From Chestnut Tree (Castanea sativa) to Flour and Foods: A Systematic Review of the Main Criticalities and Control Strategies towards the Relaunch of Chestnut Production Chain
Abstract
1. Introduction
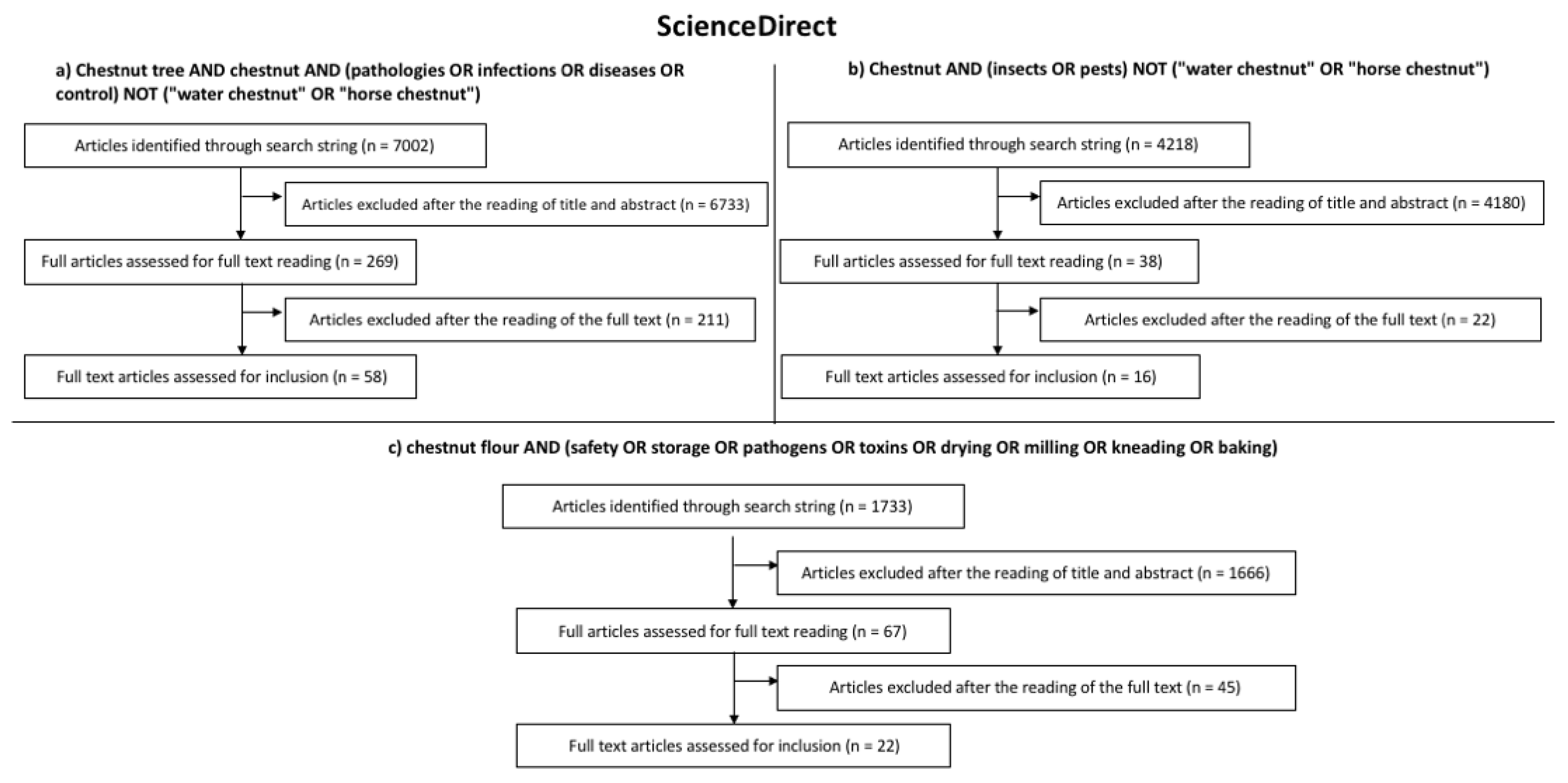
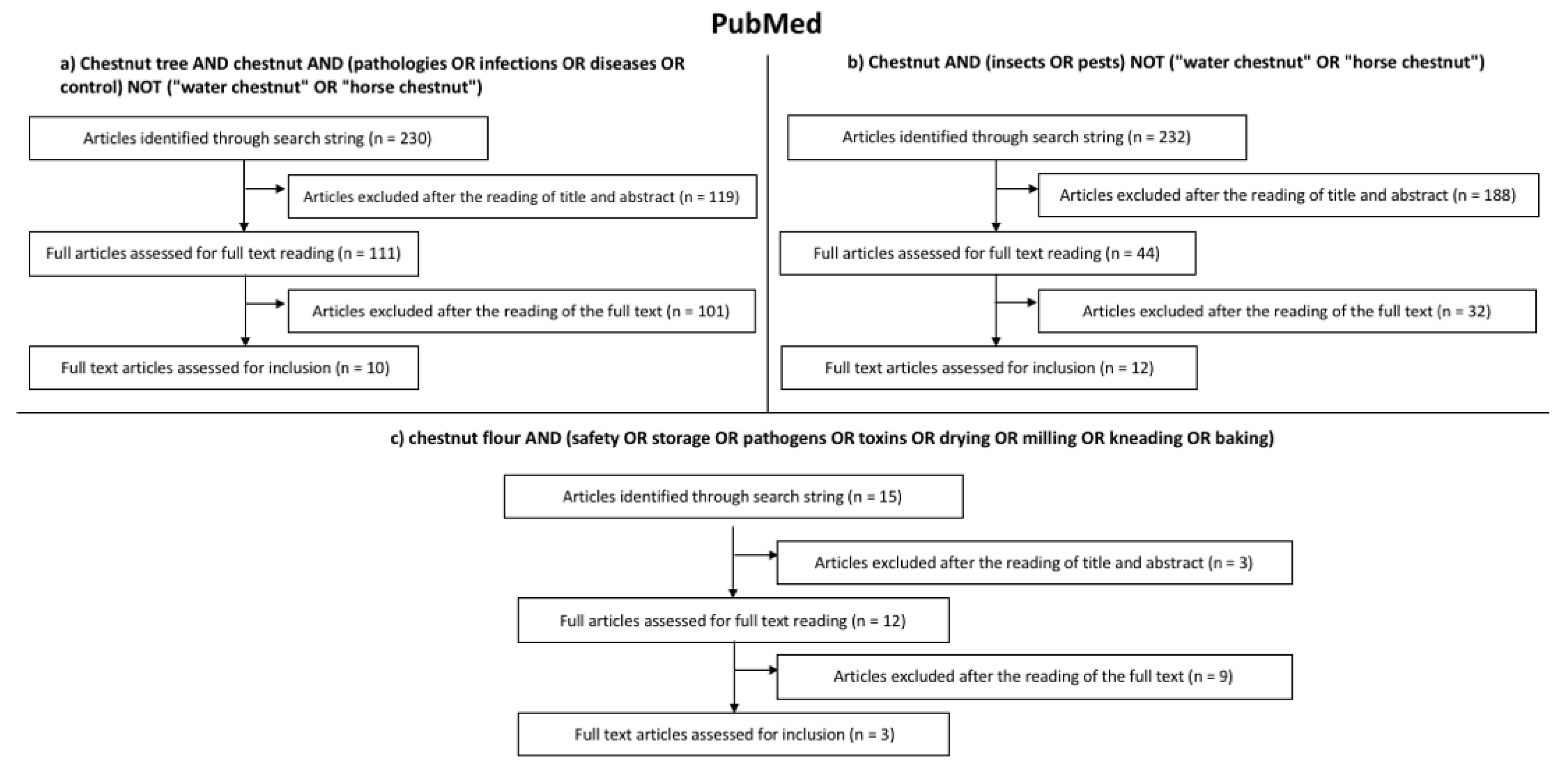
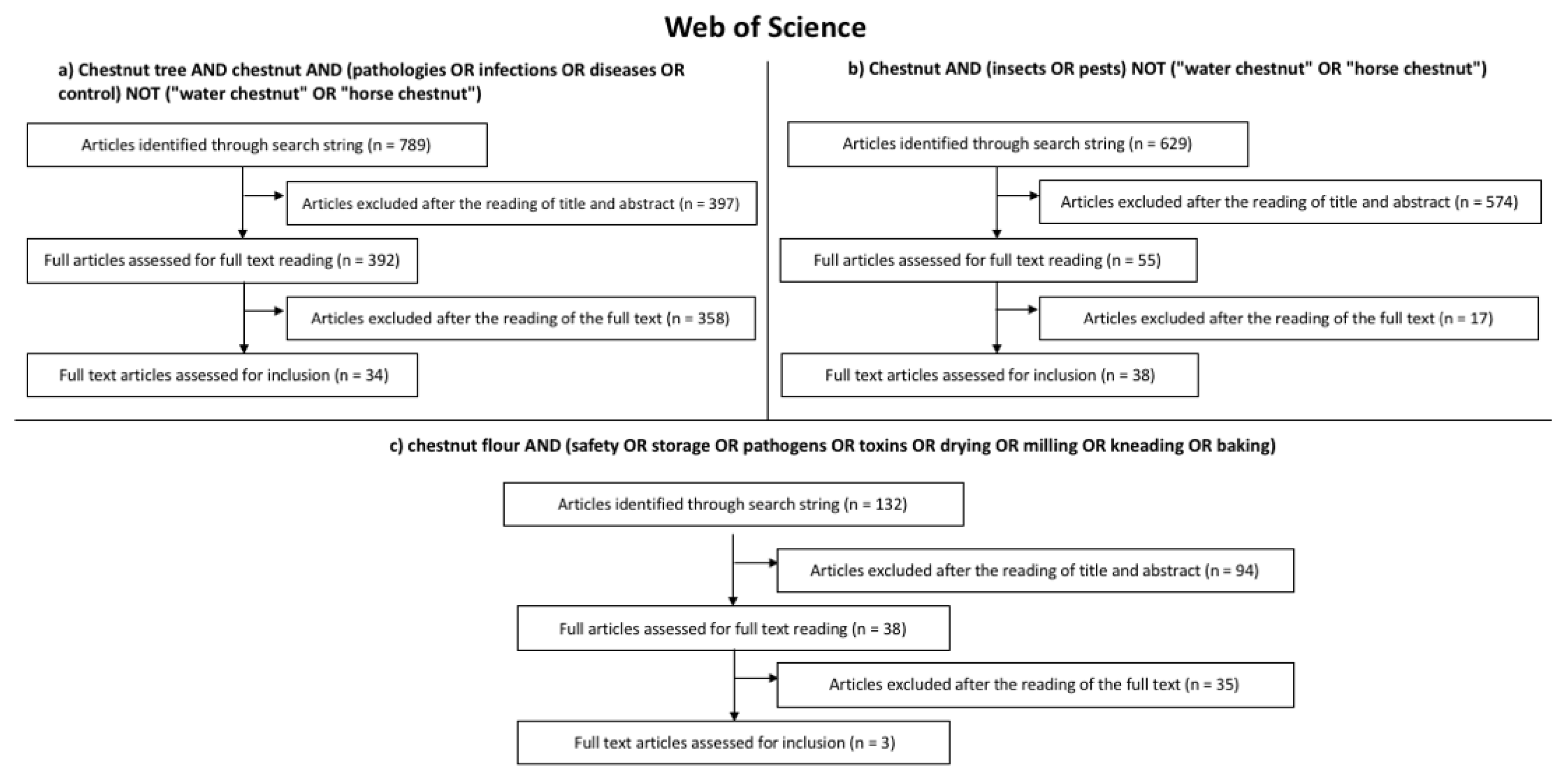
2. Search Strategy
- -
- Chestnut tree AND chestnut AND (pathologies OR infections OR diseases OR control) NOT (“water chestnut” OR “horse chestnut”);
- -
- Chestnut AND (insects OR pests) NOT (“water chestnut” OR “horse chestnut”);
- -
- Chestnut flour AND (safety OR storage OR pathogens OR toxins OR drying OR milling OR kneading OR baking).
