Prey Identification of the Little Tern, Sternula albifrons (Pallas, 1764), by Applying DNA Barcoding to Fecal Materials
Abstract
1. Introduction
2. Materials and Methods
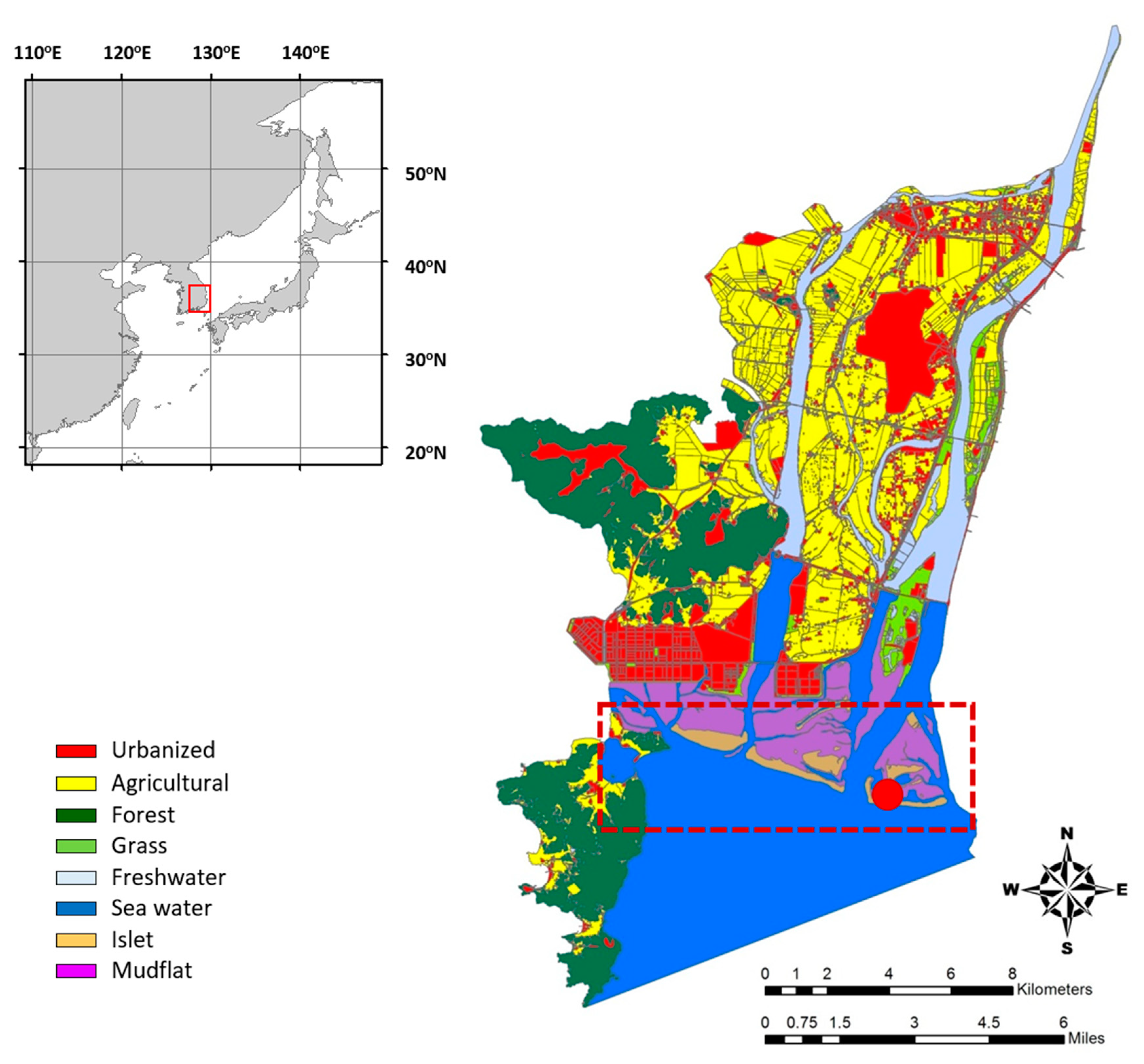
2.1. Study Site Description
2.2. Sample Collection
2.3. DNA Extraction, PCR Amplification, and Cloning
2.4. DNA Sequence Analysis and Statistics
3. Results
3.1. Identification of Prey Species by DNA Barcoding
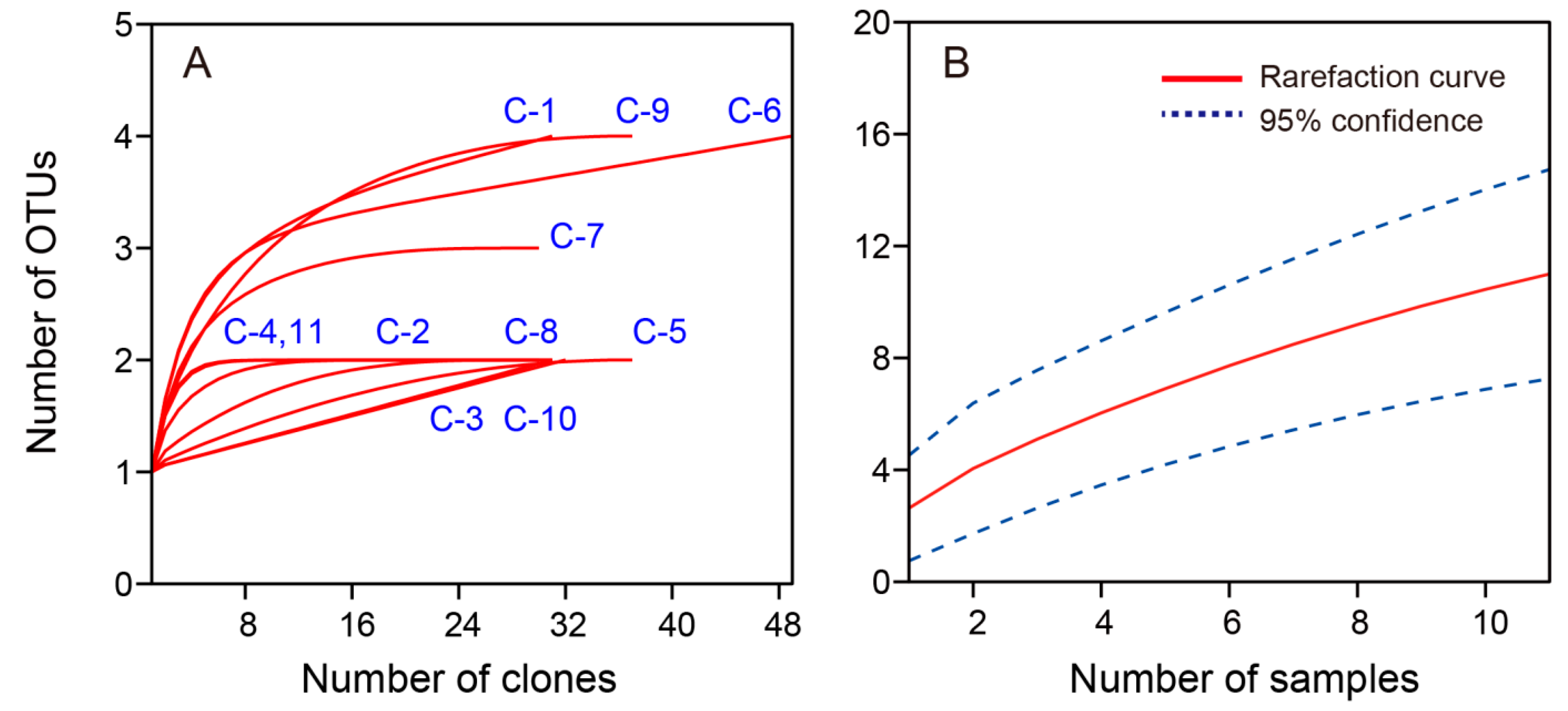
3.2. Relationship between Numbers of Clones and OTUs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jo, H.; Gim, J.A.; Jeong, K.S.; Kim, H.S.; Joo, G.J. Application of DNA barcoding for identification of freshwater carnivorous fish diets: Is number of prey items dependent on size class for Micropterus salmoides? Ecol. Evol. 2014, 4, 219–229. [Google Scholar] [CrossRef]
- Carreon-Martinez, L.; Heath, D.D. Revolution in food web analysis and trophic ecology: Diet analysis by DNA and stable isotope analysis. Mol. Ecol. 2010, 19, 25–27. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kim, S.K.; Chang, K.H.; Kim, M.C.; La, G.H.; Joo, G.J.; Jeong, K.S. Population growth of the cladoceran, Daphnia magna: A quantitative analysis of the effects of different algal food. PLoS ONE 2014, 9, e95591. [Google Scholar] [CrossRef]
- Rosel, P.E.; Kocher, T.D. DNA-based identification of larval cod in stomach contents of predatory fishes. J. Exp. Mar. Biol. Ecol. 2002, 267, 75–88. [Google Scholar] [CrossRef]
- Corse, E.; Costedoat, C.; Chappaz, R.; Pech, N.; Martin, J.F.; Gilles, A. A PCR-based method for diet analysis in freshwater organisms using 18S rDNA barcoding on faeces. Mol. Ecol. Resour. 2010, 10, 96–108. [Google Scholar] [CrossRef]
- Blankenship, L.E.; Yayanos, A.A. Universal primers and PCR of gut contents to study marine invertebrate diets. Mol. Ecol. 2005, 14, 891–899. [Google Scholar] [CrossRef]
- Valentini, A.; Miquel, C.; Nawaz, M.A.; Bellemain, E.V.A.; Coissac, E.; Pompanon, F.; Gielly, L.; Cruaud, C.; Nascetti, G.; Wincker, P.; et al. New perspectives in diet analysis based on DNA barcoding and parallel pyrosequencing: The trnL approach. Mol. Ecol. Resour. 2009, 9, 51–60. [Google Scholar] [CrossRef]
