Food Waste Originated Material as an Alternative Substrate Used for the Cultivation of Oyster Mushroom (Pleurotus ostreatus): A Review
Abstract
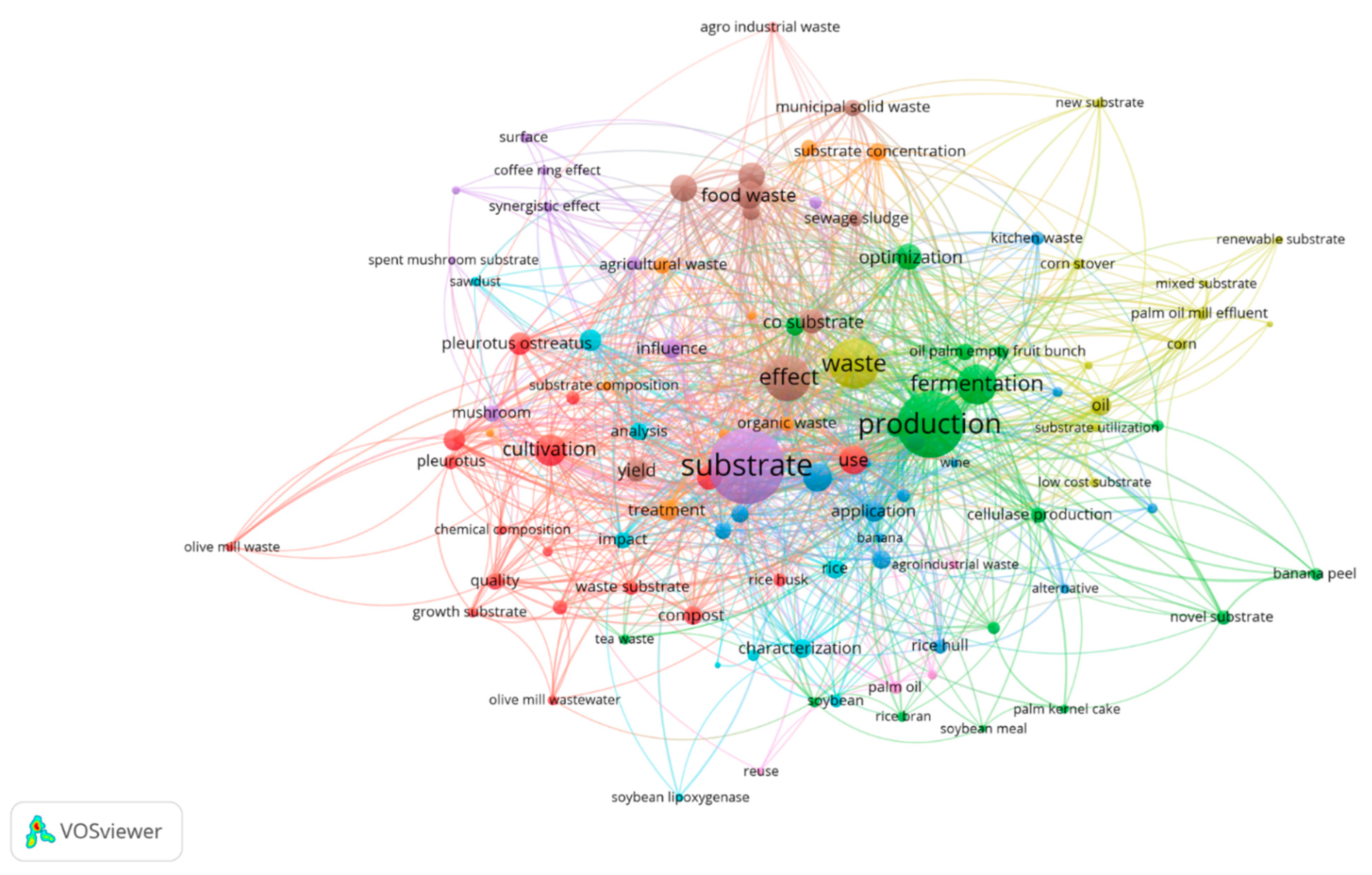
1. Introduction
2. Materials and Methods
2.1. Research Methodology
2.2. Food-Processing, Agro-Cereal and Nut–Fruit Wastes Used for Pleurotus ostreatus Cultivation: An Overview
3. Results and Discussion
3.1. Potential of Food Waste
3.2. Research Covering Substrates
3.2.1. P. ostreatus: Substrate Preparation
3.2.2. Chemical Analyzes of Lignocellulosic Substrates Originated from Food Wastes
3.2.3. Composition of Food Waste Substrates Used for Mushroom Cultivation
3.3. Research Covering P. ostreatus on Different Substrates
3.3.1. Productivity of P. ostreatus Growth on Food Waste Substrates
3.3.2. Chemical Analyzes of P. ostreatus Fruiting Bodies Cultivated on Food Waste Substrates
3.3.3. Future Challenges and Recommendations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, X.; Yan, H.; Chen, J.; Zhang, X. Bioactive Proteins from Mushrooms. Biotechnol. Adv. 2011, 29, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Kalač, P. A Review of Chemical Composition and Nutritional Value of Wild-Growing and Cultivated Mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef]
- Bellettini, M.B.; Fiorda, F.A.; Maieves, H.A.; Teixeira, G.L.; Ávila, S.; Hornung, P.S.; Júnior, A.M.; Ribani, R.H. Factors Affecting Mushroom Pleurotus spp. Saudi J. Biol. Sci. 2019, 26, 633–646. [Google Scholar] [CrossRef]
- Corrêa, R.C.G.; Brugnari, T.; Bracht, A.; Peralta, R.M.; Ferreira, I.C.F.R. Biotechnological, Nutritional and Therapeutic Uses of Pleurotus spp. (Oyster Mushroom) Related with Its Chemical Composition: A Review on the Past Decade Findings. Trends Food Sci. Technol. 2016, 50, 103–117. [Google Scholar] [CrossRef]
- El-Ramady, H.; Abdalla, N.; Fawzy, Z.; Badgar, K.; Llanaj, X.; Törős, G.; Hajdú, P.; Eid, Y.; Prokisch, J. Green Biotechnology of Oyster Mushroom (Pleurotus ostreatus L.): A Sustainable Strategy for Myco-Remediation and Bio-Fermentation. Sustainability 2022, 14, 3667. [Google Scholar] [CrossRef]
- Tsujiyama, S.-I.; Ueno, H. Performance of Wood-Rotting Fungi-Based Enzymes on Enzymic Saccharification of Rice Straw. J. Sci. Food Agric. 2013, 93, 2841–2848. [Google Scholar] [CrossRef]
- Grimm, D.; Wösten, H.A.B. Mushroom Cultivation in the Circular Economy. Appl. Microbiol. Biotechnol. 2018, 102, 7795–7803. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Estrada, A.E.; Pecchia, J. Cultivation of Pleurotus Ostreatus. Edible Med. Mushrooms 2017, 16, 339–360. [Google Scholar] [CrossRef]
- Sekan, A.S.; Myronycheva, O.S.; Karlsson, O.; Gryganskyi, A.P.; Blume, Y. Green Potential of Pleurotus spp. in Biotechnology. PeerJ 2019, 7, e6664. [Google Scholar] [CrossRef]
- Araújo, N.L.; Avelino, K.V.; Halabura, M.I.W.; Marim, R.A.; Kassem, A.S.S.; Linde, G.A.; Colauto, N.B.; do Valle, J.S. Use of Green Light to Improve the Production of Lignocellulose-Decay Enzymes by Pleurotus spp. in Liquid Cultivation. Enzyme Microb. Technol. 2021, 149, 109860. [Google Scholar] [CrossRef]
