Relationships between Organic Matter and Bulk Density in Amazonian Peatland Soils
Abstract
1. Introduction
- To find an adequate model for OM in relation to DBD in a sample of Amazonian peatlands;
- To test the significance of depth as a co-factor;
- Check for possible hydrological or landscape level effects in the site data;
- Assess the ideal mixing model.
2. Materials & Methods
2.1. Soil Sampling Process
2.2. Quantitative
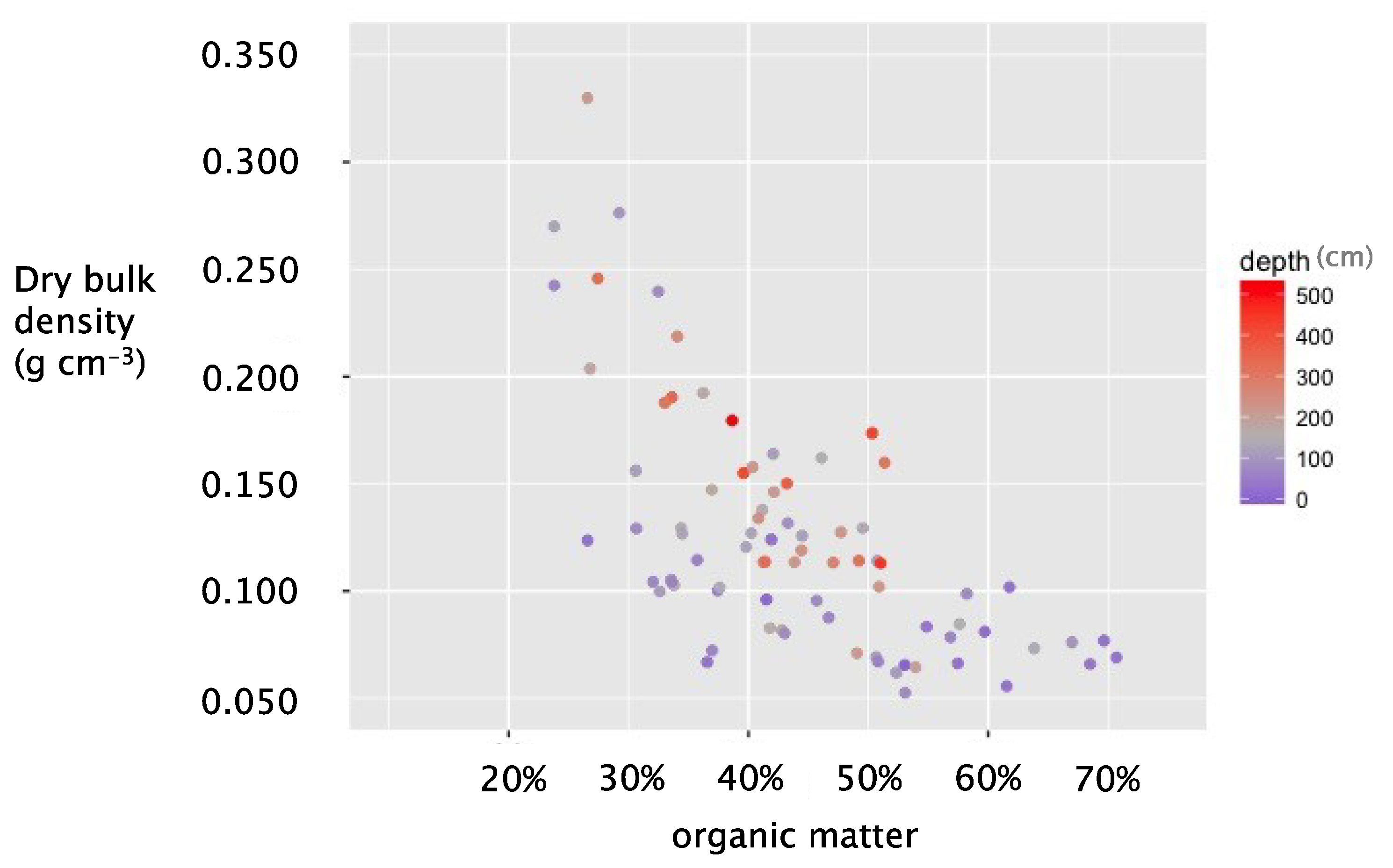
3. Results
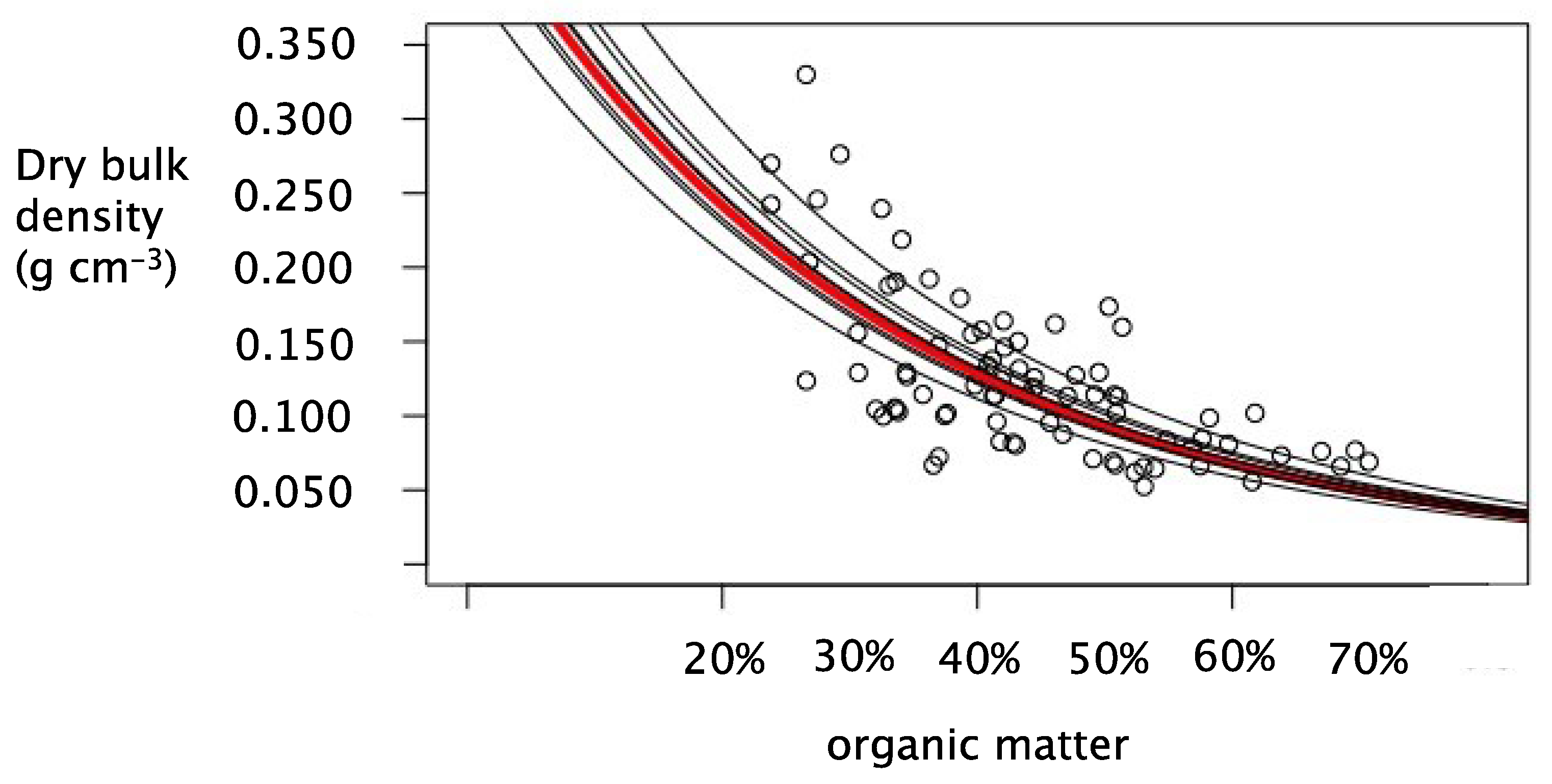
3.1. Model Fitting
3.2. The Ideal Mixing Model
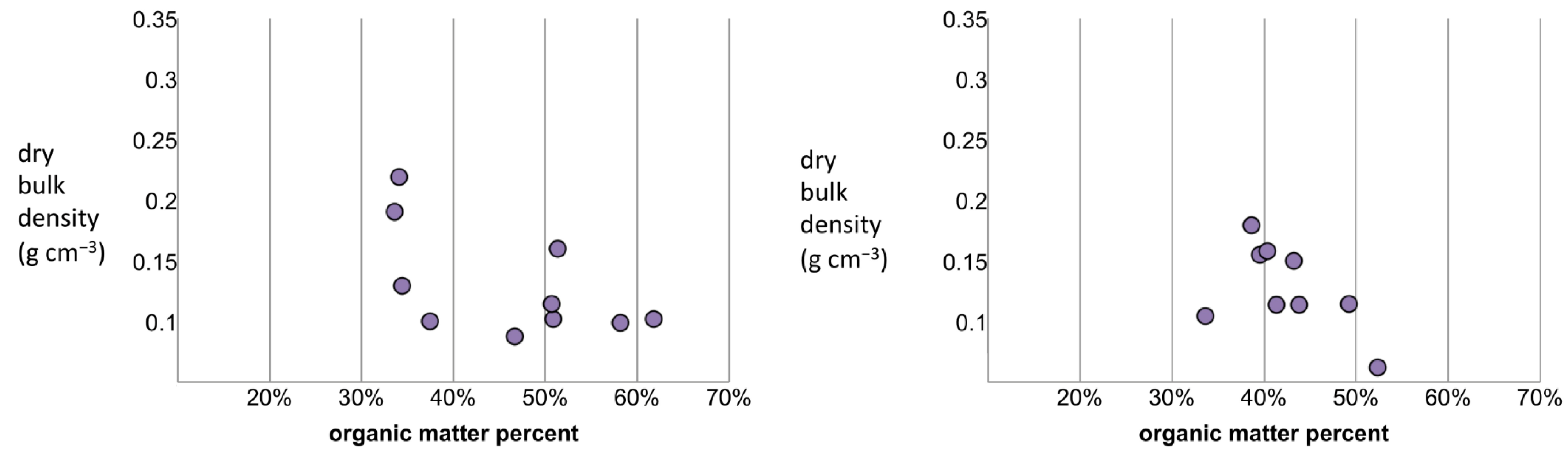
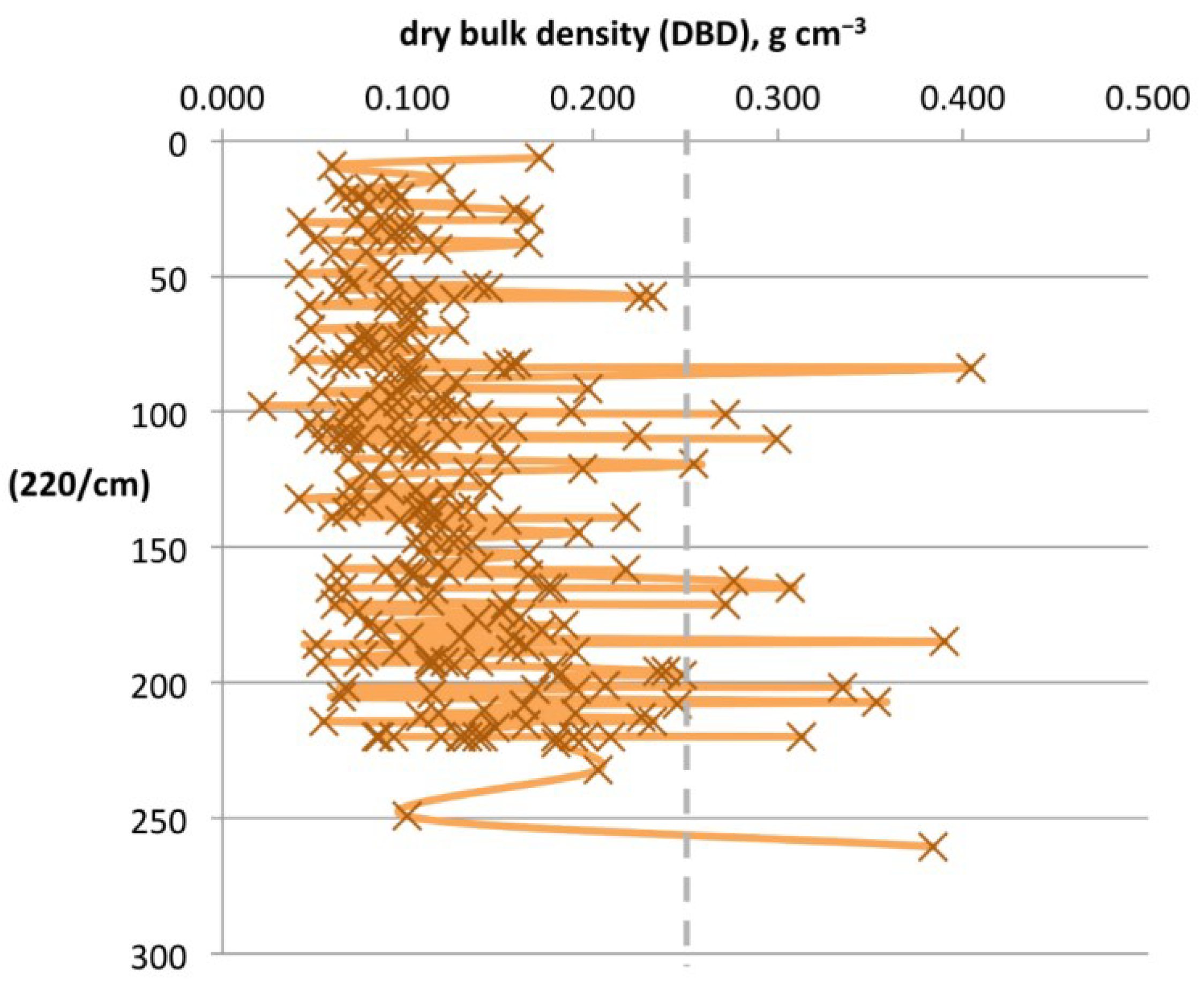
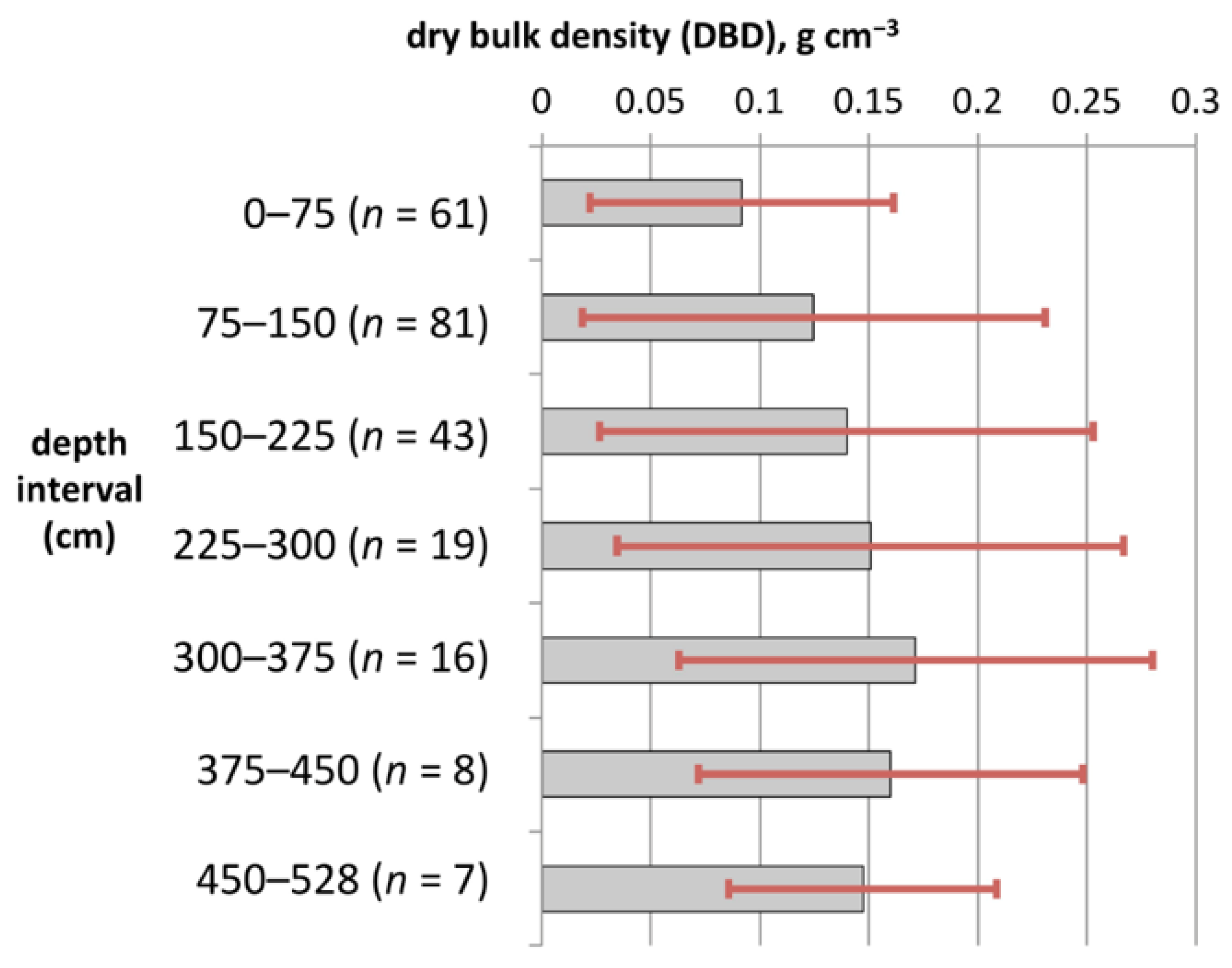
3.3. Assessing Patterns in DBD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kolka, R.; Sebestyen, S.; Verry, E.S.; Brooks, K. Peatland Biogeochemistry and Watershed Hydrology at the Marcell Experimental Forest; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Chimner, R.A.; Ott, C.A.; Perry, C.H.; Kolka, R.K. Developing and Evaluating Rapid Field Methods to Estimate Peat Carbon. Wetlands 2014, 34, 1241–1246. [Google Scholar] [CrossRef]
- Chambers, F.M.; Beilman, D.; Yu, Z. Methods for Determining Peat Humification and for Quantifying Peat Bulk Density, Organic Matter and Carbon Content for Palaeostudies of Climate and Peatland Carbon Dynamics. Mires Peat 2011, 7, 1–10. [Google Scholar]
- Minasny, B.; Berglund, Ö.; Connolly, J.; Hedley, C.; de Vries, F.; Gimona, A.; Kempen, B.; Kidd, D.; Lilja, H.; Malone, B. Digital Mapping of peatlands—A Critical Review. Earth-Sci. Rev. 2019, 196, 102870. [Google Scholar] [CrossRef]
- Jaenicke, J.; Rieley, J.O.; Mott, C.; Kimman, P.; Siegert, F. Determination of the amount of carbon stored in Indonesian peatlands. Geoderma 2008, 147, 151–158. [Google Scholar] [CrossRef]
- Page, S.E.; Rieley, J.O.; Banks, C.J. Global and regional importance of the tropical peatland carbon pool. Glob. Change Biol. 2011, 17, 798–818. [Google Scholar] [CrossRef]
- Hastie, A.; Coronado, E.N.H.; Reyna, J.; Mitchard, E.T.A.; Åkesson, C.M.; Baker, T.R.; Cole, L.E.S.; Oroche, C.J.C.; Dargie, G.; Dávila, N.; et al. Risks to carbon storage from land-use change revealed by peat thickness maps of Peru. Nat. Geosci. 2022, 15, 369–374. [Google Scholar] [CrossRef]
- Warren, M.W.; Kauffman, J.B.; Murdiyarso, D.; Anshari, G.; Hergoualc’H, K.; Kurnianto, S.; Purbopuspito, J.; Gusmayanti, E.; Afifudin, M.; Rahajoe, J.; et al. A cost-efficient method to assess carbon stocks in tropical peat soil. Biogeosciences 2012, 9, 4477–4485. [Google Scholar] [CrossRef]
- Rudiyanto, R.; Minasny, B.; Setiawan, B.I. Further results on comparison of methods for quantifying soil carbon in tropical peats. Geoderma 2016, 269, 108–111. [Google Scholar] [CrossRef]
