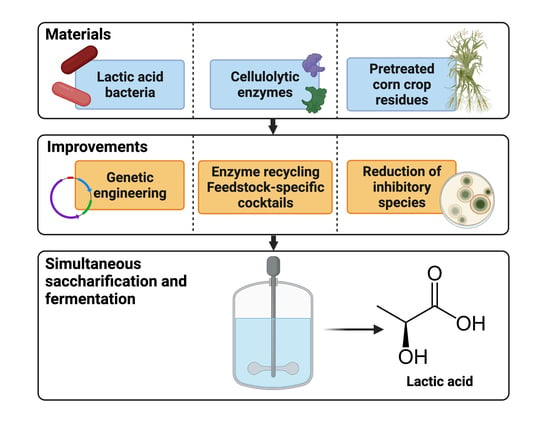
Bioconversion of Corn Crop Residues: Lactic Acid Production through Simultaneous Saccharification and Fermentation
Abstract
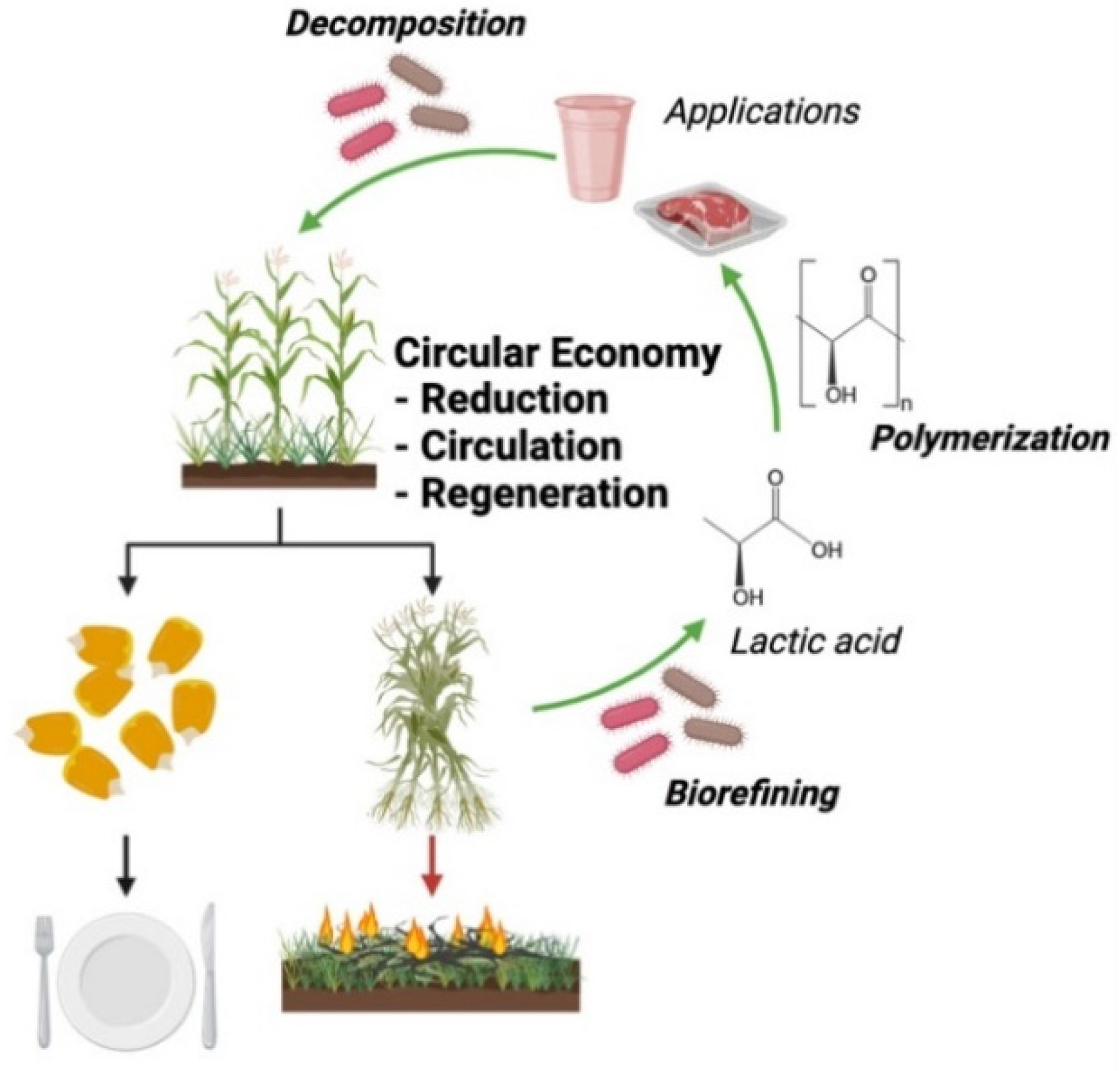
1. Introduction
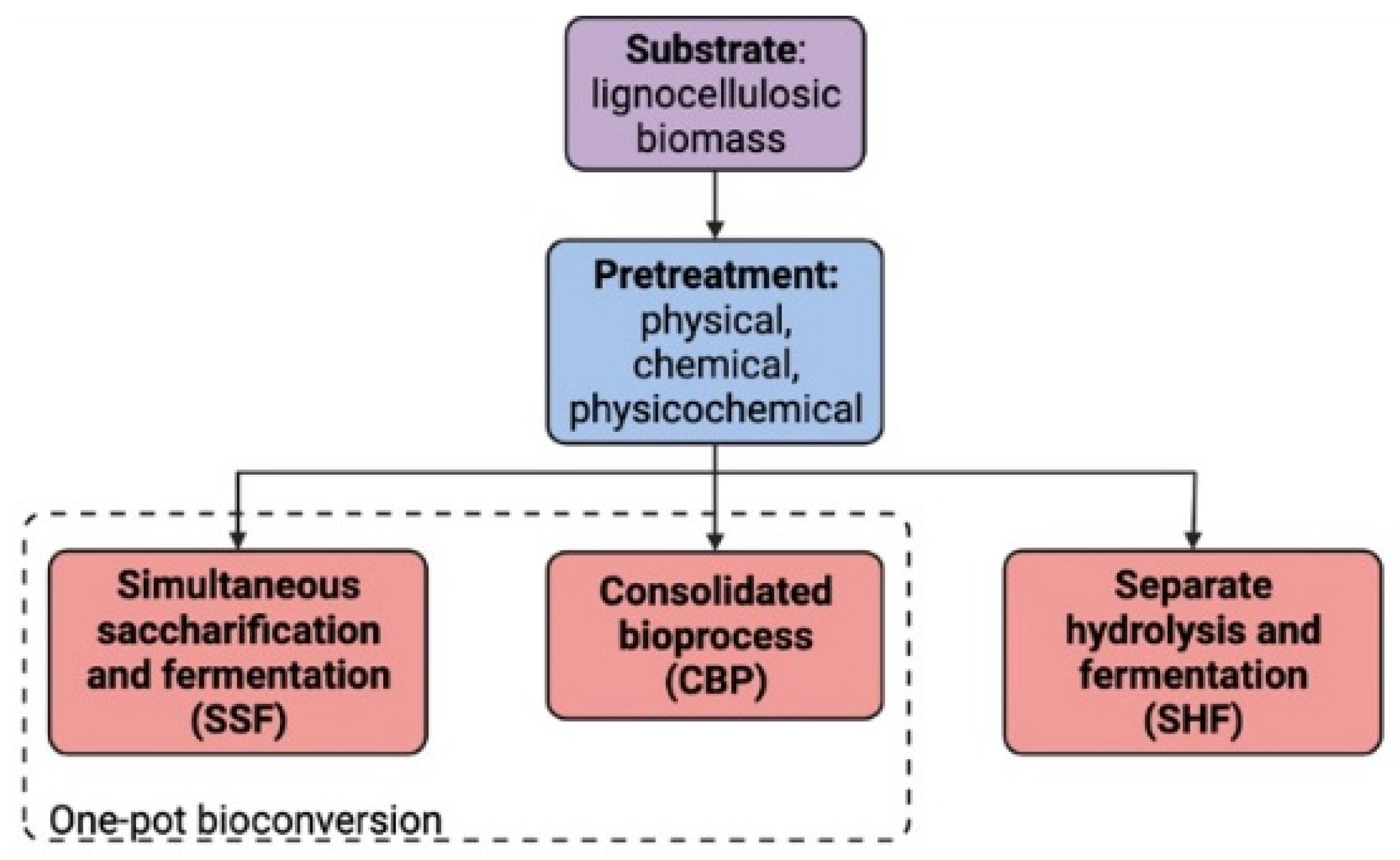
2. Bioconversion Process
2.1. Pretreatment
2.1.1. Physical Pretreatment
2.1.2. Chemical Pretreatment
2.1.3. Physicochemical Pretreatment
2.2. Simultaneous Saccharification and Fermentation
- It avoids the need to physically separate hydrolysate from biomass, an operation that would inevitably lead to sugar loss;
- In comparison to SHF, larger amounts of pretreated LB can be fed into the bioreactor at once;
- Performing two steps simultaneously in the same reactor has a direct impact on reducing overall process duration;
- Investment costs are reduced because fewer reaction vessels are needed;
2.2.1. Enzymatic Hydrolysis
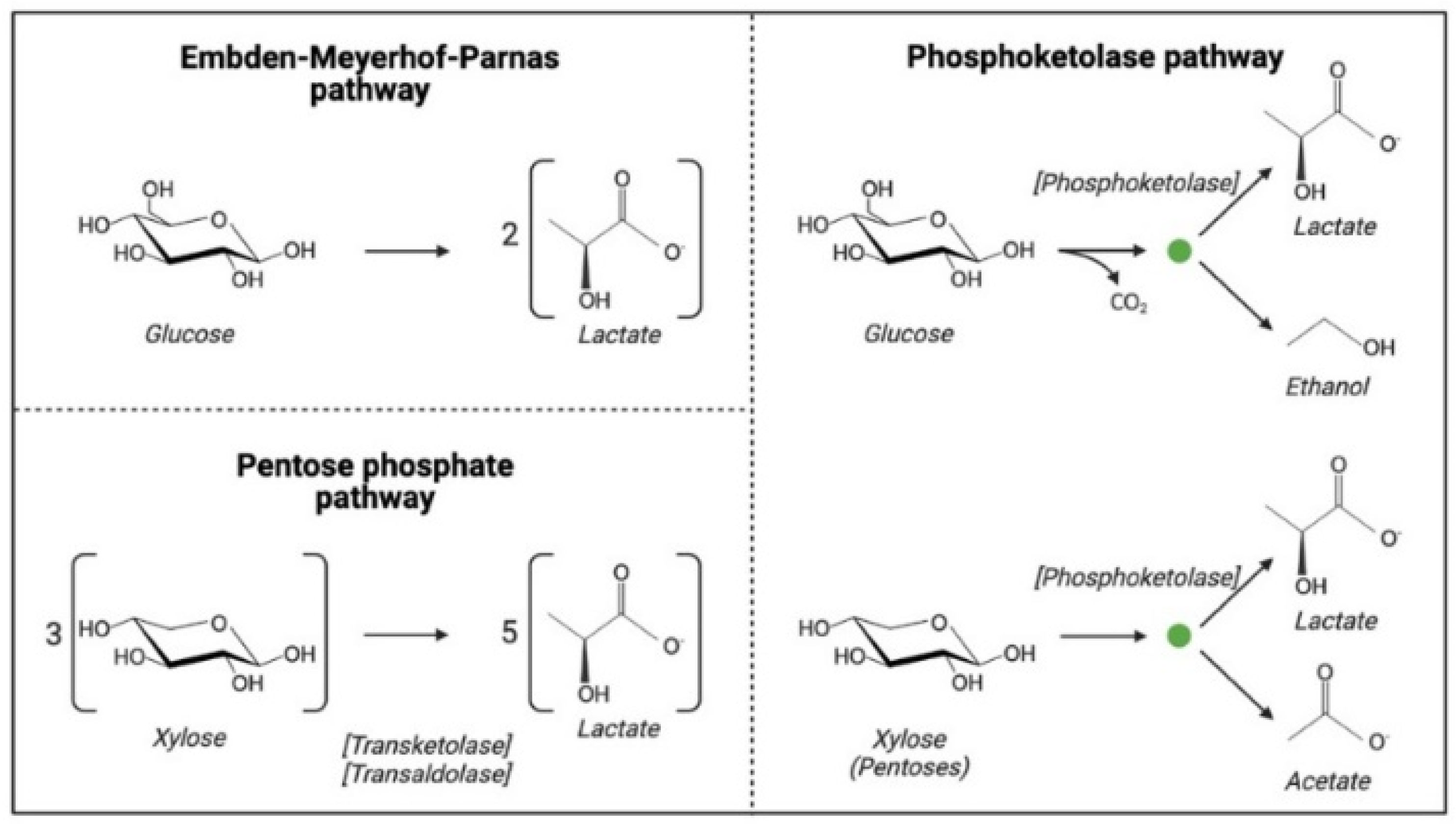
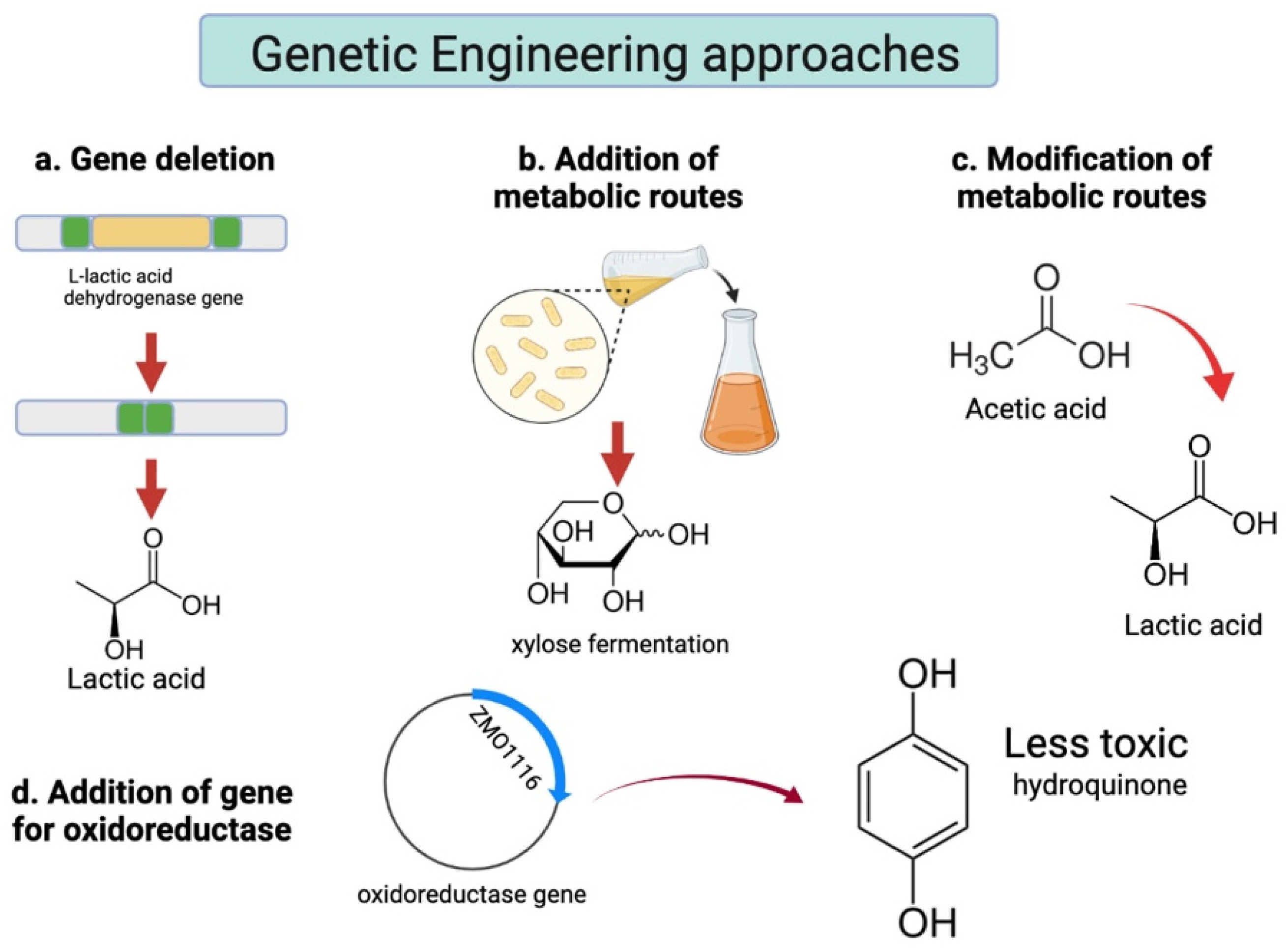
2.2.2. Fermentative Microorganisms
Wild-Type Microorganisms
| Microorganism | Feedstock | Pretreatment a | Saccharification | Fermentation Conditions | Lactic Acid | Xylose Utilization | Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Yield | Titer (g/L) | Productivity (g/L⋅h) | Optical Purity (%) | |||||||
| Bacillus coagulans H-1 | Corncob residue | Dilute acid | Cellulase cocktail (10 U/g cellulose) supplemented with β-glucosidases (15 U/g cellulose) | Batch culture at 50 °C, pH 6.0 and 10% (w/v) solids loading | 0.85 c | 68.0 | 1.89 | 100 g | No | [53] |
| Bacillus coagulans L-LA 1507 | Corn stover | NaOH pretreatment, at 118 °C for 1 h. | Commercial cocktail of cellulases from KDN Group | Sixth subsequent fed-batch cultures with recycled streams, at 50 °C and pH 6.2 | 0.396 b, d | 93.42 e | 2.14 d | N. R. | Yes | [68] |
| Bacillus coagulans MXL-9 | Corn fiber | Dilute acid (H2SO4) pretreatment, at 121°C for 1 h. | Mixture of Celluclast® 1.5 L and Novozyme 188. Saccharification 18 h prior to inoculation | Batch culture at 50°C, pH 6 and 10% (w/v) solids loading | N. R. | 45.6 | 0.21 | >99 g | Yes | [67] |
| Bacillus coagulans LA204 | Corncob | NaOH pretreatment, at 75 °C for 3 h | Cellic® CTec2 at 30 FPU/g DM | Fed-batch culture at 50 °C, pH 6 and 16% (w/w) solids loading | 0.77 b | 122.99 | 1.37 | 98 g | Yes | [32] |
