Abstract
A precise evaluation of spatial patterns in desert vegetation biomass, species richness and their environmental controls is essential for a deeper comprehension of the potential carbon preservation and sustainability of grassland ecosystems. There are widespread reports suggesting robust associations among biomass, species richness and mean annual precipitation (MAP) or temperature (MAT) at different scales. However, these reports were inconsistent, and knowledge on the desert grasslands of Central Asia remains limited. In this study, we showed that spatial patterns of biomass and species richness along the zonal climate of the northern Tianshan Mountains exhibited substantial regional differences and the relationship among biomass, richness and elevation exhibited a substantial exponential decline. We discovered that functional groups of biomass, total biomass and species richness in the desert exhibited exponential growth along the MAP gradient and a quadratic relationship with MAT. Furthermore, the biomass–species richness relationships were bell-shaped in the desert zone. Accordingly, the biomass and species richness had spatial differences. At a regional scale, the spatial variation in the desert biomass and species richness was primarily dependent on climate. Our results demonstrated the specificity between the desert vegetation and climate in arid regions of Central Asia and revealed the regularity between biomass and species richness in desert areas. The research results emphasized the impact of precipitation on desert vegetation in arid regions of Central Asia and the relationship between biomass and plant species richness, which is of great significance for understanding desert ecosystems and protecting the ecological environment.
1. Introduction
Grasslands are considered among the most important terrestrial ecosystems across the globe, encompassing 30% of the land surface and primarily distributed in both arid and semiarid regions [1]; additionally, they provide substantial and extremely effective ecosystem functions and services [2]. The vegetation biomass of grasslands is a substantial constituent of terrestrial ecosystem carbon stocks and has emerged as a critical concern in global carbon cycle research owing to the increasing global temperature [3], and the spatial patterns of grassland vegetation highlight its potential as a carbon sink [4]. In addition, the aboveground biomass of grasslands is highly spatially and temporally variable in comparison with those of forest and cropland ecosystems [5,6], which are stimulated by climatic conditions. Additionally, the variability in grassland biomass exerts an impact on the global carbon balance, ecosystem service delivery and the sustainability of grassland resources overall [7,8]. Improving our understanding of the factors affecting the spatial distribution of grassland biomass, engendered by environmental controls, is pivotal for maintaining the stability of grassland ecosystems [9,10]. As revealed by past research, precipitation and temperature are critical factors controlling vegetation biomass and ecosystem functioning, particularly in arid and semiarid grassland ecosystems [11,12]. Jiang et al. indicated that aboveground biomass exhibited exponential growth along a mean annual precipitation (MAP) gradient, ranging from 1.1 to 204.7 g·m−2 along the mean annual temperature (MAT) gradient [13]. Additionally, there are widespread reports suggesting favorable relationships between aboveground biomass and biodiversity in natural ecosystems [14,15,16,17]. The majority of the past studies on vegetation biomass emphasized meadows and steppes; conversely, less research has involved deserts that exhibit more sensitivity to environmental changes, particularly the Artemisia desert.
The Tianshan Mountains are located in the hinterland of the Eurasian continent, which crosses four countries, including China, Kazakhstan, Kyrgyzstan and Uzbekistan, while extending more than 1700 km in China. The climatic conditions and soil texture of the northern slope of the Tianshan Mountains in Xinjiang are apparently different, wherein the rainfall has gradually declined and the climate conditions worsen from west to east, gradually rendering the ecological environment severe. A fragile desert zone is formed at 800–1700 m along the northern slope of the Tianshan Mountains, which has an apparent horizontal zonality. Seriphidium transiliense, which is a hyper drought subshrub and a highly palatable forage with high nutritional value, belongs to the Seriphidium genus in the Compositae (Asteraceae) family. Additionally, the S. transiliense desert is widely distributed in the plains and low mountain areas (with elevations of 500–1700 m) on the north slope of the Tianshan Mountains, which is an extremely important pasture in the spring and autumn seasons [2]. However, the distribution pattern of the vegetation biomass and richness in the Artemisia desert is still unclear. It is both urgent and essential to develop an understanding of this pattern, together with its control factors, to provide a theoretical basis for the management and scientific utilization of this grassland.
It has been reported that the spatial dependence of desert vegetation species diversity decreases with increasing spatial scale, which is contrary to the conclusion that grassland and forest communities increase with increasing scale [18]. In addition, the population of Artemisia annua in severely degraded areas strongly deviates from a random distribution and presents an aggregated distribution. The aggregation intensity varies at different scales, and there is an obvious “aggregation wave” phenomenon on a series of spatial scales [19]. Chen Yiping et al. [20] showed that in the horizontal direction, the diversity of grassland plant communities showed a pattern of high diversity in the center and low diversity on the edge. In addition, researchers have conducted extensive research on the impact of environmental and biological factors on net primary productivity and biodiversity [21,22,23]. However, the relative contributions of abiotic and biotic factors to vegetation biomass and richness and the underlying mechanisms for their effects in the deserts of Central Asia are unknown. The northern Tianshan desert has a unique ecosystem with a simple community structure, making it an ideal place to study the effects of abiotic and external factors on biomass and abundance. Focusing on the spatial variation in spring vegetation biomass, richness and their environmental controls in the deserts of Central Asia will help us better understand not only the spatial variation in vegetation biomass and richness in different climatic zones, but also that desert vegetation ecosystems are of great significance to the protection of the ecological environment.