- Morone, P.; Papendiek, F.; Tartiu, V.E. Food Waste Reduction and Valorisation: Sustainability Assessment and Policy Analysis. Food Waste Reduct. Valoris. Sustain. Assess. Policy Anal. 2017, 1–327. [Google Scholar] [CrossRef]
- Djekic, I.; Operta, S.; Djulancic, N.; Lorenzo, J.M.; Barba, F.J.; Djordjević, V.; Tomasevic, I. Quantities, Environmental Footprints and Beliefs Associated with Household Food Waste in Bosnia and Herzegovina. Waste Manag. Res. 2019, 37, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Djekic, I.; Miloradovic, Z.; Djekic, S.; Tomasevic, I. Household Food Waste in Serbia–Attitudes, Quantities and Global Warming Potential. J. Clean. Prod. 2019, 229, 44–52. [Google Scholar] [CrossRef]
- Van Herpen, E.; van der Lans, I. A Picture Says It All? The Validity of Photograph Coding to Assess Household Food Waste. Food Qual. Prefer. 2019, 75, 71–77. [Google Scholar] [CrossRef]
- Philippidis, G.; Sartori, M.; Ferrari, E.; M’Barek, R. Waste Not, Want Not: A Bio-Economic Impact Assessment of Household Food Waste Reductions in the EU. Resour. Conserv. Recycl. 2019, 146, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Djekic, I.; Sanjuán, N.; Clemente, G.; Jambrak, A.R.; Djukić-Vuković, A.; Brodnjak, U.V.; Pop, E.; Thomopoulos, R.; Tonda, A. Review on Environmental Models in the Food Chain-Current Status and Future Perspectives. J. Clean. Prod. 2018, 176, 1012–1025. [Google Scholar] [CrossRef]
- Pfaltzgraff, L.A.; De Bruyn, M.; Cooper, E.C.; Budarin, V.; Clark, J.H. Food Waste Biomass: A Resource for High-Value Chemicals. Green Chem. 2013, 15, 307–314. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. Exploitation of Food Industry Waste for High-Value Products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef]
- Parmar, I.; Rupasinghe, H.P.V. Bio-Conversion of Apple Pomace into Ethanol and Acetic Acid: Enzymatic Hydrolysis and Fermentation. Bioresour. Technol. 2013, 130, 613–620. [Google Scholar] [CrossRef]
- Tarrés, Q.; Espinosa, E.; Domínguez-Robles, J.; Rodríguez, A.; Mutjé, P.; Delgado-Aguilar, M. The Suitability of Banana Leaf Residue as Raw Material for the Production of High Lignin Content Micro/Nano Fibers: From Residue to Value-Added Products. Ind. Crops Prod. 2017, 99, 27–33. [Google Scholar] [CrossRef]
- Avni, S.; Ezove, N.; Hanani, H.; Yadid, I.; Karpovsky, M.; Hayby, H.; Gover, O.; Hadar, Y.; Schwartz, B.; Danay, O. Olive Mill Waste Enhances α-Glucan Content in the Edible Mushroom Pleurotus Eryngii. Int. J. Mol. Sci. 2017, 18, 1564. [Google Scholar] [CrossRef]
- Huang, S.J.; Lin, C.P.; Tsai, S.Y. Vitamin D2 Content and Antioxidant Properties of Fruit Body and Mycelia of Edible Mushrooms by UV-B Irradiation. J. Food Compos. Anal. 2015, 42, 38–45. [Google Scholar] [CrossRef]
