Support is required to provide industry with tools that can be implemented effectively and economically.
Currently, several technologies have been developed for recovering CFs from CFRPs with excellent recycling yield and maintenance of critical physical properties. There are three main approaches:
- (1)
- Mechanical processes: shredding, crushing and other physical methods effect separation of CFs and raw matrix materials [2,27,28], or high voltage pressure (electrodynamic fragmentation) [29,30]. The recovery cost is low and the method is simple. Production of pollutants is minimal. However, the damage to CFs in the recovery process is high, making the recovered material unsuitable for the manufacture of typical CF-based products. As such, material recovered in this way is usually made into powder for filling or as a reinforcing material.
- (2)
- Chemical recovery: separate CFs from the matrix resin [2,9,31,32,33,34,35,36,37,38]. The damage to recovered CFs is minimal, but the cost of chemical agents is high and this technology is still very much in its infancy. Waste solutions from the process require recovery and subsequent treatment before reuse. In the absence of this step, the result is the production of secondary pollution of large volumes of liquid waste and hazardous gases, the disposal of which presents a significant hazard to the environment [6]. In addition, the method does not allow simple recovery of CFs in large quantities. At present, this method is rarely used.
- (3)
- Thermal technology: The matrix material is decomposed by heat (combustion/incineration, fluidized bed process, or pyrolysis) [2,7,39,40,41,42] or microwave radiation [43,44]. Heat is utilized to decompose the scrap composite in the thermal recycling process. Due to a higher operating temperature at ~450–700 °C [45,46,47,48], combustible materials are burnt, leaving the CF material for recovery. Generally, the process temperature is dependent on the type of resin used in the scrap composite. Irregular temperature can leave char on the undercooked fiber surface or result in a decrease in the diameter of the overheated recovered fibers [49,50].
Microwave pyrolysis is a relatively new approach, designed to replace conventional pyrolysis. Heating by microwave pyrolysis has the advantage that the rate of thermal transfer is increased, with reduced energy consumption [26,51] and heat loss reduction [52,53]. This process can recycle CFRPs, with resulting fibers maintaining desirable mechanical properties [49,54,55,56,57,58] These studies are focused on removing resin and maintaining mechanical strength to get high quality rCF, without considering the issue of residual silane on the recovered carbon fibers. However, the manufacturing process of commercial CFRPs involves adding an appropriate amount of silane [59,60]. Silanes are often used as a coupling agent, possessing differing functional groups at each molecular end. For example, trialkoxysilane can bond with inorganic substances. Alternatively, there are organofunctional groups (methacrylate, epoxy, etc.). These silanes can enhance the compatibility between carbon fibers and the resin matrix, improving the interfacial bonding strength of CFRPs. Therefore, if silane could be saved during the fiber recovery process it would reduce the need for the addition of further silane coupling agent when using rCF to manufacture new materials, reducing overall costs and waste [61]. Furthermore, Li et al. [62] compare the dispersion of sizing of carbon fiber after removed silane treatment and nontreatment, with the product intended to be added to portland cement. The results show the carbon fiber of removed silane can be efficiently dispersed to form carbon-fiber-reinforced cement, with this composite cement exhibiting higher compressive strength. The residue of silane could be a disadvantage when considering recovered carbon fibers for reinforced inorganic applications.
In this study, the residual silane content of rCF surface can be controlled by the temperature of microwave pyrolysis. The influence of the reaction temperature on the degradation of silicon on the carbon fiber surface was studied. The recovered carbon fibers were characterized by scanning electron microscopy (SEM), thermogravimetric analysis (TGA), and Energy-dispersive X-ray spectroscopy (EDS).
2. Experimental Methods
2.1. Materials
The carbon fiber reinforced composite material (CFRPs) waste from recycled bicycle frame and the original Carbon fiber (Mitsubishi TR 50) was supplied by Giant Manufacturing Co., Ltd (Taichung City, Taiwan). The waste was cut and crushed by plate-cutting and hydraulic press machine to the sample size of about 90 mm × 90 mm × 4.5 mm.
2.2. Pyrolysis
The traditional pyrolysis process is performed using a typically muffle furnace (JH-5, Taiwan, 1200 W). The CFRP waste (10 g) was loaded into a ceramic crucible and pyrolyzed at 650 °C in an air atmosphere. Figure 1 shows the micro-wave pyrolysis system (MILESTONE PYRO 260). Pyrolysis of the chips of waste (10 g) was carried out at 350–950 °C in an air atmosphere. The special microwave–transparent ceramic muffle furnace allows microwave energy to transmit through and rapidly raise the temperature of silicon carbide plates situated inside the muffle. Microwaves were generated by a single magnetron system with rotating diffuser for homogeneous microwave distribution, the power was set up to 1200 W and the frequency of the microwave was set to 2.45 GHz. The detail condition shows at Table 1.