3. Results of the Systematic Literature Review
4. Chestnut Tree
4.1. Pests and Diseases Affecting Chestnut Tree
4.1.1. Ink Disease
4.1.2. Chestnut Blight
4.1.3. Emerging Pathogens
4.1.4. Pests Affecting Chestnut Tree: The Asian Gall Wasp Dryocosmus kuriphilus
4.2. Strategies for Prevention, Control, and Management of Pests and Pathologies
4.2.1. Early Detection as Prevention Strategy
4.2.2. Control Strategies: Resistance Breeding and Biocontrol
4.2.3. Management Strategies
5. Chestnuts
5.1. Main Criticalities in Harvest, Post-Harvest, Processing, and Storage
5.1.1. Insects and Their Control Strategies
5.1.2. Molds and Toxins
5.2. Challenges and Opportunities in Chestnut Drying
6. Chestnut Flour and Applications in the Food Industry
6.1. Effects of Drying Conditions on the Safety and Characteristics of Flour, Doughs, and Products
6.2. Innovative Applications of Chestnut Flour in the Food Industry
6.3. Valorization of Chestnut by-Products
7. Conclusions and Future Trends
Author Contributions
Funding
Conflicts of Interest
References
- Pérez-Girón, J.C.; Alvarez-Alvarez, P.; Díaz-Varela, E.R.; Lopes, D.M.M. Influence of climate variations on primary production indicators and on the resilience of forest ecosystems in a future scenario of climate change: Application to sweet chestnut agroforestry systems in the Iberian Peninsula. Ecol. Indic. 2020, 113, 106199. [Google Scholar] [CrossRef]
- Pinto, D.; Braga, N.; Silva, A.M.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Chestnut. In Valorization of Fruit Processing By-Products; Academic Press: Cambridge, MA, USA, 2020; pp. 127–144. [Google Scholar]
- Massantini, R.; Moscetti, R.; Frangipane, M.T. Evaluating progress of chestnut quality: A review of recent developments. Trends Food Sci. Technol. 2021, 113, 245–254. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Luchi, N.; Rizzo, D.; Pepori, A.L.; Pecori, F.; Santini, A. Rapid diagnostics for Gnomoniopsis smithogilvyi (syn. Gnomoniopsis castaneae) in chestnut nuts: New challenges by using LAMP and real-time PCR methods. AMB Express 2021, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Pezzi, G.; Gambini, S.; Buldrini, F.; Ferretti, F.; Muzzi, E.; Maresi, G.; Nascimbene, J. Contrasting patterns of tree features, lichen, and plant diversity in managed and abandoned old-growth chestnut orchards of the northern Apennines (Italy). For. Ecol. Manag. 2020, 470, 118207. [Google Scholar] [CrossRef]
- Zhou, S.; Reddy, C.K.; Du, B.; Xu, B. Pasting, thermal, and functional properties of wheat flour and rice flour formulated with chestnut flour. Bioact. Carbohydr. Diet. Fibre 2021, 26, 100290. [Google Scholar] [CrossRef]
- Prencipe, S.; Siciliano, I.; Gatti, C.; Garibaldi, A.; Gullino, M.L.; Botta, R.; Spadaro, D. Several species of Penicillium isolated from chestnut flour processing are pathogenic on fresh chestnuts and produce mycotoxins. Food Microbiol. 2018, 76, 396–404. [Google Scholar] [CrossRef]
- Romano, A.; Aponte, M. Chestnut as source of novel ingredients for celiac people. Encycl. Food Secur. Sustain. 2019, 1, 364–368. [Google Scholar]
- Sirini, N.; Roldán, A.; Lucas-González, R.; Fernández-López, J.; Viuda-Martos, M.; Pérez-Álvarez, J.A.; Rosmini, M.R. Effect of chestnut flour and probiotic microorganism on the functionality of dry-cured meat sausages. LWT Food Sci. Technol. 2020, 134, 110197. [Google Scholar] [CrossRef]
- Paciulli, M.; Rinaldi, M.; Cavazza, A.; Ganino, T.; Rodolfi, M.; Chiancone, B.; Chiavaro, E. Effect of chestnut flour supplementation on physico-chemical properties and oxidative stability of gluten-free biscuits during storage. LWT Food Sci. Technol. 2018, 98, 451–457. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Freitas, T.R.; Santos, J.A.; Silva, A.P.; Fraga, H. Influence of climate change on chestnut trees: A review. Plants 2021, 10, 1463. [Google Scholar] [CrossRef] [PubMed]
- Ghelardini, L.; Pepori, A.L.; Luchi, N.; Capretti, P.; Santini, A. Drivers of emerging fungal diseases of forest trees. For. Ecol. Manag. 2016, 381, 235–246. [Google Scholar] [CrossRef]
- Stauber, L.; Prospero, S.; Croll, D. Comparative genomics analyses of lifestyle transitions at the origin of an invasive fungal pathogen in the genus Cryphonectria. Msphere 2020, 5, e00737-20. [Google Scholar] [CrossRef] [PubMed]