- Reverberi, M.; Di Mario, F.; Tomati, U. β-Glucan Synthase Induction in Mushrooms Grown on Olive Mill Wastewaters. Appl. Microbiol. Biotechnol. 2004, 66, 217–225. [Google Scholar] [CrossRef]
- Bumpus, J.A.; Tien, M.; Wright, D.; Aust, S.D. Oxidation of Persistent Environmental Pollutants by a White Rot Fungus. Science 1985, 228, 1434–1436. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Cabrera, C.P.; Bell, T.L.; Kertesz, M.A. Caffeine Metabolism during Cultivation of Oyster Mushroom (Pleurotus ostreatus) with Spent Coffee Grounds. Appl. Microbiol. Biotechnol. 2019, 103, 5831–5841. [Google Scholar] [CrossRef]
- Cohen, R.; Persky, L.; Hadar, Y. Biotechnological Applications and Potential of Wood-Degrading Mushrooms of the Genus Pleurotus. Appl. Microbiol. Biotechnol. 2002, 58, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Doroški, A.; Klaus, A.; Kozarski, M.; Cvetković, S.; Nikolić, B.; Jakovljević, D.; Tomasevic, I.; Vunduk, J.; Lazić, V.; Djekic, I. The Influence of Grape Pomace Substrate on Quality Characterization of Pleurotus Ostreatus—Total Quality Index Approach. J. Food Process. Preserv. 2021, 45, e15096. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Patsou, M.; Mitsou, E.K.; Bekiaris, G.; Kotsou, M.; Tarantilis, P.A.; Pletsa, V.; Kyriacou, A.; Zervakis, G.I. Valorization of Olive By-Products as Substrates for the Cultivation of Ganoderma Lucidum and Pleurotus Ostreatus Mushrooms with Enhanced Functional and Prebiotic Properties. Catalysts 2019, 9, 537. [Google Scholar] [CrossRef]
- Economou, C.N.; Economou, C.N.; Philippoussis, A.N.; Diamantopoulou, P.A. Spent Mushroom Substrate for a Second Cultivation Cycle of Pleurotus Mushrooms and Dephenolization of Agro-Industrial Wastewaters. FEMS Microbiol. Lett. 2020, 367, fnaa060. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, Ó.J.; Montoya, S. Assessment of Polysaccharide and Biomass Production from Three White-Rot Fungi by Solid-State Fermentation Using Wood and Agro-Industrial Residues: A Kinetic Approach. Forests 2020, 11, 1055. [Google Scholar] [CrossRef]
- Ferreira da Silva, I.; Rodrigues da Luz, J.M.; Oliveira, S.F.; Humberto de Queiroz, J.; Megumi Kasuya, M.C. High-Yield Cellulase and LiP Production after SSF of Agricultural Wastes by Pleurotus Ostreatus Using Different Surfactants. Biocatal. Agric. Biotechnol. 2019, 22, 101428. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Kalogeropoulos, N.; Kaliora, A.C.; Zervakis, G.I. Toward an Increased Functionality in Oyster (Pleurotus) Mushrooms Produced on Grape Marc or Olive Mill Wastes Serving as Sources of Bioactive Compounds. J. Agric. Food Chem. 2018, 66, 5971–5983. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.S.; Nene, S.N.; Joshi, K.S. A Comparative Study of Production of Hydrophobin like Proteins (HYD-LPs) in Submerged Liquid and Solid State Fermentation from White Rot Fungus Pleurotus ostreatus. Biocatal. Agric. Biotechnol. 2020, 23, 101440. [Google Scholar] [CrossRef]