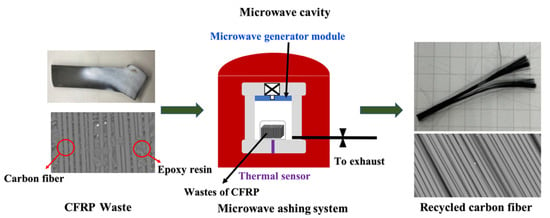
Figure 1.
CFRPs waste recycling treatment by micro-wave pyrolysis system.

Table 1.
Details of samples at different reaction conditions.
2.3. Characterization
The CFRPs wastes are characterized using Thermogravimetric Analysis (TGA), Scanning Electron Microscopy images and Energy-dispersive X-ray spectroscopy (SEM-EDS). Netzsch instrument TG 209 F3 model is used to make TGA measurements. The measurements were characterized for the thermal degradation curve of the CFRP characterized under an air atmosphere. The weight of the sample is 10 mg. Each sample was heated from room temperature to 650 °C at a heating rate of 10 °C/min. Three target areas were identified for each of the rCF samples, and taking the core layer of each sample, the surface element (C, O, and Si) of the core layer is measured using a Hitachi TM 4000 Plus instrument with Oxford AZtecOne (EDS) to assess removal capability and uniformity for silane and resin. The high magnification physical morphology of carbon fiber was detected by Hitachi Regulus 8100 field-emission scanning electron microscopes (FE-SEM).
3. Experimental Results and Discussions
3.1. TGA Analysis of CFRPs Waste
It is important to understand the thermal decomposition curve of the CFRPs’ waste from bicycle frame waste, since cause each batch of CFRP could be from different source. As shown in Figure 2, the DTG curve has a significant mass loss in the range of 250 to 450 °C and 490 to 560 °C, respectively. Therefore, it can be defined that the decomposition temperature of the first TGA stage is 220 to 450 °C and the second stage is 475 to 550 °C. The third stage of the decomposition range is between 600 and 650 °C. The thermal decomposition curve is consistent with results presented by Yatim et al. [57] and Deng et al. [55].
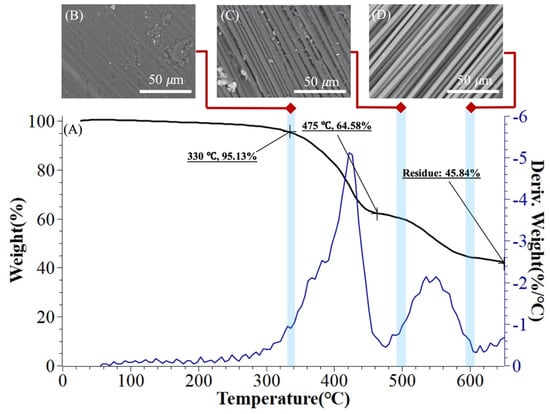
Figure 2.
TGA analysis of CFRP waste: (A) CFRP of thermal decomposition curve; and (B–D) SEM micrograph of CRFP thermal decomposition stages (B) stage 1, (C) stage 2, and (D) stage 3.
In Figure 2B–D, the SEM image also shows the extent to which CFRP waste is removed from the resin between the fibers of the CFRPs’ waste at various stages of TGA. In stage 1, at 330 °C (Figure 2B), a weight loss of approximately 5% occurred. It can be observed that there is still a large amount of epoxy residue between the fibers. In stage 2 (Figure 2C), after heating beyond the first interval, the weight loss is 30%. The gaps between the fibers can be clearly seen and there is still un-removeable resin around the carbon fiber. After 500 to 600 °C, second interval, an increasing amount of resin is removed; approximately 20%. No residual resin was observed on the surface of carbon fiber. Based on TGA thermal weight loss results, it can be speculated that the carbon fiber-to-resin weight ratio of the CFRPs waste is approximately 40:60.
3.2. Compare the Pyrolysis Result of Microwave with Traditional Treatment for CFRPs Waste
The parameters obtained from TGA thermal decomposition were used as the reference condition for the removal of the resin by high temperature furnace and microwave pyrolysis. Figure 3 presents CFRP waste samples recovered using two different heat treatment methods. Figure 3A shows SEM image and EDS analysis results of virgin waste carbon fiber. The SEM image clearly shows a significant quantity of resin wrapped around the carbon fiber. EDS elemental analysis shows the presence of carbon, oxygen, and silicon content at 85.8 ± 5.3%, 13.6 ± 4.7%, and 0.3 ± 0.6%, respectively. Figure 3B shows no significant difference between CFRP waste in high-temperature furnaces after heating up to 650 °C, except for a color change resulting in a darker appearance. And in Figure 3C, CFRP waste decomposed by microwave pyrolysis results in carbon fibers that can be separated easily into individual fibers.
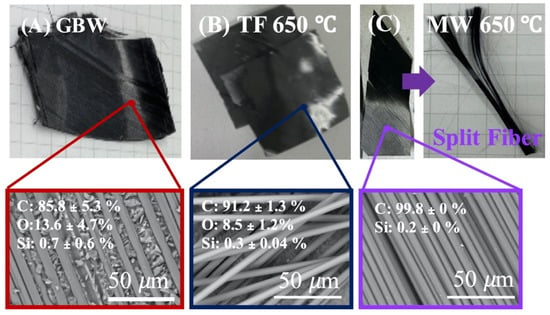
Figure 3.