Therefore, this study aims to reveal, for the first time, the critical role of environmental factors, especially MAP and MAT, in stimulating ecosystem function in deserts. This research aims to address the following specific questions: (1) Does the spatial variation in the desert vegetation biomass and richness of different climate zones in the northern Tianshan Mountains occur in the arid areas of Central Asia? (2) How do the environmental factors exert an impact on their distribution? (3) What is the relationship between biomass and species richness along the Artemisia-dominated desert transect?
2. Materials and Methods
2.1. Study Area
The study region for this research comprised the northern Tianshan Mountains in Xinjiang (43°20′~45°45′ N, 82°35′~93°20′ E, 800~1700 m altitude), a pivotal part of the arid area of Central Asia. The climate zone, which is approximately 1700 kilometers in length, is located in the hinterland of the Eurasian continent, wherein the natural conditions are quite harsh, making the desert ecosystem extremely fragile and highly sensitive to climate change. The study region has a typical temperate continental arid climate that is characterized by short, torrid summers and long, cold winters. The MAP amounts to approximately 150~350 mm, and the evaporation is greater than 2000 mm. The MAT is approximately 4.0~9.0 °C. In addition, the type of soil was gray desert soil according to the FAO/UNESCO taxonomy.
The study regions can be segregated into three climatic zones (east, middle and west sections) according to their climatic conditions, and 10 sampling sites were selected from west to east, including Gongliu County, Xinyuan County, the cities of Bole and Wusu, Manasi County, Hutubi County, the city of Urumqi, Qitai County, Mulei County and Balikun County (Figure 1). In the western and middle sections of the northern Tianshan Mountains, higher rainfall and snow were experienced in spring and winter, producing a large number of ephemeral plants that appeared in early spring. Conversely, there was less snow and rainfall in winter and spring, resulting in comparatively fewer ephemeral plants. Additionally, the altitude and soil texture shared specific differences from west to east.
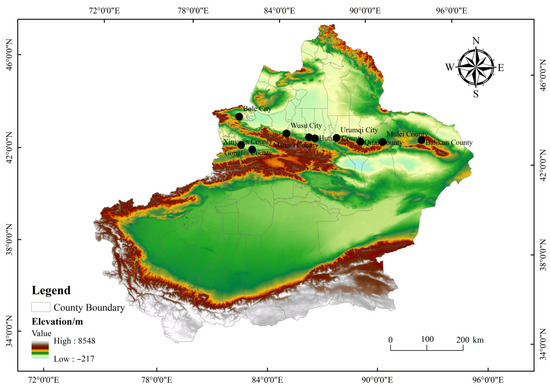
Figure 1.
The study area.
The sampling sites were grazed by sheep at a moderate grazing intensity, employed to enhance spring–autumn pasture production. The native vegetation in the ten sites included S.transiliense and Seriphidium borotalalense, which were dominant structural species, whereas the key companion species included Kochia prostrata, Carex turkestanica, Ceratocarpus arenarius, Ceratocarpus arenarius and Salsola collina. Additionally, a large number of ephemeral plants appeared in spring, for instance, Poa annua L., Ixiolirion tataricum, Trigonella arcuate, Allium mongolicum and Tulipa iliensis.
2.2. Experimental Design
A complete random design was employed in this experiment. The study sites were segregated into three climatic zones (east, middle and west sections); in addition, 3 to 4 sites with moderate grazing were selected in each zone. Each site was located at a distance of approximately 100 km, representing the entire desert area.
Within each site, three random equal-length parallel replicate transects were established at 100-m intervals, followed by ten sampling subplots (1 m × 1 m) at 10-m intervals along each transect for a total 300 samples (10 sites × 3 transects × 10 subplots).
2.3. Field Sampling
Vegetation measurements were performed at ten sampling sites in mid-May 2017, which was a typical period of high biomass and species richness due to the presence of many ephemeral plants. All the species within each subplot were identified and recorded. Plant coverage was measured by the projection method; natural heights were measured with a ruler; individual quantities (densities) of each species were recorded using statistical methods. The aboveground dry biomass of each species (only the green plant parts) was determined by clipping the whole plant from the soil surface using scissors in each sampling subplot and weighing the plants after drying them at 80 °C to a constant weight for 24 h.
2.4. Data Analysis
According to Whittaker’s scientific and comprehensive discussion on an evaluation index of plant diversity, the α-diversity index is often used to study the species diversity within a community caused by the differentiation of interspecific niches and is a commonly used evaluation index in ecological research [24]. This study used the method proposed by Whittaker. The community structure attributes were characterized by the Important value (IV), Patrick richness index (R), Simpson dominance index (D), Shannon–Wiener diversity index and Pielou index (E) [25], and they were calculated as follows:
where Hri represents the relative height, Cri indicates the relative coverage, Dri depicts the relative density, Bri is the relative biomass, S represents the number of species in the community and IVi is a representation of the importance value of species i.
Additionally, the plant community was segregated into four functional groups at these sites in accordance with Xu [26], namely, subshrub, perennial grass, annual grass and ephemeral plants. The attributes of all the functional groups are presented in Figure 2.
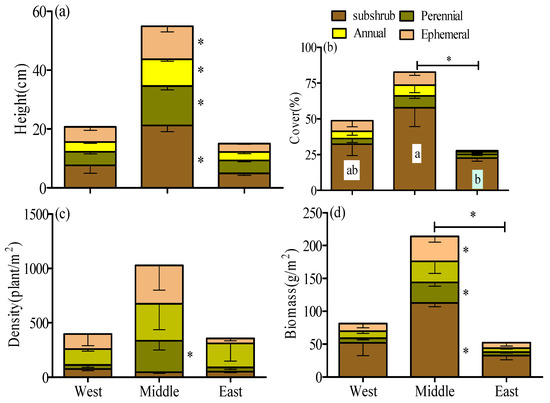
Figure 2.