- Omoni, V.T.; Ibeto, C.N.; Lag-Brotons, A.J.; Bankole, P.O.; Semple, K.T. Impact of Lignocellulosic Waste-Immobilised White-Rot Fungi on Enhancing the Development of 14C-Phenanthrene Catabolism in Soil. Sci. Total Environ. 2022, 811, 152243. [Google Scholar] [CrossRef] [PubMed]
- Sözbir Düzkale, G. Utilization of Various Lignocellulosic Substrates for Pleurotus ostreatus Mushroom Cultivation in the Manufacturing of Polycaprolactone (PCL)-Based Biocomposite Films. BioResources 2021, 16, 3783–3796. [Google Scholar] [CrossRef]
- Abou Fayssal, S.; Alsanad, M.A.; El Sebaaly, Z.; Ismail, A.I.H.; Sassine, Y.N. Valorization of Olive Pruning Residues through Bioconversion into Edible Mushroom Pleurotus ostreatus (Jacq. Ex Fr.) P. Kumm. (1871) of Improved Nutritional Value. Scientifica 2020, 2020, 3950357. [Google Scholar] [CrossRef] [PubMed]
- Cayetano-Catarino, M.; Bernabé-Villanueva, G.; Romero-Flores, A.; Bernabé-González, T. Three-Plant Stubble (Family: Fabaceae) as a Substrate for Cultivation of Pleurotus Ostreatus (Jacq.) P. Kummer., in Mexico. J. Appl. Nat. Sci. 2020, 12, 156–158. [Google Scholar] [CrossRef]
- Adebayo, E.A.; Elkanah, F.A.; Afolabi, F.J.; Ogundun, O.S.; Alabi, T.F.; Oduoye, O.T. Molecular Characterization of Most Cultivated Pleurotus Species in Sub-Western Region Nigeria with Development of Cost Effective Cultivation Protocol on Palm Oil Waste. Heliyon 2021, 7, e06215. [Google Scholar] [CrossRef] [PubMed]
- Myronycheva, O.; Bandura, I.; Bisko, N.; Gryganskyi, A.P.; Karlsson, O. Assessment of the Growth and Fruiting of 19 Oyster Mushroom Strains for Indoor Cultivation on Lignocellulosic Wastes. BioResources 2017, 12, 4606–4626. [Google Scholar] [CrossRef]
- Sundarraj, A.A.; Ranganathan, T.V. A Review on Cellulose and Its Utilization from Agro-Industrial Waste. Drug Invent. Today 2018, 10, 89–94. [Google Scholar]
- Nwanze, P.I.; Khan, A.U.; Ameh, J.B.; Umoh, V.J. The Effect of the Interaction of Various Spawn Grains with Different Culture Medium on Carpophore Dry Weights and Stipe and Pileus Diameters of Lentinus squarrosulus (Mont.) Singer. Afr. J. Biotechnol. 2005, 4, 615–619. [Google Scholar] [CrossRef]
- Chang, S.T.; Miles, P.G. Edible Mushrooms and Their Cultivation; CRC Press: Boca Raton, FL, USA, 1989. [Google Scholar]
- Elisashvili, V.I.; Kachlishvili, E.T.; Wasser, S.P. Carbon and Nitrogen Source Effects on Basidiomycetes Exopolysaccharide Production. Appl. Biochem. Microbiol. 2009, 45, 531–535. [Google Scholar] [CrossRef]
- Choi, K. Shelf Cultivation of Oyster Mushroom with Emphasis on Substrate Fermentation. In Mushroom Growers’ Handbook 1. Oyster Mushrooms Cultivation. Part II: Oyster Mushrooms; MushWorld: Seoul, Korea, 2004. [Google Scholar]
- Naraian, R.; Sahu, R.K.; Kumar, S.; Garg, S.K.; Singh, C.S.; Kanaujia, R.S. Influence of Different Nitrogen Rich Supplements during Cultivation of Pleurotus Florida on Corn Cob Substrate. Environmentalist 2009, 29, 1. [Google Scholar] [CrossRef]