Optical photo, SEM imagine, and EDS analysis: (A) CFRP waste of Giant Bicycles (GBW), (B) CFRPs waste sample (TF 650 °C) after treatment with traditional furnace pyrolysis at 650 °C, and (C) CFRPs waste (MW 650 °C) after treatment with micro-wave pyrolysis at 650 °C.
3.3. Influence of Different Temperature on Removed Resin in Large Scale Waste CFRP for Microwave Pyrolysis
In this study, the samples of waste CFRPs were obtained from recycled bicycle frames. Samples were prepared to the required sizes through smash and cut methods; the thickness being approximately 4.5 mm. The CFRP was closely composed of multilayer CF and resin. The pyrolysis of the resin requires enough oxygen and heat for carbon oxidation to occur and for effective removal of resin from CFRP wastes. The irregular and curled shape of CFRP wastes and the compact multilayer CF structure could easily result in non-uniform hot spots in the inside of each waste element and this structure also makes it difficult for the resin to make good contact with surrounding air. Therefore, the resin removal methodology requires further research [56].
To understand the uniformity of waste CFRP at large scales under microwave assisted pyrolysis, three parts of the samples in the crucible were studied via SEM and EDS as shown in Figure 4 (marked 1, 2, and 3). The effectiveness of resin removal was assessed through SEM images and with carbon and oxygen content determined using EDS element analysis. Each RCF sample was peeled off from the outside, and its core used for SEM/EDS measurement. EDS analysis results of the samples are presented in Table 2.
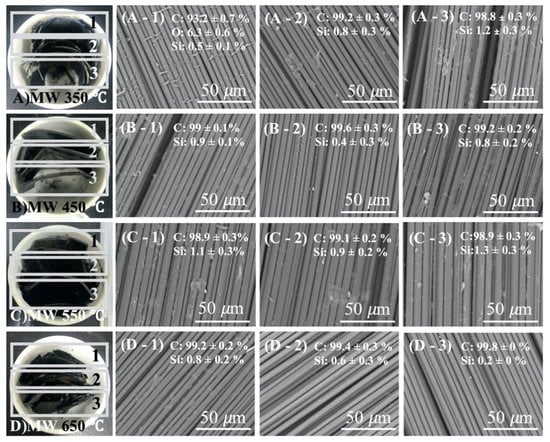
Figure 4.
Photograph and SEM micrographs of the recycled carbon fibers in different part of ceramic crucible after microwave pyrolysis with varying condition; (A) microwave pyrolysis at 350 °C, (B) at 450 °C, (C) at 550 °C and (D) at 650 °C.

Table 2.
EDS analysis of the samples.
At 350 °C pyrolysis temperature (Figure 4(A-1–A-3)), the A-1 area of SEM image shows the resin residue between the carbon fibers. EDS analysis determined the carbon, oxygen, and silicon content as 93.2 ± 0.7, 6.3 ± 0.6, and 0.5 ± 0.1%, respectively. Compared with samples GBW of Figure 3A, oxygen content decreased from 13.6 ± 4.7 to 6.3 ± 0.6% and carbon content increased from 85.8 ± 5.3 to 93.2 ± 0.7%. These results revealed that the resin is not fully pyrolyzed. In addition, the EDS and SEM results of A-2 and A-3 areas are similar. These SEM images show a significant quantity of resin adhered to the carbon fiber. Comparing the EDS results of three areas (Figure 4(A-1–A-3), Table 1), areas 2 and 3 show better resin removal. A similar situation also occurred under the different sample pyrolysis conditions applied.
At 450 °C (Figure 4(B-1–B-3), Table 1), the EDS results showed the carbon content is 99 ± 0.1, 99.6 ± 0.3, and 99.2 ± 0.2% and silicon content is 0.9 ± 0.1, 0.4 ± 0.3, and 0.8 ± 0.2%, respectively. Figure 4C,(D-1–D-3), shows the EDS and SEM results after microwave pyrolysis at 550 °C (B) and 650 °C (D). The carbon content of MW 550 °C from areas 1 to 3 is 98.9 ± 0.3, 99.1 ± 0.2, and 98.9 ± 0.3% and silicon content is 1.1 ± 0.3, 0.9 ± 0.2, and 1.3 0.3%, respectively. For MW650 °C, the carbon content is 99.2 ± 0.2, 99.4 ± 0.3, and 99.8 ± 0%, and the silicon content is 0.80.2, 0.6 ± 0.3 and 0.2 ± 0%, respectively. All of the above EDS results show that pyrolysis of waste CFRP is uniform at different crucible positions. While the pyrolysis condition is above 450 °C, the oxygen content reduced to 0%, indicating that the resin was fully carbonized. The difference between carbon content for the three areas is approximately 0.47 ± 0.01% at 550 and 650 °C. This was, perhaps, caused by the waste having a curved and irregular shape or non-uniform density. Verification of these statements requires further research. However, this pyrolysis condition had enough process time and temperature to reduce the influence caused by different crucible position.