Variation in (a) height, (b) cover, (c) density and (d) biomass of functional groups across the three climate zones of the northern Tianshan Mountains in Xinjiang. Different lowercase letters and * represent substantial differences in the treatments at the 0.05 level, whereas the error bars represent SE.
Analysis of all the data was performed using SPSS version 20.0 (IBM, Armonk, NY, USA) and expressed as the mean ± standard error of the mean (SE). The significance level for all the statistical tests was p = 0.05. An analysis of variance (ANOVA) was employed to evaluate the differences in different sites in plant community attributes as well as in plant biodiversity.
3. Results
3.1. Spatial Patterns of Desert Community Characteristics
The northern and western sections of the Tianshan Mountains had the largest number of vegetation species, which were 12.5% and 17.3% higher than those in the central and eastern parts of the Tianshan Mountains, respectively (Table 1). Overall, the plant species in the three climatic zones were broadly similar, while the eastern vegetation species differed from those in the other two climatic zones.

Table 1.
Variations in types and quantities of species across the three climate zones of the northern Tianshan Mountains in Xinjiang.
The heights of subshrub, perennial, annual and ephemeral plants in the middle section of the northern Tianshan Mountains were substantially higher by 117.2~191.2% and 202.3~332.3% than those in the western and eastern sites, respectively (Figure 2a; p < 0.05). As revealed by these findings, the heights of the functional groups exhibited sensitivity to environmental variation.
There was no observable difference in the cover of perennial, annual and ephemeral plants in the three climate zones. However, the subshrub cover was approximately 2.6 times higher in comparison with that in the east zone, which was substantially higher. In addition, the total community cover in the middle section (82.7%) was higher than that in the west (48.7%; p > 0.05) and east (27.8%; p < 0.05) sections (Figure 2b).
Perennial grass in the middle section had substantially higher values of density (288.4 plant·m−2) compared with the west (37.7 plant·m−2) and east (38.0 plant·m−2) sections (Figure 2c). However, the density of subshrub, annual and ephemeral plants was not significantly different in the three climate zones, suggesting that the density of perennial grass exhibited more sensitivity than did other functional groups.
The biomasses of the functional groups in the middle section were substantially higher by 117.4~338.0% and 246.2~474.1% than those in the western and eastern sections, respectively. In addition, the desert community in the middle section had a higher value of total biomass (213.9 g·m−2) than did the west (81.3 g·m−2; p > 0.05) and east (52.4 g·m−2; p < 0.05) sections (Figure 2d).
3.2. Spatial Patterns of Desert Plant Diversity
Along the climate zone, the Patrick richness index exhibited a slight increase at first, followed by an obvious decline (Figure 3a). However, the responses of the Simpson dominance index, Shannon–Wiener diversity index and Pielou evenness index in the desert to environmental changes were negligible (Figure 3b–d; p > 0.05).
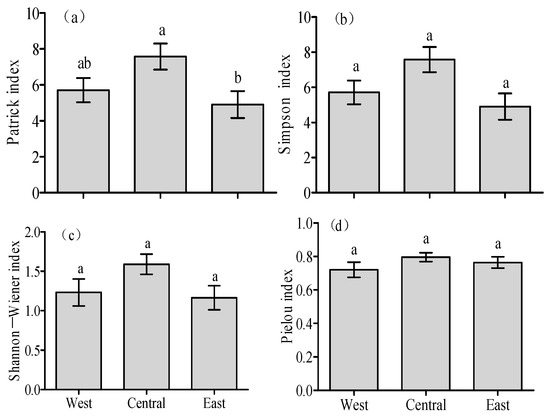
Figure 3.
Variations in the (a) Patrick index, (b) Simpson index, (c) Shannon–Wiener index and (d) Pielou index across the three climate zones of the northern Tianshan Mountains in Xinjiang. Lowercase letters indicate substantial differences in the treatments at the 0.05 level, whereas the error bars represent SE.
3.3. Effects of Environmental Variables on the Spatial Patterns in Desert Vegetation Biomass
The effects of environmental factors on the vegetation biomass are shown in Figure 4. The regressions suggest that vegetation biomass had a positive association with the MAP (Figure 4a; p < 0.001) and was negatively correlated with elevation (Figure 4c; p < 0.001). Additionally, the MAP and elevation explained 56.1% and 74.3% of the variations in the desert biomass, respectively. Along the MAT (p < 0.05) and longitude (p > 0.05) gradients, the vegetation biomass tended to increase at first and then decrease.
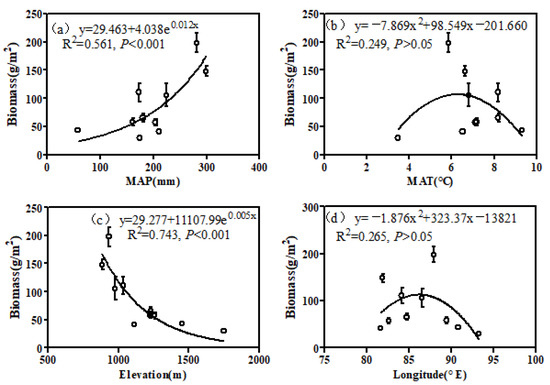
Figure 4.
Relationships between biomass in the desert zone of the northern Tianshan Mountains and environmental factors: (a) MAP, (b) MAT, (c) elevation and (d) longitude.