- Culture conditions for increasing yields of lentinula edodes. In The 4th ICMBMP; National University of Mexico: Ciudad de México, Mexico, 2002; pp. 1–9. Available online: http://www.gobe.si/dokumenti/Culture%20Conditions%20for%20Increasing%20Yields%20of%20Lentinula%20edodes.pdf (accessed on 19 September 2022).
- Rodriguez Estrada, A.E.; Royse, D.J. Yield, Size and Bacterial Blotch Resistance of Pleurotus Eryngii Grown on Cottonseed Hulls/Oak Sawdust Supplemented with Manganese, Copper and Whole Ground Soybean. Bioresour. Technol. 2007, 98, 1898–1906. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, S.; Yildiz, Ü.C.; Gezer, E.D.; Temiz, A. Some Lignocellulosic Wastes Used as Raw Material in Cultivation of the Pleurotus Ostreatus Culture Mushroom. Process Biochem. 2002, 38, 301–306. [Google Scholar] [CrossRef]
- Zervakis, G.I.; Koutrotsios, G.; Katsaris, P. Composted versus Raw Olive Mill Waste as Substrates for the Production of Medicinal Mushrooms: An Assessment of Selected Cultivation and Quality Parameters. Biomed Res. Int. 2013, 2013, 546830. [Google Scholar] [CrossRef]
- Gowthaman, M.K.; Krishna, C.; Moo-Young, M. Fungal Solid State Fermentation—An Overview. Appl. Mycol. Biotechnol. 2001, 1, 305–352. [Google Scholar] [CrossRef]
- Sakellari, A.; Karavoltsos, S.; Tagkouli, D.; Rizou, C.; Sinanoglou, V.J.; Zoumpoulakis, P.; Koutrotsios, G.; Zervakis, G.I.; Kalogeropoulos, N. Trace Elements in Pleurotus Ostreatus, P. Eryngii, and P. Nebrodensis Mushrooms Cultivated on Various Agricultural By-Products. Anal. Lett. 2019, 52, 2692–2709. [Google Scholar] [CrossRef]
- Melanouri, E.M.; Dedousi, M.; Diamantopoulou, P. Cultivating Pleurotus Ostreatus and Pleurotus Eryngii Mushroom Strains on Agro-Industrial Residues in Solid-State Fermentation. Part I: Screening for Growth, Endoglucanase, Laccase and Biomass Production in the Colonization Phase. Carbon Resour. Convers. 2022, 5, 61–70. [Google Scholar] [CrossRef]
- Zárate-Salazar, J.R.; Santos, M.N.; Caballero, E.N.M.; Martins, O.G.; Herrera, Á.A.P. Use of Lignocellulosic Corn and Rice Wastes as Substrates for Oyster Mushroom (Pleurotus ostreatus Jacq.) Cultivation. SN Appl. Sci. 2020, 2, 1904. [Google Scholar] [CrossRef]
- Ma, N.L.; Khoo, S.C.; Peng, W.; Ng, C.M.; Teh, C.H.; Park, Y.K.; Lam, S.S. Green Application and Toxic Risk of Used Diaper and Food Waste as Growth Substitute for Sustainable Cultivation of Oyster Mushroom (Pleurotus ostreatus). J. Clean. Prod. 2020, 268, 122272. [Google Scholar] [CrossRef]
- Rugolo, M.; Lechner, B.; Mansilla, R.; Mata, G.; Rajchenberg, M. Evaluation of Pleurotus Ostreatus Basidiomes Production on Pinus Sawdust and Other Agricultural and Forestry Wastes from Patagonia, Argentina. Maderas Cienc. Tecnol. 2020, 22, 517–526. [Google Scholar] [CrossRef]
- Fernandes Pereira, C.; AntÃ’nio, Z.d.M.C.; Givaldo, R.N.; José, L.B.; Ana, P.T.U.; Elizama, A.-O. Brewers Residues and Cocoa Pod Shells as a Substrate for Cultivation of Pleurotus Ostreatus CCIBt 2339 and Enzymes Production. Afr. J. Biotechnol. 2021, 20, 115–121. [Google Scholar] [CrossRef]