| NH3 for 1 day, and H2O2 for 7 days, without washing step | 0.74 b | 118.60 | 1.32 | 98 g | ||||||
| Bacillus coagulans LA204 | Corn stover | NaOH pretreatment, at 75 °C for 3 h | Cellic® CTec2 at 30 FPU/g DM | Fed-batch culture at 50 °C, pH 6 and 14.4% (w/w) solids loading | 0.68 b | 97.59 | 1.63 | 94.5 f, g | Yes | [59] |
| Bacillus coagulans LA204 | Corncob | NH3 for 2 days, and H2O2 for 7 days and biodetoxification | Enzymes isolated from a Trichoderma viride culture at 2.0 FPU/g DM | Fed-batch culture at 8% (w/w) solids loading | 0.54 b | 64.95 | 0.57 | N. R. | Yes | [57] |
| Pediococcus acidilactici DQ2 | Corn stover | Dry dilute sulphuric acid pretreatment and biodetoxification | Accellerase 1000 at 15 FPU/g DM. Saccharification 8 h prior to inoculation | Batch culture at 48 °C, pH 5.5 and 27% (w/w) solids loading | 77.2 d | 101.9 | 1.06 | 63.4 g | Yes (at specific conditions) | [24] |
| Pediococcus acidilactici PA204 | Corn stover | NaOH pretreatment, at 75 °C for 3 h | Cellic® CTec2 at 30 FPU/g DM | Fed-batch culture at 37 °C, pH 6.0 and 15% (w/w) solids loading | 0.69 b | 104.11 | 1.24 | N. R. | Yes | [38] |
| Lactobacillus pentosus FL0421 | Corn stover | NaOH pretreatment, at 75 °C for 3 h | Cellic® CTec2 at 30 FPU/g DM | Fed-batch culture at 37 °C, pH 6.0 and 14% (w/w) solids loading | 0.66 b | 92.30 | 1.92 | 98.1 g | Yes | [65] |
| Lactobacillus pentosus ATCC 8041 | Corn stover | Aqueous ammonia at 90 °C for 24 h | Spezyme CP cellulase at 5 FPU/g glucan | Fed-batch culture at 37 °C | 65 | 74.8 | 0.7 | N. R. | Yes | [40] |
| Lactobacillus delbrueckii ATCC 9649 | Corn stover | NaOH pretreatment, at 121 °C for 30 min. | Cellic® CTec2 at 8 FPU/g DM | Batch culture at 40 °C, pH 5.5, 4% (w/v) solids loading | 0.58 b | 20.1 | 0.32 | 99.9 h | No | [69] |
| Lactobacillus rhamnosus CECT-288 | Corncob | Autohydrolysis with water at 202 °C | Celluclast® at 30 FPU/g DM | Batch culture at 45 °C and 16.7% (w/v) solids loading | 86.5 d | 86.15 | N.R. | N. R. | N. R. | [41] |
| Lactobacillus rhamnosus + Lactobacillus brevis ATCC367 | Corn stover | NaOH pretreatment, at room temperature for 12 h | Spezyme CP at 25 FPU/g glucan | Batch culture at 37 °C, pH 5 and 3% (w/v) solids loading | 0.70 b | 20.95 | 0.58 | N.D. | Yes (by L. brevis) | [70] |