- Grigal, D.F.; Brovold, S.L.; Nord, W.S.; Ohmann, L.F. Bulk Density of Surface Soils and Peat in the North Central United States. Can. J. Soil Sci. 1989, 69, 895–900. [Google Scholar] [CrossRef]
- Jeffrey, D.W. A Note on the use of Ignition Loss as a Means for the Approximate Estimation of Soil Bulk Density. J. Ecol. 1970, 58, 297–299. [Google Scholar] [CrossRef]
- Périé, C.; Ouimet, R. Organic carbon, organic matter and bulk density relationships in boreal forest soils. Can. J. Soil Sci. 2008, 88, 315–325. [Google Scholar] [CrossRef]
- Sequeira, C.H.; Wills, S.A.; Seybold, C.A.; West, L.T. Predicting soil bulk density for incomplete databases. Geoderma 2014, 213, 64–73. [Google Scholar] [CrossRef]
- Vasques, G.M.; Grunwald, S.; Comerford, N.B.; Sickman, J.O. Regional modelling of soil carbon at multiple depths within a subtropical watershed. Geoderma 2010, 156, 326–336. [Google Scholar] [CrossRef]
- Yi, X.; Li, G.; Yin, Y. Pedotransfer Functions for Estimating Soil Bulk Density: A Case Study in the Three-River Headwater Region of Qinghai Province, China. Pedosphere 2016, 26, 362–373. [Google Scholar] [CrossRef]
- Upton, A.; Vane, C.H.; Girkin, N.; Turner, B.L.; Sjögersten, S. Does Litter Input Determine Carbon Storage and Peat Organic Chemistry in Tropical Peatlands? Geoderma 2018, 326, 76–87. [Google Scholar] [CrossRef]
- Quesada, C.A.; Paz, C.; Mendoza, E.O.; Phillips, O.L.; Saiz, G.; Lloyd, J. Variations in soil chemical and physical properties explain basin-wide Amazon forest soil carbon concentrations. Soil 2020, 6, 53–88. [Google Scholar] [CrossRef]
- Wiese, L.; Ros, I.; Rozanov, A.; Boshoff, A.; de Clercq, W.; Seifert, T. An approach to soil carbon accounting and mapping using vertical distribution functions for known soil types. Geoderma 2016, 263, 264–273. [Google Scholar] [CrossRef]
- Vepraskas, M.J.; Craft, C.B. Wetland Soils: Genesis, Hydrology, Landscapes, and Classification; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Bhomia, R.K.; van Lent, J.; Rios, J.M.G.; Hergoualc’H, K.; Coronado, E.N.H.; Murdiyarso, D. Impacts of Mauritia flexuosa degradation on the carbon stocks of freshwater peatlands in the Pastaza-Marañón river basin of the Peruvian Amazon. Mitig. Adapt. Strat. Glob. Change 2018, 24, 645–668. [Google Scholar] [CrossRef]
- Kalliola, R.; Salo, J.; Puhakka, M.; Rajasilta, M.; Häme, T.; Neller, R.; Räsänen, M.; Arias, W.D. Upper amazon channel migration. Naturwissenschaften 1992, 79, 75–79. [Google Scholar] [CrossRef]
- Lähteenoja, O.; Reátegui, Y.R.; Räsänen, M.; Torres, D.D.C.; Oinonen, M.; Page, S. The Large Amazonian Peatland Carbon Sink in the Subsiding Pastaza-Marañón Foreland Basin, Peru. Glob. Change Biol. 2012, 18, 164–178. [Google Scholar] [CrossRef]