The SEM images show filamentous matter on the fibers. The shape changed from the filament to a dot along with decreased silicon content. Section 3.4 will discuss silicon removal from CFRP waste through microwave pyrolysis.
3.4. Silane Removal from CFRPs Waste at Different Microwave Pyrolysis Temperature
Figure 5 shows a SEM diagram of CFRP materials that are thermally treated at different temperatures. A carbon fiber sample (pyrolysis 650) treated at 650 °C in a high-temperature oven shows significant resin filling, as shown in Figure 5A. The results of the EDS element analysis of the sample showed that the fiber surface of TF at 650 °C had an oxygen content of approximately 8.5 ± 1.2%. Based on results of the TGA analysis, the resin had been carbonized to form carbide; this means, for a traditional furnace, resin pyrolysis is not complete. For the residual resin layer wrapped on the surface of waste CFRPs, the Si element content is 0.3 ± 0.04%, while the CFRPs samples treated with microwave pyrolysis at 350 °C (Figure 5B), 450 °C (Figure 5C), and 550 °C (Figure 5D), show a cotton-like or filamentous substance on the surface of the carbon fibers. The silicon content is approximately 0.9 to 0.4%. At a treatment temperature of 650 °C (Figure 5E), the shape of filamentous substances changed to dots (silicon content: 0.2 ± 0%).
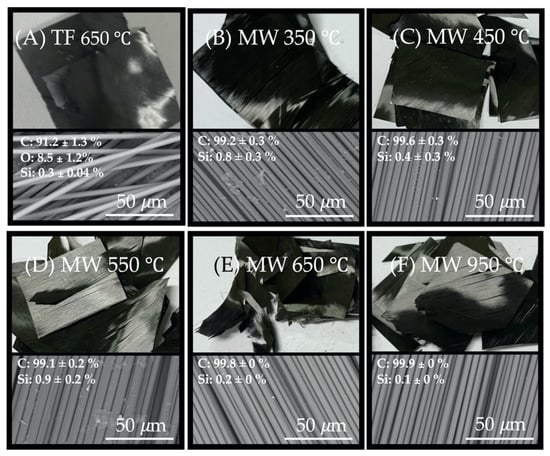
Figure 5.
SEM micrographs of the recycled carbon fibers by thermally treated at different temperatures; (A) traditional furnace pyrolysis treated at 650 °C, (B) microwave pyrolysis treated at 350 °C, (C) at 450 °C, (D) at 550 °C, (E) at 650 °C; and (F) 950 °C.
By way of understanding the effect of microwave thermal pyrolysis temperature on residual silane content on waste CFRPs surface, we also set the temperature of microwave pyrolysis at 950 °C. Following this treatment, the silicon content is 0.1%. The SEM image shows that the recovered carbon fiber surface is completely free of residues or obvious defects, as shown in Figure 5F. In Figure 6, the MW 950 °C Figure 6A,B compared with the original carbon fiber (Mitsubishi TR 50, Figure 6C,D), demonstrates they are without defects on the fiber surface, which can be further proved by high magnification FE-SEM.
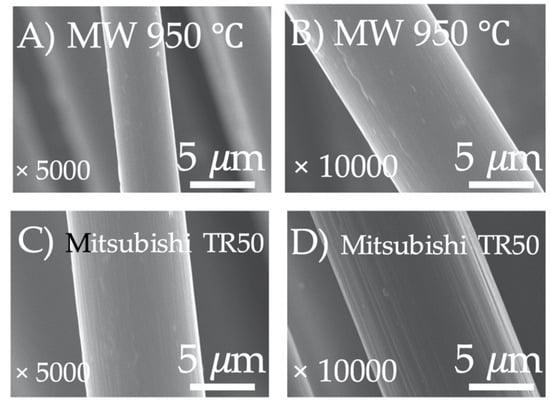
Figure 6.
High magnification FE-SEM micrographs of the recycled carbon fibers (MW 950 °C, (A); at 5000× magnification, (B); 10,000×) and the original carbon fiber (Mitsubishi TR 50, (C,D) they magnification ware respectively 5000× and 10,000×).
However, even if the microwave pyrolysis temperature increases by 300 °C, the silicon content is only slightly reduced. Therefore, the results show the silicon content is gradually reduced from the recovered carbon fiber along with breakdown of the resins, but it cannot be completely removed by microwave pyrolysis at 350 to 950 °C.
Since the organic components in the silane compounds typically degrade when heated to approximately 400 °C, only elemental silicon remains. Silicon gasification temperature is 3265 °C, which is difficult to achieve via thermal decomposition methods. Other researchers also presented SEM/EDS results of pyrolyzed carbon fiber pretreated using silane coupling agent that exhibit the same dots on the carbon fiber surface and were determined as containing elemental silicon [63,64].