- Pompanon, F.; Deagle, B.; Symondson, W.O.C.; Brown, D.S.; Jarman, S.J.; Taberlet, P. Who is eating what: Diet assessment using next generation sequencing. Mol. Ecol. 2012, 21, 1931–1950. [Google Scholar]
- Jedlicka, J.A.; Sharma, A.M.; Almeida, R.P.P. Molecular tools reveal diets of insectivorous birds from predator fecal matter. Conserv. Genet. Resour. 2013, 5, 879–885. [Google Scholar] [CrossRef]
- Alonso, H.; Granadeiro, J.P.; Waap, S.; Xavier, J.; Symondson, W.O.; Ramos, J.A.; Catry, P. An holistic ecological analysis of the diet of Cory’s shearwaters using prey morphological characters and DNA barcoding. Mol. Ecol. 2014, 23, 3719–3733. [Google Scholar] [CrossRef]
- Jang, J.D. Habitat Usage and Conservation Strategy of Waterbirds in the Nakdong River Estuary. Ph.D. Thesis, Pusan National University, Busan, Korea, 2014. [Google Scholar]
- del Hoyo, J.; Elliott, A.; Sargatal, J. Handbook of the Birds of the World (Vol.3: Hoatzin to Auks); Lynx Edicions: Barcelona, Spain, 1996. [Google Scholar]
- BirdLife International. IUCN Red List for Birds; BirdLife International: Cambridge, UK, 2013. [Google Scholar]
- Bamford, M.; Trust, N.H. Migratory Shorebirds of the East Asian-Australasian Flyway: Population Estimates and Internationally Important Sites; Wetlands International: Wageningen, The Netherlands, 2008. [Google Scholar]
- Jeong, K.S.; Jang, J.D.; Kim, D.K.; Joo, G.J. Waterfowls habitat modeling: Simulation of nest site selection for the migratory Little Tern (Sterna albifrons) in the Nakdong estuary. Ecol. Model. 2011, 222, 3149–3156. [Google Scholar] [CrossRef]
- Jang, J.D.; Chun, S.G.; Kim, K.C.; Jeong, K.Y.; Kim, D.K.; Kim, J.Y.; Joo, G.J.; Jeong, K.S. Long-term adaptations of a migratory bird (Little Tern Sternula albifrons) to quasi-natural flooding disturbance. Ecol. Inform. 2015, 29, 166–173. [Google Scholar] [CrossRef]
- Hong, S.B.; Woo, Y.T.; Higashi, S. Effects of clutch size and egg-laying order on the breeding success in the Little Tern Sterna albifrons on the Nakdong Estuary, Republic of Korea. Ibis 1998, 140, 408–414. [Google Scholar] [CrossRef]
- Lee, C.W.; Jang, J.D.; Jeong, K.S.; Kim, D.K.; Joo, G.J. Patterning habitat preference of avifaunal assemblage on the Nakdong River estuary (South Korea) using self-organizing map. Ecol. Inform. 2010, 5, 89–96. [Google Scholar] [CrossRef]