- Junk, W.J.; Piedade, M.T.F.; Schöngart, J.; Cohn-Haft, M.; Adeney, J.M.; Wittmann, F. A Classification of Major Naturally-Occurring Amazonian Lowland Wetlands. Wetlands 2011, 31, 623–640. [Google Scholar] [CrossRef]
- Aniceto, K.; Moreira-Turcq, P.; Cordeiro, R.C.; Quintana, I.; Fraizy, P.; Turcq, B. Hydrological Changes in West Amazonia over the Past 6 Ka Inferred from Geochemical Proxies in the Sediment Record of a Floodplain Lake. Procedia Earth Planet. Sci. 2014, 10, 287–291. [Google Scholar] [CrossRef]
- Räsänen, M.; Neller, R.; Salo, J.; Jungner, H. Recent and ancient fluvial deposition systems in the Amazonian foreland basin, Peru. Geol. Mag. 1992, 129, 293–306. [Google Scholar] [CrossRef]
- Lähteenoja, O.; Page, S. High Diversity of Tropical Peatland Ecosystem Types in the Pastaza-Marañón Basin, Peruvian Amazonia. J. Geophys. Res. Biogeosciences 2011, 116, G02025. [Google Scholar] [CrossRef]
- Lawson, I.T.; Kelly, T.J.; Aplin, P.; Boom, A.; Dargie, G.; Draper, F.C.H.; Hassan, P.N.Z.B.P.; Hoyos-Santillan, J.; Kaduk, J.; Large, D.J.; et al. Improving estimates of tropical peatland area, carbon storage, and greenhouse gas fluxes. Wetl. Ecol. Manag. 2014, 23, 327–346. [Google Scholar] [CrossRef]
- Limpens, J.; Berendse, F.; Blodau, C.; Canadell, J.G.; Freeman, C.; Holden, J.; Roulet, N.; Rydin, H.; Schaepman-Strub, G. Peatlands and the carbon cycle: From local processes to global implications—A synthesis. Biogeosciences 2008, 5, 1475–1491. [Google Scholar] [CrossRef]
- Neue, H.; Gaunt, J.; Wang, Z.; Becker-Heidmann, P.; Quijano, C. Carbon in tropical wetlands. Geoderma 1997, 79, 163–185. [Google Scholar] [CrossRef]
- Pastor, J.; Peckham, B.; Bridgham, S.; Weltzin, J.; Chen, J. Plant Community Dynamics, Nutrient Cycling, and Alternative Stable Equilibria in Peatlands. Am. Nat. 2002, 160, 553–568. [Google Scholar] [CrossRef]
- Kelly, T.J.; Lawson, I.T.; Roucoux, K.H.; Baker, T.R.; Jones, T.D.; Sanderson, N.K. The vegetation history of an Amazonian domed peatland. Palaeogeogr. Palaeoclim. Palaeoecol. 2017, 468, 129–141. [Google Scholar] [CrossRef]
- Blodau, C. Carbon cycling in peatlands—A review of processes and controls. Environ. Rev. 2002, 10, 111–134. [Google Scholar] [CrossRef]
- Sjögersten, S.; Cheesman, A.W.; Lopez, O.; Turner, B.L. Biogeochemical processes along a nutrient gradient in a tropical ombrotrophic peatland. Biogeochemistry 2010, 104, 147–163. [Google Scholar] [CrossRef]
- Smith, N.D.; Cross, T.A.; Dufficy, J.P.; Clough, S.R. Anatomy of an avulsion. Sedimentology 1989, 36, 1–23. [Google Scholar] [CrossRef]
- Morozova, G.S.; Smith, N.D. Organic matter deposition in the Saskatchewan River floodplain (Cumberland Marshes, Canada): Effects of progradational avulsions. Sediment. Geol. 2003, 157, 15–29. [Google Scholar] [CrossRef]