- Panzavolta, T.; Bracalini, M.; Benigno, A.; Moricca, S. Alien invasive pathogens and pests harming trees, forests, and plantations: Pathways, global consequences and management. Forests 2021, 12, 1364. [Google Scholar] [CrossRef]
- Sena, K.; Crocker, E.; Vincelli, P.; Barton, C. Phytophthora cinnamomi as a driver of forest change: Implications for conservation and management. For. Ecol. Manag. 2018, 409, 799–807. [Google Scholar] [CrossRef]
- Saiz-Fernández, I.; Milenković, I.; Berka, M.; Černý, M.; Tomšovský, M.; Brzobohatý, B.; Kerchev, P. Integrated proteomic and metabolomic profiling of Phytophthora cinnamomi attack on sweet chestnut (Castanea sativa) reveals distinct molecular reprogramming proximal to the infection site and away from it. Int. J. Mol. Sci. 2020, 21, 8525. [Google Scholar] [CrossRef]
- Jung, T.; Pérez-Sierra, A.; Durán, A.; Jung, M.H.; Balci, Y.; Scanu, B. Canker and decline diseases caused by soil-and airborne Phytophthora species in forests and woodlands. Pers. Mol. Phylogeny Evol. Fungi 2018, 40, 182–220. [Google Scholar] [CrossRef]
- Benavent-Celma, C.; López-García, N.; Ruba, T.; Ściślak, M.E.; Street-Jones, D.; van West, P.; Woodward, S.; Witzell, J. Current practices and emerging possibilities for reducing the spread of oomycete pathogens in terrestrial and aquatic production systems in the European Union. Fungal Biol. Rev. 2022, 40, 19–36. [Google Scholar] [CrossRef]
- Akilli Şimşek, S.; Katircioğlu, Y.Z.; Ulubaş Serçe, Ç.; Çakar, D.; Rigling, D.; Maden, S. Phytophthora species associated with dieback of sweet chestnut in Western Turkey. For. Pathol. 2019, 49, e12533. [Google Scholar] [CrossRef]
- Rigling, D.; Prospero, S. Cryphonectria parasitica, the causal agent of chestnut blight: Invasion history, population biology and disease control. Mol. Plant Pathol. 2018, 19, 7–20. [Google Scholar] [CrossRef]
- Cipollini, M.; Dingley, N.R.; Felch, P.; Maddox, C. Evaluation of phenotypic traits and blight-resistance in an American chestnut backcross orchard in Georgia. Glob. Ecol. Conserv. 2017, 10, 1–8. [Google Scholar] [CrossRef]
- Diamandis, S. Management of chestnut blight in Greece using hypovirulence and silvicultural interventions. Forests 2018, 9, 492. [Google Scholar] [CrossRef]
- Chandelier, A.; Massot, M.; Fabreguettes, O.; Gischer, F.; Teng, F.; Robin, C. Early detection of Cryphonectria parasitica by real-time PCR. Eur. J. Plant Pathol. 2019, 153, 29–46. [Google Scholar] [CrossRef]
- Demené, A.; Legrand, L.; Gouzy, J.; Debuchy, R.; Saint-Jean, G.; Fabreguettes, O.; Dutech, C. Whole-genome sequencing reveals recent and frequent genetic recombination between clonal lineages of Cryphonectria parasitica in western Europe. Fungal Genet. Biol. 2019, 130, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Murolo, S.; Concas, J.; Romanazzi, G. Use of biocontrol agents as potential tools in the management of chestnut blight. Biol. Control 2019, 132, 102–109. [Google Scholar] [CrossRef]
- Kolp, M.; Double, M.L.; Fulbright, D.W.; MacDonald, W.L.; Jarosz, A.M. Spatial and temporal dynamics of the fungal community of chestnut blight cankers on American chestnut (Castanea dentata) in Michigan and Wisconsin. Fungal Ecol. 2020, 45, 100925. [Google Scholar] [CrossRef]
- Sato, Y.; Shahi, S.; Telengech, P.; Hisano, S.; Cornejo, C.; Rigling, D.; Kondo, H.; Suzuki, N. A new tetra-segmented splipalmivirus with divided RdRP domains from Cryphonectria naterciae, a fungus found on chestnut and cork oak trees in Europe. Virus Res. 2022, 307, 198606. [Google Scholar] [CrossRef]
- Regué, A.; Bassié, L.; de-Miguel, S.; Colinas, C. Environmental and stand conditions related to Fistulina hepatica heart rot attack on Castanea sativa. For. Pathol. 2019, 49, e12517. [Google Scholar] [CrossRef]
- Yousefzadeh, H.; Saidi, A.; Tayebi, S.; Kartoolinejad, D.; Naghdi, R. Molecular approach to determine taxonomic status of Septoria sp. causing leaf blotch of Castanea sativa in Hyrcanian forests. J. For. Res. 2017, 28, 661–670. [Google Scholar] [CrossRef]
- Dar, M.A.; Rai, M. Gnomoniopsis smithogilvyi, a canker causing pathogen on Castanea sativa: First report. Mycosphere 2015, 6, 327–336. [Google Scholar] [CrossRef]
- Jiang, N.; Tian, C. An emerging pathogen from rotted chestnut in China: Gnomoniopsis daii sp. nov. Forests 2019, 10, 1016. [Google Scholar] [CrossRef]
- Jiang, N.; Liang, L.Y.; Tian, C.M. Gnomoniopsis chinensis (Gnomoniaceae, Diaporthales), a new fungus causing canker of Chinese chestnut in Hebei Province, China. MycoKeys 2020, 67, 19. [Google Scholar] [CrossRef] [PubMed]
