Soil Aggregate Construction: Contribution from Functional Soil Amendment Fertilizer Derived from Dolomite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil and Fertilizer in Test
2.2. Experimental Design
2.3. Culture Method
2.4. Determination of Soil Aggregate Composition
3. Results and Analysis
3.1. Composition of Elastic-Stable Macro- and Microaggregate
3.2. Composition of Water Stable Aggregate and Water Instable Aggregate
3.3. Effects of Type and Dosage of Functional Fertilizer on Elastic-Stable Macro- and Microaggregate
3.3.1. Influence on the Elastic Stable Macroaggregate
3.3.2. Influence on the Water Stable Macroaggregate
3.4. Effects of the Functional Amendment Fertilizer on MWD, GWD and PAD of Soil Aggregate
3.4.1. Effects on the MWD and GMD of Elastic-Stable Aggregate
3.4.2. Effects on the MWD and GMD of Water Stable Aggregate
3.4.3. Effect on Dispersion of the Aggregate
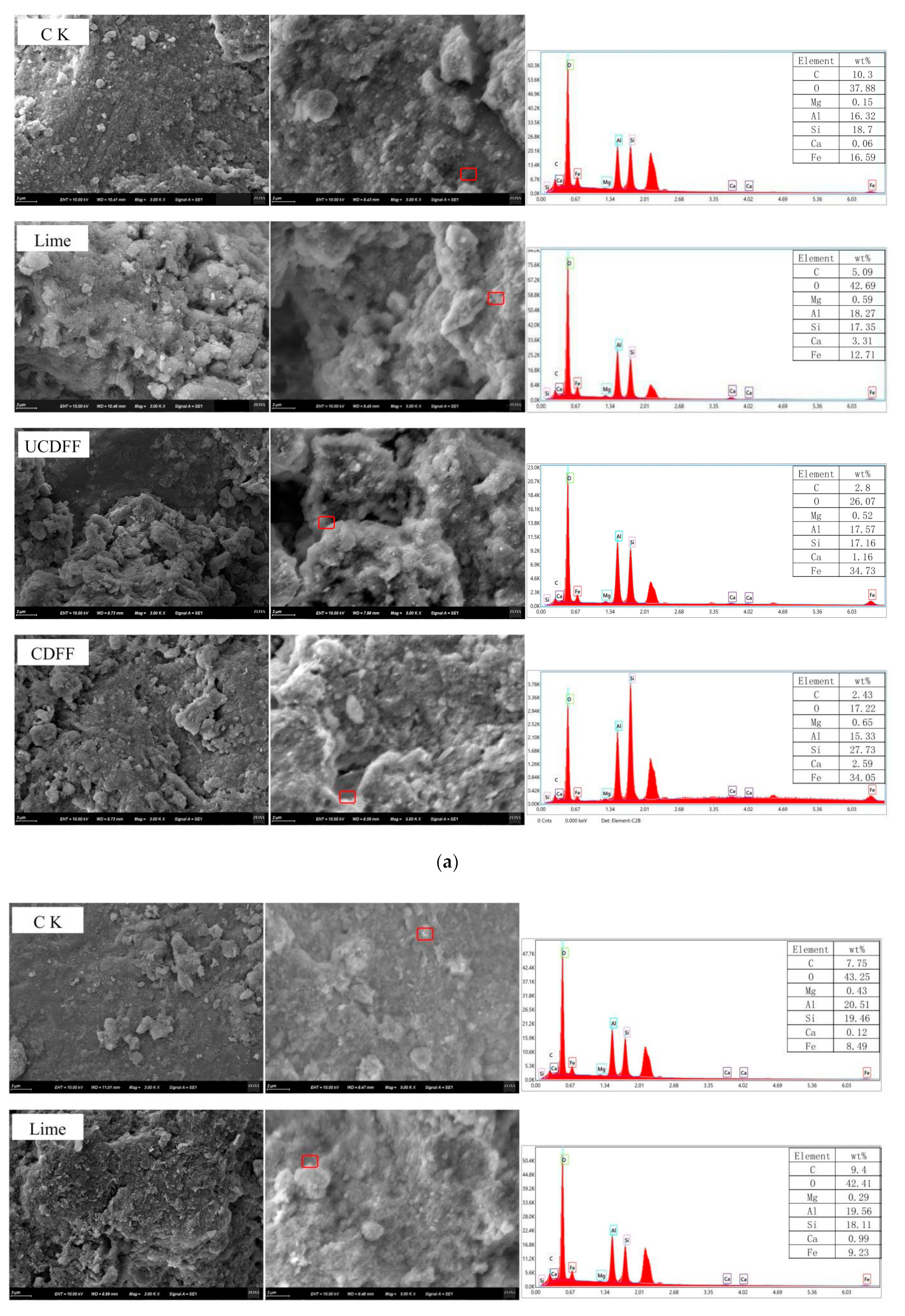
3.5. Effect of the Functional Fertilizers and Lime on Soil Aggregate Microstructure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mueller, L.; Shepherd, G.; Schindler, U.; Ball, B.C.; Munkholm, L.J.; Hennings, V.; Smolentseva, E.; Rukhovic, O.; Lukin, S.; Hu, C. Evaluation of soil structure in the framework of an overall soil quality rating. Soil Tillage Res. 2013, 127, 74–84. [Google Scholar]
- Bissett, A.; Richardson, A.E.; Baker, G.; Thrall, P.H. Long-term land use effects on soil microbial community structure and function. Appl. Soil Ecol. 2011, 51, 66–78. [Google Scholar] [CrossRef]
- Six, J.; Paustian, K. Aggregate-associated soil organic matter as an ecosystem property and a measurement tool. Soil Biol. Biochem. 2014, 68, A4–A9. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Lehmann, A.; Zheng, W.; Ryo, M.; Soutschek, K.; Roy, J.; Rongstock, R.; Maaß, S.; Rillig, M.C. Fungal Traits Important for Soil Aggregation. Front. Microbiol. 2020, 10, 2904. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar]
- Barthès, B.; Roose, E. Aggregate stability as an indicator of soil susceptibility to runoff and erosion; validation at several levels. Catena 2002, 47, 133–149. [Google Scholar] [CrossRef]
- Šimanský, V.; Balashov, E.; Horák, J. Water stability of soil aggregates and their ability to sequester carbon in soils of vineyards in Slovakia. Arch. Agron. Soil Sci. 2015, 62, 177–197. [Google Scholar]
- Lwin, C.S.; Seo, B.; Kim, H.; Owens, G.; Kim, K. Application of soil amendments to contaminated soils for heavy metal immobilization and improved soil quality-a critical review. Soil Sci. Plant Nutr. 2018, 64, 156–167. [Google Scholar] [CrossRef]