- Ban, Y.B. A landform change of barrier islands around the Nakdong River Estuary. In Proceedings of the 8th International Symposium on Marine Engineering: Advanced Strategies toward the Green Growth of Marine Engineering Industries, The Korean Society of Marine Engineering, Busan, Korea, 17 June 2009; pp. 452–455. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotech. 1994, 3, 294–299. [Google Scholar]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Benson, D.A.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Wheeler, D.L. GenBank. Nucleic Acids Res. 2005, 33, D34–D38. [Google Scholar] [CrossRef] [PubMed]
- Ratnasingham, S.; Hebert, P.D.N. BOLD: The Barcode of Life Data System (www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Jarman, S.N.; Deagle, B.E.; Gales, N.J. Group-specific polymerase chain reaction for DNA-based analysis of species diversity and identity in dietary samples. Mol. Ecol. 2004, 13, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Clare, E.L.; Fraser, E.E.; Braid, H.E.; Fenton, M.B.; Hebert, P.D.N. Species on the menu of a generalist predator, the eastern red bat (Lasiurus borealis): Using a molecular approach to detect arthropod prey. Mol. Ecol. 2009, 18, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological Statistics Software Package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Hajibabaei, M.; Smith, M.; Janzen, D.H.; Rodriguez, J.J.; Whitfield, J.B.; Hebert, P.D. A minimalist barcode can identify a specimen whose DNA is degraded. Mol. Ecol. Notes 2006, 6, 959–964. [Google Scholar] [CrossRef]
- Deagle, B.E.; Gales, N.J.; Evans, K.; Jarman, S.N.; Robinson, S.; Trebilco, R.; Hindell, M.A. Studying seabird diet through genetic analysis of faeces: A case study on macaroni penguins (Eudyptes chrysolophus). PLoS ONE 2007, 2, e831. [Google Scholar] [CrossRef] [PubMed]
| Phylum | Class (Subclass) | Order | Family | Genus + Species | C-1 | C-2 | C-3 | C-4 | C-5 | C-6 | C-7 | C-8 | C-9 | C-10 | C-11 | Sum | Genebank | QC | ID (%) | IL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chordata | Actinopterygii | Clupeiformes | Engraulidae | Engraulis japonicus | 1 | 1 | AB040676.1 | 99 | 99 | Sp | ||||||||||