- Lione, G.; Danti, R.; Fernandez-Conradi, P.; Ferreira-Cardoso, J.V.; Lefort, F.; Marques, G.; Meyer, J.B.; Prospero, S.; Radócz, L.; Robin, C.; et al. The emerging pathogen of chestnut Gnomoniopsis castaneae: The challenge posed by a versatile fungus. Eur. J. Plant Pathol. 2019, 153, 671–685. [Google Scholar] [CrossRef]
- Lione, G.; Giordano, L.; Sillo, F.; Brescia, F.; Gonthier, P. Temporal and spatial propagule deposition patterns of the emerging fungal pathogen of chestnut Gnomoniopsis castaneae in orchards of north-western Italy. Plant Pathol. 2021, 70, 2016–2033. [Google Scholar] [CrossRef]
- Trapiello, E.; Feito, I.; González, A.J. First report of Gnomoniopsis castaneae causing canker on hybrid plants of Castanea sativa× C. crenata in Spain. Plant Dis. 2018, 102, 1040. [Google Scholar] [CrossRef]
- Turco, S.; Bastianelli, G.; Morales-Rodrìguez, C.; Vannini, A.; Mazzaglia, A. Development of a TaqMan qPCR assay for the detection and quantification of Gnomoniopsis castaneae in chestnut tissues. For. Pathol. 2021, 51, e12701. [Google Scholar] [CrossRef]
- Lewis, A.; Gorton, C.; Rees, H.; Webber, J.; Perez-Sierra, A. First report of Gnomoniopsis smithogilvyi causing lesions and cankers of sweet chestnut in the United Kingdom. New Dis. Rep. 2017, 35, 20. [Google Scholar] [CrossRef]
- Pasche, S.; Crovadore, J.; Pelleteret, P.; Jermini, M.; Mauch-Mani, B.; Oszako, T.; Lefort, F. Biological control of the latent pathogen Gnomoniopsis smithogylvyi in European chestnut grafting scions using Bacillus amyloliquefaciens and Trichoderma atroviride. Dendrobiology 2016, 75, 113–122. [Google Scholar] [CrossRef]
- Sillo, F.; Giordano, L.; Zampieri, E.; Lione, G.; De Cesare, S.; Gonthier, P. HRM analysis provides insights on the reproduction mode and the population structure of Gnomoniopsis castaneae in Europe. Plant Pathol. 2017, 66, 293–303. [Google Scholar] [CrossRef]
- Vannini, A.; Vettraino, A.; Martignoni, D.; Morales-Rodriguez, C.; Contarini, M.; Caccia, R.; Paparatti, B.; Speranza, S. Does Gnomoniopsis castanea contribute to the natural biological control of chestnut gall wasp? Fungal Biol. 2017, 121, 44–52. [Google Scholar] [CrossRef]
- Shuttleworth, L.A.; Guest, D.I. The infection process of chestnut rot, an important disease caused by Gnomoniopsis smithogilvyi (Gnomoniaceae, Diaporthales) in Oceania and Europe. Australas. Plant Pathol. 2017, 46, 397–405. [Google Scholar] [CrossRef]
- Fernandez-Conradi, P.; Fort, T.; Castagneyrol, B.; Jactel, H.; Robin, C. Fungal endophyte communities differ between chestnut galls and surrounding foliar tissues. Fungal Ecol. 2019, 42, 100876. [Google Scholar] [CrossRef]
- Gehring, E.; Bellosi, B.; Reynaud, N.; Conedera, M. Chestnut tree damage evolution due to Dryocosmus kuriphilus attacks. J. Pest Sci. 2020, 93, 103–115. [Google Scholar] [CrossRef]
- Quinto, J.; Wong, M.E.; Boyero, J.R.; Vela, J.M.; Aguirrebengoa, M. Population Dynamics and Tree Damage of the Invasive Chestnut Gall Wasp, Dryocosmus kuriphilus, in Its Southernmost European Distributional Range. Insects 2021, 12, 900. [Google Scholar] [CrossRef] [PubMed]
- Bonsignore, C.P.; Vizzari, G.; Vono, G.; Bernardo, U. Short-term cold stress affects parasitism on the Asian chestnut gall wasp Dryocosmus kuriphilus. Insects 2020, 11, 841. [Google Scholar] [CrossRef] [PubMed]
- Gil-Tapetado, D.; Cabrero-Sañudo, F.J.; Gómez, J.F.; Askew, R.R.; Nieves-Aldrey, J.L. Differences in native and introduced chalcid parasitoid communities recruited by the invasive chestnut pest Dryocosmus kuriphilus in two Iberian territories. Bull. Entomol. Res. 2021, 111, 307–322. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Panja, A. Different mechanisms of signaling pathways for plant protection from diseases by fungi. In Biocontrol Agents and Secondary Metabolites; Woodhead Publishing: Sawston, UK, 2021; pp. 591–630. [Google Scholar]
- Ray, M.; Ray, A.; Dash, S.; Mishra, A.; Achary, K.G.; Nayak, S.; Singh, S. Fungal disease detection in plants: Traditional assays, novel diagnostic techniques and biosensors. Biosens. Bioelectron. 2017, 87, 708–723. [Google Scholar] [CrossRef]
- Prospero, S.; Botella, L.; Santini, A.; Robin, C. Biological control of emerging forest diseases: How can we move from dreams to reality? For. Ecol. Manag. 2021, 496, 119377. [Google Scholar] [CrossRef]