- Ramos, M.C.; Nacci, S.; Pla, I. Effect of raindrop impact and its relationship with aggregate stability to different disaggregation forces. Catena 2003, 53, 365–376. [Google Scholar]
- Zhang, J.; Wei, Y.; Liu, J.; Yuan, J.; Liang, Y.; Ren, J.; Cai, H. Effects of maize straw and its biochar application on organic and humic carbon in water-stable aggregates of a Mollisol in Northeast China: A five-year field experiment. Soil Tillage Res. 2019, 190, 1–9. [Google Scholar] [CrossRef]
- Abiven, S.; Menasseri, S.; Chenu, C. The effects of organic inputs over time on soil aggregate stability—A literature analysis. Soil Biol. Biochem. 2009, 41, 1–12. [Google Scholar]
- Ren, J.; Zou, Y.; Liu, B.; Zhang, C. Study on aggregate formation mechanism of soil in limestone. Carsologica Sinica. Carsologica Sin. 2019, 38, 722–728. [Google Scholar]
- Ye, L.; Zhao, X.; Bao, E.; Li, J.; Zou, Z.; Cao, K. Bio-organic fertilizer with reduced rates of chemical fertilization improves soil fertility and enhances tomato yield and quality. Sci. Rep. 2020, 10, 117. [Google Scholar]
- Zhang, H.; Wang, B.; Xu, M.; Fan, T. Crop Yield and Soil Responses to Long-Term Fertilization on a Red Soil in Southern China. Pedosphere 2009, 19, 199–207. [Google Scholar] [CrossRef]
- Song, H.; Che, Z.; Cao, W.; Huang, T.; Wang, J.; Dong, Z. Changing roles of ammonia-oxidizing bacteria and archaea in a continuously acidifying soil caused by over-fertilization with nitrogen. Environ. Sci. Pollut. Res. Int. 2016, 23, 11964–11974. [Google Scholar] [CrossRef]
- Pan, X.; Baquy, M.A.; Guan, P.; Yan, J.; Wang, R.; Xu, R.; Xie, L. Effect of soil acidification on the growth and nitrogen use efficiency of maize in Ultisols. J. Soil Sediment 2020, 20, 1435–1445. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Zhang, S.; Wang, Y. What could promote farmers to replace chemical fertilizers with organic fertilizers? J. Clean. Prod. 2018, 199, 882–890. [Google Scholar]
- Wang, T.; Cao, X.; Chen, M.; Lou, Y.; Wang, H.; Yang, Q.; Pan, H.; Zhuge, Y. Effects of Soil Acidification on Bacterial and Fungal Communities in the Jiaodong Peninsula, Northern China. Agronomy 2022, 12, 927. [Google Scholar]
- Martínez-Hidalgo, P.; Maymon, M.; Pule-Meulenberg, F.; Hirsch, A.M. Engineering root microbiomes for healthier crops and soils using beneficial, environmentally safe bacteria. Can. J. Microbiol. 2019, 65, 91–104. [Google Scholar]
- Saride, S.; Puppala, A.J.; Chikyala, S.R. Swell-shrink and strength behaviors of lime and cement stabilized expansive organic clays. Appl. Clay Sci 2013, 85, 39–45. [Google Scholar] [CrossRef]