3.4. Effects of Environmental Variables on Spatial Patterns in Species Richness
We discovered a robust effect of MAP and elevation on the species richness along the climate zone of the northern Tianshan Mountains. Specifically, the Patrick richness index showed an exponential increase along the precipitation gradient (Figure 5a; p < 0.001) while exhibiting an exponential decline along the elevation gradient (Figure 5c; p < 0.001). Additionally, the Patrick richness index explained 36.8% and 50.7% of the variation in desert species richness. In addition, the species richness first increased and then decreased with increasing temperature (Figure 5b; p < 0.05) and increased with longitude in the desert of the northern Tianshan Mountains (Figure 5c; p > 0.05).
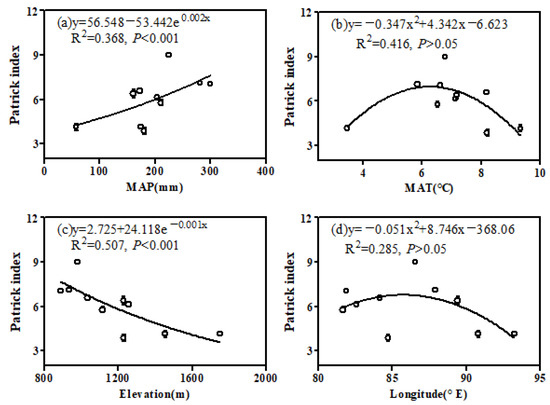
Figure 5.
Relationships between species richness in the desert zone of the northern Tianshan Mountains and environmental factors: (a) MAP, (b) MAT, (c) elevation and (d) longitude.
3.5. The Relationships between Biomass and Species Richness in the Desert Zone
The biomass of the desert zones in the northern Tianshan Mountains ranged from 34.3 g·m−2 to 245.0 g·m−2 along the MAP gradient. The average height, total cover and total density ranged from 3.0 to 15.5 cm, 21.2 to 99.9% and 87.6 to 1137.4 plants·m−2, respectively. These parameters were significantly correlated with each other (r > 0.70; p < 0.05; Table 2). Likewise, there was a substantial positive correlation between species richness and the MAP, Simpson dominance index, Shannon–Wiener diversity index and Pielou evenness index (r > 0.64; p < 0.05; Table 2), while biomass and richness had a significant negative correlation with elevation (r > 0.61; p < 0.05; Table 2).

Table 2.
Correlation coefficients among community characteristics, biodiversity and environmental factors.
As revealed by the results of the regression analysis, the relationship between the total biomass and species richness of vegetation presented a quadratic function (Figure 6). The species richness had “updown” tendency as a whole, with a preliminary increase and subsequent decrease along a productivity gradient and suggested that the vegetation biomass had a positive correlation with species richness (R2 = 0.537; p < 0.05, Figure 6).
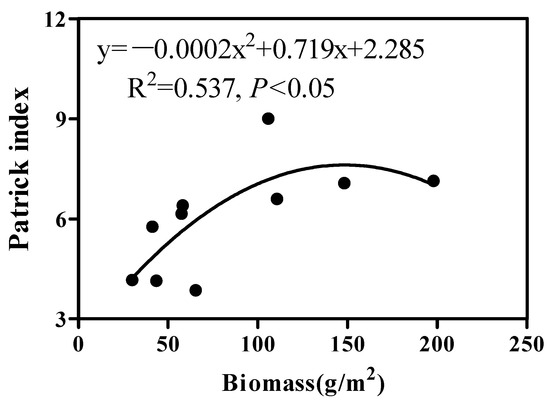
Figure 6.
Relationship between species richness and vegetation biomass in the desert zone of the northern Tianshan Mountains.
4. Discussion
By determining the spatial pattern of the desert vegetation biomass and species richness in the northern Tianshan Mountains of Xinjiang, this study proposed some fundamental insights into the factors that control the variability in biomass and richness in this ecoregion, representing the arid area of Central Asia. One of our key findings suggested that the spatial variability of biomass and species richness were dependent on elevation and the longitudinal transect along the climate zonal that was controlled by the climate condition, in particular, precipitation and temperature. In the paragraphs below, we discuss the potential mechanisms underlying our research findings.
4.1. Spatial Patterns of Biomass and Species Richness in the Desert Zone of the Northern Tianshan Mountains
Both biomass and biodiversity are important indicators of grassland ecosystem health [27]. The biomass and species richness in the desert zones of the northern Tianshan Mountains revealed an updown tendency from west to east, implying that the biomass and richness in the middle section were the highest. One of the key findings suggests that when the climate is drier (the east section), dominant species with low growth rates may limit the biomass responses to precipitation changes, while simultaneously, there is relatively less water in the soil when plants turn green in spring, resulting in fewer numbers and species of annual herbs and ephemeral, ephemeral-like plants [28], which further affects species richness. Conversely, in the comparatively more humid zone (the western and middle sections), the biomass and richness were higher due to abundant precipitation and substantial ephemeral plants. Therefore, climatic differences were the main factors affecting the changes in vegetation biomass and species richness in the three regions.