- Treat, C.C.; Kleinen, T.; Broothaerts, N.; Dalton, A.S.; Dommain, R.; Douglas, T.A.; Drexler, J.Z.; Finkelstein, S.A.; Grosse, G.; Hope, G.; et al. Widespread global peatland establishment and persistence over the last 130,000 y. Proc. Natl. Acad. Sci. USA 2019, 116, 4822–4827. [Google Scholar] [CrossRef]
- Dubroeucq, D.; Volkoff, B. From Oxisols to Spodosols and Histosols: Evolution of the soil mantles in the Rio Negro basin (Amazonia). CATENA 1998, 32, 245–280. [Google Scholar] [CrossRef]
- Junk, W.J.; Furch, K. A general review of tropical South American floodplains. Wetl. Ecol. Manag. 1993, 2, 231–238. [Google Scholar] [CrossRef]
- Dargie, G.C.; Lewis, S.L.; Lawson, I.T.; Mitchard, E.T.A.; Page, S.E.; Bocko, Y.E.; Ifo, S.A. Age, extent and carbon storage of the central Congo Basin peatland complex. Nature 2017, 542, 86–90. [Google Scholar] [CrossRef]
- Morris, J.T.; Barber, D.C.; Callaway, J.C.; Chambers, R.; Hagen, S.C.; Hopkinson, C.S.; Johnson, B.J.; Megonigal, P.; Neubauer, S.C.; Troxler, T.; et al. Contributions of organic and inorganic matter to sediment volume and accretion in tidal wetlands at steady state. Earth’s Future 2016, 4, 110–121. [Google Scholar] [CrossRef]
- Villa, J.A.; Bernal, B. Carbon sequestration in wetlands, from science to practice: An overview of the biogeochemical process, measurement methods, and policy framework. Ecol. Eng. 2018, 114, 115–128. [Google Scholar] [CrossRef]
- Dirección de Gestión del Territorio, Autoridad Reigonal Ambiental de Ucayali. Estudio De Clima Y Zonas De Vida. In Zonificacion Ecologica y Economica de la Region Ucayali; Dirección de Gestión del Territorio, Autoridad Reigonal Ambiental de Ucayali: Ucayali, Peru, 2016; pp. 1–64. [Google Scholar]
- Sanchez Huaman, S.; Torres, L.C.; Llamocca Huamani, J. Ucayali: Sinergias Por El Clima; Centro de Conservación Investigación y Manejo de Áreas Naturales: Lima, Peru, 2017; p. 25. [Google Scholar]
- Dirección de Gestión del Territorio, Autoridad Reigonal Ambiental de Ucayali. Suelos Y Capacidad De Uso Mayor De Las Tierras. In Zonificacion Ecologica y Economica de la Region Ucayali; Dirección de Gestión del Territorio, Autoridad Reigonal Ambiental de Ucayali: Ucayali, Peru, 2016; 181p. [Google Scholar]
- Quesada, C.A.; Phillips, O.L.; Schwarz, M.; Czimczik, C.I.; Baker, T.R.; Patiño, S.; Fyllas, N.M.; Hodnett, M.G.; Herrera, R.; Almeida, S.; et al. Basin-wide variations in Amazon forest structure and function are mediated by both soils and climate. Biogeosciences 2012, 9, 2203–2246. [Google Scholar] [CrossRef]
- Nascimento, N.R.D.; Fritsch, E.; Bueno, G.T.; Bardy, M.; Grimaldi, C.; Melfi, A.J. Podzolization as a deferralitization process: Dynamics and chemistry of ground and surface waters in an Acrisol—Podzol sequence of the upper Amazon Basin. Eur. J. Soil Sci. 2008, 59, 911–924. [Google Scholar] [CrossRef]