- Rubio, S.; Barnes, A.; Webb, K.; Hodgetts, J. A real-time PCR assay for improved rapid, specific detection of Cryphonectria parasitica. Ann. Appl. Biol. 2017, 171, 52–61. [Google Scholar] [CrossRef]
- Westbrook, J.W.; Holliday, J.A.; Newhouse, A.E.; Powell, W.A. A plan to diversify a transgenic blight-tolerant American chestnut population using citizen science. Plants People Planet 2020, 2, 84–95. [Google Scholar] [CrossRef]
- Javed, M.R.; Ijaz, A.; Shahid, M.; Nadeem, H.; Shokat, Z.; Raziq, A. Fungal genome editing using CRISPR-Cas nucleases: A new tool for the management of plant diseases. In CRISPR and RNAi Systems; Elsevier: Amsterdam, The Netherlands, 2021; pp. 333–360. [Google Scholar]
- Ferracini, C.; Bertolino, S.; Bernardo, U.; Bonsignore, C.P.; Faccoli, M.; Ferrari, E.; Lupi, D.; Maini, S.; Mazzon, L.; Nugnes, F.; et al. Do Torymus sinensis (Hymenoptera: Torymidae) and agroforestry system affect native parasitoids associated with the Asian chestnut gall wasp? Biol. Control 2018, 121, 36–43. [Google Scholar] [CrossRef]
- Dorado, F.J.; Pujade-Villar, J.; Muñoz-Adalia, E.J.; Vinagrero, J.C.; Diez-Casero, J.J.; Fernández-Fernández, M.M. Characterization of native parasitoid community associated with the invasive pest Dryocosmus kuriphilus (Hymenoptera: Cynipidae) in Cantabria (northern Spain). Scand. J. For. Res. 2020, 35, 334–340. [Google Scholar] [CrossRef]
- Panzavolta, T.; Croci, F.; Bracalini, M.; Melika, G.; Benedettelli, S.; Tellini Florenzano, G.; Tiberi, R. Population dynamics of native parasitoids associated with the Asian chestnut gall wasp (Dryocosmus kuriphilus) in Italy. Psyche J. Entomol. 2018, 2, 8078049. [Google Scholar] [CrossRef]
- Avtzis, D.N.; Melika, G.; Matošević, D.; Coyle, D.R. The Asian chestnut gall wasp Dryocosmus kuriphilus: A global invader and a successful case of classical biological control. J. Pest Sci. 2019, 92, 107–115. [Google Scholar] [CrossRef]
- Loru, L.; Mannu, R.; Guerrieri, E.; Pantaleoni, R.A. Disentangling the effects of the invasive pest, Dryocosmus kuriphilus, and the introduction of the biocontrol agent, Torymus sinensis, on native parasitoids in an isolated insular chestnut-growing area. Biol. Control 2021, 162, 104724. [Google Scholar] [CrossRef]
- Sartor, C.; Dini, F.; Torello Marinoni, D.; Mellano, M.G.; Beccaro, G.L.; Alma, A.; Quacchia, A.; Botta, R. Impact of the Asian wasp Dryocosmus kuriphilus (Yasumatsu) on cultivated chestnut: Yield loss and cultivar susceptibility. Sci. Hortic. 2015, 197, 454–460. [Google Scholar] [CrossRef]
- Nugnes, F.; Gualtieri, L.; Bonsignore, C.P.; Parillo, R.; Annarumma, R.; Griffo, R.; Bernardo, U. Resistance of a local ecotype of Castanea sativa to Dryocosmus kuriphilus (Hymenoptera: Cynipidae) in Southern Italy. Forests 2018, 9, 94. [Google Scholar] [CrossRef]
- Castedo-Dorado, F.; Álvarez-Álvarez, P.; Cuenca Valera, B.; Lombardero, M.J. Local-scale dispersal patterns and susceptibility to Dryocosmus kuriphilus in different Castanea species and hybrid clones: Insights from a field trial. New For. 2021, 1–20. [Google Scholar] [CrossRef]
- Şahin, Y.S.; Gençer, N.; Susurluk, A.S. Control potentials of some entomopathogenic nematodes against Asian chestnut gall wasp, Dryocosmus kuriphilus Yasumatsu, 1951 (Hymenoptera: Cynipidae). Turk. J. Entomol. 2020, 44, 529–537. [Google Scholar] [CrossRef]
- Maltoni, A.; Mariotti, B.; Jacobs, D.F.; Tani, A. Pruning methods to restore Castanea sativa stands attacked by Dryocosmus kuriphilus. New For. 2012, 43, 869–885. [Google Scholar] [CrossRef]
- Fernandez-Conradi, P.; Borowiec, N.; Capdevielle, X.; Castagneyrol, B.; Maltoni, A.; Robin, C.; Selvi, F.; Van Halder, I.; Vétillard, F.; Jactel, H. Plant neighbour identity and invasive pathogen infection affect associational resistance to an invasive gall wasp. Biol. Invasions 2018, 20, 1459–1473. [Google Scholar] [CrossRef]
- Ciordia, M.; García, J.C.; Loureiro, M.D. Hot water treatment: An effective method for disinfecting Castanea sativa mill. dormant scions against Dryocosmus kuriphilus Yasumatsu. Pest Manag. Sci. 2020, 76, 1944–1948. [Google Scholar] [CrossRef] [PubMed]
- Menu, F.; Debouzie, D. Coin-flipping plasticity and prolonged diapause in insects: Example of the chestnut weevil Curculio elephas (Coleoptera: Curculionidae). Oecologia 1993, 93, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Sieber, T.N.; Jermini, M.; Conedera, M. Effects of the harvest method on the infestation of chestnuts (Castanea sativa) by insects and moulds. J. Phytopathol. 2007, 155, 497–504. [Google Scholar] [CrossRef]