- Bolan, N.; Adriano, D.; Mahimairaja, S. Distribution and Bioavailability of Trace Elements in Livestock and Poultry Manure By-Products. Crit. Rev. Environ. Sci. Technol. 2004, 34, 291–338. [Google Scholar] [CrossRef]
- Yang, S.X.; Li, J.T.; Yang, B.; Liao, B.; Zhang, J.T.; Shu, W.S. Effectiveness of amendments on re-acidification and heavy metal immobilization in an extremely acidic mine soil. J. Environ. Monit. 2011, 13, 1876–1883. [Google Scholar] [CrossRef] [PubMed]
- Haynes, R.J. What effect does liming have on silicon availability in agricultural soils? Geoderma 2019, 337, 375–383. [Google Scholar] [CrossRef]
- Xiao, K.; Yu, L.; Xu, J.; Brookes, P.C. pH, nitrogen mineralization, and KCl-extractable aluminum as affected by initial soil pH and rate of vetch residue application: Results from a laboratory study. J. Soil. Sediment 2014, 14, 1513–1525. [Google Scholar] [CrossRef]
- Liu, J.; Cui, J.; Liu, H.; Pan, Q.; He, X. Research progress of soil amelioration of acidified soil by soil amendments. J. Environ. Eng. Technol. 2014, 14, 1513–1525. [Google Scholar]
- Jiang, J.; Zhou, L.; Zhang, X.; Wei, B.; Li, Y.; Fan, X. Effects of calcinated dolomite on the amendment of acid soil and release kinetics of Ca-Mg. Trans. Chin. Soc. Agric. Eng. 2020, 36, 235–244. [Google Scholar]
- Cao, Q.; Jiang, J.; Wang, X.; Fan, J.; Zhan, Y.; Zhang, L.; Li, F.; Sun, S.; Chou, R.; Fan, X. A Novel Alkaline Fertilizer and Its Function as well as Mechanism to Remediation Soil Acid and Cd Pollution. Acta Pedol. Sin. 2022, 21, 1–15. [Google Scholar]
- Savinov, N.O. Soil Physics; Sielchozgiz Press: Moscow, Russia, 1936. [Google Scholar]
- Barreto, R.C.; Madari, B.E.; Maddock, J.E.L.; Machado, P.L.O.A.; Torres, E.; Franchini, J.; Costa, A.R. The impact of soil management on aggregation, carbon stabilization and carbon loss as CO2 in the surface layer of a Rhodic Ferralsol in Southern Brazil. Agric. Ecosyst. Environ. 2009, 132, 243–251. [Google Scholar] [CrossRef]
- Bu, C.; Gale, W.J.; Cai, Q.; Wu, S. Process and Mechanism for the Development of Physical Crusts in Three Typical Chinese Soils. Pedosphere 2013, 23, 321–332. [Google Scholar] [CrossRef]
- Wei, B.; Jiang, J.; Gao, C.; Zhang, L.; Zhan, Y.; Jiang, S.; Li, Y.; Sun, S.; Xie, J.; Fan, X. Revealing channel controlled nutrient release mechanism of bio-oil polymer coated controlled-release fertilizer. Ind. Crop. Prod. 2021, 173, 114096. [Google Scholar] [CrossRef]