- de Assis, R.L.; Wittmann, F.; Bredin, Y.K.; Schöngart, J.; Quesada, C.A.N.; Piedade, M.T.F.; Haugaasen, T. Above-ground woody biomass distribution in Amazonian floodplain forests: Effects of hydroperiod and substrate properties. For. Ecol. Manag. 2019, 432, 365–375. [Google Scholar] [CrossRef]
- Goulding, M.; Cañas, C.; Barthem, R.; Forsberg, B.; Ortega, H. Amazon Headwaters: Rivers, Wildlife, and Conservation in Southeastern Peru; Asociacion para la Conservacion de la Cuenca Amazonica, ACCA: Lima, Peru, 2003; p. 117. [Google Scholar]
- García-Soria, D.; Honorio-Coronado, E.N.; Del Castillo-Torres, D. Determinación del Stock de Carbono en Aguajales de la Cuenca del Río Aguaytía, Ucayali—Perú. Folia Amaz. 2012, 21, 153–160. [Google Scholar] [CrossRef]
- Junk, W.J.; Piedade, M.T.; Wittmann, F.; Schöngart, J.; Parolin, P. Amazonian Floodplain Forests: Ecophysiology, Biodiversity and Sustainable Management; Springer: Dordrecht, The Netherlands, 2010; p. 618. [Google Scholar]
- Jowsey, P.C. An Improved Peat Sampler. New Phytol. 1966, 65, 245–248. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models using Lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar]
- Elzhov, T.V.; Mullen, K.M.; Spiess, A.; Bolker, B. R Package, Version 1.1-8; Minpack.Lm: R Interface to the Levenberg-Marquardt Nonlinear Least-Squares Algorithm found in MINPACK, Plus Support for Bounds; R Core: R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Hergoualc’H, K.; Verchot, L.V. Stocks and fluxes of carbon associated with land use change in Southeast Asian tropical peatlands: A review. Glob. Biogeochem. Cycles 2011, 25, GB2001. [Google Scholar] [CrossRef]
- Hooijer, A.; Page, S.; Canadell, J.G.; Silvius, M.; Kwadijk, J.; Wösten, H.; Jauhiainen, J. Current and future CO2 emissions from drained peatlands in Southeast Asia. Biogeosciences 2010, 7, 1505–1514. [Google Scholar] [CrossRef]
- Konecny, K.; Ballhorn, U.; Navratil, P.; Jubanski, J.; Page, S.E.; Tansey, K.; Hooijer, A.; Vernimmen, R.; Siegert, F. Variable carbon losses from recurrent fires in drained tropical peatlands. Glob. Change Biol. 2016, 22, 1469–1480. [Google Scholar] [CrossRef]
- Ingram, H.A.P. Size and shape in raised mire ecosystems: A geophysical model. Nature 1982, 297, 300–303. [Google Scholar] [CrossRef]
- Adams, W.A. The Effect of Organic Matter on the Bulk and True Densities of Some Uncultivated Podzolic Soils. Eur. J. Soil Sci. 1973, 24, 10–17. [Google Scholar] [CrossRef]
- Farmer, J.; Matthews, R.; Smith, P.; Langan, C.; Hergoualc’H, K.; Verchot, L.; Smith, J.U. Comparison of methods for quantifying soil carbon in tropical peats. Geoderma 2014, 214, 177–183. [Google Scholar] [CrossRef]