| Lactobacillus plantarum ATCC 21028 + Lactobacillus brevis ATCC 367 | Corn stover | NaOH pretreatment at 121 °C for 30 min | Cellic ® CTec2 at 8 FPU/g DM | Batch culture at 37 °C, pH 6.0 and 4% (w/v) solids loading | 0.78 b | 31.2 | 0.43 | 56.8 g | Yes (by L. brevis) | [71] |
| Acremonium thermophilus ATCC 24622 + Rhizopus sp. MK-96-1196 | Corncob | Milled only | Cellulase produced on-site by A. thermophilus | Batch culture at 30 °C, initial pH 4.5, aeration rate of 2 vvm and 10% solids loading | N. R. | 24 | N.R. | N.R. | Yes (by Rhizopus sp. MK-96-1196) | [30] |
| Rhizopus oryzae NLX-M-1 | Corncob | NaOH at 85–90 °C for 1 h, and neutralized H2SO4 solution | Cellic ® CTec2 at 60 mg protein/g DM | Batch culture at 40 °C, pH > 6.0 and aeration rate of 1 vvm and 10% (w/v) solids loading | 0.60 b | 60.3 | 1.0 | 100 g | Yes | [74] |
| Trichoderma koningii | Corn straw | Milled and dipped in an ammonia liquor (8%) | Cellulase produced by T. koningii in a citric acid–sodium citrate buffer | Batch culture | 0.204 c | 20.4 | 0.283 | N.R. | Yes | [75] |
Genetically Modified Strains
| Microorganism | Feedstock | Pretreatment | Enzymes | Fermentation Conditions | Lactic Acid | Xylose Utilization | Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Yield | Titer (g/L) | Productivity (g/L⋅h) | Optical Purity (%) | |||||||
| Lactobacillus plantarum NCIMB 8826 𝛥IdhL1 -pLEM-xylAB | Corn stover | NaOH pretreatment at 121 °C for 30 min and water washed | Cellic® CTec2 at 5.6 FPU/g DM | Fed-batch culture at 37 °C and pH 5 | 0.77 a | 61.4 | 0.32 | >99 e, g | Yes g | [60] |
| Pediococcus acidilactici TY112 | Corn stover | Dry dilute sulphuric acid pretreatment and biodetoxification | Cellulase Youtell #6 at 15 FPU/g DM Saccharification 6 h prior to inoculation | Batch culture at 45 °C, pH 5.5 and 30% (w/w) solids loading | 71.5 b | 104.5 | 1.45 | N. R. | No | [23] |
| Pediococcus acidilactici ZY271 | Ensiled corn stover | Dry dilute sulphuric acid pretreatment, biodetoxification and partial saccharification. | Cellic ® CTec2 at 5 mg protein/g DM. Saccharification 6 h prior to inoculation | Batch culture at 42 °C, pH 5.5 and 30% (w/w) solids loading | N. R. | 139.0 | 1.93 f | 99.7 d | Yes g | [33] |