- Demir, S.; Karagoz, M.; Hazir, S.; Kaya, H.K. Evaluation of entomopathogenic nematodes and their combined application against Curculio elephas and Polyphylla fullo larvae. J. Pest Sci. 2015, 88, 163–170. [Google Scholar] [CrossRef]
- Asan, C.; Hazir, S.; Cimen, H.; Ulug, D.; Taylor, J.; Butt, T.; Karagoz, M. An innovative strategy for control of the chestnut weevil Curculio elephas (Coleoptera: Curculionidae) using Metarhizium brunneum. Crop Prot. 2017, 102, 147–153. [Google Scholar] [CrossRef]
- Hummadi, E.H.; Dearden, A.; Generalovic, T.; Clunie, B.; Harrott, A.; Cetin, Y.; Demirbek, M.; Khoja, S.; Eastwood, D.; Dudley, E.; et al. Volatile organic compounds of Metarhizium brunneum influence the efficacy of entomopathogenic nematodes in insect control. Biol. Control 2021, 155, 104527. [Google Scholar] [CrossRef]
- Hou, L.; Hou, J.; Li, Z.; Johnson, J.A.; Wang, S. Validation of radio frequency treatments as alternative non-chemical methods for disinfesting chestnuts. J. Stored Prod. Res. 2015, 63, 75–79. [Google Scholar] [CrossRef]
- Pino-Hernández, E.; Pereira, R.N.; Ballesteros, L.F.; Vicente, A.A.; Abrunhosa, L.; Teixeira, J.A. Effects of moderate electric fields on the post-harvest preservation of chestnuts. Food Bioprocess Technol. 2021, 14, 920–934. [Google Scholar] [CrossRef]
- Pino-Hernández, E.; Pinto, C.A.; Abrunhosa, L.; Teixeira, J.A.; Saraiva, J.A. Hydrothermal and high-pressure processing of chestnuts-Dependence on the storage conditions. Postharvest Biol. Technol. 2022, 185, 111773. [Google Scholar] [CrossRef]
- Pietri, A.; Rastelli, S.; Mulazzi, A.; Bertuzzi, T. Aflatoxins and ochratoxin A in dried chestnuts and chestnut flour produced in Italy. Food Control 2012, 25, 601–606. [Google Scholar] [CrossRef]
- Bertuzzi, T.; Rastelli, S.; Pietri, A. Aspergillus and Penicillium toxins in chestnuts and derived products produced in Italy. Food Control 2015, 50, 876–880. [Google Scholar] [CrossRef]
- Paciulli, M.; Mert, I.D.; Rinaldi, M.; Pugliese, A.; Chiavaro, E. Chestnut and breads: Nutritional, functional, and technological qualities. In Flour and Breads and Their Fortification in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2019; pp. 237–247. [Google Scholar]
- Correia, P.; Leitão, A.; Beirão-da-Costa, M.L. The effect of drying temperatures on morphological and chemical properties of dried chestnuts flours. J. Food Eng. 2009, 90, 325–332. [Google Scholar] [CrossRef]
- Ahmed, J.; Al-Attar, H. Effect of drying method on rheological, thermal, and structural properties of chestnut flour doughs. Food Hydrocoll. 2015, 51, 76–87. [Google Scholar] [CrossRef]
- Cappelli, A.; Bini, A.; Cini, E. The Effects of Storage Time and Environmental Storage Conditions on Flour Quality, Dough Rheology, and Biscuit Characteristics: The Case Study of a Traditional Italian Biscuit (Biscotto di Prato). Foods 2022, 11, 209. [Google Scholar] [CrossRef]
- Sharma, R.; Singh, A.; Yemmireddy, V. Effect of storage relative humidity on the survival kinetics of salmonella spp., in different tree nut flours. LWT Food Sci. Technol. 2021, 152, 112365. [Google Scholar] [CrossRef]
- Moreira, R.; Chenlo, F.; Torres, M.D.; Rama, B. Influence of the chestnuts drying temperature on the rheological properties of their doughs. Food Bioprod. Process. 2013, 91, 7–13. [Google Scholar] [CrossRef]
- Correia, P.; Beirão-da-Costa, M.L. Effect of drying temperatures on starch-related functional and thermal properties of chestnut flours. Food Bioprod. Process. 2012, 90, 284–294. [Google Scholar] [CrossRef]
- Wani, I.A.; Hamid, H.; Hamdani, A.M.; Gani, A.; Ashwar, B.A. Physico-chemical, rheological and antioxidant properties of sweet chestnut (Castanea sativa Mill.) as affected by pan and microwave roasting. J. Adv. Res. 2017, 8, 399–405. [Google Scholar] [CrossRef]
- Littardi, P.; Paciulli, M.; Carini, E.; Rinaldi, M.; Rodolfi, M.; Chiavaro, E. Quality evaluation of chestnut flour addition on fresh pasta. LWT Food Sci. Technol. 2020, 126, 109303. [Google Scholar] [CrossRef]
- Cappelli, A.; Lupori, L.; Cini, E. Baking technology: A systematic review of machines and plants and their effect on final products, including improvement strategies. Trends Food Sci. Technol. 2021, 115, 275–284. [Google Scholar] [CrossRef]