- Page, S.E.; Rieley, J.O.; Shotyk, W.; Weiss, D. Interdependence of Peat and Vegetation in a Tropical Peat Swamp Forest. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 1885–1897. [Google Scholar] [CrossRef] [PubMed]
- De Vleeschouwer, F.; Chambers, F.M.; Swindles, G.T. Coring and Sub-Sampling of Peatlands for Palaeoenvironmental Research. Mires Peat 2010, 7, 1–10. [Google Scholar]
- US Forest Service. Forest Inventory and Analysis, US. Soil measurements and sampling. In Field Methods for Forest Health (Phase 3) Measurements; US Forest Service, Ed.; US Forest Service: Logan, UT, USA, 2011; pp. 1–29. [Google Scholar]
- Federer, C.A.; Turcotte, D.E.; Smith, C.T. The organic fraction–bulk density relationship and the expression of nutrient content in forest soils. Can. J. For. Res. 1993, 23, 1026–1032. [Google Scholar] [CrossRef]
- Stewart, V.; Adams, W.; Abdulla, H. Quantitative Pedological Studies on Soils Derived from Silurian Mudstones: II. the Relationship between Stone Content and the Apparent Density of the Fine Earth. J. Soil Sci. 1970, 21, 248–255. [Google Scholar] [CrossRef]
- Roucoux, K.H.; Lawson, I.T.; Jones, T.D.; Baker, T.R.; Jones, T.D.; Gosling, W.D.; Lähteenoja, O. Vegetation development in an Amazonian peatland. Palaeogeogr. Palaeoclim. Palaeoecol. 2013, 374, 242–255. [Google Scholar] [CrossRef]
- Davis, J.L.; Currin, C.A.; O’Brien, C.; Raffenburg, C.; Davis, A. Living Shorelines: Coastal Resilience with a Blue Carbon Benefit. PLoS ONE 2015, 10, e0142595. [Google Scholar] [CrossRef]
- Neubauer, S.C. Contributions of mineral and organic components to tidal freshwater marsh accretion. Estuar. Coast. Shelf Sci. 2008, 78, 78–88. [Google Scholar] [CrossRef]
- Morris, J.T.; Bowden, W.B. A Mechanistic, Numerical Model of Sedimentation, Mineralization, and Decomposition for Marsh Sediments 1. Soil Sci. Soc. Am. J. 1986, 50, 96–105. [Google Scholar] [CrossRef]
- Furch, K.; Junk, W.J. Physicochemical Conditions in the Floodplains. In The Central Amazon Floodplain; Junk, W.J., Ed.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 69–108. [Google Scholar] [CrossRef]
- Roucoux, K.H.; Lawson, I.T.; Baker, T.R.; Del Castillo Torres, D.; Draper, F.C.; Lähteenoja, O.; Gilmore, M.P.; Honorio Coronado, E.N.; Kelly, T.J.; Mitchard, E.T.A. Threats to intact tropical peatlands and opportunities for their conservation. Conserv. Biol. 2017, 31, 1283–1292. [Google Scholar] [CrossRef]
- Posa, M.R.C.; Wijedasa, L.S.; Corlett, R.T. Biodiversity and Conservation of Tropical Peat Swamp Forests. Bioscience 2011, 61, 49–57. [Google Scholar] [CrossRef]
- Leifeld, J.; Menichetti, L. The underappreciated potential of peatlands in global climate change mitigation strategies. Nat. Commun. 2018, 9, 1071. [Google Scholar] [CrossRef] [PubMed]