In the natural grassland, biodiversity was always correlated with geographic factors (elevation, longitude, latitude), and the vegetation biomass was significantly impacted by the geographic position that determined the impact on the climatic variation in grasslands [29]. As observed by our findings, there was a quadratic function among the biomass, richness and longitude, which was consistent with the findings of Broun et al. [30]. Longitude affects the variability in biomass, and richness determines the key differences in microclimates [31]. Furthermore, past research findings have shown that the relationships among biomass, richness and elevation are linear and substantially negative due to decreases in soil nutrients and temperatures with increasing altitude [32,33]. These results were inconsistent with the exponentially decreasing biomass and species richness with increasing elevation in our research. There are three possible reasons for the differences in the results. The first may be the existence of spatial differences at different scales. This study is located in the arid region of Central Asia, which is a special geographical location. Other inconsistent factors that may affect the results include phylogeny, biogeography, geohistorical evolution and environmental change processes. The second possibility is that there are differences in climate. Species biomass and richness are closely related to the climatic conditions of the study area. Under different humidity levels, species diversity has different distribution patterns. Studies have shown that plant species diversity in arid areas decreases with increasing altitude [34]. The third may be caused by different life forms of plant species. Because the diversity of life forms in the plant community does not respond consistently to the environment, plant species with different life forms show different distribution patterns with altitude. Relevant studies have shown that a negative correlation between species diversity of woody plants and increasing altitude generally exists in different ecosystems [35,36,37], while the plants studied in this study were mostly subshrubs, which led to the different results. Vegetation biomass and species richness decreased exponentially with elevation.
4.2. Environmental Factors Influencing Biomass and Species Richness at a Regional Scale
Precipitation was considered a crucial factor that controlled the biomass in most of the grassland, since it impacted the availability of the soil water [11,38,39]. As revealed by our research, the functional group biomass, total biomass and species richness of the desert exhibited an exponential increase along the MAP gradient, and MAP was the primary factor that constrained vegetation growth and species richness. These results were consistent with those of previous studies [40,41]. In the Artemisia-dominated desert area, the biomass was comparatively lower than that in other grasslands owing to less precipitation, higher evaporation, lower soil fertility and fewer species, resulting in precipitation being the strongest environmental determinant of biomass and richness variation. MAP explained 56.1% and 36.8% of the overall variation in the biomass and richness of the desert, respectively. Another reasonable explanation for the biomass response to increasing precipitation could be the long-term plant adaptation to indigenous dry habitats; moreover, it is fully understood that plants have several mechanisms for tolerating various levels of soil water availability.
In addition, our research results also illustrated that the relationship among the vegetation total biomass, species richness and MAT presented a quadratic function in the desert, implying that the biomass and species richness first increased then decreased with increasing temperature. Low temperature was termed a key climatic factor restricting grassland productivity because it inhibited the biogeochemical cycle and reduced the soil nitrogen availability [13], resulting in a lowered response of biomass to precipitation [42,43]. In contrast, high temperature was likely to result in increased evaporation and reduced biomass and species richness by increasing the drying level of the grassland [40,44]. High precipitation, together with moderate temperature, is likely to favor an increase in biomass and species richness [11]. Accordingly, the vegetation biomass and species richness exhibited an exponential increase with precipitation in the desert zone of the northern Tianshan Mountains. Together with MAP and MAT, factors such as nutrient availability, soil pH, bulk density and C and N contents are likely to affect vegetation and species richness, which will be further studied and evaluated in the future.
4.3. Relationships between Biomass and Species Richness in the Desert of the Northern Tianshan Mountains
The relationship between productivity and biodiversity is a critical topic in ecology at present and has been deemed to be essential for sustainable pasture management. However, the nature of causality in the various diversity–productivity relationships remains unclear [45], though it has been gaining increasing attention in recent years [46]. Gillman and Wright also suggested that the positive species diversity relationship, the aboveground net primary productivity (ANPP) relationship, was predominant over the unimodal relationship [47]. Wang et al. observed a unimodal biomass–richness relationship along an altitudinal transect in an alpine meadow zone [48]. These results were consistent with the unimodal biomass–richness relationship along the climate transect in our research, with a coefficient of determination of 53.7%. In addition to unimodal models, monotonous (positive or negative linear) and unrelated patterns have also been observed in a great number of empirical studies involving different spatial scales and ecological levels [49,50,51].
In the natural grassland, the biomass–species richness relationships changed with spatial scale. A meta-analysis by Mittelbach et al. indicated that the unimodal model is applicable at the regional to landscape scale, while monotonous patterns typically take place at finer scales [52]. Our study region, a climate zone of approximately 1700 km, was at a large regional scale, wherein the biomass–species richness relationships were bell-shaped. Two mechanisms support the unimodal model of the biomass–species richness relationships: (1) Variation in species richness is likely to be dependent on grassland ecosystem productivity as well as the size of dominant plant species [53]. Deleglise et al. concluded that the changes in species richness in the spatial patterns were community-dependent, and richness increased with less productivity [54], which likely explained the changes in richness along the biomass gradient in our results. However, the richness declined with the high productivity and/or large size of local species. The dominant species size, for S. transiliense for instance, was larger in high precipitation areas, allowing species to occupy an absolute dominant position and seize a large amount of space and nutrient resources, inhibiting other species’ growth and development sometimes to the point of extinction. This is likely to explain the relationship between high biomass and lower species richness. (2) The ecological compensation mechanism of the whole species assembly played a critical role in the regulation of the relationship of biomass–species richness in both arid and semiarid grasslands [16]. Desert communities in arid sites were capable of showing a low vegetation biomass and low species richness. Accordingly, the species diversity was critically significant for maintaining grassland ecosystem resilience at these sites.