- Goulding, K. Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manag. 2016, 32, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Verchot, L.V.; Dutaur, L.; Shepherd, K.D.; Albrecht, A. Organic matter stabilization in soil aggregates: Understanding the biogeochemical mechanisms that determine the fate of carbon inputs in soils. Geoderma 2011, 161, 182–193. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; He, J.; Wang, Q.; Golabi, M.H. Influence of conservation tillage practices on soil properties and crop yields for maize and wheat cultivation in Beijing, China. Soil Res. 2009, 47, 362. [Google Scholar]
- Nimmo, J.R.; Perkins, K.S. 2.6 Aggregate Stability and Size Distribution. Methods Soil Anal. Part Phys. Methods 2002, 5, 317–328. [Google Scholar]
- Zou, C.; Li, Y.; Huang, W.; Zhao, G.; Pu, G.; Su, J.; Coyne, M.S.; Chen, Y.; Wang, L.; Hu, X.; et al. Rotation and manure amendment increase soil macro-aggregates and associated carbon and nitrogen stocks in flue-cured tobacco production. Geoderma 2018, 325, 49–58. [Google Scholar] [CrossRef]
- Oades, J.M.; Waters, A.G. Aggregate hierarchy in soils. Soil Res. 1991, 29, 815–828. [Google Scholar] [CrossRef]
- Aitken, R.L.; Dickson, T.; Hailes, K.J.; Moody, P.W. Response of field-grown maize to applied magnesium in acidic soils in north-eastern Australia. Aust. J. Agric. Res. 1999, 50, 191. [Google Scholar] [CrossRef]
- Mavi, M.S.; Marschner, P. Salinity affects the response of soil microbial activity and biomass to addition of carbon and nitrogen. Soil Res. 2013, 51, 68. [Google Scholar] [CrossRef]
- Jalali, M.; Arian, T.M.; Ranjbar, F. Selectivity coefficients of K, Na, Ca, and Mg in binary exchange systems in some calcareous soils. Environ. Monit. Assess. 2020, 192, 80. [Google Scholar]
- Reddy, P.S.; Mohanty, B.; Rao, B.H. Investigations for Chemical Parameters Effect on Swelling Characteristics of Expansive Soils. KSCE J. Civ. Eng. 2021, 25, 4088–4105. [Google Scholar] [CrossRef]
- Rowley, M.C.; Grand, S.; Verrecchia, É.P. Calcium-mediated stabilisation of soil organic carbon. Biogeochemistry 2017, 137, 27–49. [Google Scholar] [CrossRef]
- Boiteau, R.M.; Kukkadapu, R.; Cliff, J.B.; Smallwood, C.R.; Kovarik, L.; Wirth, M.G.; Engelhard, M.H.; Varga, T.; Dohnalkova, A.; Perea, D.E.; et al. Calcareous organic matter coatings sequester siderophores in alkaline soils. Sci. Total Environ. 2020, 724, 138250. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, A.H.; Usman, A.R.A.; Al-Omran, A.; Ok, Y.S.; Ahmad, M.; Al-Wabel, M.I. Carbon mineralization and nutrient availability in calcareous sandy soils amended with woody waste biochar. Chemosphere 2015, 138, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Muneer, M.; Oades, J. The role of Ca-organic interactions in soil aggregate stability. III. Mechanisms and models. Aust. J. Soil Res. 1989, 27, 411–423. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Wang, X. Structures, Formation and Transformation, and Surface Physicochemical Properties of Several Meta-Stable Iron Oxides. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2015. [Google Scholar]
- Porter, H.; Dhami, N.K.; Mukherjee, A. Synergistic chemical and microbial cementation for stabilization of aggregates. Cem. Concr. Compos. 2017, 83, 160–170. [Google Scholar] [CrossRef]
- Jozefaciuk, G.; Czachor, H. Impact of organic matter, iron oxides, alumina, silica and drying on mechanical and water stability of artificial soil aggregates. Assessment of new method to study water stability. Geoderma 2014, 221–222, 1–10. [Google Scholar] [CrossRef]
- Czachor, H.; Charytanowicz, M.; Gonet, S.; Niewczas, J.; Jozefaciuk, G.; Lichner, L. Impact of long-term mineral and organic fertilizer application on the water stability, wettability and porosity of aggregates obtained from two loamy soils. Eur. J. Soil Sci. 2015, 66, 577–588. [Google Scholar] [CrossRef]




| Treatment | Composition of Macro- and Microaggregate (%) | |||
|---|---|---|---|---|
| Exp. 1 | Exp. 2 | |||
| >0.25 mm | <0.25 mm | >0.25 mm | <0.25 mm | |
| CK | 61.2 ± 0.4 d | 38.8 ± 0.4 a ** | 61.2 ± 1.4 e | 38.8 ± 1.4 a ** |
| Lime 4 | 72.3 ± 0.6 c | 27.7 ± 0.6 bc ** | 72.3 ± 2.7 abc | 27.7 ± 2.7 d ** |
| Lime 6 | 72.4 ± 1.1 c | 27.6 ± 1.1 bc ** | 72.4 ± 2.6 d | 27.6 ± 2.6 bc ** |
| Lime 8 | 74.8 ± 1.8 bd | 25.2 ± 1.8 bc ** | 74.8 ± 1.2 cd | 25.2 ± 1.2 c ** |
| UCDFF 4 | 77.6 ± 1.5 ab | 22.4 ± 1.5 cd ** | 77.6 ± 1.3 c | 22.4 ± 1.3 cd ** |
| UCDFF 6 | 76.2 ± 1.3 bc | 23.8 ± 1.3 cd ** | 76.2 ± 0.0 ab | 23.8 ± 0.0 d ** |
| UCDFF 8 | 80.5 ± 1.1 ab | 19.5 ± 1.1 de ** | 80.5 ± 0.9 ab | 19.5 ± 0.9 de ** |
| CDFF 4 | 76.6 ± 0.1 bc | 23.4 ± 0.1 c ** | 76.6 ± 1.9 c | 23.4 ± 1.9 bc ** |
| CDFF 6 | 79.7 ± 1.8 ab | 20.3 ± 1.8 c ** | 79.7 ± 0.2 a | 20.3 ± 0.2 de ** |
| CDFF 8 | 82.9 ± 0.4 ab | 17.1 ± 0.4 e ** | 82.9 ± 2.1 a | 17.1 ± 2.1 e ** |
| Treatment | Composition of Macro- and Microaggregate (%) | |||
|---|---|---|---|---|
| Exp. 1 | Exp. 2 | |||
| >0.25 mm | <0.25 mm | >0.25 mm | <0.25 mm | |
| CK | 35.7 ± 0.2 e | 64.3 ± 0.2 a ** | 34.6 ± 0.6 e | 65.4 ± 0.6 a ** |
| Lime 4 | 34.6 ± 0.6 e | 65.4 ± 0.6 a ** | 39.2 ± 2.3 abc | 60.8 ± 2.3 ab * |
| Lime 6 | 45.8 ± 3.7 c | 54.2 ± 3.7 c | 42.5 ± 2.7 d | 57.5 ± 2.7 bc * |
| Lime 8 | 49.5 ± 0.9 a | 50.5 ± 0.9 d | 48.9 ± 1.3 cd | 51.1 ± 1.3 cd |