| Pediococcus acidilactici TY112 | Corn stover | Dry dilute sulphuric acid pretreatment and biodetoxification | Cellulase Youtell #6 at 15 FPU/g DM. Saccharification 6 h prior to inoculation | Batch culture at 45 °C, pH 5.5 and 25% (w/w) solids loading | 65 b | 77.66 | 1.06 | 99.89 d, g | No | [34] |
| Pediococcus acidilactici ZP26 | 58 b | 76.76 | 1.02 | 99.3 e, g | No | |||||
| Pediococcus acidilactici ZY15 | Corn stover | Dry dilute sulphuric acid pretreatment and biodetoxification | Cellic ® CTec2 at 10 mg protein/g cellulose. Saccharification 6 h prior to inoculation | Batch culture at 42 °C, pH 5.5 and 25% (w/w) solids loading | 64.7 c | 97.3 | 1.01 | 99.2 e, g | Yes sg | [35] |
| Pediococcus acidilactici ZY15-𝛥ackA2::ZMO1116 | Corn stover | Dry dilute sulphuric acid pretreatment and biodetoxification | Cellic ® CTec2 at 10 mg protein/g cellulose. Saccharification 6 h prior to inoculation | Batch culture at 42 °C, pH 5.5 and 30% (w/w) solids loading | N.R. | 123.8 | N.R. | N.R. | Yes g | [61] |
3. Challenges of Scalability and Trends
3.1. Scientific and Engineering Challenges in Bioconversion
3.1.1. Wastewater Generation
3.1.2. Enzyme Efficiency and Cost
3.2. Challenges Associated with SSF as Bioconversion Platform
4. Feasibility Analysis of SSF of Corn Crop Residues for LA Production
4.1. Technology Readiness Assessment
| Description | Maturity | TRL | Reference | |||
|---|---|---|---|---|---|---|
| Description | General Project | Engineering | Capacity | |||
| SS(C)F of corn crop residues for LA manufacture | Batch culture at high solids loadings Low wastewater generation High optical purity, titer, and bioconversion yield Mini-plant scale fermentation Patents | 5 | 5 | 4 | 3 | [24,33,102,103] |
| Homofermentation of 1G feedstock | Commercial production by NatureWorks LLC, Galactic, Corbion, etc. | 9 | 9 | 9 | 9 | |
4.2. Review on Techno-Economic Evaluation
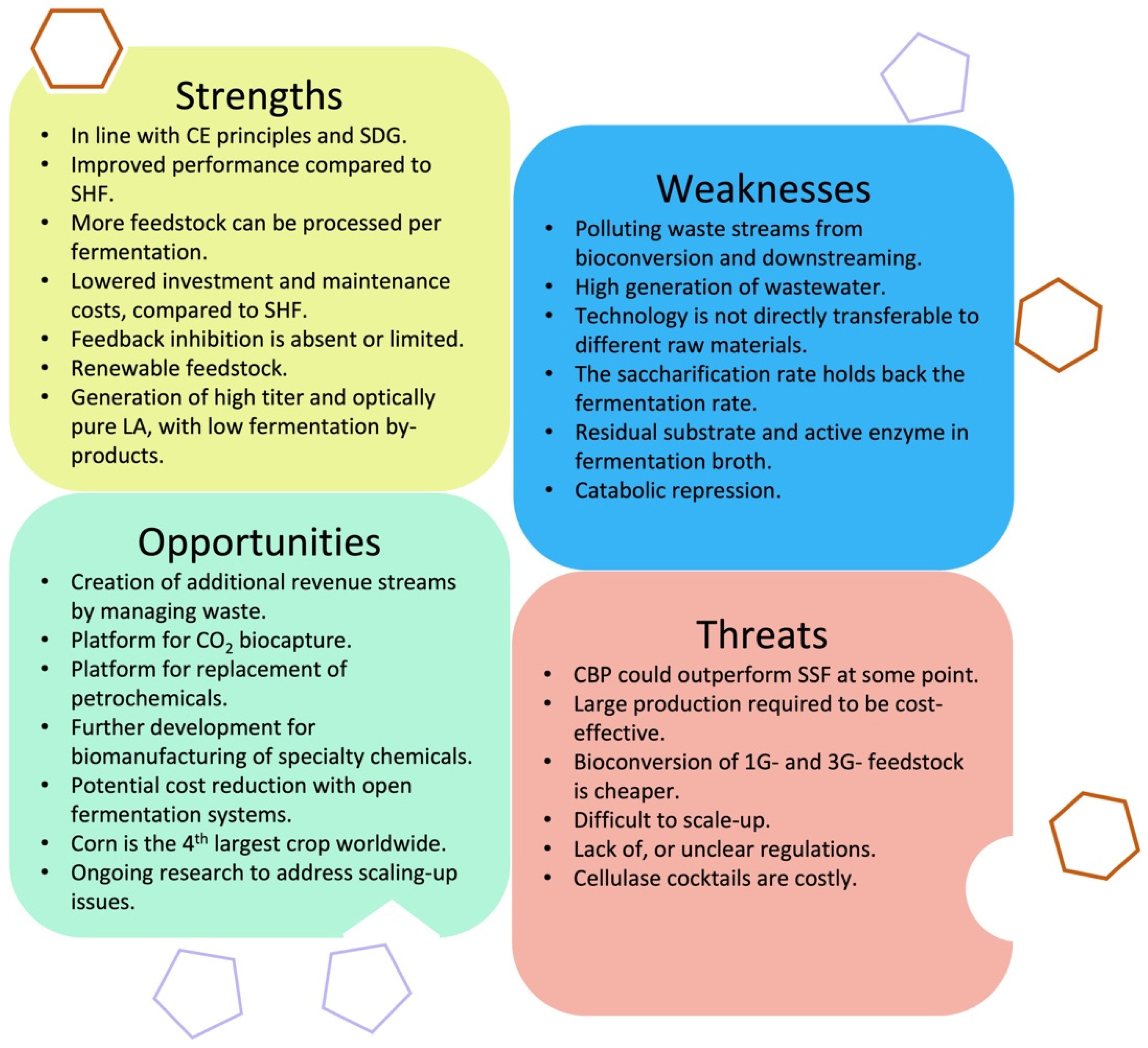
4.3. SWOT Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- The World Bank Agriculture, Forestry and Fishing, Value Added (% of GDP). Available online: https://data.worldbank.org/indicator/NV.AGR.TOTL.ZS (accessed on 30 August 2022).