- Narváez, L.; Bolaños, A.C.; Chaurra, A.; Escobar, O.Z. Changes in Macronutrients and Physical Properties during the Growth of Lentinula edodes and Pleurotus ostreatus in a Compost Based on Sugarcane Bagasse Agricultural Waste. Chil. J. Agric. Anim. Sci. 2021, 37, 301–312. [Google Scholar] [CrossRef]
- Zakil, F.A.; Sueb, M.S.M.; Isha, R. Growth and Yield Performance of Pleurotus ostreatus on Various Agro-Industrial Wastes in Malaysia. AIP Conf. Proc. 2019, 2155, 020055. [Google Scholar] [CrossRef]
- Huang, W.; Chufo Wachemo, A.; Yuan, H.; Li, X. Full Utilization of Nutrients in Rice Straw by Integrating Mushroom Cultivation, Biogas Production, and Fertilizer Use. Int. J. Agric. Biol. Eng. 2019, 12, 174–183. [Google Scholar] [CrossRef]
- Economou, C.N.; Diamantopoulou, P.A.; Philippoussis, A.N. Valorization of Spent Oyster Mushroom Substrate and Laccase Recovery through Successive Solid State Cultivation of Pleurotus, Ganoderma, and Lentinula Strains. Appl. Microbiol. Biotechnol. 2017, 101, 5213–5222. [Google Scholar] [CrossRef]
- Tagkouli, D.; Bekiaris, G.; Pantazi, S.; Anastasopoulou, M.E.; Koutrotsios, G.; Mallouchos, A.; Zervakis, G.I.; Kalogeropoulos, N. Volatile Profiling of Pleurotus Eryngii and Pleurotus Ostreatus Cultivated on Agricultural and Agro-Industrial by-Products. Foods 2021, 10, 1287. [Google Scholar] [CrossRef]
- Tsiantas, K.; Tsiaka, T.; Koutrotsios, G.; Siapi, E.; Zervakis, G.I.; Kalogeropoulos, N.; Zoumpoulakis, P. On the Identification and Quantification of Ergothioneine and Lovastatin in Various Mushroom Species: Assets and Challenges of Different Analytical Approaches. Molecules 2021, 26, 1832. [Google Scholar] [CrossRef]
- Melanouri, E.M.; Dedousi, M.; Diamantopoulou, P. Cultivating Pleurotus Ostreatus and Pleurotus Eryngii Mushroom Strains on Agro-Industrial Residues in Solid-State Fermentation. Part II: Effect on Productivity and Quality of Carposomes. Carbon Resour. Convers. 2022, 5, 52–60. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Ranamukhaarachchi, S.L. Effect of Different Culture Media, Grain Sources and Alternate Substrates on the Mycelial Growth of Pleurotus Eryngii and Pleurotus Ostreatus. Pak. J. Biol. Sci. 2020, 23, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Lowor, S.T.; Ofori, E. Evaluation of Cashew Pulp and Shell, Kola, Cocoa and Coffee Husk as Substrates for the Cultivation of Pleurotus Ostreatus. J. Adv. Biol. Biotechnol. 2018, 19, 1–10. [Google Scholar] [CrossRef]
- Da Silva, R.M.; do Carmo, C.O.; de Oliveira, T.A.S.; de Figueirêdo, V.R.; Duarte, E.A.A.; Soares, A.C.F. Biological Efficiency and Nutritional Value of Pleurotus Ostreatus Cultivated in Agroindustrial Wastes of Palm Oil Fruits and Cocoa Almonds. Arq. Inst. Biol. 2020, 87, 1–10. [Google Scholar] [CrossRef]