- Ozcan, T.; Yilmaz-Ersan, L.; Akpinar-Bayizit, A.; Delikanli, B. Antioxidant properties of probiotic fermented milk supplemented with chestnut flour (Castanea sativa Mill). J. Food Process. Preserv. 2017, 41, e13156. [Google Scholar] [CrossRef]
- Alinovi, M.; Rinaldi, M.; Paciulli, M.; Littardi, P.; Chiavaro, E. Chestnut peels and wheat bran at different water level influence the physical properties of pan bread. Eur. Food Res. Technol. 2022, 248, 1227–1237. [Google Scholar] [CrossRef]
- Rinaldi, M.; Paciulli, M.; Caligiani, A.; Scazzina, F.; Chiavaro, E. Sourdough fermentation and chestnut flour in gluten-free bread: A shelf-life evaluation. Food Chem. 2017, 224, 144–152. [Google Scholar] [CrossRef]
- Venturi, M.; Cappelli, A.; Pini, N.; Galli, V.; Lupori, L.; Granchi, L.; Cini, E. Effects of kneading machine type and total element revolutions on dough rheology and bread characteristics: A focus on straight dough and indirect (biga) methods. LWT–Food Sci. Technol. 2022, 153, 112500. [Google Scholar] [CrossRef]
- Torra, M.; Belorio, M.; Ayuso, M.; Carocho, M.; Ferreira, I.C.; Barros, L.; Gómez, M. Chickpea and chestnut flours as non-gluten alternatives in cookies. Foods 2021, 10, 911. [Google Scholar] [CrossRef]
- Silav-Tuzlu, G.; Tacer-Caba, Z. Influence of chia seed, buckwheat and chestnut flour addition on the overall quality and shelf life of the gluten-free biscuits. Food Technol. Biotechnol. 2021, 59, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Ham, J.S.; Kim, H.Y.; Lim, S.T. Antioxidant and deodorizing activities of phenolic components in chestnut inner shell extracts. Ind. Crops Prod. 2015, 73, 99–105. [Google Scholar] [CrossRef]
- Squillaci, G.; Apone, F.; Sena, L.M.; Carola, A.; Tito, A.; Bimonte, M.; Morana, A. Chestnut (Castanea sativa Mill.) industrial wastes as a valued bioresource for the production of active ingredients. Process Biochem. 2018, 64, 228–236. [Google Scholar] [CrossRef]
- Pinto, D.; De la Luz Cádiz-Gurrea, M.; Vallverdú-Queralt, A.; Delerue-Matos, C.; Rodrigues, F. Castanea sativa shells: A review on phytochemical composition, bioactivity and waste management approaches for industrial valorization. Food Res. Int. 2021, 144, 110364. [Google Scholar] [CrossRef]
- Vella, F.M.; Laratta, B.; La Cara, F.; Morana, A. Recovery of bioactive molecules from chestnut (Castanea sativa Mill.) by-products through extraction by different solvents. Nat. Prod. Res. 2018, 32, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Munekata, P.E.S.; Franco, D.; Trindade, M.A.; Lorenzo, J.M. Characterization of phenolic composition in chestnut leaves and beer residue by LC-DAD-ESI-MS. LWT-Food Sci. Technol. 2016, 68, 52–58. [Google Scholar] [CrossRef]
- Gulsunoglu-Konuskan, Z.; Karbancioglu-Guler, F.; Kilic-Akyilmaz, M. Development of a bioprocess for production of ellagic acid from chestnut (Castanea sativa Mill.) waste by fermentation with Aspergillus spp. Food Biosci. 2021, 42, 101058. [Google Scholar] [CrossRef]
- Gullón, B.; Eibes, G.; Dávila, I.; Moreira, M.T.; Labidi, J.; Gullón, P. Hydrothermal treatment of chestnut shells (Castanea sativa) to produce oligosaccharides and antioxidant compounds. Carbohydr. Polym. 2018, 192, 75–83. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aglietti, C.; Cappelli, A.; Andreani, A. From Chestnut Tree (Castanea sativa) to Flour and Foods: A Systematic Review of the Main Criticalities and Control Strategies towards the Relaunch of Chestnut Production Chain. Sustainability 2022, 14, 12181. https://doi.org/10.3390/su141912181
Aglietti C, Cappelli A, Andreani A. From Chestnut Tree (Castanea sativa) to Flour and Foods: A Systematic Review of the Main Criticalities and Control Strategies towards the Relaunch of Chestnut Production Chain. Sustainability. 2022; 14(19):12181. https://doi.org/10.3390/su141912181
Chicago/Turabian StyleAglietti, Chiara, Alessio Cappelli, and Annalisa Andreani. 2022. "From Chestnut Tree (Castanea sativa) to Flour and Foods: A Systematic Review of the Main Criticalities and Control Strategies towards the Relaunch of Chestnut Production Chain" Sustainability 14, no. 19: 12181. https://doi.org/10.3390/su141912181
APA StyleAglietti, C., Cappelli, A., & Andreani, A. (2022). From Chestnut Tree (Castanea sativa) to Flour and Foods: A Systematic Review of the Main Criticalities and Control Strategies towards the Relaunch of Chestnut Production Chain. Sustainability, 14(19), 12181. https://doi.org/10.3390/su141912181