- Sjögersten, S.; de la Barreda-Bautista, B.; Brown, C.; Boyd, D.; Lopez-Rosas, H.; Hernández, E.; Monroy, R.; Rincón, M.; Vane, C.; Moss-Hayes, V.; et al. Coastal wetland ecosystems deliver large carbon stocks in tropical Mexico. Geoderma 2021, 403, 115173. [Google Scholar] [CrossRef]


| Site Number | Number of Points in Transect | Sample Size | Above 65% Thres- Hold | Above 40% Thres- Hold | Number of Cores over 40% | Average OM | Min. OM | Max. OM | Average DBD(g cm−3) | Average Depth (cm) | Min Depth | Max Depth |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | n = 8 | 0 | 2 | 2 | 34.8% | 23.8% | 54.9% | 0.123 | 136 | 50 | 210 |
| 2 | 7 | n = 8 | 0 | 4 | 4 | 37.3% | 23.8% | 57.5% | 0.191 | 150 | 10 | 345 |
| 3 | 6 | n = 14 | 1 | 11 | 5 | 46.4% | 33.0% | 68.5% | 0.116 | 312 | 145 | 528 |
| 4 | 6 | n = 10 | 0 | 6 | 4 | 45.9% | 33.6% | 61.8% | 0.130 | 148 | 10 | 375 |
| 5 | 2 | n = 9 | 0 | 6 | 2 | 42.5% | 33.6% | 52.3% | 0.128 | 503 | 480 | 525 |
| 8 | 4 | n = 4 | 1 | 2 | 2 | 45.9% | 26.8% | 67.0% | 0.100 | 112 | 60 | 190 |
| 9 | 3 | n = 4 | 0 | 3 | 2 | 51.0% | 39.8% | 61.5% | 0.084 | 100 | 0 | 215 |
| 10 | 7 | n = 15 | 0 | 11 | 7 | 44.8% | 27.4% | 63.8% | 0.106 | 259 | 190 | 380 |
| 13 | 4 | n = 8 | 2 | 5 | 4 | 47.1% | 32.5% | 70.7% | 0.134 | 92 | 20 | 220 |
| All sites | 44 | n = 80 | 4 | 50 | 32 | 43.8% | 23.8% | 70.7% | 0.125 | 165 | 0 | 528 |
| 0–75 cm interval | n/a | n = 23 | n/a | n/a | n/a | 48.1% | 23.8% | 70.7% | 0.093 | 43 | n/a | n/a |
| 75–150 cm interval | n/a | n = 27 | n/a | n/a | n/a | 43.2% | 23.8% | 67.0% | 0.126 | 114 | n/a | n/a |
| 150–300 cm interval | n/a | n = 20 | n/a | n/a | n/a | 42.7% | 26.6% | 63.8% | 0.132 | 192 | n/a | n/a |
| Equation | Coefficient of Determination (R2) | Mean Error (ME) | Root Mean Square Error (RMSE) | Akaike Information Criterion (AIC) | |
|---|---|---|---|---|---|
| Logarithmic decay | DBD = ln(OM) | 0.478 | 0.0000 | 0.40 | −281.61 |
| Power-law decrease | DBD = xOMy | 0.519 | 0.0003 | 0.038 | −288.16 |
| Power w/intercept | DBD = xOMy + z | 0.528 | 0.0000 | 0.038 | −287.7 |
| Ideal mixing model | DBD = 1/ [OM/k1 + (1 − OM)/k2] | 0.524 | 0.0004 | 0.038 | −289.03 |
| Site Number | Sample Size | R2: Logarithmic Decay | AIC: Logarithmic Decay | R2: Power- Law Decrease | AIC: Power- Law Decrease | R2: Power w/Intercept | AIC: Power w/Intercept | R2: Ideal Mixing Model | AIC: Ideal Mixing Model |
|---|---|---|---|---|---|---|---|---|---|
| 1 | n = 8 | 0.467 | −24.63 | 0.605 | −27.01 | 0.810 * | −30.88 | 0.665 | −28.34 |
| 2 | n = 8 | 0.791 | −23.37 | 0.777 | −22.83 | 0.792 | −21.388 | 0.767 | −22.48 |
| 3 | n = 14 | 0.307 | −53.41 | 0.303 | −53.34 | 0.307 * | −51.41 | 0.298 | −53.23 |