- Singh, C.K.; Kumar, A.; Roy, S.S. Quantitative analysis of the methane gas emissions from municipal solid waste in India. Sci. Rep. 2018, 8, 2913. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Wang, Z.; Cui, J.; Ferreira, L.F.R.; Bharagava, R.N.; Iqbal, H.M.N. Environmental impact of lignocellulosic wastes and their effective exploitation as smart carriers—A drive towards greener and eco-friendlier biocatalytic systems. Sci. Total Environ. 2020, 722, 137903. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Trinh, L.T.P.; Song, Y.; Lee, Y.G.; Bae, H.-J. Bioconversion of biomass waste into high value chemicals. Bioresour. Technol. 2020, 298, 122386. [Google Scholar] [CrossRef] [PubMed]
- Gunarathne, D.S.; Udugama, I.A.; Jayawardena, S.; Gernaey, K.V.; Mansouri, S.S.; Narayana, M. Resource recovery from bio-based production processes in developing Asia. Sustain. Prod. Consum. 2019, 17, 196–214. [Google Scholar] [CrossRef]
- Ellen Macarthur Foundation Circular Economy Introduction. Available online: https://ellenmacarthurfoundation.org/topics/circular-economy-introduction/overview (accessed on 16 April 2022).
- Kumar, A.; Thakur, A.; Panesar, P.S. Lactic acid and its separation and purification techniques: A review. Rev. Environ. Sci. Biotechnol. 2019, 18, 823–853. [Google Scholar] [CrossRef]
- Tan, J.; Abdel-Rahman, M.A.; Sonomoto, K. Biorefinery-Based Lactic Acid Fermentation: Microbial Production of Pure Monomer Product. Adv. Polym. Sci. 2018, 279, 27–66. [Google Scholar] [CrossRef]
- Castillo Martinez, F.A.; Balciunas, E.M.; Salgado, J.M.; Domínguez González, J.M.; Converti, A.; de S. Oliveira, R.P. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar] [CrossRef]
- Ameen, S.M.; Caruso, G. The Importance of Lactic Acid in the Current Food Industry. An Introduction. In Lactic Acid in the Food Industry; Parisi, S., Ed.; SpringerBriefs in Molecular Science; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–5. ISBN 978-3-319-58144-6. [Google Scholar]
- Rawoof, S.A.A.; Kumar, P.S.; Vo, D.V.N.; Devaraj, K.; Mani, Y.; Devaraj, T.; Subramanian, S. Production of optically pure lactic acid by microbial fermentation: A review. Environ. Chem. Lett. 2020, 18, 539–556. [Google Scholar] [CrossRef]
- Abedi, E.; Mohammad, S.; Hashemi, B. Lactic acid production—Producing microorganisms and substrates sources-state of art. Heliyon 2020, 6, e04974. [Google Scholar] [CrossRef]
- Ahmad, A.; Banat, F.; Taher, H. A review on the lactic acid fermentation from low-cost renewable materials: Recent developments and challenges. Environ. Technol. Innov. 2020, 20, 101138. [Google Scholar] [CrossRef]
- Ajala, E.O.; Olonade, Y.O.; Ajala, M.A.; Akinpelu, G.S. Lactic Acid Production from Lignocellulose—A Review of Major Challenges and Selected Solutions. ChemBioEng Rev. 2020, 7, 38–49. [Google Scholar] [CrossRef]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef]
- Lassalle, V.; Ferreira, M.L. PLA nano- and microparticles for drug delivery: An overview of the methods of preparation. Macromol. Biosci. 2007, 7, 767–783. [Google Scholar] [CrossRef]
- Zhang, Z.; Tsapekos, P.; Alvarado-Morales, M.; Zhu, X.; Zervas, A.; Jacobsen, C.S.; Angelidaki, I. Enhanced fermentative lactic acid production from source-sorted organic household waste: Focusing on low-pH microbial adaptation and bio-augmentation strategy. Sci. Total Environ. 2022, 808, 152129. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Hasunuma, T.; Ogino, C.; Kondo, A. Bioprocessing of bio-based chemicals produced from lignocellulosic feedstocks. Curr. Opin. Biotechnol. 2016, 42, 30–39. [Google Scholar] [CrossRef]
- Carrillo-Nieves, D.; Rostro Alanís, M.J.; de la Cruz Quiroz, R.; Ruiz, H.A.; Iqbal, H.M.N.; Parra-Saldívar, R. Current status and future trends of bioethanol production from agro-industrial wastes in Mexico. Renew. Sustain. Energy Rev. 2019, 102, 63–74. [Google Scholar] [CrossRef]
- Gauss, W.F.; Suzuki, S.; Takagi, M. Manufacture of Alcohol from Cellulosic Materials Using Plural Ferments. U.S. Patent No. 3990944, 1976. [Google Scholar]
- Zaini, N.A.B.M.; Chatzifragkou, A.; Charalampopoulos, D. Microbial production of D-lactic acid from dried distiller’s grains with solubles. Eng. Life Sci. 2019, 19, 21–30. [Google Scholar] [CrossRef]
- Li, Y.; Bhagwat, S.S.; Cortés-Penã, Y.R.; Ki, D.; Rao, C.V.; Jin, Y.S.; Guest, J.S. Sustainable Lactic Acid Production from Lignocellulosic Biomass. ACS Sustain. Chem. Eng. 2021, 9, 1341–1351. [Google Scholar] [CrossRef]
- Liu, G.; Sun, J.; Zhang, J.; Tu, Y.; Bao, J. High titer l-lactic acid production from corn stover with minimum wastewater generation and techno-economic evaluation based on Aspen plus modeling. Bioresour. Technol. 2015, 198, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Qiao, Q.; Chu, D.; Gu, H.; Dao, T.H.; Zhang, J.; Bao, J. Simultaneous saccharification and high titer lactic acid fermentation of corn stover using a newly isolated lactic acid bacterium Pediococcus acidilactici DQ2. Bioresour. Technol. 2013, 135, 481–489. [Google Scholar] [CrossRef]
- FAO FAOSTAT: Crops. Available online: http://www.fao.org/faostat/es/?#data/QC/visualize (accessed on 29 November 2020).