- Nam, W.L.; Su, M.H.; Phang, X.Y.; Chong, M.Y.; Liew, R.K.; Ma, N.L.; Lam, S.S. Production of Bio-Fertilizer from Microwave Vacuum Pyrolysis of Waste Palm Shell for Cultivation of Oyster Mushroom (Pleurotus ostreatus). Sci. Total Environ. 2017, 624, 22. [Google Scholar] [CrossRef]
- De Aguiar, L.V.B.; Sales-Campos, C.; Gouvêa, P.R.D.S.; Vianez, B.F.; Dias, E.S.; Chevreuil, L.R. Substrate Disinfection Methods on the Production and Nutritional Composition of a Wild Oyster Mushroom from the Amazon. Cienc. Agrotecnologia 2021, 45, e010321. [Google Scholar] [CrossRef]
- Zied, D.C.; Prado, E.P.; Dias, E.S.; Pardo, J.E.; Pardo-Gimenez, A. Use of Peanut Waste for Oyster Mushroom Substrate Supplementation—Oyster Mushroom and Peanut Waste. Braz. J. Microbiol. 2019, 50, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Halawani, R.F.; Aloufi, F.A.; Taleb, M.A.; Akter, S.; Mahmood, S. Utilization of Agro-Industrial Wastes for the Production of Quality Oyster Mushrooms. Sustainability 2022, 14, 994. [Google Scholar] [CrossRef]
- Wiafe-Kwagyan, M.; Odamtten, G.T.; Kortei, N.K. Influence of Substrate Formulation on Some Morphometric Characters and Biological Efficiency of Pleurotus ostreatus EM-1 (Ex. Fr) Kummer Grown on Rice Wastes and “Wawa” (Triplochiton scleroxylon) Sawdust in Ghana. Food Sci. 2022, 10, 1854–1863. [Google Scholar] [CrossRef]
- Iwuagwu, M.; Nwaukwa, D.; Nwaru, C. Use of Different Agro-Wastes in the Cultivation of Pleurotus ostreatus (Jacq.) Kummer. J. Bioresour. Manag. 2020, 7, 29–38. [Google Scholar] [CrossRef]
- Fufa, B.K.; Tadesse, B.A.; Tulu, M.M. Cultivation of Pleurotus ostreatus on Agricultural Wastes and Their Combination. Int. J. Agron. 2021, 2021, 1465597. [Google Scholar] [CrossRef]
- Djekic, I.; Vunduk, J.; Tomašević, I.; Kozarski, M.; Petrovic, P.; Niksic, M.; Pudja, P.; Klaus, A. Total Quality Index of Agaricus Bisporus Mushrooms Packed in Modified Atmosphere. J. Sci. Food Agric. 2017, 97, 3013–3021. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doroški, A.; Klaus, A.; Režek Jambrak, A.; Djekic, I. Food Waste Originated Material as an Alternative Substrate Used for the Cultivation of Oyster Mushroom (Pleurotus ostreatus): A Review. Sustainability 2022, 14, 12509. https://doi.org/10.3390/su141912509
Doroški A, Klaus A, Režek Jambrak A, Djekic I. Food Waste Originated Material as an Alternative Substrate Used for the Cultivation of Oyster Mushroom (Pleurotus ostreatus): A Review. Sustainability. 2022; 14(19):12509. https://doi.org/10.3390/su141912509
Chicago/Turabian StyleDoroški, Ana, Anita Klaus, Anet Režek Jambrak, and Ilija Djekic. 2022. "Food Waste Originated Material as an Alternative Substrate Used for the Cultivation of Oyster Mushroom (Pleurotus ostreatus): A Review" Sustainability 14, no. 19: 12509. https://doi.org/10.3390/su141912509
APA StyleDoroški, A., Klaus, A., Režek Jambrak, A., & Djekic, I. (2022). Food Waste Originated Material as an Alternative Substrate Used for the Cultivation of Oyster Mushroom (Pleurotus ostreatus): A Review. Sustainability, 14(19), 12509. https://doi.org/10.3390/su141912509