For these reasons, these filamentous substances are inferred to be residual silicon and carbonized resin produced during the CFRPs pyrolysis process. The residual silicon is removed as the carbonized resin pyrolyzed to form carbon dioxide or carbon monoxide by air atmosphere. Despite the evidence for the above conclusions, further research is required to affirm this interpretation.
4. Conclusions
The silane controllable recycled carbon fiber can effectively simplify the process of rCF reuse in suitable applications. Through adjusted silane content, the dispersion of rCF in organic or inorganic materials was improved for use in the manufacture of high-performance composite materials.
This study successfully demonstrates control of the silane content on the rCF surface. Different microwave pyrolysis methods and temperature conditions were compared. The results show that the microwave pyrolysis method achieves better resin removal when compared with traditional thermal treatment methods at the same temperature. Over 450 °C, the SEM/EDS results show the recovered surface of the carbon fibers are absent of oxygen. Compared with the GBW sample, no additional defects were observed. Moreover, the resin and silane removal uniformities were examined from recovered carbon fiber in large scale wastes. At different crucible position, the difference of maximum silane content is about ±0.33%. Moreover, a higher treatment temperature can reduce this difference.
The results during microwave pyrolysis could be reproduced for this CFRP waste of Giant Bicycles discussed in this article. However, a multitude of influencing parameters affect the reclamation of carbon fibers, such as matrix material, temperatures, atmosphere composition, etc. This complicates the recycling process control to reclaim high quality fibers. Further research into the decomposition behavior of CFRPs recovered from other, commonly recycled industrial composite materials is required.
Author Contributions
ConceptualizationConceptualization, K.-Y.C. and S.-M.C.; data curation, K.-Y.C. and Y.-J.W.; formal analysis, K.-Y.C. and Y.-J.W.; investigation, K.-Y.C. and A.S.; methodology, K.-Y.C., Y.-J.W. and S.-M.C.; project administration, K.-Y.C. and Y.-J.W.; supervision, A.S., S.-M.C., Y.-F.L. and M.-Y.S.; writing—original draft, K.-Y.C. and A.S.; writing—review and editing, A.S., G.L., Y.-F.L., S.-M.C. and M.-Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Technology of Taiwan (108-2218-E-027-005, 109-2221-E-027-113), the Ministry of Education in Taiwan, and the Topkey Corp. Taiwan.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Asmatulu, E.; Twomey, J.M.; Overcash, M. Recycling of fiber-reinforced composites and direct structural composite recycling concept. J. Compos. Mater. 2013, 48, 593–608. [Google Scholar] [CrossRef]
- Oliveux, G.; Dandy, L.O.; Leeke, G. Current status of recycling of fibre reinforced polymers: Review of technologies, reuse and resulting properties. Prog. Mater. Sci. 2015, 72, 61–99. [Google Scholar] [CrossRef] [Green Version]
- Jensen, J.; Skelton, K. Wind turbine blade recycling: Experiences, challenges and possibilities in a circular economy. Renew. Sustain. Energy Rev. 2018, 97, 165–176. [Google Scholar] [CrossRef]
- Pimenta, S.; Pinho, S. Recycling carbon fibre reinforced polymers for structural applications: Technology review and market outlook. Waste Manag. 2011, 31, 378–392. [Google Scholar] [CrossRef] [Green Version]
- Cousins, D.S.; Suzuki, Y.; Murray, R.; Samaniuk, J.R.; Stebner, A.P. Recycling glass fiber thermoplastic composites from wind turbine blades. J. Clean. Prod. 2018, 209, 1252–1263. [Google Scholar] [CrossRef]
- Sun, H.; Guo, G.; Memon, S.A.; Xu, W.; Zhang, Q.; Zhu, J.-H.; Xing, F. Recycling of carbon fibers from carbon fiber reinforced polymer using electrochemical method. Compos. Part A Appl. Sci. Manuf. 2015, 78, 10–17. [Google Scholar] [CrossRef]
- Oliveux, G.; Bailleul, J.-L.; Gillet, A.; Mantaux, O.; Leeke, G. Recovery and reuse of discontinuous carbon fibres by solvolysis: Realignment and properties of remanufactured materials. Compos. Sci. Technol. 2017, 139, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Liu, J.; Liu, W.; Wang, J.; Tang, T. Recycling of carbon fibre reinforced epoxy resin composites under various oxygen concentrations in nitrogen–oxygen atmosphere. J. Anal. Appl. Pyrolysis 2015, 112, 253–261. [Google Scholar] [CrossRef]