- Qing, Q.; Wyman, C.E. Supplementation with xylanase and β-xylosidase to reduce xylo-oligomer and xylan inhibition of enzymatic hydrolysis of cellulose and pretreated corn stover. Biotechnol. Biofuels 2011, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Zoghlami, A.; Paës, G. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S. A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process. Technol. 2017, 160, 196–206. [Google Scholar] [CrossRef]
- Miura, S.; Arimura, T.; Itoda, N.; Dwiarti, L.; Feng, J.B.; Bin, C.H.; Okabe, M. Production of L-lactic acid from corncob. J. Biosci. Bioeng. 2004, 97, 153–157. [Google Scholar] [CrossRef]
- Oyedeji, O.; Gitman, P.; Qu, J.; Webb, E. Understanding the Impact of Lignocellulosic Biomass Variability on the Size Reduction Process: A Review. ACS Sustain. Chem. Eng. 2020, 8, 2327–2343. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, Y.; He, X.; Li, X.; Hu, J.; Ruan, Z.; Zhao, S.; Peng, N.; Liang, Y. Comparison of high-titer lactic acid fermentation from NaOH-and NH3-H2O2-pretreated corncob by Bacillus coagulans using simultaneous saccharification and fermentation. Sci. Rep. 2016, 6, 37245. [Google Scholar] [CrossRef]
- Han, X.; Hong, F.; Liu, G.; Bao, J. An Approach of Utilizing Water-Soluble Carbohydrates in Lignocellulose Feedstock for Promotion of Cellulosic L-Lactic Acid Production. J. Agric. Food Chem. 2018, 66, 10225–10232. [Google Scholar] [CrossRef]
- Yi, X.; Zhang, P.; Sun, J.; Tu, Y.; Gao, Q.; Zhang, J.; Bao, J. Engineering wild-type robust Pediococcus acidilactici strain for high titer l- and d-lactic acid production from corn stover feedstock. J. Biotechnol. 2016, 217, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Gao, Q.; Bao, J. Constructing xylose-assimilating pathways in Pediococcus acidilactici for high titer D-lactic acid fermentation from corn stover feedstock. Bioresour. Technol. 2017, 245, 1369–1376. [Google Scholar] [CrossRef]
- Li, H.; Ye, C.; Liu, K.; Gu, H.; Du, W.; Bao, J. Analysis of particle size reduction on overall surface area and enzymatic hydrolysis yield of corn stover. Bioprocess Biosyst. Eng. 2015, 38, 149–154. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Zhang, J.; Peng, N.; Liang, Y.; Zhao, S. High-titer lactic acid production by Pediococcus acidilactici PA204 from corn stover through fed-batch simultaneous saccharification and fermentation. Microorganisms 2020, 8, 1491. [Google Scholar] [CrossRef]
- Qing, Q.; Zhou, L.; Guo, Q.; Gao, X.; Zhang, Y.; He, Y.; Zhang, Y. Mild alkaline presoaking and organosolv pretreatment of corn stover and their impacts on corn stover composition, structure, and digestibility. Bioresour. Technol. 2017, 233, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lee, Y.Y.; Elander, R.T. Conversion of aqueous ammonia-treated corn stover to lactic acid by simultaneous saccharification and cofermentation. Appl. Biochem. Biotechnol. 2007, 137–140, 721–738. [Google Scholar] [CrossRef]
- Rivas, B.; Moldes, A.B.; Domínguez, J.M.; Parajó, J.C. Lactic acid production from corn cobs by simultaneous saccharification and fermentation: A mathematical interpretation. Enzyme Microb. Technol. 2004, 34, 627–634. [Google Scholar] [CrossRef]
- Gao, J.; Yang, X.; Wan, J.; He, Y.; Chang, C.; Ma, X.; Bai, J. Delignification Kinetics of Corn Stover with Aqueous Ammonia Soaking Pretreatment. BioResources 2016, 11, 2403–2416. [Google Scholar] [CrossRef]
- Zhao, C.; Shao, Q.; Chundawat, S.P.S. Recent advances on ammonia-based pretreatments of lignocellulosic biomass. Bioresour. Technol. 2020, 298, 122446. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.; Mancini, E.; Garg, N.; Woodley, J.M.; Gernaey, K.V.; Pinelo, M.; Liu, J.; Mansouri, S.S. Sustainable bio-succinic acid production: Superstructure optimization, techno-economic, and lifecycle assessment. Energy Environ. Sci. 2021, 14, 3542–3558. [Google Scholar] [CrossRef]
- Hans, M.; Kumar, S.; Chandel, A.K.; Polikarpov, I. A review on bioprocessing of paddy straw to ethanol using simultaneous saccharification and fermentation. Process Biochem. 2019, 85, 125–134. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Qi, W.; Wang, F.; Li, Q.; Su, R.X.; He, Z.M. Ethanol From Corn Stover Using SSF: An Economic Assessment. Energy Sources Part B Econ. Plan. Policy 2011, 6, 136–144. [Google Scholar] [CrossRef]
- Singh, J.; Kundu, D.; Das, M.; Banerjee, R. Enzymatic Processing of Juice from Fruits/Vegetables: An Emerging Trend and Cutting Edge Research in Food Biotechnology; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128132807. [Google Scholar]
- Sindhu, R.; Binod, P.; Pandey, A. Biological pretreatment of lignocellulosic biomass—An overview. Bioresour. Technol. 2016, 199, 76–82. [Google Scholar] [CrossRef]
- Sun, F.F.; Hong, J.; Hu, J.; Saddler, J.N.; Fang, X.; Zhang, Z.; Shen, S. Accessory enzymes influence cellulase hydrolysis of the model substrate and the realistic lignocellulosic biomass. Enzyme Microb. Technol. 2015, 79–80, 42–48. [Google Scholar] [CrossRef]
- Okeke, B.C.; Obi, S.K.C. Lignocellulose and sugar compositions of some agro-waste materials. Bioresour. Technol. 1994, 47, 283–284. [Google Scholar] [CrossRef]
- Van Dyk, J.S.; Pletschke, B.I. A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes—Factors affecting enzymes, conversion and synergy. Biotechnol. Adv. 2012, 30, 1458–1480. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Chang, Y.; Lee, D.J. Enzymatic saccharification of lignocellulosic biorefinery: Research focuses. Bioresour. Technol. 2018, 252, 198–215. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, P.; Tao, F. l-Lactic acid production by Bacillus coagulans through simultaneous saccharification and fermentation of lignocellulosic corncob residue. Bioresour. Technol. Rep. 2019, 6, 131–137. [Google Scholar] [CrossRef]
- Contreras, F.; Pramanik, S.; Rozhkova, A.M.; Zorov, I.N.; Korotkova, O.; Sinitsyn, A.P.; Schwaneberg, U.; Davari, M.D. Engineering robust cellulases for tailored lignocellulosic degradation cocktails. Int. J. Mol. Sci. 2020, 21, 1589. [Google Scholar] [CrossRef]
- Culbertson, A.; Jin, M.; Da Costa Sousa, L.; Dale, B.E.; Balan, V. In-house cellulase production from AFEXTM pretreated corn stover using Trichoderma reesei RUT C-30. RSC Adv. 2013, 3, 25960–25969. [Google Scholar] [CrossRef]
- Balan, V.; Jin, M.; Culbertson, A.; Uppugundla, N. The Saccharification Step: Trichoderma Reesei Cellulase Hyper Producer Strains. In Lignocellulose Conversion; Springer: Berlin, Heidelberg, 2013; pp. 65–91. ISBN 9783642378614. [Google Scholar]
- Liu, J.; Cai, Y.; Liu, L.; Zhan, R.; Zhao, S.; Liang, Y.; Peng, N.; Zhu, J.; Li, H.; Xiao, N.; et al. Enhanced lactic acid production by Bacillus coagulans through simultaneous saccharification, biodetoxification, and fermentation. Biofuels Bioprod. Biorefining 2020, 14, 533–543. [Google Scholar] [CrossRef]