| 4 | n = 10 | 0.370 | −33.50 | 0.393 | −33.88 | 0.503 * | −33.88 | 0.392 | −33.87 |
| 5 | n = 9 | 0.237 | −31.86 | 0.192 | −31.35 | 0.236 * | −29.85 | 0.186 | −31.28 |
| 8 | n = 4 | 0.573 | −8.48 | 0.761 | −10.82 | 0.977 * | −18.25 | 0.836 | −12.30 |
| 9 | n = 4 | 0.626 | −16.68 | 0.646 | −16.89 | 0.681 * | −15.32 | 0.650 | −16.95 ** |
| 10 | n = 15 | 0.579 | −56.92 | 0.655 | −59.89 | 0.712 | −60.63 | 0.682 | −61.14 ** |
| 13 | n = 8 | 0.576 | −24.40 | 0.620 | −25.27 | 0.657 | −24.10 | 0.636 | −25.61 ** |
| 0–75 cm interval * | n = 23 | 0.480 | −89.82 | 0.576 | −94.32 | 0.800 | −108.85 | 0.588 | −94.95 |
| 75–150 cm interval | n = 27 | 0.518 | −84.23 | 0.583 | −87.81 | 0.603 | −87.07 | 0.594 | −88.52 ** |
| 150–300 cm interval | n = 20 | 0.659 | −71.26 | 0.683 | −72.73 | 0.683 | −70.73 | 0.683 | −72.72 |
| Site Number | Sample Size | Average OM | Average DBD | k1 (g cm−3) | k2 (g cm−3) | x-Intercept | Interbedded Clay Layers Observed |
|---|---|---|---|---|---|---|---|
| 4 | n = 10 | 37.3% | 0.130 | 0.057 | 0.312 | y | |
| 5 | n = 9 | 42.5% | 0.128 | 0.053 | 0.193 | y | |
| 2 | n = 8 | 44.8% | 0.191 | 0.053 | −0.611 | 0.417 | y |
| 3 | n = 14 | 45.9% | 0.116 | 0.052 | 0.179 | y | |
| 13 | n = 8 | 51.0% | 0.134 | 0.043 | −0.245 | 0.312 | y |
| 9 | n = 4 | 47.1% | 0.084 | 0.037 | −0.391 | n | |
| 10 | n = 15 | 45.9% | 0.106 | 0.033 | −0.190 | 0.061 | y |
| 1 | n = 8 | 34.8% | 0.123 | 0.025 | −0.155 | −0.095 | n |
| 8 | n = 4 | 46.4% | 0.100 | 0.021 | −0.094 | −0.032 | n |
| all sites | n = 80 | 43.8% | 0.125 | 0.044 | −0.591 | n/a | |
| 0–75 cm interval | n = 22 | 48.1% | 0.093 | 0.036 | −0.641 | n/a | n/a |
| 75–150 cm interval | n = 27 | 43.2% | 0.126 | 0.040 | −0.335 | n/a | n/a |
| 150–300 cm interval | n = 26 | 42.7% | 0.132 | 0.040 | −0.245 | n/a | n/a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crnobrna, B.; Llanqui, I.B.; Cardenas, A.D.; Panduro Pisco, G. Relationships between Organic Matter and Bulk Density in Amazonian Peatland Soils. Sustainability 2022, 14, 12070. https://doi.org/10.3390/su141912070
Crnobrna B, Llanqui IB, Cardenas AD, Panduro Pisco G. Relationships between Organic Matter and Bulk Density in Amazonian Peatland Soils. Sustainability. 2022; 14(19):12070. https://doi.org/10.3390/su141912070
Chicago/Turabian StyleCrnobrna, Brian, Irbin B. Llanqui, Anthony Diaz Cardenas, and Grober Panduro Pisco. 2022. "Relationships between Organic Matter and Bulk Density in Amazonian Peatland Soils" Sustainability 14, no. 19: 12070. https://doi.org/10.3390/su141912070
APA StyleCrnobrna, B., Llanqui, I. B., Cardenas, A. D., & Panduro Pisco, G. (2022). Relationships between Organic Matter and Bulk Density in Amazonian Peatland Soils. Sustainability, 14(19), 12070. https://doi.org/10.3390/su141912070