5. Conclusions
As revealed by the data from our study, the spatial patterns of biomass and species richness along the zonal climate of the northern Tianshan Mountains exhibited substantial regional differences. MAP and MAT were the critical environmental determinants of biomass and richness. The higher the MAP was, the higher the biomass and species richness, suggesting that along the precipitation gradient, the biomass and species richness showed an exponential increase. Furthermore, the relationship of the total biomass and species richness of vegetation presented a quadratic function. The species richness had “updown” tendency as a whole, with a preliminary increase and subsequent decrease along a productivity gradient and suggested that the vegetation biomass had a positive correlation with species richness. Most importantly, our results also highlighted the fact that the biomass–species richness relationships were bell-shaped at the regional scale. These results showed that paying attention to the spatial variation in desert spring vegetation biomass, richness and their environmental controls in the arid region of Central Asia will help us better understand not only the spatial variation in vegetation biomass and abundance in different climatic zones, but also in the desert ecology system and help to protect the ecological environment, which is also of great significance.
Author Contributions
Conceptualization, S.Z.; Data curation, S.Z.; Formal analysis, S.Z.; Investigation, Y.D.; Methodology, Y.D.; Resources, A.J. (Asitaiken Julihaiti) and A.J. (Anjing Jiang); Software, A.J. (Asitaiken Julihaiti) and T.N.; Supervision, S.A.; Validation, T.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Natural Science Foundation of the Xinjiang Uygur Autonomous Region (2020D01B29) and National Basic Resources Survey Project of China (2017FY100201).
Acknowledgments
The authors would like to thank Huixia Liu from the School of Grass Industry at the Xinjiang Agricultural University for her support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shi, Y.; Wang, Y.; Ma, Y.; Ma, W.; Liang, C.; Flynn, D.F.B.; Schmid, B.; Fang, J.; He, J.S. Field-based observations of regional-scale, temporal variation in net primary production in Tibetan alpine grasslands. Biogeosciences 2014, 11, 16843–16878. [Google Scholar] [CrossRef]
- Dong, Y.Q.; Sun, Z.J.; An, S.Z.; Yang, H.L.; Yang, J.; Ma, L. Natural restoration of degraded grassland on the northern Xinjiang, China: The restoration difference between lightly and moderately degraded deserts under grazing exclusion. Fresenius Environ. Bull. 2017, 26, 3845–3855. [Google Scholar]
- Jiao, C.; Yu, G.; He, N.; Ma, A.; Ge, J.P.; Hu, Z.M. Spatial pattern of grassland aboveground biomass and its environmental controls in the Eurasian steppe. J. Geogr. Sci. 2017, 27, 3–22. [Google Scholar] [CrossRef]
- Gao, T.; Xu, B.; Yang, X.; Deng, S.Q.; Liu, Y.C.; Jin, Y.X.; Ma, H.L.; Li, J.Y.; Yu, H.D.; Zheng, X.; et al. Aboveground net primary productivity of vegetation along a climate-related gradient in a Eurasian temperate grassland: Spatiotemporal patterns and their relationships with climate factors. Environ. Earth Sci. 2017, 76, 56. [Google Scholar] [CrossRef]
- Liu, J.H.; Wu, J.J.; Su, H.B.; Gao, Z.H.; Wu, Z.T. Effects of grazing exclusion in Xilin Gol grassland differ between regions. Ecol. Eng. 2017, 99, 271–281. [Google Scholar] [CrossRef]
- Fang, J.; Piao, S.; Tang, Z.; Peng, C.; Ji, W. Interannual variability in net primary production and precipitation. World Sci. 2001, 2, 224–227. [Google Scholar] [CrossRef]
- Sala, O.E.; Gherardil, L.A.; Reichmann, L.; Jobbagye, E.; Peters, D. Legacies of precipitation fluctuations on primary production: Theory and data synthesis. Philos. Philos. Trans. R. Soc. Lond. 2012, 367, 3135–3144. [Google Scholar] [CrossRef]
- Guo, L.; Cheng, J.; Luedeling, E.; Sally, E.K.; He, J.S.; Xu, J.C.; Gang, C.C.; Li, W.; Luo, R.M.; Peng, C.H. Critical climate periods for grassland productivity on China’s Loess Plateau. Agric. For. Meteorol. 2017, 233, 101–109. [Google Scholar] [CrossRef]
- Tyukavina, A.; Baccini, A.; Hansen, M.C.; Potapov, P.V.; Stehman, S.V.; Houghton, R.A.; Krylov, A.M.; Turubanova, S.; Goetz, S.J. Aboveground carbon loss in natural and managed tropical forests from 2000 to 2012. Environ. Res. Lett. 2015, 10, 074002. [Google Scholar] [CrossRef]
- Pelletier, J.; Siampale, A.; Legendre, P.; Patrick, J.; Nadine, T.; Laporte, E.; Scott, J.G. Human and natural controls of the variation in aboveground tree biomass in African dry tropical forests. Ecol. Appl. A Publ. Ecol. Soc. Am. 2017, 27, 1578. [Google Scholar] [CrossRef]