- Yang, J.; Kim, J.E.; Kim, J.K.; Lee, S.H.; Yu, J.H.; Kim, K.H. Evaluation of commercial cellulase preparations for the efficient hydrolysis of hydrothermally pretreated empty fruit bunches. BioResources 2017, 12, 7834–7840. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Z.; Lin, Y.; Zhao, S.; Mei, Y.; Liang, Y.; Peng, N. High-titer lactic acid production from NaOH-pretreated corn stover by Bacillus coagulans LA204 using fed-batch simultaneous saccharification and fermentation under non-sterile condition. Bioresour. Technol. 2015, 182, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Vadlani, P.V.; Kumar, A.; Hardwidge, P.R.; Govind, R.; Tanaka, T.; Kondo, A. Enhanced D-lactic acid production from renewable resources using engineered Lactobacillus plantarum. Appl. Microbiol. Biotechnol. 2016, 100, 279–288. [Google Scholar] [CrossRef]
- Qiu, Z.; Fang, C.; He, N.; Bao, J. An oxidoreductase gene ZMO1116 enhances the p-benzoquinone biodegradation and chiral lactic acid fermentability of Pediococcus acidilactici. J. Biotechnol. 2020, 323, 231–237. [Google Scholar] [CrossRef]
- Chekushina, A.V.; Dotsenko, G.S.; Sinitsyn, A.P. Comparing the efficiency of plant material bioconversion processes using biocatalysts based on Trichoderma and Penicillium verruculosum enzyme preparations. Catal. Ind. 2013, 5, 98–104. [Google Scholar] [CrossRef]
- Salvetti, E.; Harris, H.M.B.; Felis, G.E.; O’Toole, P.W. Comparative genomics of the genus Lactobacillus reveals robust phylogroups that provide the basis for reclassification. Appl. Environ. Microbiol. 2018, 84, AEM-00993. [Google Scholar] [CrossRef] [PubMed]
- Salvetti, E.; Torriani, S.; Felis, G.E. The Genus Lactobacillus: A Taxonomic Update. Probiotics Antimicrob. Proteins 2012, 4, 217–226. [Google Scholar] [CrossRef]
- Hu, J.; Lin, Y.; Zhang, Z.; Xiang, T.; Mei, Y.; Zhao, S.; Liang, Y.; Peng, N. High-titer lactic acid production by Lactobacillus pentosus FL0421 from corn stover using fed-batch simultaneous saccharification and fermentation. Bioresour. Technol. 2016, 214, 74–80. [Google Scholar] [CrossRef]
- Altuntas, E.G.; Cosansu, S.; Ayhan, K. Some growth parameters and antimicrobial activity of a bacteriocin-producing strain Pediococcus acidilactici 13. Int. J. Food Microbiol. 2010, 141, 28–31. [Google Scholar] [CrossRef]
- Bischoff, K.M.; Liu, S.; Hughes, S.R.; Rich, J.O. Fermentation of corn fiber hydrolysate to lactic acid by the moderate thermophile Bacillus coagulans. Biotechnol. Lett. 2010, 32, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Su, Z.; Wang, Y.; Wang, B.; Si, Z.; Lu, J.; Su, C.; Ren, W.; Chen, H.; Cai, D.; et al. Lactic acid production from pretreated corn stover with recycled streams. Process Biochem. 2020, 91, 132–140. [Google Scholar] [CrossRef]
- Zhang, Y.; Vadlani, P.V. D-lactic acid biosynthesis from biomass-derived sugars via lactobacillus delbrueckii fermentation. Bioprocess Biosyst. Eng. 2013, 36, 1897–1904. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Li, Y.; Wan, C. Lactic acid production from corn stover using mixed cultures of Lactobacillus rhamnosus and Lactobacillus brevis. Bioresour. Technol. 2011, 102, 1831–1836. [Google Scholar] [CrossRef]
- Zhang, Y.; Vadlani, P.V. Lactic acid production from biomass-derived sugars via co-fermentation of Lactobacillus brevis and Lactobacillus plantarum. J. Biosci. Bioeng. 2015, 119, 694–699. [Google Scholar] [CrossRef]
- Maheshwari, R.; Bharadwaj, G.; Bhat, M.K. Thermophilic Fungi: Their Physiology and Enzymes. Microbiol. Mol. Biol. Rev. 2000, 64, 461–488. [Google Scholar] [CrossRef]
- Bhatt, S.M. Economical Lactic Acid Production and Optimization Strategies. In Fungal Biorefineries; Springer: Cham, Switzerland, 2018; pp. 85–105. [Google Scholar]
- Zhang, L.; Li, X.; Yong, Q.; Yang, S.T.; Ouyang, J.; Yu, S. Simultaneous saccharification and fermentation of xylo-oligosaccharides manufacturing waste residue for l-lactic acid production by Rhizopus oryzae. Biochem. Eng. J. 2015, 94, 92–99. [Google Scholar] [CrossRef]
- Wang, Q.; Zou, D.; Ma, H.; Ji, Y.; Wang, X. Simultaneous Saccharification and Fermentation of Corn Straw to Lactic Acid. Chem. Biochem. Eng. Q. 2010, 24, 371–376. [Google Scholar]
- Tripathi, R.M.; Gupta, R.K.; Shrivastav, A.; Singh, M.P.; Shrivastav, B.R.; Singh, P. Trichoderma koningii assisted biogenic synthesis of silver nanoparticles and evaluation of their antibacterial activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 35005. [Google Scholar] [CrossRef]
- Okano, K.; Zhang, Q.; Shinkawa, S.; Yoshida, S.; Tanaka, T.; Fukuda, H.; Kondo, A. Efficient production of optically pure D-lactic acid from raw corn starch by using a genetically modified L-lactate dehydrogenase gene-deficient and α-amylase-secreting Lactobacillus plantarum strain. Appl. Environ. Microbiol. 2009, 75, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Gao, Q.; Bao, J. Engineering Pediococcus acidilactici with xylose assimilation pathway for high titer cellulosic L-lactic acid fermentation. Bioresour. Technol. 2018, 249, 9–15. [Google Scholar] [CrossRef]
- Mahendrasinh Kosamia, N.; Samavi, M.; Piok, K.; Kumar Rakshit, S. Perspectives for scale up of biorefineries using biochemical conversion pathways: Technology status, techno-economic, and sustainable approaches. Fuel 2022, 324, 124532. [Google Scholar] [CrossRef]
- Ouyang, J.; Ma, R.; Zheng, Z.; Cai, C.; Zhang, M.; Jiang, T. Open fermentative production of l-lactic acid by Bacillus sp. strain NL01 using lignocellulosic hydrolyzates as low-cost raw material. Bioresour. Technol. 2013, 135, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, Z.; Wang, X.; Wang, N.; Wang, W.; Bao, J. Biodetoxification of toxins generated from lignocellulose pretreatment using a newly isolated fungus, Amorphotheca resinae ZN1, and the consequent ethanol fermentation. Biotechnol. Biofuels 2010, 3, 26. [Google Scholar] [CrossRef]
- Liu, T.; Williams, D.L.; Pattathil, S.; Li, M.; Hahn, M.G.; Hodge, D.B. Coupling alkaline pre-extraction with alkaline-oxidative post-treatment of corn stover to enhance enzymatic hydrolysis and fermentability. Biotechnol. Biofuels 2014, 7, 48. [Google Scholar] [CrossRef]
- Mansouri, S.S.; Udugama, I.A.; Cignitti, S.; Mitic, A.; Flores-Alsina, X.; Gernaey, K.V. Resource recovery from bio-based production processes: A future necessity? Curr. Opin. Chem. Eng. 2017, 18, 1–9. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kumar, B.; Agrawal, K.; Verma, P. Current perspective on production and applications of microbial cellulases: A review. Bioresour. Bioprocess. 2021, 8, 95. [Google Scholar] [CrossRef]
- Teter, S.A. DECREASE Final Technical Report: Development of a Commercial Ready Enzyme Application System for Ethanol; U.S. Department of Energy Office of Scientific and Technical Information: Golden, CO, USA, 2012.