- Xu, P.; Li, J.; Ding, J. Chemical recycling of carbon fibre/epoxy composites in a mixed solution of peroxide hydrogen and N,N-dimethylformamide. Compos. Sci. Technol. 2013, 82, 54–59. [Google Scholar] [CrossRef]
- Frohs, W.; Jaeger, H. Carbon fiber & composite material—Landscape Germany. Carbon 2012, 50, 737. [Google Scholar]
- Yao, S.S.; Jin, F.L.; Rhee, K.Y.; Hui, D.; Park, S.J. Recent advances in carbonfiber-reinforced thermoplastic composites: A review. Compos. Part B Eng. 2018, 142, 241–250. [Google Scholar] [CrossRef]
- Bachmann, J.; Hidalgo, C.; Bricout, S. Environmental analysis of innovative sustainable composites with potential use in avia-tion sector—A life cycle assessment review. Sci. China Technol. Sci. 2017, 60, 1301–1317. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.-H.; Wei, L.; Moahmoud, H.; Redaelli, E.; Xing, F.; Bertolini, L. Investigation on CFRP as dual-functional material in chloride-contaminated solutions. Constr. Build. Mater. 2017, 151, 127–137. [Google Scholar] [CrossRef]
- Zhu, J.-H.; Su, M.-N.; Huang, J.-Y.; Ueda, T.; Xing, F. The ICCP-SS technique for retrofitting reinforced concrete compressive members subjected to corrosion. Constr. Build. Mater. 2018, 167, 669–679. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Shi, Q.; Dunn, M.L.; Wang, T.T.; Qi, H.J. Carbon Fiber Reinforced Thermoset Composite with Near 100% Recyclability. Adv. Funct. Mater. 2016, 26, 6098. [Google Scholar] [CrossRef]
- Hadigheh, S.; Kashi, S. Effectiveness of vacuum consolidation in bonding fibre reinforced polymer (FRP) composites onto concrete surfaces. Constr. Build. Mater. 2018, 187, 854–864. [Google Scholar] [CrossRef]
- Yoshinori Kakichi, Y.; Yamaguchi, A.; Hashimoto, T.; Urushisaki, M.; Sakaguchi, T.; Kawabe, K.; Kondo, K.; Iyo, H. Development of recyclable carbon fiber-reinforced plastics (CFRPs) with controlled degradability and stability using acetal link-age-containing epoxy resins. Polym. J. 2017, 49, 851–859. [Google Scholar] [CrossRef]
- Kalpakjian, S.; Schmid, S.R. Manufacturing Engineering and Technology, 4th ed.; Prentice Hall: Hoboken, NJ, USA, 2000. [Google Scholar]
- Lau, K.-T.; Hung, P.-Y.; Zhu, M.-H.; Hui, D. Properties of natural fibre composites for structural engineering applications. Compos. Part B Eng. 2018, 136, 222–233. [Google Scholar] [CrossRef]
- Kraus, T.; Kühnel, M.; Witten, E. Composites Market Report 2014Market Developments, Trends, Challenges and Opportunities; Federation of Reinforced Plastics: Frankfurt, Germany, 2014. [Google Scholar]
- Roberts, T. The Carbon Fibre Industry: Global Strategic Market Evaluation 2006–2010; Materials Technology Publications: Watford, UK, 2006; Volume 10. [Google Scholar]
- Krauklis, A.E.; Karl, C.W.; Gagani, A.I.; Jørgensen, J.K. Composite material recycling technology—State-of-the-art and sustainable development for the 2020s. J. Compos. Sci. 2021, 5, 28. [Google Scholar] [CrossRef]
- Jiang, G.; Pickering, S.; Walker, G.; Wong, K.; Rudd, C. Surface characterisation of carbon fibre recycled using fluidised bed. Appl. Surf. Sci. 2007, 254, 2588–2593. [Google Scholar] [CrossRef]
- Witik, R.A.; Teuscher, R.; Michaud, V.; Ludwig, C.; Månson, J.A.E. Carbon fibre reinforced composite waste: An environmental assessment of recycling, energy recovery and landfilling. Compos. Part A Appl. Sci. Manuf. 2013, 49, 89–99. [Google Scholar] [CrossRef]
- Meng, F. Environmental and Cost Analysis of Carbon Fibre Composites Recycling. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2017. [Google Scholar]
- Gopalraj, S.K.; Kärki, T. A review on the recycling of waste carbon fibre/glass fibre-reinforced composites: Fibre recovery, properties and life-cycle analysis. SN Appl. Sci. 2020, 2, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Kouparitsas, C.E.; Kartalis, C.N.; Varelidis, P.C.; Tsenoglou, C.J.; Papaspyrides, C.D. Recycling of the fibrous fraction of rein-forced thermoset composites. Polym. Compos. 2010, 23, 682–689. [Google Scholar] [CrossRef]