| UCDFF 4 | 38.9 ± 3.4 d | 61.1 ± 3.4 ab ** | 40.3 ± 3.0 c | 59.7 ± 3.0 ab ** |
| UCDFF 6 | 39.5 ± 1.3 d | 60.5 ± 1.3 ab ** | 42.8 ± 3.3 ab | 57.2 ± 3.3 bc ** |
| UCDFF 8 | 50.1 ± 2.5 a | 49.9 ± 2.5 d | 47.7 ± 4.4 ab | 52.3 ± 4.4 cd |
| CDFF 4 | 42.6 ± 1.4 c | 57.4 ± 1.4 bc * | 42.1 ± 2.1 c | 57.9 ± 2.1 bcd ** |
| CDFF 6 | 49.2 ± 2.1 b | 50.8 ± 2.1 d | 49.0 ± 2.2 a | 51.0 ± 2.2 cd |
| CDFF 8 | 51.9 ± 0.7 a | 48.1 ± 0.7 d | 53.5 ± 1.8 a | 46.5 ± 1.8 d * |
| Experiment | Treatment | MWD (mm) | GWD (mm) | ||
|---|---|---|---|---|---|
| Exp. 1 | CK | 0.71 ± 0.010 | b | 0.57 ± 0.002 | c |
| Lime | 0.64 ± 0.019 | c | 0.56 ± 0.014 | cd | |
| UCDFF | 0.72 ± 0.029 | ab | 0.61 ± 0.022 | ab | |
| CDFF | 0.75 ± 0.010 | a | 0.62 ± 0.011 | a | |
| Exp. 2 | CK | 0.71 ± 0.004 | b | 0.59 ± 0.003 | a |
| Lime | 0.69 ± 0.010 | c | 0.59 ± 0.009 | a | |
| UCDFF | 0.71 ± 0.006 | b | 0.59 ± 0.004 | a | |
| CDFF | 0.72 ± 0.001 | a | 0.59 ± 0.004 | a | |
| Experiment | Treatment | MWD (mm) | GWD (mm) |
|---|---|---|---|
| Exp. 1 | CK | 0.82 ± 0.019 d | 0.70 ± 0.003 d |
| Lime | 0.90 ± 0.010 c | 0.76 ± 0.015 c | |
| UCDFF | 0.92 ± 0.023 b | 0.78 ± 0.015 b | |
| CDFF | 0.95 ± 0.005 a | 0.82 ± 0.013 a | |
| Exp. 2 | CK | 0.80 ± 0.005 d | 0.69 ± 0.001 d |
| Lime | 0.82 ± 0.036 c | 0.71 ± 0.028 c | |
| UCDFF | 0.99 ± 0.005 b | 0.82 ± 0.002 b | |
| CDFF | 1.00 ± 0.016 a | 0.84 ± 0.016 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, Y.; Jiang, K.; Jiang, J.; Zhang, L.; Gao, C.; Qi, X.; Fan, J.; Li, Y.; Sun, S.; Fan, X. Soil Aggregate Construction: Contribution from Functional Soil Amendment Fertilizer Derived from Dolomite. Sustainability 2022, 14, 12287. https://doi.org/10.3390/su141912287
Zhan Y, Jiang K, Jiang J, Zhang L, Gao C, Qi X, Fan J, Li Y, Sun S, Fan X. Soil Aggregate Construction: Contribution from Functional Soil Amendment Fertilizer Derived from Dolomite. Sustainability. 2022; 14(19):12287. https://doi.org/10.3390/su141912287
Chicago/Turabian StyleZhan, Yaowei, Kaixin Jiang, Jiaquan Jiang, Lidan Zhang, Chengxiang Gao, Xiuxiu Qi, Jiayan Fan, Yuechen Li, Shaolong Sun, and Xiaolin Fan. 2022. "Soil Aggregate Construction: Contribution from Functional Soil Amendment Fertilizer Derived from Dolomite" Sustainability 14, no. 19: 12287. https://doi.org/10.3390/su141912287
APA StyleZhan, Y., Jiang, K., Jiang, J., Zhang, L., Gao, C., Qi, X., Fan, J., Li, Y., Sun, S., & Fan, X. (2022). Soil Aggregate Construction: Contribution from Functional Soil Amendment Fertilizer Derived from Dolomite. Sustainability, 14(19), 12287. https://doi.org/10.3390/su141912287