- Yarbrough, J.M.; Mittal, A.; Mansfield, E.; Taylor, L.E.; Hobdey, S.E.; Sammond, D.W.; Bomble, Y.J.; Crowley, M.F.; Decker, S.R.; Himmel, M.E.; et al. New perspective on glycoside hydrolase binding to lignin from pretreated corn stover. Biotechnol. Biofuels 2015, 8, 214. [Google Scholar] [CrossRef]
- Gao, D.; Chundawat, S.P.S.; Sethi, A.; Balan, V.; Gnanakaran, S.; Dale, B.E. Increased enzyme binding to substrate is not necessary for more efficient cellulose hydrolysis. Proc. Natl. Acad. Sci. USA 2013, 110, 10922–10927. [Google Scholar] [CrossRef]
- Nemmaru, B.; Ramirez, N.; Farino, C.J.; Yarbrough, J.M.; Kravchenko, N.; Chundawat, S.P.S. Reduced type-A carbohydrate-binding module interactions to cellulose I leads to improved endocellulase activity. Biotechnol. Bioeng. 2021, 118, 1141–1151. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Raj, T.; Tsai, M.-L.; Chen, C.-W.; Dong, C. Advances and Challenges in Biocatalysts Application for High Solid-Loading of Biomass for 2nd Generation Bio-Ethanol Production. Catalysts 2022, 12, 615. [Google Scholar] [CrossRef]
- Huang, R.; Guo, H.; Su, R.; Qi, W.; He, Z. Enhanced cellulase recovery without β-glucosidase supplementation for cellulosic ethanol production using an engineered strain and surfactant. Biotechnol. Bioeng. 2017, 114, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Arantes, V.; Pribowo, A.; Gourlay, K.; Saddler, J.N. Substrate factors that influence the synergistic interaction of AA9 and cellulases during the enzymatic hydrolysis of biomass. Energy Environ. Sci. 2014, 7, 2308–2315. [Google Scholar] [CrossRef]
- Hu, J.; Chandra, R.; Arantes, V.; Gourlay, K.; Susan van Dyk, J.; Saddler, J.N. The addition of accessory enzymes enhances the hydrolytic performance of cellulase enzymes at high solid loadings. Bioresour. Technol. 2015, 186, 149–153. [Google Scholar] [CrossRef]
- Yenenler, A.; Sezerman, O.U. Design and characterizations of two novel cellulases through single-gene shuffling of Cel12A (EG3) gene from Trichoderma reseei. Protein Eng. Des. Sel. 2016, 29, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Strobel, K.L.; Pfeiffer, K.A.; Blanch, H.W.; Clark, D.S. Engineering Cel7A carbohydrate binding module and linker for reduced lignin inhibition. Biotechnol. Bioeng. 2016, 113, 1369–1374. [Google Scholar] [CrossRef]
- Kari, J.; Molina, G.A.; Schaller, K.S.; Schiano-di-Cola, C.; Christensen, S.J.; Badino, S.F.; Sørensen, T.H.; Røjel, N.S.; Keller, M.B.; Sørensen, N.R.; et al. Physical constraints and functional plasticity of cellulases. Nat. Commun. 2021, 12, 3847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Cao, C.; Bi, J.; Li, Y. Fungal cellulases: Protein engineering and post-translational modifications. Appl. Microbiol. Biotechnol. 2022, 106, 1–24. [Google Scholar] [CrossRef]
- Olofsson, K.; Bertilsson, M.; Lidén, G. A short review on SSF—An interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol. Biofuels 2008, 1, 7. [Google Scholar] [CrossRef]
- Althuri, A.; Chintagunta, A.D.; Sherpa, K.C.; Banerjee, R. Simultaneous Saccharification and Fermentation of Lignocellulosic Biomass. In Biorefining of Biomass to Biofuels; Springer: Cham, Switzerland, 2018; pp. 265–285. [Google Scholar] [CrossRef]
- Cannella, D.; Jørgensen, H. Do new cellulolytic enzyme preparations affect the industrial strategies for high solids lignocellulosic ethanol production? Biotechnol. Bioeng. 2014, 111, 59–68. [Google Scholar] [CrossRef]
- Müller, G.; Kalyani, D.C.; Horn, S.J. LPMOs in cellulase mixtures affect fermentation strategies for lactic acid production from lignocellulosic biomass. Biotechnol. Bioeng. 2017, 114, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Buchner, G.A.; Stepputat, K.J.; Zimmermann, A.W.; Schomäcker, R. Specifying Technology Readiness Levels for the Chemical Industry. Ind. Eng. Chem. Res. 2019, 58, 6957–6969. [Google Scholar] [CrossRef]
- Wei, C.; Liu, G.; Zhang, J.; Bao, J. Elevating fermentation yield of cellulosic lactic acid in calcium lactate form from corn stover feedstock. Ind. Crops Prod. 2018, 126, 415–420. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, L.; Shi, W. Method for Producing L-Lactic Acid by Simultaneous Saccharification and Fermentation 2021. Available online: https://patents.google.com/patent/CN102776248A/en (accessed on 25 July 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malacara-Becerra, A.; Melchor-Martínez, E.M.; Sosa-Hernández, J.E.; Riquelme-Jiménez, L.M.; Mansouri, S.S.; Iqbal, H.M.N.; Parra-Saldívar, R. Bioconversion of Corn Crop Residues: Lactic Acid Production through Simultaneous Saccharification and Fermentation. Sustainability 2022, 14, 11799. https://doi.org/10.3390/su141911799
Malacara-Becerra A, Melchor-Martínez EM, Sosa-Hernández JE, Riquelme-Jiménez LM, Mansouri SS, Iqbal HMN, Parra-Saldívar R. Bioconversion of Corn Crop Residues: Lactic Acid Production through Simultaneous Saccharification and Fermentation. Sustainability. 2022; 14(19):11799. https://doi.org/10.3390/su141911799
Chicago/Turabian StyleMalacara-Becerra, Alonso, Elda M. Melchor-Martínez, Juan Eduardo Sosa-Hernández, L. María Riquelme-Jiménez, Seyed Soheil Mansouri, Hafiz M. N. Iqbal, and Roberto Parra-Saldívar. 2022. "Bioconversion of Corn Crop Residues: Lactic Acid Production through Simultaneous Saccharification and Fermentation" Sustainability 14, no. 19: 11799. https://doi.org/10.3390/su141911799
APA StyleMalacara-Becerra, A., Melchor-Martínez, E. M., Sosa-Hernández, J. E., Riquelme-Jiménez, L. M., Mansouri, S. S., Iqbal, H. M. N., & Parra-Saldívar, R. (2022). Bioconversion of Corn Crop Residues: Lactic Acid Production through Simultaneous Saccharification and Fermentation. Sustainability, 14(19), 11799. https://doi.org/10.3390/su141911799