| Clupeidae | Clupea pallasii | 11 | 23 | 35 | 9 | 9 | 27 | 25 | 1 | 140 | JF693636.1 | 100 | 100 | Sp | ||||||
| Cypriniformes | Cyprinidae | Hemibarbus labeo | 3 | 3 | HQ536371.1 | 100 | 99 | Sp | ||||||||||||
| Pseudorasbora parva | 1 | 1 | HQ536453.1 | 100 | 98 | Sp | ||||||||||||||
| Perciformes | Centrarchidae | Micropterus salmoides | 15 | 15 | DQ536425.1 | 98 | 99 | Sp | ||||||||||||
| Gobiidae | Tridentiger brevispinis | 7 | 1 | 18 | 2 | 23 | 18 | 7 | 31 | 15 | 122 | HQ536531.1 | 100 | 100 | Sp | |||||
| Arthropoda | Malacostraca | Isopoda | Asellidae | Asellus sp. | 4 | 2 | 16 | 22 | AY531829.1 | 93 | 89 | Ge | ||||||||
| Decapoda | Palaemonidae | Macrobrachium nipponense | 16 | 16 | JN874528.1 | 94 | 99 | Sp | ||||||||||||
| Maxillopoda | Sessilia | Chthamalidae | Chthamalus challengeri | 30 | 3 | 33 | EU304447.1 | 91 | 99 | Sp | ||||||||||
| ETC | Homo sapiens | 13 | 3 | 16 | KF540983.1 | 100 | 99 | Sp | ||||||||||||
| Number of clones | 31 | 30 | 31 | 31 | 37 | 49 | 30 | 30 | 37 | 32 | 31 | 369 | ||||||||
| Number of OTUs | 4 | 2 | 2 | 2 | 2 | 4 | 3 | 2 | 4 | 2 | 2 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.; Jang, J.-D.; Jeong, K.-Y.; Gim, J.-A.; Joo, G.-J.; Jeong, K.-S. Prey Identification of the Little Tern, Sternula albifrons (Pallas, 1764), by Applying DNA Barcoding to Fecal Materials. Sustainability 2022, 14, 11945. https://doi.org/10.3390/su141911945
Jo H, Jang J-D, Jeong K-Y, Gim J-A, Joo G-J, Jeong K-S. Prey Identification of the Little Tern, Sternula albifrons (Pallas, 1764), by Applying DNA Barcoding to Fecal Materials. Sustainability. 2022; 14(19):11945. https://doi.org/10.3390/su141911945
Chicago/Turabian StyleJo, Hyunbin, Ji-Deok Jang, Keon-Young Jeong, Jeong-An Gim, Gea-Jae Joo, and Kwang-Seuk Jeong. 2022. "Prey Identification of the Little Tern, Sternula albifrons (Pallas, 1764), by Applying DNA Barcoding to Fecal Materials" Sustainability 14, no. 19: 11945. https://doi.org/10.3390/su141911945
APA StyleJo, H., Jang, J.-D., Jeong, K.-Y., Gim, J.-A., Joo, G.-J., & Jeong, K.-S. (2022). Prey Identification of the Little Tern, Sternula albifrons (Pallas, 1764), by Applying DNA Barcoding to Fecal Materials. Sustainability, 14(19), 11945. https://doi.org/10.3390/su141911945