- Howarth, J.; Mareddy, S.S.; Mativenga, P. Energy intensity and environmental analysis of mechanical recycling of carbon fibre composite. J. Clean. Prod. 2014, 81, 46–50. [Google Scholar] [CrossRef]
- Vo Dong, P.A.; Azzaro-Pantel, C.; Cadene, A.L. Economic and environmental assessment of recovery and disposal pathways for CFRP waste management. Resour. Conserv. Recycl. 2018, 133, 63–75. [Google Scholar] [CrossRef] [Green Version]
- Mativenga, P.; Shuaib, N.A.; Howarth, J.; Pestalozzi, F.; Woidasky, J. High voltage fragmentation and mechanical recycling of glass fibre thermoset composite. CIRP Ann. 2016, 65, 45–48. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Z.; Feng, L. Chemical recycling of carbon fibers reinforced epoxy resin composites in oxygen in supercritical water. Mater. Des. 2010, 31, 999–1002. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Jiang, Z.; Tang, T. Chemical recycling of carbon fibre reinforced epoxy resin composites in subcritical water: Synergistic e_ect of phenol and koh on the decomposition efficiency. Polym. Degrad. Stab. 2012, 97, 214–220. [Google Scholar] [CrossRef]
- Yan, H.; Lu, C.-X.; Jing, D.-Q.; Chang, C.-B.; Liu, N.-X.; Hou, X.-L. Recycling of carbon fibers in epoxy resin composites using supercritical 1-propanol. Carbon 2016, 100, 710–711. [Google Scholar] [CrossRef]
- Yildirir, E.; Onwudili, J.A.; Williams, P.T. Recovery of carbon fibres and production of high quality fuel gas from the chemical recycling of carbon fibre reinforced plastic wastes. J. Supercrit. Fluids 2014, 92, 107–114. [Google Scholar] [CrossRef]
- Oshima, K.; Fujii, H.; Morita, K.; Hosaka, M.; Muroi, T.; Satokawa, S. Selective Phenol Recovery by Catalytic Cracking of Thermal Decomposition Gas from Epoxy-Based Carbon-Fiber-Reinforced Plastic. Ind. Eng. Chem. Res. 2020, 59, 13460–13466. [Google Scholar] [CrossRef]
- Lo, J.N.; Nutt, S.R.; Williams, T.J. Recycling Benzoxazine–Epoxy Composites via Catalytic Oxidation. ACS Sustain. Chem. Eng. 2018, 6, 7227–7231. [Google Scholar] [CrossRef]
- Das, M.; Chacko, R.; Varughese, S. An Efficient Method of Recycling of CFRP Waste Using Peracetic Acid. ACS Sustain. Chem. Eng. 2018, 6, 1564–1571. [Google Scholar] [CrossRef]
- Borjan, D.; Knez, Ž.; Knez, M. Recycling of carbon fiber-reinforced composites—Difficulties and future perspectives. Materials 2021, 14, 4191. [Google Scholar] [CrossRef] [PubMed]
- López, F.; Martín, M.; Alguacil, F.; Rincón, J.M.; Centeno, T.; Romero, M. Thermolysis of fibreglass polyester composite and reutilisation of the glass fibre residue to obtain a glass–ceramic material. J. Anal. Appl. Pyrolysis 2012, 93, 104–112. [Google Scholar] [CrossRef] [Green Version]
- López, F.A.; Rodríguez, O.; Alguacil, F.J.; García-Díaz, I.; Centeno, T.A.; García-Fierro, J.L.; González, C. Recovery of carbon fibres by the thermolysis and gasification of waste prepreg. J. Anal. Appl. Pyrolysis 2013, 104, 675–683. [Google Scholar] [CrossRef]
- Yip, H.L.H.; Pickering, S.; Rudd, C. Characterisation of carbon fibres recycled from scrap composites using fluidised bed process. Plast. Rubber Compos. 2002, 31, 278–282. [Google Scholar] [CrossRef]
- Ye, S.Y.; Bounaceur, A.; Soudais, Y.; Barna, R. Parameter Optimization of the Steam Thermolysis: A Process to Recover Carbon Fibers from Polymer-Matrix Composites. Waste Biomass Valorization 2013, 4, 73–86. [Google Scholar] [CrossRef]
- Akesson, D.; Foltynowicz, Z.; Christeen, J.; Skrifvars, M. Products obtained from decomposition of glass fibre-reinforced composites using microwave pyrolysis. Polimery 2013, 58, 582–586. [Google Scholar] [CrossRef]
- Obunai, K.; Fukuta, T.; Ozaki, K. Carbon fiber extraction from waste CFRP by microwave irradiation. Compos. Part A Appl. Sci. Manuf. 2015, 78, 160–165. [Google Scholar] [CrossRef]
- De Moraes, V.T.; Jermolovicius, L.A.; Tenório, J.A.S.; Lebrão, S.M.G.; Lebrãoa, G.W. Microwave-assisted recycling process to recover fiber from fiberglass polyester composites. Mater. Res. 2019, 22, 1–8. [Google Scholar] [CrossRef]