- Hu, Z.; Yu, G.; Fan, J.; Zhong, H.P.; Wang, S.Q.; Li, S.G. Precipitation-use efficiency along a 4500-km grassland transect. Glob. Ecol. Biogeogr. 2010, 19, 842–851. [Google Scholar]
- Bai, Y.; Wu, J.; Xing, Q.; Pan, Q.M.; Huang, J.H.; Yang, D.L.; Han, X.G. Primary production and rain use efficiency across a precipitation gradient on the Mongolia Plateau. Ecology 2008, 89, 2140–2153. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, Y.; Zhu, J.; Tao, J.; Zhang, T.; Xi, Y. Effects of community structure on precipitation-use efficiency of grasslands in northern Tibet. J. Veg. Sci. 2017, 28, 281–290. [Google Scholar] [CrossRef]
- Ali, A.; Mattsson, E. Individual tree size inequality enhances aboveground biomass in homegarden agroforestry systems in the dry zone of Sri Lanka. Sci. Total Environ. 2017, 575, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Prado-junior, J.A.; Schiavini, I.; Vale, V.S.; Arantes, C.S.; Sande, M.T.; Lohbeck, M.; Poorter, L. Conservative species drive biomass productivity in tropical dry forests. J. Ecol. 2016, 104, 817–827. [Google Scholar] [CrossRef]
- Wang, G.; Li, H.; An, M.; Ni, J.; Ji, S.; Wang, J. A regional-scale consideration of the effects of species richness on above-ground biomass in temperate natural grasslands of China. J. Veg. Sci. 2011, 22, 414–424. [Google Scholar] [CrossRef]
- Zhang, Q.; Buyantuev, A.; Li, F.Y.; Jiang, L.; Niu, J.; Ding, Y.; Kang, S.; Ma, W. Functional dominance rather than taxonomic diversity and functional diversity mainly affects community aboveground biomass in the Inner Mongolia grassland. Ecol. Evol. 2017, 7, 1605. [Google Scholar] [CrossRef]
- Zhibin, H.; Wenzhi, Z.; Xuexiang, C.; Xueli, C. Scale dependence in desert plant biodiversity. Acta Ecol. Sin. 2004, 24, 1146–1149. [Google Scholar]
- Lu, W.; Zhu, J.; Wang, D.; Jin, G.; Yu, B. Point pattern analysis of Seriphidium transiliense populations under two degradation gradients in the Northern Tianshan Mountains. Acta Prataculturae Sin. 2009, 18, 142–149. [Google Scholar]
- Chen, Y.P.; Jiang, C.; Jian, X.M.; Shui, W.; Hu, Y.; Ma, T.; Xiang, Z.Y. Spatial distribution characteristics of grassland plant communities in a moderately degraded Tiankeng in Zhanyi, Yunnan. Acta Ecol. Sin. 2018, 38, 8008–8021. [Google Scholar]
- Guo, Q.F. The diversity-biomass-productivity relationships in grassland management and restoration. Basic Appl. Ecol. 2007, 8, 199–208. [Google Scholar] [CrossRef]
- O’Brien, E.M.; Field, R.; Whittaker, R.J. Climatic gradients in woody plant (tree and shrub) diversity: Water-energy dynamics, residual variation, and topography. OIKOS 2000, 89, 588–600. [Google Scholar] [CrossRef]
- Xu, C.D.; Feng, J.M.; Wang, X.P.; Yang, X. Vertical distribution patterns of plant species diversity in northern Mt. Gaoligong, Yunan Province. Chin. J. Ecol. 2008, 27, 323–327. [Google Scholar]
- Whittaker, R.H. Evolution of species diversity in land communities. Evol. Biol. 1977, 10, 1–67. [Google Scholar]
- Wu, G.L.; Du, G.Z.; Liu, Z.H. Effect of grazing exclusion and grazing on a Kobresia-dominated meadow in the Qinghai–Tibetan Plateau. Plant Soil 2009, 319, 115–126. [Google Scholar] [CrossRef]
- Xu, P. Grassland Investigation and Planning; Publishing House of Agriculture in China: Beijing, China, 1994; Volume 10, p. 30. [Google Scholar]
- Gao, H.Y.; Hong, M.; Huo, L.X.; Liu, P.F.; Chang, F. Effect of water and nitrogen interaction on plant species diversity and biomass in a desert grassland. Pratacultural Sci. 2018, 35, 36. [Google Scholar]
- Duan, C.; Wu, L.; Wang, S.M.; He, L.Y. Analysis of spatio-temporal patterns of ephemeral plants in the Gurbantünggüt Desert over the last 30 years. Acta Ecol. Sin. 2017, 37, 2642. [Google Scholar]
- Briggs, J.M.; Knapp, A.K. Inter annual variability in primary production in tall grass prairie: Climate, soil moisture, topographic position and fire as determinants of aboveground productivity. Am. J. Bot. 1995, 82, 1024–1030. [Google Scholar] [CrossRef]
- Bruun, H.H.; Moen, R.J.; Virtanen, J.A.; Grytnes, L.; Oksanen, A.; Angerborn, A. Effects of altitude and topography on species richness of vascular plants, bryophytes and lichens in alpine communities. J. Veg. Sci. 2006, 17, 37–46. [Google Scholar] [CrossRef]
- Asaeda, T.; Hai, D.N.; Manatunge, J.; Williams, D.; Roberts, J. Latitudinal characteristics of below-and above-ground productivity of typha: A modelling approach. Ann. Bot. 2005, 96, 299–312. [Google Scholar] [CrossRef]
- Roem, W.J.; Berendse, F. Soil acidity and nutrient supply ratio as possible factors determining changes in plant species diversity in grassland and heathland communities. Biol. Conserv. 2000, 92, 151–161. [Google Scholar] [CrossRef]
- Rastetter, E.B.; Kwiatkowski, B.L.; Dizes, S.L.; Hobbie, J.E. The role of down-slope water and nutrient fluxes in the response of Arctic hill slopes to climate change. Biogeochemistry 2004, 69, 37–62. [Google Scholar] [CrossRef]
- Willis, K.J.; Whittaker, R.J. Ecology Species diversity—Scale matters. Science 2002, 295, 1245–1248. [Google Scholar] [CrossRef] [PubMed]
- Gentry, A.H. Changes in plants communities diversity and floristic composition on environment and geographical gradients. Ann. Mo. Bot. Gard. 1982, 75, 1–34. [Google Scholar] [CrossRef]
- Glenn-Lewin, D.C. Species diversity in North American temperate forests. Plant Ecol. 1977, 33, 153–162. [Google Scholar] [CrossRef]
- Hamilton, A.C.; Perrott, R.A. A study of altitudinal zonation in the montane forest belt of Mt. Elgon, Kenya/Uganda. Plant Ecol. 1981, 47, 107–125. [Google Scholar] [CrossRef]
- Huxman, T.E.; Smith, M.D.; Fay, P.A.; Knapp, A.K.; Shaw, M.R.; Loik, M.E.; Smith, S.D.; Tissue, D.T.; Zak, J.C.; Weltzin, J.F.; et al. Convergence across biomes to a common rain-use efficiency. Nature 2004, 429, 651–655. [Google Scholar] [CrossRef]
- O’connor, T.G.; Haines, L.M.; Snyman, H.A. Influence of precipitation and species composition on phytomass of a semiarid African grassland. J. Ecol. 2001, 89, 850–860. [Google Scholar] [CrossRef]
- Yang, Y.H.; Fang, J.Y.; Pan, Y.D. Aboveground biomass in Tibetan grasslands. J. Arid Environ. 2009, 73, 91–95. [Google Scholar] [CrossRef]
- Bernhardt-romermann, M.; Romernmann, C.; Sperlich, S.; Wolfgang, S. Explaining grassland biomass—the contribution of climate, species and functional diversity depends on fertilization and mowing frequency. J. Appl. Ecol. 2011, 48, 1088–1097. [Google Scholar] [CrossRef]
- Du, M.Y.; Kawashima, S.; Yonemura, S.; Zhang, X.Z.; Chen, S.B. Mutual influence between human activities and climate change in the Tibetan Plateau during recent years. Glob. Planet. Chang. 2010, 41, 241–249. [Google Scholar] [CrossRef]
- Yang, Y.H.; Fang, J.Y.; Ma, W.H.; Guo, D.L.; Mohammat, A. Large-scale pattern of biomass partitioning across China’s grasslands. Glob. Ecol. Biogeogr. 2010, 19, 268–277. [Google Scholar] [CrossRef]
- Epstein, H.E.; Lauenroth, W.K.; Burke, I.C. Effects of temperature and soil texture on ANPP in the US Great Plains. Ecology 1997, 78, 2628–2631. [Google Scholar] [CrossRef]
- Gross, K.; Cardinale, B.J. Does species richness drive community production or vice versa? Reconciling historical and contemporary paradigms in competitive communities. Am. Nat. 2007, 170, 207. [Google Scholar] [CrossRef]
- Wu, J.S.; Shen, Z.X.; Zhang, X.Z. Precipitation and species composition primarily determine the diversity-productivity relationship of alpine grasslands on the Northern Tibetan Plateau. Alp. Bot. 2014, 124, 13–25. [Google Scholar] [CrossRef]
- Gillman, L.N.; Wright, S.D. The influence of productivity on the species richness of plants: A critical assessment. Ecology 2006, 87, 1234–1243. [Google Scholar] [CrossRef]
- Wang, C.T.; Long, R.J.; Wang, Q.J.; Ding, L.M.; Wang, M.P. Effects of altitude on plant-species diversity and productivity in an alpine meadow, Qinghai-Tibetan Plateau. Aust. J. Bot. 2007, 55, 110–117. [Google Scholar] [CrossRef]
- Naeem, S.; Hakansson, K.; Lawton, J.H.; Crawley, M.J.; Thompson, L.J. Biodiversity and plant productivity in a model assemblage of plant species. Oikos 1996, 76, 259–264. [Google Scholar] [CrossRef]
- Bai, Y.F.; Wu, J.G.; Pan, Q.M.; Huang, J.H.; Wang, Q.B.; Li, F.S.; Buyantuyev, A.; Han, X.G. Positive linear relationship between productivity and diversity: Evidence from the Eurasian Steppe. J. Appl. Ecol. 2007, 44, 1023–1034. [Google Scholar] [CrossRef]
- Ma, W.H.; He, J.S.; Yang, Y.H.; Wang, X.P.; Liang, C.Z.; Anwar, M.; Zeng, H.; Fang, J.Y.; Schmid, B. Environmental factors covary with plant diversity–productivity relationships among Chinese grassland sites. Glob. Ecol. Biogeogr. 2010, 19, 233–243. [Google Scholar] [CrossRef]
- Mittelbach, G.G.; Steiner, C.F.; Scheiner, S.M.; Gross, K.L.; Reynolfs, H.L.; Waide, R.B.; Willig, M.R.; Dodson, S.I.; Gough, L. What is the observed relationship between species richness and productivity? Ecology 2001, 82, 2381. [Google Scholar] [CrossRef]
- Osem, Y.; Perevolotsky, A.; Kigel, J. Site productivity and plant size explain the response of annual species to grazing exclusion in a Mediterranean semi-arid rangeland. J. Ecol. 2004, 92, 297–309. [Google Scholar] [CrossRef]
- Deleglise, C.; Loucougaray, G.; Alard, D. Spatial patterns of species and plant traits in response to 20 years of grazing exclusion in subalpine grassland communities. J. Veg. Sci. 2011, 22, 402–413. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).