- Limburg, M.; Stockschläder, J.; Quicker, P. Thermal treatment of carbon fibre reinforced polymers (Part 1: Recycling). Waste Manag. Res. 2019, 37, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Yatim, N.M.; Shamsudin, Z.; Shaaban, A.; Sani, N.A.; Jumaidin, R.; Shariff, E.A. Thermal analysis of carbon fibre reinforced polymer decomposition. Mater. Res. Express 2020, 7, 015615. [Google Scholar] [CrossRef] [Green Version]
- Naqvi, S.R.; Prabhakara, H.M.; Bramer, E.; Dierkes, W.; Akkerman, R.; Brem, G. A critical review on recycling of end-of-life carbon fibre/glass fibre reinforced composites waste using pyrolysis towards a circular economy. Resour. Conserv. Recycl. 2018, 136, 118–129. [Google Scholar] [CrossRef] [Green Version]
- Leclerc, P.; Doucet, J.; Chaouki, J. Development of a microwave thermogravimetric analyzer and its application on polystyrene microwave pyrolysis kinetics. J. Anal. Appl. Pyrolysis 2018, 130, 249–255. [Google Scholar] [CrossRef]
- Rodrigues, G.G.M.; Paiva, J.M.F.; Carmo, J.B.D.; Botaro, V. Recycling of carbon fibers inserted in composite of DGEBA epoxy matrix by thermal degradation. Polym. Degrad. Stab. 2014, 109, 50–58. [Google Scholar] [CrossRef]
- Kesson, D.; Foltynowicz, Z.; Jonas Christe´en, J.; Skrifvars, M. Microwave pyrolysis as a method of recycling glass fibre from used blades of wind turbines. J. Reinf. Plast. Compos. 2012, 31, 1136–1142. [Google Scholar] [CrossRef]
- Delvere, I.; Iltina, M.; Shanbayev, M.; Abildayeva, A.; Kuzhamberdieva, S.; Blumberga, D. Evaluation of Polymer Matrix Composite Waste Recycling Methods. Environ. Clim. Technol. 2019, 23, 168–187. [Google Scholar] [CrossRef] [Green Version]
- Gharde, S.; Kandasubramanian, B. Mechanothermal and chemical recycling methodologies for the Fibre Reinforced Plastic (FRP). Environ. Technol. Innov. 2019, 14, 100311. [Google Scholar] [CrossRef]
- Lester, E.; Kingman, S.; Wong, K.H.; Rudd, C.; Pickering, S.; Hilal, N. Microwave heating as a means for carbon fibre re-covery from polymer composites: A technical feasibility study. Mater. Res. Bull. 2004, 39, 1549–1556. [Google Scholar] [CrossRef]
- Deng, J.; Xu, L.; Zhang, L.; Peng, J.; Guo, S.; Liu, J.; Koppala, S. Recycling of Carbon Fibers from CFRP Waste by Microwave Thermolysis. Processes 2019, 7, 207. [Google Scholar] [CrossRef] [Green Version]
- Fernández, Y.; Arenillas, A.; Angel, J. Microwave Heating Applied to Pyrolysis. In Advances in Induction and Microwave Heating of Mineral and Organic Materials; Grundas, S., Ed.; InTech: Rijeka, Croatia, 2011; pp. 724–752. ISBN 978-953-307-522-8. [Google Scholar]
- Shamsuddin, S.-R.; Chee Ho, K.K.; Lee, K.-Y.; Hodgkinson, J.M.; Bismarck, A. Carbon Fiber: Properties, Testing, and Analysis. In Wiley Encyclopedia of Composites; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 1–17. [Google Scholar] [CrossRef]
- Yatim, N.M.; Shamsudin, Z.; Shaaban, A.; Chafar, J.A.; Khan, M.J.H. Recovery of carbon fiber from carbon fiber reinforced polymer waste via pyrolysis. J. Adv. Manuf. Technol. 2020, 14, 37–47. [Google Scholar]
- Zhu, W.; Xiao, H.; Wang, J.; Li, X. Effect of Different Coupling Agents on Interfacial Properties of Fibre-Reinforced Aluminum Laminates. Materials 2021, 14, 1019. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Shen, H.; Han, W.; Chen, L.; Li, J.; Tang, Y. Hybrid polyurethane and silane sized carbon fibre/epoxy composites with enhanced impact resistance. Compos. Part A Appl. Sci. Manuf. 2018, 118, 49–56. [Google Scholar] [CrossRef]
- Matinlinna, J.P.; Lung, C.Y.K.; Tsoi, J.K.H. Silane adhesion mechanism in dental applications and surface treatments: A review. Dent. Mater. 2018, 34, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-F.; Yang, T.-H.; Kuo, C.-Y.; Tsai, Y.-K. A Study on Improving the Mechanical Performance of Carbon-Fiber-Reinforced Cement. Materials 2019, 12, 2715. [Google Scholar] [CrossRef] [Green Version]
- Onischuk, A.; Panfilov, V.N. Mechanism of thermal decomposition of silanes. Russ. Chem. Rev. 2001, 70, 321–332. [Google Scholar] [CrossRef]
- Jiang, B.; Zhao, L.; Guo, J.; Yan, X.; Ding, D.; Zhu, X.; Huang, Y.; Guo, Z. Improved thermal stability of methylsilicone resins by compositing with N-doped graphene oxide/Co3O4 nanoparticles. J. Nanoparticle Res. 2016, 18, 145. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).